Introduction
Breast cancer is the most commonly diagnosed invasive cancer among women both globally and within the United States and the number one cause of cancer-related death among women globally [1, 2]. Less than one percent of diagnosed breast cancers occur in men [2] and, therefore, male breast cancer is not included in this report. Breast cancer is an etiologically and clinically heterogeneous disease. Many risk factors, primarily hormone related, have been identified and these associations can vary by breast cancer subtype. Survival has increased over the past few decades, with the introduction of screening mammography and improved treatments. However, progress has not been seen equally among all ethnicities/ races or with all breast cancer subtypes (e.g., triple-negative).
Descriptive Epidemiology
Incidence
Breast cancer accounts for 25% of new cancer cases in women globally, with an estimated 2,088,849 female breast cancer cancers occurring worldwide in 2018 (46.3 per 100,000 women) [1, 3]. Based on available data, incidence rates are highest in Australia, New Zealand, much of Europe, and North America, intermediate in South America and Eastern Europe, and lowest in the majority of Asia and Africa (Figure 1) [3]. Within the United States, breast cancer accounts for 30% of female cancer diagnoses with an estimated 268,600 new invasive breast cancers and an additional 62,930 cases of in situ breast cancer documented in 2019 (124.7 per 100,000 women) [2]. One in eight women will be diagnosed with breast cancer during their lifetime [2]. Diagnosis is rare before the age of 40 (probability <1%), after which incidence rates increase until about age 70 (median age at diagnosis: 62 years), before decreasing (Figure 2) [2, 4].
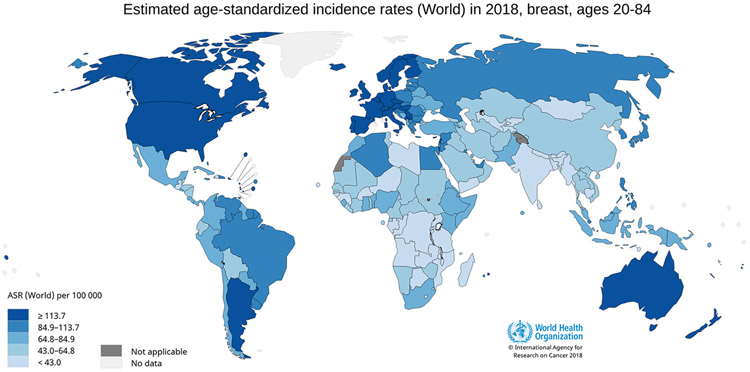
Figure 1.
World age-standardized female breast cancer incidence rates for ages 20-84. Figure 1 shows age-standardized incidence rates for female breast cancer worldwide using data from GLOBOCAN, 2018. Breast cancer incidence is highest in Australia, New Zealand, Northern Europe, and North America, intermediate in Central and South America and Eastern Europe, and lowest in the majority of Asia and Africa. Data source: GLOBOCAN, 2018; Graph production: IARC (http://gco.iarc.fr/today) World Health Organization.
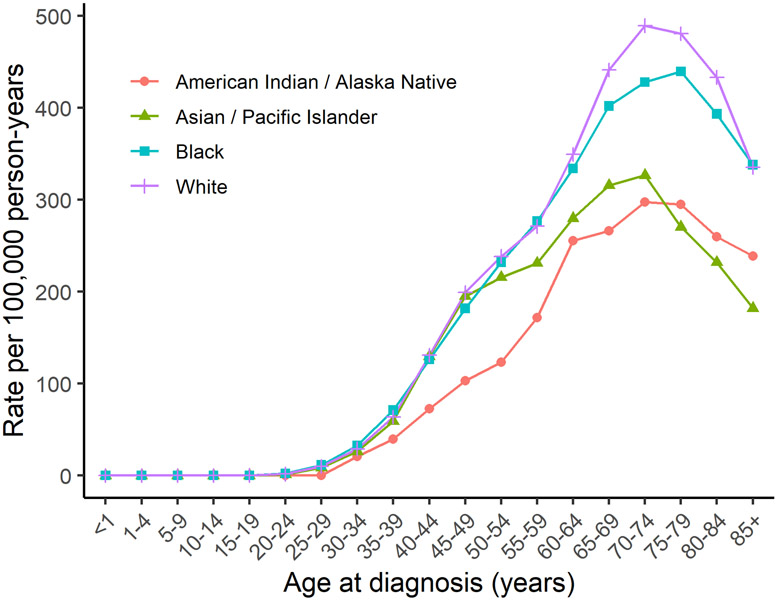
Figure 2.
United States female breast cancer incidence rates by age of diagnosis and race/ethnicity. Figure 2 shows breast cancer incidence rates by age of diagnosis in the United States among White, Black, American Indian/ Alaskan Native, and Asian/ Pacific Islander women using data from the SEER program (SEER 21, 2013-2017).
In many westernized countries including the United States (Figure 3), breast cancer incidence rates increased during the 1980s and 1990s due to changes in reproductive patterns, hormone therapy use, and increased mammographic screening [1, 5]. Incidence rates then dropped in the early 2000s, particularly among women over 45 and for estrogen receptor-positive (ER) breast tumors, following a decline in hormone therapy use after the publication of the Women’s Health Initiative findings and declines in mammography screening rates [1, 5-9]. Since 2004, incidence rates in the US have been increasing slowly (0.3% per year), potentially due to increasing obesity and declining birth rates [4, 5]. Increases in ER+ tumors, particularly in situ, and decreases in ER− tumors are projected to continue according to forecasting models [9-11]. However, incidence rates have continued to decline or stabilize in multiple other westernized countries (e.g., Canada, UK, France, Australia) [1, 8]. In contrast, incidence rates have been rapidly increasing in historically lower risk areas (e.g., Latin America, Africa, Asia) likely from increased life expectancy due to reductions in infectious diseases, increasing prevalence of overweight and obesity, changes in reproductive patterns, and increased breast cancer screening [1, 8].
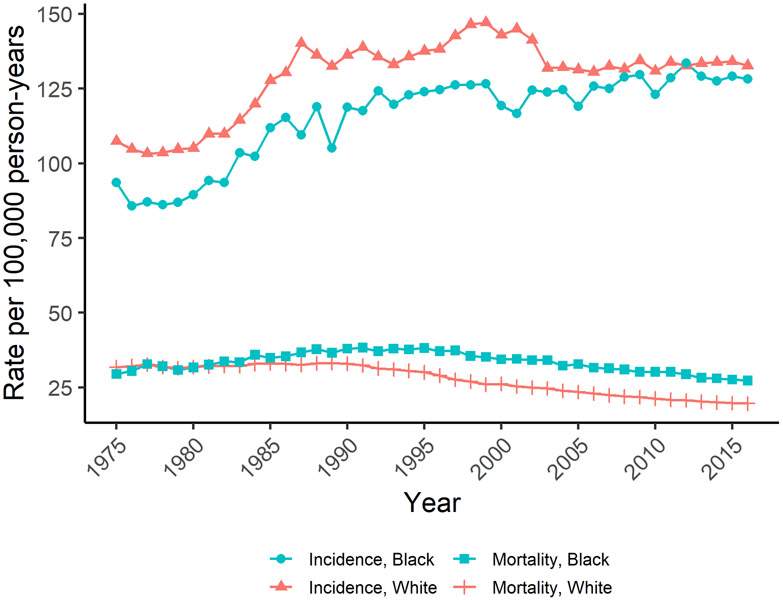
Figure 3.
United States trends in age-adjusted female breast cancer incidence and mortality. Figure 3 shows trends in age-adjusted breast cancer incidence and mortality among White and Black United States women using data from the SEER program (SEER 9, 1975-2016).
Mortality
Breast cancer is the most common cancer death in women globally, accounting for 15% of cancer deaths, with an estimated 626,700 breast cancer deaths among women in 2018 (13.0 per 100,000 women) [1, 3]. Mortality rates are lowest in Eastern Asia (8.6 per 100,000 women) and highest in Fiji (36.9 per 100,000 women) (Figure 4) [3, 12]. In the US, where the lifetime risk of dying from breast cancer is 1 in 39 women, an estimated 41,760 women will die from breast cancer in 2019 (12.7 per 100,000 women) [5, 12]. Mortality rates in the US increased between 1975 and 1989, then decreased through 2017 due to improvements in detection and treatment (Figure 3) [5, 13]. Similar trends have been observed in Canada and European countries [1, 8], whereas mortality rates have increased in Asia, Africa, and Latin America [1, 8].
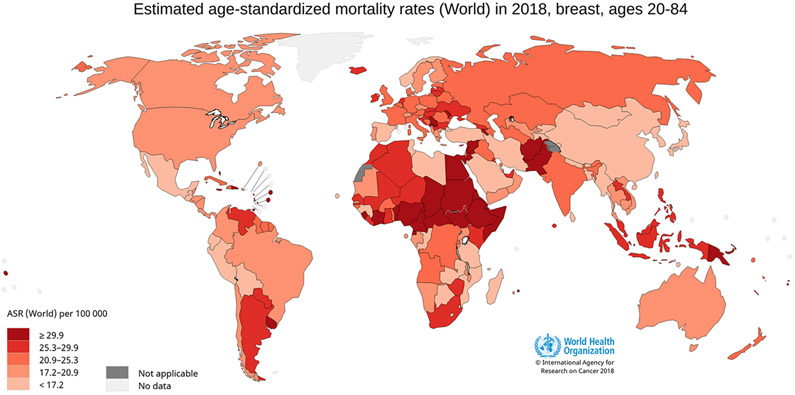
Figure 4.
World age-standardized female breast cancer mortality rates for ages 20-84. Figure 4 shows age-standardized mortality rates for female breast cancer worldwide using data from GLOBOCAN, 2018. Breast cancer mortality is highest in parts of Africa and South-Eastern Asia, intermediate in Europe, and lowest in Eastern Asia. Data source: GLOBOCAN, 2018; Graph production: IARC (http://gco.iarc.fr/today) World Health Organization.
Survival among women with breast cancer
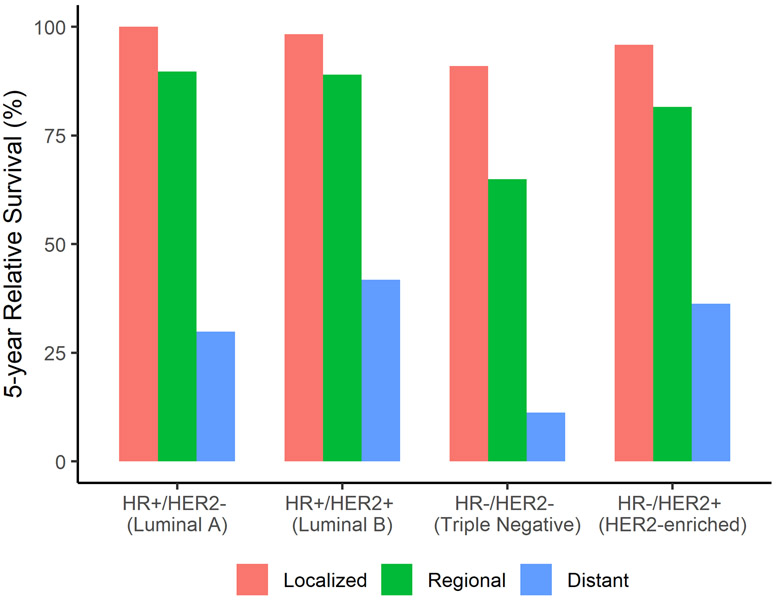
In the US, the 5-year relative survival is 91%, and after 10 and 15 years, the survival rates are 84% and 80%, respectively, for all stages combined [5]. The 5-year survival rate is 99% when the tumor is diagnosed at a local stage, 86% at a regional stage, and 17% when metastatic [5]. Survival rates by stage and subtype are shown in Figure 5. Survival rates in the US have been increasing over time (74.8% in 1975 vs. 91.3% in 2015) likely due to earlier detection through mammographic screening and improved treatments such as the use of more targeted therapies [4, 5, 13-16].
Figure 5.
United States five-year relative female breast cancer subtype survival rates by stage at diagnosis. Figure 5 shows female breast cancer five-year relative survival rates for women at local, regional, and distant stages for each breast cancer subtype using data from the SEER program (SEER 18, 2000-2015).
Etiologic Heterogeneity
Breast cancer is a heterogeneous disease with considerable genetic and clinical heterogeneity [17]. Breast cancers, the majority of which are adenocarcinomas, are often classified by invasiveness (i.e., in situ or invasive), morphology, expression of immunohistochemical markers, and more recently through genetic panels. In turn, these features have been associated with differing responsiveness to treatment and prognosis [18]. In situ breast cancers are confined to the ducts or lobules [17, 19]. Ductal carcinoma in situ (DCIS) is more common than lobular carcinoma in situ (LCIS), and while both are considered risk factors for invasive breast cancer, LCIS is not considered to be a lesion capable of becoming malignant [19, 20]. However, the etiology and natural history of in situ tumors is not well known.
Invasive breast cancer is classified by histology, to guide clinical treatment, into invasive ductal (70-80% of breast cancer), invasive lobular (5-15% of breast cancer), and other less common types such as papillary tumors [17, 19]. Immunohistochemical (IHC) staining for the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) guide the use of targeted therapies. The use of other tests such as gene array profiling or other IHC markers are not as commonly used clinically [17, 21-23]. Several main intrinsic molecular subtypes have been identified using gene micro-arrays: Luminal A, Luminal B, HER2-enriched, Basal Like, and Normal Like [17, 24-26]. Further classifications of primarily triple negative tumors have identified the Claudin-low subtype and six triple-negative molecular subtypes [27, 28]. Several gene panels (e.g., PAM50) have been developed as less expensive options with a similar ability to classify tumors into molecular subtypes [17, 29, 30]. In 2013, the St. Gallen consensus agreed on surrogate definitions of the intrinsic subtypes that could be approximated by IHC staining of ER, PR, and HER2 as well as grade and proliferation [31] (Table 1), though several studies have noted that the agreement may be low [18, 32].
Table 1.
Breast cancer intrinsic molecular subtypes
| Intrinsic Molecular Subtype |
St. Gallen Surrogate classification |
% of breast cancer |
Grade | Proliferation | Prognosis |
|---|---|---|---|---|---|
| Luminal A | ER+/PR+/HER2− and low Ki-67 | 50% | Low | Low | Good |
| Luminal B | 1.ER+/HER2− and high Ki-67 or PR− 2.ER+/HER2+ and any Ki67 or any PR |
20% | Higher than Luminal A | Low, but higher than Luminal A | Good, but slightly worse than Luminal A |
| HER2-enriched | ER−/PR−HER2+ | 15% | High | High | Moderate |
| Basal-like | ER−/PR−/HER2− | 15% | High | High | Moderate, but worse than other subtypes |
Disparities in the United States
Prior to age 40, US Black women have the highest breast cancer incidence rates, after which rates are highest among White women (Figure 2). American Indian/ Alaska Native women have the lowest rates until age 74. Asian/ Pacific Islander women have similar incidence rates as White and Black women until age 45, after which they have the lowest incidence rates. Black women have the highest mortality rates at all ages followed by White women (e.g., 68.2 per 100,000 in Blacks vs 46.5 per 100,000 in Whites at age 60-64). Mortality rates for American Indian/ Alaska Native and Asian/ Pacific Islander women are similar until about age 60, at which point Asian/ Pacific Islander women have the lowest mortality rates (e.g., 30.0 per 100,000 in Asian/ Pacific Islander vs 33.2 per 100,000 in American Indian/ Alaska Native at age 60-64).
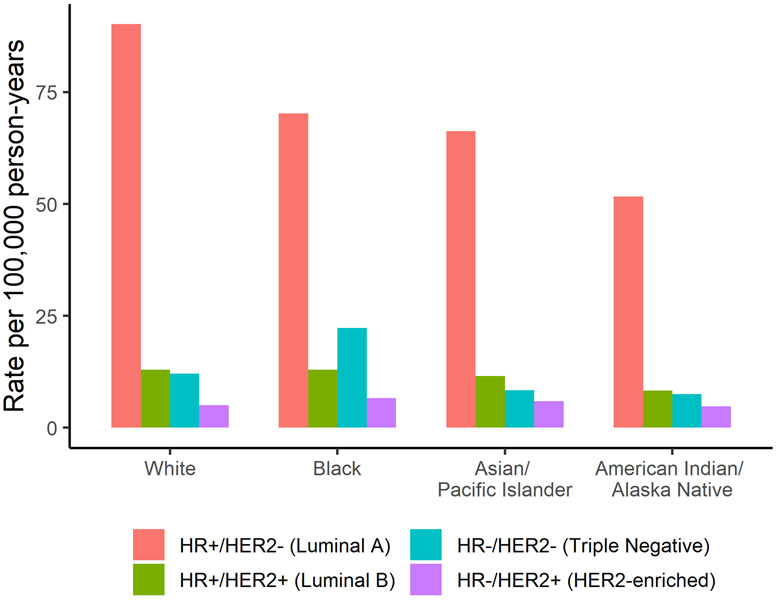
While breast cancer incidence rates have either declined or remained stable since the early 2000s among White women, incidence rates among Black women have continued to increase (Figure 3) [33]. In 2016, the age-standardized incidence rate was 128.2 per 100,000 among Black women versus 132.7 per 100,000 among White women [4]. While incidence rates are lower, Black women experience higher breast cancer mortality than White women; further, that gap has continued to widen even as survival has increased overall (Figure 3) [33]. The 2016 age-standardized mortality rate was 27.3 per 100,000 Black women versus 19.6 per 100,000 White women [4]. Compared to White women, Black women also have a higher incidence of the more aggressive triple-negative (ER−/PR−/HER2−) tumor subtype (Figure 6) and, among those with ER+ tumors, Black women are more likely to be diagnosed at a later stage, potentially due to lower screening rates and barriers to health care access [33-35]. Further, disparities are still present within a similar stage and subtype of breast cancer [33]. For example, among women with ER+/HER2− tumors, Black women have lower survival at local (98.7% vs. 100.0%) and regional (82.2% vs. 90.6%) stages than White women, suggesting that racial disparities in treatment may play a role [4, 33]. Studies have shown that Black women are more likely to experience delayed or inadequate surgery, radiation therapy, chemotherapy, and targeted therapies [33, 35-37].
Figure 6.
United States five-year age-adjusted female breast cancer incidence rates for each breast cancer subtype by race/ethnicity. Figure 6 shows five-year age-adjusted female breast cancer incidence rates by breast cancer subtype for White, Black, American Indian/ Alaskan Native, and Asian/ Pacific Islander women using data from the SEER program (SEER 21, 2012-2016).
Familial and genetic factors
Having a first-degree relative with breast cancer increases a woman’s risk of developing breast cancer two to three-fold [38, 39]. Approximately 10-15% of breast cancers are thought to be hereditary, though a known pathogenic mutation is identified in only about 30% of those with hereditary breast cancer [40]. It is estimated that 5-10% of breast cancer is associated with a highly penetrant germ-line mutation, including in the BRCA1 and BRCA2 genes [41]. Those of Ashkenazi heritage (vs. without this heritage) have a higher prevalence of BRCA1/2 founder mutations (2.0-2.5% vs. 0.1-0.2%) [42-44]. A higher prevalence of BRCA1 mutations are found in women with early onset or triple-negative/ basal-like tumors ([45]). Additional genes with moderately and highly penetrant mutations associated with breast cancer are shown in Table 2. Other similar genes that have been less consistently associated with breast cancer include BRIP1, BARD1, NBN, NF1, and RAD50 [46-51]. Several hundred common gene polymorphisms have been identified through genome-wide association studies (GWAS), which, cumulatively, currently explain about 18% of the two-fold familial relative risk [52].
Table 2.
Moderate to high penetrance genes associated with breast cancer
| Penetrance | Gene | Gene Function | RR of BRCA |
|---|---|---|---|
| High | BRCA1 | Tumor suppressor, DNA Repair | >10 |
| BRCA2 | Tumor suppressor, DNA Repair | >10 | |
| TP53 | Tumor suppressor, Cell cycle regulation | 5 to >10 | |
| Moderate | PALB2 | Tumor suppressor | 2 to >10 |
| CDH1 | Tumor suppressor, Cell adhesion | 2 to >10 * | |
| PTEN | Tumor suppressor, Apoptosis | 2 to 10 | |
| STK11 | Tumor suppressor, Apoptosis | 2 to 10 | |
| ATM | DNA Repair, Cell cycle regulation, | 2 to 7 | |
| CHEK2 | Tumor suppressor, DNA Repair, Cell cycle regulation | 2 to 5 |
Risk Factors
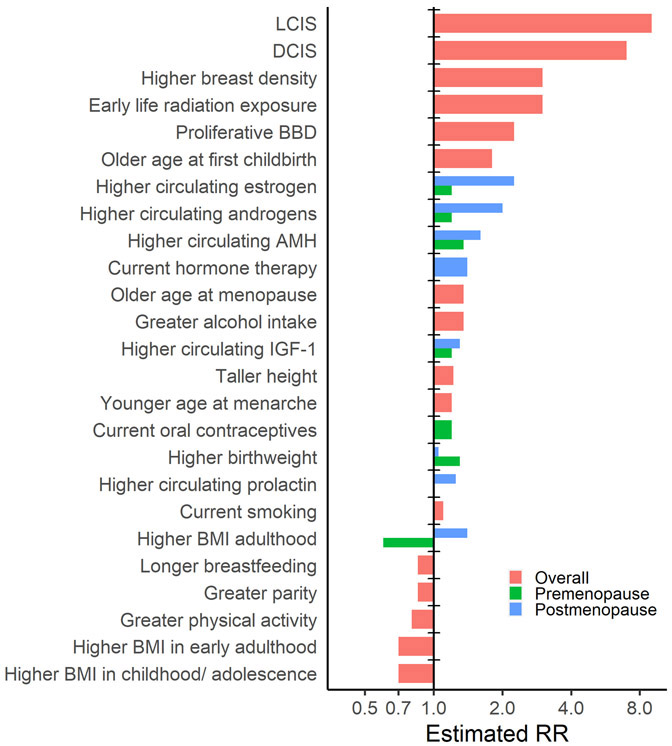
Breast cancer etiology is influenced by multiple exposures occurring over the life course, including early life factors during childhood and adolescence that can affect risk later in adulthood. Many of the risk factors identified, and some that still need to be elucidated by additional well-conducted research are shown in Table 3. The approximate magnitude of risk associated with each of the established risk factors is presented in Figure 7. Further, research has suggested that associations between breast cancer risk factors and breast cancer may vary by breast cancer subtype (Table 4).
Table 3.
Summary of risk factors and serum biomarkers with breast cancer risk
| Domain | Risk Factor | BRCA Association |
Heterogeneity of risk | Comments | Highest level of evidence |
Refs. |
|---|---|---|---|---|---|---|
| Anthropometrics | Birthweight, high | + | Association stronger for premenopausal; Association possibly stronger for ER+ | PL | [53, 54, 355-357] | |
| Birth length, longer | + | Limited assessment of heterogeneity | PL | [53, 76] | ||
| Height, taller | + | Association stronger for ER+ | PL Prospective | [66, 74, 75] | ||
| Childhood & adolescent BMI/ somatotype, heavier | − | Limited assessment of heterogeneity | Cohort Prospective | [55-60] | ||
| Early adult (18-30) body mass index (BMI)/ weight, heavier | − | No substantial heterogeneity observed | PL Prospective | [61-63, 358] | ||
| Premenopausal body mass index (BMI)/ weight, heavier | − | Association may vary by ethnicity; No heterogeneity observed by ER | MA Prospective | [61, 63-69] | ||
| Postmenopausal body mass index (BMI)/ weight, heavier | + | Association may vary by ethnicity; Association stronger for never users of hormone therapy; Association stronger for ER+ | MA Prospective | [63-69] | ||
| Waist circumference/ waist-to-hip ratio, greater | + | Association stronger for never users of hormone therapy; Limited assessment of ER heterogeneity | Potential confounding by BMI | MA Prospective | [63, 71] | |
| Weight gain | + | Association possibly limited to postmenopausal; Association stronger for ER+ | MA | [63, 72, 73, 359] | ||
| Breast density, high | + + | Association possibly stronger for premenopausal (percent density); Association possibly stronger for ER− (premenopausal only) | Association stronger for percent density vs. dense area | PL | [77-79, 360, 361] | |
| Bone mineral density (BMD), high | N/A | Association observed in several studies but not seen in prospective meta-analysis; Potential confounding by BMI, HT use, estrogen levels | MA Prospective | [81, 82] | ||
| Reproductive | Age at menarche, younger | + | Association stronger for ER+ | PL Prospective | [67, 68, 83, 87, 362] | |
| Age at menopause, older | + | Association stronger for ER+ | PL Prospective | [67, 83, 363] | ||
| Parity, greater | − | Association consistent for ER+ and inconsistent for ER− (probable positive association) | Initial increased risk for up to 10-15 years, but inverse association long term | PL Prospective | [67, 68, 84-87, 363] | |
| Age at first childbirth, younger | − | Association limited to ER+ | MA | [67, 68, 86-88, 362, 363] | ||
| Breastfeeding, longer duration | − | Association consistent for ER− and suggestive for ER+; No substantial heterogeneity by menopause | PL Prospective | [84, 86, 87, 364] | ||
| Endogenous hormones and other circulating biomarkers | Estrogens, high | +/+ + | Association stronger for postmenopausal; Association stronger for ER+; Association stronger for never users of hormone therapy | PL Prospective | [91-93] | |
| Androgens, high | +/+ + | Association possibly stronger for ER+ | PL Prospective | [91-93] | ||
| Prolactin, high | + | Association possibly limited to postmenopausal; Association possibly limited to ER+ | MA | [94] | ||
| Anti-Mullerian hormone (AMH), high | + | Association possibly stronger for ER+; No substantial heterogeneity observed by menopause at diagnosis | PL Prospective | [98] | ||
| Sex hormone binding globulin (SHBG), high | − | No association observed for premenopausal, inverse association for postmenopausal; Association possibly stronger for ER+ (postmenopausal) | PL Prospective (Premenopausal); MA Prospective (Postmenopausal) | [91, 92, 99] | ||
| Progesterone, high (premenopausal only) | No association observed for premenopausal; Limited assessment of postmenopausal | PL Prospective (premenopausal) | [91] | |||
| Insulin-like growth factor 1 (IGF-1), high | + | No substantial heterogeneity observed by menopause; Association limited to ER+ | PL Prospective | [101] | ||
| Insulin/ C-peptide, high | + | Association possibly limited to postmenopausal; Limited assessment of ER heterogeneity | Null in meta-analysis, associations observed in several cohort studies published afterwards; May be mediated through BMI | MA Prospective | [103-107] | |
| Adipokines (low adiponectin/ high leptin) | + | Associations possibly stronger among Asian women | Possible association of lower adiponectin levels with breast cancer; Possible positive association for leptin | MA | [111, 112] | |
| C-reactive protein, high | + | Limited assessment of heterogeneity | MA Prospective | [108-110] | ||
| Melatonin, high | Association possibly limited to postmenopausal; Association possibly limited to ER+ | Possible inverse association for urinary aMT6s among subgroups; May vary by timing/ method of melatonin assessment | MA Prospective | [113, 114] | ||
| Exogenous Hormones | Oral contraceptives | + | Limited assessment of heterogeneity | Association stronger for current/ recent longer duration users; Limited assessment of dose/formulation | MA Prospective | [101, 115-117] |
| Levonorgestrel-releasing intrauterine system | + | Limited assessment of heterogeneity | Possible positive association, may be confounded by prior OC use | MA | [118] | |
| Postmenopausal hormone therapy | +/+ + | Association stronger for ER+ | Association stronger for estrogen and progestin vs. estrogen only; Association stronger for current vs. past users and associations increase (or become apparent for estrogen only) with increased duration of use; Limited assessment of dose/formulation | PL Prospective | [119] | |
| Dietary | Alcohol | + | Heterogeneity by ER inconsistent; | Association possibly stronger if initiated prior to first birth | PL Prospective | [178-183] |
| Carbohydrates | Association possibly limited to ER− | MA | [127] | |||
| Carotenoids | − | Association possibly stronger for ER− (α-carotene, β-carotene, and lutein/zeaxanthin) | Association stronger for β-carotene and lycopene; Association observed for β-carotene plasma levels but not supplement trials | PL Prospective (RCT MA for β-carotene) | [154-157] | |
| Coffee/ Tea/ Caffeine | N/A | Caffeine likely not associated; Associations for coffee/tea inconsistent but possible among subgroups | MA Prospective | [142-146] | ||
| Dairy/ Calcium | − | Association stronger for premenopausal (low-fat dairy/milk, calcium); Limited assessment of ER heterogeneity | Association possible for low-fat dairy/ milk; Association observed for calcium from dietary intake and plasma levels but not in supplement trials | MA Prospective (RCT MA for calcium) | [136-138, 147-149, 365-368] | |
| Dietary fat | N/A | PL Prospective | [121-126] | |||
| Dietary Inflammatory Index (DII) | N/A | Possible positive association, weak in prospective studies and may be cofounded by other lifestyle factors | MA Prospective | [369-372] | ||
| Dietary patterns | N/A | Suggestive inverse associations for ‘Prudent’ diets or Mediterranean diets and suggestive positive for ‘Western’ diets; Associations weak in prospective studies and results may be confounded by other lifestyle factors | MA Prospective | [184-188] | ||
| Fiber | − | Limited assessment of ER heterogeneity | Association stronger for soluble fiber | MA prospective | [129-131, 373-377] | |
| Folate/ folic acid | Association varies by alcohol consumption (folate intake), MTHFR status, and possibly menopause status; Association possibly inverse for ER− (folate intake) but may vary by dietary or supplement folate | Possible J-shaped association (folate intake); Possible differences by dietary intake (null/inverse) vs. supplemental intake (suggestive positive); No association observed in RCT (supplemental) | RCT MA | [164-168] | ||
| Fruits/ vegetables | − | Association limited to ER− | Association possibly stronger for vegetables | PL Prospective | [139-141] | |
| Glycemic index/ glycemic load | + | Association stronger for postmenopausal (glycemic index); Association stronger for ER− (glycemic load) | MA Prospective | [127, 128] | ||
| Iron | + | Association possibly stronger for premenopausal (heme iron); No heterogeneity observed by ER | Possible positive association for heme iron intake and plasma iron levels | MA | [158, 378, 379] | |
| Meat | + | No heterogeneity observed by ER | Possible positive association for processed meat | MA Prospective | [132-136, 366, 368, 378, 380-382] | |
| Multivitamins | N/A | MA | [177] | |||
| Phytoestrogens | N/A | Possible inverse association for enterolignan intake but not seen with blood/urine levels | MA | [171, 172] | ||
| Selenium | N/A | Methods of selenium assessment heterogeneous; No association observed in RCT | MA | [169, 170] | ||
| Soy/ Isoflavones | N/A | MA Prospective | [134, 173-176] | |||
| Vitamin A | N/A | Possible inverse association for dietary intake (not seen in prospective and no dose-response), not seen with supplements or plasma levels | MA | [159] | ||
| B-Vitamins (B2, B6, B12) | N/A | MA | [160, 161] | |||
| Vitamin C | N/A | Possible inverse association for dietary intake (not seen in prospective), not seen with supplements (positive) or plasma levels | PL Prospective | [159, 162, 163] | ||
| Vitamin D | − | No heterogeneity observed | Possible inverse association for 25(OH)D levels; Intake and supplement inconsistent | RCT MA | [150-153] | |
| Vitamin E | N/A | Possible inverse association for dietary intake (not seen in prospective and no dose-response) and plasma alpha-tocopherol levels | MA | [159, 383, 384] | ||
| Environmental | Air pollution | N/A | Possible positive association for NO2 and NOx, though weak and often borderline significant | MA | [194, 195] | |
| Acrylamides (dietary) | N/A | MA | [203] | |||
| Electromagnetic fields | N/A | Possible positive association, though weak and often borderline significant | MA | [196-198] | ||
| Organochlorine blood levels | N/A | MA | [199-202] | |||
| Secondhand smoke | + | Association possibly stronger for premenopausal; Limited assessment of ER heterogeneity | Possible positive association, though weak and often borderline significant | MA Prospective | [190-193, 385-388] | |
| Lifestyle | Light at night and shift work/ Circadian rhythm disruptions | + | Association possibly stronger for premenopausal; Association possibly stronger for ER+ | Possible positive association for shift work or light at night; Associations stronger in case-control studies | MA Prospective | [217-221] |
| Hair dyes/ relaxers | + | Association possibly stronger for Black women | Possible positive association for hair dye/ relaxers; results mostly case-control studies and 1 cohort | MA | [215, 216] | |
| Physical activity | − | No substantial heterogeneity observed by menopause or ER | MA Prospective | [63, 206-210] | ||
| Physical Inactivity/ Sedentary behavior | + | Association possibly limited to postmenopausal women | Possible increased association with greater sedentary behavior; methodology varies widely | MA | [63, 211, 212] | |
| Stressful life events | + | Limited assessment of heterogeneity | Possible increased association with stressful life events; methodology varies widely | MA Prospective | [224] | |
| Sleep duration | N/A | MA | [220, 222, 223] | |||
| Smoking | + | Associations may vary by ethnicity; No heterogeneity observed by menopause; Associations possibly limited to ER+ among those initiated prior to first birth | Association stronger if initiated 10+ years prior to first birth | PL Prospective | [182, 190, 213, 214] | |
| Medications | Antibiotics | N/A | MA | [225] | ||
| Antidepressants | N/A | MA | [226] | |||
| Aspirin/ Nonsteroidal anti-inflammatory drugs (NSAIDs) | N/A | Possible inverse association for long term, consistent aspirin use among subgroups; Associations stronger in case-control studies | MA Prospective | [227-229, 389] | ||
| Bisphosphonates | N/A | Possible inverse association but not seen in RCT and possible confounding by indication | MA | [230-232] | ||
| Diethylstilbestrol (DES) | + | Limited assessment of heterogeneity | Probable association among women who took DES during pregnancy; Possible association among daughters exposed in utero, but possible surveillance bias | MA (in utero); Cohorts (maternal use) | [76, 236-244] | |
| Fertility drugs | N/A | Issues with confounding (e.g., parity, underlying cause) | MA | [233, 234] | ||
| Statins | N/A | Heterogeneous results among various subgroups | MA | [235] | ||
| Personal Health | Abortion | N/A | N/A | MA Prospective | [245-248] | |
| Diabetes | N/A | Association may vary by type of diabetes | MA | [261-265] | ||
| Ductal carcinoma in situ (DCIS) | + + | Limited assessment of heterogeneity | MA | [251-253] | ||
| Benign breast disease | +/+ + | Limited assessment of heterogeneity | Association varies by type of benign breast disease – strongest associations with atypical proliferation | MA | [249, 250] | |
| Ionizing Radiation | + + | Limited assessment of heterogeneity | Association stronger if exposed early in life | PL Prospective | [204, 205, 269] | |
| Lobular carcinoma in situ (LCIS) | + + | Limited assessment of heterogeneity | Long term follow-up studies Prospective | [254-260] | ||
| Metabolic syndrome | Possible variation by menopause | MA | [266] | |||
| Migraine headaches | N/A | N/A | MA Prospective | [267, 268] |
ABBREVIATIONS: Refs, references; PL, pooled analysis; MA, meta-analysis; RCT, randomized trials; ER, estrogen receptor
NOTE: Color used to indicate consistency of association: Dark red, established/ probable positive association; light red, possible positive association; dark green, established/probable inverse association; light green, possible inverse association; grey, inconsistent/ no association.
Symbols used to indicate magnitude of association: + + + strong positive association (RR/OR >5); + + moderate positive association (RR/OR 2-5), + modest positive association (RR/OR <2); − − − strong inverse association (RR/OR <0.2); − − moderate inverse association (RR/OR 0.2-0.5), − modest inverse association (RR/OR >0.5).
Magnitude of association may vary by race/ethnicity, tumor subtype.
Association directions are for increasing exposure unless otherwise specified.
Figure 7.
Magnitude of association for established breast cancer risk factors. Figure 7 shows the magnitude of association for each established breast cancer risk factor; if the association has been shown to consistently vary by menopause status both the premenopausal and postmenopausal associations are shown. Risks are approximate and can vary depending on the extent of exposure, breast cancer subtype, and menopause status.
Table 4.
Risk factors of breast cancer by subtypes
| Risk factor | Luminal A | Luminal B | HER2-enriched | Triple negative |
|---|---|---|---|---|
| Age at menarche, younger | + | + | ||
| Parity, greater | − | − | + | |
| Age at 1st birth, older | + | + | ||
| Breastfeeding, longer duration | − | − | − | |
| Age at menopause, older | + | |||
| Oral contraceptives use | + | |||
| Hormone therapy use | + | + | ||
| BMI premenopausal, heavier | − | + | ||
| BMI postmenopausal, heavier | ||||
| Benign breast disease | + | + | + | + |
| Family history of breast cancer | + | + | + | + |
| Alcohol intake | + | + |
NOTE: Color used to indicate consistency of association: Dark red, established/ probable positive association; light red, possible positive association; dark green, established/probable inverse association; light green, possible inverse association; grey, inconsistent/ no association.
Symbols used to indicate magnitude of association: + + + strong positive association (RR/OR >5); + + moderate positive association (RR/OR 2-5), + modest positive association (RR/OR <2); − − − strong inverse association (RR/OR <0.2); − − moderate inverse association (RR/OR 0.2-0.5), − modest inverse association (RR/OR >0.5).
Anthropometrics
The relationship between adiposity and breast cancer risk is complex and varies by the timing of body size assessment over the life course. Greater birthweight is associated with modestly higher risk of breast cancer in adulthood [53, 54]. In contrast, a higher body mass index (BMI), indicating greater adiposity, measured in childhood or in early adulthood (18-30 years) is associated with decreased risk of breast cancer [55-63]. Premenopausal adult BMI is inversely associated with risk [61, 63-66], while postmenopausal BMI is positively associated [63-66], particularly among never hormone therapy users and ER+ tumors [64, 67-69]. The differing associations by menopausal status have been hypothesized to be due to the differences in estrogen levels and its primary sources (i.e., ovary vs. adipose tissue). In a large Mendelian randomization study using data from two breast cancer consortia, a polygenic risk score (PRS) of adult BMI was inversely associated with breast cancer risk regardless of menopausal status, suggesting the PRS may reflect early life obesity [70]. Larger central adiposity (e.g., waist circumference, waist-to-hip ratio) is associated with higher postmenopausal breast cancer risk and possibly greater risk of premenopausal breast cancer, though premenopausal studies have been limited and may be influenced by additional BMI-adjustment [63, 71]. Adult weight gain is positively associated with postmenopausal breast cancer risk [63, 72, 73].
Other non-modifiable anthropometrics that increase breast cancer risk, particularly ER+ tumors, includes taller height [66, 74, 75] and larger birth lengths [53, 76]. Having dense breasts, as assessed radiologically (e.g., digital mammogram), substantially increases risk regardless of menopausal status or hormone receptor status of the tumor [77-79]. For this reason, 38 states require breast density notifications after a mammogram, though language varies by state and may only state that there is an association in general and not contextualize the individual woman’s risk [80]. In March 2019, the FDA proposed a rule to extend this to all mammograms [80]. Associations have been much less consistent for bone mineral density. Early case-control studies observed increased risk; however, more recent meta-analyses of prospective studies do not see a significant association [81, 82].
Reproductive factors
The associations of multiple reproductive factors with breast cancer risk have been well established. Younger age at menarche [83] and older age at menopause [83] are associated with increased breast cancer risk, potentially reflecting the number of ovulatory cycles over a woman's lifetime and estrogen exposure. Parous women initially have a higher risk of breast cancer after delivery compared to nulliparous women which peaks about 5 years after birth and remains elevated for approximately 20 years. Overall, women with greater parity have a lower risk of breast cancer long-term, and risk is further reduced with each subsequent birth [84, 85]. However, this relationship does appear to vary by ER status [68, 86, 87]. Additionally, the younger age at which a woman has her first child [88] and longer durations of breastfeeding [84] further reduces breast cancer risk independent of parity.
Endogenous hormones and other circulating biomarkers
Sex hormones are integral in the etiology of breast cancer, supported by laboratory studies, epidemiologic evidence (e.g., reproductive risk factors and postmenopausal BMI) and the use of selective estrogen receptor modulators (e.g., Tamoxifen) to prevent breast cancer [89, 90]. Higher circulating levels of estrogens [91-93], androgens [91-93], and prolactin [94], primarily in postmenopausal women, are established to increase breast cancer risk. Estrogen metabolites may also play a role [95-97] though evidence remains limited. Higher anti-mullerian hormone (AMH), measured premenopausally, is positively associated with breast cancer [98], though it is also strongly and directly related to age at menopause. Higher sex hormone binding globulin (SHBG) may decrease breast cancer risk [91, 92, 99]. Progesterone levels are not related to premenopausal breast cancer [91], possibly due to challenges in characterizing long-term hormone levels, and only one prospective study has examined postmenopausal progesterone levels, finding no association [100]. Circulating concentrations of insulin-like growth factor-1 are modestly positively associated with risk of ER+ breast cancer [101], which is further supported by a mendelian randomization study [102].
Other circulating biomarkers also may play a role in breast cancer etiology. Studies examining insulin or c-peptide, a byproduct of insulin, have suggested an increased risk among postmenopausal women only [103-107]. Potentially, chronic low levels of inflammation indicated by c-reactive protein may increase risk [108-110]. There is a suggestive increase in breast cancer risk for greater leptin and lower adiponectin levels [111, 112]. Studies examining melatonin levels, which may be affected by light at night or shiftwork, have been relatively inconsistent perhaps due to differences in sample collection and timing, showing either inverse or null associations between the main melatonin metabolite, 6-sulfatoxymelatonin (aMT6s) in urine and breast cancer [113, 114].
Exogenous hormones
Use of oral contraceptives increases breast cancer risk for up to 10 years after stopping use, and this is most consistently observed among current and recent users [115-117]. However, as oral contraceptive use occurs during the reproductive years, at ages when breast cancer incidence is low, the impact on population rates of breast cancer is minimal. Levonorgestrel-releasing intrauterine devices, also are suggestively associated with increased risk [118]. Other forms of hormonal contraceptives have been less studied.
The use of postmenopausal hormone therapy has been evaluated in multiple observational studies and randomized trials [119]. Combined estrogen and progestin use substantially increases risk, and associations are strongest for current/recent use and among those with the longest durations of use [119]. Long duration use of estrogen only is associated with more modest increases in risk; the major clinical trial did not observe increases in risk with estrogen only use, though timing of treatment (i.e. years after menopause) likely played a role [119, 120]. Relatively few studies have examined doses, formulations, and changing use patterns.
Dietary
There has been an interest in diet as a risk factor for breast cancer since early ecologic studies of fat and breast cancer mortality. Dietary fat has had considerable interest and controversy; however, most studies indicate no association overall with total fat [121-126]. Studies of carbohydrate intake have been inconsistent [127], though glycemic index/load may be associated with increased risk [127, 128], and soluble fiber with decreased risk [129-131].
Assessments of specific foods have suggested an increased risk with processed meats [132-136] and decreases in risk with low-fat dairy [136-138] and fruit and vegetable intake [139-141]. Coffee and tea associations have been inconsistent, but there may be associations in subgroups [142-146]. Several nutrients have also been assessed as potential risk factors. Nutrients with suggestive decreased risk include calcium [147-149], vitamin D [150-153], and carotenoids, particularly β-carotene [154-157]. Higher heme-iron intake and plasma iron levels may increase risk [158]. However, most other nutrients are inconsistent or have not been found to be associated with breast cancer, including vitamin A [159], B-vitamins [160, 161], vitamin C [159, 162, 163], vitamin E [159], folate [164-168], selenium [169, 170], phytoestrogens [171, 172], and isoflavones [173-176]. Additionally, multivitamins have not been associated with breast cancer risk [177]. Alcohol is the dietary factor most consistently associated with breast cancer, conferring a moderate increase in risk [178-183].
As foods and nutrients are not eaten in isolation and interactions between nutrients and foods are likely (e.g., folate and alcohol), the examination of dietary patterns is important. Some studies have suggested that dietary patterns such as the “prudent”, “western”, and Mediterranean may be associated with breast cancer [184-188]. Apart from the Mediterranean pattern, including olive oil, decreasing risk [189], studies are inconsistent or weakly associated, and there may be substantial confounding with other lifestyle risk factors.
Environmental
Although many environmental factors have been evaluated, most have limited or inconsistent evidence linking them to breast cancer, leading to some controversy. Obtaining valid exposure measures during susceptible periods of life continues to be a challenge. Exposure to secondhand smoke has been suggestively associated with increases in breast cancer risk [190-193]. Others, such as air pollution [194, 195], electromagnetic fields [196-198], organochlorines (e.g., DDT/DDE, PCBs) [199-202], and acrylamides in food [203] have been inconsistent. Non-medical radiation exposure (e.g., atomic bombs) has been associated with increased breast cancer risk, particularly among those exposed at younger ages [204, 205].
Lifestyle factors
Higher levels of physical activity have been consistently linked with decreases in breast cancer risk [63, 206-210] and greater sedentary behavior or physical inactivity may be associated with increased risk [63, 211, 212]. Smoking has recently become quite well established to increase breast cancer risk, particularly if initiated prior to first pregnancy and for long durations [182, 190, 213, 214]. The use of hair dye or relaxers may possibly be associated with increased risk among Black women in particular [215, 216]. Exposure to light at night or shift work has been suggested to be positively associated with breast cancer, but the mechanism is unclear [217-221]. Sleeping duration is unlikely to be associated with risk [220, 222, 223]. Stressful life events may also be associated with increased risk, though definitions of stress or stressful life events have varied widely [224].
Medications/ other health conditions
The association of many medications with breast cancer risk (e.g., antibiotics [225], antidepressants [226], aspirin/NSAIDs [227-229], bisphosphonates [230-232], infertility drugs [233, 234], statins [235]) has been inconsistent or null, and likely limited by confounding or ascertainment biases. Diethylstilbestrol (DES) has been found to increase breast cancer risk among women who took the drug during pregnancy [236-240], and associations among the daughters exposed in utero are less consistent but also may be positive [76, 239, 241-244]. Early case-control studies on abortion reported an increased risk; however, prospective studies have overall observed no association [245-248]. Prior history of breast conditions such as proliferative benign breast disease [249, 250] or in situ tumors [251-260] has been established to substantially increase breast cancer. Obesity-related disorders, such as diabetes [261-265] and metabolic syndrome [266], have been generally inconsistent. Migraine headaches are likely not related, as observed associations have been limited to case-control studies [267, 268]. Lastly, ionizing radiation for medical reasons (e.g., for lymphoma) has been established to increase breast cancer risk, which increases with greater doses and the highest risk occurs among those exposed before puberty similar to those exposed due to radiation from atomic bombs [204, 205, 269].
Other factors
Additional risk factors not included in Table 3 with very limited or insufficient evidence include a number of chemical or biologic agents (i.e., polycyclic aromatic hydrocarbons [270], parabens [271], BPA [272, 273], phthalates [274-276], perfluorocarbons[277-279], human papillomavirus [280], Epstein–Barr virus [281]), breast size [282], blood pressure [283-287], under-wire bras [288, 289], breast implants [290], cellphone use [291], deodorant/ antiperspirant use [292-294], and trauma to the breast [295, 296].
Risk Prediction Models
Multiple risk prediction models have been developed to predict future risk of breast cancer (e.g., Gail/ Breast Cancer Risk Assessment Tool [BCRAT], Breast Cancer Surveillance Consortium [BCSC], Rosner-Colditz, Claus, BRCAPRO, Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm [BOADICEA], Tyrer-Cuszik/ International Breast Cancer Study [IBIS]), some of which can also predict BRCA carrier status (e.g., BRCAPRO, BOADICEA, IBIS) [297, 298]. Risk prediction models are increasingly being used clinically to help guide decisions related to screening and prevention (e.g., timing and frequency of screening, use of chemoprevention) [299-301]. Risk prediction models vary in terms of factors included, with several including hormonal, environmental, or pathologic factors, high-risk genetic mutations, and more recently mammographic density [297, 298]. While all models include family history assessment, the level of detail varies widely ranging from only first-degree relatives with breast cancer to all relatives with breast, ovarian, pancreas, and prostate cancers and their ages of onset [297, 298]. The risk prediction models also vary in terms of type of population the model is suitable for (i.e., general screening population, women with a family history of breast or ovarian cancer) [297, 298].
The Gail, Rosner-Colditz, Claus, BCSC, BRCAPRO, BOADICEA, and IBIS models have all been validated in external datasets [298, 302, 303]. A recent comparison of several of the most commonly used clinical models (i.e., Gail, BRCAPRO, BCSC, Claus, IBIS) in a large, predominantly White US screening population indicated that the models were generally well-calibrated (O/E range: 0.78-0.97) but with only moderate discrimination (AUC range: 0.61-0.64) [304]. Expansion of these models to include multiple biomarkers (e.g., mammographic density and/or other imaging features, polygenic risk scores, endogenous hormones, epigenetics, metabolomics), and the development and validation of models across race/ethnicity and by tumor subtype is ongoing, and likely to lead to model improvement [305-316].
Prevention
Modifiable risk factors often targeted for breast cancer prevention include maintaining a healthy weight, participating in regular physical activity, moderating or avoiding alcohol intake, and minimizing or avoiding postmenopausal hormone therapy [317]. Women who most adhered to the American Cancer Society prevention guidelines had a 22% lower risk of breast cancer compared to women with the lowest adherence [318]. Other risk factors that could be targeted for prevention include healthy eating (e.g., increased intake of fruits and vegetables) [319] and, when possible, breastfeeding [320]. For example, among African American women, who often have lower rates of breastfeeding and higher rates of triple-negative breast cancer [317, 321], facilitating increased breastfeeding may importantly lower the risk of triple-negative breast cancers [86, 322-324]. Additionally, prevention programs (e.g., avoidance of smoking) in adolescents and young adults, particularly prior to first pregnancy, may be important, as these have been shown to be etiologically important periods.
For high-risk women, the American Society of Clinical Oncology recommends the use of selective estrogen receptor modulators (e.g., Tamoxifen, Raloxifene) or aromatase inhibitors (Exemestane, Anastrozole) [300]. While no single threshold for being high risk has been defined, women who are most likely to benefit from endocrine therapy are those with one or more of the following: diagnosis of atypical hyperplasia or LCIS, an estimated 5-year risk ≥3% (BCRAT) or 10-year risk ≥5% (IBIS), or a relative risk ≥4 times the population risk for ages 40-44 years or ≥2 times the population risk for ages 45-69 years. Tamoxifen can be used regardless of menopausal status, whereas Raloxifene, Exemestane, and Anastrozole can only be used by postmenopausal women [300]. Trials of Tamoxifen and Raloxifene have shown a 50% reduction in breast cancer risk, primarily due to a reduction of hormone receptor-positive breast cancers [317, 325-327] and trials of aromatase inhibitors have shown similar reductions [328, 329]. However, less than 10% of women eligible for chemoprevention use these drugs [330], primarily due to lack of provider recommendations and concerns about potential side effects. In the future, better targeting of women who may benefit, the use of newer selective estrogen receptor modulators, or low-dose of topical Tamoxifen may help mitigate these issues [331-333].
Additionally, in recent years there has been an increase in women electing to undergo surgical prevention for breast cancer [334]. The NCCN recommends prophylactic salpingo-oophorectomy for women with a known BRCA1/2 mutation between the ages of 35-40 that have completed childbearing and that prophylactic mastectomy should be discussed as an option for risk reduction (NCCN guidelines high risk). Bilateral prophylactic mastectomy has been shown to reduce breast cancer risk and increase survival [335]. Contralateral prophylactic mastectomy after the diagnosis of cancer in the other breast, has also been shown to reduce the breast cancer risk in the other breast; however, evidence for increased survival remains limited [335]. Prophylactic bilateral oophorectomy, the removal of both ovaries (generally with bilateral salpingectomy) reduces the risk of ovarian cancer and may also reduce the risk of breast cancer, though this is less clear [336].
Screening and Early Detection
Mammography is the most common modality for breast cancer screening in the United States; however, organizations vary in the recommended screening age range and frequency of mammograms. For example, for average-risk women, the US Preventive Services Task Force and the American Academy of Family Physicians now recommend biennial screening for women 50-74 years. In contrast, the American College of Radiology and the National Comprehensive Cancer Network recommend annual screening starting at age 40 [337-339]. In other high-income countries, the screening age range is most commonly 50 to 69 or 70 with a two-year screening interval [337, 340]. However, in 2018 only 58-74% of women ages 50-74 had at least one screening mammogram in the prior two years [341]. Mammographic screening can identify breast cancer at earlier, more treatable stages, and thus reduce breast cancer mortality [337, 342, 343]. However, evidence has been inconsistent on whether this has occurred in all age groups, and concerns exist that screening increases the number of false positives, and may lead to the over detection of breast cancer [342, 344]. Due to the difficulty of defining and estimating over detection, estimates have ranged widely from 1 to 60% in trials and 1–12% in studies with a low risk of bias [342]. While several studies have suggested that breast cancer mortality rates and advanced breast cancer diagnoses have been reduced with screening mammography [343], others have suggested that other factors are additionally responsible (e.g., better treatments) [344]. Among women with a 20% or greater lifetime risk of breast cancer, several organizations recommend annual supplemental MRIs [337]. Additionally, as mammography is less sensitive for women with dense breasts [345], alternative and supplemental screenings have been proposed including more frequent mammograms, supplemental ultrasound, MRI, and digital breast tomosynthesis (i.e., 3D mammography) [337, 345]. Several trials are currently underway examining breast cancer screening intervals and start ages (i.e., WISDOM) and the use of digital breast tomosynthesis or ultrasound (i.e., TMIST, DBTUST, ASTOUND) [346-349].
Future Directions
While earlier detection and improved treatments have reduced breast cancer mortality, breast cancer continues to be the most common cancer among women, and incidence is projected to continue to increase in the next few decades. To combat this, multiple strategies are needed. Further research into etiologic heterogeneity should be conducted, such as risk factors among different ethnicities and tumor subtypes, particularly for ER-negative/ basal-like tumors where fewer effective treatments are currently available. Underlying mechanisms of known risk factors should be examined, including reasons for heterogeneity by menopausal status or tumor subtype, potentially using emerging technologies (e.g., metabolomics, proteomics) to assess local and systemic biomarkers and tumor heterogeneity. Further improvement in and validation of risk prediction models is needed, for example, by the addition of both biomarkers (e.g., breast imaging, genetics, hormones) and lifestyle factors and further development of models that better model risk at both the youngest and oldest ages, among different ethnicities, and for subtypes particularly ER−, for which models perform less well. Finally, additional efforts to determine how to successfully implement known (e.g., weight maintenance or reduction) and future preventive strategies, during susceptible periods, is critical.
Continued research on alternative screening modalities that may increase adherence or be more effective (e.g., more sensitive and specific) is needed. In addition to increasing prevention activities and awareness, further development or trials of chemopreventives with lower doses or better side effect profiles to increase adherence or uptake when appropriate and chemopreventives for ER-negative breast cancers may be beneficial. In addition to improvements in screening and treatments, research on improving access and equity in cancer care is needed to address existing disparities. Lastly, with the observed increases in breast cancer survival rates over time, additional research is needed regarding survivorship to improve quality of life.
Acknowledgments
Financial support: SCH was supported through National Research Service Awards F32 CA224677 by the National Cancer Institute. SEH was supported through R01 CA207369 by the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society (2018) Global Cancer Facts & Figures 4th Edition. American Cancer Society, Atlanta, GA [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.SEER*Explorer. In: SEER. https://seer.cancer.gov/explorer/index.html. Accessed 16 Dec 2019 [Google Scholar]
- 5.American Cancer Society (2019) Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Atlanta, GA [Google Scholar]
- 6.Kerlikowske K, Miglioretti DL, Buist DSM, et al. (2007) Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst 99:1335–1339. 10.1093/jnci/djm111 [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Kuller LH, Prentice RL, et al. (2009) Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 360:573–587. 10.1056/NEJMoa0807684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torre LA, Islami F, Siegel RL, et al. (2017) Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 26:444–457. 10.1158/1055-9965.EPI-16-0858 [DOI] [PubMed] [Google Scholar]
- 9.Glass AG, Lacey JV, Carreon JD, Hoover RN (2007) Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst 99:1152–1161. 10.1093/jnci/djm059 [DOI] [PubMed] [Google Scholar]
- 10.Anderson WF, Katki HA, Rosenberg PS (2011) Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst 103:1397–1402. 10.1093/jnci/djr257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg PS, Barker KA, Anderson WF (2015) Estrogen Receptor Status and the Future Burden of Invasive and In Situ Breast Cancers in the United States. J Natl Cancer Inst 107:. 10.1093/jnci/djv159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GLOBOCAN. http://gco.iarc.fr/today/home. Accessed 16 Dec 2019
- 13.Berry DA, Cronin KA, Plevritis SK, et al. (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792. 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- 14.Kristeleit H, Parton M, Beresford M, et al. (2016) Long-term Follow-up Data from Pivotal Studies of Adjuvant Trastuzumab in Early Breast Cancer. Target Oncol 11:579–591. 10.1007/s11523-016-0438-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet 386:1341–1352. 10.1016/S0140-6736(15)61074-1 [DOI] [PubMed] [Google Scholar]
- 16.(2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet 365:1687–1717. 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 17.Malhotra GK, Zhao X, Band H, Band V (2010) Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther 10:955–960. 10.4161/cbt.10.10.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat A, Pineda E, Adamo B, et al. (2015) Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast Edinb Scotl 24 Suppl 2:S26–35. 10.1016/j.breast.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Makki J (2015) Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin Med Insights Pathol 8:23–31. 10.4137/CPath.S31563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliano AE, Connolly JL, Edge SB, et al. (2017) Breast Cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:290–303. 10.3322/caac.21393 [DOI] [PubMed] [Google Scholar]
- 21.Gradishar WJ, Anderson BO, Balassanian R, et al. (2018) Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:310–320. 10.6004/jnccn.2018.0012 [DOI] [PubMed] [Google Scholar]
- 22.Lester T, Wang J, Bourne P, et al. (2009) Different panels of markers should be used to predict mammary Paget’s disease associated with in situ or invasive ductal carcinoma of the breast. Ann Clin Lab Sci 39:17–24 [PubMed] [Google Scholar]
- 23.Walker LC, Harris GC, Holloway AJ, et al. (2007) Cytokeratin KRT8/18 expression differentiates distinct subtypes of grade 3 invasive ductal carcinoma of the breast. Cancer Genet Cytogenet 178:94–103. 10.1016/j.cancergencyto.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Perou CM, Sørlie T, Eisen MB, et al. (2000) Molecular portraits of human breast tumours. Nature 406:747–752. 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 25.Sorlie T, Tibshirani R, Parker J, et al. (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100:8418–8423. 10.1073/pnas.0932692100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørlie T, Perou CM, Tibshirani R, et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98:10869–10874. 10.1073/pnas.191367098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prat A, Parker JS, Karginova O, et al. (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res BCR 12:R68. 10.1186/bcr2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann BD, Bauer JA, Chen X, et al. (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767. 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker JS, Mullins M, Cheang MCU, et al. (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol Off J Am Soc Clin Oncol 27:1160–1167. 10.1200/JCO.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross JS, Hatzis C, Symmans WF, et al. (2008) Commercialized multigene predictors of clinical outcome for breast cancer. The Oncologist 13:477–493. 10.1634/theoncologist.2007-0248 [DOI] [PubMed] [Google Scholar]
- 31.Goldhirsch A, Winer EP, Coates AS, et al. (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol Off J Eur Soc Med Oncol 24:2206–2223. 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundgren C, Bendahl P-O, Borg Å, et al. (2019) Agreement between molecular subtyping and surrogate subtype classification: a contemporary population-based study of ER-positive/HER2-negative primary breast cancer. Breast Cancer Res Treat 178:459–467. 10.1007/s10549-019-05378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeder-Hayes KE, Anderson BO (2017) Breast Cancer Disparities at Home and Abroad: A Review of the Challenges and Opportunities for System-Level Change. Clin Cancer Res Off J Am Assoc Cancer Res 23:2655–2664. 10.1158/1078-0432.CCR-16-2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed AT, Welch BT, Brinjikji W, et al. (2017) Racial Disparities in Screening Mammography in the United States: A Systematic Review and Meta-analysis. J Am Coll Radiol JACR 14:157–165.e9. 10.1016/j.jacr.2016.07.034 [DOI] [PubMed] [Google Scholar]
- 35.Yedjou CG, Tchounwou PB, Payton M, et al. (2017) Assessing the Racial and Ethnic Disparities in Breast Cancer Mortality in the United States. Int J Environ Res Public Health 14:. 10.3390/ijerph14050486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green AK, Aviki EM, Matsoukas K, et al. (2018) Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 172:247–263. 10.1007/s10549-018-4909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts MC, Weinberger M, Dusetzina SB, et al. (2016) Racial Variation in the Uptake of Oncotype DX Testing for Early-Stage Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol 34:130–138. 10.1200/JCO.2015.63.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiyanbola OO, Arao RF, Miglioretti DL, et al. (2017) Emerging Trends in Family History of Breast Cancer and Associated Risk. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 26:1753–1760. 10.1158/1055-9965.EPI-17-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collaborative Group on Hormonal Factors in Breast Cancer (2001) Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet Lond Engl 358:1389–1399. 10.1016/S0140-6736(01)06524-2 [DOI] [PubMed] [Google Scholar]
- 40.Samadder NJ, Giridhar KV, Baffy N, et al. (2019) Hereditary Cancer Syndromes-A Primer on Diagnosis and Management: Part 1: Breast-Ovarian Cancer Syndromes. Mayo Clin Proc 94:1084–1098. 10.1016/j.mayocp.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 41.Bennett IC, Gattas M, Teh BT (1999) The genetic basis of breast cancer and its clinical implications. Aust N Z J Surg 69:95–105. 10.1046/j.1440-1622.1999.01515.x [DOI] [PubMed] [Google Scholar]
- 42.Ford D, Easton DF, Peto J (1995) Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 57:1457–1462 [PMC free article] [PubMed] [Google Scholar]
- 43.Oddoux C, Struewing JP, Clayton CM, et al. (1996) The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet 14:188–190. 10.1038/ng1096-188 [DOI] [PubMed] [Google Scholar]
- 44.Struewing JP, Abeliovich D, Peretz T, et al. (1995) The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 11:198–200. 10.1038/ng1095-198 [DOI] [PubMed] [Google Scholar]
- 45.Hahnen E, Hauke J, Engel C, et al. (2017) Germline Mutations in Triple-Negative Breast Cancer. Breast Care 12:15–19. 10.1159/000455999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Easton DF, Pharoah PDP, Antoniou AC, et al. (2015) Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 372:2243–2257. 10.1056/NEJMsr1501341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hauke J, Horvath J, Groß E, et al. (2018) Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancer Med 7:1349–1358. 10.1002/cam4.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Couch FJ, Shimelis H, Hu C, et al. (2017) Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol 3:1190–1196. 10.1001/jamaoncol.2017.0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B, Beeghly-Fadiel A, Long J, Zheng W (2011) Genetic variants associated with breast-cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Lancet Oncol 12:477–488. 10.1016/S1470-2045(11)70076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aloraifi F, Boland MR, Green AJ, Geraghty JG (2015) Gene analysis techniques and susceptibility gene discovery in non-BRCA1/BRCA2 familial breast cancer. Surg Oncol 24:100–109. 10.1016/j.suronc.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 51.Angeli D, Salvi S, Tedaldi G (2020) Genetic Predisposition to Breast and Ovarian Cancers: How Many and Which Genes to Test? Int J Mol Sci 21:. 10.3390/ijms21031128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michailidou K, Lindström S, Dennis J, et al. (2017) Association analysis identifies 65 new breast cancer risk loci. Nature 551:92–94. 10.1038/nature24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva I dos S, De Stavola B, McCormack V, Collaborative Group on Pre-Natal Risk Factors and Subsequent Risk of Breast Cancer (2008) Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med 5:e193. 10.1371/journal.pmed.0050193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, Dailey AB, Peoples-Sheps M, et al. (2009) Birth weight as a risk factor for breast cancer: a meta-analysis of 18 epidemiological studies. J Womens Health 2002 18:1169–1178. 10.1089/jwh.2008.1034 [DOI] [PubMed] [Google Scholar]
- 55.Warner ET, Hu R, Collins LC, et al. (2016) Height and Body Size in Childhood, Adolescence, and Young Adulthood and Breast Cancer Risk According to Molecular Subtype in the Nurses’ Health Studies. Cancer Prev Res Phila Pa 9:732–738. 10.1158/1940-6207.CAPR-16-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keinan-Boker L, Levine H, Derazne E, et al. (2016) Measured adolescent body mass index and adult breast cancer in a cohort of 951,480 women. Breast Cancer Res Treat 158:157–167. 10.1007/s10549-016-3860-6 [DOI] [PubMed] [Google Scholar]
- 57.Fagherazzi G, Guillas G, Boutron-Ruault M-C, et al. (2013) Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 22:29–37. 10.1097/CEJ.0b013e328355ec04 [DOI] [PubMed] [Google Scholar]
- 58.Andersen ZJ, Baker JL, Bihrmann K, et al. (2014) Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res BCR 16:R4. 10.1186/bcr3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horn-Ross PL, Canchola AJ, Bernstein L, et al. (2016) Lifetime body size and estrogen-receptor-positive breast cancer risk in the California Teachers Study cohort. Breast Cancer Res BCR 18:132. 10.1186/s13058-016-0790-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiderpass E, Braaten T, Magnusson C, et al. (2004) A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 13:1121–1127 [PubMed] [Google Scholar]
- 61.Premenopausal Breast Cancer Collaborative Group, Schoemaker MJ, Nichols HB, et al. (2018) Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol 4:e181771. 10.1001/jamaoncol.2018.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hidayat K, Yang C-M, Shi B-M (2018) Body fatness at a young age, body fatness gain and risk of breast cancer: systematic review and meta-analysis of cohort studies. Obes Rev Off J Int Assoc Study Obes 19:254–268. 10.1111/obr.12627 [DOI] [PubMed] [Google Scholar]
- 63.Chan DSM, Abar L, Cariolou M, et al. (2019) World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control CCC 30:1183–1200. 10.1007/s10552-019-01223-w [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Liu L, Zhou Q, et al. (2017) Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: a dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health 17:936. 10.1186/s12889-017-4953-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu K, Zhang W, Dai Z, et al. (2018) Association between body mass index and breast cancer risk: evidence based on a dose-response meta-analysis. Cancer Manag Res 10:143–151. 10.2147/CMAR.S144619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Brandt PA, Spiegelman D, Yaun SS, et al. (2000) Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 152:514–527 [DOI] [PubMed] [Google Scholar]
- 67.Gaudet MM, Gierach GL, Carter BD, et al. (2018) Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res 78:6011–6021. 10.1158/0008-5472.CAN-18-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang XR, Chang-Claude J, Goode EL, et al. (2011) Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst 103:250–263. 10.1093/jnci/djq526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki R, Orsini N, Saji S, et al. (2009) Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status—A meta-analysis. Int J Cancer 124:698–712. 10.1002/ijc.23943 [DOI] [PubMed] [Google Scholar]
- 70.Guo Y, Warren Andersen S, Shu X-O, et al. (2016) Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent. PLoS Med 13:e1002105. 10.1371/journal.pmed.1002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen G-C, Chen S-J, Zhang R, et al. (2016) Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev Off J Int Assoc Study Obes 17:1167–1177. 10.1111/obr.12443 [DOI] [PubMed] [Google Scholar]
- 72.Teras LR, Patel AV, Wang M, et al. (2019) Sustained weight loss and risk of breast cancer in women ≥50 years: a pooled analysis of prospective data. J Natl Cancer Inst. 10.1093/jnci/djz226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoemaker MJ, Nichols HB, Wright LB, et al. (2020) Adult weight change and premenopausal breast cancer risk: A prospective pooled analysis of data from 628,463 women. Int J Cancer. 10.1002/ijc.32892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wirén S, Häggström C, Ulmer H, et al. (2014) Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes Control CCC 25:151–159. 10.1007/s10552-013-0317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang B, Shu X-O, Delahanty RJ, et al. (2015) Height and Breast Cancer Risk: Evidence From Prospective Studies and Mendelian Randomization. J Natl Cancer Inst 107:. 10.1093/jnci/djv219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue F, Michels KB (2007) Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol 8:1088–1100. 10.1016/S1470-2045(07)70377-7 [DOI] [PubMed] [Google Scholar]
- 77.Bertrand KA, Scott CG, Tamimi RM, et al. (2015) Dense and nondense mammographic area and risk of breast cancer by age and tumor characteristics. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 24:798–809. 10.1158/1055-9965.EPI-14-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pettersson A, Graff RE, Ursin G, et al. (2014) Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 106:. 10.1093/jnci/dju078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bae J-M, Kim EH (2016) Breast Density and Risk of Breast Cancer in Asian Women: A Meta-analysis of Observational Studies. J Prev Med Public Health Yebang Uihakhoe Chi 49:367–375. 10.3961/jpmph.16.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kyanko KA, Hoag J, Busch SH, et al. (2020) Dense Breast Notification Laws, Education, and Women’s Awareness and Knowledge of Breast Density: a Nationally Representative Survey. J Gen Intern Med. 10.1007/s11606-019-05590-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen J-H, Yuan Q, Ma Y-N, et al. (2019) Relationship between bone mineral density and the risk of breast cancer: a systematic review and dose-response meta-analysis of ten cohort studies. Cancer Manag Res 11:1453–1464. 10.2147/CMAR.S188251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagel G, Peter RS, Klotz E, et al. (2017) Bone mineral density and breast cancer risk: Results from the Vorarlberg Health Monitoring & Prevention Program and meta-analysis. Bone Rep 7:83–89. 10.1016/j.bonr.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collaborative Group on Hormonal Factors in Breast Cancer (2012) Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13:1141–1151. 10.1016/S1470-2045(12)70425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collaborative Group on Hormonal Factors in Breast Cancer (2002) Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet Lond Engl 360:187–195. 10.1016/S0140-6736(02)09454-0 [DOI] [PubMed] [Google Scholar]
- 85.Nichols HB, Schoemaker MJ, Cai J, et al. (2019) Breast Cancer Risk After Recent Childbirth: A Pooled Analysis of 15 Prospective Studies. Ann Intern Med 170:22–30. 10.7326/M18-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambertini M, Santoro L, Del Mastro L, et al. (2016) Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev 49:65–76. 10.1016/j.ctrv.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 87.Ma H, Bernstein L, Pike MC, Ursin G (2006) Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res BCR 8:R43. 10.1186/bcr1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacMahon B, Cole P, Lin TM, et al. (1970) Age at first birth and breast cancer risk. Bull World Health Organ 43:209–221 [PMC free article] [PubMed] [Google Scholar]
- 89.Mallick S, Benson R, Julka PK (2016) Breast cancer prevention with anti-estrogens: review of the current evidence and future directions. Breast Cancer Tokyo Jpn 23:170–177. 10.1007/s12282-015-0647-2 [DOI] [PubMed] [Google Scholar]
- 90.Samavat H, Kurzer MS (2015) Estrogen metabolism and breast cancer. Cancer Lett 356:231–243. 10.1016/j.canlet.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, et al. (2013) Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14:1009–1019. 10.1016/S1470-2045(13)70301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.(2002) Endogenous Sex Hormones and Breast Cancer in Postmenopausal Women: Reanalysis of Nine Prospective Studies. JNCI J Natl Cancer Inst 94:606–616. 10.1093/jnci/94.8.606 [DOI] [PubMed] [Google Scholar]
- 93.Key TJ, Appleby PN, Reeves GK, et al. (2015) Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: Reanalysis of eighteen prospective studies. Steroids 99:49–55. 10.1016/j.steroids.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang M, Wu X, Chai F, et al. (2016) Plasma prolactin and breast cancer risk: a meta- analysis. Sci Rep 6:25998. 10.1038/srep25998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziegler RG, Fuhrman BJ, Moore SC, Matthews CE (2015) Epidemiologic studies of estrogen metabolism and breast cancer. Steroids 99:67–75. 10.1016/j.steroids.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sampson JN, Falk RT, Schairer C, et al. (2017) Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Res 77:918–925. 10.1158/0008-5472.CAN-16-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dallal CM, Stone RA, Cauley JA, et al. (2013) Urinary estrogen metabolites and breast cancer: a combined analysis of individual level data. Int J Biol Markers 28:3–16. 10.5301/JBM.2012.9353 [DOI] [PubMed] [Google Scholar]
- 98.Ge W, Clendenen TV, Afanasyeva Y, et al. (2018) Circulating anti-Müllerian hormone and breast cancer risk: A study in ten prospective cohorts. Int J Cancer 142:2215–2226. 10.1002/ijc.31249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He XY, Liao YD, Yu S, et al. (2015) Sex hormone binding globulin and risk of breast cancer in postmenopausal women: a meta-analysis of prospective studies. Horm Metab Res Horm Stoffwechselforschung Horm Metab 47:485–490. 10.1055/s-0034-1395606 [DOI] [PubMed] [Google Scholar]
- 100.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE (2004) Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst 96:1856–1865. 10.1093/jnci/djh336 [DOI] [PubMed] [Google Scholar]
- 101.Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, et al. (2010) Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol 11:530–542. 10.1016/S1470-2045(10)70095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy N, Knuppel A, Papadimitriou N, et al. (2020) Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, and breast cancer risk: observational and Mendelian randomization analyses with ~430 000 women. Ann Oncol 31:641–649. 10.1016/j.annonc.2020.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Autier P, Koechlin A, Boniol M, et al. (2013) Serum insulin and C-peptide concentration and breast cancer: a meta-analysis. Cancer Causes Control CCC 24:873–883. 10.1007/s10552-013-0164-6 [DOI] [PubMed] [Google Scholar]
- 104.Hernandez AV, Guarnizo M, Miranda Y, et al. (2014) Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis. PloS One 9:e99317. 10.1371/journal.pone.0099317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen J, Hernandez D, Ye Y, et al. (2019) Metabolic hormones and breast cancer risk among Mexican American Women in the Mano a Mano Cohort Study. Sci Rep 9:9989. 10.1038/s41598-019-46429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahern TP, Hankinson SE, Willett WC, et al. (2013) Plasma C-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 22:1786–1796. 10.1158/1055-9965.EPI-13-0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaudet MM, Patel AV, Teras LR, et al. (2013) Obesity-related markers and breast cancer in CPS-II Nutrition Cohort. Int J Mol Epidemiol Genet 4:156–166 [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Lee I-M, Tworoger SS, et al. (2015) Plasma C-reactive protein and risk of breast cancer in two prospective studies and a meta-analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 24:1199–1206. 10.1158/1055-9965.EPI-15-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan DSM, Bandera EV, Greenwood DC, Norat T (2015) Circulating C-Reactive Protein and Breast Cancer Risk-Systematic Literature Review and Meta-analysis of Prospective Cohort Studies. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 24:1439–1449. 10.1158/1055-9965.EPI-15-0324 [DOI] [PubMed] [Google Scholar]
- 110.Guo L, Liu S, Zhang S, et al. (2015) C-reactive protein and risk of breast cancer: A systematic review and meta-analysis. Sci Rep 5:10508. 10.1038/srep10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pan H, Deng L-L, Cui J-Q, et al. (2018) Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine (Baltimore) 97:e11345. 10.1097/MD.0000000000011345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu Z, Tang S, Ma H, et al. (2019) Association of serum adiponectin with breast cancer: A meta-analysis of 27 case-control studies. Medicine (Baltimore) 98:e14359. 10.1097/MD.0000000000014359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu J, Huang L, Sun G-P (2017) Urinary 6-sulfatoxymelatonin level and breast cancer risk: systematic review and meta-analysis. Sci Rep 7:5353. 10.1038/s41598-017-05752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Veiga EC de A, Simões R, Valenti VE, et al. (2019) Repercussions of melatonin on the risk of breast cancer: a systematic review and meta-analysis. Rev Assoc Medica Bras 1992 65:699–705. 10.1590/1806-9282.65.5.699 [DOI] [PubMed] [Google Scholar]
- 115.Ji L-W, Jing C-X, Zhuang S-L, et al. (2019) Effect of age at first use of oral contraceptives on breast cancer risk: An updated meta-analysis. Medicine (Baltimore) 98:e15719. 10.1097/MD.0000000000015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu H, Lei X, Feng J, Wang Y (2012) Oral contraceptive use and risk of breast cancer: a meta-analysis of prospective cohort studies. Eur J Contracept Reprod Health Care Off J Eur Soc Contracept 17:402–414. 10.3109/13625187.2012.715357 [DOI] [PubMed] [Google Scholar]
- 117.(1996) Breast cancer and hormonal contraceptives: further results. Collaborative Group on Hormonal Factors in Breast Cancer. Contraception 54:1S–106S. 10.1016/s0010-7824(15)30002-0 [DOI] [PubMed] [Google Scholar]
- 118.Conz L, Mota BS, Bahamondes L, et al. (2020) Levonorgestrel-releasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 10.1111/aogs.13817 [DOI] [PubMed] [Google Scholar]
- 119.Collaborative Group on Hormonal Factors in Breast Cancer (2019) Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet Lond Engl 394:1159–1168. 10.1016/S0140-6736(19)31709-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jordan VC (2015) The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer. Endocr Relat Cancer 22:R1–31. 10.1530/ERC-14-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Key TJ, Appleby PN, Cairns BJ, et al. (2011) Dietary fat and breast cancer: comparison of results from food diaries and food-frequency questionnaires in the UK Dietary Cohort Consortium. Am J Clin Nutr 94:1043–1052. 10.3945/ajcn.111.015735 [DOI] [PubMed] [Google Scholar]
- 122.Smith-Warner SA, Spiegelman D, Adami HO, et al. (2001) Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int J Cancer 92:767–774. [DOI] [PubMed] [Google Scholar]
- 123.Anjom-Shoae J, Sadeghi O, Larijani B, Esmaillzadeh A (2020) Dietary intake and serum levels of trans fatty acids and risk of breast cancer: A systematic review and dose-response meta-analysis of prospective studies. Clin Nutr Edinb Scotl 39:755–764. 10.1016/j.clnu.2019.03.024 [DOI] [PubMed] [Google Scholar]
- 124.Cao Y, Hou L, Wang W (2016) Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int J Cancer 138:1894–1904. 10.1002/ijc.29938 [DOI] [PubMed] [Google Scholar]
- 125.Alexander DD, Morimoto LM, Mink PJ, Lowe KA (2010) Summary and meta-analysis of prospective studies of animal fat intake and breast cancer. Nutr Res Rev 23:169–179. 10.1017/S095442241000003X [DOI] [PubMed] [Google Scholar]
- 126.Hunter DJ, Spiegelman D, Adami HO, et al. (1996) Cohort studies of fat intake and the risk of breast cancer--a pooled analysis. N Engl J Med 334:356–361. 10.1056/NEJM199602083340603 [DOI] [PubMed] [Google Scholar]
- 127.Schlesinger S, Chan DSM, Vingeliene S, et al. (2017) Carbohydrates, glycemic index, glycemic load, and breast cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Nutr Rev 75:420–441. 10.1093/nutrit/nux010 [DOI] [PubMed] [Google Scholar]
- 128.Mullie P, Koechlin A, Boniol M, et al. (2016) Relation between Breast Cancer and High Glycemic Index or Glycemic Load: A Meta-analysis of Prospective Cohort Studies. Crit Rev Food Sci Nutr 56:152–159. 10.1080/10408398.2012.718723 [DOI] [PubMed] [Google Scholar]
- 129.Chen S, Chen Y, Ma S, et al. (2016) Dietary fibre intake and risk of breast cancer: A systematic review and meta-analysis of epidemiological studies. Oncotarget 7:80980–80989. 10.18632/oncotarget.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aune D, Chan DSM, Greenwood DC, et al. (2012) Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol Off J Eur Soc Med Oncol 23:1394–1402. 10.1093/annonc/mdr589 [DOI] [PubMed] [Google Scholar]
- 131.Dong J-Y, He K, Wang P, Qin L-Q (2011) Dietary fiber intake and risk of breast cancer: a meta-analysis of prospective cohort studies. Am J Clin Nutr 94:900–905. 10.3945/ajcn.111.015578 [DOI] [PubMed] [Google Scholar]
- 132.Farvid MS, Stern MC, Norat T, et al. (2018) Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int J Cancer 143:2787–2799. 10.1002/ijc.31848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anderson JJ, Darwis NDM, Mackay DF, et al. (2018) Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur J Cancer Oxf Engl 1990 90:73–82. 10.1016/j.ejca.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 134.Wu J, Zeng R, Huang J, et al. (2016) Dietary Protein Sources and Incidence of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 8:. 10.3390/nu8110730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han MA, Zeraatkar D, Guyatt GH, et al. (2019) Reduction of Red and Processed Meat Intake and Cancer Mortality and Incidence: A Systematic Review and Meta-analysis of Cohort Studies. Ann Intern Med 171:711–720. 10.7326/M19-0699 [DOI] [PubMed] [Google Scholar]
- 136.Missmer SA, Smith-Warner SA, Spiegelman D, et al. (2002) Meat and dairy food consumption and breast cancer: a pooled analysis of cohort studies. Int J Epidemiol 31:78–85. 10.1093/ije/31.1.78 [DOI] [PubMed] [Google Scholar]
- 137.Dong J-Y, Zhang L, He K, Qin L-Q (2011) Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat 127:23–31. 10.1007/s10549-011-1467-5 [DOI] [PubMed] [Google Scholar]
- 138.Chen L, Li M, Li H (2019) Milk and yogurt intake and breast cancer risk: A meta-analysis. Medicine (Baltimore) 98:e14900. 10.1097/MD.0000000000014900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jung S, Spiegelman D, Baglietto L, et al. (2013) Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst 105:219–236. 10.1093/jnci/djs635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aune D, Chan DSM, Vieira AR, et al. (2012) Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 134:479–493. 10.1007/s10549-012-2118-1 [DOI] [PubMed] [Google Scholar]
- 141.Smith-Warner SA, Spiegelman D, Yaun SS, et al. (2001) Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA 285:769–776. 10.1001/jama.285.6.769 [DOI] [PubMed] [Google Scholar]
- 142.Jiang W, Wu Y, Jiang X (2013) Coffee and caffeine intake and breast cancer risk: an updated dose-response meta-analysis of 37 published studies. Gynecol Oncol 129:620–629. 10.1016/j.ygyno.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 143.Lafranconi A, Micek A, De Paoli P, et al. (2018) Coffee Intake Decreases Risk of Postmenopausal Breast Cancer: A Dose-Response Meta-Analysis on Prospective Cohort Studies. Nutrients 10:. 10.3390/nu10020112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y, Zhao Y, Chong F, et al. (2020) A dose-response meta-analysis of green tea consumption and breast cancer risk. Int J Food Sci Nutr 1–12. 10.1080/09637486.2020.1715353 [DOI] [PubMed] [Google Scholar]
- 145.Bhoo-Pathy N, Peeters PHM, Uiterwaal CSPM, et al. (2015) Coffee and tea consumption and risk of pre- and postmenopausal breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Breast Cancer Res BCR 17:15. 10.1186/s13058-015-0521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu S, Zhu L, Wang K, et al. (2019) Green tea consumption and risk of breast cancer: A systematic review and updated meta-analysis of case-control studies. Medicine (Baltimore) 98:e16147. 10.1097/MD.0000000000016147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bristow SM, Bolland MJ, MacLennan GS, et al. (2013) Calcium supplements and cancer risk: a meta-analysis of randomised controlled trials. Br J Nutr 110:1384–1393. 10.1017/S0007114513001050 [DOI] [PubMed] [Google Scholar]
- 148.Hidayat K, Chen G-C, Zhang R, et al. (2016) Calcium intake and breast cancer risk: meta-analysis of prospective cohort studies. Br J Nutr 116:158–166. 10.1017/S0007114516001768 [DOI] [PubMed] [Google Scholar]
- 149.Wulaningsih W, Sagoo HK, Hamza M, et al. (2016) Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies. Int J Mol Sci 17:. 10.3390/ijms17091487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Estébanez N, Gómez-Acebo I, Palazuelos C, et al. (2018) Vitamin D exposure and Risk of Breast Cancer: a meta-analysis. Sci Rep 8:9039. 10.1038/s41598-018-27297-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sperati F, Vici P, Maugeri-Saccà M, et al. (2013) Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PloS One 8:e69269. 10.1371/journal.pone.0069269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hossain S, Beydoun MA, Beydoun HA, et al. (2019) Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin Nutr ESPEN 30:170–184. 10.1016/j.clnesp.2018.12.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Song D, Deng Y, Liu K, et al. (2019) Vitamin D intake, blood vitamin D levels, and the risk of breast cancer: a dose-response meta-analysis of observational studies. Aging 11:12708–12732. 10.18632/aging.102597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eliassen AH, Hendrickson SJ, Brinton LA, et al. (2012) Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst 104:1905–1916. 10.1093/jnci/djs461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X, Spiegelman D, Baglietto L, et al. (2012) Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr 95:713–725. 10.3945/ajcn.111.014415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aune D, Chan DSM, Vieira AR, et al. (2012) Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 96:356–373. 10.3945/ajcn.112.034165 [DOI] [PubMed] [Google Scholar]
- 157.Druesne-Pecollo N, Latino-Martel P, Norat T, et al. (2010) Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer 127:172–184. 10.1002/ijc.25008 [DOI] [PubMed] [Google Scholar]
- 158.Chang VC, Cotterchio M, Khoo E (2019) Iron intake, body iron status, and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer 19:543. 10.1186/s12885-019-5642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fulan H, Changxing J, Baina WY, et al. (2011) Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control CCC 22:1383–1396. 10.1007/s10552-011-9811-y [DOI] [PubMed] [Google Scholar]
- 160.Wu W, Kang S, Zhang D (2013) Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: a dose-response meta-analysis. Br J Cancer 109:1926–1944. 10.1038/bjc.2013.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu L, Tan Y, Zhu L (2017) Dietary vitamin B2 intake and breast cancer risk: a systematic review and meta-analysis. Arch Gynecol Obstet 295:721–729. 10.1007/s00404-016-4278-4 [DOI] [PubMed] [Google Scholar]
- 162.Hu F, Wu Z, Li G, et al. (2015) The plasma level of retinol, vitamins A, C and α-tocopherol could reduce breast cancer risk? A meta-analysis and meta-regression. J Cancer Res Clin Oncol 141:601–614. 10.1007/s00432-014-1852-7 [DOI] [PubMed] [Google Scholar]
- 163.Hutchinson J, Lentjes M a. H, Greenwood DC, et al. (2012) Vitamin C intake from diary recordings and risk of breast cancer in the UK Dietary Cohort Consortium. Eur J Clin Nutr 66:561–568. 10.1038/ejcn.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen P, Li C, Li X, et al. (2014) Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br J Cancer 110:2327–2338. 10.1038/bjc.2014.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Y-F, Shi W-W, Gao H-F, et al. (2014) Folate intake and the risk of breast cancer: a dose-response meta-analysis of prospective studies. PloS One 9:e100044. 10.1371/journal.pone.0100044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qin X, Cui Y, Shen L, et al. (2013) Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials. Int J Cancer 133:1033–1041. 10.1002/ijc.28038 [DOI] [PubMed] [Google Scholar]
- 167.Tio M, Andrici J, Eslick GD (2014) Folate intake and the risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 145:513–524. 10.1007/s10549-014-2969-8 [DOI] [PubMed] [Google Scholar]
- 168.Zeng J, Wang K, Ye F, et al. (2019) Folate intake and the risk of breast cancer: an up-to-date meta-analysis of prospective studies. Eur J Clin Nutr 73:1657–1660. 10.1038/s41430-019-0394-0 [DOI] [PubMed] [Google Scholar]
- 169.Cai X, Wang C, Yu W, et al. (2016) Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Sci Rep 6:19213. 10.1038/srep19213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Babaknejad N, Sayehmiri F, Sayehmiri K, et al. (2014) The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biol Trace Elem Res 159:1–7. 10.1007/s12011-014-9998-3 [DOI] [PubMed] [Google Scholar]
- 171.Velentzis LS, Cantwell MM, Cardwell C, et al. (2009) Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies. Br J Cancer 100:1492–1498. 10.1038/sj.bjc.6605003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Buck K, Zaineddin AK, Vrieling A, et al. (2010) Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr 92:141–153. 10.3945/ajcn.2009.28573 [DOI] [PubMed] [Google Scholar]
- 173.Chen M, Rao Y, Zheng Y, et al. (2014) Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PloS One 9:e89288. 10.1371/journal.pone.0089288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao T-T, Jin F, Li J-G, et al. (2019) Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: A meta-analysis of prospective cohort studies. Clin Nutr Edinb Scotl 38:136–145. 10.1016/j.clnu.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 175.Wei Y, Lv J, Guo Y, et al. (2020) Soy intake and breast cancer risk: a prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur J Epidemiol 35:567–578. 10.1007/s10654-019-00585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Q, Liu X, Ren S (2020) Tofu intake is inversely associated with risk of breast cancer: A meta-analysis of observational studies. PloS One 15:e0226745. 10.1371/journal.pone.0226745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chan ALF, Leung HWC, Wang S-F (2011) Multivitamin supplement use and risk of breast cancer: a meta-analysis. Ann Pharmacother 45:476–484. 10.1345/aph.1P445 [DOI] [PubMed] [Google Scholar]
- 178.Smith-Warner SA, Spiegelman D, Yaun SS, et al. (1998) Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279:535–540. 10.1001/jama.279.7.535 [DOI] [PubMed] [Google Scholar]
- 179.Bagnardi V, Rota M, Botteri E, et al. (2015) Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 112:580–593. 10.1038/bjc.2014.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jung S, Wang M, Anderson K, et al. (2016) Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol 45:916–928. 10.1093/ije/dyv156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Y, Nguyen N, Colditz GA (2015) Links between alcohol consumption and breast cancer: a look at the evidence. Womens Health Lond Engl 11:65–77. 10.2217/whe.14.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hamajima N, Hirose K, Tajima K, et al. (2002) Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer 87:1234–1245. 10.1038/sj.bjc.6600596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Keogh RH, Park JY, White IR, et al. (2012) Estimating the alcohol-breast cancer association: a comparison of diet diaries, FFQs and combined measurements. Eur J Epidemiol 27:547–559. 10.1007/s10654-012-9693-7 [DOI] [PubMed] [Google Scholar]
- 184.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 9:. 10.3390/nu9101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van den Brandt PA, Schulpen M (2017) Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer 140:2220–2231. 10.1002/ijc.30654 [DOI] [PubMed] [Google Scholar]
- 186.Grosso G, Bella F, Godos J, et al. (2017) Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev 75:405–419. 10.1093/nutrit/nux012 [DOI] [PubMed] [Google Scholar]
- 187.Xiao Y, Xia J, Li L, et al. (2019) Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res BCR 21:16. 10.1186/s13058-019-1096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pot GK, Stephen AM, Dahm CC, et al. (2014) Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK Dietary Cohort Consortium. Eur J Clin Nutr 68:1353–1358. 10.1038/ejcn.2014.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Toledo E, Salas-Salvadó J, Donat-Vargas C, et al. (2015) Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern Med 175:1752–1760. 10.1001/jamainternmed.2015.4838 [DOI] [PubMed] [Google Scholar]
- 190.Macacu A, Autier P, Boniol M, Boyle P (2015) Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat 154:213–224. 10.1007/s10549-015-3628-4 [DOI] [PubMed] [Google Scholar]
- 191.Lee PN, Hamling JS (2016) Environmental tobacco smoke exposure and risk of breast cancer in nonsmoking women. An updated review and meta-analysis. Inhal Toxicol 28:431–454. 10.1080/08958378.2016.1210701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim A-S, Ko H-J, Kwon J-H, Lee J-M (2018) Exposure to Secondhand Smoke and Risk of Cancer in Never Smokers: A Meta-Analysis of Epidemiologic Studies. Int J Environ Res Public Health 15:. 10.3390/ijerph15091981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang Y, Zhang F, Skrip L, et al. (2013) Lack of an association between passive smoking and incidence of female breast cancer in non-smokers: evidence from 10 prospective cohort studies. PloS One 8:e77029. 10.1371/journal.pone.0077029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Andersen ZJ, Stafoggia M, Weinmayr G, et al. (2017) Long-Term Exposure to Ambient Air Pollution and Incidence of Postmenopausal Breast Cancer in 15 European Cohorts within the ESCAPE Project. Environ Health Perspect 125:107005. 10.1289/EHP1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang Z, Yan W, Chen Q, et al. (2019) The relationship between exposure to particulate matter and breast cancer incidence and mortality: A meta-analysis. Medicine (Baltimore) 98:e18349. 10.1097/MD.0000000000018349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen Q, Lang L, Wu W, et al. (2013) A meta-analysis on the relationship between exposure to ELF-EMFs and the risk of female breast cancer. PloS One 8:e69272. 10.1371/journal.pone.0069272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhao G, Lin X, Zhou M, Zhao J (2014) Relationship between exposure to extremely low-frequency electromagnetic fields and breast cancer risk: a meta-analysis. Eur J Gynaecol Oncol 35:264–269 [PubMed] [Google Scholar]
- 198.Zhang Y, Lai J, Ruan G, et al. (2016) Meta-analysis of extremely low frequency electromagnetic fields and cancer risk: a pooled analysis of epidemiologic studies. Environ Int 88:36–43. 10.1016/j.envint.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 199.Guo J-Y, Wang M-Z, Wang M-S, et al. (2020) The Undervalued Effects of Polychlorinated Biphenyl Exposure on Breast Cancer. Clin Breast Cancer 20:12–18. 10.1016/j.clbc.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 200.Zhang J, Huang Y, Wang X, et al. (2015) Environmental Polychlorinated Biphenyl Exposure and Breast Cancer Risk: A Meta-Analysis of Observational Studies. PloS One 10:e0142513. 10.1371/journal.pone.0142513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leng L, Li J, Luo X-M, et al. (2016) Polychlorinated biphenyls and breast cancer: A congener-specific meta-analysis. Environ Int 88:133–141. 10.1016/j.envint.2015.12.022 [DOI] [PubMed] [Google Scholar]
- 202.Ingber SZ, Buser MC, Pohl HR, et al. (2013) DDT/DDE and breast cancer: a meta-analysis. Regul Toxicol Pharmacol RTP 67:421–433. 10.1016/j.yrtph.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Adani G, Filippini T, Wise LA, et al. (2020) Dietary Intake of Acrylamide and Risk of Breast, Endometrial, and Ovarian Cancers: A Systematic Review and Dose–Response Meta-analysis. Cancer Epidemiol Prev Biomark 29:1095–1106. 10.1158/1055-9965.EPI-19-1628 [DOI] [PubMed] [Google Scholar]
- 204.Barcellos-Hoff MH (2013) New biological insights on the link between radiation exposure and breast cancer risk. J Mammary Gland Biol Neoplasia 18:3–13. 10.1007/s10911-013-9272-x [DOI] [PubMed] [Google Scholar]
- 205.Preston DL, Mattsson A, Holmberg E, et al. (2002) Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res 158:220–235. 10.1667/0033-7587(2002)158[0220:reobcr]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 206.Pizot C, Boniol M, Mullie P, et al. (2016) Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. Eur J Cancer Oxf Engl 1990 52:138–154. 10.1016/j.ejca.2015.10.063 [DOI] [PubMed] [Google Scholar]
- 207.Neilson HK, Farris MS, Stone CR, et al. (2017) Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: a systematic review and meta-analysis. Menopause N Y N 24:322–344. 10.1097/GME.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 208.Chen X, Wang Q, Zhang Y, et al. (2019) Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health J Int Soc Pharmacoeconomics Outcomes Res 22:104–128. 10.1016/j.jval.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 209.Gong Z, Hong C-C, Bandera EV, et al. (2016) Vigorous physical activity and risk of breast cancer in the African American breast cancer epidemiology and risk consortium. Breast Cancer Res Treat 159:347–356. 10.1007/s10549-016-3936-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hidayat K, Zhou H-J, Shi B-M (2020) Influence of physical activity at a young age and lifetime physical activity on the risks of 3 obesity-related cancers: systematic review and meta-analysis of observational studies. Nutr Rev 78:1–18. 10.1093/nutrit/nuz024 [DOI] [PubMed] [Google Scholar]
- 211.Jochem C, Wallmann-Sperlich B, Leitzmann MF (2019) The Influence of Sedentary Behavior on Cancer Risk: Epidemiologic Evidence and Potential Molecular Mechanisms. Curr Nutr Rep 8:167–174. 10.1007/s13668-019-0263-4 [DOI] [PubMed] [Google Scholar]
- 212.Zhou Y, Zhao H, Peng C (2015) Association of sedentary behavior with the risk of breast cancer in women: update meta-analysis of observational studies. Ann Epidemiol 25:687–697. 10.1016/j.annepidem.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 213.Gaudet MM, Carter BD, Brinton LA, et al. (2017) Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int J Epidemiol 46:881–893. 10.1093/ije/dyw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Connor AE, Baumgartner KB, Baumgartner RN, et al. (2016) Cigarette Smoking and Breast Cancer Risk in Hispanic and Non-Hispanic White Women: The Breast Cancer Health Disparities Study. J Womens Health 25:299–310. 10.1089/jwh.2015.5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gera R, Mokbel R, Igor I, Mokbel K (2018) Does the Use of Hair Dyes Increase the Risk of Developing Breast Cancer? A Meta-analysis and Review of the Literature. Anticancer Res 38:707–716. 10.21873/anticanres.12276 [DOI] [PubMed] [Google Scholar]
- 216.Eberle CE, Sandler DP, Taylor KW, White AJ (2020) Hair dye and chemical straightener use and breast cancer risk in a large US population of black and white women. Int J Cancer 147:383–391. 10.1002/ijc.32738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cordina-Duverger E, Menegaux F, Popa A, et al. (2018) Night shift work and breast cancer: a pooled analysis of population-based case-control studies with complete work history. Eur J Epidemiol 33:369–379. 10.1007/s10654-018-0368-x [DOI] [PubMed] [Google Scholar]
- 218.Travis RC, Balkwill A, Fensom GK, et al. (2016) Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of Published Studies. J Natl Cancer Inst 108:. 10.1093/jnci/djw169 [DOI] [Google Scholar]
- 219.Pahwa M, Labrèche F, Demers PA (2018) Night shift work and breast cancer risk: what do the meta-analyses tell us? Scand J Work Environ Health 44:432–435. 10.5271/sjweh.3738 [DOI] [PubMed] [Google Scholar]
- 220.Yang W-S, Deng Q, Fan W-Y, et al. (2014) Light exposure at night, sleep duration, melatonin, and breast cancer: a dose-response analysis of observational studies. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 23:269–276. 10.1097/CEJ.0000000000000030 [DOI] [PubMed] [Google Scholar]
- 221.He C, Anand ST, Ebell MH, et al. (2015) Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health 88:533–547. 10.1007/s00420-014-0986-x [DOI] [PubMed] [Google Scholar]
- 222.Lu C, Sun H, Huang J, et al. (2017) Long-Term Sleep Duration as a Risk Factor for Breast Cancer: Evidence from a Systematic Review and Dose-Response Meta-Analysis. BioMed Res Int 2017:4845059. 10.1155/2017/4845059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Qin Y, Zhou Y, Zhang X, et al. (2014) Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer 134:1166–1173. 10.1002/ijc.28452 [DOI] [PubMed] [Google Scholar]
- 224.Bahri N, Fathi Najafi T, Homaei Shandiz F, et al. (2019) The relation between stressful life events and breast cancer: a systematic review and meta-analysis of cohort studies. Breast Cancer Res Treat 176:53–61. 10.1007/s10549-019-05231-x [DOI] [PubMed] [Google Scholar]
- 225.Sergentanis TN, Zagouri F, Zografos GC (2010) Is antibiotic use a risk factor for breast cancer? A meta-analysis. Pharmacoepidemiol Drug Saf 19:1101–1107. 10.1002/pds.1986 [DOI] [PubMed] [Google Scholar]
- 226.Eom C-S, Park SM, Cho K-H (2012) Use of antidepressants and the risk of breast cancer: a meta-analysis. Breast Cancer Res Treat 136:635–645. 10.1007/s10549-012-2307-y [DOI] [PubMed] [Google Scholar]
- 227.Lu L, Shi L, Zeng J, Wen Z (2017) Aspirin as a potential modality for the chemoprevention of breast cancer: A dose-response meta-analysis of cohort studies from 857,831 participants. Oncotarget 8:40389–40401. 10.18632/oncotarget.16315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhong S, Chen L, Zhang X, et al. (2015) Aspirin use and risk of breast cancer: systematic review and meta-analysis of observational studies. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 24:1645–1655. 10.1158/1055-9965.EPI-15-0452 [DOI] [PubMed] [Google Scholar]
- 229.de Pedro M, Baeza S, Escudero M-T, et al. (2015) Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res Treat 149:525–536. 10.1007/s10549-015-3267-9 [DOI] [PubMed] [Google Scholar]
- 230.Liu Y, Zhang X, Sun H, et al. (2019) Bisphosphonates and primary breast cancer risk: an updated systematic review and meta-analysis involving 963,995 women. Clin Epidemiol 11:593–603. 10.2147/CLEP.S194056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fournier A, Mesrine S, Gelot A, et al. (2017) Use of Bisphosphonates and Risk of Breast Cancer in a French Cohort of Postmenopausal Women. J Clin Oncol Off J Am Soc Clin Oncol 35:3230–3239. 10.1200/JCO.2016.71.4337 [DOI] [PubMed] [Google Scholar]
- 232.Rouach V, Goldshtein I, Buch A, et al. (2019) The association between adherence with oral bisphosphonates and the risk of breast cancer in post-menopausal women. J Bone Oncol 16:100202. 10.1016/j.jbo.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gennari A, Costa M, Puntoni M, et al. (2015) Breast cancer incidence after hormonal treatments for infertility: systematic review and meta-analysis of population-based studies. Breast Cancer Res Treat 150:405–413. 10.1007/s10549-015-3328-0 [DOI] [PubMed] [Google Scholar]
- 234.Sergentanis TN, Diamantaras A-A, Perlepe C, et al. (2014) IVF and breast cancer: a systematic review and meta-analysis. Hum Reprod Update 20:106–123. 10.1093/humupd/dmt034 [DOI] [PubMed] [Google Scholar]
- 235.Islam MM, Yang H-C, Nguyen P-A, et al. (2017) Exploring association between statin use and breast cancer risk: an updated meta-analysis. Arch Gynecol Obstet 296:1043–1053. 10.1007/s00404-017-4533-3 [DOI] [PubMed] [Google Scholar]
- 236.Colton T, Greenberg ER, Noller K, et al. (1993) Breast cancer in mothers prescribed diethylstilbestrol in pregnancy. Further follow-up. JAMA 269:2096–2100 [PubMed] [Google Scholar]
- 237.Greenberg ER, Barnes AB, Resseguie L, et al. (1984) Breast cancer in mothers given diethylstilbestrol in pregnancy. N Engl J Med 311:1393–1398. 10.1056/NEJM198411293112201 [DOI] [PubMed] [Google Scholar]
- 238.Hadjimichael OC, Meigs JW, Falcier FW, et al. (1984) Cancer risk among women exposed to exogenous estrogens during pregnancy. J Natl Cancer Inst 73:831–834 [PubMed] [Google Scholar]
- 239.Al Jishi T, Sergi C (2017) Current perspective of diethylstilbestrol (DES) exposure in mothers and offspring. Reprod Toxicol Elmsford N 71:71–77. 10.1016/j.reprotox.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 240.Titus-Ernstoff L, Hatch EE, Hoover RN, et al. (2001) Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer 84:126–133. 10.1054/bjoc.2000.1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Palmer JR, Wise LA, Hatch EE, et al. (2006) Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 15:1509–1514. 10.1158/1055-9965.EPI-06-0109 [DOI] [PubMed] [Google Scholar]
- 242.Troisi R, Hatch EE, Titus L, et al. (2019) Prenatal diethylstilbestrol exposure and cancer risk in women. Environ Mol Mutagen 60:395–403. 10.1002/em.22155 [DOI] [PubMed] [Google Scholar]
- 243.Cohn BA, La Merrill M, Krigbaum NY, et al. (2015) DDT Exposure in Utero and Breast Cancer. J Clin Endocrinol Metab 100:2865–2872. 10.1210/jc.2015-1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tournaire M, Devouche E, Espié M, et al. (2015) Cancer Risk in Women Exposed to Diethylstilbestrol in Utero. Therapie 70:433–441. 10.2515/therapie/2015030 [DOI] [PubMed] [Google Scholar]
- 245.Guo J, Huang Y, Yang L, et al. (2015) Association between abortion and breast cancer: an updated systematic review and meta-analysis based on prospective studies. Cancer Causes Control CCC 26:811–819. 10.1007/s10552-015-0536-1 [DOI] [PubMed] [Google Scholar]
- 246.Deng Y, Xu H, Zeng X (2018) Induced abortion and breast cancer: An updated meta-analysis. Medicine (Baltimore) 97:e9613. 10.1097/MD.0000000000009613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Beral V, Bull D, Doll R, et al. (2004) Breast cancer and abortion: collaborative reanalysis of data from 53 epidemiological studies, including 83?000 women with breast cancer from 16 countries. Lancet Lond Engl 363:1007–1016. 10.1016/S0140-6736(04)15835-2 [DOI] [PubMed] [Google Scholar]
- 248.Tong H, Wu Y, Yan Y, et al. (2020) No association between abortion and risk of breast cancer among nulliparous women. Medicine (Baltimore) 99:. 10.1097/MD.0000000000020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Salamat F, Niakan B, Keshtkar A, et al. (2018) Subtypes of Benign Breast Disease as a Risk Factor of Breast Cancer: A Systematic Review and Meta Analyses. Iran J Med Sci 43:355–364 [PMC free article] [PubMed] [Google Scholar]
- 250.Dyrstad SW, Yan Y, Fowler AM, Colditz GA (2015) Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat 149:569–575. 10.1007/s10549-014-3254-6 [DOI] [PubMed] [Google Scholar]
- 251.Visser LL, Elshof LE, Schaapveld M, et al. (2018) Clinicopathological Risk Factors for an Invasive Breast Cancer Recurrence after Ductal Carcinoma In Situ-A Nested Case-Control Study. Clin Cancer Res Off J Am Assoc Cancer Res 24:3593–3601. 10.1158/1078-0432.CCR-18-0201 [DOI] [PubMed] [Google Scholar]
- 252.Zhang X, Dai H, Liu B, et al. (2016) Predictors for local invasive recurrence of ductal carcinoma in situ of the breast: a meta-analysis. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 25:19–28. 10.1097/CEJ.0000000000000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mannu GS, Wang Z, Broggio J, et al. (2020) Invasive breast cancer and breast cancer mortality after ductal carcinoma in situ in women attending for breast screening in England, 1988-2014: population based observational cohort study. BMJ 369:. 10.1136/bmj.m1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dania V, Liu Y, Ademuyiwa F, et al. (2019) Associations of race and ethnicity with risk of developing invasive breast cancer after lobular carcinoma in situ. Breast Cancer Res BCR 21:120. 10.1186/s13058-019-1219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Levi F, Randimbison L, Te V-C, La Vecchia C (2005) Invasive breast cancer following ductal and lobular carcinoma in situ of the breast. Int J Cancer 116:820–823. 10.1002/ijc.20870 [DOI] [PubMed] [Google Scholar]
- 256.Chuba PJ, Hamre MR, Yap J, et al. (2005) Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol Off J Am Soc Clin Oncol 23:5534–5541. 10.1200/JCO.2005.04.038 [DOI] [PubMed] [Google Scholar]
- 257.McDivitt RW, Hutter RV, Foote FW, Stewart FW (1967) In situ lobular carcinoma. A prospective follow-up study indicating cumulative patient risks. JAMA 201:82–86. 10.1001/jama.201.2.82 [DOI] [PubMed] [Google Scholar]
- 258.King TA, Pilewskie M, Muhsen S, et al. (2015) Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol Off J Am Soc Clin Oncol 33:3945–3952. 10.1200/JCO.2015.61.4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wong SM, King T, Boileau J-F, et al. (2017) Population-Based Analysis of Breast Cancer Incidence and Survival Outcomes in Women Diagnosed with Lobular Carcinoma In Situ. Ann Surg Oncol 24:2509–2517. 10.1245/s10434-017-5867-6 [DOI] [PubMed] [Google Scholar]
- 260.Page DL, Kidd TE, Dupont WD, et al. (1991) Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 22:1232–1239. 10.1016/0046-8177(91)90105-x [DOI] [PubMed] [Google Scholar]
- 261.Sona MF, Myung S-K, Park K, Jargalsaikhan G (2018) Type 1 diabetes mellitus and risk of cancer: a meta-analysis of observational studies. Jpn J Clin Oncol 48:426–433. 10.1093/jjco/hyy047 [DOI] [PubMed] [Google Scholar]
- 262.Boyle P, Boniol M, Koechlin A, et al. (2012) Diabetes and breast cancer risk: a meta-analysis. Br J Cancer 107:1608–1617. 10.1038/bjc.2012.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hope C, Robertshaw A, Cheung KL, et al. (2016) Relationship between HbA1c and cancer in people with or without diabetes: a systematic review. Diabet Med J Br Diabet Assoc 33:1013–1025. 10.1111/dme.13031 [DOI] [PubMed] [Google Scholar]
- 264.Xie C, Wang W, Li X, et al. (2019) Gestational diabetes mellitus and maternal breast cancer risk: a meta-analysis of the literature. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 32:1022–1032. 10.1080/14767058.2017.1397117 [DOI] [PubMed] [Google Scholar]
- 265.Tang GH, Satkunam M, Pond GR, et al. (2018) Association of Metformin with Breast Cancer Incidence and Mortality in Patients with Type II Diabetes: A GRADE-Assessed Systematic Review and Meta-analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 27:627–635. 10.1158/1055-9965.EPI-17-0936 [DOI] [PubMed] [Google Scholar]
- 266.Guo M, Liu T, Li P, et al. (2019) Association Between Metabolic Syndrome and Breast Cancer Risk: An Updated Meta-Analysis of Follow-Up Studies. Front Oncol 9:1290. 10.3389/fonc.2019.01290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wu X, Wang M, Li S, Zhang Y (2016) Migraine and breast cancer risk: a meta-analysis of observational studies based on MOOSE compliant. Medicine (Baltimore) 95:e4031. 10.1097/MD.0000000000004031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Winter AC, Rice MS, Fortner RT, et al. (2015) Migraine and breast cancer risk: a prospective cohort study and meta-analysis. J Natl Cancer Inst 107:381. 10.1093/jnci/dju381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Preston DL, Kitahara CM, Freedman DM, et al. (2016) Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983-2008. Br J Cancer 115:1105–1112. 10.1038/bjc.2016.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rodgers KM, Udesky JO, Rudel RA, Brody JG (2018) Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ Res 160:152–182. 10.1016/j.envres.2017.08.045 [DOI] [PubMed] [Google Scholar]
- 271.Parada H, Gammon MD, Ettore HL, et al. (2019) Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ Int 130:104890. 10.1016/j.envint.2019.05.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Trabert B, Falk RT, Figueroa JD, et al. (2014) Urinary bisphenol A-glucuronide and postmenopausal breast cancer in Poland. Cancer Causes Control CCC 25:1587–1593. 10.1007/s10552-014-0461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Morgan M, Deoraj A, Felty Q, Roy D (2017) Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol Cell Endocrinol 457:89–102. 10.1016/j.mce.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 274.Ahern TP, Broe A, Lash TL, et al. (2019) Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study. J Clin Oncol Off J Am Soc Clin Oncol 37:1800–1809. 10.1200/JCO.18.02202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Reeves KW, Díaz Santana M, Manson JE, et al. (2019) Urinary Phthalate Biomarker Concentrations and Postmenopausal Breast Cancer Risk. J Natl Cancer Inst 111:1059–1067. 10.1093/jnci/djz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zuccarello P, Oliveri Conti G, Cavallaro F, et al. (2018) Implication of dietary phthalates in breast cancer. A systematic review. Food Chem Toxicol 118:667–674. 10.1016/j.fct.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 277.Ghisari M, Long M, Røge DM, et al. (2017) Polymorphism in xenobiotic and estrogen metabolizing genes, exposure to perfluorinated compounds and subsequent breast cancer risk: A nested case-control study in the Danish National Birth Cohort. Environ Res 154:325–333. 10.1016/j.envres.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 278.Mancini FR, Cano-Sancho G, Gambaretti J, et al. (2020) Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. Int J Cancer 146:917–928. 10.1002/ijc.32357 [DOI] [PubMed] [Google Scholar]
- 279.Hurley S, Goldberg D, Wang M, et al. (2018) Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: a case-control study nested in the California Teachers Study. Environ Health Glob Access Sci Source 17:83. 10.1186/s12940-018-0426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bae J-M, Kim EH (2016) Human papillomavirus infection and risk of breast cancer: a meta-analysis of case-control studies. Infect Agent Cancer 11:14. 10.1186/s13027-016-0058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Farahmand M, Monavari SH, Shoja Z, et al. (2019) Epstein-Barr virus and risk of breast cancer: a systematic review and meta-analysis. Future Oncol Lond Engl 15:2873–2885. 10.2217/fon-2019-0232 [DOI] [PubMed] [Google Scholar]
- 282.Jansen LA, Backstein RM, Brown MH (2014) Breast size and breast cancer: a systematic review. J Plast Reconstr Aesthetic Surg JPRAS 67:1615–1623. 10.1016/j.bjps.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 283.Manjer J, Kaaks R, Riboli E, Berglund G (2001) Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: a prospective study within the Malmö Preventive Project. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 10:33–42. 10.1097/00008469-200102000-00004 [DOI] [PubMed] [Google Scholar]
- 284.Törnberg SA, Holm LE, Carstensen JM (1988) Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol Stockh Swed 27:31–37. 10.3109/02841868809090315 [DOI] [PubMed] [Google Scholar]
- 285.Yang Y, Lynch BM, Hodge AM, et al. (2017) Blood pressure and risk of breast cancer, overall and by subtypes: a prospective cohort study. J Hypertens 35:1371–1380. 10.1097/HJH.0000000000001372 [DOI] [PubMed] [Google Scholar]
- 286.Peeters PH, van Noord PA, Hoes AW, et al. (2000) Hypertension and breast cancer risk in a 19-year follow-up study (the DOM cohort). Diagnostic investigation into mammarian cancer. J Hypertens 18:249–254. 10.1097/00004872-200018030-00002 [DOI] [PubMed] [Google Scholar]
- 287.Agnoli C, Berrino F, Abagnato CA, et al. (2010) Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis NMCD 20:41–48. 10.1016/j.numecd.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chen L, Malone KE, Li CI (2014) Bra wearing not associated with breast cancer risk: a population based case-control study. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 23:2181–2185. 10.1158/1055-9965.EPI-14-0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hsieh CC, Trichopoulos D (1991) Breast size, handedness and breast cancer risk. Eur J Cancer Oxf Engl 1990 27:131–135. 10.1016/0277-5379(91)90469-t [DOI] [PubMed] [Google Scholar]
- 290.Noels EC, Lapid O, Lindeman JHN, Bastiaannet E (2015) Breast implants and the risk of breast cancer: a meta-analysis of cohort studies. Aesthet Surg J 35:55–62. 10.1093/asj/sju006 [DOI] [PubMed] [Google Scholar]
- 291.Schüz J, Jacobsen R, Olsen JH, et al. (2006) Cellular Telephone Use and Cancer Risk: Update of a Nationwide Danish Cohort. JNCI J Natl Cancer Inst 98:1707–1713. 10.1093/jnci/djj464 [DOI] [PubMed] [Google Scholar]
- 292.McGrath KG (2003) An earlier age of breast cancer diagnosis related to more frequent use of antiperspirants/deodorants and underarm shaving. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 12:479–485. 10.1097/00008469-200312000-00006 [DOI] [PubMed] [Google Scholar]
- 293.Fakri S, Al-Azzawi A, Al-Tawil N (2006) Antiperspirant use as a risk factor for breast cancer in Iraq. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit 12:478–482 [PubMed] [Google Scholar]
- 294.Mirick DK, Davis S, Thomas DB (2002) Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst 94:1578–1580. 10.1093/jnci/94.20.1578 [DOI] [PubMed] [Google Scholar]
- 295.Krieger N (2015) Breast bruises and breast cancer. Breast Cancer Res 17:118. 10.1186/s13058-015-0631-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Rigby JE, Morris JA, Lavelle J, et al. (2002) Can physical trauma cause breast cancer? Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 11:307–311. 10.1097/00008469-200206000-00014 [DOI] [PubMed] [Google Scholar]
- 297.Engel C, Fischer C (2015) Breast Cancer Risks and Risk Prediction Models. Breast Care 10:7–12. 10.1159/000376600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cintolo-Gonzalez JA, Braun D, Blackford AL, et al. (2017) Breast cancer risk models: a comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res Treat 164:263–284. 10.1007/s10549-017-4247-z [DOI] [PubMed] [Google Scholar]
- 299.Saslow D, Boetes C, Burke W, et al. (2007) American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin 57:75–89. 10.3322/canjclin.57.2.75 [DOI] [PubMed] [Google Scholar]
- 300.Visvanathan K, Fabian CJ, Bantug E, et al. (2019) Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol 37:3152–3165. 10.1200/JCO.19.01472 [DOI] [PubMed] [Google Scholar]
- 301.US Preventive Services Task Force, Owens DK, Davidson KW, et al. (2019) Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 322:652–665. 10.1001/jama.2019.10987 [DOI] [PubMed] [Google Scholar]
- 302.Tice JA, Bissell MCS, Miglioretti DL, et al. (2019) Validation of the breast cancer surveillance consortium model of breast cancer risk. Breast Cancer Res Treat 175:519–523. 10.1007/s10549-019-05167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Vachon CM, Pankratz VS, Scott CG, et al. (2015) The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst 107:. 10.1093/jnci/dju397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.McCarthy AM, Guan Z, Welch M, et al. (2019) Performance of breast cancer risk assessment models in a large mammography cohort. J Natl Cancer Inst. 10.1093/jnci/djz177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ming C, Viassolo V, Probst-Hensch N, et al. (2019) Machine learning techniques for personalized breast cancer risk prediction: comparison with the BCRAT and BOADICEA models. Breast Cancer Res BCR 21:75. 10.1186/s13058-019-1158-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Gabrielson M, Ubhayasekera KA, Acharya SR, et al. (2020) Inclusion of Endogenous Plasma Dehydroepiandrosterone Sulfate and Mammographic Density in Risk Prediction Models for Breast Cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 29:574–581. 10.1158/1055-9965.EPI-19-1120 [DOI] [PubMed] [Google Scholar]
- 307.Wong EM, Southey MC, Terry MB (2020) Integrating DNA methylation measures to improve clinical risk assessment: are we there yet? The case of BRCA1 methylation marks to improve clinical risk assessment of breast cancer. Br J Cancer 122:1133–1140. 10.1038/s41416-019-0720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhang X, Rice M, Tworoger SS, et al. (2018) Addition of a polygenic risk score, mammographic density, and endogenous hormones to existing breast cancer risk prediction models: A nested case-control study. PLoS Med 15:e1002644. 10.1371/journal.pmed.1002644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Clendenen TV, Ge W, Koenig KL, et al. (2019) Breast cancer risk prediction in women aged 35-50 years: impact of including sex hormone concentrations in the Gail model. Breast Cancer Res BCR 21:42. 10.1186/s13058-019-1126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Shieh Y, Hu D, Ma L, et al. (2016) Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat 159:513–525. 10.1007/s10549-016-3953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mavaddat N, Michailidou K, Dennis J, et al. (2019) Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am J Hum Genet 104:21–34. 10.1016/j.ajhg.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rice MS, Tworoger SS, Hankinson SE, et al. (2017) Breast cancer risk prediction: an update to the Rosner-Colditz breast cancer incidence model. Breast Cancer Res Treat 166:227–240. 10.1007/s10549-017-4391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lécuyer L, Victor Bala A, Deschasaux M, et al. (2018) NMR metabolomic signatures reveal predictive plasma metabolites associated with long-term risk of developing breast cancer. Int J Epidemiol 47:484–494. 10.1093/ije/dyx271 [DOI] [PubMed] [Google Scholar]
- 314.Fung SM, Wong XY, Lee SX, et al. (2019) Performance of Single-Nucleotide Polymorphisms in Breast Cancer Risk Prediction Models: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 28:506–521. 10.1158/1055-9965.EPI-18-0810 [DOI] [PubMed] [Google Scholar]
- 315.Hüsing A, Fortner RT, Kühn T, et al. (2017) Added Value of Serum Hormone Measurements in Risk Prediction Models for Breast Cancer for Women Not Using Exogenous Hormones: Results from the EPIC Cohort. Clin Cancer Res Off J Am Assoc Cancer Res 23:4181–4189. 10.1158/1078-0432.CCR-16-3011 [DOI] [PubMed] [Google Scholar]
- 316.Lee A, Mavaddat N, Wilcox AN, et al. (2019) BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med Off J Am Coll Med Genet 21:1708–1718. 10.1038/s41436-018-0406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Colditz GA, Bohlke K, Berkey CS (2014) Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat 145:567–579. 10.1007/s10549-014-2993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Thomson CA, McCullough ML, Wertheim BC, et al. (2014) Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer Prev Res Phila Pa 7:42–53. 10.1158/1940-6207.CAPR-13-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin n/a: 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 320.Sauter ER (2018) Breast Cancer Prevention: Current Approaches and Future Directions. Eur J Breast Health 14:64–71. 10.5152/ejbh.2018.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Anstey EH, Shoemaker ML, Barrera CM, et al. (2017) Breastfeeding and Breast Cancer Risk Reduction: Implications for Black Mothers. Am J Prev Med 53:S40–S46. 10.1016/j.amepre.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Islami F, Liu Y, Jemal A, et al. (2015) Breastfeeding and breast cancer risk by receptor status--a systematic review and meta-analysis. Ann Oncol Off J Eur Soc Med Oncol 26:2398–2407. 10.1093/annonc/mdv379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.John EM, Hines LM, Phipps AI, et al. (2018) Reproductive history, breast-feeding and risk of triple negative breast cancer: The Breast Cancer Etiology in Minorities (BEM) study. Int J Cancer 142:2273–2285. 10.1002/ijc.31258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ma H, Ursin G, Xu X, et al. (2017) Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: a pooled analysis. Breast Cancer Res BCR 19:6. 10.1186/s13058-016-0799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Fisher B, Costantino JP, Wickerham DL, et al. (2005) Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 97:1652–1662. 10.1093/jnci/dji372 [DOI] [PubMed] [Google Scholar]
- 326.Martino S, Costantino J, McNabb M, et al. (2004) The role of selective estrogen receptor modulators in the prevention of breast cancer: comparison of the clinical trials. The Oncologist 9:116–125. 10.1634/theoncologist.9-2-116 [DOI] [PubMed] [Google Scholar]
- 327.Cuzick J, Sestak I, Thorat MA (2015) Impact of preventive therapy on the risk of breast cancer among women with benign breast disease. Breast Edinb Scotl 24 Suppl 2:S51–55. 10.1016/j.breast.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Goss PE, Ingle JN, Alés-Martínez JE, et al. (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364:2381–2391. 10.1056/NEJMoa1103507 [DOI] [PubMed] [Google Scholar]
- 329.Cuzick J, Sestak I, Forbes JF, et al. (2014) Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet Lond Engl 383:1041–1048. 10.1016/S0140-6736(13)62292-8 [DOI] [PubMed] [Google Scholar]
- 330.Ropka ME, Keim J, Philbrick JT (2010) Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol Off J Am Soc Clin Oncol 28:3090–3095. 10.1200/JCO.2009.27.8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Mocellin S, Pilati P, Briarava M, Nitti D (2016) Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials. J Natl Cancer Inst 108:. 10.1093/jnci/djv318 [DOI] [PubMed] [Google Scholar]
- 332.Crew KD, Albain KS, Hershman DL, et al. (2017) How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention? NPJ Breast Cancer 3:20. 10.1038/s41523-017-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Cummings SR, Ensrud K, Delmas PD, et al. (2010) Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 362:686–696. 10.1056/NEJMoa0808692 [DOI] [PubMed] [Google Scholar]
- 334.Metcalfe K, Eisen A, Senter L, et al. (2019) International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br J Cancer 121:15–21. 10.1038/s41416-019-0446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Carbine NE, Lostumbo L, Wallace J, Ko H (2018) Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev 4:CD002748. 10.1002/14651858.CD002748.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Eleje GU, Eke AC, Ezebialu IU, et al. (2018) Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev 2018:. 10.1002/14651858.CD012464.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Jordan V, Khan M, Prill D (2019) Breast Cancer Screening: Why Can’t Everyone Agree? Prim Care 46:97–115. 10.1016/j.pop.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 338.Monticciolo DL, Newell MS, Hendrick RE, et al. (2017) Breast Cancer Screening for Average-Risk Women: Recommendations From the ACR Commission on Breast Imaging. J Am Coll Radiol 14:1137–1143. 10.1016/j.jacr.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 339.Bevers TB, Helvie M, Bonaccio E, et al. (2018) Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:1362–1389. 10.6004/jnccn.2018.0083 [DOI] [PubMed] [Google Scholar]
- 340.Ebell MH, Thai TN, Royalty KJ (2018) Cancer screening recommendations: an international comparison of high income countries. Public Health Rev 39:7. 10.1186/s40985-018-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Breast Cancer Screening. In: NCQA. https://www.ncqa.org/hedis/measures/breast-cancer-screening/. Accessed 15 Jun 2020 [Google Scholar]
- 342.Nelson HD, Pappas M, Cantor A, et al. (2016) Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 164:256–267. 10.7326/M15-0970 [DOI] [PubMed] [Google Scholar]
- 343.Nelson HD, Fu R, Cantor A, et al. (2016) Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 164:244–255. 10.7326/M15-0969 [DOI] [PubMed] [Google Scholar]
- 344.Harding C, Pompei F, Burmistrov D, Wilson R (2019) Long-term relationships between screening rates, breast cancer characteristics, and overdiagnosis in US counties, 1975-2009. Int J Cancer 144:476–488. 10.1002/ijc.31904 [DOI] [PubMed] [Google Scholar]
- 345.Vourtsis A, Berg WA (2019) Breast Density Implications and Supplemental Screening. Eur Radiol 29:1762–1777. 10.1007/s00330-018-5668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Esserman LJ, WISDOM Study and Athena Investigators (2017) The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 3:34. 10.1038/s41523-017-0035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.(2017) TMIST Breast Screening Study - National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/tmist. Accessed 15 Jun 2020 [Google Scholar]
- 348.Berg WA (2016) Current Status of Supplemental Screening in Dense Breasts. J Clin Oncol Off J Am Soc Clin Oncol 34:1840–1843. 10.1200/JCO.2015.65.8674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Assessment of Periodic Screening of Women With Denser Breast Using WBUS and DBT - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02643966. Accessed 15 Jun 2020
- 350.Turnbull C, Rahman N (2008) Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet 9:321–345. 10.1146/annurev.genom.9.081307.164339 [DOI] [PubMed] [Google Scholar]
- 351.Kurian AW, Griffith KA, Hamilton AS, et al. (2017) Genetic Testing and Counseling Among Patients With Newly Diagnosed Breast Cancer. JAMA 317:531–534. 10.1001/jama.2016.16918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ripperger T, Gadzicki D, Meindl A, Schlegelberger B (2009) Breast cancer susceptibility: current knowledge and implications for genetic counselling. Eur J Hum Genet EJHG 17:722–731. 10.1038/ejhg.2008.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Slavin TP, Maxwell KN, Lilyquist J, et al. (2017) The contribution of pathogenic variants in breast cancer susceptibility genes to familial breast cancer risk. NPJ Breast Cancer 3:22. 10.1038/s41523-017-0024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Tung N, Battelli C, Allen B, et al. (2015) Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 121:25–33. 10.1002/cncr.29010 [DOI] [PubMed] [Google Scholar]
- 355.Hurley S, Goldberg D, Von Behren J, et al. (2011) Birth size and breast cancer risk among young California-born women. Cancer Causes Control CCC 22:1461–1470. 10.1007/s10552-011-9821-9 [DOI] [PubMed] [Google Scholar]
- 356.Michels KB, Xue F, Terry KL, Willett WC (2006) Longitudinal study of birthweight and the incidence of breast cancer in adulthood. Carcinogenesis 27:2464–2468. 10.1093/carcin/bgl105 [DOI] [PubMed] [Google Scholar]
- 357.Barber LE, Bertrand KA, Rosenberg L, et al. (2019) Pre- and perinatal factors and incidence of breast cancer in the Black Women’s Health Study. Cancer Causes Control CCC 30:87–95. 10.1007/s10552-018-1103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ma H, Ursin G, Xu X, et al. (2018) Body mass index at age 18 years and recent body mass index in relation to risk of breast cancer overall and ER/PR/HER2-defined subtypes in white women and African-American women: a pooled analysis. Breast Cancer Res BCR 20:5. 10.1186/s13058-017-0931-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Vrieling A, Buck K, Kaaks R, Chang-Claude J (2010) Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat 123:641–649. 10.1007/s10549-010-1116-4 [DOI] [PubMed] [Google Scholar]
- 360.Shieh Y, Scott CG, Jensen MR, et al. (2019) Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res BCR 21:48. 10.1186/s13058-019-1129-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Antoni S, Sasco AJ, dos Santos Silva I, McCormack V (2013) Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat 137:337–347. 10.1007/s10549-012-2362-4 [DOI] [PubMed] [Google Scholar]
- 362.Aktipis CA, Ellis BJ, Nishimura KK, Hiatt RA (2014) Modern reproductive patterns associated with estrogen receptor positive but not negative breast cancer susceptibility. Evol Med Public Health 2015:52–74. 10.1093/emph/eou028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Anderson KN, Schwab RB, Martinez ME (2014) Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 144:1–10. 10.1007/s10549-014-2852-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Unar-Munguía M, Torres-Mejía G, Colchero MA, González de Cosío T (2017) Breastfeeding Mode and Risk of Breast Cancer: A Dose-Response Meta-Analysis. J Hum Lact Off J Int Lact Consult Assoc 33:422–434. 10.1177/0890334416683676 [DOI] [PubMed] [Google Scholar]
- 365.Farvid MS, Eliassen AH, Cho E, et al. (2018) Dairy Consumption in Adolescence and Early Adulthood and Risk of Breast Cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 27:575–584. 10.1158/1055-9965.EPI-17-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Genkinger JM, Makambi KH, Palmer JR, et al. (2013) Consumption of dairy and meat in relation to breast cancer risk in the Black Women’s Health Study. Cancer Causes Control CCC 24:675–684. 10.1007/s10552-013-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.McCullough ML, Rodriguez C, Diver WR, et al. (2005) Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 14:2898–2904. 10.1158/1055-9965.EPI-05-0611 [DOI] [PubMed] [Google Scholar]
- 368.Larsson SC, Bergkvist L, Wolk A (2009) Long-term meat intake and risk of breast cancer by oestrogen and progesterone receptor status in a cohort of Swedish women. Eur J Cancer Oxf Engl 1990 45:3042–3046. 10.1016/j.ejca.2009.04.035 [DOI] [PubMed] [Google Scholar]
- 369.Namazi N, Larijani B, Azadbakht L (2018) Association between the dietary inflammatory index and the incidence of cancer: a systematic review and meta-analysis of prospective studies. Public Health 164:148–156. 10.1016/j.puhe.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 370.Jayedi A, Emadi A, Shab-Bidar S (2018) Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv Nutr Bethesda Md 9:388–403. 10.1093/advances/nmy015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Moradi S, Issah A, Mohammadi H, Mirzaei K (2018) Associations between dietary inflammatory index and incidence of breast and prostate cancer: a systematic review and meta-analysis. Nutr Burbank Los Angel Cty Calif 55–56:168–178. 10.1016/j.nut.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 372.Wang L, Liu C, Zhou C, et al. (2019) Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. Eur J Clin Nutr 73:509–517. 10.1038/s41430-018-0196-9 [DOI] [PubMed] [Google Scholar]
- 373.Narita S, Inoue M, Saito E, et al. (2017) Dietary fiber intake and risk of breast cancer defined by estrogen and progesterone receptor status: the Japan Public Health Center-based Prospective Study. Cancer Causes Control CCC 28:569–578. 10.1007/s10552-017-0881-3 [DOI] [PubMed] [Google Scholar]
- 374.Ferrari P, Rinaldi S, Jenab M, et al. (2013) Dietary fiber intake and risk of hormonal receptor-defined breast cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr 97:344–353. 10.3945/ajcn.112.034025 [DOI] [PubMed] [Google Scholar]
- 375.Zhang C-X, Ho SC, Cheng S-Z, et al. (2011) Effect of dietary fiber intake on breast cancer risk according to estrogen and progesterone receptor status. Eur J Clin Nutr 65:929–936. 10.1038/ejcn.2011.57 [DOI] [PubMed] [Google Scholar]
- 376.Park Y, Brinton LA, Subar AF, et al. (2009) Dietary fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr 90:664–671. 10.3945/ajcn.2009.27758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Suzuki R, Rylander-Rudqvist T, Ye W, et al. (2008) Dietary fiber intake and risk of postmenopausal breast cancer defined by estrogen and progesterone receptor status--a prospective cohort study among Swedish women. Int J Cancer 122:403–412. 10.1002/ijc.23060 [DOI] [PubMed] [Google Scholar]
- 378.Inoue-Choi M, Sinha R, Gierach GL, Ward MH (2016) Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer 138:1609–1618. 10.1002/ijc.29901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Graff RE, Cho E, Lindström S, et al. (2014) Premenopausal plasma ferritin levels, HFE polymorphisms, and risk of breast cancer in the nurses’ health study II. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 23:516–524. 10.1158/1055-9965.EPI-13-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Alexander DD, Morimoto LM, Mink PJ, Cushing CA (2010) A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr Res Rev 23:349–365. 10.1017/S0954422410000235 [DOI] [PubMed] [Google Scholar]
- 381.Cho E, Chen WY, Hunter DJ, et al. (2006) Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med 166:2253–2259. 10.1001/archinte.166.20.2253 [DOI] [PubMed] [Google Scholar]
- 382.Linos E, Willett WC, Cho E, et al. (2008) Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 17:2146–2151. 10.1158/1055-9965.EPI-08-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Cui Y, Shikany JM, Liu S, et al. (2008) Selected antioxidants and risk of hormone receptor-defined invasive breast cancers among postmenopausal women in the Women’s Health Initiative Observational Study. Am J Clin Nutr 87:1009–1018. 10.1093/ajcn/87.4.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Roswall N, Olsen A, Christensen J, et al. (2010) Micronutrient intake and breast cancer characteristics among postmenopausal women. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 19:360–365. 10.1097/cej.0b013e32833ade68 [DOI] [PubMed] [Google Scholar]
- 385.Rosenberg L, Boggs DA, Bethea TN, et al. (2013) A prospective study of smoking and breast cancer risk among African-American women. Cancer Causes Control CCC 24:2207–2215. 10.1007/s10552-013-0298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Kakugawa Y, Kawai M, Nishino Y, et al. (2015) Smoking and survival after breast cancer diagnosis in Japanese women: A prospective cohort study. Cancer Sci 106:1066–1074. 10.1111/cas.12716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Tong J, Li Z, Shi J, et al. (2014) Passive smoking exposure from partners as a risk factor for ER+/PR+ double positive breast cancer in never-smoking Chinese urban women: a hospital-based matched case control study. PloS One 9:e97498. 10.1371/journal.pone.0097498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Dossus L, Boutron-Ruault M-C, Kaaks R, et al. (2014) Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int J Cancer 134:1871–1888. 10.1002/ijc.28508 [DOI] [PubMed] [Google Scholar]
- 389.Algra AM, Rothwell PM (2012) Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 13:518–527. 10.1016/S1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- 390.Barnard ME, Boeke CE, Tamimi RM (2015) Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta 1856:73–85. 10.1016/j.bbcan.2015.06.002 [DOI] [PubMed] [Google Scholar]