To the Editor: Male androgenic alopecia (MAA) is the most common hair loss disorder characterized by miniaturization of hair follicles and progressive hair loss.[1] Although oral drugs, topical agents, and laser therapy can promote hair growth, minoxidil is the only topical therapy approved by the United States Food and Drug Administration. Fibroblast growth factors (FGFs) have a wide range of biological functions. The basic FGF (bFGF) promotes the growth of the hair follicle in mice, indicating its potential as a therapeutic agent for MAA.[2] The nano-microneedle is a new device that can promote transdermal absorption of topical drugs,[3] and increase drug penetration by up to 10 or 20 times. In this study, we compared the safety and efficacy of topical minoxidil, nano-microneedle-assisted FGF, and a combination of the two.
After approval by the Medical Ethics and Human Research Committee of First Hospital of China Medical University, registration with the China Clinical Trials Registry (http://www.chictr.org.cn, No. ChiCTR1900021107) and written informed consent from each patient, 40 Chinese male patients aged 22 to 50 years, diagnosed with MAA, were enrolled (Supplementary Table 1). They did not undergo any treatment affecting the hair cycle within the 6 months before enrollment. Those with other hair disorders or known systemic diseases were excluded.
Participants were randomized into four groups of 10 each using the random number table method. Groups S, M, F, and MF were administered saline, 5% topical minoxidil solution (Mandi; Wansheng Medication Manufacture Corporation Ltd., Hangzhou, China), FGF (Beifushu; Zhuhai Yisheng Biological Pharmaceutical Company Ltd., Zhuhai, China); and a combination of 5% topical minoxidil and FGF, respectively, for 16 weeks.
Saline (group S) and FGF (groups F and MF) were applied once weekly using a nano-microneedle (Suzhou Natong Biological Nanotechnology Corporation Ltd., Suzhou, China). After spraying 1 mL of saline or FGF on the balding region, the nano-microneedle was moved smoothly and slowly on the scalp. Topical minoxidil (groups M and MF, seven drops per treatment) was applied to the balding region every morning and evening, followed by local massage for 5 to 10 minutes.
Patients were evaluated monthly for safety and efficacy. At each visit, standardized global photographs were obtained using a digital camera (Nikon D40s, Tokyo, Japan). To ensure that the same location was observed each time, we divided the patient's head into four quadrants, the line between two earlobes was the abscissa, the vertical line through the tip of nose was the ordinate. We selected the same coordinate mark and marked a 10-mm-wide circle for measurement at each visit. Hair in that area was trimmed and a hair microscope (CBS-1717, Taiwan, China) was used to measure the hair diameter and amount of hair or hair follicles in unit area (hair density and hair unit follicular).[4]
At the end of the 16-week treatment period, participants and two dermatologists blinded to the treatment evaluated the treatment efficacy based on the photographs taken at baseline and at 16 weeks. The assessment was measured on a six-point scale: −1 = worsened, 0 = unchanged, 1 = slight improvement, 2 = moderate improvement, 3 = good improvement, and 4 = excellent improvement.
Adverse reactions were recorded during follow-up visits, and pain experienced during nano-microneedle treatment was evaluated using a numerical rating scale, where 0 indicated no pain, while 10 indicated intolerable pain.
Statistical analysis was conducted using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
All patients completed their respective treatments. No significant differences in patient age, severity of baldness, or duration of symptoms were observed among the groups. At baseline, no significant differences were found in hair density, hair diameter, or follicular unit density among the groups (P > 0.05). After 16 weeks of treatment, hair density increased significantly in groups M, F, and MF, from 104.12 ± 30.54, 91.53 ± 37.98, 100.92 ± 27.79/cm2 to 129.07 ± 27.52, 118.88 ± 41.41, 155.01 ± 24.64/cm2, respectively (P < 0.05) (Supplementary Table 2). Significant improvements in hair diameter were seen in groups M and MF from 70.00 ± 16.33 and 63.00 ± 16.36 μm to 77.00 ± 13.37 and 70.00 ± 15.63 μm (Supplementary Table 3) (P < 0.05) and in follicular unit density from 109.20 ± 32.80 and 109.93 ± 19.80/cm2 to 139.95 ± 24.48 and 130.02 ± 15.65/cm2 in groups F and MF, respectively (Supplementary Table 4) (P < 0.05).
Hair density increased in group F but not in group S. This suggests that the mechanical stimulus of the nano-microneedle does not produce hair growth but it promotes the penetration of FGF. Hair density was also higher in group MF than that in groups M and F (both P < 0.05), indicating that combination treatment is superior to individual treatments.
Both the investigators (70%, 21/30) and participants (90%, 27/30) reported subjective improvements following all treatments except saline at the end of the study. The Kruskal-Wallis H-test showed a significant difference among the four groups (P < 0.05), although not among groups M, F, and MF (P > 0.05), probably because of the small number of participants in each group (Supplementary Table 5) [Figure 1].
Figure 1.
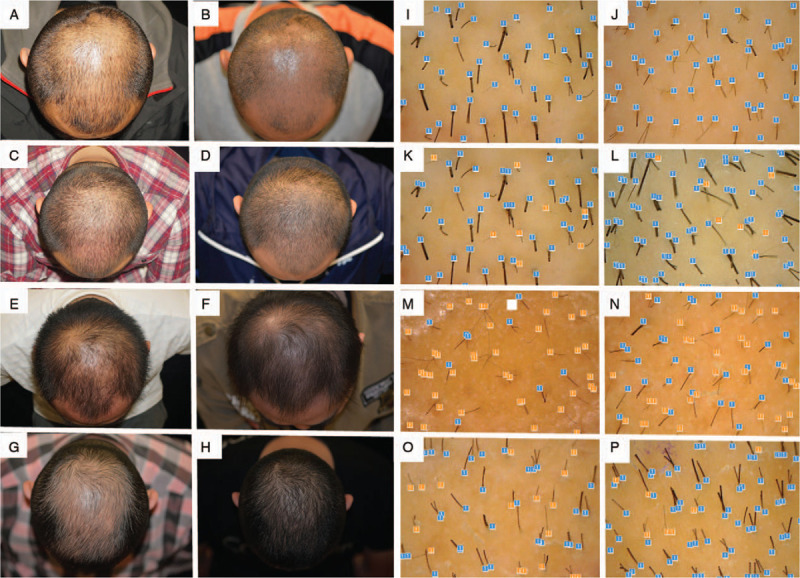
Photographs of patients’ hair at baseline (left) and after the 16-week treatment (right). (A, B): Nano-microneedle-assisted normal saline. (C, D) 5% topical minoxidil. (E, F): Nano-microneedle-assisted fibroblast growth factor. (G, H): 5% topical minoxidil and nano-microneedle-assisted fibroblast growth factor. Photographs showing hair diameter at baseline (left) and after 16-week treatment (right) using a hair microscope. (I, J): Nano-microneedle-assisted saline. (K, L): 5% topical minoxidil. (M, N): Nano-microneedle-assisted fibroblast growth factor. (O, P): Topical minoxidil and nano-microneedle-assisted fibroblast growth factor.
No serious adverse effects were encountered during the treatment period. Pain during nano-microneedle treatment was well tolerated by patients in groups S, F, and MF. Three participants developed mild erythema, which disappeared within 24 h.
In the present study, we found that hair density of patients receiving minoxidil, nano-microneedle-assisted FGF, or both increased notably over baseline, with patients treated using combined treatments achieving the most satisfactory results. Hair diameter significantly improved in patients receiving minoxidil with or without FGF for 16 weeks, whereas follicular unit density significantly improved in patients receiving FGF with or without minoxidil. These findings show that minoxidil increases hair diameter and density, whereas FGF increases hair and follicle density. Therefore, combination therapy is superior to monotherapies in terms of hair density, hair diameter, and follicular unit density.
Thus, the combination of nano-microneedle-assisted FGF and topical minoxidil is safe and reliable for treatment of MAA and better than monotherapy. Further research is required to evaluate the long-term efficacy and safety of this combination therapy in a larger group of subjects.
Funding
This work was supported by grants from the National Key Research and Development Program of China (No. 2016YFC0901504) and the 111 Project (No. D18011).
Acknowledgements
We appreciate Prof. Yin-Sheng Wan, Prof. Bing Song, Prof. Marco Stoller for their helpful discussion and review of this manuscript.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yu CQ, Zhang H, Guo ME, Li XK, Chen HD, Li YH, Xu XG. Combination therapy with topical minoxidil and nano-microneedle-assisted fibroblast growth factor for male androgenetic alopecia: a randomized controlled trial in Chinese patients. Chin Med J 2021;134:851–853. doi: 10.1097/CM9.0000000000001195
Cheng-Qian Yu and Hui Zhang contributed equally to this manuscript.
Supplemental digital content is available for this article.
References
- 1.Zhuo FL, Li LF, Cai LQ, Hua Y. Effects of CO2 Fractional laser on hair growth in C57BL/6 mice and potential underlying mechanisms. Chin Med J 2019; 20:1257–1260. doi: 10.1097/CM9.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiso M, Hamazaki TS, Itoh M, Kikuchi S, Nakagawa H, Okochi H. Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J Dermatol Sci 2015; 79:110–118. doi: 10.1016/j.jdermsci.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol 2017; 32:56–59. doi: 10.1111/jdv.14722. [DOI] [PubMed] [Google Scholar]
- 4.Shen X, Yu RX, Shen CB, Li CX, Jing Y, Zheng YJ, et al. Dermoscopy in China current status and future prospective. Chin Med J 2019; 5:2096–2104. doi: 10.1097/CM9.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


