Abstract
Chromosomal aberrations are generally considered to have a remarkable impact on the outcome of multiple myeloma. Bortezomib helps to achieve complete responses and leads to longer life expectancy in many multiple myeloma patients. This study was designed to clarify whether bortezomib can improve the poor prognosis resulting from del(17q13), del(13q14), amp(1q21), t(4,14), t(14,16) in patients with multiple myeloma. A total of 255 MM patients treated with bortezomib-based regimens were included in this study. All chromosomal aberrations were detected by interphase fluorescence in situ hybridization. Kaplan–Meier survival and Multivariable Cox regression analysis were employed to assess the prognostic situation in progression-free survival and overall survival. The result showed that the progression-free survival and overall survival of patients with del(17q13) were shorter than those without del(17q13) in multivariate analysis and patients with del(13q14), amp(1q21), t(4,14), t(14,16) were similar to patients without these chromosomal aberrations in progression-free survival and overall survival after receiving bortezomib-based regimens.
In conclusion Bortezomib-based regimens can overcome the poor prognosis derived from del(13q14), amp(1q21), t(4,14), t(14,16) but not del(17q13).
Keywords: bortezomib, chromosomal aberrations, multiple myeloma, survival
1. Introduction
Multiple myeloma (MM) is a common plasma cell neoplasm characterized by a malignant clone of plasma cells derived from a diverse range of genetic events, such as chromosomal translocations, deletions, amplifications that contribute to the onset, progression, and prognosis.[1] Today, the genetic events are mainly detected using interphase fluorescence in situ hybridization (FISH).[2] Of these adverse events, it is generally acknowledged that del(17q13), del(13q14), amp(1q21), t(4,14), t(14,16) play an important role in poor prognosis.[3–8] These events have been introduced in the guidelines developed by the International Myeloma Working Group as well as the Mayo Stratification of Myeloma and Risk-Adapted Therapy to stratify MM patients.[9,10] Patients with the chromosomal aberrations are intermediate and high risk, usually evolving to a poor outcome when treated with traditional chemotherapy.[11]
Currently, the treatments for MM have already stepped into an era of novel agents. All kinds of novel drugs emerge endlessly. Proteasome inhibitors, such as bortezomib, and immunomodulatory drugs, such as lenalidomide and pomalidomide, dramatically improved therapeutic efficacy.[12] Additionally, Daratumumab, a targeted drug, was also approved in patients with relapsed and refractory MM. Today, bortezomib, as the most frequently used drug for MM, even helps many patients achieve complete responses,[13] and incorporating bortezomib in MM treatment can lead to longer life expectancy in some patients.[14]
With the development of novel drugs, increasing clinical trials have demonstrated that the poor prognosis of MM patients could be overcome. Bortezomib, as the most successful drug for MM ever, was also employed in many MM patients with chromosomal aberrations in numerous studies. However, of these studies, some did not discuss all the loci mentioned in the guidelines and some suggested controversial conclusions. Therefore, we collected and analyzed the data of MM patients treated with bortezomib-based regimens who were analyzed for del(17q13), del(13q14), amp(1q21), t(4,14), t(14,16) in our hospital, aiming to figure out the effect of bortezomib on MM patients with the abnormal chromosomes.
2. Materials and methods
2.1. Patients and materials
We have reviewed all the materials of MM patients in West China Hospital. A total of 255 patients with results from interphase FISH from 2009 to 2018 were included in the retrospective study. All patients had a confirmed diagnosis of MM according to the International Myeloma Working Group's latest criteria.[15] Additionally, all patients received bortezomib-based regimens, including bortezomib united with dexamethasone, bortezomib combined with dexamethasone and thalidomide or lenalidomide and bortezomib plus melphalan and prednisone, with 2 to 12 cycles. Patients were divided into following groups based on the results of interphase FISH: “normal,” “p53” (17q13del), “RB1” (13q14del), “D13S319” (13q14.3del), “IGH translocations” (t(4,14) and t(14,16)), “1q21” (1q21 gain). The follow-up time ranged from 2 to 114 months. Additionally, patients’ characteristics, such as gender, age, immunoglobulin isotype, calcium, creatinine, hemoglobin count, bone disease status, lactate dehydrogenase and β2 microglobulin were also collected. Bone disease status was classified based on X-ray results of whole body flat bones. The study was approved by the Ethics Committee of West China Hospital of Sichuan University. The ethics committee waived informed consent due to a retrospective study. All of the data were anonymized prior to access by authors.
2.2. FISH experiment
We used the interphase FISH method to detect abnormal chromosomes. Verdure bone marrow from patients was first purified using a magnetic bead of CD138, and then these purified cells were hybridized with specific probes (RB1, D13S319, IGH, 1q21, p53). Four hundred cells were stained for each probe. A ratio of aberration exceeding 10% was considered positive. The FISH analysis was performed again if the result value was close to the threshold.
2.3. Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were employed to evaluate the survival of patients. PFS was defined as the duration from the initial treatment of bortezomib to the relapse of MM. OS was defined as the months between the initial treatment of bortezomib and death. The survival analysis was conducted using the Kaplan–Meier method to evaluate the influence of del(13q14), del(13q14.3), t(4,14), t(14,16), amp(1q21), and del(17q13) on PFS and OS. A log-rank test was used to assess the results with a significant difference of P < .05. The multivariable Cox regression analyses were employed to assess the effect of del(13q14), del(13q14.3), t(4,14), t(14,16), amp(1q21), del(17q13), gender, age, International Staging System stage, immunoglobulin isotype, hypercalcemia, renal dysfunction, hemoglobin count, bone disease status, and elevated β2 microglobulin on PFS and OS. P < .05 was considered significant. All statistical analyses were performed with SPSS 23.0 software (SPSS, Inc, Chicago, IL).
3. Results
3.1. Difference between RB1, D13S319, IGH, 1q21, p53 positive and negative groups in PFS and OS in the FISH study
The baseline characteristics of the patients were listed in Table 1. The percentage of the clone harboring the abnormal chromosome ranged from 11% to 96%. The RB1 and D13S319 were the most common chromosomal abnormality and occurred almost simultaneously while p53 deletion was the least common aberration and also occurred with other abnormal loci. 1q21 gain occurred most frequently alone. In addition, the number of concomitant abnormal chromosomal sites was summarized. Each locus with one or more other loci was described (Table 2).
Table 1.
Baseline characteristics of included patients.
| Total patients (N = 255) | |
| Gender | |
| Male | 142 |
| Female | 113 |
| Age | |
| Median (range) | 60 (28–82) |
| ISS stage | |
| I | 17 |
| II | 120 |
| III | 118 |
| Immunoglobulin isotype | |
| IgG | 135 |
| IgA | 62 |
| IgD | 1 |
| Light chain | 57 |
| Bone disease status | |
| Absent | 80 |
| Present | 175 |
| Hypercalcemia | |
| Absent | 216 |
| Present | 39 |
| Renal dysfunction | |
| Absent | 179 |
| Present | 76 |
| Lactate dehydrogenase (LDH) | |
| >220 UI/L | 85 |
| β2 microglobulin | |
| ≥5.5 mg/L | 118 |
| Hemoglobin | |
| < 90 g/L | 113 |
Table 2.
Characteristics of FISH results.
| Combination | ||||||
| Probes | Total | Alone | Two | Three | Four | Five |
| RB1 | 114 | 0 | 20 | 33 | 52 | 9 |
| D13S319 | 115 | 0 | 20 | 34 | 52 | 9 |
| IGH | 106 | 18 | 13 | 18 | 48 | 9 |
| 1q21 | 103 | 19 | 12 | 21 | 42 | 9 |
| p53 | 25 | 1 | 2 | 3 | 10 | 9 |
All the 255 patients had the five probes tested. Eighty-seven patients were all normal in these five probes and others had at least one normal locus.
The results of the Kaplan–Meier survival analysis showed that the PFS for the D13S319 (P = .051), IGH (P = .184), and 1q21 (P = .697) positive groups were similar to the negative groups. Similarly, there was no difference between the RB1 (P = .231), D13S319 (P = .267), IGH (P = .267), and 1q21 (P = .475) positive groups and the negative groups in OS. However, the PFS for the RB1 positive group was significantly shorter than the RB1 negative group (P = .038). Additionally, in the locus of p53, the del(17q13) positive group was much shorter than the del(17q13) negative group in the PFS (P < .001) and OS (P = .003), respectively (Fig. 1).
Figure 1.
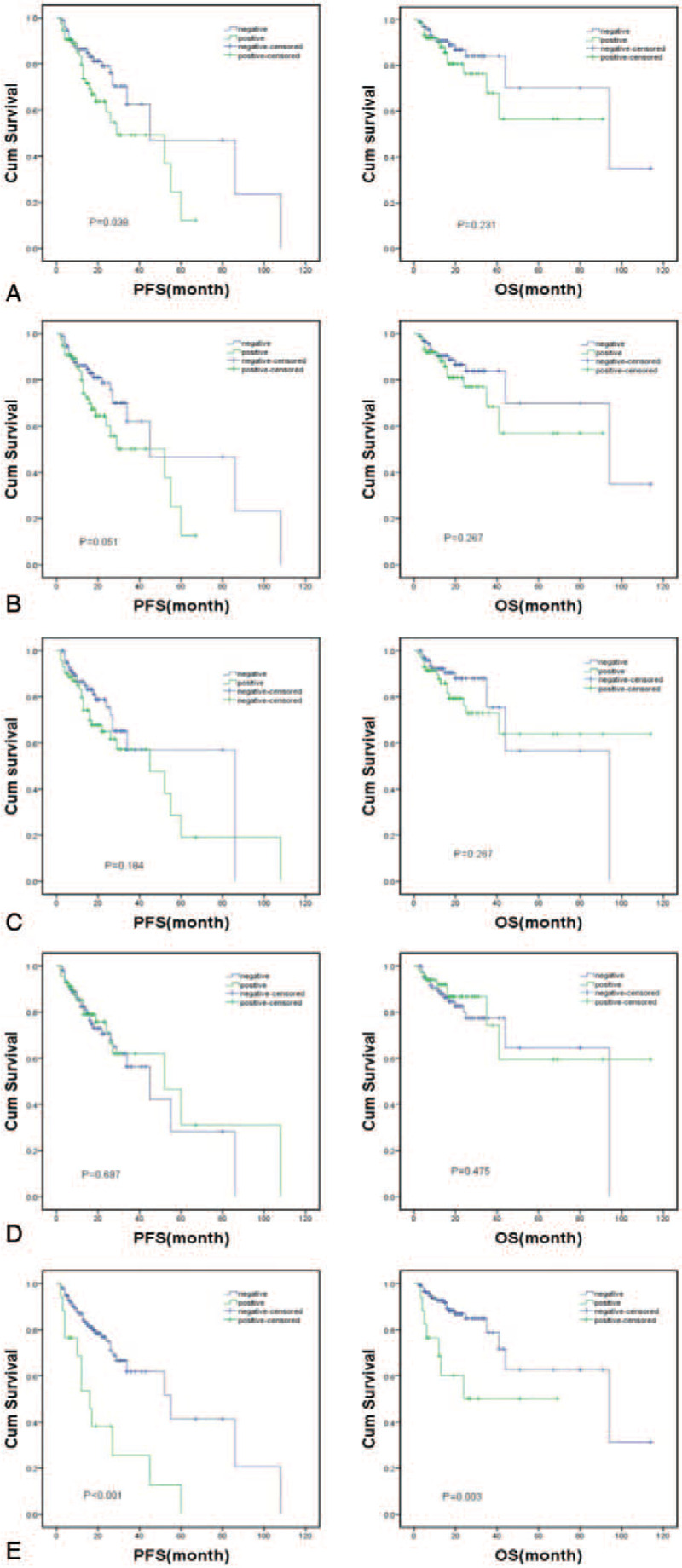
Kaplan–Meier survival curve of normal and abnormal FISH results for PFS and OS. (A) RB1; (B) D13S319; (C) IGH; (D) 1q21; (E) p53.
Then, subgroup analysis by each group of abnormal chromosome was conducted. All patients with abnormal chromosomes were classified in stage II or III. The result showed that no difference was found between stage II and III of each group of abnormal chromosome (see Figure, Supplemental Content, which illustrates the PFS and OS of patients at different stages of each group of abnormal chromosomes).
Next, multivariable Cox regression analyses were conducted to exclude the effects of the variables of RB1, D13S319, IGH, 1q21, and p53, gender, age, International Staging System stage, immunoglobulin isotype, hypercalcemia, renal dysfunction, hemoglobin count, bone disease status, and elevated β2 microglobulin on each result of the Kaplan–Meier analysis. The results suggested the PFS of the RB1 positive group was not different from the RB1 negative group (P = .285), while del(17q13) positive was significantly independent of other factors in both PFS (P = .002) and OS (0.045) as in the Kaplan–Meier analysis (Table 3).
Table 3.
Results of multivariable Cox regression analyses for PFS and OS.
| PFS | OS | |||||
| Variables | HR | 95% CI | P | HR | 95% CI | P |
| Age (>60 y vs ≤60 y) | 1.471 | 0.811–2.669 | .204 | 1.669 | 0.732–3.805 | .223 |
| Gender (female vs male) | 1.187 | 0.647–2.176 | .580 | 1.454 | 0.638–3.310 | .373 |
| Creatinine (>106 umol/L vs ≤106 umol/L) | 0.669 | 0.324–1.384 | .279 | 0.855 | 0.324–2.256 | .365 |
| Calcium (>2.75 mmol/L vs ≤ 2.75 mmol/L) | 0.970 | 0.406–2.318 | .945 | 1.145 | 0.353–3.717 | .821 |
| Isotype (light chain vs non-light chain) | 1.544 | 0.722–3.303 | .263 | 1.183 | 0.451–3.105 | .733 |
| Bone disease status (absent vs present) | 0.720 | 0.388–1.334 | .306 | 0.495 | 0.227–1.079 | .085 |
| β2 microglobulin (<5.5 mg/L vs ≥ 5.5 mg/L) | 1.790 | 0.900–3.562 | .097 | 2.451 | 0.985–6.099 | .054 |
| Hemoglobin (<90 g/L vs ≥90 g/L) | 2.107 | 0.866–5.127 | .100 | 2.111 | 0.614–7.256 | .236 |
| Stage II (vs stage I) | 2.763 | 0.348–21.920 | .336 | 1.264 | 0.146–10.970 | .832 |
| Stage III (vs stage I) | 4.586 | 0.570–36.877 | .152 | 2.695 | 0.306–23.699 | .372 |
| RB1 (positive vs negative) | 0.684 | 0.341–1.372 | .285 | 0.812 | 0.324–2.040 | .658 |
| D13S319 (positive vs negative) | 0.763 | 0.378–1.544 | .452 | 0.902 | 0.356–2.285 | .828 |
| IGH (positive vs negative) | 1.205 | 0.572–2.538 | .625 | 0.982 | 0.358–2.688 | .971 |
| 1q21 (positive vs negative) | 1.355 | 0.709–2.591 | .358 | 1.449 | 0.599–3.505 | .410 |
| P53 (positive vs negative) | 0.276 | 0.125–0.611 | .002 | 0.335 | 0.115–0.974 | .045 |
CI = confidence interval, HR = hazard ratio, OS = overall survival, PFS = progression-free survival.
3.2. Difference between normal, other abnormal and del(17q13) positive groups in PFS and OS in the FISH study
A total of 87 patients who were normal in the loci of RB1, D13S319, IGH, 1q21, and p53 were defined as a normal group. RB1, D13S319, IGH, and 1q21 positive groups were formed as the other abnormal group, totaling 143 patients. Then, the normal, other abnormal and del(17q13) positive FISH results were established. The results of the Kaplan–Meier survival analysis showed the normal group was similar to the other abnormal group in PFS (P = .889) and OS (P = .749) but better than the del(17q13) group in PFS (P = .001) and OS (P = .008) (Fig. 2).
Figure 2.
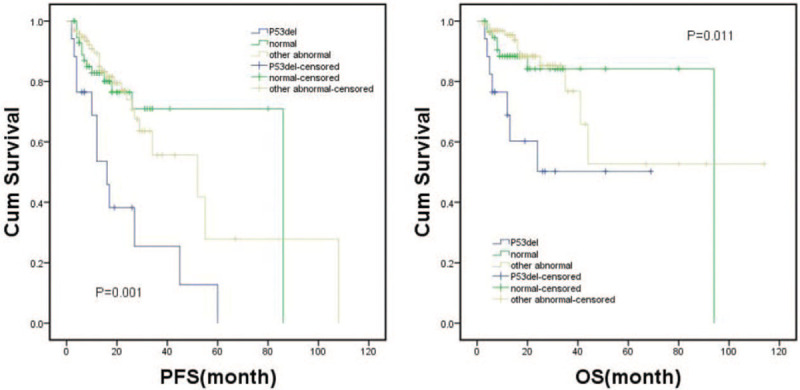
Kaplan–Meier survival curve of normal, other abnormal and del(17q13) groups for PFS and OS.
4. Discussion
MM patients with abnormal FISH results, such as del(13q14), amp(1q21), t(4,14), t(14,16), and del(17q13), are usually considered to have an inferior outcome.[9,10] Since novel agents like bortezomib were continually applied in the clinic, more MM patients achieved a very good partial response or even complete response and simultaneously a relatively good quality of life.[16–18] However, whether bortezomib can overcome the poor prognosis from these chromosomal aberrations and prolong PFS and OS remains dismal. Previous studies did not reach an accordant and complete conclusion. In our study, we analyzed five bad loci of chromosomal aberrations included in the MM guideline and concluded that bortezomib can indeed improve the poor prognosis resulting from del(13q14), amp(1q21), t(4,14), and t(14,16) but not for del(17q13).
In the first part of the results, the del(13q14) was considered an poor prognostic factor for PFS that could not be improved by bortezomib. However, we noted that this result was different from documental records which advised that multivariate analysis should be taken into consideration.[19,20] Then, in the multivariate analysis, del(13q14) was no longer a poor prognostic factor in the patients treated with bortezomib, which is identical to previous studies. Therefore, we thought that bortezomib could overcome the adverse effect of del(13q14).
In 2003, Zavrski et al demonstrated that proteasome inhibitors can induce apoptosis in human bone marrow myeloma cells with chromosomal aberrations in vitro.[21] Since then many studies were performed and inferred that intermediate and high-risk MM patients responded well to bortezomib. Next, medical scientists started focusing on the impact of bortezomib on the outcomes of intermediate and high-risk MM patients. Cumulative studies have been conducted to investigate the problem. Jagannath et al concluded there was no difference in OS among patients with or without del(13) after being treated with bortezomib according to the SUMMIT and APEX matched-pairs analysis.[22] A study from Sagaster et al also showed relapsed patients received bortezomib and achieved a similar OS in both the del(13q14) and non-del(13q14) group.[23] In the study of Kiyota et al, patients treated with bortezomib were divided into four groups based on the FISH results of del(13q), t(4,14), t(14,16), 1q21 abnormality. They found that patients with each aberration had no difference in PFS and OS from the negative group and thought bortezomib can overcome adverse events brought by these abnormalities.[24]
Mateos et al reported that patients with del(13) and IGH translocations treated with bortezomib were similar to those without these chromosomal aberrations in event-free survival and PFS in their two articles.[25,26] In another study,[27] Mateos et al defined the patients with t(4,14), t(14,16), del(17q) as a high-risk group and the rest were defined as a standard risk group using a larger sample size with patients 65 years of age and older. However, they found that the high-risk group still had shorter survival than the standard group when treated with bortezomib. Thus, they summarized that bortezomib does not overcome the negative prognosis of high-risk chromosomal aberrations. In addition, Biran et al reported that patients with the amp(1q21) harbored a shorter PFS and OS, even when treated with bortezomib, indicating that bortezomib did not benefit high-risk patients in survival.[28] The results of these studies were not accordant with ours. However, Mateos's latter study had several differences from ours in design. The subjects were all elderly and they did not analyze every single abnormal locus in prognosis. Additionally, the del(17q) was in the high-risk group, and we suspect the del(17q) interfered with the real result according to our findings. In Biran's study, chromosomal aberrations were detected using two methods of FISH and conventional cytogenetics in a small population (N = 28). However, in our study, abnormal chromosomes were all detected by FISH in a larger sample size.
Avet-Loiseau et al designed a study including del(17q) and discovered that patients with del(17q) did not have improved survival in EFS and OS by using bortezomib.[29] Cohen et al also found that the inferior outcome associated with del(17q13) was not corrected by bortezomib.[30] Additionally, Byun et al demonstrated that in the Asian population, patients with del(17q13) did not benefit from bortezomib in survival.[31] These studies are consistent with our results. However, some other articles reported that bortezomib could improve the survival of patients with del(17q). In the design of Sonneveld et al's study, bortezomib was used as induction and maintenance therapy for patients aged 65 years or younger. Over the duration of disease, high-dose melphalan and autologous stem-cell transplantation were also performed in all patients. They concluded that bortezomib significantly improved PFS and OS in patients with del(17p13).[32] Similarly, El-Ghammaz et al also supposed that bortezomib-based induction benefits patients harboring del(17q) in PFS but not in OS.[33] Their study was designed with Sonneveld's methodology, excluding bortezomib maintenance therapy, and the included patients were younger (<62 years old). We attributed the differences in results to younger subjects and the treatment of high-dose melphalan and autologous stem-cell transplantation.
However, there is a remarkable limitation in our study. Our algorithm was conducted at a single medical center, where the only database was used to analyze the prognosis of MM patients. The survival curve of patients receiving bortezomib-based regimens may not generalize to other populations. Despite practical challenges, multi-centers cohorts can extend the issue. Other medical centers are not willing to provide their data due to patient privacy and contrast with each other. A sharing database and experimental technique built in multi-centers may solve the problem.
5. Conclusions
In summary, we presented comprehensive data of 255 MM patients treated with bortezomib-based regimens. The result suggested that bortezomib-based regimens could overcome the poor prognosis resulting from del(13q14), amp(1q21), t(4,14) and t(14,16) but not del(17q13). This indicated us that bortezomib was the first choice for intermediate and high-risk patients without del(17q13). Novel approaches or allogeneic hematopoietic stem cell transplantation should be taken into consideration for patients with del(17q13). Continuous efforts are needed to improve the outcome of MM patients with del(17q13) in the future.
Author contributions
Zhigang Liu and Qiang Zeng contributed equally.
Zhigang Liu wrote the main manuscript text;
Qiang Zeng prepared all the figures and tables;
Both Zhigang Liu and Qiang Zeng analyzed the data and contributed to the materials and tools;
Bing Xiang revised the manuscript text and supervised the methods part.
Conceptualization: Zhigang Liu.
Data curation: Zhigang Liu, Qiang Zeng.
Funding acquisition: Zhigang Liu.
Investigation: Qiang Zeng.
Methodology: Qiang Zeng.
Project administration: Zhigang Liu, Bing Xiang.
Resources: Bing Xiang.
Supervision: Bing Xiang.
Writing – original draft: Qiang Zeng.
Writing – review & editing: Bing Xiang.
Supplementary Material
Footnotes
Abbreviations: CR = complete response, FISH = fluorescence in situ hybridization, LDH = lactate dehydrogenase, MM = multiple myeloma, OS = overall survival, PFS = progression-free survival.
How to cite this article: Liu Z, Zeng Q, Xiang B. Bortezomib-based regimens improve the prognosis of newly diagnosed MM patients with chromosomal aberrations except for del(17q13): A retrospective study from a single center. Medicine. 2021;100:18(e25834).
ZL and QZ contributed equally to this work.
This work was supported by key research and development program of Science and Technology Department of Sichuan Province (2019YFS0104).
Ethics Approval and Consent to participate: The study was approved by the Ethics Committee of West China Hospital of Sichuan University. The ethics committee waived informed consents due to a retrospective study.
Availability of data and material: The datasets supporting the conclusions of this article are included in the article.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet (London, England) 2015;385:2197–208. [DOI] [PubMed] [Google Scholar]
- [2].Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 2016;127:2955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Neben K, Jauch A, Bertsch U, et al. Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica 2010;95:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Narita T, Inagaki A, Kobayashi T, et al. t(14;16)-positive multiple myeloma shows negativity for CD56 expression and unfavorable outcome even in the era of novel drugs. Blood Cancer Journal 2015;5:e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hebraud B, Magrangeas F, Cleynen A, et al. Role of additional chromosomal changes in the prognostic value of t(4;14) and del(17p) in multiple myeloma: the IFM experience. Blood 2015;125:2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chang H, Qi X, Jiang A, et al. 1p21 deletions are strongly associated with 1q21 gains and are an independent adverse prognostic factor for the outcome of high-dose chemotherapy in patients with multiple myeloma. Bone Marrow Transplant 2010;45:117–21. [DOI] [PubMed] [Google Scholar]
- [7].Boyd KD, Ross FM, Chiecchio L, et al. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia 2012;26:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Teoh PJ, Chung TH, Sebastian S, et al. p53 haploinsufficiency and functional abnormalities in multiple myeloma. Leukemia 2014;28:2066–74. [DOI] [PubMed] [Google Scholar]
- [9].Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014;28:269–77. [DOI] [PubMed] [Google Scholar]
- [10].Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 2013;88:360–76. [DOI] [PubMed] [Google Scholar]
- [11].Binder M, Rajkumar SV, Ketterling RP, et al. Prognostic implications of abnormalities of chromosome 13 and the presence of multiple cytogenetic high-risk abnormalities in newly diagnosed multiple myeloma. Blood Cancer J 2017;7:e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saad AA, Sharma M, Higa GM. Treatment of multiple myeloma in the targeted therapy era. Ann Pharmacother 2009;43:329–38. [DOI] [PubMed] [Google Scholar]
- [13].Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Brit J Haematol 2008;140:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014;28:1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–548. [DOI] [PubMed] [Google Scholar]
- [16].San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008;359:906–17. [DOI] [PubMed] [Google Scholar]
- [17].Naymagon L, Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol 2016;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gandolfi S, Paba Prada C, Richardson P. How I treat the young patient with multiple myeloma. Blood 2018;132:1114–24. [DOI] [PubMed] [Google Scholar]
- [19].Merz M, Jauch A, Hielscher T, et al. Longitudinal fluorescence in situ hybridization reveals cytogenetic evolution in myeloma relapsing after autologous transplantation. Haematologica 2017;102:1432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Merz M, Jauch A, Hielscher T, et al. Prognostic significance of cytogenetic heterogeneity in patients with newly diagnosed multiple myeloma. Blood Adv 2018;2:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zavrski I, Naujokat C, Niemoller K, et al. Proteasome inhibitors induce growth inhibition and apoptosis in myeloma cell lines and in human bone marrow myeloma cells irrespective of chromosome 13 deletion. J Cancer Res Clin Oncol 2003;129:383–91. [DOI] [PubMed] [Google Scholar]
- [22].Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia 2007;21:151–7. [DOI] [PubMed] [Google Scholar]
- [23].Sagaster V, Ludwig H, Kaufmann H, et al. Bortezomib in relapsed multiple myeloma: response rates and duration of response are independent of a chromosome 13q-deletion. Leukemia 2007;21:164–8. [DOI] [PubMed] [Google Scholar]
- [24].Kiyota M, Kobayashi T, Fuchida S, et al. Monosomy 13 in metaphase spreads is a predictor of poor long-term outcome after bortezomib plus dexamethasone treatment for relapsed/refractory multiple myeloma. Int J Hematol 2012;95:516–26. [DOI] [PubMed] [Google Scholar]
- [25].Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood 2006;108:2165–72. [DOI] [PubMed] [Google Scholar]
- [26].Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: updated time-to-events results and prognostic factors for time to progression. Haematologica 2008;93:560–5. [DOI] [PubMed] [Google Scholar]
- [27].Mateos MV, Gutierrez NC, Martin-Ramos ML, et al. Outcome according to cytogenetic abnormalities and DNA ploidy in myeloma patients receiving short induction with weekly bortezomib followed by maintenance. Blood 2011;118:4547–53. [DOI] [PubMed] [Google Scholar]
- [28].Biran N, Malhotra J, Bagiella E, et al. Patients with newly diagnosed multiple myeloma and chromosome 1 amplification have poor outcomes despite the use of novel triplet regimens. Am J Hematol 2014;89:616–20. [DOI] [PubMed] [Google Scholar]
- [29].Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol 2010;28:4630–4. [DOI] [PubMed] [Google Scholar]
- [30].Cohen YC, Saranga A, Gatt ME. Treatment patterns and clinical outcomes in high-risk newly diagnosed multiple myeloma patients carrying the 17p deletion: an observational multi-center retrospective study. Am J Hematol 2018;93:810–5. [DOI] [PubMed] [Google Scholar]
- [31].Byun JM, Shin DY. Distinct predictive impact of FISH abnormality in proteasome inhibitors and immunomodulatory agents response: redefining high-risk multiple myeloma in Asian patients. Cancer Med 2018;7:831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol 2012;30:2946–55. [DOI] [PubMed] [Google Scholar]
- [33].El-Ghammaz AM, Abdelwahed E. Bortezomib-based induction improves progression-free survival of myeloma patients harboring 17p deletion and/or t(4;14) and overcomes their adverse prognosis. Ann Hematol 2016;95:1315–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


