Abstract
Tocilizumab (TCZ), a monoclonal recombinant antibody against IL-6 receptor, is currently used in managing the cytokine release syndrome (CRS) that occurred in coronavirus disease 2019 (COVID-19) selected cases. The primary objective of our study was to establish the effectiveness of TCZ in patients with severe or critical severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pneumonia.
We retrospectively analyzed 25 consecutive patients, admitted in the Academic Emergency Hospital Sibiu, Romania from April 1, 2020 until May 25, 2020, all with confirmed SARS-CoV-2 infection and severe pneumonia. All patients were treated off-label with TCZ, beside their standard care. Adjuvant iron chelator was associated in 11 patients.
Six female and 19 male patients admitted in our hospital all with confirmed SARS-CoV-2 infection and severe pneumonia as defined by Chinese Centers for Disease Control and Prevention were enrolled in this study. Seventeen of the 25 enrolled patients (68%) were seriously ill requiring noninvasive ventilation or oxygen mask, and 8 cases (32%) were critically ill requiring invasive mechanical ventilation. All patients received TCZ, and also received hydroxychloroquine, and lopinavir/ritonavir 200/50 mg for 10 days. Adjuvant iron chelator (deferasirox – marketed as Exjade) was associated in 11 patients who had ferritin serum levels above 1000 ng/mL. No side effects were encountered during infusions or after TCZ. We observed a rapid increase in arterial oxygen saturation for 20 of the 25 cases (80%) with a favorable evolution toward healing. Survivors were younger than 60 years old (80%), had less comorbidities (10% no comorbidities, 70% with 1 or 2 comorbidities), lower serum ferritin levels (30% under 1000 ng/mL), and 50% had no serum glucose elevation. Our patients with CRS had no response to corticosteroid therapy. Five out of the 25 patients had an unfavorable evolution to death. The off-label use of TCZ in patients with severe or critically ill form of SARS-CoV-2 infection had good results in our study.
Off-label use of TCZ in severe and critical cases of COVID-19 pneumonia is effective in managing the “cytokine storm.” Better outcomes were noted in younger patients. Associated adjuvant iron chelators may contribute to a good outcome and needs to be confirmed in larger studies.
Keywords: COVID-19, critically ill patients, cytokine release syndrome, effectiveness, SARS-CoV-2, severe patients, tocilizumab
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, that emerged at the end of 2019, related to the exposure and direct animal-human transmission to persons exposed to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the Huanan Seafood Wholesale Market of Wuhan is produced by the SARS-CoV-2, a strain of the Coronaviridae family, Orthocoronavirinae subfamily, and betacoronavirus (betaCoV) family.[1] Based on of the whole-genome sequence analyzes of the virus, from the phylogenetic tree based, the SARS-CoV-2 has a parallel structure to the SARS-like bat CoVs, while the severe acute respiratory syndrome coronavirus (SARS-CoV) has descended from the SARS-like bat CoV lineage, this assessment indicates that SARS-CoV-2 is closer to the SARS-like bat CoVs than it is to the SARS-CoVs.[2]
Due to the interhuman transmission of SARS-CoV-2 that allowed the infection, at the time of the writing (June 3, 2020) of this manuscript, the pandemic rapidly spread and caused 6,441,023 (infected) patients worldwide, resulting in 380,940 deaths. At the time of the revision (February 15, 2021), 109,505,562 cases were diagnosed worldwide, resulting in 2,413,903 deaths.[3] SARS-CoV-2 virulence involves nonstructural proteins responsible for blocking the innate immunity,[4] structural proteins, and the viral envelope which ensures the assembly and release of virions. SARS-CoV-2 infection may be also responsible for an excessive inflammatory response (the “cytokine storm”), associated with the release of serum pro-inflammatory cytokines, such as tumour necrosis factor alpha (TNF-alpha), IL-2, IL-7 and IL-10, G-CSF, MIP-1 alpha, and others, which are responsible for the progression of the lesions caused by the direct cytopathic action of the virus. In clinically severe forms of the disease, there is a significant decrease in circulating T lymphocytes and monocytes, with a possible increase in their concentration in the lungs, causing extreme local inflammation in critically ill patients with SARS-CoV-2. IL-6 is involved in the activation and differentiation of B lymphocytes and the synthesis of acute phase proteins, as well as in the cytokine release syndrome (CRS), and it is responsible for multiple organ dysfunction syndrome. Different pattern of cytokines and chemokines were found in the bronchoalveolar lavage of severe (IL-6, TNF, IL-1β and CCL24, and CCL7 chemokines) vs moderate types of COVID-19 pneumonia (CXCR3 and CXCR6 chemokines involved in T cell activation and attraction).[5]
Tocilizumab (TCZ), a monoclonal recombinant antibody against IL-6 Receptor (IL-6R), is currently used in rheumatoid arthritis, juvenile idiopathic arthritis, vasculitis (giant cell arteritis, and Takayasu arteritis), and some other new indications in local and general autoimmune diseases. Inhibition of IL-6 has both specific and pleiotropic effect.[6] Among other anticytokine therapy, TCZ was effectively used for managing CRS that occurred as a common adverse event associated with chimeric antigen receptor T cells therapies,[7,8] and more recently for other secondary hemophagocyte lymphohistiocytosis (HLH) syndromes in children and adults,[9] as well as in COVID-19 selected cases.[10]
Over the last couple of months in Sibiu, Romania, 509 cases (among 19,669 cases nationwide) were hospitalized to date with COVID-19 (June 3, 2020). At the time of the revision (February 15, 2021), in Sibiu, 19,173 cases were diagnosed, resulting in 694 deaths. Coordinated successful containment efforts were implemented on March 16, 2020, and continued nationwide for 9 weeks, thus limiting the virus transmission.[11]
In this setting, the Academic Emergency Hospital Sibiu, Romania, was involved from the beginning in the treatment of COVID-19 patients. This retrospective observational study describes the clinical characteristics, laboratory data, the treatment, and the clinical outcome of the patients with laboratory confirmed COVID-19 admitted into our hospital. Patients received the standard of care according to the national guideline, including lopinavir/ritonavir, hydroxychloroquine, and corticosteroid, other symptom relievers and oxygen therapy associated with TCZ and adjuvant iron chelator therapy (for selected patients who had ferritin serum levels above 1000 ng/mL). We aimed to present treatment responses of TCZ, and to verify that the targeted IL-6 therapy, is an effective and safe way to reduce the mortality of SARS-CoV-2 associated with adjuvant iron chelator therapy. At the beginning of this study Food and Drug Administration recently approved a Phase III Clinical Trial of TCZ for COVID-19 pneumonia, previously published data was from studies that were evaluating its effectiveness in a very small number of patients.
2. Materials and methods
A single-center observational cohort ongoing study on SARS-CoV-2 infected patients is conducted in the Academic Emergency Hospital Sibiu, Romania, a county hospital with 1054 beds, dedicated for the treatment of COVID-19 patients. In this study, we retrospectively analyzed 25 consecutive patients (6 female and 19 male patients), admitted in our hospital from April 1, 2020 until May 25, 2020, all with confirmed SARS-CoV-2 infection (by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) from nasal and pharyngeal swabs) and severe pneumonia as defined by Chinese Centers for Disease Control and Prevention: dyspnea, tachypnea (respiratory rate over 30/min), SpO2 below 93%, PaO2/FiO2 ratio <300, and increase in size of lung lesions by over 50% in 24 to 48 hours. Patients presenting respiratory distress, septic shock and/or multiple organ dysfunction syndrome were diagnosed as critically ill.[12,13] Written informed consent was obtained from the patients.
The primary objective of our study was to establish the effectiveness of TCZ in patients with severe or critical form of SARS-CoV-2 pneumonia, uninfluenced by the therapy with lopinavir/ritonavir, hydroxychloroquine, and corticosteroid, patients with CRS. The secondary objective was to assess the evolution of patients with concomitant hyperferritinemia, under treatment with TCZ and deferasirox (SARS-CoV-2 decreases the production of heme with the accumulation of metabolites like porphyrin, δ-aminolevulinic acid and porphobilinogen aggravating the respiratory but also the neurological, digestive, and muscular impairment).
All patients were treated off-label with TCZ, beside their standard of care. All patients received hydroxychloroquine, 200 mg q12 hours for 5 to 7 days and lopinavir/ritonavir 200/50 mg, q12 hours for 10 days. Adjuvant iron chelator (deferasirox – marketed as Exjade) was associated in 11 patients who had ferritin serum levels above 1000 ng/mL (reference range 22–322 ng/mL). Cases with sepsis, as well as patients with respiratory bacterial infections, received appropriate antibiotic therapy after identified isolated bacteria (VITEK 2 Compact analyzer bioMérieux, Marcy-l’Étoile, France) and the assessed MICs according to the EUCAST breakpoints.
Patient outcome and treatment effectiveness were assessed by clinical (central body temperature, oxygen saturation of arterial blood, and clinical status) and biologic markers of inflammation, both before and after TCZ administration. Laboratory examinations included full blood count, the neutrophil-to-lymphocyte ratio, C-reactive protein (CRP), and serum IL-6 levels, fibrinogen, erythrocyte sedimentation rate but also lactate dehydrogenase and ferritin levels, markers of coagulopathy, liver and kidney function tests, and bacteriological examinations (selected cases where a bacterial superinfection was suspected). Serum Il-6 levels were detected using an IL-6 electro-chemiluminescence immunoassay kit.
Detailed information was abstracted from the medical records of the patients using a standardized collection form. All data were available for all the enrolled patients. Correlations between different clinical parameters and statistical analysis were performed using the IBM SPSS Statistics version 26 software. Patient follow-up ended at discharge (improved/cured or deceased). Long-term prospective follow-up of our COVID-19 patients is not yet available; it will be reported at the end of another ongoing study.
Written informed consent was obtained from the patients for publication of their case report and any accompanying images. The study was accepted by the Ethics Committee of the hospital and they encouraged publishing the article. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
3. Results
Demographic and main clinical characteristics of the 25 enrolled patients are shown in Table 1. Mean age was 52.56 years (ranged from 35 to 89 years). The average time from COVID-19 symptoms onset and admission was 4 days (ranged between 2 and 14 days). Most frequent comorbidities were obesity (18/25 = 72%), hypertension (10/25 = 40%), and type 2 diabetes mellitus (4/25 = 16%). Less frequent comorbidities were endocrine disorders (1 case of thyroid nodules, hypothyroidism, and Addison disease), inflammatory bowel disease (Crohn disease), hematological malignancy (chronic lymphocytic leukemia), psoriasis, chronic obstructive pulmonary disease (2 cases), chronic kidney disease (2 cases), chronic hepatitis B virus infection, and coronary artery disease. According to the COVID-19 pneumonia severity grade classification, 17 of the 25 enrolled patients (68%) were seriously ill requiring noninvasive ventilation or oxygen mask, and 8 cases (32%) were critically ill requiring invasive mechanical ventilation.
Table 1.
Demographic and clinical characteristics of patients.
| Case number | Gender | Age (years) | Clinical classification | Comorbidities | Days from TCZ until final outcome | Final clinical outcomes |
| 1 | M | 89 | Critically ill requiring invasive mechanical ventilation | EHBP, CAD, newly discovered type 2 diabetes mellitus during hospitalization | 11 | Death |
| 2 | M | 48 | Critically ill requiring invasive mechanical ventilation | 22 | Clinical stabilization and favorable outcome | |
| 3 | F | 54 | Seriously ill | Obesity, thyroid nodules | 8 | Clinical stabilization and favorable outcome |
| 4 | M | 38 | Critically ill requiring invasive mechanical ventilation | Obesity | 26 | Clinical stabilization and favorable outcome |
| 5 | F | 36 | Seriously ill | Obesity | 24 | Clinical stabilization and favorable outcome |
| 6 | M | 68 | Seriously ill | EHBP, obesity | 7 | Clinical stabilization and favorable outcome |
| 7 | M | 54 | Seriously ill | Obesity | 8 | Clinical stabilization and favorable outcome |
| 8 | F | 36 | Seriously ill | Obesity | 14 | Clinical stabilization and favorable outcome |
| 9 | M | 29 | Seriously ill | Crohn disease, corticosteroid therapy, obesity | 13 | Clinical stabilization and favorable outcome |
| 10 | M | 28 | Seriously ill | Obesity | 13 | Clinical stabilization and favorable outcome |
| 11 | M | 35 | Critically ill requiring invasive mechanical ventilation | Obesity | 28 | Clinical stabilization and favorable outcome |
| 12 | F | 74 | Seriously ill | COPD, EHBP | 15 | Clinical stabilization and favorable outcome |
| 13 | M | 57 | Critically ill requiring invasive mechanical ventilation | Chronic lymphocytic leukemia, EHBP, obesity, chronic hepatitis B virus infection | 8 | Death |
| 14 | M | 61 | Critically ill requiring invasive mechanical ventilation | Diabetes mellitus type 2, psoriasis, chronic kidney disease | 4 | Death |
| 15 | M | 51 | Seriously ill | EHBP, obesity | 10 | Clinical stabilization and favorable outcome |
| 16 | M | 44 | Seriously ill | COPD, EHBP, diabetes mellitus type 2, obesity | 11 | Clinical stabilization and favorable outcome |
| 17 | M | 70 | Seriously ill | Addison disease, hypothyroidism | 25 | Clinical stabilization and favorable outcome |
| 18 | M | 52 | Seriously ill | Obesity | 15 | Clinical stabilization and favorable outcome |
| 19 | F | 58 | Critically ill requiring invasive mechanical ventilation | Obesity | 34 | Death |
| 20 | M | 52 | Seriously ill | Obesity | 12 | Clinical stabilization and favorable outcome |
| 21 | M | 51 | Seriously ill | Obesity, EHBP | 12 | Clinical stabilization and favorable outcome |
| 22 | M | 44 | Seriously ill | 19 | Clinical stabilization and favorable outcome | |
| 23 | M | 70 | Critically ill requiring invasive mechanical ventilation | EHBP, diabetes mellitus type 2, CAD | 2 | Death |
| 24 | F | 39 | Seriously ill | Stage 5 chronic kidney disease, EHBP, obesity | 19 | Clinical stabilization and favorable outcome |
| 25 | M | 76 | Seriously ill | EHBO, obesity, abdominal aortic aneurysm | 16 | Clinical stabilization and favorable outcome |
CAD = coronary artery disease, COPD = chronic obstructive pulmonary disease, critically ill = critical disease: respiratory failure, septic shock, and/or multiple organ dysfunction or failure, EHBP = essential high blood pressure, F = female. M = male, seriously ill = severe disease: dyspnea, respiratory frequency ≥30/min, SpO2 ≤93%, PaO2/FiO2 ratio < 300, and/or lung infiltrates >50% within 24 to 48 hours, TCZ = tocilizumab.
Of the critically ill cases, 2 patients presented sepsis (with Enterococcus faecalis and Streptococcus gallolyticus, respectively), 1 patient presented macrophage activation syndrome (MAS). In 4 cases, respiratory infections with Acinetobacter baumanii, Klebsiella pneumoniae + Haemophilus influenzae, Stenotrophomonas maltophilia + Acinetobacter baumanii, and Klebsiella pneumoniae + Acinetobacter baumanii were associated.
All but 1 patient received parenteral corticosteroid (methylprednisolone – 22 cases or dexamethasone – 2 cases), with no improvement, so TCZ was initiated. The mean time from admission to off-label TCZ administration was 7.92 days, since for most of the patients (n = 21, 84%), the CRS onset was noted in the first 5 hospitalization days. The decision of off-label TCZ administration was taken for with patients with serum IL-6 level 5-fold above normal range (>35 pg/mL) for most of the patients. In 1 case, we decided the use of off-label TCZ, without determining the serum IL-6 level prior to infusion, for a patient that was under mechanical ventilation, and hemodynamically unstable. The response was favorable in 20 of the 25 enrolled cases (80%). An unfavorable outcome was noted in 5 patients, all with invasive mechanical ventilation. Death occurred between day 13 and day 38 after admission into the hospital. The other 3 critically ill patients were extubated at 3 and 4, respectively 7 days after TCZ administration. No side effects were encountered during infusions or after TCZ. The mean IL-6 serum level prior to TCZ administration was 1069.33 (±1876.08 pg/mL, range 38.00–8507.00). The mean IL-6 serum level after TCZ was 1201.22 (±1894.72 pg/mL, range 23.00–6000.00). An increase in the serum IL-6 level after TCZ was observed in 7 cases. As expected, an important decrease in serum CRP levels was noted after TCZ, from 159.75 (±116.71 mg/L, range 17.93–521.54) to 64.48 (± 46/05 mg/L, range 3.92–164.30); normal levels were reached in only 2 (8%) cases after TCZ. The monoclonal antibody against IL-6R treatment was followed by a rapid increase in arterial oxygen saturation for 20 of the 25 cases (80%) who had a favorable outcome and were finally discharged when clinical state permitted and viral clearance was achieved (repeated negative RT-PCR swab test for SARS CoV-2 at 24 h). Clinical response to TCZ was rapidly noted: 72 hours after the first dose of TCZ, most of the patients no longer required additional oxygen supplementation (respiratory support or oxygen mask). Five of the 25 enrolled patients (20%) had an unfavorable evolution which led to death, and for 2 of them an increase of the serum IL-6 level after TCZ was noted.
When the deceased and survivors’ subgroups were compared, the mean CRP decrease after TCZ was not significantly lower (73.932 vs 97.66955, P = .15). Still, there was a difference in the mean age (67 vs 48.95 years, P = .01) and time from symptoms onset to treatment (9.8 vs 7.45 days, P = .02), respectively. We also found a statistically significant trend to increasing number of comorbidities, increasing age, higher levels of serum glucose, and serum ferritin levels, data shown in Table 2. Among deceased, 80% of patients had multiple (3 or 4) comorbidities, 60% were older than 60 years and had elevated levels of serum glucose (80%) (>125 mg/dL) and ferritin (60% above 1000 ng/mL). Survivors were younger than 60 years old (80%), had less comorbidities (10% no comorbidities and 70% with 1 or 2 comorbidities), lower serum ferritin levels (30% under 1000 ng/mL), and 50% had no serum glucose elevation (Table 3; Figures 1 and 2).
Table 2.
Baseline characteristics of the enrolled patients.
| Variable | Strata | Deceased % | Survivors % | Significance |
| Number of comorbidities | 0 | 0 | 10 | P < .001 |
| 1 | 20 | 45 | ||
| 2 | 0 | 25 | ||
| 3 | 60 | 15 | ||
| 4 | 20 | 5 | ||
| Age (years) | <50 | 0 | 50 | P < .001 |
| 50–60 | 40 | 30 | ||
| 60–70 | 40 | 10 | ||
| >70 | 20 | 10 | ||
| Serum ferritin (ng/mL) | <400 | 20 | 15 | P < .001 |
| 400–1000 | 40 | 15 | ||
| 1000–1500 | 60 | 45 | ||
| 1500–2000 | 0 | 10 | ||
| >2000 | 0 | 15 | ||
| Serum glucose (mg/dL) | <125 | 20 | 50 | P < .001 |
| 125–200 | 20 | 25 | ||
| >200 | 60 | 25 |
Table 3.
Characteristics of the proinflammatory markers of the enrolled patients.
| Parameter | Before TCZ (mean, SD, range) | After TCZ (mean, SD, range) |
| Serum IL-6 | 1069.33 (±1876.08 pg/mL, range 38.00–8507.00) | 1201.22 (±1894.72 pg/mL, range 23.00– 6000.00). |
| Serum C-reactive protein | 159.75 (±116.71 mg/L, range 17.93–521.54) | 64.48 (±46/05 mg/L, range 3.92–164.30) |
SD = standard deviation, TCZ = tocilizumab.
Figure 1.
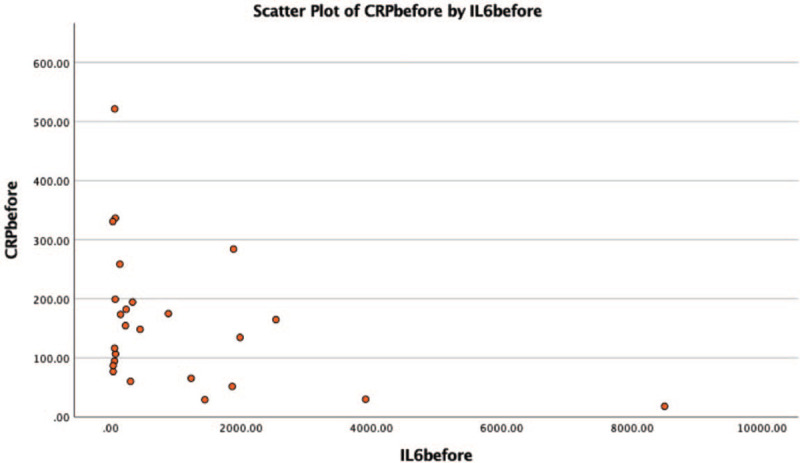
Scatter plot of IL-6 and CRP level for each patient before tocilizumab. CRP = C-reactive protein.
Figure 2.
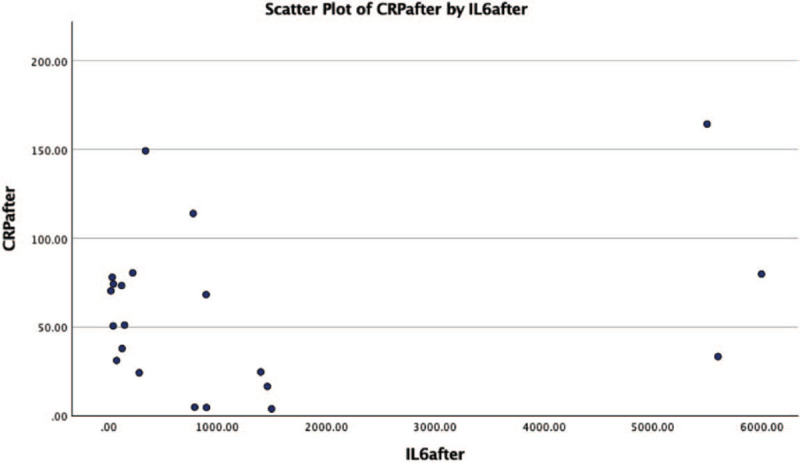
Scatter plot of IL-6 and CRP level for each patient after tocilizumab. CRP = C-reactive protein.
Positive correlations were found between the evolution after the administration of TCZ and the final outcome of the case (r = .89, P = .00, n = 25), between the CRP level prior to the administration of TCZ and d-dimer serum concentration (r = .59, P = .007, n = 25), and between the CRP level prior to the administration of TCZ and the ferritin level (r = .68, P = .00, n = 25). We also noted a positive correlation between the neutrophil-to-lymphocyte ratio and the d-dimer level (r = .77, P = .00, n = 25). There was a negative correlation between the platelet count and d-dimer level (r = −.46, p = .045, n = 25).
Also, a positive correlation was found between the serum IL-6 level after the administration of TCZ and the extubation of the patient (r = .564, P = .006, n = 25). There were no correlation between the serum IL-6 level before the administration of TCZ, the serum IL-6 level after the administration of TCZ or the difference between the serum IL-6 level before and after the administration of TCZ and the final outcome of the case (r = −.067, P = .794, n = 25; r = −.114, P = .614, n = 25; and respectively r = −.063, P = .764, n = 25).
Nine of the 11 cases (81.81%) that received TCZ and adjuvant therapy with deferasirox, an oral iron chelator, progressed to a favorable outcome, and 2 unfortunately toward death. Using the Kaplan–Meier estimator for patient's survival at 30 days, we found a slightly still non-significant increase in the adjuvant therapy (deferasirox) subgroup (80 vs 75%, P = .67) (Table 4; Figure 3).
Table 4.
Correlations between different parameters.
| Correlations | |||||||||||
| Final outcome | Evolution after TCZ | Serum C-reactive protein level before TCZ | d-dimer serum concentration | Serum ferritin level | Neutrophil to lymphocyte ratio | Platelet count | Serum IL-6 level after TCZ | Extubation | Serum IL-6 level before TCZ | IL-6 difference | |
| Final outcome | |||||||||||
| Pearson correlation | 1 | .890∗ | 0.206 | 0.085 | 0.097 | 0.084 | 0.052 | −0.114 | −.647∗ | −0.067 | −0.063 |
| Sig. (2-tailed) | 0.000 | 0.323 | 0.731 | 0.652 | 0.690 | 0.806 | 0.614 | 0.000 | 0.749 | 0.764 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Evolution after TCZ | |||||||||||
| Pearson correlation | .890∗ | 1 | 0.282 | 0.165 | 0.148 | 0.051 | −0.174 | −0.121 | −.657∗ | −0.105 | −0.044 |
| Sig. (2-tailed) | 0.000 | 0.173 | 0.499 | 0.491 | 0.810 | 0.405 | 0.590 | 0.000 | 0.619 | 0.835 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Serum C-reactive protein level before TCZ | |||||||||||
| Pearson correlation | 0.206 | 0.282 | 1 | .595∗ | .684∗ | 0.139 | −0.046 | −0.299 | −0.196 | −0.385 | 0.081 |
| Sig. (2-tailed) | 0.323 | 0.173 | 0.007 | 0.000 | 0.506 | 0.826 | 0.176 | 0.348 | 0.058 | 0.702 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| d-dimer serum concentration | |||||||||||
| Pearson correlation | 0.085 | 0.165 | .595∗ | 1 | 0.140 | .771∗ | −.465† | −0.404 | −0.153 | −0.240 | −0.081 |
| Sig. (2-tailed) | 0.731 | 0.499 | 0.007 | 0.567 | 0.000 | 0.045 | 0.121 | 0.531 | 0.322 | 0.741 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Serum ferritin level | |||||||||||
| Pearson correlation | 0.097 | 0.148 | .684∗ | 0.140 | 1 | 0.038 | 0.211 | −0.195 | −0.221 | −0.181 | 0.004 |
| Sig. (2-tailed) | 0.652 | 0.491 | 0.000 | 0.567 | 0.861 | 0.323 | 0.398 | 0.299 | 0.396 | 0.984 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Neutrophil to lymphocyte ratio | |||||||||||
| Pearson correlation | 0.084 | 0.051 | 0.139 | .771∗ | 0.038 | 1 | −0.075 | −0.071 | −0.109 | −0.003 | −0.030 |
| Sig. (2-tailed) | 0.690 | 0.810 | 0.506 | 0.000 | 0.861 | 0.721 | 0.754 | 0.604 | 0.988 | 0.887 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Platelet count | |||||||||||
| Pearson correlation | 0.052 | −0.174 | −0.046 | −.465† | 0.211 | −0.075 | 1 | −0.139 | −0.117 | 0.361 | −0.293 |
| Sig. (2-tailed) | 0.806 | 0.405 | 0.826 | 0.045 | 0.323 | 0.721 | 0.537 | 0.579 | 0.076 | 0.154 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Serum IL-6 level after TCZ | |||||||||||
| Pearson correlation | −0.114 | −0.121 | −0.299 | −0.404 | −0.195 | −0.071 | −0.139 | 1 | .564∗ | 0.093 | .668∗ |
| Sig. (2-tailed) | 0.614 | 0.590 | 0.176 | 0.121 | 0.398 | 0.754 | 0.537 | 0.006 | 0.681 | 0.001 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Extubation | |||||||||||
| Pearson correlation | −.647∗ | −.657∗ | −0.196 | −0.153 | −0.221 | −0.109 | −0.117 | .564∗ | 1 | 0.091 | 0.377 |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.348 | 0.531 | 0.299 | 0.604 | 0.579 | 0.006 | 0.666 | 0.063 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Serum IL-6 level before TCZ | |||||||||||
| Pearson correlation | −0.067 | −0.105 | −0.385 | −0.240 | −0.181 | −0.003 | 0.361 | 0.093 | 0.091 | 1 | −.663∗ |
| Sig. (2-tailed) | 0.749 | 0.619 | 0.058 | 0.322 | 0.396 | 0.988 | 0.076 | 0.681 | 0.666 | 0.000 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| IL-6 Difference | |||||||||||
| Pearson correlation | −0.063 | −0.044 | 0.081 | −0.081 | 0.004 | −0.030 | −0.293 | .668∗ | 0.377 | −.663∗ | 1 |
| Sig. (2-tailed) | 0.764 | 0.835 | 0.702 | 0.741 | 0.984 | 0.887 | 0.154 | 0.001 | 0.063 | 0.000 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
TCZ = tocilizumab.
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Figure 3.
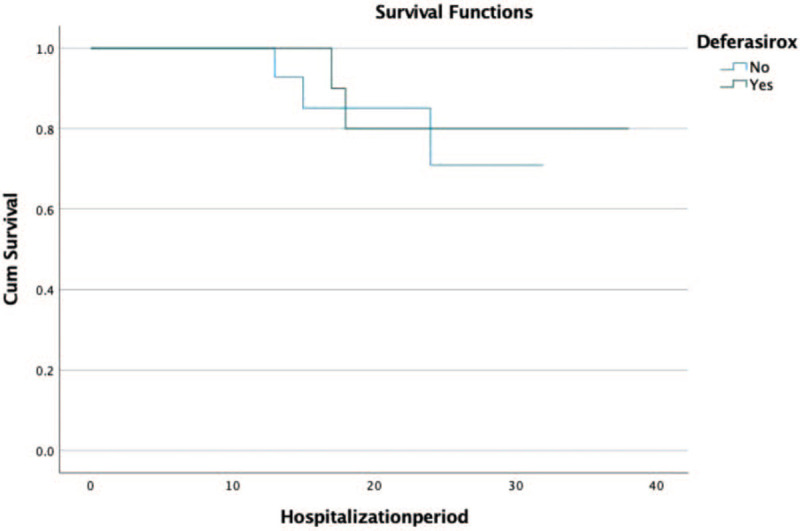
Kaplan–Meier plot for patient survival under tocilizumab with and without deferasirox. Number of patients that received deferasirox 11. Number of patients that did not received deferasirox 14; 81.81% of the patients that received TCZ and adjuvant therapy with deferasirox, progressed to a favorable outcome.
4. Discussions
Since December 2019, COVID-19 has become a pandemic affecting more than 6 million people worldwide. Different treatment schemes and protocols were applied in each country. TCZ was used for COVID-19 associated “cytokine storm” or CRS associated with a severe or critically course of disease, that occurred in a low number of internationally reported cases, 5% to 29%.[14,15] Most of the cases in our region, similar to others, had a mild or moderate course.
It is already known the COVID-19 evolves through 3 successive stages of disease: an early phase (penetration of the virus to the cells), a viremic phase and eventually the most severe “cytokine storm” phase occurring in a subset of patients. The last 1 could have a catastrophic course and patients often died or needed prolonged mechanical ventilation with uncertain outcome.
CRS was also described during previous Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus outbreaks.[16] In terms of immune mechanisms and clinical course, the “cytokine storm” or CRS described in some COVID-19 patients is very similar to a severe life-threatening complication of some rheumatologic diseases, known as MAS, which is currently explained by a defective lyse of activated antigen presenting cells resulting in the amplification of a proinflammatory cascade, with macrophage activation and emerging hemophagocytosis and organ damage.[17] MAS is considered a particular type of HLH in patients with rheumatic disorders. Clinically, MAS is characterized by high fever, disseminated intravascular coagulation, hypofibrinogenemia, high ferritin, hypertriglyceridemia, pancytopenia, hepatosplenomegaly, lymphadenopathy, and hepatic dysfunction. The excessive proinflammatory cytokines found in MAS are about the same as in COVID-19-induced CRS, predominantly IL-1, IL-6, TNF-alpha, IL-2, IL-10, and other.
Mehta et al[18] suggested that the pathogenesis of COVID-19 acute respiratory distress syndrome (ARDS) is similar to that of secondary HLH, leading to fulminant hypercytokinemia with multiple organ failure. SARS-CoV-2 is associated with extensive lung injuries and high levels of IL-6 making IL-6 a possible “culprit” in ARDS onset.[19,20]
From a rheumatologist's perspective, at least 2 common points can be distinguished between COVID-19 and inflammatory rheumatologic diseases. On one hand, common symptoms of rheumatologic diseases were described in SARS-CoV-2 infection (such as arthralgias, myalgias, myocarditis, leuko-/thrombocytopenia, interstitial pulmonary disease/pneumonitis, haemophagocytic lympho-hystiocitosis, and increased thromboembolic risk). On the other hand, some drugs currently used by rheumatologists, especially biologic anti-cytokine drugs, including TCZ, are used off-label in COVID-19 treatment protocols worldwide.
Recent and emerging data from previous and concomitant pandemic sites focused on the multifaceted complex systemic pattern of COVID-19 disease. Few studies suggested that immunotherapy treatment can be crucial and lifesaving in selected patients with COVID-19-induced CRS, with a rapid clinical and biochemical improvement following TCZ administration. TCZ therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients, highlighted in approximately 80 reports.[21–23]
Using TCZ in all indications, we need to be aware of its serious side effects: the risk of serious infection, neutropenia or thrombocytopenia, hyperlipidemia, liver, and intestinal potential damage, but there is no need to adjust the dose of TCZ in patients with mild or moderate renal impairment.[24] Inhibiting IL-6, through 2 different and divergent pathways is a balance between the trans-signaling transduction, harmful (pro-inflammatory response), and the classic protective signaling transduction (anti-inflammatory response) involved in the initial phase of disease.[25] Thus, the moment of clinical TCZ intervention has to be carefully chosen to minimize potential harm.[26]
Our patients with CRS had no response to corticosteroid therapy, as it was demonstrated in several other SARS-CoV and MERS-CoV studies[27,28] or SARS-CoV-2 studies[29,30] where serum level of IL-6 increased after administration of methylprednisolone or dexamethasone.
The off-label use of TCZ in patients with severe or critically ill form of SARS-CoV-2 infection had good results in 80% of our patients, similar to other results from already published studies;[31,32] in 1 recent report on a group of 15 patients, most patients no longer required additional respiratory support like oxygen mask at 72 hours after infusion.[33]
In our group, 13 of 20 (65%) obese or overweight patients had a good outcome and good response to maximum dose (800 mg) of IL-6 inhibition with TCZ, a dose that might have been inferior to the optimal therapeutic dose for rheumatoid arthritis or other CRS similar patients.[34,35] No hepatotoxic side effects were observed in our group.
Hyperglycemia found in some of our patients may be attributed to SARS-CoV-2 infection (pancreatic lesions due to the direct cytopatic lesions or indirect hyperthrombotic state and cytokine-induced multiple organ lesions), host condition (diabetes), or treatment (corticosteroid therapy, TCZ). Any of these could be a confounder for the unfavorable response to TCZ in high levels glycemia patients from our group.
The association of iron chelators as an adjuvant therapy to SARS-CoV-2 current antiviral therapy, with and without TCZ in severe/critical pneumonia seems to be a good alternative in patients with significant hyperferritinemia and needs to be demonstrated by larger studies.[35]
The main limitations of our observational study are the small sample size and the short period of observation. Nevertheless, our results on TCZ effectiveness in managing the “cytokine storm” in severe/critical cases of COVID-19 pneumonia and ARDS, are in line with some other retrospective observational studies on this medication from different pandemic sites. All these studies support the need to introduce this monoclonal antibody against interleukin-6 receptor in the SARS-CoV-2 antiviral armamentarium for carefully selected clinical conditions.
To date, TCZ is included in 45 ongoing registered studies (among 286 studies on COVID-19 medication) from different parts of the world (Italy, Spain, United Kingdom, United States, Malaysia, and others) and emerging results are needed to confirm our results and to state the appropriate patient profile and the best timing for a successful therapeutic intervention.
5. Conclusion
Off-label use of TCZ in severe and critical cases of COVID-19 pneumonia is effective in managing the “cytokine storm,” with rapid improvement of oxygen requirements and switch to a better clinical course and disease resolution. Better outcomes were noted in younger patients with less comorbidities and moderately increased markers of inflammation (ferritin, d-dimer) and serum glucose. Associated adjuvant iron chelators may contribute to a good outcome and needs to be confirmed in larger studies. Awaited results from prospective randomized clinical trials, together with long-term prospective follow-up of COVID-19 patients are needed to confirm our encouraging results and to definitely recommend this treatment in COVID-19 patients.
Author contributions
All authors contributed equally to this manuscript in terms of acquisition, analysis and interpretation of data, conception and design, drafting the manuscript. All authors read and approved the final manuscript.
Conceptualization: Victoria Birlutiu, Liana Chicea.
Data curation: Victoria Birlutiu, Rares Mircea Birlutiu, Liana Chicea.
Formal analysis: Victoria Birlutiu, Rares Mircea Birlutiu, Liana Chicea.
Investigation: Victoria Birlutiu, Liana Chicea.
Methodology: Victoria Birlutiu, Liana Chicea.
Project administration: Victoria Birlutiu.
Resources: Victoria Birlutiu, Rares Mircea Birlutiu, Liana Chicea.
Software: Rares Mircea Birlutiu.
Supervision: Victoria Birlutiu.
Validation: Victoria Birlutiu, Liana Chicea.
Visualization: Victoria Birlutiu, Rares Mircea Birlutiu, Liana Chicea.
Writing – original draft: Victoria Birlutiu, Liana Chicea.
Writing – review & editing: Rares Mircea Birlutiu.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, betaCoV = betacoronavirus, CCL = chemokine ligand, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein, CRS = cytokine release syndrome, CXCR = chemokine receptor, G-CSF = granulocyte-colony stimulating factor, HLH = hemophagocyte lymphohistiocytosis, IL = interleukin, IL-6R = IL-6 receptor, MAS = macrophage activation syndrome, MERS-CoV = Middle East respiratory syndrome coronavirus, MIP-1 alpha = macrophage inflammatory protein-1 alpha, RT-PCR = real-time reverse transcriptase-polymerase chain reaction, SARS-CoV = severe acute respiratory syndrome coronavirus, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, TCZ = tocilizumab, TNF-alpha = tumour necrosis factor alpha.
How to cite this article: Birlutiu V, Birlutiu RM, Chicea L. Off-label tocilizumab and adjuvant iron chelator effectiveness in a group of severe COVID-19 pneumonia patients: a single center experience. Medicine. 2021;100:18(e25832).
The authors declare no conflicts of interest.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Chan JF, To KK, Tse H, et al. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol 2013;21:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 2020;27:325–8. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Available at: http://coronavirus.jhu.edu/map.html. [Google Scholar]
- [4].Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antiviral Res 2018;149:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- [6].González-Gay MA, Mayo J, Castañeda S, et al. Tocilizumab: from the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces. Expert Opin Biol Ther 2020;doi: 10.1080/14712598.2020.1770222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018;23:943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cron RQ, Chatham WW. The question of whether to remain on therapy for chronic rheumatic diseases in the setting of the covid-19 pandemic. J Rheumatol 2020;jrheum.200492doi:10.3899/jrheum.200492. [Google Scholar]
- [10].Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect 2020;80:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Streinu-Cercel A. SARS-CoV-2 in Romania – situation update and containment strategies. Germs 2020;10:08.doi: 10.18683/germs.2020.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- [13].Stat Pearls Publishing, Marco Cascella, Michael Rajnik, Arturo Cuomo, et al. Features, Evaluation and Treatment Coronavirus (COVID-19). 2020. [PubMed] [Google Scholar]
- [14].Quartuccio L, Sonaglia A, McGonagle D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol 2020;104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. doi:10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017;39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yasin S, Schulert GS. Systemic juvenile idiopathic arthritis and macrophage activation syndrome: update on pathogenesis and treatment. Curr Opin Rheumatol 2018;30:514–20. [DOI] [PubMed] [Google Scholar]
- [18].Mehta PM, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ’culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. ytokine X 2020; 10.1016/j.cytox.2020.100029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Magro G. SARS-CoV-2 and COVID-19: what are our options? Where should we focus our attention on to find new drugs and strategies? Travel Med Infect Dis 2020;101685.doi: 10.1016/j.tmaid.2020.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease. J Med Virol 2020;doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020;102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med 2020;doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang S, Li L, Shen A, et al. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig 2020;01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaur S, Bansal Y, Kumar R, et al. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem 2020;28:115327. [DOI] [PubMed] [Google Scholar]
- [26].Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest 2020;158:e15–9. doi:10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3:e343.doi:10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197:757–67. [DOI] [PubMed] [Google Scholar]
- [29].Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature published online Feb 3. DOI:10.1038/s41586-020-2008-3;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fu B, Xu X, Wei H. Why tocilizumab could be an elective treatment for severe COVID-19? J Transl Med 2020;18:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv 2020;doi:10.12074/202003.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pan L, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020;01–5. doi.org/10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Available at: www.ema.europa.eu>documents>product-information. [Google Scholar]
- [35].Liu W, Zhang S, Nekhai S, et al. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr Clin Microbiol Rep 2020;01–7. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


