Abstract
BACKGROUND:
Smooth muscle cells (SMC) play significant roles in atherosclerosis via phenotypic switching, a pathological process in which SMC dedifferentiation, migration and transdifferentiation into other cell types. Yet, how SMC contribute to pathophysiology of atherosclerosis remains elusive.
METHODS:
To reveal the trajectories of SMC transdifferentiation during atherosclerosis and to identify molecular targets for disease therapy, we combined SMC fate mapping and single-cell RNA sequencing of both mouse and human atherosclerotic plaques. We also performed cell biology experiments on isolated SMC-derived cells, conducted integrative human genomics, and employed pharmacological studies targeting SMC-derived cells both in vivo and in vitro.
RESULTS:
We found that SMC transitioned to an intermediate cell state during atherosclerosis, which was also found in human atherosclerotic plaques of carotid and coronary arteries. SMC-derived intermediate cells, termed “SEM” cells, were multipotent and could differentiate into macrophage-like and fibrochondrocyte-like cells, as well as return towards SMC phenotype. Retinoic acid (RA) signaling was identified as a regulator of SMC to SEM cell transition and RA signaling was dysregulated in symptomatic human atherosclerosis. Human genomics revealed enrichment of genome wide association study (GWAS) signals for coronary artery disease (CAD) in RA signaling target gene loci and correlation between CAD risk alleles and repressed expression of these genes. Activation of RA signaling by all-trans retinoic acid (ATRA), an anti-cancer drug for acute promyelocytic leukemia, blocked SMC transition to SEM cells, reduced atherosclerotic burden and promoted fibrous cap stability.
CONCLUSIONS:
Integration of cell-specific fate mapping, single-cell genomics and human genetics adds novel insights into the complexity of SMC biology and reveals regulatory pathways for therapeutic targeting of SMC transitions in atherosclerotic cardiovascular disease.
Keywords: smooth muscle cell, single-cell RNA sequencing, atherosclerosis, cardiovascular disease, retinoic acid signaling
INTRODUCTION
Atherosclerosis is a complex disease involving pathophysiological activation of multiple cell types, such as smooth muscle cells (SMC), endothelial cells (EC) and immune cells1. With the development of single-cell genomic technologies, emerging studies of both murine atherosclerotic models and human atherosclerotic plaques have revealed the heterogeneity of cell composition in lesions2–5. However, the regulation of involved cell types, their dynamics during atherosclerosis and their relationship with risk of human cardiovascular disease (CVD) remain unclear.
SMC are proposed to play central roles in plaque development, progression and stability through “phenotypic switching”, a process of medial SMC proliferation, dedifferentiation and migration into the intimal lesions in response to atherogenic stimuli6, 7. Recent SMC-lineage tracing studies in mouse models provided evidence that SMC-derived cells contributed a large proportion of cells within lesions8, 9. Human genetic studies have also refocused attention on genes that regulate SMC functions as directly causal in coronary artery disease (CAD)10. Yet, most current treatments for atherosclerosis target low-density lipoprotein cholesterol and have little direct impact on SMC per se11. Thus, directly targeting SMC during atherosclerosis provides therapeutic promise particularly for those patients with CAD who have relatively normal cholesterol level or recurrent CAD despite lipid lowering therapy. Therapies that target SMC-derived cells, however, could be beneficial or harmful depending on the trajectories of these cells. SMC transdifferentiation into ‘inflammatory’ macrophage-like cells might promote instability of atherosclerotic lesions, while SMC transition to ‘synthetic’ fibrotic SMC could stabilize lesions by increasing thickness of the protective fibrous cap6, 7.
A recent study reported that SMC transdifferentiated predominantly into fibroblast-like cells in atherosclerosis contributing to fibrous cap stability, whereas few SMC-derived macrophage-like cells were detected12. Yet, this finding argues against several other previous reports of SMC-derived macrophage-like cells in mouse and human lesions4, 8, 9, 13. It remains uncertain, therefore, whether SMC phenotypic switching is predominantly atheroprotective or harmful, or perhaps both depending on disease microenvironment. To probe these uncertainties, we utilized SMC-lineage tracing and single-cell genomics to interrogate the trajectories of SMC transdifferentiation during atherosclerosis, performed cell biology experiments on isolated SMC-derived cells, conducted integrative human genomics, and employed pharmacological studies both in vivo and in vitro.
Here we report that SMC can transdifferentiate into multiple cell types during atherosclerosis, that a multipotent SMC-derived intermediate cell state can differentiate ex vivo into macrophage-like and fibrochondrocyte-like cells, and even back towards SMC, and that retinoic acid (RA) signaling is one of main regulators of SMC transition to other cell states. Human genomics revealed dysregulated RA signaling in symptomatic atherosclerotic plaques, overlap of RA signaling target genes with GWAS loci for CAD, and convergence of CAD risk alleles with lower expression of RA regulated genes. Experimentally, activation of RA signaling by all-trans retinoic acid (ATRA), an anti-cancer drug used to treat acute promyelocytic leukemia (APL), blocked SMC transition to the intermediate cell state coincident with attenuation of atherosclerotic severity and promotion of lesion stability.
METHODS
The authors declare that all data that support the findings of this study are available within the article and its online supplementary files. Detailed descriptions of materials and methods used in this study, including single cell preparation of mouse arterial specimens, fluorescence-activated cell sorting and flow cytometry analysis, single cell preparation of human atherosclerotic carotid arteries, single-cell RNA sequencing (scRNA-seq), analysis of mouse scRNA-seq data, GO enrichment analysis, IPA analysis, analysis of human atherosclerotic carotid artery scRNA-seq data, integration analysis of human and mouse scRNA-seq data, analysis of human atherosclerotic carotid artery RNA-seq data, master regulators analysis, human GWAS and eQTLs analyses, preparation of frozen sections, immunohistochemistry staining, RNAscope assay, differentiation of SEM cells to macrophage-like cells, fibroblast-like cells and SMC phenotype, macrophage efferocytosis, tissue and cell imaging, quantitative RT-PCR, in vitro studies of ATRA effects on mouse SMC, in vivo studies of ATRA effects on atherosclerosis, SMC transition and vascular injury, hematoxylin and eosin (H&E) staining, and serum cholesterol measurement, can be found in the online Data Supplement.
Mouse studies
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University. Myh11-CreERT2 (Stock No: 019079), Ldlr−/− (Stock No:002207), ApoE−/− (Stock No: 002052) and B6.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J (Ai6(RCL-ZsGreen), Stock No: 007906) mice were obtained from the Jackson Laboratory and were bred onto the C57BL/6J (Stock No: 000664) background. To generate SMC-lineage tracing murine model, the Myh11-CreERT2 mice were bred with Ai6(RCL-ZsGreen) mice, and then crossed to atheroprone backgrounds (Ldlr−/− or ApoE−/−). The Gt(ROSA)26Sor locus in Ai6(RCL-ZsGreen) mice was designed to carry a loxP-flanked STOP cassette preventing the transcription of a CAG promoter-driven ZsGreen114, which enabled SMC-lineage tracing under the condition of Myh11 promoter-driven CreERT2 activation induced by tamoxifen. To induce the specific expression of ZsGreen1 in SMC, SMC-lineage tracing mice were fed tamoxifen diet for 2 days, then chow diet for 2 days. After induction, ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 and ROSA26ZsGreen1/+; ApoE−/−; Myh11-CreERT2 mice at the age of 8 weeks were fed Western diet (Envigo Teklad, TD.88137) for various timepoints as indicated. As BAC transgene expressing CreERT2 driven by the Myh11 promoter was integrated into the Y chromosome15, all Myh11-CreERT2 mice and SMC lineage tracing mice used in the study are male.
Statistical analysis
For atherosclerosis studies, unpaired Student’s t-test was used to compare atherosclerotic lesion areas of aortic sinuses from control (n=6) and ATRA-treated (n=7) mice. For carotid artery ligation study, unpaired Student’s t-test was used to compare media area, intima area, and the ratio of intima-to-media in control (n=6 mice) and ATRA-treated (n=6 mice) groups. For flow cytometry analysis of SEM cell proportions in control (n=6) and ATRA-treated (n=8 mice) mice aortas, unpaired Student’s t-test was applied. Unpaired Student’s t-test was also used to compare SEM cells, and ZsGreen1+ cells in lesion, intima, and fibrous cap between control (n=6 mice) and ATRA (n=7 mice) groups. Analysts were blinded to group allocation (vehicle or ATRA-treated) during analyses. Differentiation of SEM cells to macrophage-like cells, fibroblast-like cells and SMC phenotype, and RT-qPCR assays were performed in biological triplicates. Two-way analysis of variance (ANOVA) was applied for SEM cells to SMC phenotype differentiation analysis, while unpaired Student’s t-test was applied for RT-qPCR data analysis. Statistical significances were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. GraphPad Prism 7 software was used for all statistical analysis.
RESULTS
Single-cell genomics combined with SMC-lineage tracing reveals multiple SMC-derived cell states during atherosclerosis
To track SMC transdifferentiation in atherogenesis, we generated a SMC-lineage tracing murine model by crossing ROSA26ZsGreen1/+ mice14 with Myh11-CreERT2 mice15. In this model, SMC and their progenies permanently express ZsGreen1 after induction by tamoxifen (Figure 1A). In tamoxifen-induced ROSA26ZsGreen1/+; Myh11-CreERT2 mice, ZsGreen1 overlapped well with SMC marker, ACTA2, in normal brachiocephalic artery (BCA) sections (Figure IA in the Supplement). Subsequently, ROSA26ZsGreen1/+; Myh11-CreERT2 mice were crossed onto Ldlr−/− background to track SMC behaviors during atherosclerosis. No ZsGreen1+ cell was detected in flow cytometry analysis of blood and bone marrow (BM) cells from the SMC-lineage tracing mice induced with tamoxifen for various timepoints, and ZsGreen1 was restricted to MYH11+ blood vessels in spleen sections (Figure IB and IC in the Supplement). These results suggest that the SMC-lineage tracing murine model is both sensitive and specific for tracking SMC behaviors during atherosclerosis.
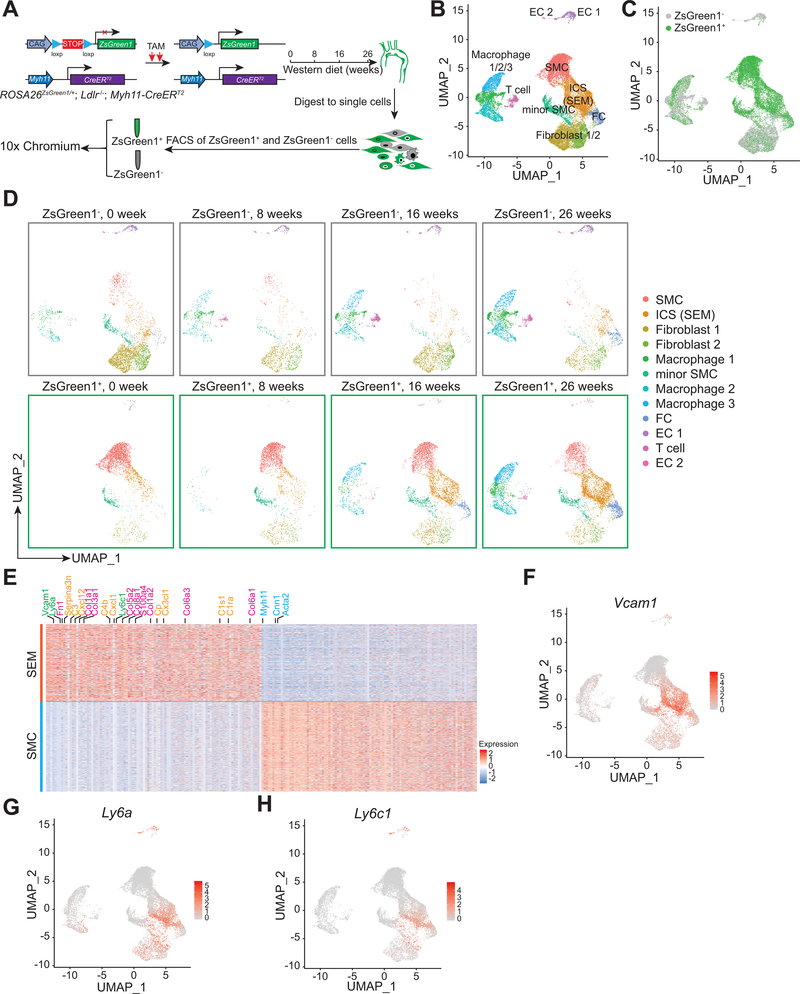
Figure 1. scRNA-seq identified multiple SMC-derived cell types/states, especially SEM cell, during atherosclerosis.
A, Schematic of scRNA-seq using 10x Chromium with both ZsGreen1+ and ZsGreen1− cells from aortas of mice fed with Western diet (WD) for various timepoints (0, 8, 16, 26 weeks). ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice at 7.5-week old are induced with tamoxifen (TAM) for 2 days, followed by chow diet. Afterwards, mice at 8-week old are fed WD for various timepoints as indicated. Arterial tissues (including ascending aorta, BCA and thoracic aorta) with atherosclerotic lesions are isolated and digested to single cells for fluorescence activated cell sorting (FACS) of ZsGreen1+ and ZsGreen1− cells. Single cells are subsequently loaded to 10x Chromium for scRNA-seq. B-D, UMAP visualization of all scRNA-seq data from ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice, including both ZsGreen1+ and ZsGreen1− cells. For combined data of all timepoints (0, 8, 16, 26 weeks), representative cell type/state for each cluster (B) and ZsGreen1 status (ZsGreen1+ and ZsGreen1−) of cell clusters (C) are indicated. For each timepoint, representative cell types/states for cell clusters stratified by ZsGreen1 status are shown in D. SMC, smooth muscle cell; ICS, intermediate cell state, which is afterwards termed “SEM” cell; FC, fibrochondrocyte; EC, endothelial cell. E, Heatmap showing top 100 upregulated and top 100 downregulated DEGs in SEM cells versus SMC identified in ZsGreen1+ scRNA-seq data of Ldlr−/− mice fed 16-week WD. SEM cell markers (Vcam1, Ly6a, Ly6c1) (green), FC-related genes (pink), complement and inflammation-related genes (orange) and SMC markers (Myh11, Cnn1, Acta2) (blue) are indicated. F-H, Expression levels of Vcam1 (F), Ly6a (G) and Ly6c1 (H) in each ZsGreen1+ cell type/state are indicated by color scales.
Following Western diet (WD) in ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice, abundant ZsGreen1+ cells lacking SMC marker expression in atherosclerotic lesions were found in both intima and some regions of arterial media (Figure ID and IE in the Supplement), which is consistent with previous reports8 and suggests that those cells lose canonical SMC phenotype and transition to other cell states. To probe the progression of SMC transdifferentiation, we performed scRNA-seq of both ZsGreen1+ and ZsGreen1− cells from aortas of ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice fed WD for various timepoints (0, 8, 16 and 26 weeks) (Figure 1A and Figure IF, IG in the Supplement). Clustering of combined datasets for ZsGreen1+ and ZsGreen1− cells visualized by Uniform Manifold Approximation and Projection (UMAP)16 revealed multiple cell populations in atherosclerotic aortas (Figure 1B). Cell clusters based on ZsGreen1 status for all timepoints combined (Figure 1C) and for each timepoint (Figure 1D) indicated that multiple SMC-derived cell types/states emerged over time during atherosclerosis, including SMC-derived intermediate cell state (ICS), fibrochondrocyte (FC) and three macrophage-like subtypes. These results demonstrate that SMC undergo transdifferentiation into multiple cell states/types during atherosclerosis.
SMC transition to macrophage-like cells in both Ldlr−/− and ApoE−/− mouse models
Consistent with several previous reports4, 8, 9, 13, our single-cell profiling identified SMC-derived macrophage-like cells and their proportion increased at flow cytometry analysis as atherosclerosis progressed (Figure IIA and IIB in the Supplement). However, the finding contrasts with a recent study, using scRNA-seq and a different SMC-lineage tracing model (tdTomato-transgenic mouse on ApoE−/−; Myh11-CreERT2 background), which reported few SMC-derived macrophages in atherogenesis12. To address the conflict, scRNA-seq was repeated with both ZsGreen1+ and ZsGreen1− cells from aortas of ROSA26ZsGreen1/+; ApoE−/−; Myh11-CreERT2 mice fed WD for various timepoints to determine if genetic backgrounds mattered. This is not the case as clustering results of ApoE−/− scRNA-seq data indicated all counterparts of SMC-derived ICS, FC and macrophage-like subtypes observed in Ldlr−/− mouse model (Figure IIC–IIE in the Supplement).
Efferocytosis is a classic function of macrophages whereby they “eat” apoptotic cells and debris17. One potential concern, therefore, is that apparent SMC-derived ZsGreen1+ macrophage-like cells may be ZsGreen1− macrophages engulfing ZsGreen1+ SMC-lineage cells in vivo or during single cell preparation, and thus be mistakenly assigned as SMC-derived macrophages. To address this, we sorted SMC-derived macrophage-like cells (ZsGreen1+CD11b+) (Figure IIA in the Supplement) and found that almost all sorted ZsGreen1+CD11b+ cells (>90%) remained ZsGreen1+ even after 3-day ex vivo culture (Figure IIF in the Supplement). In parallel, a well-established in vitro efferocytosis assay applying wild-type mouse BM-derived macrophages incubated with PKH26-labeled Jurkat cells18 indicated that almost all engulfed Jurkat cells had been degraded by macrophages within 2.5 days (Figure IIG in the Supplement), which was in stark contrast to persistence of diffuse bright ZsGreen1 in cultured ZsGreen1+CD11b+ cells. These data suggest that ZsGreen1+ macrophage-like cells are derived from SMC during atherosclerosis and are unlikely to be produced through efferocytosis of ZsGreen1+ cells by ZsGreen1− macrophages.
SMC-derived intermediate cell state, SEM cell, possesses multipotent molecular and cellular features
SMC-derived ICS accounted for the largest proportion among SMC lineages in advanced atherosclerosis (Figure 1D). To address molecular and functional characteristics of this cell state, we analyzed differentially expressed genes (DEGs) between ICS and SMC. Notably, Ly6a, Vcam1 and Ly6c1, known markers for stem cell19, endothelial cell20 and monocytes/macrophage differentiation21, respectively, were enriched within SMC-derived intermediate cells (Figure 1E–1H). Hence, we termed these ICS cells as SMC-derived “SEM” cells reflecting their multicellular features. As expected, canonical SMC markers were downregulated in SEM cells (Figure 1E, 2A and Figure IIIA in the Supplement), suggesting loss of SMC phenotype during transition. Conversely, continuously increasing expression of FC-related genes (e.g., Fn1, Col1a1, Col1a2) was found across SMC transition to SEM cells to FC (Figure 2B and Figure IIIB in the Supplement). Moreover, Lgals3, a previously deemed macrophage marker, was upregulated in the SEM cells, albeit with lower expression level than that in one macrophage subtype (Figure IIIC in the Supplement). Notably, Nt5e (encoding CD73) and Eng (encoding CD105), typical makers of mesenchymal stem cells (MSC)22, were absent in SMC-derived SEM cells (Figure IIID and IIIE in the Supplement), suggesting that SEM cells are a unique SMC-derived cell state, instead of a MSC-like cell type.
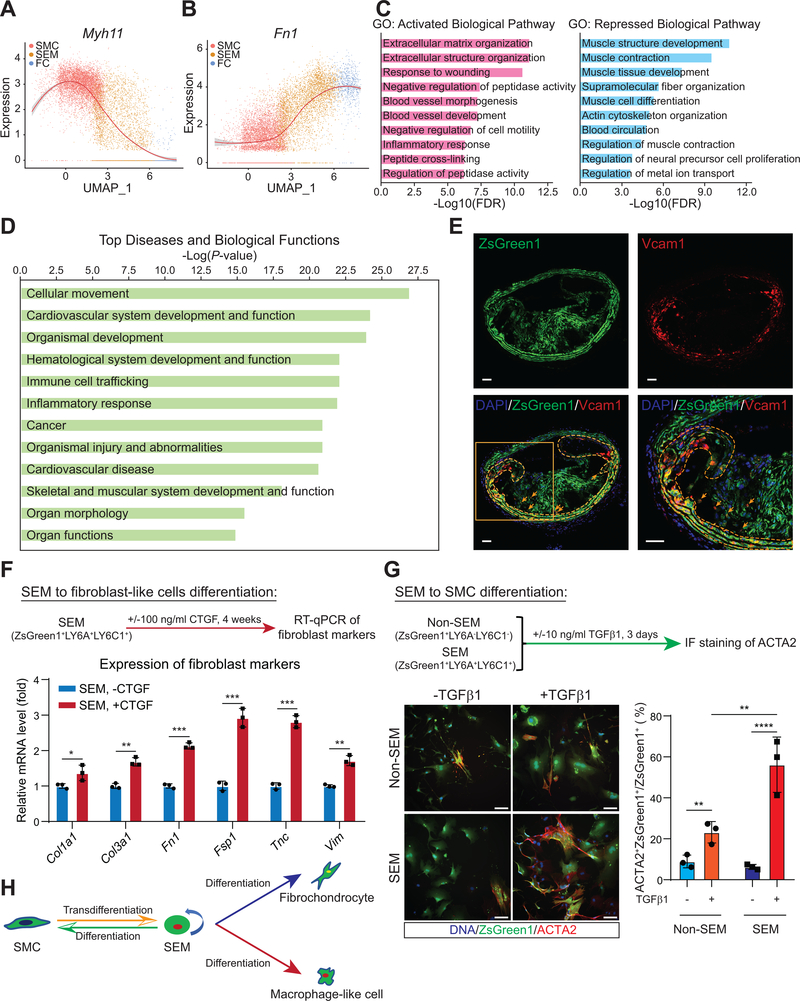
Figure 2. SMC-derived intermediate SEM cells exhibit complex molecular and cellular features.
A and B, Gene expression tendencies of SMC marker, Myh11 (A) and FC-related gene, Fn1 (B) through SMC-SEM-FC axis shown as normalized expression versus UMAP_1. C, GO pathway analysis of DEGs (n = 149 upregulated and 159 downregulated genes in SEM cells, fold change ≥ 1.5, Bonferroni corrected P-value < 0.05) upregulated (pink) or downregulated (blue) in SEM cells versus SMC. D, IPA analysis of DEGs (the same with those in C) upregulated and downregulated in SEM cells versus SMC. Top 20 ranking “Diseases and Biological Functions” significantly altered in SEM cells from SMC are shown. E, RNAscope assay using probe set targeting the mRNA of SEM cell marker, Vcam1. BCA sections are from ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice fed WD for 16 weeks. DAPI (blue), ZsGreen1 protein (green) and Vcam1 mRNA (red) are indicated. Yellow broken lines and arrows highlight ZsGreen1+Vcam1+ cells in media and intima regions, respectively. Scale bars, 50 μm. F, Induction of SEM cells to fibroblast-like cells. FACS sorted ZsGreen1+LY6A+LY6C1+ SEM cells are induced by CTGF (100 ng/mL; +CTGF) or control (PBS; -CTGF) for 4 weeks. The cells were from mice fed WD for 26 weeks. Relative mRNA levels of fibroblast markers (Col1a1, Col3a1, Fn1, Fsp1, Tnc, Vim) are measured by RT-qPCR and normalized against Actb. Values are shown as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001, n=3. G, Induction of SEM cells towards SMC phenotype. FACS sorted ZsGreen1+LY6A+LY6C1+ SEM cells and ZsGreen1+LY6A−LY6C1− non-SEM cells from mice fed WD for 26 weeks are incubated with TGFβ1 (10 ng/mL; +TGFβ1) or control (PBS; -TGFβ1) for 3 days. Immunofluorescence (IF) stained ACTA2+ cells are presented. Two-way ANOVA analysis indicates greater increase in the proportion of ZsGreen1+ACTA2+/ZsGreen1+ cells in SEM cells compared to that in non-SEM cells after 3-day TGFβ1 treatment. Scale bars, 100 μm. Values are shown as mean ± s.d. **P < 0.01, ****P < 0.0001, n=3. H, Schematic of SMC transdifferentiation into SEM cell state during atherosclerosis and the SEM cells differentiation into SMC-derived fibrochondrocyte and SMC-derived macrophage-like cell, and back towards SMC phenotype depending on conditions.
Consistent with response to atherogenic stresses, “extracellular matrix organization”, “extracellular structure organization” and “response to wounding” were among the top biological pathways identified in Gene Ontology (GO) analysis of upregulated DEGs in SEM cells versus SMC, while “muscle contraction”, “actin cytoskeleton organization” and “regulation of muscle contraction” related pathways were repressed in SEM cells, suggesting a loss of contractile features during SMC transition to SEM cells (Figure 2C). Moreover, Ingenuity Pathway Analysis (IPA) of DEGs revealed enrichment of multiple diseases and biological functions, such as “cellular movement”, “cardiovascular system development and function”, “organismal development”, “inflammatory response” and “cardiovascular disease” in SEM cells (Figure 2D). “Cancer” related functions were also dysregulated, suggesting that these cells may share some molecular commonalities with cancer cells, noteworthy because of recent finding that SMC undergo tumorigenesis-like clonal expansion during atherosclerosis9. These bioinformatic analyses suggest that SEM cells have transitioned to a complex multipotent cell state, which is much different, at the molecular and functional levels, from their SMC precursors but also from classical MSC.
To localize SEM cells within atherosclerotic lesions, we performed RNAscope with a probe set targeting Vcam1 mRNA, one of SEM markers (Figure 1F and Figure IG in the Supplement). In BCA sections of 16-week WD fed mice, ZsGreen1+Vcam1+ cells were mainly localized in media of the lesion, with some cells localized in intima overlying the media (Figure 2E), consistent with the finding that some medial ZsGreen1+ cells lost MYH11 expression (Figure ID and IE in the Supplement). The spatial localization pattern of SEM cells, combining with single-cell genomic analyses, suggests that SEM cells might differentiate into multiple other SMC-derived cell types, thus contributing to progression of atherosclerosis. To address this, we isolated ZsGreen1+ SEM cells co-expressing LY6A and LY6C1 (Figure 1G, 1H and Figure IIIF in the Supplement) from mouse atherosclerotic aortas and investigated the differentiation potential of SEM cells to two SMC-derived cell types, macrophage-like cells and FC. Indeed, SEM cells were induced to CD68+ macrophages by macrophage colony stimulating factor (M-CSF)23, while very few CD68+ cells were found in simultaneously induced non-SEM cells (Figure IIIG in the Supplement). Additionally, multiple fibroblast markers, including Col1a1, Col3a1, Fn1, Fsp1, Tnc and Vim, were significantly upregulated in connective tissue growth factor (CTGF)24-treated SEM cells versus control (Figure 2F), producing an in vitro gene expression pattern with similarities to that of SEM cell transition to FC found in atherosclerosis. We also probed whether SMC transition to SEM cells is reversible. As expected, cells expressing ACTA2 were infrequent in cultured ZsGreen1+ SEM and non-SEM (mostly SMC-derived FC) cells in basal culture medium. Following 3-day induction with transforming growth factor beta 1 (TGFβ1), a factor promoting SMC differentiation25, the proportion of ACTA2+ cells in induced SEM cells was markedly increased relative to that in non-SEM cells (Figure 2G). Taken together, these data demonstrate that SMC-derived SEM cells, but not non-SEM cells, harbor potential to differentiate into SMC-derived macrophage-like and FC-like cells and revert towards SMC-like phenotype (Figure 2H).
Counterparts of mouse intermediate SEM cells exist in human atherosclerosis
To determine whether similar SMC transition, especially SMC to intermediate SEM cell state, occur in human atherosclerosis, we jointly analyzed scRNA-seq data of mouse atherosclerotic aortas and human carotid artery atherosclerotic plaques (n=3 patients). Reference-based integration analysis was employed to interrogate mouse atherosclerotic cell counterparts in human by projecting human data onto the mouse clusters (Figure 3A and 3B). The analysis demonstrated a cell population in human atherosclerotic carotid arteries that overlaid mouse SEM cells (Figure 3B), confirming human counterparts of these intermediate cells. Furthermore, applying the same approach, we analyzed public scRNA-seq data of human atherosclerotic coronary arteries (n=4 patients)12, which have a different developmental origin than that of carotid arteries26, 27, and observed intermediate SEM cell counterparts in human atherosclerotic lesions of coronary arteries as well (Figure IVA and IVB in the Supplement). Intriguingly, we found that the broadly defined “fibromyocyte” population previously identified through scRNA-seq and SMC fate mapping12 was inclusive of both the SEM cell (~26% of fibromyocytes) and the fibrochondrocyte (~16% of fibromyocytes) clusters identified in our study (Figure IVC in the Supplement). Overall, these data indicate that SEM-like cells are induced in human atherosclerotic plaques and that this is independent of arterial developmental origins.
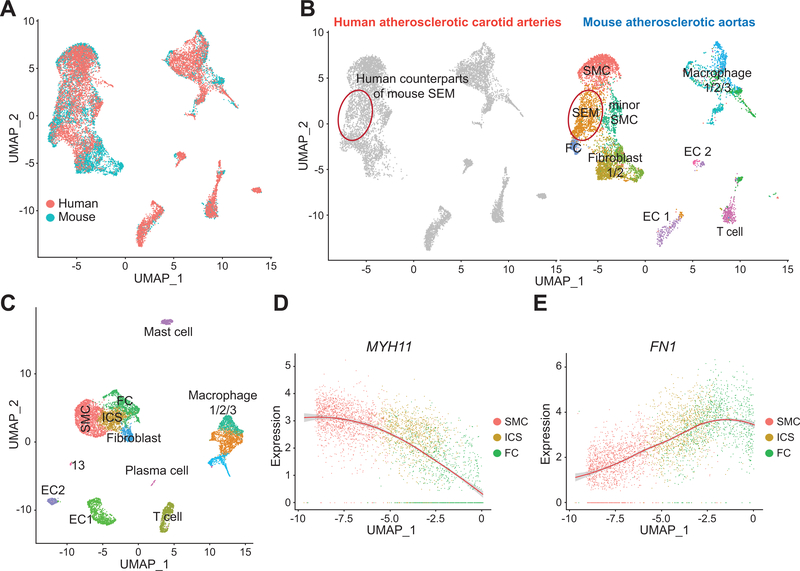
Figure 3. Counterpart of mouse intermediate SEM cell state is identified in human atherosclerotic carotid arteries.
A and B, Reference-based integration analysis of combined scRNA-seq data of Ldlr−/− mice fed 16-week WD (n=3 mice; 7029 cells) and human atherosclerotic carotid arteries scRNA-seq data (n=3 patients; 8867 cells). Mouse cell clusters are used as reference and human scRNA-seq data are then projected onto the mouse data. Combined mouse and human scRNA-seq data are visualized by UMAP (A). Representative mouse cell types/states are indicated, and a cell population (red circled) in human scRNA-seq data overlaps with the intermediate SEM cell state found in mouse atherosclerotic scRNA-seq data (B). C, Joint analysis of human atherosclerotic carotid arteries scRNA-seq data (n=3 patients) demonstrates an intermediate cell state (ICS) between SMC and fibrochondrocyte (FC). Representative cell type/state for each cluster is indicated. D and E, Gene expression tendencies of SMC marker, MYH11 (D) and FC-related gene, FN1 (E) through SMC-ICS-FC axis are shown as normalized expression versus UMAP_1.
To interrogate molecular features of human SEM-like cell counterparts, scRNA-seq data of human atherosclerotic carotid arteries were individually analyzed and a human intermediate cell state (ICS) between SMC and FC was identified (Figure 3C). SMC markers were marked downregulated in ICS (Figure 3D and Figure IVD in the Supplement), while the gene expression pattern of multiple FC-related genes (e.g., FN1, COL1A2, COL3A1) was very similar in human to mouse with continuous increase from SMC through ICS to FC (Figure 3E and Figure IVE in the Supplement). Thus, this complex ICS in human atherosclerotic plaques shares extensive molecular commonalities with mouse SEM cells.
Master regulator analysis of SMC transition reveals multiple signaling pathways, especially retinoic acid signaling, in atherogenesis
To interrogate the modulation of SMC transition, we analyzed mouse scRNA-seq data of SMC lineages using Virtual Inference of Protein activity by Enriched Regulon (VIPER), and more specifically, its tissue-independent single-cell-based extension, metaVIPER28, 29. VIPER was designed to measure the differential activities of regulatory proteins, between two cellular states, by assessing the enrichment of their transcriptional targets in DEGs (Figure VA in the Supplement)28, 29. Multiple master regulators (MRs) were identified as significantly activated/repressed in SEM versus SMC, and the regulatory network for the top 50 MRs is shown in Figure 4A. Several top MRs-modulated signaling pathways are known to be important for SMC differentiation (e.g., TGFβ signaling)32, while some are better known as important regulators of other cell types in atherosclerosis (e.g., PI3K/Akt signaling in macrophage)33. These analyses suggest that they may also be prominent modulators of SMC transition during atherosclerosis.
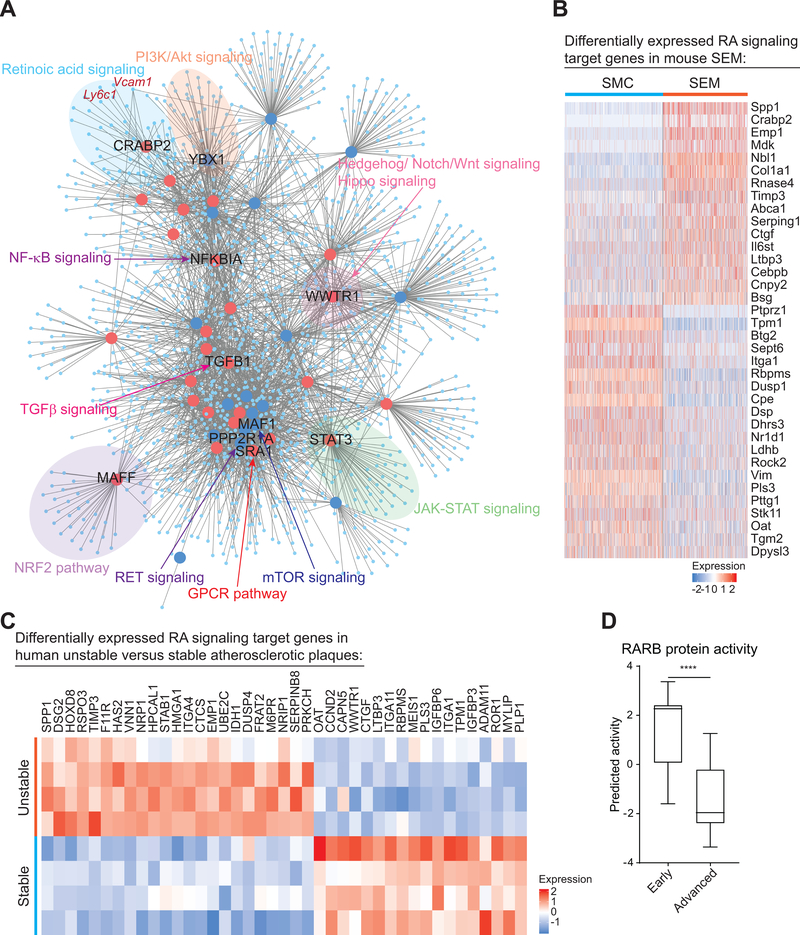
Figure 4. metaVIPER analysis identifies multiple master regulators of SMC to SEM cell transition during atherosclerosis, among which RA signaling is also found to be dysregulated in human atherosclerosis development and progression.
A, ARACNe network showing top 50 activated (red large dots) and repressed (blue large dots) MRs and their predicted target genes (light blue small dots) identified via metaVIPER using ZsGreen1+ scRNA-seq data of Ldlr−/− mice fed 16-week WD. Some MRs-involved canonical cell signaling pathways are highlighted. Vcam1 and Ly6c1, in dark red text, are shown as predicted target genes of CRABP2, a transducer of retinoic acid (RA) signaling. B, Heatmap showing significantly up and downregulated (fold change ≥ 1.2, Bonferroni corrected P-value < 0.05) RA target genes (36 genes) in SEM cells relative to SMC. C, Heatmap showing 41 RA signaling target genes that were differentially expressed (fold change ≥ 1.2, FDR adjusted P-value < 0.05) in human unstable plaques (visible zone of plaque rupture) of atherosclerotic carotid arteries compared to stable plaques (macroscopically normal adjacent areas). Paired unstable and stable plaques were from 4 individual patients30. D, Predicted protein activity of RA signaling transducer, RARB (retinoic acid receptor beta), in early (intimal thickening and xanthoma, n=13) and advanced (fibrous cap atheroma, n=16) human atherosclerotic lesions of carotid arteries31. Protein activity was estimated using metaVIPER. Values are shown as box and whisker plot (min to max). ****P < 0.0001.
A novel finding is that CRABP2 was identified as one of the top MRs during SMC to SEM transition (Figure 4A and Figure VB in the Supplement). Strikingly, two SEM markers, Vcam1 and Ly6c1, were identified as target genes of CRABP2 (Figure 4A). Retinoic acid (RA) signaling, for which CRABP2 is a transducer, is an important pathway in development, suggesting that it may contribute to the broad alteration of development-related functions during SMC to SEM transition indicated by IPA (Figure 2D). Indeed, RA signaling target genes34–37 were extensively up or downregulated in SEM cells relative to SMC (Figure 4B). Moreover, computational analysis revealed significantly altered expression of RA signaling target genes and reduced protein activity of RARB, another RA signaling transducer, in human unstable and advanced atherosclerotic plaques of carotid arteries30, 31 (Figure 4C, 4D and Figure VC in the Supplement), indicating human disease relevance of RA signaling. Taken together, these analyses suggest that RA signaling regulates SMC phenotypic switching and that RA signaling is associated with clinically important plaque phenotypes in human.
Genetic variations in retinoic acid signaling modulated genes are associated with risk of human atherosclerotic CVD
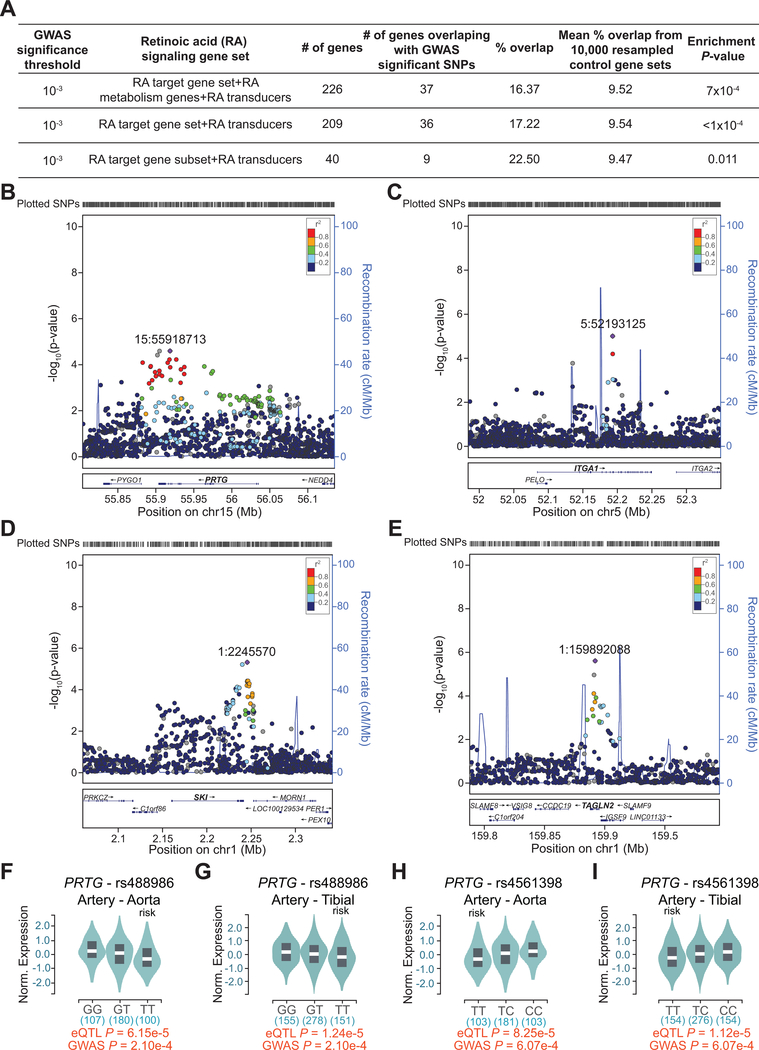
To further understand the impact of RA signaling on human CAD, we interrogated CAD associated single nucleotide polymorphisms (SNPs) in CARDIoGRAMplusC4D, a public meta-analysis database of summary results from multiple large-scale genetic studies identifying risk loci for CAD and myocardial infarction (MI)38. Focusing on various sets of reported RA signaling target genes34–37 (hereafter RA genes), we found a robust and consistent enrichment of CAD/MI-associated SNPs in multiple loci containing RA genes (Figure 5A). Multiple RA genes were found at loci with moderate to strong, but sub genome-wide significant, signals for CAD, such as PRTG, ITGA1, SKI and TAGLN2 (Figure 5B–5E), as well as IGFBP3, LTBP3 and IFRD1 (Figure VIA–VIC in the Supplement). Of note, ITGA1, IGFBP3 and LTBP3 were significantly downregulated in unstable human atherosclerotic plaques compared with stable plaques (Figure 4C), suggesting a correlation between repression of these genes and severity of atherosclerosis. Indeed, expression quantitative trait loci (eQTLs) analysis of RA genes in the Genotype-Tissue Expression (GTEx) database showed that CAD associated SNPs for several RA genes, such as PRTG and IGFBP3, were also eQTLs in CAD-relevant tissues (e.g., aorta and tibial artery) with the risk alleles associated with decreased expression (Figure 5F–5I and Figure VID, VIE in the Supplement). Taken together, these integrative human genomic analyses provide multiple lines of evidence for association of RA signaling genes with atherosclerotic CVD and hint at a link between repression of RA signaling and increased CVD risk.
Figure 5. Multiple RA signaling target gene loci are associated with risk of human CAD.
A, CAD GWAS enrichment summary based on a set of 226 RA signaling genes and its subsets. B-E, Regional association plots showing 1000-Genomes based GWAS of CAD/MI in four of RA signaling target gene loci, PRTG (B), ITGA1 (C), SKI (D) and TAGLN2 (E). −log10(p-value) of SNP association for CAD/MI is shown for each plot. Linkage disequilibrium (LD) with the top SNP from each region is color coded by r2. F-I, eQTL data from GTEx for SNPs, rs488986 (F and G) and rs4561398 (H and I), localized within the PRTG locus. Representative violin plots showing normalized expression of PRTG by genotype in CAD-relevant tissues (aorta (F and H) and tibial artery (G and I)). Numbers below the genotypes indicate sample size. Risk genotypes and eQTL and GWAS P-values are indicated. Reduced expression of PRTG is associated with risk alleles in both CAD-relevant tissues.
Retinoic acid signaling modulates SMC to SEM cell transition and atherosclerosis progression
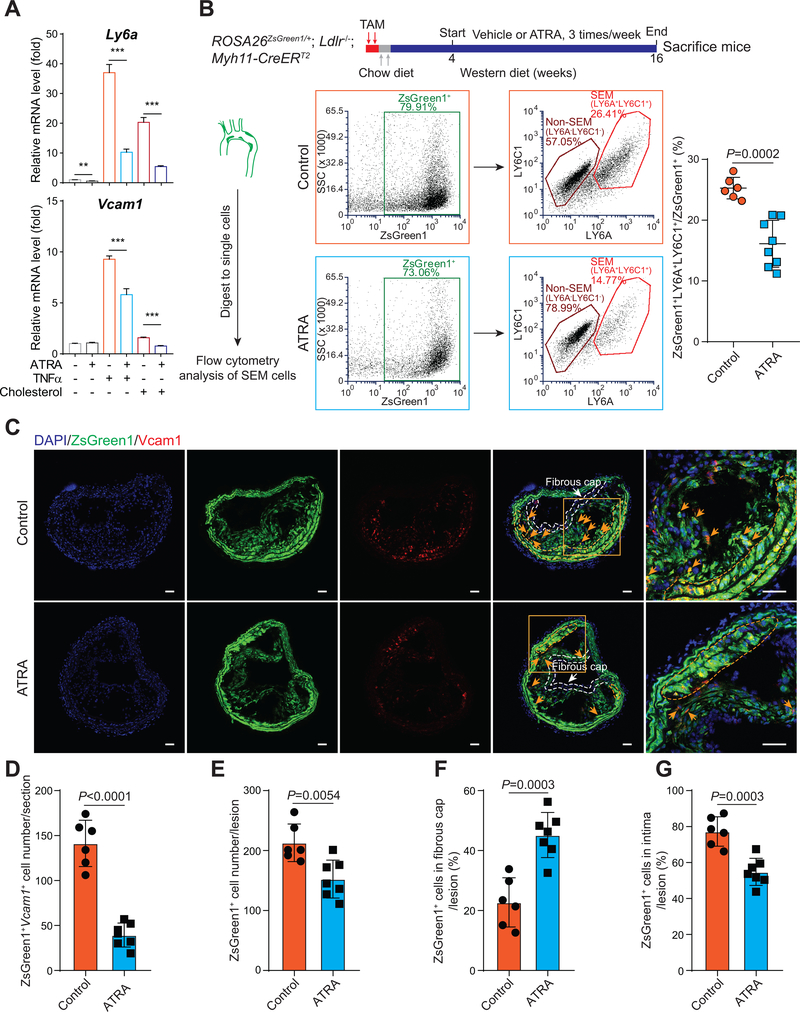
To build on human genetic studies and to interrogate the function and mechanism of RA signaling in SMC transition and atherosclerosis, we employed ATRA, an activator of RA signaling, in both in vitro and in vivo systems, in particular because of its therapeutic and clinical relevance – it is the main component of an FDA-approved anti-cancer drug for treatment of APL39. Pre-treatment of cultured mouse SMC with ATRA blocked the induction of SEM markers, Ly6a and Vcam1, by pro-atherogenic stimuli, cholesterol loading and TNFα (Figure 6A). To examine the effects of ATRA on SMC-SEM cell transition during atherosclerosis, we administrated ATRA to ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice (Figure 6B). In mice treated with ATRA, atherosclerotic lesion area was reduced by ~40% in aortic sinus (Figure VIIA in the Supplement). Flow cytometry analysis of single cells prepared from atherosclerotic aortas revealed that ATRA reduced by ~40% the proportion of ZsGreen1+LY6A+LY6C1+ SEM cells among total ZsGreen1+ cells (Figure 6B). Similarly, RNAscope of Vcam1 in atherosclerotic BCA sections showed a marked decrease (~70%) of Vcam1-stained SEM cells by ATRA (Figure 6C and 6D). Although ATRA decreased the total number of lesion SMC-derived cells (ZsGreen1+) by ~30% (Figure 6C and 6E), the percentage of ZsGreen1+ cells in the protective fibrous cap was increased to ~47% in ATRA-treated mice versus ~23% in control (Figure 6C and 6F). The proportion of ZsGreen1+ cells in intima, containing scattered SEM cells and other SMC-derived cell types (i.e., FC and macrophage-like cell), was ~77% in control and ~53% in ATRA group (Figure 6C and 6G). To probe whether ATRA might blunt differentiation of SEM cells into more differentiated SMC-derived cell types, we analyzed SMC-derived macrophage-like cells in control and ATRA-treated mice and found that ATRA reduced the proportion of SMC-derived macrophages (Figure VIIB in the Supplement). These findings suggest that ATRA inhibits SEM cells generation from SMC and may block their differentiation towards more differentiated SMC-derived cell types in atherosclerosis.
Figure 6. Activation of RA signaling via all-trans retinoic acid (ATRA) inhibits SEM cell marker expression in vitro and suppresses SMC to SEM cell transition and atherosclerosis in vivo.
A, Relative mRNA levels of SEM cell markers, Ly6a and Vcam1, in cultured mouse SMC treated with TNFα (25 ng/mL), cholesterol (40 μg/mL) or vehicle control (PBS) in the absence or presence of ATRA (10 μM) for 72 hours, are measured by RT-qPCR and normalized against Actb. Values are shown as mean ± s.d. **P < 0.01, ***P < 0.001, n=3. B, ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice induced by 2-day TAM are fed chow diet for 2 days, followed by WD. Vehicle (corn oil, control) or ATRA (2.5 mg/kg mice) administration is started after 4 weeks of WD, 3 times/week. Mice are sacrificed after 16-week WD. Arterial tissues (including ascending aorta, BCA and thoracic aorta) with atherosclerotic lesions are isolated and digested to single cells for flow cytometry analysis of the proportion of ZsGreen1+LY6A+LY6C1+ SEM cells among total ZsGreen1+ cells (control, n=6 mice; ATRA-treated, n=8 mice). Values are shown as mean ± s.d. P-value is indicated. C, RNAscope stained representative BCA sections from control (n=6) and ATRA-treated (n=7) mice indicate ZsGreen1+Vcam1+ SEM cells, regions of media, intima and fibrous cap (defined as the region within 30 μm of the luminal surface). Scale bars, 50 μm. D-G, ZsGreen1+Vcam1+ SEM cell number in BCA sections (D), ZsGreen1+ cell number within lesions (intima+fibrous cap) (E), percentage of ZsGreen1+ cells in fibrous cap/lesion (F) and percentage of ZsGreen1+ cells in intima/lesion (G) are calculated. Values are shown as mean ± s.d. P-values are indicated.
ATRA treatment, however, modestly reduced serum cholesterol in WD-fed ROSA26ZsGreen1/+; Ldlr−/−; Myh11-CreERT2 mice (801±314 mg/dL, n=6 ATRA-treated mice versus 1192±40mg/dL, n=7 control mice; P < 0.05), similar to a prior report in a rabbit model40. This partially confounds interpretation of whether ATRA modulates atherosclerosis by directly targeting SMC transition or indirectly via circulating lipoproteins. To address this, we performed carotid artery ligation and sham surgeries on non-hyperlipidemic ROSA26ZsGreen1/+; Myh11-CreERT2 mice and treated the ligated mice with ATRA (Figure VIIC in the Supplement). ATRA-treated mice exhibited a ~70% reduction in intima area and SMC-derived ZsGreen1+ cell composition in the intima, and ~60% reduction in ratios of intima/medial area and of ZsGreen1+ SMC/SMC-derived cell areas in intima/media (Figure VIIC–VIIJ in the Supplement). Taken together, these findings suggest that ATRA reduces atherosclerosis progression and increases fibrous cap thickness coincident with suppression of SMC transition to SEM cells.
DISCUSSION
SMC modulate atherosclerotic plaque progression and stability through phenotypic switching in response to atherogenic stressors. Here, utilizing SMC fate mapping and single-cell genomics to interrogate the dynamics of SMC transdifferentiation during atherosclerosis, we report that SMC-derived cells account for an increasing proportion of cells in progressing atherosclerotic lesions and that SMC can transdifferentiate into an intermediate “SEM” cell state, which is also found in human atherosclerotic plaques. SEM cells are multipotent and can differentiate into macrophage-like and FC-like cells, and even back towards SMC phenotype, suggesting clinical relevance of this intermediate cell state. RA signaling is a prominent regulator of SMC to SEM cell transition and repression of multiple RA signaling target genes may be associated with severity of human atherosclerosis. Human genomic interrogation reveals enrichment of GWAS signals for CAD in multiple RA signaling target gene loci and overlap of CAD risk alleles with eQTLs for reduced expression of several RA signaling targets. Experimentally, activation of RA signaling in vitro and in vivo by ATRA, an anti-cancer drug used to treat APL, blocks SMC transition to SEM cells, reduces atherosclerotic burden and promotes fibrous cap stability.
Integration of cell-specific fate mapping, single-cell genomics and human genetics is helpful to uncover cell complexity and novel genetic regulation of complex diseases, including atherosclerosis5, 12. Our main finding that the SMC-derived SEM cell state has potential to differentiate into multiple cell types and can be targeted therapeutically to reduce atherosclerosis provides proof of principle for this integrative genomics strategy. Previous studies reported SMC-derived Sca1+ cells, which were assumed to be MSC, in atherosclerotic and vascular injury models4, 8, but the roles of such cells in disease were largely uncharacterized. Through comprehensive bioinformatic analyses of scRNA-seq data, we did not detect notable expression of any typical MSC markers (e.g. Nt5e, Eng)22 in SEM cells (Figure IIID and IIIE in the Supplement). Actually, the molecular features of SEM cells are complex, suggesting that these cells are not simply MSC-like, but a unique transition state from SMC found in the milieu of atherosclerosis. We identified multiple disease-related pathways significantly dysregulated in SEM cells, including multiple developmental and “cancer” pathways. Such features might contribute to the reported phenotype of monoclonal or oligoclonal expansion of SMC in vascular pathologies9. Importantly, the SEM cells appear to be at the crossroad of SMC phenotypic switching by serving as precursors for other SMC-derived cell types, such as macrophages and FC. In this context, our integrative analyses suggest that the modulated SMC, “fibromyocytes” previously identified by Wirka et al.12 contain both the SEM cell and fibrochondrocyte clusters identified in our study.
Through VIPER-based MR analysis, we identified many MRs that may modulate SMC transition. Several signaling pathways, in which identified MRs are involved, have been reported to control SMC functions and atherosclerosis. For instance, Hippo signaling modulates SMC proliferation41, while inhibition of NF-κB signaling may attenuate SMC proliferation both in vitro and in mouse models42. These examples provide support for VIPER-inferred MRs not previously related to these phenotypes. We focused on RA signaling, considering its limited mechanistic study in SMC biology and atherosclerosis and its potential clinical relevance. As a critical human translational support, integrative human genomic analysis revealed genetic association between RA signaling regulation and clinical complications of human atherosclerosis. Enrichment of GWAS signals for CAD were observed in multiple RA signaling target gene loci (e.g., PRTG, ITGA1, SKI, TAGLN2, IGFBP3, LTBP3 and IFRD1), providing support for a broader and unrecognized enrichment of multiple RA signaling-regulated genes in human atherosclerotic CAD. Moreover, downregulation of several of these RA signaling targets (e.g., ITGA1, IGFBP3 and LTBP3) was found in unstable human atherosclerotic plaques and overlap of CAD risk alleles with eQTL alleles for decreased expression of several RA signaling target genes in human CAD-relevant tissues suggest a correlation between repression of RA signaling and risk of human atherosclerotic CVD. These data support further exploration of RA signaling in SMC lineages as a potential mechanism-based therapeutic target for atherosclerosis.
To probe therapeutic targeting of RA signaling, we used ATRA, an anti-cancer drug used to treat APL. Although the precise mechanisms are unclear, previous work does suggest that ATRA might inhibit SMC proliferation43–46, and activation of RA signaling by 9-cis-RA and ATRA can reduce atherosclerotic lesions in mouse47 and rabbit40 models, respectively. Effect of ATRA on plasma lipoproteins is one hypothesized mechanism for effects on atherosclerosis. Further, studies dating back about two decades ago have shown that ATRA can modulate SMC growth and vascular response to injury in rodent models43, 44, yet the cellular and molecular mechanisms have remained uncertain. Our studies both in vitro and in vivo using mouse models of atherosclerosis and vascular injury provide novel mechanistic insights that suggest that one mechanism by which ATRA-activated RA signaling reduces atherosclerosis by blunting SMC to SEM cell transition and SEM functions during development and progression of atherosclerosis. This finding, coupled with the marked dysregulation of RA signaling in unstable human atherosclerosis and genetic implication of repressed RA signaling in human CAD, provides a mechanistic basis and human context for further clinical translation. Indeed, these novel findings on RA signaling in atherosclerosis have both clinical relevance and therapeutic implications, especially because ATRA therapy is already approved for treatment of APL in clinic. Finally and of broad translational relevance, other regulatory pathways, with limited prior data for roles in atherosclerosis, were revealed by metaVIPER as master regulators of SMC transition to intermediate SEM cells providing many opportunities for novel translation towards novel clinical therapies.
Some controversies remain regarding the occurrence of SMC transition to macrophages in mouse and human atherosclerosis. Our work is consistent with most previous papers4, 8, 9, 13, but not all12, and suggests that SMC-derived macrophage-like cells emerge later in lesions than myeloid-derived or resident authentic macrophages. These controversies may result from various mouse models and different single cell preparation procedures. An open question remains whether SMC-derived macrophage-like cells have similar or defective functions relative to primary macrophages and if they contribute uniquely to the risk of clinical events.
In conclusion, we find that SMC transition via an intermediate SEM cell state to multiple cell types during atherosclerosis, that SEM cells can differentiate into macrophage-like and FC-like cells, and also return towards SMC phenotype. Human genomics provides strong support for the roles of RA signaling in human atherosclerotic CVD. Activation of RA signaling, using a clinically established anti-cancer therapeutic strategy, blocks the transition of SMC to SEM cells, reduces atherosclerotic burden and promotes lesion stability. Our findings add novel insights to the complexity of SMC biology in atherogenesis and reveal the potential to target novel regulatory pathways of SMC phenotypic switching in atherosclerotic CVD. Several questions remain to be addressed including the specific molecular mechanisms that evoke SMC transdifferentiation and the ability to dissect and target all molecular and cellular regulons of diverse SMC transitions during atherosclerosis.
Supplementary Material
Clinical Perspective.
1). What is new?
We revealed new and comprehensive knowledge of SMC phenotypic switching during atherosclerosis by identifying multiple SMC-derived cell states/types in both mouse and human atherosclerotic plaques through SMC fate mapping and single-cell genomics.
SMC-derived “SEM” cells were found as an intermediate cell state and precursors for other SMC-derived cell types, including fibrochondrocyte-like and macrophage-like cells.
Using novel computational approaches for single cell data, we identified multiple master regulators, including retinoic acid (RA) signaling, that control SMC-SEM cell transition and found evidence for genetic link between RA signaling and coronary artery disease risk through integrative human genomics.
2). What are the clinical implications?
A SMC-derived intermediate “SEM” cell state is identified as a promising cellular target for new therapeutic strategies in atherosclerotic cardiovascular disease.
The identification of multiple potential master regulators of SMC to SEM cell transition provides a valuable and broad resource for exploring therapeutic modalities for atherosclerosis via modulation of SMC phenotypic switching.
We provide proof of principle that all-trans retinoic acid (ATRA), an anti-cancer drug for acute promyelocytic leukemia treatment, has promising therapeutic effects on atherosclerosis by blocking SMC transition to SEM cells, reducing atherosclerotic burden, and promoting fibrous cap formation.
Acknowledgements
We thank Dr. Thomas Quertermous for his suggestion on single cell preparation from human atherosclerotic tissues. The scRNA-seq (10x Genomics) was performed in JP Sulzberger Columbia Genome Center, supported in part through the NIH/NCI Cancer Center Support Grant P30CA013696 and used the Genomics and High Throughput Screening Shared Resource. Flow cytometry analysis and FACS sorting were performed in the Flow Cytometry Core at Columbia Center for Translational Immunology, supported in part by the NIH award S10OD020056. Images were collected in the Confocal and Specialized Microscopy Shared Resource of Herbert Irving Comprehensive Cancer Center (HICCC) at Columbia University, supported by NIH grant #P30 CA013696 (National Cancer Institute). Block sectioning and H&E staining were performed in the Molecular Pathology Shared Resource of HICCC, supported by NIH grant #P30 CA013696 (National Cancer Institute).
Sources of Funding
H.P. is supported by American Heart Association (19POST34450233). J.W., L.V., P.L. and A.C. are supported by the NIH grants nos. R35CA197745, 1S10OD012351 and 1S10OD021764. H.Z. is funded by R00HL130574 and the National Center for Advancing Translational Sciences (NCATS) and National Institutes of Health (NIH) through grant number UL1TR001873. M.L. is supported by NIH grants R01-GM-125301, R01-HL-113147 and R01-HL-150359. M.P.R. is supported for this work by NIH grants R01-HL-113147, R01-HL-150359, and K24-HL-107643.
Non-standard Abbreviations and Acronyms:
- ATRA
All-trans retinoic acid
- BCA
Brachiocephalic artery
- BM
Bone marrow
- CAD
Coronary artery disease
- CTGF
Connective tissue growth factor
- CVD
Cardiovascular disease
- DEG
Differentially expressed gene
- EC
Endothelial cell
- eQTLs
Expression quantitative trait loci
- FC
Fibrochondrocyte
- GO
Gene Ontology
- GTEx
Genotype-Tissue Expression
- GWAS
Genome wide association study
- ICS
Intermediate cell state
- IPA
Ingenuity Pathway Analysis
- MR
Master regulator
- RA
Retinoic acid
- scRNA-seq
Single-cell RNA sequencing
- SEM
Stem cell, endothelial cell, monocyte
- SMC
Smooth muscle cell
- SNP
Single nucleotide polymorphism
- VIPER
Virtual Inference of Protein activity by Enriched Regulon
Footnotes
Disclosures
Pasquale Laise is Director of Single Cell Systems Biology at DarwinHealth Inc., a company that has licensed some of the algorithms used in this manuscript from Columbia University.
REFERENCES
- 1.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., Rosenfeld ME, Schwartz CJ, Wagner WD and Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. [DOI] [PubMed] [Google Scholar]
- 2.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE and Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 3.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M and Jorgensen HF. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun. 2018;9:4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez D and Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR and Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–744. [DOI] [PubMed] [Google Scholar]
- 8.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR and Jorgensen HF. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ Res. 2016;119:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong D, Turner AW and Miller CL. Genetic Insights Into Smooth Muscle Cell Contributions to Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2019;39:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. [DOI] [PubMed] [Google Scholar]
- 12.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M and Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. [DOI] [PubMed] [Google Scholar]
- 14.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. [DOI] [PubMed] [Google Scholar]
- 16.Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F and Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2019;37:38–44. [DOI] [PubMed] [Google Scholar]
- 17.Elliott MR, Koster KM and Murphy PS. Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J Immunol. 2017;198:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Subramanian M, Yurdagul A Jr., Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, Ryan TA, Nomura M, Maxfield FR and Tabas I. Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell. 2017;171:331–345 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spangrude GJ, Heimfeld S and Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. [DOI] [PubMed] [Google Scholar]
- 20.McHale JF, Harari OA, Marshall D and Haskard DO. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-alpha. J Immunol. 1999;162:1648–1655. [PubMed] [Google Scholar]
- 21.Rivollier A, He J, Kole A, Valatas V and Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 23.Manzanero S Generation of mouse bone marrow-derived macrophages. Methods Mol Biol. 2012;844:177–181. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Shah B, Moioli EK and Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S and Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004;94:1195–1202. [DOI] [PubMed] [Google Scholar]
- 26.Peace A, Van Mil A, Jones H and Thijssen DHJ. Similarities and Differences Between Carotid Artery and Coronary Artery Function. Curr Cardiol Rev. 2018;14:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez MJ, Shen Y, Giorgi FM, Lachmann A, Ding BB, Ye BH and Califano A. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat Genet. 2016;48:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding H, Douglass EF Jr., Sonabend AM, Mela A, Bose S, Gonzalez C, Canoll PD, Sims PA, Alvarez MJ and Califano A. Quantitative assessment of protein activity in orphan tissues and single cells using the metaVIPER algorithm. Nat Commun. 2018;9:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoud AD, Ballantyne MD, Miscianinov V, Pinel K, Hung J, Scanlon JP, Iyinikkel J, Kaczynski J, Tavares AS, Bradshaw AC, et al. The Human-Specific and Smooth Muscle Cell-Enriched LncRNA SMILR Promotes Proliferation by Regulating Mitotic CENPF mRNA and Drives Cell-Cycle Progression Which Can Be Targeted to Limit Vascular Remodeling. Circ Res. 2019;125:535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125:1673–1683. [DOI] [PubMed] [Google Scholar]
- 32.Guo X and Chen SY. Transforming growth factor-beta and smooth muscle differentiation. World J Biol Chem. 2012;3:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K and Tsatsanis C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J Immunol. 2017;198:1006–1014. [DOI] [PubMed] [Google Scholar]
- 34.Freemantle SJ, Kerley JS, Olsen SL, Gross RH and Spinella MJ. Developmentally-related candidate retinoic acid target genes regulated early during neuronal differentiation of human embryonal carcinoma. Oncogene. 2002;21:2880–2889. [DOI] [PubMed] [Google Scholar]
- 35.Delacroix L, Moutier E, Altobelli G, Legras S, Poch O, Choukrallah MA, Bertin I, Jost B and Davidson I. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol Cell Biol. 2010;30:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al Tanoury Z, Piskunov A, Andriamoratsiresy D, Gaouar S, Lutzing R, Ye T, Jost B, Keime C and Rochette-Egly C. Genes involved in cell adhesion and signaling: a new repertoire of retinoic acid receptor target genes in mouse embryonic fibroblasts. J Cell Sci. 2014;127:521–533. [DOI] [PubMed] [Google Scholar]
- 37.Savory JG, Edey C, Hess B, Mears AJ and Lohnes D. Identification of novel retinoic acid target genes. Dev Biol. 2014;395:199–208. [DOI] [PubMed] [Google Scholar]
- 38.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osman AEG, Anderson J, Churpek JE, Christ TN, Curran E, Godley LA, Liu H, Thirman MJ, Odenike T, Stock W et al. Treatment of Acute Promyelocytic Leukemia in Adults. J Oncol Pract. 2018;14:649–657. [DOI] [PubMed] [Google Scholar]
- 40.Zhou B, Pan Y, Hu Z, Wang X, Han J, Zhou Q, Zhai Z and Wang Y. All-trans-retinoic acid ameliorated high fat diet-induced atherosclerosis in rabbits by inhibiting platelet activation and inflammation. J Biomed Biotechnol. 2012;2012:259693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Hu G, Liu F, Wang X, Wu M, Schwarz JJ and Zhou J. Deletion of yes-associated protein (YAP) specifically in cardiac and vascular smooth muscle cells reveals a crucial role for YAP in mouse cardiovascular development. Circ Res. 2014;114:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida T, Yamashita M, Horimai C and Hayashi M. Smooth muscle-selective inhibition of nuclear factor-kappaB attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. J Am Heart Assoc. 2013;2:e000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miano JM, Topouzis S, Majesky MW and Olson EN. Retinoid receptor expression and all-trans retinoic acid-mediated growth inhibition in vascular smooth muscle cells. Circulation. 1996;93:1886–1895. [DOI] [PubMed] [Google Scholar]
- 44.Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R and Blaner WS. all-Trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation. 1998;98:1219–1227. [DOI] [PubMed] [Google Scholar]
- 45.Wiegman PJ, Barry WL, McPherson JA, McNamara CA, Gimple LW, Sanders JM, Bishop GG, Powers ER, Ragosta M, Owens GK, et al. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit : a favorable effect on vessel remodeling. Arterioscler Thromb Vasc Biol. 2000;20:89–95. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Deng B, Jiang X, Cai M, Liu N, Zhang S, Tan Y, Huang G, Jin W, Liu B et al. All-Trans-Retinoic Acid Suppresses Neointimal Hyperplasia and Inhibits Vascular Smooth Muscle Cell Proliferation and Migration via Activation of AMPK Signaling Pathway. Front Pharmacol. 2019;10:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou W, Lin J, Chen H, Wang J, Liu Y and Xia M. Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in apoE-deficient mice. Br J Nutr. 2015;114:509–518. [DOI] [PubMed] [Google Scholar]
- 48.Butler A, Hoffman P, Smibert P, Papalexi E and Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blomhoff R and Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. [DOI] [PubMed] [Google Scholar]
- 50.Ghyselinck NB and Duester G. Retinoic acid signaling pathways. Development. 2019;146:dev167502. doi: 10.1242/dev.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.