Abstract
Background:
Children, parents, older adults, and caregivers routinely use sensor technology as a source of health information and health monitoring.
Purpose:
The purpose of this paper is to describe three exemplars of research that used a human-centered approach to engage participants in the development, design, and usability of interventions that integrate technology to promote health.
Methods:
The exemplars are based on current research studies that integrate sensor technology into pediatric, adult, and older adult populations living with a chronic health condition. Lessons learned and considerations for future studies are discussed.
Discussion:
Nurses have successfully implemented interventions that use technology to improve health and detect, prevent, and manage diseases in children, families, individuals and communities. Nurses are key stakeholders to inform clinically relevant health monitoring that can support timely and personalized intervention and recommendations.
Keywords: human centered design, health technology, smart homes, self and shared-management, sleep, diabetes
INTRODUCTION
Children, parents, older adults, caregivers, and families routinely use technology in the form of smart watches and wireless-enabled activity trackers, such as Fitbit or the Apple Watch, and health dialog systems such as ChatBots, as a source of health information and health monitoring. The design of health interventions that integrate such technology to meet the needs of end users often involves a participatory design approach. As shown in Figure 1, human-centered approach (HCA) is a framework that integrates theory, research, practice, and end users into the design and development of products,1 and guides the creation of products or solutions to problems by engaging stakeholders through-out the entire design process.2,3 HCA is commonly used during the development of health technologies to support the needs of children, caregivers, patients and their families in various settings, including home and hospice care.4–9 Involving stakeholders in the design and development ensures that a product solves pragmatic problems and addresses real-world user priorities and needs.10
Figure 1.

Human-Centered Approach
Integrating technology into health and psychosocial interventions can reduce barriers for children, families, and caregivers. For example, traditional face-to-face interventions often have long waiting lines, require weekly transportation to and from the clinic, implying missed work and school, and may not occur due to lack of insurance coverage, and financial concerns. HCA can be a powerful method in the development of solutions, including web or mobile-health based interventions and smart homes to support the health of children, adults, older adults and their caregivers by providing tools to track and manage their health. HCA incorporates two phases:1–3 1) a generative phase aimed at understanding end user needs and priorities, and brainstorming design ideas through an evidence review, user research, and ideation and selection; and 2) an evaluative phase to narrow, evaluate, test, and refine the design of the final solutions or products. This includes multiple rounds of concept testing (e.g., elicit feedback from dyads on the concepts that emerged from the generative phase), prototyping (e.g., low fidelity testing [paper drawings, sketching] to high fidelity testing [functional technology or devices]), and usability testing (e.g., think aloud sessions where end users talk through their engagement with the prototypes, what they like, dislike, and additional feedback and suggestions) until the final product is arrived at. This paper describes three exemplars of research that engage children with Juvenile Idiopathic Arthritis (JIA) and their caregivers, adults with diabetes, and older adults with multiple chronic health conditions, to assess the use and usability of interventions that integrate technology to support their wellbeing. These examples address the value of taking a human-centered approach to developing interventions.
Exemplar #1 Shared Sleep Self-Management Intervention (SLEEPSMART) for Children with Juvenile Idiopathic Arthritis and their Caregivers
Sleep deficiency, defined as inadequate quantity or quality of sleep, 11 is comorbid in children with juvenile idiopathic arthritis (JIA),12–15affecting an estimated 20 to 30% of all children. Comorbid sleep deficiency in JIA has been associated with increased arthritis symptoms, poorer health-related quality of life, and increased healthcare utilization. Despite this prevalence and the deleterious consequences of sleep deficiency, children with JIA are not routinely screened. Further, pediatric providers have limited knowledge and skills to manage sleep deficiency, low reimbursement rates, and high caseloads; all of these are barriers to treating this condition.16 The underlying contributing factors for inadequate sleep are often behavioral in nature (e.g., lack of a consistent bedtime routine, variable bedtimes and waketimes, media use at bedtime, unhealthy beliefs about sleep [e.g., I need the TV to fall asleep]), and multi-faceted including child characteristics (e.g., temperament, health status), parent characteristics (e.g., parenting style, stress, sleep habits), family context (e.g., work/school schedules, family support, family conflict, home environment) and sociocultural variables.17–19 Given this complexity and inherent variability, it is important that interventions are flexible in their design and scope. The traditional approach to managing sleep problems is a barrier for families in that it requires in-person sessions that are costly, time intensive, and require parents and children to miss work and school. Parents and children routinely use technology as a source of health information. Thus, these readily available tools offer a novel way to treat sleep deficiency that meets the needs of end users - JIA children and their parents.
This exemplar describes the design of a web-based SLEEPSMART intervention to support sleep in JIA children by targeting self-management behaviors (patient activation, motivation, self-efficacy), and partnering with parents and children to provide them with the knowledge, motivation, and skills for setting and achieving goals, adapting to setbacks, and problem-solving in an iterative fashion. SLEEPSMART is informed by Bandura’s Social Cognitive Theory and a transdiagnostic approach to treat sleep problems.20–22
Initial Development Phase.
The content development team included a biomedical informatician and pediatric behavioral sleep experts with knowledge in cognitive behavioral therapy, treating sleep deficiency, intervention design and development, and HCA. The evidence-based clinical practice guidelines to treat behavioral sleep problems in children 23 and an existing WebMAP program for youth with chronic pain guided the overall structure of the SLEEPSMART program and use of multimedia elements.24 Content was developed to address core components for treating sleep deficiency including sleep education, sleep skills training (consistent sleep schedule, bedtime routine), lifestyle changes (activities prior to bed), relaxation techniques, positive coping skills, communication with parents (reinforce and modeling of positive coping skills, reward systems for activity participation, communication with children), and self-management skills (e.g., goal setting, engagement, motivation, [see Figure 2]). The content team met weekly to review content. Subsequently, intervention materials, multimedia elements, structure of the program, and paper-based prototypes were created for the participatory design sessions.
Figure 2:

SLEEPSMART Content
Participatory Design (PD) Sessions.
Three in-person PD sessions were completed with JIA children and their caregivers. The goal of each session was to use generative and evaluative techniques to better understand: a) What content types are most engaging for and appealing to 8 to 12 year-old children and their parents (e.g. video, audio, text); b) What theme/design is most engaging for and appealing to children; and c) What will help motivate children with JIA and sleep issues to go through a sequence of learning modules. The first PD session was several hours with parent-child dyads, child-child dyads, and parent-parent dyads who used paper protypes and sketching to generate ideas. Based on the feedback a user flow was developed that consisted of the key parts to navigate users across the SLEEEPSMART intervention. First, parent-child dyads would receive from the research team weekly emails notifying them to access reading materials; these emails would be automatically sent to registered users every Sunday. Each email described steps to be done in the week and included a link that connected to the SLEEPSMART website that provided a general guide about that week’s lesson and activities. The weekly learning modules covered different content that parents and children would need to review. After the user reviewed the content, they would then go back to the Instructional Website to engage in activities such as listening to a power point presentation, taking a short knowledge quiz, applying what they learned to set 1–2 sleep goals and plans to fulfill the goals for the coming week.
Design and Development of the Intervention high-fidelity Prototype.
Based on the initial development, paper prototypes, PD sessions results, and user flows, ahigh-fidelity prototypes of the SLEEPSMART intervention was developed that included an email that would provide users a link to the SLEEPSMART website to learn new modules, set goals, and track their progress. Page structures including layout and navigation and designed pages with Sketch, a graphics editor tool were developed. Finalized page designs were implemented in WIX and REDCap (Research Electronic Data Capture) was used to capture participant data (Figure 3). The Intervention included the SLEEPSMART Website that provided basic information about the SLEEPSMART study, and weekly learning modules that were tailored to the individual child needs based on actigraphy, sleep diary, and the child’s sleep goals and struggles.
Figure 3:

SLEEPSMART Weekly Module Components
Next steps and Lessons Learned.
With respect to lessons learned, it was critical to include JIA children and parent in the development of the SLEEPSMART intervention to gain a better understanding of their needs, likes, and dislikes for the website, content, and weekly activities. JIA children had a strong preference for a “soothing” color palette for the website- one that would be associated with calmness, and relaxation. They also preferred a “space” theme for the website because it is a nighttime element tied back into the idea of sleep. It was also important that the space theme depictions “felt” accurate, in that they did not want clouds because there are no clouds in space. This also raises the question of whether these preferences matter for compliance rate and/or adoption of the intervention. The weekly modules were designed for both parents and children to work together to manage sleep; although participants liked this idea, some children preferred to go through the lessons first on their own, and then discuss with their parent, whereas, others wanted to work simultaneously with their parent. Thus, the instructions for the weekly emails to participants provided them a choice to work together or separately, and the learning modules were structured accordingly. Children and parents enjoyed the weekly modules, brief voiceover power point presentations (<20 minutes), and short quizzes. The weekly assignments were modified and/or decreased due to the length of time it took to complete. For example, the time tracker that required participants to record their activities every 45 minutes when school ended (e.g., 3:30pm- walked home from school; 4:15pm -ate a chocolate bar and started my homework.). Participants also wanted a method to track their assignments and progress; thus, a progress bar was included in the weekly modules, and an option to print out their weekly goals. Currently, a pilot randomized control trial (RCT) of SLEEPSMART in 60 parents-children with JIA is underway. It will require refinements and further testing in a larger RCT.
Exemplar #2: Engaging Adults in Physical Activity Diabetes Self-Management Data Collection: Diabetes LIVE™© Study
Chronic illness management among adults often includes self-monitoring of behaviors such as physical activity. Wireless activity trackers provide a consumer-based means to monitor physical activity. While there is ongoing debate about the reliability of consumer device data as compared to research grade accelerometers, several validation studies have shown these devices to be comparable on step counts and sedentary time, and mixed findings regarding activity levels (i.e., moderate or vigorous activity time).25,26 In the Diabetes LIVE study, the primary outcome measures were behavioral – dietary intake and physical activity. In order to collect objective data, wireless activity trackers (Fitbit devices) were used to collect longitudinal physical activity data throughout the course of the study period. Participants were randomized to receive diabetes self-management education and support through either the web-based virtual environment intervention or the control website for a period of 12 months. Activity data was tracked for 18 months to record the trajectories following intervention participation.27 To facilitate collation and download of the Fitbit data, Fitabase was contracted to provide a study dashboard and data downloads. Fitabase is a secure platform for monitoring, tracking, and managing device data. The advantage of this approach was that study team members could monitor Fitbit use, syncing and charge remotely on a single dashboard. Participant data could also be downloaded for various study periods.
Wireless trackers as intervention and data collection.
Although the use of the Fitbits allowed for objective data collection, the tracking became part of the overall intervention as well. It is known that routine tracking or self-monitoring of health behaviors can result in an improvement in behaviors.28 However, many studies that apply wireless activity trackers are short-term with only a few applications at 12 months or greater.29,30 In the Diabetes LIVE© study, Fitbits were used to determine participant steps per month and this data was posted in both the control and intervention group sites on leaderboards to highlight the three most active participants each month. The data for the intervention group was also collated each month and the total ‘team’ step counts were used to map out ‘walk around the world’ to show group progress and achievement. These two approaches address potentially varying participant preferences for either competitive or cooperative motivation.
It is difficult in the context of a multi-pronged behavioral intervention to determine the effects of each component on behavioral outcomes. In addition to tracking using the Fitbit devices, other aspects of the diabetes education and support focused on physical activity for improved diabetes care. However, there may have been an effect regardless of that intervention of the self-monitoring alone. Future analyses will disentangle this influence based on activity in the intervention site and physical activity tracking over the different phases of the study period. In further development of the virtual environment, an automated wireless activity tracker will be integrated into the site in using appropriate application program interfaces (APIs) and connections.
Adherence to tracker use and data collection.
The full sample of 211 adults with type 2 diabetes were given a Fitbit (Flex or Zip) to wear and sync for a total of 18 months. Very few devices were replaced due to dysfunction, loss or damage. The study protocol allowed for one replacement for each participant during study enrollment if needed. Rate of wear and data sync was high in the total sample with only 31.2% of all study days not having valid step count data over the 18-month period for all participants. Interestingly, the rate of use was fairly consistent across the study period, while other follow-up measures and intervention participation showed a downward trend over time.
Next Steps and Lessons learned.
In future studies, additional usability testing and human centered approach will be used to determine the best wireless trackers for physical activity data collection or monitoring, particularly for long-term use. Although participants wore the wireless trackers consistently throughout the study, additional assessment upfront would have been beneficial to better understand long-term use in wearing Fitbit, adherence to study protocol, syncing, and participant attrition. Wear time (i.e., hours per day and days per week) influences the validity of the assessment of physical activity as a primary study outcome and therefore, determining a feasible yet accurate measurement time and duration is important in future studies. As mentioned above, integration of the Fitbit data into the intervention site may reduce the potential for participants to perceive the Fitbit and associated account as a standalone monitoring intervention, separate from use of the intervention site. Data cleaning may be more straightforward if a tracker integrates a measure of heart rate which would help determine valid days of data when the sensor was worn as compared to not worn or functional. Future studies should consider using Fitabase or other software to track use, syncing, and battery life on the tracking devices. It was beneficial to review participant activity regularly on one dashboard and for troubleshooting. Currently, the next steps include exploring the use or log-in to the virtual environment intervention as compared to the Fitbit to assess the relative impact of each device.
Exemplar #3: Supporting Older Adults with Smart Sensor Technology
Chronic illness management and early detection of health changes are keys to promoting health and function of older adults, assisting them to age independently.31,32 Smart sensing technology can recognize early signs of health changes and thus, facilitate early interventions before problems escalate and reduce hospitalizations and relocations to assisted living or nursing homes. This exemplar describes the development of an in-home system with sensors and automated health alerts for detecting early signs of illness and functional decline in older adults, to aid nursing care coordination. The initial work was conducted in TigerPlace, a senior living site with 54 apartments, designed as a living laboratory and operated to promote aging in place with a care coordination model.33–35 The sensor system was initially designed as a clinical decision support system for staff nurses and social workers and has been developed and refined during a period of 15 years.36,37 Three main components include: (1) a set of non-wearable sensors mounted in each apartment, (2) a collection of algorithms for logging and processing sensor data streams and generating alerts, and (3) interfaces for visualizing sensor data trends and displaying alert messages. Health alerts are generated as possible indicators of health change; the system relies on the clinical staff receiving the alerts to determine if any intervention is needed. The strategy for recognizing early signs of health changes is to learn a pattern for each individual resident (based on the sensor data) and then recognize when the pattern changes in a way that may indicate a health change.
Different sensors have been tested. The nursing researchers suggested non-wearable sensors at the beginning of the study because of the concern for older adults not being able to keep the wearable devices on and charged. The current sensors (see Figure 4) have been effective in capturing health changes which include a depth sensor for capturing walking gait and detecting falls,38,39 a bed sensor for capturing heart rate, respiration rate, restlessness in bed, and general sleep patterns;40 and passive infrared motion sensors for capturing room specific movement (e.g., bathroom activity) and overall activity patterns (e.g., daily sedentary vs. active patterns).41 The system includes numerous pattern recognition and machine learning algorithms.42–51 Here, the focus will include the overall development and deployment using a human-centered approach, including the challenges faced as the system was adapted and tested in new settings. The project has included an interdisciplinary team of engineers and computer scientists along with the clinical team of nurses, social workers, physical therapists, and physicians, essential to the success of the system.
Figure 4:
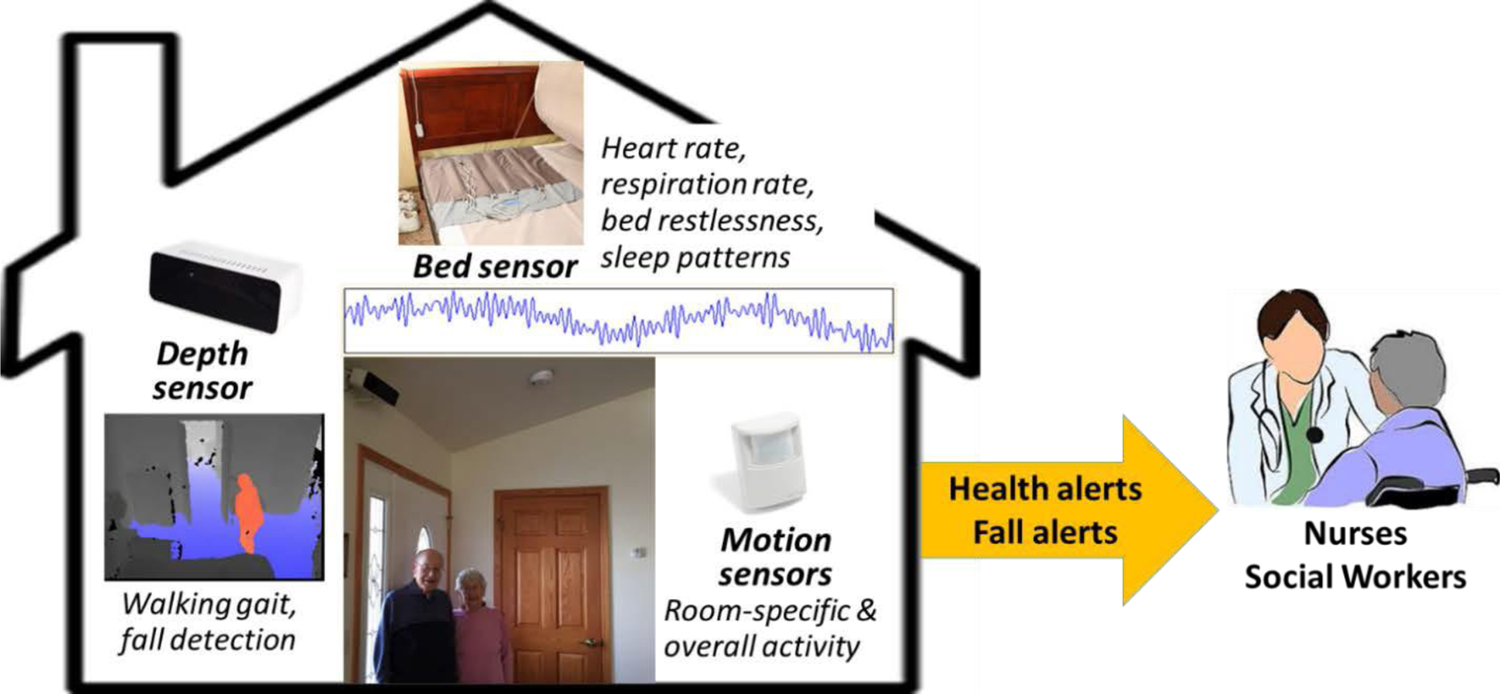
The smart in-home sensor system for older adults with alerts for health changes and falls.
Initial Phase – Capturing and Visualizing Sensor Data.
The project began in TigerPlace, with the aim of setting up an infrastructure to support exploration of possible links between passive sensor data collected in the home and the health status of the residents living there. Sensors were installed in a small number of apartments that functioned as case studies, with approval from the University of Missouri IRB. The health status of the study participants was available through an electronic health record (EHR). In addition, the TigerPlace clinical staff were highly engaged as part of the research team to provide current and past health status. The team of clinical and technical researchers met weekly to discuss the project and review the data. An infrastructure was set up to transfer the sensor data to a server, and web-based visualizations were developed for viewing the data according to the preferences and feedback of the clinical team using participatory design practice.51
Investigating Sensor Data Trends for Detecting Health Changes.
The team began investigating possible approaches for generating health change alerts with a retrospective analysis of the data, using incidents of hospitalizations, emergency visits, and falls, and then looking at preceding sensor data trends that might show early indicators of the health events. With feedback from the clinical team, the technical team developed test algorithms on the retrospective data, and the group reviewed the results, iteratively developing more effective alert algorithms.52–55 Analysis showed that the sensor system detected health changes 10 to 14 days before usual assessment methods, which provided time for earlier interventions. The health alerts included a brief description of the parameter that prompted the alert along with a link to the website showing visualizations of the sensor data. The clinical workflow for acting on a health alert was streamlined by linked the alert interface with the EHR56 Next, the health alert system was tested prospectively in a pilot study with 20 TigerPlace residents living with sensors compared to a control group of 22 TigerPlace residents without sensors. After one year, the residents with sensors and health alerts showed better health outcomes in gait speed and grip strength, suggestive of better functionality and lower frailty as a result of earlier interventions compared to the control group. 57
Further Testing and Refinement in New Sites.
Longitudinal studies of the smart sensor system were also conducted in Assisted Living (AL) communities. Some challenges arose in moving to these new sites, in part because of the differences in infrastructure and clinical users. The original system was developed with input from the clinical research faculty and the experienced RN and social worker at TigerPlace. In contrast, the AL communities had an LPN with oversight provided remotely by an RN who served several AL sites. The health alerts were sent to the local LPN’s and the remote RN supervisor. However, the LPN’s lacked the time or training to interpret the health alerts. Additionally, the new sites lacked the EHR in use at TigerPlace, which made it difficult for the remote RN supervisor to effectively provide care suggestions. To address the challenge, training was added for the local LPN’s, to provide guidance on the interpretation of the health alerts based on a protocol developed by a TigerPlace nurse. A longitudinal RCT was conducted in 13 AL communities with 86 residents randomly assigned to intervention (sensors and health alerts) and 85 as a comparison group receiving usual care. Results showed significant differences between the two groups in walking speed and fall risk, with the comparison group declining more rapidly than the intervention group.58
Next steps and Lessons Learned.
As the smart sensor system has been tested over time, there have been some notable results in detecting early signs of illness and functional decline. Findings from TigerPlace showed that a decrease in average in-home walking speed of 5.1 cm/sec over 7 days (as captured by the in-home depth sensor) suggestive of a 86% probability of falling within the next three weeks.59 In another study, in-home gait data along with monthly assessments of validated fall risk measurements allowed for a mapping of average in-home walking speed into fall risk.38 The fall risk indicators provide valuable lead time for interventions to prevent falls. The studies also show the importance of the accompanying nursing care coordination and the importance of integrating the health alert system into the clinical workflow. The effect of the health alerts was more pronounced in TigerPlace, where the system was better integrated into the clinical care process and the clinical staff were better trained.60 Current studies are underway to further test the usability of the system in AL, including new methods for displaying alert messages in the form of linguistic summaries.72
DISCUSSION
Nurses are at the frontlines in the delivery of healthcare in acute, primary care, and community health settings and are the largest workforce in healthcare. Thus, they are in a unique position to inform the development of interventions that leverage technology. Regardless of nurse education or the clinical or community setting, nurses routinely engage with patients, families, and communities in monitoring symptoms and comorbidities thru EHR, social and economic factors, community needs and barriers, and care coordination which is critical to address in the design of interventions that use technology. Below we describe how technology has informed nursing research and practice, posed challenges to healthcare providers, and recommendations for policy initiatives.
How has nursing science contributed to the development and use of sensor technology?
The NINR has been pivotal in providing funding to nurse scientists to develop and test technology to improve symptom and self-management and to reduce health disparities as evidenced by the P20 Exploratory Centers, P30 Centers of Excellence, and Omics Nursing Science and Education Network.61–63 Nurses have integrated wearable technology (actigraphy, smart phones, Fitbit) to capture physiological monitoring such as heart rate, sleep patterns, physical activity 61–70 with machine learning to characterize changes in self-management to promote communication between the patient and healthcare provider.5,71–73 Biological technologies such as genomics and metabolomics have been used by nurses to characterize symptom phenotypes and to identify biomarkers for early detection of disease onset and new strategies for intervention.63,67,74–78 Nurses provide engineers and computer scientists with the needed expertise to ensure that sensor technology captures clinically relevant parameters (e.g., sleep duration, physical activity, blood pressure parameters; type of data to track; frequency);, and collecting data in unstructured environments (e.g., home, school settings, AL).79 Furthermore, nurses have informed algorithms that allow the sensor technology to be useful in unstructured environments, such as noisy home rather than in controlled clinical settings.
What are some of the challenges nurses and other health care providers face when relying on sensor technology?
Liability in monitoring of additional patient data and how this data is integrated into EHR is a challenge. Another one is the uncertainty around the validation of the devices and algorithms, and whether or not it has been tested against the gold standard. Technology changes rapidly and regular updates and training of healthcare providers are needed. This will require resources for continuing education courses and for training the trainer programs to ensure all healthcare providers are comfortable in using technology. Health and technology literacy needs to be taken into account to make informed decisions to understand basic health information and services, and to communicate information. Although smartphones, Alexa, and FitBit devices are commonly used, patients or communities may be reluctant to have sensors in the home because of concerns about privacy, costs, trust, and uncertainty about who will see their data and/or what happens to the data. The testing of consumer health technologies among underserved populations is low and may further perpetuate health disparities.80–82 Sociotechnical interventions hold promise for reducing disparities and improving the health of marginalized populations, but this potential is yet to be fully realized as outlined in the Computing Community consortium.83 Further work that integrates community based participatory research is needed to create inclusive sociotechnical health intervention for underserved individuals and/or communities.5,83,84
What policy initiatives are needed to drive and sustain advantages that sensor technology might make to personalized prevention and intervention?
New models of digital education and workforce development are needed for students (high school, undergraduate and graduate) and employees to have not only the skills in how to use technology, but also skills in critical thinking, design, and collaboration. For example, at the college level, the development of courses that consist of STEM and non-STEM students with an emphasis on interdisciplinary collaboration to use sensor, biological and machine learning technologies and community based participatory research would move this work forward. Within the healthcare systems, a common EHR across hospital agencies could leverage large data bases to support biological (omics) and sensor technology. Support from hospital administration and information technology to develop policies that promote the integration of sensor and biologic health information into health care operations (e.g., clinical validity and clinical utility, laboratory standards sensitivity and specificity) is needed.
Inclusion of nurses on sensor technology advisory committees (e.g., Health Information Technology Advisory Committee, tech companies [Amazon, Apple, Google, Microsoft], Institution Review Boards, community advisory boards), and engagement with local and state officials such as Public Health departments who develop policy recommendations and can enact effective policies are necessary. For example, Bekemeier and colleagues in the Schools of Nursing and Public Health at the University of Washington embarked on the SHARE-NW project: a five-year effort to identify, gather and visualize data in four Northwest states to help rural communities more effectively address health disparities and achieve health equity.85 Hirsch and colleagues from the University of Washington, School of Nursing, collaborated with Premera Blue Cross, a leading health plan in the Pacific Northwest, on a grant to establish the Rural Nursing Health Initiative to place current students in rural practices in Washington state. Additionally, nurses could integrate faculty who, preceptors, and graduate students who are well versed in telehealth into local tech companies interested in providing this benefit to their employees.
Additional work is needed to maintain mechanisms for protection of personal data, privacy, discrimination, and bias in the data and algorithms from being used against employment and health insurance, and in obtaining informed consent to store and use biological (e.g., omics) or other health related information. Current policies in the U.S. do not address these important issues, and nurses are well positioned to lead in this area. Lastly, funding from federal and non-federal agencies is critical to drive and sustain advantages that sensor technology has with respect to personalized health promotion and prevention. Research training with support from NIH bootcamps, funding of P20/P30 Center grants, National Science Foundation, and non-federal funding (Gates Foundation, RWJ) is critical for students and the next generation of nurse scientists to continue to advance the science and change practice to improve health promotion, disease prevention, detection, and management.
Conclusion
In conclusion, nurses have successfully used technology to improve health and prevent, detect and manage in children, families, individuals and communities. Nurses are key stakeholders to inform clinically relevant health monitoring that supports timely and personalized intervention and recommendations.
ACKNOWLEDGEMENT
Funding: This work was supported by the National Institute of Health/National Institute of Nursing Research (P30NR016585, R21NR01747, R21NR011197, R01NR014255, NHLBI 1R01HL118189-01); the Agency for Healthcare Research and Quality (R01HS018477); the National Science Foundation (IIS-1115956, IIS-0428420, IIS-0703692, CNS-0931607, CNS-1237970), and the Barbara Snider Sleep Endowment, Center for Innovation in Sleep Self-Management, University of Washington, Seattle, WA. We would like to thank the families who participated in this study; graduate students-Christina Hussain, Maeve Edstrom, and Jeff Matarrese from the Human Centered Design and Engineering and Jonika Hash, Weichoa Yuwen, Shumenghui Zhai at the University of Washington; and the Eldertech team at the University of Missouri; and the ARA/Virtual Heroes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Teresa M. Ward, School of Nursing, University of Washington.
Seattle Marjorie Skubic, Electrical Engineering and Computer Science, University of Missouri.
Marilyn Rantz, Sinclair School of Nursing, University of Missouri.
Allison Vorderstrasse, College of Nursing, New York University, NY.
REFERENCES
- 1.Nastasi BK, Varjas K, Schensul SL, Silva KT, Schensul JJ, Ratnayake P. The participatory intervention model: A framework for conceptualizing and promoting intervention acceptability School Psychology Quarterly,15 (2000), pp. 207–232. [Google Scholar]
- 2.Arsand E, Demiris G. User-centered methods for designing patient-centric self-help tools. Inform Health Soc Care. 2008. September;33(3):158–69. [DOI] [PubMed] [Google Scholar]
- 3.Eikey EV, Reddy MC, Kuziemsky CE Examining the role of collaboration in studies of health information technologies in biomedical informatics: A systematic review of 25 years of research. J Biomed Inform. 2015. October; 57:263–77. [DOI] [PubMed] [Google Scholar]
- 4.Yuwen W, Backonja B, Bromberg MH, Garrison MM, Ringold S, Ward TM. Usability testing of a web-based intervention for parents to improve sleep in young children with arthritis. Poster presentation, Pediatric Sleep Medicine Tenth Bi-Annual Conference, Naples, FL. November, 2019 [Google Scholar]
- 5.Kearns WR, Kaura N, Divina M, Vo C, Si D, Ward TM, & Yuwen W (2020). A Wizard-of-Oz interface and persona-based methodology for collecting health counseling dialog. Proceedings of the ACM CHI 2020 Conference on Human Factors in Computing Systems [Google Scholar]
- 6.Law EF, Beals-Erickson SE, Noel M, Claar R, Palermo TM. Headache. Pilot Randomized Controlled Trial of Internet-Delivered Cognitive-Behavioral Treatment for Pediatric Headache. 2015. Nov-Dec;55(10):1410–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bock C, Demiris G, Choi Y, Le T, Thompson HJ, Samuel A, Huang D. Engaging older adults in the visualization of sensor data facilitated by an open platform for connected devices. Technol Health Care. 2016. March 11;24(4):541–50. [DOI] [PubMed] [Google Scholar]
- 8.Kearns WR, Kaura N, Divina M, Vo C, Si D, Ward TM, & Yuwen W A Wizard-of-Oz interface and persona-based methodology for collecting health counseling dialog. Proceedings of the ACM CHI 2020 Conference on Human Factors in Computing Systems. 10.1145/3334480.3382902 [DOI] [Google Scholar]
- 9.Backonja U, Kneale L, Demiris G, Thompson HJ. Senior Tech: The Next Generation: Health Informatics Solutions for Older Adults Living in the Community. J Gerontol Nurs. 2016;42(1):2–3 [DOI] [PubMed] [Google Scholar]
- 10.Schuler and Namioka, 2009. Schuler D, Namioka A (Eds.), Participatory design principles and practices, CRC Press, Boca Raton, FL: (2009). [Google Scholar]
- 11.NCSDR. The National Center on Sleep Disorders Research (NCSDR) of the National Institutes of Health (NIH). June 10, 1993; Available from http://www.nhlbi.nih.gov/about/org/ncsdr/. Accessed December 29, 2019.
- 12.Ward TM, Yuwen W, Voss J, & Ringold S. (2016). Sleep fragmentation and protein biomarkers in pain in children with juvenile idiopathic arthritis. Biological Research for Nursing, 18(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward TM, Sonney J, Ringold S, Stockfish S, Wallace CA, Landis CA. Sleep disturbances and behavior problems in children with and without arthritis. J Pediatr Nurs. 2014; 29(4):321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aviel Y, Stremler R, Benseler SM, et al. , Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology. 2011; 50, 2051–206. [DOI] [PubMed] [Google Scholar]
- 15.Shyen S, Amine B, Rostom S, E L Badri D, Ezzahri M, Mawani N, Moussa F, Gueddari S, Wabi M, Abouqal R, Chkirate B, Hajjaj-Hassouni N. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile idiopathic arthritis. Clin Rheumatol. 2014;33(10):1425–31. [DOI] [PubMed] [Google Scholar]
- 16.Sorscher AJ. How is your sleep: a neglected topic for health care screening. J Am Board Fam Med. 2008. 21(2):141–8. [DOI] [PubMed] [Google Scholar]
- 17.Stremler R, McMurray J, Brennenstuhl S. Self-Reported Sleep Quality and Actigraphic Measures of Sleep in New Mothers and the Relationship to Postpartum Depressive Symptoms. Behav Sleep Med. 2019. 1–10. [DOI] [PubMed]
- 18.Ward TM, Rankin S, & Lee KA Caring for children with sleep problems. J Pediatr Nurs. 2007. 22(4), 283–296. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell BA, Ordway MR, Sadler LS, Redeker NS. (2019). Parent Perspective on Sleep and Sleep Habits Among Young Children Living With Economic Adversity. J Pediatr Health Care. 2020 Jan - Feb;34(1):10–22. [DOI] [PubMed] [Google Scholar]
- 20.Harvey AG, & Buysse DJ (2018). Treating sleep problems: A transdiagnostic approach. The Guilford Press. [Google Scholar]
- 21.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Annals of behavioral medicine. 2003;26(1):1–7. [DOI] [PubMed] [Google Scholar]
- 22.Miller WR, Lasiter S, Bartlett Ellis R, Buelow JM. Chronic disease self-management: a hybrid concept analysis. Nurs Outlook. 2015;63(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens JA, Mindell JA. Pediatric Insomnia. Pediatr Clin North Am. 2011; 58(3):555–69 [DOI] [PubMed] [Google Scholar]
- 24.Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T, Tai G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain. 2016.157, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S48–S65. 10.2337/dc20-S005. [DOI] [PubMed] [Google Scholar]
- 26.Diaz KM, Krupka DJ, Chang MJ, Shaffer JA, Ma Y, Goldsmith J, Schwartz JE, Davidson KW. Validation of the Fitbit One® for physical activity measurement at an upper torso attachment site. BMC Res Notes. 2016. 12;9:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015. 18;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vorderstrasse AA, Melkus GD, Pan W, Lewinski AA, Johnson CM. Diabetes Learning in Virtual Environments: Testing the Efficacy of Self-Management Training and Support in Virtual Environments (Randomized Controlled Trial Protocol).Nurs Res. 2015;64(6):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueiredo M, Caldeira C, Chen Y, Zheng K. Routine self-tracking of health: reasons, facilitating factors, and the potential impact on health management practices. AMIA Annu Symp Proc. 2018;2017:706–714. Published 2018 Apr 16. [PMC free article] [PubMed] [Google Scholar]
- 30.Phan K, Mobbs RJ. Long-Term Objective Physical Activity Measurements using a Wireless Accelerometer Following Minimally Invasive Transforaminal Interbody Fusion Surgery. Asian Spine J. 2016;10(2):366–369. 10.4184/asj.2016.10.2.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rantz M (2003). Aging in place. Nurseweek, Midwest/Heartland Edition, 4(7) [Google Scholar]
- 32.Rantz MJ, Phillips L, Aud M, Popejoy L, Marek KD, Hicks LL, Zaniletti I, & Miller SJ. Evaluation of aging in place model with home care services and registered nurse care coordination in senior housing. Nursing Outlook. 2011. 59(1), 37–46. [DOI] [PubMed] [Google Scholar]
- 33.Rantz MJ, Marek KD, Aud M, Tyrer HW, Skubic M, Demiris G, & Hussam A. (2005). A technology and nursing collaboration to help older adults age in place. Nursing Outlook, 53(1), 40–45. [DOI] [PubMed] [Google Scholar]
- 34.Rantz MJ, Porter RT, Cheshier D, Otto D, Servey CH 3rd, Johnson RA, Aud M, Skubic M, Tyrer H, He Z, Demiris G, Alexander GL, & Taylor G. (2008). TigerPlace, A State-Academic-Private Project to Revolutionize Traditional Long-Term Care. Journal of Housing For the Elderly, 22(1–2), 66–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. https://www.americareusa.net/senior-living/mo/columbia/tiger-place/
- 36.Skubic M, Alexander G, Popescu M, Rantz M and Keller J (2009), “A Smart Home Application to Eldercare: Current Status and Lessons Learned,” Technology and Health Care 17(3):183–201. [DOI] [PubMed] [Google Scholar]
- 37.Skubic M, Guevara RD & Rantz M, “Automated Health Alerts Using In-Home Sensor Data for Embedded Health Assessment,” IEEE Journal of Translational Engineering in Health and Medicine, 2015, 3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone E & Skubic M, “Unobtrusive, Continuous, In-HomeGait Measurement Using the Microsoft Kinect,” IEEE Transactions on Biomedical Engineering, 2013, 60(10):2925–2932 [DOI] [PubMed] [Google Scholar]
- 39.Stone E & Skubic M, “Fall Detection in Homes of Older Adults Using the Microsoft Kinect,” IEEE Journal of Biomedical and Health Informatics, 2015, 19(1):290–301. [DOI] [PubMed] [Google Scholar]
- 40.Rosales L, Bo-Yu Su, Skubic M, and Ho KC, “Heart Rate Estimation from Hydraulic Bed Sensor Ballistocardiogram,” Journal of Ambient Intelligence and Smart Environments, vol. 9, no. 2, pp. 193–207, 2017. [Google Scholar]
- 41.Wang S, Skubic M & Zhu Y, “Activity Density Map Visualization and Dis-similarity Comparison for Eldercare Monitoring,” IEEE Journal of Biomedical and Health Informatics, 2012, 16(4):607–614. [DOI] [PubMed] [Google Scholar]
- 42.Popescu M & Mahnot A, “Early Illness Recognition in Older Adults Using In-Home Monitoring Sensors and Multiple Instance Learning,” Methods of Informatics in Medicine, 2012, 51(4):359–67. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee T, Skubic M, Keller JM & Abbott CC, “Sit-To-Stand Measurement For In Home Monitoring Using Voxel Analysis,” IEEE Journal of Biomedical and Health Informatics, 2014, 18(4):1502–1509. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee T, Keller J, Skubic M & Stone E, “Day or Night Activity Recognition from Video Using Fuzzy Clustering Techniques,” IEEE Transactions on Fuzzy Systems, 2014, 22(3):483–493. [Google Scholar]
- 45.Stone E, Skubic M, Rantz M, Abbott C & Miller S, “Average In-Home Gait Speed: Investigation of a New Metric for Mobility and Fall Risk Assessment of Elders,” Gait and Posture, 2015, 41:57–62. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee T, Keller JM, Popescu M & Skubic M, “Recognizing Complex Instrumental Activities of Daily Living Using Scene Information and Fuzzy Logic,” Computer Vision and Image Understanding, 2015, 140:68–82. [Google Scholar]
- 47.Banerjee T, Yefimova M, Keller J, Skubic M, Woods DL & Rantz M, “Exploratory analysis of older adults’ sedentary behavior in the primary living area using Kinect depth data,” Journal of Ambient Intelligence and Smart Environments, Vol. 9, pp. 163–179, 2017. [Google Scholar]
- 48.Wallace R, Abbott C, Gibson-Horn C, Rantz M, & Skubic M. “Metrics from In-Home Sensor Data to Assess Gait Change Due to Weighted Vest Therapy,” Smart Health, vol. 3–4, pp. 1–19, 2017. [Google Scholar]
- 49.Jiao C, Su BY, Lyons P, Zare A, Ho KH, & Skubic M. “Multiple Instance Dictionary Learning for Beat-to-Beat Heart Rate Monitoring from Ballistocardiograms,” IEEE Trans. on Biomedical Engineering, 65(11), 2634–2648, November. 2018. 10.1109/TBME.2018.2812602. [DOI] [PubMed] [Google Scholar]
- 50.Su B-Y, Enayati M, Ho K-C, Skubic M, Despins D, Keller J, Popescu M, Guidoboni G & Rantz M, “Monitoring the Relative Blood Pressure Using a Hydraulic Bed Sensor System,” IEEE Trans. on Biomedical Engineering, 66(3): 740–748, March, 2019. 10.1109/TBME.2018.2855639. [DOI] [PubMed] [Google Scholar]
- 51.Demiris G, Skubic M, Keller J, Rantz M, Parker Oliver D, Aud M, Lee J, Burks K & Green N, “Nurse Participation in the Design of User Interfaces for a Smart Home System,” Proceedings, International Conference on Smart Homes and Health Telematics, Belfast, N Ireland, June 26–28, 2006, pp 66–73. [Google Scholar]
- 52.Rantz MJ, Skubic M, Miller SJ, & Krampe J. “Using Technology to Enhance Aging in Place,” In Proc. of the 6th International Conference on Smart Homes and Health Telematics, Ames, IA, July, 2008, pp. 169–176. [Google Scholar]
- 53.Alexander G, Rantz M, Skubic M, Aud MA, Wakefield B, Florea E, and Paul A, “Sensor Systems for Monitoring Functional Status in Assisted Living Facility Residents,” Research in Gerontological Nursing, 1(10):238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rantz MJ, Skubic M, Alexander GL, Aud MA, Wakefield BJ, Galambos C, Koopman RJ, & Miller SJ (2010), “Improving Nurse Care Coordination with Technology,” Computers, Informatics, Nursing, 28(6):325–332. [DOI] [PubMed] [Google Scholar]
- 55.Rantz MJ, Skubic M, Koopman R, Phillips L, Alexander GL & Miller SJ, “Using Sensor Networks to Detect Urinary Tract Infections in Older Adults,” Proceedings, 13th IEEE International Conference on e-Health Networking, Application, & Services, Columbia, MO, June 13–15, 2011, pp 142–149. [Google Scholar]
- 56.Rantz MJ, Skubic M, Alexander G, Popescu M, Aud M, Koopman R & Miller S. “Developing a Comprehensive Electronic Health Record to Enhance Nursing Care Coordination, Use of Technology, and Research,” Journal of Gerontological Nursing, 36(1):13–17, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Rantz MJ, Skubic M, Koopman RJ, Alexander G, Phillips L, Musterman KI, Back JR, Aud MA, Galambos C, Guevara RD & Miller SJ, “Automated Technology to Speed Recognition of Signs of Illness in Older Adults,” Journal Gerontological Nursing, 2012, 38(4):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rantz M, Lane K, Phillips LJ, Despins LA, Galambos C, Alexander GL, Koopman RJ, Hicks L, Skubic M & Miller SJ, “Enhanced RN Care Coordination with Sensor Technology: Impact on Length of Stay and Cost in Aging in Place Housing,” Nursing Outlook, 2015, 63(650–655). [DOI] [PubMed] [Google Scholar]
- 59.Phillips LJ, Deroche C, Rantz M, Alexander GL, Skubic M, Despins L, Abbott C, Harris BH, Galambos C, & Koopman R. (2016). “Using Embedded Sensors in Independent Living to Predict Gait Changes and Falls,” Western Journal of Nursing Research, published online 7/27/16. [DOI] [PMC free article] [PubMed]
- 60.Rantz M, Skubic M, Popescu M, Galambos C, Koopman RJ, Alexander GL, Phillips LJ, Musterman K, Back J, & Miller SJ, “A New Paradigm of Technology Enabled “Vital Signs” for Early Detection of Health Change for Older Adults,” Gerontology, 2015, 61(3):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu MR, Kurnat-Thoma E, Starkweather A, Henderson WA, Cashion AK, Williams JK, Katapodi MC, Reuter-Rice K, Hickey KT, Barcelona de Mendoza, Calzone K, Conley YP, Anderson CM, Lyon DE, Weaver MT, Shiao PK, Constantino RE, Wung SF, Hammer MJ, … Coleman B (2020). Precision health: A nursing perspective. International Journal of Nursing Sciences,7(1), 5–12. 10.1016/j.ijnss.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starkweather A, Jacelon CS, Bakken S, Barton DL, DeVito Dabbs A, Dorsey SG, Guthrie BJ, Heitkemper MM, Hickey KT, Kelechi TJ, Kim MT, Marquard J, Moore SM, Redeker NS, Schiffman RF, Ward TM, Adams LS, Kehl KA, Miller JL. The Use of Technology to Support Precision Health in Nursing Science. J Nurs Scholarsh. 2019;51(6):614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Omics Nursing Science and education network. ONSEN. Accessed March 16, 2020 https://omicsnursingnetwork.net/ [DOI] [PMC free article] [PubMed]
- 64.Van Blarigan EL, Kenfield SA, Chan JM, Van Loon K, Paciorek A, Zhang L, Chan H, Savoie MB, Bocobo AG, Liu VN, Wong LX, Laffan A, Atreya CE, Miaskowski C, Fukuoka Y, Meyerhardt JA, Venook AP. Feasibility and Acceptability of a Web-Based Dietary Intervention with Text Messages for Colorectal Cancer: A Randomized Pilot Trial. Cancer Epidemiol Biomarkers Prev. 2020; 29(4):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pina LR, Sien SW, Song C, Ward TM, Fogarty J, Munson SM, Kientz J. 2020. DreamCatcher: Exploring How Parents and School-Age Children Can Track and Review Sleep Information Together. In Proceedings of the ACM on Human-Computer Interaction, Vol. 4, CSCW1, Article 46 (May 2020), 10.1145/3392882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi YK, Demiris G, Lin SY, Iribarren SJ, Landis CA, Thompson HJ, McCurry SM, Heitkemper MM,Ward TM. Smartphone Applications to Support Sleep Self-Management: Review and Evaluation. J Clin Sleep Med. 2018. 14(10):1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickey KT, Bakken S, Byrne MW, Bailey DCE, Demiris G, Docherty SL, Dorsey SG, Guthrie BJ, Heitkemper MM, Jacelon CS, Kelechi TJ, Moore SM, Redeker NS, Renn CL, Resnick B, Starkweather A, Thompson H, Ward TM, McCloskey DJ, Austin JK, Grady PA. Precision health: Advancing symptom and self-management science. Nurs Outlook. 2019;67(4):462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnall R, Bakken S, Rojas M et al. mHealth Technology as a Persuasive Tool for Treatment, Care and Management of Persons Living with HIV. AIDS Behav. 2015;19, 81–89. 10.1007/s10461-014-0984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breitenstein SM, Shane J, Julion W, Gross D. Developing the eCPP: adapting an evidence-based parent training program for digital delivery in primary care settings. Worldviews Evid Based Nurs. 2015;12(1):31–40. [DOI] [PubMed] [Google Scholar]
- 70.Whittemore R, Jeon S, Grey M. An internet obesity prevention program for adolescents. J Adolesc Health. 2013;52(4):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.,Koleck TA, Dreisbach C, Bourne PE, Bakken S Natural language processing of symptoms documented in free-text narratives of electronic health records: a systematic review.J Am Med Inform Assoc. 2019;26(4):364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain A, Popescu M, Keller J, Rantz M, Markway B. Linguistic Summarization of In-Home Sensor Data. Journal of biomedical informatics. 2019. June 28:103240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu MR, Wang Y, Li C, Qiu Z, Axelrod D, Guth AA, Scagliola J, Conley Y, Aouizerat BE, Qiu JM, Yu G, Van Cleave JH, Haber J, Cheung YK. Machine learning for detection of lymphedema among breast cancer survivors. Mhealth. 2018; 29;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorsey SG, Renn CL, Griffioen M, Lassiter CB, Zhu S, Huot-Creasy H, McCracken C, Mahurkar A, Shetty AC, Jackson-Cook CK, Kim H, Henderson WA, Saligan L, Gill J, Colloca L, Lyon DE, Starkweather AR. Whole blood transcriptomic profiles can differentiate vulnerability to chronic low back pain. PLoS One. 2019.16;14(5):e0216539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knisely MR, Maserati M, Heinsberg LW, Shah LL, Li H, Zhu Y, Ma Y, Graves LY, Merriman JD, Conley YP. Symptom Science: Advocating for Inclusion of Functional Genetic Polymorphisms. Biol Res Nurs. 2019; 21(4):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shulman RJ, Öhman L, Stridsberg M, Cain K, Simrén M, Heitkemper M. Evidence of increased fecal granins in children with irritable bowel syndrome and correlates with symptoms. Neurogastroenterol Motil. 2019; 31(1):e13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maguire R, Fox PA, McCann L, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open 2017;7:e015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S, Dunlop AL, Jones DP, Corwin EJ . High-Resolution Metabolomics: Review of the Field and Implications for Nursing Science and the Study of Preterm Birth. Biol Res Nurs. 2016; 18(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeVito Dabbs A, Song MK, Myers B, Hawkins RP, Aubrecht J, Begey A, Connolly M, Li R, Pilewski JM, Bermudez CA, Dew MA. Clinical trials of health information technology interventions intended for patient use: unique issues and considerations. Clin Trials. 2013;10(6):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veinot TC, Ancker JS, Cole-Lewis H, Mynatt ED, Parker AG, Siek KA, Mamykina L. (2019). Leveling Up: On the Potential of Upstream Health Informatics Interventions to Enhance Health Equity.Med Care. 57 Suppl 6 Suppl 2:S108–S114. 10.1097/MLR.0000000000001032 [DOI] [PubMed] [Google Scholar]
- 81.Veinot TC, Mitchell H, Ancker JS. (2018). Good intentions are not enough: how informatics interventions can worsen inequality. J Am Med Inform Assoc. 1;25(8):1080–1088. 10.1093/jamia/ocy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unertl KM, Schaefbauer CL, Campbell TR,Senteio C, Siek KA, Bakken S, Veinot TC. Integrating community-based participatory research and informatics approaches to improve the engagement and health of underserved populations. J Am Med Inform Assoc. 2016. 23(1):60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siek K, Veinot T, & Mynatt B. (2019). Research Opportunities in Sociotechnical Interventions for Health Disparity Reduction. A Computing Community Consortium. June 2019. Accessed January 14, 2020. https://arxiv.org/ftp/arxiv/papers/1908/1908.01035.pdf
- 84.Webel A, Prince-Paul M, Ganocy S, DiFranco E, Wellman C, Avery A, Daly B, Slomka J. Randomized clinical trial of a community navigation intervention to improve well-being in persons living with HIV and other co-morbidities. AIDS Care. 2019;31(5):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bekemeier B, Park S, Backonja U, Ornelas I, Turner AM. Data, capacity-building, and training needs to address rural health inequities in the Northwest United States: a qualitative study. J American Medical Informatics Association, 2019; 26 (8–9): 825. [DOI] [PMC free article] [PubMed] [Google Scholar]


