Abstract
Previous studies have shown that serum total cholesterol (TC) and serum alanine aminotransferase (ALT) are associated with liver cancer risk. However, the common contribution of TC and normal-high ALT to primary liver cancer (PLC) has not been reported. We aim to assess the separate and joint effect of low TC level and normal-high ALT level on the risk of PLC, a large prospective cohort was conducted in our study.
The participants were divided into 4 groups via the cross-matching method according to TC [low level (−)/non-low level (+)] and ALT [normal level (−)/normal-high level(+)] status, and using the lower quartile value of TC and the upper quartile value of ALT as a threshold, respectively. Incident PLC was confirmed by review of medical records. Cox proportional hazards regression models and interactive additive models were used to evaluate whether the joint effect of low TC level and normal-high ALT level is associated with the risk of PLC.
During 1,248,895 person-years follow-up, 298 participants were diagnosed with PLC among 114,972 subjects. In male population, TC < 4.24 mmol/L was group “TC (−)”; TC ≥ 4.24 mmol/L was group “TC (+)”; ALT < 23 U/L was group “ALT (−)”: 33 U/L ≥ ALT ≥ 23 U/L was group “ALT (+)”. Compared with the group “TC (+)”, group “ALT (−)”, respectively, the adjusted hazard ratio (HR) and 95% confidence interval (95%CI) for PLC risk was 1.74 (1.36–2.25) in group “TC (−)” and 1.49 (1.15–1.94) in group “ALT (+)”. In combinatorial analysis, compared with group “TC (+) and ALT (−)”, the significant increased risk of PLC were observed in group “TC (+) and ALT (+)” (HR = 1.41; 95% confidence intervals [CI]: 1.02–1.95), group “TC (−) and ALT (−)” (HR = 1.67; 95%CI: 1.24–2.27) and group “TC (−) and ALT (+)” (HR = 2.72; 95%CI: 1.81–4.09), respectively. However, no statistical significance was found among female.
The separate and joint effect of low TC level and normal-high ALT level was observed for PLC risk in males. When combined, individuals with coexistence of low TC level and normal-high ALT level significantly increase the risk of PLC.
Keywords: joint effect, low total cholesterol level, normal-high alanine aminotransferase level, primary liver cancer, prospective study, risk
1. Introduction
Liver cancer, a heavy disease burden worldwide, is one of malignant tumors which causes serious harm to human life and health. According to the cancer data produced by the International Agency for Research on Cancer, liver cancer was predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death around the world.[1] Particularly, the study estimated that annually half of new cases occur in China and the incidence of liver cancer has been on the rise.[2] In the past decade, the age-standardized incidence of liver cancer has increased from 8.1 per 100,000 person-years[3] to 13.9 per 100,000 person-years[1] worldwide. Although some new progress has been made in the treatment of liver cancer in the past 30 years, it still remains the poor outcome for advanced liver cancer.[4] Positively seeking and avoiding the risk factors for liver cancer are the most effective approaches to decrease the liver cancer risk.
Currently, the aging,[5] male,[6] obesity,[7] elevated fasting plasma glucose,[8] and especially infection of hepatitis B virus[9] and Hepatitis C Virus (HCV)[9] are well-established risk factors for development of liver cancer, respectively. Additionally, the dyslipidemia and inflammation related to chronic liver damage are also associated with the incidence of liver cancer.[10] Study has suggested that the deregulation of cholesterol homeostasis could affect the cancer development.[11] The observational studies from Japan[12] and Korea[13] found that low total cholesterol (TC) level was associated with an increased risk of liver cancer. Wen et al.[14] by using risk prediction model indicated that transaminase was best able to predict liver cancer risk. As we know, elevated alanine aminotransferase is one of most markers for the hepatocyte injury and necrosis.[15] Although some researches have confirmed the close association between obviously elevated alanine aminotransferase (ALT) level and the increased liver cancer risk,[16,17] the normal-high ALT level remains unclear for liver cancer risk to date.
Previous studies only assessed the role of low TC alone or elevated ALT alone in the risk of liver cancer, and none of them had investigated the combined effect of both with the risk of primary liver cancer (PLC). Based on Kailuan study, we examine the separate and joint effects of low TC level and normal-high ALT level on the risk of PLC.
2. Methods
2.1. Research design and participants
The Kailuan study is a prospective cohort study based on the community population in Tangshan city, northern China (Trial Registration Number: ChiCTR-TNRC-11001489).[18,19] Since 2006, the employees (≥18 years, including the retired) of the Kailuan Group, Tangshan City, were invited to participate in biennial health check-up at 11 affiliated hospitals. The Kailuan Study was conducted to estimate the prevalence chronic disease, nutritional disorders and major risk factors for these diseases. The details of the study design and procedures are available elsewhere.[18,19] From 2006 to 2007, 101,510 participants completed the survey, which constituted Kailuan Study I. From 2008 to 2009, 2010 to 2011, both 25,337 adults and 10,519 adults formed the Kailuan Study II and Kailuan Study III, respectively. And all participants (137,366) underwent questionnaire survey, clinical and laboratory examinations.
In our current study, we excluded 463 subjects who had PLC and a history of malignant tumors at the baseline, excluded 1,830 and 490 subjects with missing information of TC and ALT, respectively. According to the adult standard of American College of Gastroenterology,[20] 19,611 subjects with abnormal data of ALT were excluded (serum ALT > 33 U/L in male or serum ALT > 25 U/L in female). A total of 114,972 individuals were finally included in the current analyses (Fig. 1). This study was approved by Ethics Committee of Kailuan General Hospital and in compliance with the Declaration of Helsinki. Informed consent was obtained from the participants.
Figure 1.
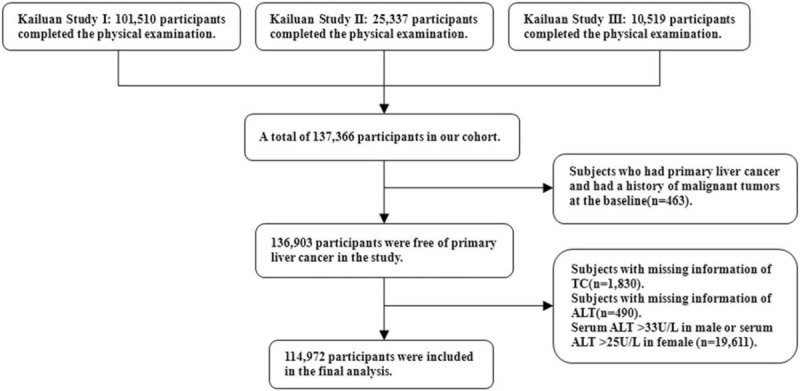
Flow chart of participants screening.
2.2. Assessment of exposure factor and other related laboratory
At 7:00–9:00 a.m., the fasting (8h-12 h) elbow venous blood of all participants was collected about 5 ml and placed in a vacuum tube that containing EDTA. The upper serum was taken after centrifugating for 10 minutes at 3000 rotations per minute at 24 °C. The serum samples were assuredthat completed the detection within 4 hours. Serum TC and serum ALT were determined by professional laboratory physicians using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) and strictly following the instructions of reagents. TC was measured enzymatically (CHOD-PAP) with an upper limit of detection of 20.68 mmol/L. ALT (ALT, in U/L) was measured by enzymatic rate methed. The upper limit of detection of ALT was 1000 U/L. Other biochemical parameters, including serum high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), hemoglobin, fasting blood glucose, hypersensitive C-reactive protein (hs-CRP) were determined by automatic biochemical analyzer (Hitachi 747; Hitachi, Tokyo, Japan). All plasma samples were analyzed at the central laboratory of Kailuan General Hospital.
2.3. Assessment of other relevant variables
On the day of physical examination, the trained medical and nursing personnel would assist the participants to fill in the questionnaires via face-to-face interviews. The information of the questionnaire included: age, gender, smoking habits, drinking status, physical activity, past medical history and so on (eg, hypertension, diabetes mellitus, malignant tumors, etc.).[21,22] Height and weight were measured by professionally trained staff. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). Hypertension was defined as systolic blood pressure ≥ 140 mmHg, and/or diastolic blood pressure ≥ 90 mmHg, or using antihypertensive medication. Diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L or use of oral hypoglycemic agent. Smoking was defined as having smoked at least 1 cigarette per day on average for at least 1 year. Alcohol consumption was defined as having taken alcohol of 100 mL/day (alcohol contents > 50%) of alcohol for more than 1 year. Physical activity was defined as taking exercises more than four times a week, each time lasting at least 30 minutes.[23] Ultrasound diagnosis standard of fatty liver: Comparing the liver echogenicity with the kidney, the diffuse echo enhancement in liver, image of intrahepatic blood vessels and the diaphragm was blurry or invisible.[24] The diagnostic criterias of cirrhosis in ultrasound: the coarse tissue and nodularity in liver surface or parenchyma, with or without ascites and splenomegaly; or subjects with medical history of cirrhosis.[24,25]
2.4. Definition and ascertainment of outcome events
During the period from participants’ first physical examination to December 31, 2018, subjects which were first diagnosed with hepatocellular carcinoma, intrahepatic cholangiocarcinoma and other liver cancers with unclear types (excluding the liver metastasis), we defined as PLC. Follow-up began at the first physical examination, and ended at occurrence of cancer, death, or December 31, 2018, whichever event came first. In our cohort, cancer events were confirmed via biennially health screening with face-to-face questionnaires and medical examinations. Additionally, medical records from Municipal Medical Service System (including medical insurance system and social security system) were checked yearly in detail to obtain outcome information of participants that may have been missed.[8] The outcome information was collected by professionally trained staff, and the CanReg 4.0 software that provided by the International Agency for Research on Cancer of the World Health Organization was used to input and logically verify new cases of LC. According to the International Classification of Diseases, Tenth Revision, and PLC was defined as C22.
2.5. Statistical analysis
Participants were divided into 4 groups according to TC (low level/non-low level) and ALT(normal level/normal-high level) status and using the lower quartile value (4.24 mmol/L) of TC and the upper quartile value (22 U/L) of ALT as a threshold, respectively.[26,27] Low TC level was defined as TC less than 4.24 mmol/L as group “TC (−)”; non-low TC level was defined as TC greater than or equal to 4.24 mmol/L as group “TC (+)”. Normal ALT level was defined as ALT less than 22 U/L as group “ALT (−)”; normal-high ALT level was defined as ALT greater than or equal to 22 U/L as group “ALT (+)”. Four groups were obtained by cross-matching method: “TC (−) + ALT (+)”, “TC (−) + ALT (−)”, “TC (+) + ALT (+)” and “TC (+) + ALT (−)”. Quantitative data with normal distribution was expressed as mean ± standard deviation, one-way analysis of variance was used for multiple comparison between groups. The measurement data with skewed distribution were described as median (interquartile range)(), the nonparametric Kruskal-Wallis test of variance was used for multiple comparison between groups. Categorical variables were described by percentage and compared using the Chi-square test. Incidence rates were calculated by dividing the number of events by person years of following up in each group. To investigate the joint effect of TC and ALT for PLC, three dummy variables were included in the models, and “TC (+) + ALT (−)” with minimum incidence in all groups was used as the reference group. The Cox proportional hazards model was used to estimate the hazard ratios and 95% confidence intervals (CIs) for the separate and joint effect of TC and ALT on PLC. Furthermore, interactive additive model was constructed to further test the joint effect of TC and ALT for PLC risk. We calculated the relative excess risk due to interaction, proportion of disease attributable to interaction, synergy index and P value for interaction.[28,29]
As sensitivity analyses, we further excluded 2,488 HBsAg positive participants, 231 participants in cirrhosis, 31,567 fatty liver participants, 38 participants who took statins, 11,127 ALT ≥ 40 U/L participants during follow-up, and 13 participants who occurred PLC within 1 year after entry to the cohort, respectively. And the Cox proportional hazards model was repeated again. The data management and all analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant for 2-sided tests.
3. Results
Total of 114,972 participants were included in this study with the mean age of 49.65 ± 13.68 years (males: n = 92522, 84.66%; females: n = 22450, 15.34%). The lower quartile value of TC is 4.24mmol/L, TC < 4.24 mmol/L was group “TC (−)”; TC ≥ 4.24 mmol/L was group “TC (+)”. The upper quartile value of ALT is 22U/L, ALT < 22U/L was group “ALT (−)”; ALT ≥ 22 U/L was group “ALT (+)”. The general baseline characteristics of the participants according to mismatch combinations of TC and ALT status are presented in Table 1.
Table 1.
Baseline characteristics by TC and ALT status.
| Variable | TC (−) + ALT (+) | TC (−) + ALT (−) | TC (+) + ALT (+) | TC(+) + ALT (−) | F/X2 | P Value |
| N | 6,669 | 22,070 | 22,962 | 63,271 | ||
| Male, % | 6263 (94.15) | 16584 (75.22) | 21169 (92.56) | 48506 (76.72) | X2 = 3890.93 | <.0001 |
| Age, y | 47.91 ± 14.14 | 48.25 ± 15.58 | 50.16 ± 11.96 | 52.29 ± 13.03 | F = 642.38 | <.0001 |
| BMI, kg/m2 | 25.22 ± 3.48 | 23.98 ± 3.47 | 25.62 ± 3.30 | 24.63 ± 3.39 | F = 932.07 | <.0001 |
| HDL-C, mmol/L | 1.38 ± 0.61 | 1.39 ± 0.36 | 1.53 ± 0.40 | 1.58 ± 0.45 | F = 1192.65 | <.0001 |
| HGB, g/L | 152 (143–161) | 146 (133–156) | 153 (144–162) | 148 (136–158) | X2 = 3256.49 | <.0001 |
| FBG, mmol/L | 5.36 ± 1.49 | 5.19 ± 1.35 | 5.61 ± 1.72 | 5.51 ± 1.73 | F = 283.36 | <.0001 |
| Hs-CRP, mg/L | 0.85 (0.34–2.20) | 0.80 (0.30–2.30) | 0.96 (0.40–2.20) | 0.90 (0.33–2.34) | X2 = 102.05 | <.0001 |
| TG, mmol/L | 1.22 (0.85–1.96) | 0.99 (0.70–1.46) | 1.43 (1.05–2.15) | 1.23 (0.88–1.79) | X2 = 4854.86 | <.0001 |
| TC, mmol/L | 3.58 ± 0.77 | 3.64 ± 0.67 | 5.37 ± 0.97 | 5.31 ± 0.89 | F = 28092.10 | <.0001 |
| ALT, U/L | 25.00 (23.00–28.00) | 14.00 (10.00–18.00) | 25.00 (23.00–28.00) | 14.00 (11.00–18.00) | X2 = 66124.85 | <.0001 |
| Fatty liver, % | 2257 (34.43) | 4130 (19.38) | 9254 (41.38) | 15926 (25.97) | X2 = 3006.89 | <.0001 |
| Hypertension, % | 2734 (41.00) | 7471 (33.85) | 10935 (47.62) | 27064 (42.77) | X2 = 918.07 | <.0001 |
| Diabetes mellitus, % | 505 (7.57) | 1275 (5.78) | 2275 (9.91) | 5504 (8.70) | X2 = 279.96 | <.0001 |
| Alcohol consumption, % | 1008 (15.11) | 2488 (11.27) | 4389 (19.11) | 10447 (16.51) | X2 = 550.06 | <.0001 |
| Smoking, % | 2018 (30.26) | 5154 (23.35) | 7348 (32.00) | 17647 (27.89) | X2 = 436.15 | <.0001 |
| Physical activity, % | 975 (14.62) | 3078 (13.95) | 3396 (14.79) | 10246 (16.19) | X2 = 76.10 | <.0001 |
TC (+): TC ≥4.24 mmol/L, TC (−): TC <4.24 mmol/L; ALT (+): ALT ≥22 U/L, ALT(−): ALT <22 U/L; ALT = alanine aminotransferase, BMI = body mass index, FBG = fasting blood glucose, HDL-C = high-density lipoprotein cholesterol, HGB = hemoglobin, hs-CRP = hypersensitive C-reactive protein, TC = total cholesterol, TG = triglyceride.
3.1. Incidence and risk of PLC in groups by TC and ALT status
During total 1,248,895 person-years (average 10.86 ± 2.11 years) follow-up, 298 PLC (278 male and 20 female) occurred, and the incidence of PLC was 24.00 per 100,000 person-years (28.00 per 100,000 person-years for male and 8.00 per 100,000 person-years for female). The incidence of PLC for group “TC (+) + ALT (−)”, group “TC (+) + ALT (+)”, group “TC (−) + ALT (−)” and group “TC (−) + ALT (+)” were 19.00 per 100,000 person-years, 28.00 per 100,000 person-years, 27.00 per 100,000 person-years and 50.00 per 100,000 person-years, respectively. The group “TC (−) + ALT (+)” had the highest incidence of PLC (Table 2).
Table 2.
Hazard ratios and 95% confidence interval for Risk of PLC in Groups by TC and ALT Status.
| Cases | Follow-up time, person-years | Incidence rate, per 100,000 person-years | Model 1 | Model 2 | Model 3 | |
| TC a alone | ||||||
| TC (+) | 197 | 939,206 | 21 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (−) | 101 | 309,689 | 33 | 1.56 (1.23–1.98) | 1.67 (1.32–2.13) | 1.71 (1.34–2.19) |
| ALT b alone | ||||||
| ALT (−) | 193 | 927,859 | 21 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| ALT (+) | 105 | 321,036 | 33 | 1.58 (1.24–2.00) | 1.56 (1.23–1.99) | 1.52 (1.18–1.95) |
| Combinations of TC and ALTc | ||||||
| TC (+) + ALT (−) | 128 | 690,346 | 19 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (+) + ALT (+) | 69 | 248,861 | 28 | 1.50 (1.12–2.01) | 1.51 (1.13–2.04) | 1.45 (1.07–1.97) |
| TC (−) + ALT (−) | 65 | 237,513 | 27 | 1.48 (1.10–2.00) | 1.62 (1.20–2.18) | 1.64 (1.21–2.22) |
| TC (−) + ALT (+) | 36 | 72,176 | 50 | 2.70 (1.86–3.90) | 2.81 (1.93–4.07) | 2.70 (1.84–3.96) |
TC(+): TC ≥4.24mmol/L, TC(−): TC <4.24mmol/L; ALT(+): ALT ≥22U/L, ALT(−): ALT <22U/L. CI = confidence interval, HR = hazard ratios, Ref = reference.
Model 1: Univariate analysis.
Model 2: Adjusted for age, gender.
Model 3: c Adjusted for age, gender, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity; a Further adjusted for ALT based on c; b Further adjusted for TC based on c.
In the multivariable adjusted analysis, the adjusted hazard ratio (HR) and 95% confidence interval (95%CI) for the risk of PLC in group “TC (−)” alone and group “ALT (+)” alone were 1.71 (1.34–2.19) and 1.52 (1.18–1.95), respectively, after adjustment for gender, age, BMI, TC, ALT, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity. And the adjusted HR (95%CI) for PLC risk increased from 1.45 (1.07–1.97) to 1.64 (1.21–2.22) and 2.70 (1.84–3.96) in each combination group of “TC (+) + ALT (+)”, “TC (−) + ALT (−)”, “TC (−) + ALT (+)”, respectively, after adjustment for gender, age, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity (Table 2).
3.2. Sex-specific analysis and PLC risk
A strong association was observed for PLC by different TC and ALT status in males. The adjusted HR (95%CI) for the risk of PLC in group “TC (−)” alone and group “ALT (+)” alone were 1.74 (1.36–2.25) and 1.49 (1.15–1.94), respectively, after adjustment for age, BMI, TC, ALT, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity. The adjusted HR (95%CI) for PLC risk increased from 1.41 (1.02–1.95) to 1.67 (1.24–2.27) and 2.72 (1.81–4.09) in each combination group of “TC (+) + ALT (+)”, “TC (−) + ALT (−)”, “TC (−) + ALT (+)”, respectively, after adjustment for age, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity (Table 3). However, no statistical significance was found among female (Table 4).
Table 3.
Hazard ratios and 95% confidence interval for Risk of PLC in Groups by TC and ALT Status in male.
| Cases | Follow-up time, person-years | Incidence rate, per 100,000 person-years | Model 1 | Model 2 | Model 3 | |
| TC #1 alone | ||||||
| TC (+) | 181 | 755,399 | 24 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (−) | 97 | 245,404 | 40 | 1.65 (1.29–2.11) | 1.73 (1.36–2.22) | 1.74 (1.36–2.25) |
| ALT #2 alone | ||||||
| ALT (−) | 190 | 745,091 | 26 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| ALT (+) | 88 | 255,711 | 34 | 1.35 (1.05–1.74) | 1.51 (1.17–1.95) | 1.49 (1.15–1.94) |
| Combinations of TC and ALT† | ||||||
| TC (+) + ALT (−) | 124 | 557,055 | 22 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (+) + ALT (+) | 57 | 198,344 | 29 | 1.30 (0.95–1.77) | 1.46 (1.06–2.00) | 1.41 (1.02–1.95) |
| TC (−) + ALT (−) | 66 | 188,036 | 35 | 1.58 (1.17–2.13) | 1.67 (1.24–2.25) | 1.67 (1.24–2.27) |
| TC (−) + ALT (+) | 31 | 573,67 | 54 | 2.43 (1.64–3.61) | 2.85 (1.92–4.23) | 2.72 (1.81–4.09) |
TC (+): TC ≥4.24 mmol/L, TC (−): TC <4.24 mmol/L; ALT (+): 33 U/L ≥ ALT ≥23 U/L, ALT (−): ALT <23 U/L. CI = confidence interval, HR = Hazard ratios, Ref = reference.
Model 1: Univariate analysis.
Model 2: Adjusted for age.
Model 3: †Adjusted for age, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity; #1 Further adjusted for ALT based on †; #2 Further adjusted for TC based on †.
Table 4.
Hazard ratios and 95% confidence interval for risk of PLC in groups by TC and ALT status in female.
| Cases | Follow-up time, person-yr | Incidence rate, per 100,000 person-yr | Model 1 | Model 2 | Model 3 | |
| TC #1 alone | ||||||
| TC (+) | 16 | 186,213 | 9 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (−) | 4 | 601,40 | 7 | 0.78 (0.26–2.34) | 1.36 (0.45–4.13) | 1.80 (0.57–5.68) |
| ALT #2 alone | ||||||
| ALT (−) | 14 | 179,901 | 8 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| ALT (+) | 6 | 664,52 | 9 | 1.15 (0.44–3.00) | 1.12 (0.43–2.92) | 0.98 (0.35–2.76) |
| Combinations of TC and ALT† | ||||||
| TC (+) + ALT (−) | 10 | 132,298 | 8 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (+) + ALT (+) | 6 | 53915 | 11 | 1.47 (0.53–4.03) | 1.57 (0.57–4.32) | 1.39 (0.46–4.18) |
| TC (−) + ALT(−) | 4 | 476,03 | 8 | 1.12 (0.35–3.58) | 2.17 (0.66–7.10) | 2.73 (0.80–9.29) |
| TC (−) + ALT (+) | 0 | 125,37 | NA | NA | NA | NA |
TC (+): TC ≥4.20 mmol/L, TC (−): TC <4.20 mmol/L; ALT (+): 25 U/L ≥ ALT ≥18 U/L, ALT (−): ALT <18 U/L. CI = confidence interval, HR = Hazard ratios, NA = not available, Ref = reference.
Model 1: Univariate analysis.
Model 2: Adjusted for age.
Model 3: †Adjusted for age, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity; #1 Further adjusted for ALT based on †; #2 Further adjusted for TC based on †.
3.3. Interaction between TC and ALT for PLC
Fig. 2 shows the adjusted HR (95%CI) and interaction terms for PLC in different status of TC and ALT. The results showed that there was no evidence of interaction effect between “TC (−)” and “ALT (+).” Relative excess risk due to interaction (95%CI), proportion of disease attributable to interaction (95%CI) and SI (95%CI) were 0.61 (−0.45–1.67), 0.23 (−0.11–0.56) and 1.56 (0.73–3.33), respectively, indicating that the parameters of interaction effect between “TC (−)” and “ALT (+)” were not statistically significant (Pinteraction > 0.05).
Figure 2.
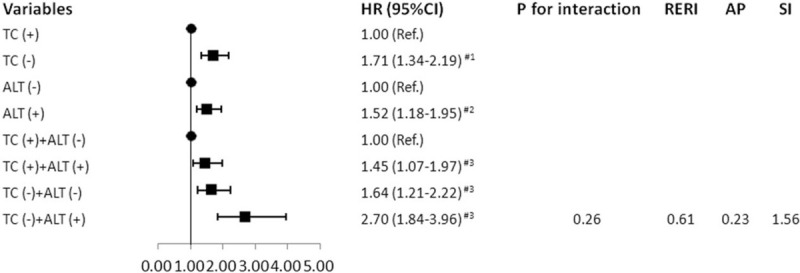
The adjusted HR (95%CI) and interaction terms for PLC in different status of TC and ALT. #3: Adjusted for age, gender, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity; #2: Further adjusted for TC based on #3; #1: Further adjusted for ALT based on #3. AP = proportion of disease attributable to interaction, CI = confidence interval, HR = Hazard ratios, Ref = reference, RERI = relative excess risk due to interaction, SI = synergy index.
3.4. Sensitivity analysis
To further determine the stability of the results, we excluded HBsAg positive participants, participants in cirrhosis, fatty liver participants, participants who took statins, ALT ≥ 40 U/L participants during follow-up and participants who occurred PLC within 1 year after entry to the cohort, respectively. We found that group “TC (−) + ALT (+)” still had a highest risk of PLC events in all models (Table 5). The results of sensitivity analyses concerning the major potential confounders cannot alter the main findings.
Table 5.
Sensitivity analysis of hazard ratios and 95% confidence interval for the risk of LC in groups by TC and ALT status.
| Sensitivity Analysis I | Sensitivity Analysis II | Sensitivity Analysis III | Sensitivity Analysis IV | Sensitivity Analysis V | Sensitivity Analysis VI | |
| TC a alone | ||||||
| TC (+) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (−) | 1.45 (1.06–1.98) | 1.64 (1.27–2.13) | 1.82 (1.37–2.43) | 1.69 (1.32–2.16) | 1.72 (1.32–2.23) | 1.56 (1.19–2.05) |
| ALT b alone | ||||||
| ALT (−) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| ALT (+) | 1.19 (0.87–1.64) | 1.48 (1.14–1.92) | 2.12 (1.61–2.79) | 1.52 (1.18–1.95) | 1.65 (1.27–2.13) | 1.45 (1.11–1.90) |
| Combinations of TC and ALTc | ||||||
| TC (+) + ALT (−) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| TC (+) + ALT (+) | 1.18 (0.81–1.72) | 1.43 (1.04–1.97) | 2.03 (1.45–2.84) | 1.43 (1.06–1.94) | 1.59 (1.17–2.17) | 1.41 (1.03–1.91) |
| TC (−) + ALT (−) | 1.44 (1.00–2.08) | 1.59 (1.16–2.19) | 1.72 (1.18–2.51) | 1.59 (1.17–2.16) | 1.64 (1.17–2.31) | 1.54 (1.12–2.10) |
| TC (−) + ALT (+) | 1.76 (1.03–3.00) | 2.50 (1.66–3.78) | 4.02 (2.66–6.09) | 2.71 (1.85–3.96) | 2.93 (1.99–4.33) | 2.50 (1.68–3.72) |
TC (+): TC ≥4.24 mmol/L, TC (−): TC <4.24 mmol/L; ALT (+): ALT ≥22 U/L, ALT (−): ALT <22 U/L. CI = confidence interval, HR = hazard ratios, Ref = reference.
Sensitivity Analysis I: Excluding HBsAg positive participants, c Adjusted for age, gender, BMI, HDL-C, hs-CRP, TG, hypertension, diabetes, alcohol consumption, smoking and physical activity; a Further adjusted for ALT based on c; b Further adjusted for TC based onc.
Sensitivity Analysis II: Excluding participants in cirrhosis, the adjusted factors are the same asa,b,c.
Sensitivity Analysis III: Excluding fatty liver participants, the adjusted factors are the same asa,b,c.
Sensitivity Analysis IV: Excluding participants who took statins, the adjusted factors are the same asa,b,c.
Sensitivity Analysis V: Excluding ALT ≥40 U/L participants during follow-up, the adjusted factors are the same asa,b,c.
Sensitivity Analysis VI: Excluding participants who occurred liver cancer within 1 year after entry to the cohort, the adjusted factors are the same asa,b,c.
4. Discussion
In this study, we have confirmed previous studies that low TC level is associated with an increased risk of liver cancer. And we also found that normal-high ALT level could increase the risk of PLC. Furthermore, with significantly higher relative risk for PLC would be seen in subject who both keep low TC level and normal-high ALT level.
While the association between low TC level and the risk of liver cancer was rarely reported, our results are basically consistent with the previous studies. A large prospective study including 1,189,719 adults based on NHIC (National Health Insurance Corporation) cohort in Korea had clarified that the inverse association between the concentration of TC and incident liver cancer.[13] Additionally, Tanaka et al.[16] also found that low TC level (TC < 3.59 mmol/L) was significantly inversely associated with the risk of liver cancer (RR = 6.16; 95% CI: 1.39–27.35) based on data of voluntary blood donors in Japan. Our findings agree with this. In our study, we observed that low TC level (TC < 4.24 mmol/L) increased 1.71-fold risk of PLC (HR = 1.71; 95%CI: 1.34–2.19) compared with group non-low TC level. Even after excluding statins in our studies, there were no significant changes in our results (HR = 1.69; 95%CI: 1.32–2.16). Decreased TC concentration is significantly associated with the risk of PLC.
Transaminase has a strong power to predict the risk of liver cancer.[14] Although the JPHC Study (The Japan Public Health Center-based Prospective Study) has confirmed that elevated ALT would increase the risk of liver cancer (HR = 13.5; 95% CI: 8.0–22.0),[30] this slightly different from our research. Indeed, the association between normal-high ALT level and PLC risk is our focus. We found that normal-high ALT level alone increased 1.52-fold risk of PLC (HR = 1.52; 95%CI: 1.18–1.95) compared with normal ALT level after adjustment of potential confounders. This means that ALT in normal range is adverse for development of PLC. And we should paid enough attention to this phenomenon.
More importantly, by using cross-classification method, our study indicated that low TC level and normal-high ALT level have a combined effect on the risk of PLC. After adjusting confounders, we observed that combination of low TC level and normal-high ALT level showed 2.70-fold increased risk of PLC (HR = 2.70; 95%CI: 1.84–3.96) compared with combination of non-low TC level and normal ALT level. Futhermore, the joint effect of these two factors was greater than their separate contribution. To the best of our knowledge, this is the first time to prospectively evaluate the association of joint effect of low TC level and normal-high ALT level with PLC risk to date. These observations actually indicated that low TC level and normal-high ALT level had conjoint impact on PLC risk. This will remind us that in screening for early PLC, besides focusing on chronic liver diseases such as hepatitis, cirrhosis and fatty liver, dyslipidemia and slight increase of transaminase also play roles in PLC risk.
According to prior clinical and epidemiological studies, the development of liver cancer is usually accompanied with liver cirrhosis, and have probably lowered serum cholesterol level before hepatocarcinogenesis.[31] However, in sensitivity analysis, after excluding HBsAg positive participants, participants in cirrhosis, fatty liver participants, participants who used statins, ALT ≥ 40 U/L participants during follow-up, respectively. We found that the results of sensitivity analysis are consistent with the overall results. This could speculate that the roles of low TC level and normal-high ALT level on the risk of PLC may be independent of chronic liver disease. In consideration of the prediagnostic PLC might influence the level of TC or ALT, thus, this result of PLC was slightly attenuated after excluding participants who occurred PLC within 1 year after entry to the cohort.
The mechanisms that low TC level and normal-high ALT level increased the risk of PLC remain uncertain. Omer F et al.[11] have reported that cancer development is associated with modulation of cholesterol homeostasis. Several carcinogenic signals, such as PI3K/AKT/mTOR, RTK/RAS and TP53, play important roles in modulating cholesterol synthesis in tumor cells.[11] Especially TP53, a key tumor suppressor, could affect the development of cancers via modulating cholesterol homeostasis.[32] Furthermore, serum ALT levels were easily available marker of chronic liver inflammation. The OhdG, a parameter of genetic risk for hepatocarcinogenesis, acts as a pro-mutagenic DNA lesion produced by oxygen (hydroxy) radicals.[33–35] Shimoda et al[36] found that the OhdG is positively related to serum ALT levels in patients without liver cancer, and speculated that oxidative DNA damage is produced by chronic liver tissue inflammation, which would increase the risk of genomic alterations causing liver cancer. The high incidence of PLC in male may be related to the interaction between androgen and the hepatitis B virus X protein.[37] The imbalance of cholesterol homeostasis and oxidative DNA damage will accelerate this process.
In fact, the interventions for the development of liver cancer can be achieved. Such as effective therapy or lifestyle changes are available to reduce the incidence and mortality for high-risk individuals. Meanwhile, it should be noted that the association between correct use of cholesterol-lowering drugs and the potential health risks.
Our study has several limitations. Firstly, HCV is a known independent risk factor for PLC.[38] Around 170 million people worldwide were infected with HCV.[39] Our research lacked this information, and the influence of HCV on the risk of PLC cannot be verified. Secondly, in this study, there were still a part of potential unmeasured factors which we did not consider, such as Aflatoxin, dietary habit et al. Thirdly, serum TC and ALT levels fluctuate daily, our study needs to be measured several times to ensure the accuracy of the results. Finally, our data did not differentiate between hepatocellular carcinoma and intrahepatic cholangiocarcinoma, the risk factors of both might be different.
In conclusion, based on Kailuan Study, we have confirmed that low TC level is associated with an increased risk of liver cancer. In addition, the novel evidence was provided that normal-high ALT level is associated with the risk of PLC. And individuals with coexistence of low TC level and normal-high ALT level would significantly increase the risk of PLC. The prediction of disease risk by mismatch should be recommended. The joint effect is beneficial to screening high-risk population for chronic disease, and the effectiveness of it will be further evaluated in the future.
Acknowledgments
We thank the staff and participants of Kailuan study for their important contributions. And we also thank all professors who contributed to this manuscript.
Author contributions
Miaomiao Sun and Siqing Liu conceived and designed the work; Wanchao Wang, Xining Liu and Yiming Wang have performed data acquisition; Miaomiao Sun, Wanchao Wang and Yiming Wang have analyzed the data; Miaomiao Sun and Wanchao Wang wrote the paper; Siqing Liu and Liying Cao reviewed the manuscript. All authors read and approved the final manuscript.
Data curation: Miaomiao Sun, Siqing Liu, Liying Cao, Xining Liu, Yiming Wang.
Formal analysis: Miaomiao Sun, Wanchao Wang, Yiming Wang.
Funding acquisition: Siqing Liu.
Investigation: Wanchao Wang, Xining Liu, Yiming Wang, Haozhe Cui.
Methodology: Miaomiao Sun, Siqing Liu, Haozhe Cui.
Project administration: Siqing Liu.
Software: Miaomiao Sun, Wanchao Wang, Haozhe Cui.
Supervision: Siqing Liu, Liying Cao.
Writing – original draft: Miaomiao Sun, Wanchao Wang.
Writing – review & editing: Siqing Liu, Liying Cao.
Footnotes
Abbreviations: ALT = alanine aminotransferase, BMI = body mass index, CI = confidence intervals, HCV = hepatitis C virus, HDL-C = high-density lipoprotein cholesterol, hs-CRP = hypersensitive C-reactive protein, PLC = primary liver cancer, TC = total cholesterol, TG = triglyceride.
How to cite this article: Sun M, Wang W, Liu X, Wang Y, Cui H, Liu S, Cao L. Total cholesterol, alanine aminotransferase and the risk of primary liver cancer: a population-based prospective study. Medicine. 2021;100:18(e25746).
MS, WW, SL, and LC contributed equally to this work.
This research protocol was approved by the Ethics Committee of Kailuan General Hospital, and it was in compliance with the Declaration of Helsinki. Informed consent was obtained from the participants.
This study was supported by a grant from the Key scientific and technological research program of Health and Family Planning Commission of Hebei Province (No. 20191339).
Trial registration: ChiCTR-TNRC-11001489. Registered August 24, 2011 (retrospectively registered).
The authors have no conflicts of interest to disclose.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Bray F, Center MM, et al. Global cancer statistics [published correction appears in CA Cancer J Clin. 2011 Mar-Apr;61(2):134]. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [4].Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol 2013;10:34–42. [DOI] [PubMed] [Google Scholar]
- [5].Sun CA, Wu DM, Lin CC, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol 2003;157:674–82. [DOI] [PubMed] [Google Scholar]
- [6].Montalto G, Cervello M, Giannitrapani L, et al. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci 2002;963:13–20. [DOI] [PubMed] [Google Scholar]
- [7].Vanni E, Bugianesi E. Obesity and liver cancer. Clin Liver Dis 2014;18:191–203. [DOI] [PubMed] [Google Scholar]
- [8].Feng X, Wang G, Li N, et al. The association between fasting blood glucose and the risk of primary liver cancer in Chinese males: a population-based prospective study. Br J Cancer 2017;117:1405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323–31. [DOI] [PubMed] [Google Scholar]
- [10].Bakiri L, Hamacher R, Graña O, et al. Liver carcinogenesis by FOS-dependent inflammation and cholesterol dysregulation. J Exp Med 2017;214:1387–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kuzu OF, Noory MA, Robertson GP. The role of cholesterol in cancer. Cancer Res 2016;76:2063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iso H, Ikeda A, Inoue M, et al. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer 2009;125:2679–86. [DOI] [PubMed] [Google Scholar]
- [13].Kitahara CM, Berrington de González A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011;29:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wen CP, Lin J, Yang YC, et al. Hepatocellular carcinoma risk prediction model for the general population: the predictive power of transaminases. J Natl Cancer Inst 2012;104:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zelman S, Wang CC. Transaminases in serum and liver correlated with liver cell necrosis in needle aspiration biopsies. Am J Med Sci 1959;237:323–34. [DOI] [PubMed] [Google Scholar]
- [16].Tanaka H, Tsukuma H, Yamano H, et al. Prospective study on the risk of hepatocellular carcinoma among hepatitis C virus-positive blood donors focusing on demographic factors, alanine aminotransferase level at donation and interaction with hepatitis B virus. Int J Cancer 2004;112:1075–80. [DOI] [PubMed] [Google Scholar]
- [17].Suruki R, Hayashi K, Kusumoto K, et al. Alanine aminotransferase level as a predictor of hepatitis C virus-associated hepatocellular carcinoma incidence in a community-based population in Japan. Int J Cancer 2006;119:192–5. [DOI] [PubMed] [Google Scholar]
- [18].Wu SL, Jin C, Li SS, et al. Aging, arterial stiffness, and blood pressure association in Chinese adults. Hypertension 2019;73:893–9. [DOI] [PubMed] [Google Scholar]
- [19].Wu SL, Huang ZR, Yang XC, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes 2012;5:487–93. [DOI] [PubMed] [Google Scholar]
- [20].Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18–35. [DOI] [PubMed] [Google Scholar]
- [21].Jin C, Chen S, Vaidya A, et al. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care 2017;40:1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang S, Li J, Shearer GC, et al. Longitudinal study of alcohol consumption and HDL concentrations: a community-based study. Am J Clin Nutr 2017;105:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu T, Wang W, Ji Y, et al. Association between different combination of measures for obesity and new-onset gallstone disease. PLoS One 2018;13:e0196457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tchelepi H, Ralls PW, Radin R, et al. Sonography of diffuse liver disease. J Ultrasound Med 2002;21:1023–34. [DOI] [PubMed] [Google Scholar]
- [25].Di Lelio A, Cestari C, Lomazzi A, et al. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989;172:389–92. [DOI] [PubMed] [Google Scholar]
- [26].Cohen HW, Hailpern SM, Alderman MH. Glucose-cholesterol interaction magnifies coronary heart disease risk for hypertensive patients. Hypertension 2004;43:983–7. [DOI] [PubMed] [Google Scholar]
- [27].Cohen HW, Sloop GD. PDAY Study. Glucose interaction magnifies atherosclerotic risk from cholesterol. Findings from the PDAY Study. Atherosclerosis 2004;172:115–20. [DOI] [PubMed] [Google Scholar]
- [28].Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17:227–36. [DOI] [PubMed] [Google Scholar]
- [29].Li CI, Chen HJ, Lai HC, et al. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes--National cohort of Taiwan Diabetes Study. Int J Cancer 2015;136:2668–79. [DOI] [PubMed] [Google Scholar]
- [30].Ishiguro S, Inoue M, Tanaka Y, et al. Serum aminotransferase level and the risk of hepatocellular carcinoma: a population-based cohort study in Japan. Eur J Cancer Prev 2009;18:26–32. [DOI] [PubMed] [Google Scholar]
- [31].Cicognani C, Malavolti M, Morselli-Labate AM, et al. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med 1997;157:792–6. [PubMed] [Google Scholar]
- [32].Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 2008;9:125–38. [DOI] [PubMed] [Google Scholar]
- [33].Kasai H, Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res 1984;12:2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991;349:431–4. [DOI] [PubMed] [Google Scholar]
- [35].Shiota G, Maeta Y, Mukoyama T, et al. Effects of Sho-Saiko-to on hepatocarcinogenesis and 8-hydroxy-2’-deoxyguanosine formation. Hepatology 2002;35:1125–33. [DOI] [PubMed] [Google Scholar]
- [36].Shimoda R, Nagashima M, Sakamoto M, et al. Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res 1994;54:3171–2. [PubMed] [Google Scholar]
- [37].Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology 2010;78: Suppl 1: 172–9. [DOI] [PubMed] [Google Scholar]
- [38].Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol 2010;28:4587–93. [DOI] [PubMed] [Google Scholar]
- [39].Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41–52. [DOI] [PubMed] [Google Scholar]


