Abstract
Coronavirus disease-2019 (COVID-19) pneumonia is one of the severe and most dreaded forms of illness caused by severe acute respiratory syndrome coronavirus 2. It often progresses to respiratory failure and acute respiratory distress syndrome (ARDS) requiring mechanical ventilation. ARDS can lead to multiple complications while on mechanical ventilation due to positive airway pressures in a fibrotic lung, one such complication is the development of alveolopleural fistula. Alveolopleural fistula has high morbidity and mortality. We used endobronchial valve in a patient with COVID-19-related ARDS with persistent air leak (alveolopleural fistula), which allowed us to remove the chest tube and wean the patient successfully off mechanical ventilation.
KEY WORDS: Alveopleural fistula, acute respiratory distress syndrome, bronchopleural fistula, COVID-19, endobronchial valves, SARS-CoV-2
INTRODUCTION
Coronavirus disease-2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It can affect any organ and has wide range of manifestations but respiratory system is involved in majority of the patients. In some patients, it can lead to severe pneumonia followed by acute respiratory distress syndrome (ARDS).[1,2]
We report a patient with COVID-19 pneumonia leading to ARDS and subsequent development of an alveolopleural fistula who was successfully treated with multiple endobronchial valves (EBVs).
CASE REPORT
The patient was a 55-year-old hispanic male with no prior past medical history who presented with 2 days of dry cough and shortness of breath. Vital signs on admission showed an oxygen saturation of 68% on ambient air, respiratory rate of 30 breaths per min, heart rate of 95 beats per min with a temperature of 36.7°C. On physical examination, he was alert and oriented, in moderate respiratory distress with rales and mild expiratory wheezes, he was tachycardic in sinus rhythm, soft abdomen, and anxious mood. He was admitted with acute hypoxic respiratory failure. He was tested for SARS-Cov2 on admission and was found to be positive.
Initial computed tomography (CT) chest without contrast showed diffuse bilateral ground-glass opacities [Figure 1]. His oxygen requirement increased as well as his work of breathing requiring Bilevel Positive Airway Pressure, and was transferred to the medical intensive care unit (ICU) 1 day after admission requiring intubation. His inflammatory markers were elevated, with C-reactive protein 183 mg/L, ferritin 1723 ng/ml, lactate dehydrogenase 993 IU/L, and D-dimer >34 mg/L. He received 2 doses of tocilizumab, as well as a 5-day course of Remdesivir. He had worsening oxygenation, significant coughing paroxysms, and ventilator asynchrony, with PF ratio of 90, requiring high-dose sedation and neuromuscular blockade, as well as prone positioning. On day 4, he developed hemoptysis with >200 mL of bright red blood, and emergent bedside bronchoscopy was completed, which noted normal bronchial mucosa which do not appear to be friable or inflamed, no endobronchial lesions, and no active bleeding. On the 10th day of admission, he developed a right-sided pneumothorax, requiring chest tube placement, likely secondary to ongoing severe ARDS, and lung infection with Stenotrophomonas maltophilia. A tracheostomy was completed on day 14 for further ventilator weaning. On day 20, he developed persistent air leak concerning for a alveolopleural fistula, repeat CT chest concerning for a moderate-sized pneumothorax and findings concerning for post ARDS fibrotic lungs (particularly on the right side) [Figure 1]. He had difficulty with tracheostomy collar trial secondary to severe cough paroxysms. He continued to have a loculated pneumothorax despite chest tube, with persistent alveolopleural fistula. On day 34, he had a repeat chest CT scan small-to-medium size right pneumothorax with well-positioned chest tube in place. After interdisciplinary conference with cardiothoracic surgery, pulmonary, and the ICU team, it was decided that patient had fibrotic right lower and middle lobe from ARDS and the leak is from multiple lobe. He was not deemed to be a surgical candidate hence interventional pulmonology was consulted for EBV placement to facilitate chest tube removal and ventilator weaning.
Figure 1.
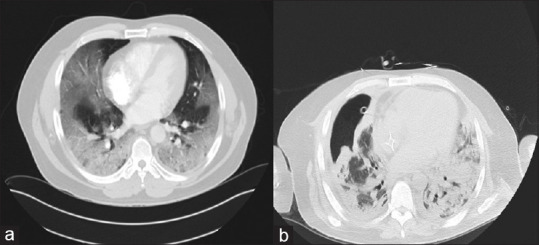
Computed tomography chest: On the day of admission (a) and 4 weeks later (b)
Procedure details
Bronchoscopy was done on day 41 of admission. Pulmonary balloon was used to sequentially block the right mainstem, bronchus intermedius, and basilar segments. The air leak was recognized to be coming from right middle lobe (RML) and right lower lobe (RLL). Sequential EBV placement was planned to make sure the patient does not become hypoxic from multiple lobar blocks (RLL and RML), although both lobes were fibrotic on the CT scan. On day 41, he had a total 4 Spiration EBV placed. One each in RML lateral segment (9 mm), RML medial segment (9 mm), RLL superior segment (9 mm) and RLL medial basilar segment (7 mm) [Figures 2 and 3]. The air leak improved significantly but still had small leak. Bronchoscopy was repeated 4 days later, and two additional Spiration EBVs were placed, 9 mm each, one in anterolateral and another in posterior basilar segment. Over next few days, his leak completely resolved. The patient was weaned off of positive pressure a week later to trach collar, and the chest tube was subsequently removed. A week later, he was completely liberated from ventilator, tracheostomy tube was downsized and eventually decannulated. He was transferred out of the ICU to long-term acute care for rehabilitation, and then ultimately was discharged to home. Three months later, he followed up in the pulmonary clinic. His tracheostomy site was completely healed. He was able to walk without shortness of breath, saturating 96 on ambient air and 91% on 6-min walk test. Repeat imaging showed hydropneumothorax which was an expected finding.
Figure 2.
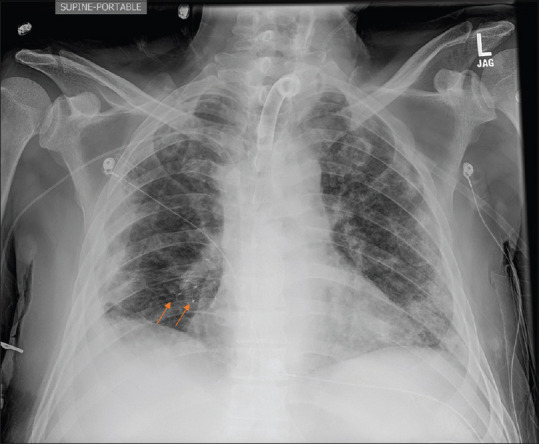
Arrows showing endobronchial valves
Figure 3.
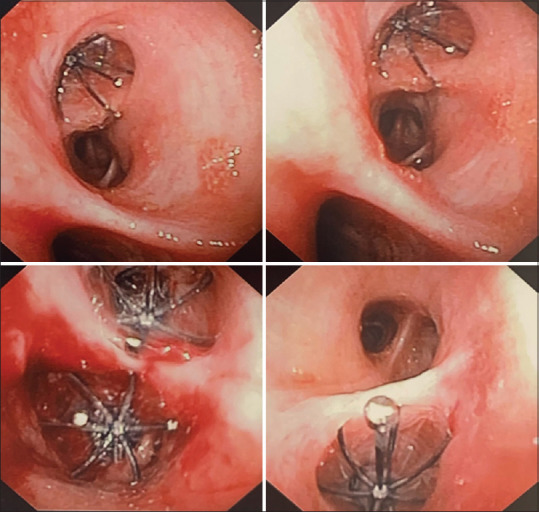
Bronchoscopic view of endobronchial valves
DISCUSSION
Alveolopleural fistula is a communication or fistula between an alveoli and the pleural space. We called it alveolopleural fistula instead of bronchopleural fistula because the patient had lung parenchyma necrosis followed by fistula development; in addition, the fistula was distal to the segmental bronchi.[3,4] Patient's with ARDS secondary to COVID-19 requiring high amounts of positive end expiratory pressure (PEEP) and are at higher risk in developing a pneumothorax. Parenchymal necrosis followed by fibrotic lung can increase the risk when patient is on positive pressure ventilation for long time. Many patients with a prolonged ventilator course are at risk of developing a secondary bacterial infection, which also increases the risk of pneumothorax and bronchopleural and alveolopleural fistula.[5] Significant coughing paroxysms add additional risk of pneumothorax. Positive pressure and higher amounts of PEEP can make it challenging to heal a pneumothorax, allowing complications of alveolopleural fistula to arise.
EBVs have been used since 2005 to treat alveolopleural and bronchopleural fistula in patients who are not considered a good surgical candidates.[6] EBV can treat these fistulas by allowing air to exit through the valve but not enter the lower segment. This is the first documented use of an EBV in the setting of COVID-19 that we could find. The placement of the valves, allowed a significant reduction in the air leak. This assisted in the patient's breathing trials on the ventilator and tracheostomy collar trials by reducing the overall volume loss through the fistula, ultimately allowing the patient to successfully liberated from the ventilator and have his chest tubes removed.
CONCLUSION
EBV can safely be used in patients with alveolopleural fistula secondary to COVID-19 ARDS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bos LD. COVID-19-related acute respiratory distress syndrome: Not so atypical. Am J Respir Crit Care Med. 2020;202:622–4. doi: 10.1164/rccm.202004-1423LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos LD, Paulus F, Vlaar AP, Beenen LF, Schultz MJ. Subphenotyping acute respiratory distress syndrome in patients with COVID-19: Consequences for ventilator management. Ann Am Thorac Soc. 2020;17:1161–3. doi: 10.1513/AnnalsATS.202004-376RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feller-Kopman D, Bechara R, Garland R, Ernst A, Ashiku S. Use of a removable endobronchial valve for the treatment of bronchopleural fistula. Chest. 2006;130:273–5. doi: 10.1378/chest.130.1.273. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Agarwal R. Bronchopleural fistula or alveolopleural fistula? Not just semantics. Chest. 2006;130:1948. doi: 10.1378/chest.130.6.1948. [DOI] [PubMed] [Google Scholar]
- 5.Salik I, Vashisht R, Abramowicz AE. Bronchopleural Fistula. Treasure Island, FL: In Statpearls; 2020. [PubMed] [Google Scholar]
- 6.Gaspard D, Bartter T, Boujaoude Z, Raja H, Arya R, Meena N, et al. Endobronchial valves for bronchopleural fistula: Pitfalls and principles. Ther Adv Respir Dis. 2017;11:3–8. doi: 10.1177/1753465816672132. [DOI] [PMC free article] [PubMed] [Google Scholar]


