Abstract
Background
Tuberculosis (TB) presents a global threat in the world and the lung is the frequent site of metastatic focus. A previous study demonstrated that TB might increase primary lung cancer risk by two-fold for more than 20 years after the TB diagnosis. However, no large-scale study has evaluated the risk of TB and secondary lung cancer. Thus, we evaluated the risk of secondary lung cancer in patients with or without tuberculosis (TB) using a nationwide population-based dataset.
Methods
In a cohort study of 1,936,512 individuals, we selected 6934 patients among patients with primary cancer and TB infection, based on the International Classification of Disease (ICD-p-CM) codes 010–011 from 2000 to 2015. The control cohort comprised 13,868 randomly selected, propensity-matched patients (by age, gender, and index date) without TB exposure. Using this adjusted date, a possible association between TB and the risk of developing secondary lung cancer was estimated using a Cox proportional hazards regression model.
Results
During the follow-up period, secondary lung cancer was diagnosed in 761 (10.97%) patients with TB and 1263 (9.11%) patients without TB. After adjusting for covariates, the risk of secondary lung cancer was 1.67 times greater among primary cancer in the cohort with TB than in the cohort without TB. Stratification revealed that every comorbidity (including diabetes, hypertension, cirrhosis, congestive heart failure, cardiovascular accident, chronic kidney disease, chronic obstructive pulmonary disease) significantly increased the risk of secondary lung cancer when comparing the TB cohort with the non-TB cohort. Moreover, the primary cancer types (including head and neck, colorectal cancer, soft tissue sarcoma, breast, kidney, and thyroid cancer) had a more significant risk of becoming secondary lung cancer.
Conclusion
A significant association exists between TB and the subsequent risk for metastasis among primary cancers and comorbidities. Therefore, TB patients should be evaluated for the subsequent risk of secondary lung cancer.
Introduction
Tuberculosis (TB) presents a global threat in both developing and developed countries. TB is caused by bacteria (Mycobacterium tuberculosis) and most often affects the lungs. According to the World Health Organization, 10 million people become ill with TB annually. Despite being a preventable and curable disease, 1.5 million people die from TB each year–making it the world’s top infectious killer [1].
The lung is the frequent site of metastatic focus. About 20% to 54% of malignant tumors developing elsewhere in our body have pulmonary metastasis [2, 3]. This is so-called secondary lung cancer when cancer cells have spread to the lungs from cancer that started elsewhere in the body. It is also called metastatic cancer to the lungs and differs from the definition of primary lung cancer that has originated in the lungs. A previous study demonstrated that TB might increase primary lung cancer risk by two-fold for more than 20 years after the TB diagnosis [4]. However, no large-scale study has evaluated the risk of TB and secondary lung cancer. Thus, a nationwide, population-based, matched cohort study is needed to clarify the association between TB infection and secondary lung cancer. Furthermore, we have conducted this study to investigate whether comorbidities could attenuate the risk of developing secondary lung cancer after TB infection.
Material and methods
Data source and ethics statement
The National Health Insurance (NHI) program began in Taiwan in 1995 and covers more than 99% of the entire population (or more than 23 million people). The data for this study were collected from the NHI Research Database (NHIRD) of Taiwan, which uses the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to record diagnoses. Therefore, we used the NHIRD inpatient and outpatient databases and the Registry of Beneficiaries. Patient confidentiality was ensured by double-encrypted identifiers in the NHIRD. The Institutional Review Board of Tri-Service General Hospital approved this study (TSGHIRB No.B-109-44), and the committee waived the need for written informed consent.
Study design and population
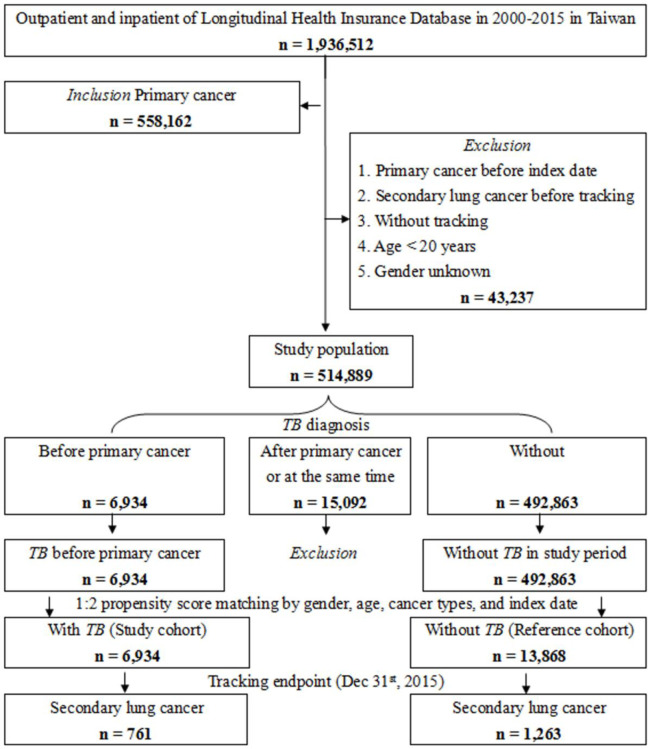
The study design and specific patient characteristics, including inclusion and exclusion criteria, are shown in Fig 1. The control cohort (non-TB patients) was randomly matched with TB patients according to age, sex, and index date (two controls for each TB patient) using the same exclusion criteria. The study cohort included 514,889 patients aged ≥20 years who had been diagnosed with cancer except for lung origin (ICD-9-CM codes in S1 Table) from 2000 to 2015. The index date was designated as the first clinical visit for primary cancer. The exclusion criteria were: diagnosis with primary cancer before 2000, secondary lung cancer before tracking, patients without tracking, age <20 years, and unknown gender. The ratio of primary cancer patients with TB to patients without TB in the study period was maintained at 1:2 to enhance the power of the statistical tests employed, particularly regarding the stratification analysis. Using these criteria, 6934 patients with TB infection and 13,868 patients without TB infection were identified.
Fig 1. Flowchart of study sample selection.
Covariates
We examined the sociodemographic factors in the case and control groups, such as age, monthly income, comorbidity, urbanization level, and hospital level. The patients were divided into three groups: 20–44 years, 45–69 years, and ≥70 years. Their monthly income in New Taiwan Dollars was divided into three groups: <18,000, 18,000–34,999, and ≥35,000. Seven comorbidities (ICD codes as in S1 Table), such as diabetes (DM)、hypertension (HTN)、cirrhosis、congestive heart failure (CHF)、cardiovascular accident (CVA)、chronic kidney disease (CKD)、and chronic obstructive pulmonary disease (COPD), were also considered into our study (using ICD9 codes in S1 Table). The patients were categorized into four urbanization levels. The three hospital levels where patients sought medical attention were also considered: medical centers, regional hospitals, and local hospitals.
Study outcome
All study participants were followed from the index date until the onset of secondary lung cancer (ICD-9-CM codes: 197.0), withdrawal from the NHI program, or the end of 2015. The covariates included were included those mentioned previously.
Statistical analysis
We performed all analyses using SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA). χ2 and t-tests were used to evaluate the distribution of categorical and continuous variables, respectively. Fisher’s exact test was used for categorical variables to examine the statistical differences between the two cohorts. The multivariate Cox proportional hazards regression analysis was used to determine the risk of secondary lung cancer. The results were presented as a hazard ratio with a 95% confidence interval (CI). The difference in the risk of secondary lung cancer between TB-infected subjects and control groups was estimated using the Kaplan-Meier method with the log-rank test. A two-tailed p-value < .05 was considered statistically significant.
From 2000 to 2015, a total of 558,162 patients with primary cancer were enrolled in this study in accordance with our inclusion criteria. Secondary lung cancer was observed in 761 of 6934 TB-infected patients, and in 1263 of 13,868 non-TB-infected patients. Table 1 lists demographic characteristics and comorbidities of the TB (6934) and non-TB cohorts (13,868) during this time. In both cohorts, approximately 90% were older than 45 years of age, 72% were male, and the proportion by age and sex were similar. All comorbidities, except CVA, were significantly different in the TB cohort than in the endpoint non-TB cohort: DM (17.41% vs 13.36%; p < .001), HTN (16.33% vs 18.73%; p < .001), cirrhosis (4.12% vs 2.40%; p < .001), CHF (3.87% vs 2.63%; p < .001), CKD (8.48% vs 5.70%; p < .001), and COPD (11.52% vs 4.25%; p < .001). The TB cohorts had a higher proportion of individuals living at the lowest urbanization level city (19.12% vs 14.08%; p < .001) and call for treatment in local hospital (15.10% vs 11.85%; p < .001).
Table 1. Demographic characteristics and comorbidities of study participants.
| TB | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Overall | 20,802 | 6934 | 33.33 | 13,868 | 66.67 | ||
| Secondary lung cancer | < .001 | ||||||
| Without | 18,778 | 90.27 | 6173 | 89.03 | 12,605 | 90.89 | |
| With | 2024 | 9.73 | 761 | 10.97 | 1263 | 9.11 | |
| Gender | .999 | ||||||
| Male | 15,069 | 72.44 | 5023 | 72.44 | 10,046 | 72.44 | |
| Female | 5733 | 27.56 | 1911 | 27.56 | 3,822 | 27.56 | |
| Age (yrs) | 67.50 ± 14.19 | 67.05 ± 13.63 | 67.73 ± 14.46 | < .001 | |||
| Age groups (yrs) | < .001 | ||||||
| 20–44 | 1713 | 8.23 | 473 | 6.82 | 1240 | 8.94 | |
| 45–69 | 9577 | 46.04 | 3125 | 45.07 | 6452 | 46.52 | |
| ≧70 | 9512 | 45.73 | 3336 | 48.11 | 6,176 | 44.53 | |
| Insurance premium (NT$) | .936 | ||||||
| <18,000 | 20,450 | 98.31 | 6818 | 98.33 | 13,632 | 98.30 | |
| 18,000–34,999 | 291 | 1.40 | 97 | 1.40 | 194 | 1.40 | |
| ≧35,000 | 61 | 0.29 | 19 | 0.27 | 42 | 0.30 | |
| DM | < .001 | ||||||
| Without | 17,742 | 85.29 | 5727 | 82.59 | 12,015 | 86.64 | |
| With | 3060 | 14.71 | 1207 | 17.41 | 1853 | 13.36 | |
| HTN | < .001 | ||||||
| Without | 17,072 | 82.07 | 5802 | 83.67 | 11,270 | 81.27 | |
| With | 3730 | 17.93 | 1132 | 16.33 | 2598 | 18.73 | |
| Cirrhosis | < .001 | ||||||
| Without | 20,183 | 97.02 | 6648 | 95.88 | 13,535 | 97.60 | |
| With | 619 | 2.98 | 286 | 4.12 | 333 | 2.40 | |
| CHF | < .001 | ||||||
| Without | 20,169 | 96.96 | 6666 | 96.13 | 13,503 | 97.37 | |
| With | 633 | 3.04 | 268 | 3.87 | 365 | 2.63 | |
| CVA | .629 | ||||||
| Without | 19,807 | 95.22 | 6608 | 95.30 | 13,199 | 95.18 | |
| With | 995 | 4.78 | 326 | 4.70 | 669 | 4.82 | |
| CKD | < .001 | ||||||
| Without | 19,423 | 93.37 | 6346 | 91.52 | 13,077 | 94.30 | |
| With | 1379 | 6.63 | 588 | 8.48 | 791 | 5.70 | |
| COPD | < .001 | ||||||
| Without | 19,413 | 93.32 | 6135 | 88.48 | 13,278 | 95.75 | |
| With | 1389 | 6.68 | 799 | 11.52 | 590 | 4.25 | |
| Urbanization level | < .001 | ||||||
| 1 (The highest) | 7125 | 34.25 | 2183 | 31.48 | 4942 | 35.64 | |
| 2 | 9382 | 45.10 | 3067 | 44.23 | 6315 | 45.54 | |
| 3 | 1017 | 4.89 | 358 | 5.16 | 659 | 4.75 | |
| 4 (The lowest) | 3278 | 15.76 | 1326 | 19.12 | 1952 | 14.08 | |
| Level of care | < .001 | ||||||
| Hospital center | 9793 | 47.08 | 2898 | 41.79 | 6895 | 49.72 | |
| Regional hospital | 8318 | 39.99 | 2989 | 43.11 | 5329 | 38.43 | |
| Local hospital | 2691 | 12.94 | 1047 | 15.10 | 1,644 | 11.85 |
P: Chi-square / Fisher’s exact test for categorical variables and t-test for continuous variables
Secondary lung cancer incidence and risk
Table 2 presents factors of secondary lung cancer using Cox regression and Fine & Gray’s competing risk model. According to our study, the risk of secondary lung cancer was 1.671 times greater in the TB cohort than in the non-TB cohort (aHR = 1.671; 95% CI = 1.525–1.832; p < .001) after adjusting for gender, age, insurance premium, related comorbidities, urbanization level, and level of care. All comorbidities were significantly higher in the TB cohort than in the non-TB cohort: DM (aHR = 1.472; 95% CI = 1.271–1.705; p < .001), HTN (aHR = 2.318; 95% CI = 1.993–2.696; p < .001), cirrhosis (aHR = 1.334; 95% CI = 1.017–1.750; p = .038), CHF (aHR = 3.017; 95% CI = 1.979–4.600; p < .001), CVA (aHR = 3.866; 95% CI = 2.676–5.584; p < .001), CKD (aHR = 2.562; 95% CI = 1.990–3.298; p < .001), and COPD (aHR = 2.238; 95% CI = 1.770–2.830; p < .001). Compared with the local hospital, the risk of secondary lung cancer was higher in the medical center (aHR = 2.332; 95% CI = 1.926–2.823; p < .001) and in the regional hospital (aHR = 1.728; 95% CI = 1.443–2.070; p < .001).
Table 2. Factors of secondary lung cancer using Cox regression and Fine & Gray’s competing risk model.
| Model | Competing risk in the model | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Crude HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
| TB | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.478 | 1.350 | 1.618 | < .001 | 1.671 | 1.525 | 1.832 | < .001 |
| Gender | ||||||||
| Male | 1.083 | 0.983 | 1.194 | .108 | 1.088 | 0.986 | 1.201 | .093 |
| Female | Reference | Reference | ||||||
| Age groups (yrs) | ||||||||
| 20–44 | Reference | Reference | ||||||
| 45–69 | 1.004 | 0.664 | 1.124 | .074 | 1.088 | 0.986 | 1.201 | .093 |
| ≧70 | 1.018 | 0.865 | 1.197 | .833 | 1.109 | 0.942 | 1.306 | .215 |
| Insured premium (NT$) | ||||||||
| <18,000 | Reference | Reference | ||||||
| 18,000–34,999 | 1.096 | 0.528 | 1.201 | .278 | 1.073 | 0.512 | 1.166 | .220 |
| ≧35,000 | 1.512 | 0.755 | 3.027 | .244 | 1.352 | 0.674 | 2.712 | .396 |
| DM | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.849 | 1.601 | 2.136 | < .001 | 1.472 | 1.271 | 1.705 | < .001 |
| HTN | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.638 | 2.277 | 3.057 | < .001 | 2.318 | 1.993 | 2.696 | < .001 |
| Cirrhosis | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.127 | 0.860 | 1.478 | .385 | 1.334 | 1.017 | 1.750 | .038 |
| CHF | ||||||||
| Without | Reference | Reference | ||||||
| With | 3.533 | 2.320 | 5.380 | < .001 | 3.017 | 1.979 | 4.600 | < .001 |
| CVA | ||||||||
| Without | Reference | Reference | ||||||
| With | 4.848 | 3.360 | 6.995 | < .001 | 3.866 | 2.676 | 5.584 | < .001 |
| CKD | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.463 | 1.915 | 3.168 | < .001 | 2.562 | 1.990 | 3.298 | < .001 |
| COPD | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.249 | 1.786 | 2.833 | < .001 | 2.238 | 1.770 | 2.830 | < .001 |
| Urbanization level | ||||||||
| 1 (The highest) | 0.779 | 0.600 | 1.011 | .061 | 0.797 | 0.614 | 1.034 | .088 |
| 2 | 0.859 | 0.702 | 1.439 | .091 | 0.881 | 0.749 | 1.037 | .127 |
| 3 | 0.938 | 0.779 | 1.474 | .300 | 0.957 | 0.826 | 1.109 | .559 |
| 4 (The lowest) | Reference | Reference | ||||||
| Level of care | ||||||||
| Hospital center | 2.465 | 2.071 | 2.933 | < .001 | 2.332 | 1.926 | 2.823 | < .001 |
| Regional hospital | 1.814 | 1.518 | 2.167 | < .001 | 1.728 | 1.443 | 2.070 | < .001 |
| Local hospital | Reference | Reference | ||||||
HR = Hazard Ratio, CI = Confidence Interval, Adjusted HR: Adjusted variables listed in the Table
P: Chi-square/Fisher’s exact test for categorical variables and t-test for continuous variables
CI: Confidence Interval
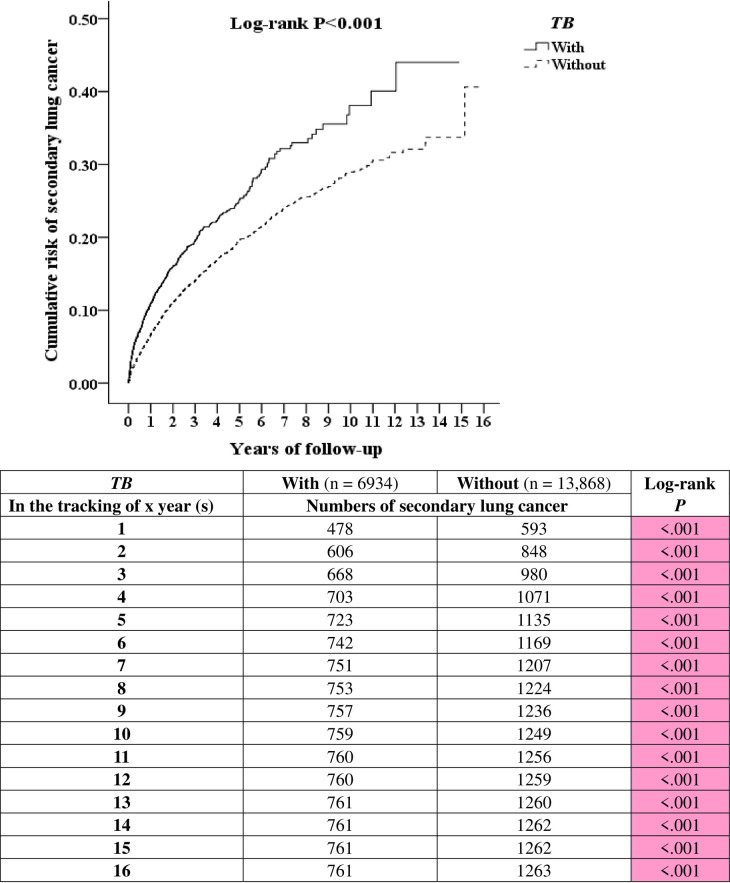
Fig 2 compares the Kaplan-Meier curves for the cumulative incidence of secondary lung cancer between the TB and non-TB cohorts after 16 years of follow-up. The 1-, 5-, 11-, and 15-year actuarial rates of secondary lung cancer were 6.89%, 10.42%, 10.96%, and 10.97% in the TB cohort and 4.27%, 8.18%, 9.05%, and 9.10% in the non-TB cohort, respectively. This geography revealed that TB-infected patients had a significantly higher risk of developing secondary lung cancer than non-TB patients among primary cancer patients, even in the first year of tracking.
Fig 2. Kaplan-Meier for cumulative risk of secondary lung cancer among primary cancer patients aged 20 and over stratified by TB with the log-rank test.
We found that 761 (10.97%) TB cohort members progressed to secondary lung cancer with 57,340 person-years of follow-up over 16 years, for an incidence rate of 1327 per 100,000 person-years. Conversely, only 1263 (9.10%) of the non-TB cohort members progressed to secondary lung cancer over the 124,884 person-years of follow-up for 16 years, for an incidence rate of 1011 per 100,000 person-years. Therefore, the incidence rate of osteoporosis was 1.671-fold higher in the TB cohort than in the non-TB cohort.
Table 3 shows the factors of secondary lung stratified by the variables listed using Cox regression and Fine & Gray’s competing risk model. All the factors show a significantly higher risk in TB-infected patients at every stratified level than in non-TB-infected patients.
Table 3. Factors of secondary lung cancer stratified by variables listed using Cox regression and Fine & Gray’s competing risk model.
| TB | With | Without (Reference) | Competing risk in the model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | P |
| Overall | 761 | 57,340.90 | 1327.15 | 1263 | 124,884.91 | 1011.33 | 1.671 | 1.525 | 1.832 | < .001 |
| Gender | ||||||||||
| Male | 537 | 39,444.69 | 1361.40 | 908 | 89,000.07 | 1020.22 | 1.699 | 1.550 | 1.863 | < .001 |
| Female | 224 | 17,896.21 | 1251.66 | 355 | 35,884.84 | 989.28 | 1.611 | 1.470 | 1.766 | < .001 |
| Age group (yrs) | ||||||||||
| 20–44 | 57 | 3026.89 | 1883.12 | 134 | 8816.02 | 1519.96 | 1.578 | 1.439 | 1.729 | < .001 |
| 45–69 | 314 | 22,904.34 | 1370.92 | 559 | 52,083.65 | 1073.27 | 1.627 | 1.484 | 1.783 | < .001 |
| ≧70 | 390 | 31,409.67 | 1241.66 | 570 | 63,985.24 | 890.83 | 1.775 | 1.619 | 1.946 | < .001 |
| Insured premium (NT$) | ||||||||||
| <18,000 | 746 | 56,337.98 | 1324.15 | 1247 | 122,847.46 | 1015.08 | 1.661 | 1.516 | 1.821 | < .001 |
| 18,000–34,999 | 11 | 865.00 | 1271.67 | 12 | 1715.19 | 699.63 | 2.315 | 2.112 | 2.537 | < .001 |
| ≧35,000 | 4 | 137.92 | 2900.30 | 4 | 322.26 | 1241.22 | 2.976 | 2.715 | 3.262 | < .001 |
| DM | ||||||||||
| Without | 663 | 45,624.00 | 1453.18 | 1,144 | 104,122.51 | 1098.71 | 1.684 | 1.537 | 1.846 | < .001 |
| With | 98 | 11,716.90 | 836.40 | 119 | 20,762.40 | 573.15 | 1.858 | 1.695 | 2.037 | < .001 |
| HTN | ||||||||||
| Without | 687 | 44,820.23 | 1532.79 | 1,139 | 96,117.53 | 1185.01 | 1.647 | 1.503 | 1.806 | < .001 |
| With | 74 | 12,520.67 | 591.02 | 124 | 28,767.38 | 431.04 | 1.746 | 1.593 | 1.914 | < .001 |
| Cirrhosis | ||||||||||
| Without | 731 | 55,081.69 | 1327.12 | 1,239 | 121,468.64 | 1020.02 | 1.657 | 1.512 | 1.816 | < .001 |
| With | 30 | 2259.21 | 1327.90 | 24 | 3416.27 | 702.52 | 2.407 | 2.196 | 2.638 | < .001 |
| CHF | ||||||||||
| Without | 748 | 54,419.08 | 1374.52 | 1,254 | 119,772.93 | 1046.98 | 1.672 | 1.525 | 1.833 | < .001 |
| With | 13 | 2921.82 | 444.93 | 9 | 5111.98 | 176.06 | 3.218 | 2.936 | 3.528 | < .001 |
| CVA | ||||||||||
| Without | 746 | 53,561.46 | 1392.79 | 1244 | 115,641.45 | 1075.74 | 1.649 | 1.504 | 1.807 | < .001 |
| With | 15 | 3779.44 | 396.88 | 19 | 9243.46 | 205.55 | 2.459 | 2.243 | 2.695 | < .001 |
| CKD | ||||||||||
| Without | 723 | 51,376.75 | 1407.25 | 1228 | 115,637.41 | 1061.94 | 1.688 | 1.540 | 1.850 | < .001 |
| With | 38 | 5964.16 | 637.14 | 35 | 9247.50 | 378.48 | 2.144 | 1.956 | 2.350 | < .001 |
| COPD | ||||||||||
| Without | 711 | 49,078.54 | 1448.70 | 1238 | 117,246.40 | 1055.90 | 1.747 | 1.594 | 1.915 | < .001 |
| With | 50 | 8262.37 | 605.15 | 25 | 7638.51 | 327.29 | 2.355 | 2.148 | 2.581 | < .001 |
| Pneumoconiosis | ||||||||||
| Without | 759 | 56,747.49 | 1337.50 | 1,263 | 124,776.18 | 1012.21 | 1.683 | 1.535 | 1.844 | < .001 |
| With | 2 | 593.41 | 337.04 | 0 | 108.73 | 0.00 | ∞ | - | - | .970 |
| Sarcoidosis | ||||||||||
| Without | 761 | 57,340.90 | 1327.15 | 1263 | 124,877.32 | 1011.39 | 1.671 | 1.524 | 1.832 | < .001 |
| With | 0 | 0.00 | - | 0 | 7.59 | 0.00 | - | - | - | - |
| HIV | ||||||||||
| Without | 759 | 57,233.06 | 1326.16 | 1263 | 124,864.57 | 1011.50 | 1.670 | 1.523 | 1.830 | < .001 |
| With | 2 | 107.85 | 1854.49 | 0 | 20.34 | 0.00 | ∞ | - | - | .990 |
| Urbanization level | ||||||||||
| 1 (The highest) | 239 | 17,642.70 | 1354.67 | 460 | 42,581.05 | 1080.29 | 1.597 | 1.457 | 1.750 | < .001 |
| 2 | 348 | 24,791.59 | 1403.70 | 608 | 57,101.20 | 1,064.78 | 1.679 | 1.532 | 1.840 | < .001 |
| 3 | 30 | 3228.48 | 929.23 | 41 | 6408.10 | 639.82 | 1.849 | 1.687 | 2.027 | < .001 |
| 4 (The lowest) | 144 | 11,678.13 | 1,233.07 | 154 | 18,794.56 | 819.39 | 1.916 | 1.748 | 2.101 | < .001 |
| Level of care | ||||||||||
| Hospital center | 379 | 22,314.91 | 1698.42 | 694 | 55,941.34 | 1240.59 | 1.743 | 1.591 | 1.911 | < .001 |
| Regional hospital | 316 | 25,700.37 | 1229.55 | 471 | 51,400.65 | 916.33 | 1.709 | 1.559 | 1.873 | < .001 |
| Local hospital | 66 | 9325.62 | 707.73 | 98 | 17,542.91 | 558.63 | 1.613 | 1.472 | 1.768 | < .001 |
HR = Hazard Ratio, CI = Confidence Interval, Adjusted HR: Adjusted variables listed in the table
P: Chi-square/Fisher’s exact test for categoricaly variables and t-test for continuous variables
CI: Confidence Interval
PYs: Person-years
Table 4 shows the factors of secondary lung cancer among different primary cancer types. The primary cancer types, including head and neck, colorectal, soft tissue sarcoma, breast, kidney, and thyroid cancer, have a significantly higher risk of developing secondary lung cancer in TB-infected patients. Nevertheless, bone, melanoma, and testicular cancer show no difference.
Table 4. Factors of secondary lung cancer among different primary cancer types using Cox regression and Fine & Gray’s competing risk model.
| TB | With | Without (Reference) | Competing risk in the model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary cancer types | Population | Events | PYs | Rate (per 105 PYs) | Population | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | P |
| Overall | 6934 | 761 | 57,340.90 | 1327.15 | 13,868 | 1263 | 124,884.91 | 1011.33 | 1.671 | 1.525 | 1.832 | < .001 |
| Head and neck | 2563 | 236 | 18,422.48 | 1281.04 | 5126 | 356 | 44,308.93 | 803.45 | 1.922 | 1.623 | 2.276 | < .001 |
| Colorectal | 2560 | 312 | 21,330.60 | 1462.69 | 5120 | 571 | 45,984.63 | 1241.72 | 1.521 | 1.321 | 1.750 | < .001 |
| Bone | 54 | 4 | 367.11 | 1089.59 | 108 | 14 | 1083.85 | 1291.70 | 1.366 | 0.397 | 4.703 | .621 |
| Soft tissue sarcoma | 121 | 19 | 970.68 | 1957.40 | 242 | 28 | 1712.78 | 1634.77 | 2.104 | 1.103 | 4.013 | .024 |
| Melanoma | 63 | 10 | 462.20 | 2163.58 | 126 | 21 | 1153.24 | 1820.96 | 1.081 | 0.475 | 2.464 | .852 |
| Breast | 705 | 94 | 6728.86 | 1396.97 | 1410 | 131 | 13,787.25 | 950.15 | 2.058 | 1.569 | 2.700 | < .001 |
| Testicular | 18 | 0 | 190.42 | 0.00 | 36 | 2 | 261.48 | 764.88 | 0.000 | - | - | .963 |
| Kidney | 628 | 58 | 6306.78 | 919.65 | 1256 | 106 | 12,601.34 | 841.18 | 1.391 | 1.001 | 1.932 | .049 |
| Thyroid | 222 | 28 | 2561.77 | 1092.99 | 444 | 34 | 3991.41 | 851.83 | 1.642 | 1.007 | 2.787 | .046 |
HR = Hazard Ratio, CI = Confidence Interval, Adjusted HR: Adjusted variables listed in the Table
P: Chi-square/Fisher’s exact test for categorical variables and t-test for continuous variables
CI: Confidence Interval
PYs: Person-years
Discussion
Previous studies conducted by the National Cancer Institute found the pulmonary TB was associated with an increased risk of lung cancer after adjusting for socioeconomic status and active smoking (odds ratio 2.1, 95% CI = 1.4–3.1) [5]. Epidemiological evidence concerning the association between pre-existing pulmonary TB and lung cancer has been documented [6–11]. Similarly, TB was associated with a 1.78-fold increase in lung cancer risk among nonsmokers and adenocarcinoma (relative risk: 1.6; 95% CI = 1.2–2.1) [4]. However, there is no large-scale study to discuss the association between TB-infected patients with subsequent metastatic cancer from other origins.
In our study, TB was associated with a 1.67-fold increase in the risk of secondary lung cancer compared with the non-TB cohort after adjusting for numerous potential confounders. The underlying mechanism of increasing cancer risk after TB infection had been reported. TB is thought to increase lung cancer risk through chronic pulmonary inflammation and fibrosis. TB infection may cause a profound and host immune response, with inflammatory cells in the lung producing extensive cytokine signaling cascades, oxygen species, reactive nitrogen, prostaglandins, and tissue-destructive proteases [12, 13]. The cell wall component of Mycobacterium tuberculosis can induce the production of nitric oxide and reactive oxygen species, which have been implicated in DNA damage leading to carcinogenesis [14].
It should be noted that nitrative DNA damage and oxidative DNA damage have been implicated in inflammation-related carcinogenesis [15]. Some data revealed that Mycobacterium tuberculosis might also enhance the synthesis of BCL-2, potentially leading to increased anti-apoptotic activity [16]. Chronic inflammation may also enhance lung fibrosis, which may be associated with decreased clearance of lymph and lymph-associated particles from the infected region [17]. Overall, the combination of DNA damage, anti-apoptosis, and the perpetuation of chronic inflammation may enhance progeny cell mutagenesis. These effects may lead to an increased risk of primary or secondary lung cancer.
We also found that all comorbidities increased the risk of secondary lung cancer significantly. This finding may be because more severe comorbidities were associated with the increased toxicity of specific treatments or the use of less aggressive or optimal treatment. These possibilities would thereby reduce the patient’s remaining life expectancy [18, 19]. In recent studies, the presence of comorbidities was significantly associated with elevated all-cause mortality in patients diagnosed with lung cancer, even after adjusting for sex, age, and cancer stage [20].
In our study, the study endpoint, the level of care, showed a significant difference. Compared with the non-TB cohort, TB-infected patients went to the local hospital (15.10% vs 11.85%; p < .001) and regional hospital (43.11% vs 38.43%) for treatments rather than to the medical center (41.79% vs 49.72%). This is because TB control and elimination relied on the early detection of active TB cases that prompted anti-TB treatment, identified persons at risk of exposure, and prevented secondary TB cases [21]. All of this depends on good diagnostic methods and effective treatments for TB. Thus, apart from medical care, the epidemiology of TB is increasing [22–24]. Outside of cities, most care is provided at the level of hospitals or lower at the local and regional levels. In these latter two instances, facilities may not be equipped to provide acute diagnoses and deliver effective treatment regimens. Thus, these patients’ characteristics are more at the lower urbanization level and go to their nearby local and regional hospital in the TB-infected study than non-TB patients. However, after adjusting for other risks (such as gender, age, insurance premium, related comorbidities, and urbanization level), the risk of secondary lung cancer was higher at the medical center (aHR = 2.332; 95% CI = 1.926–2.823; p < .001) and regional hospital (aHR = 1.728; 95% CI = 1.443–2.070; p < .001). The reason for this is that the NHI in Taiwan is a government-administered insurance-based national healthcare system. It is characterized by good accessibility, comprehensive population coverage, and relatively low costs [25].
Nevertheless, only the medical center and regional hospital having negative-pressure isolation wards that can isolate TB-infected patients. After they were discharged and developed secondary lung cancer, they went to their previous and familiar hospitals for help. This makes it a significantly high risk to “find” secondary lung cancer at medical centers and regional hospitals by 2.332- and 1.728 times than local hospitals, respectively.
After stratifying by variable factors using Cox regression and Fine & Gray’s competing risk model, we found that all factors increased the risk in TB-infected patients to develop lung cancer compared with non-infected patients. The Kaplan-Meier analysis revealed that TB-infected patients had a significantly higher risk of developing secondary lung cancer among primary cancer patients, even during the first year of tracking. The reasons for all the above phenomena are similar to those mentioned before. Also, these results demonstrated that TB-exposure is a risk for facilitating primary cancer to metastasize to the lung.
The primary cancer types, including head and neck, colorectal, soft tissue sarcoma, breast, kidney, and thyroid tumors, have a significantly higher risk of developing secondary lung cancer in TB-infected patients. Bone, melanoma, and testicular cancer show no difference. These results are similar to a previous study conducted on 228 cases with lung nodules. Most of the primary sites are colorectal in 25.8%, head and neck in 19.4%, urological organ in 14.7%, breast cancer in 10.5%, melanoma in 6.5%, and other primary sites (sarcoma, thyroid, squamous cell) in 6.1% [26]. Because the incidence of melanoma in Taiwan is relatively lower than that of Europe and America, the metastatic rate may not differ significantly. Also, bone and testicular tumors are more recurrent tumors and not distal metastases, so both did not show a difference.
Although this was a large-scale population-based nationwide study conducted from 2000 to 2015, it had some limitations. First, the patients’ ethnic background in this study was predominantly Asian, limiting the generalizability of these results. Second, the health insurance data we utilized did not include the histological stage and severity of primary cancer that may affect the metastatic ability. Third, our study also excluded laboratory results, such as sputum culture, exercise capacity, lifestyle data, nutrition supplements, and family history of systemic disease.
Conclusions
In this study, TB was associated with a 1.67-fold increase in risk of secondary lung cancer compared with non-TB cohorts among the primary cancer. All comorbidities may increase the risk of developing secondary lung cancer. Therefore, clinicians should consider this in TB-infected patients, since TB leads to secondary lung cancer more easily among patients with primary cancer.
Supporting information
(DOCX)
Acknowledgments
We would like to thank the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) for their support. This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-B-110012), and the sponsor has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-B-110012), and the sponsor has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization 2020. Available from: https://www.who.int/health-topics/tuberculosis-tab=tab_1.
- 2.Stella GM, Kolling S, Benvenuti S, Bortolotto C. Lung-Seeking Metastases. Cancers (Basel). 2019;11(7). Epub 2019/07/25. 10.3390/cancers11071010 ; PMCID: PMC6678078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Wen X, Wei W, Chen Y, Zhu J, Wang C. Clinical characteristics and prognoses of patients treated surgically for metastatic lung tumors. Oncotarget. 2017;8(28):46491–7. Epub 2017/02/06. 10.18632/oncotarget.14822 ; PMCID: PMC5542284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He QC, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer. 2009;125(12):2936–44. Epub 2009/06/13. 10.1002/ijc.24636 . [DOI] [PubMed] [Google Scholar]
- 5.Brenner AV, Wang Z, Kleinerman RA, Wang L, Zhang S, Metayer C, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol. 2001;30(1):118–24. Epub 2001/02/15. 10.1093/ije/30.1.118 . [DOI] [PubMed] [Google Scholar]
- 6.Shiels MS, Albanes D, Virtamo J, Engels EA. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):672–8. Epub 2011/02/22. 10.1158/1055-9965.EPI-10-1166 ; PMCID: PMC3076700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer. 2011;117(3):618–24. Epub 2010/10/05. 10.1002/cncr.25616 . [DOI] [PubMed] [Google Scholar]
- 8.Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, Muo CH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. 2011;6(1):32–7. Epub 2010/12/15. 10.1097/JTO.0b013e3181fb4fcc . [DOI] [PubMed] [Google Scholar]
- 9.Brenner DR, Boffetta P, Duell EJ, Bickeböller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol. 2012;176(7):573–85. Epub 2012/09/19. 10.1093/aje/kws151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everatt R, Kuzmickiene I, Davidaviciene E, Cicenas S. Incidence of lung cancer among patients with tuberculosis: a nationwide cohort study in Lithuania. Int J Tuberc Lung Dis. 2016;20(6):757–63. Epub 2016/05/09. 10.5588/ijtld.15.0783 . [DOI] [PubMed] [Google Scholar]
- 11.Hong S, Mok Y, Jeon C, Jee SH, Samet JM. Tuberculosis, smoking and risk for lung cancer incidence and mortality. Int J Cancer. 2016;139(11):2447–55. Epub 2016/08/16. 10.1002/ijc.30384 . [DOI] [PubMed] [Google Scholar]
- 12.el-Ahmady O, Mansour M, Zoeir H, Mansour O. Elevated concentrations of interleukins and leukotriene in response to Mycobacterium tuberculosis infection. Ann Clin Biochem. 1997;34 (Pt 2):160–4. Epub 1997/03/01. 10.1177/000456329703400205 . [DOI] [PubMed] [Google Scholar]
- 13.Rangel Moreno J, Estrada García I, De La Luz García Hernández M, Aguilar Leon D, Marquez R, Hernández Pando R. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology. 2002;106(2):257–66. Epub 2002/06/06. 10.1046/j.1365-2567.2002.01403.x ; PMCID: PMC1782721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falagas ME, Kouranos VD, Athanassa Z, Kopterides P. Tuberculosis and malignancy. Qjm. 2010;103(7):461–87. Epub 2010/05/28. 10.1093/qjmed/hcq068 . [DOI] [PubMed] [Google Scholar]
- 15.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387(4):365–72. Epub 2006/04/12. 10.1515/BC.2006.049 . [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Jiang R, Takayama H, Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbiol Immunol. 2005;49(9):845–52. Epub 2005/09/21. 10.1111/j.1348-0421.2005.tb03673.x . [DOI] [PubMed] [Google Scholar]
- 17.Ardies CM. Inflammation as cause for scar cancers of the lung. Integr Cancer Ther. 2003;2(3):238–46. Epub 2004/03/24. 10.1177/1534735403256332 . [DOI] [PubMed] [Google Scholar]
- 18.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. Jama. 2005;294(6):716–24. Epub 2005/08/11. 10.1001/jama.294.6.716 . [DOI] [PubMed] [Google Scholar]
- 19.Lee M, Cronin KA, Gail MH, Feuer EJ. Predicting the absolute risk of dying from colorectal cancer and from other causes using population-based cancer registry data. Stat Med. 2012;31(5):489–500. Epub 2011/12/16. 10.1002/sim.4454 . [DOI] [PubMed] [Google Scholar]
- 20.Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J Epidemiol. 2019;29(3):110–5. Epub 2018/07/18. 10.2188/jea.JE20170241 ; PMCID: PMC6375811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lönnroth K, Migliori GB, Abubakar I, D’Ambrosio L, de Vries G, Diel R, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45(4):928–52. Epub 2015/03/21. 10.1183/09031936.00214014 ; PMCID: PMC4391660 article at erj.ersjournals.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–62. Epub 2011/02/19. 10.2105/AJPH.2010.199505 ; PMCID: PMC3052350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375(9728):1814–29. Epub 2010/05/22. 10.1016/S0140-6736(10)60483-7 . [DOI] [PubMed] [Google Scholar]
- 24.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–6. Epub 2009/04/28. 10.1016/j.socscimed.2009.03.041 . [DOI] [PubMed] [Google Scholar]
- 25.Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London J Prim Care (Abingdon). 2010;3(2):115–9. Epub 2010/12/01. 10.1080/17571472.2010.11493315 ; PMCID: PMC3960712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caparica R, Mak MP, Rocha CH, Velho PHI, Viana P, Moura MRL, et al. Pulmonary Nodules in Patients With Nonpulmonary Cancer: Not Always Metastases. J Glob Oncol. 2016;2(3):138–44. Epub 2016/02/03. 10.1200/JGO.2015.002089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.