Abstract
Purpose of review:
Survivorship” or addressing impaired quality of life (QoL) in ICU survivors has been named “the defining challenge of critical care” for this century to address this challenge, in addition to optimal nutrition, we must learn to employ targeted metabolic/muscle assessment techniques and utilize structured, progressive ICU rehabilitative strategies.
Recent Findings:
Objective measurement tools such as ccardiopulmonary exercise testing (CPET) and muscle-specific ultrasound show great promise to assess/treat post-ICU physical dysfunction. CPET is showing systemic mitochondrial dysfunction may underlie development and persistence of poor post-ICU functional recovery. Finally, recent data indicates we are poor at delivering effective, early ICU rehabilitation and that there is limited benefit of currently-employed later ICU rehabilitation on ICU-acquired weakness and QoL outcomes
Summary:
The combination of nutrition with effective, early rehabilitation is highly likely to be essential to optimize muscle mass/strength and physical function in ICU survivors. Currently technologies such as muscle-specific ultrasound and CPET testing show great promise to guide ICU muscle/functional recovery. Further, we must evolve improved ICU-rehabilitation strategies, as current methods are not consistently improving outcomes. In conclusion, we must continue to look to other areas of medicine and to athletes if we hope to ultimately improve “ICU Survivorship”.
Keywords: muscle, cardiopulmonary exercise testing, ultrasound, rehabilitation, critical illness
Introduction:
Modern ICU care now allows prolonged survival from ARF by providing life-sustaining support for extended periods of time, making previously nonsurvivable ICU insults, survivable. In fact, innovations in ICU care have resulted in yearly reductions in hospital mortality from sepsis and critical care(1–3). However, these same data reveal many ICU “survivors” are not returning home to functional lives post-ICU; but, instead to post-acute care facilities for prolonged periods where they incur substantial costs of ~$3.5 million/functioning survivor(4, 5),. Tragically, ICU survivors experience a high burden of muscle weakness, functional impairment and activity limitation(5, 6). ICU follow-up data consistently show persistent functional limitation resulting in only 50% of patients returning to employment at 1 year post-ICU (7), difficulty performing activities of daily living, and only reaching 60%−65% of functional exercise capacity at 12 months,(8). Survivors report that prolonged weakness and loss of function associated with ICU are the most concerning disabilities they experience8. Unfortunately, post-ICU functional disability or ICU-acquired weakness (ICU-AW) is most common and most severe in ICU survivors, where recent data shows 2 out of 3 ICU survivors with lung injury (65%) suffer significant functional limitations (5). It must be asked in modern ICU care- “are we creating survivors…or victims?”. “Survivorship”, or addressing impaired quality of life (QoL) in ICU survivors has been named “the defining challenge of critical care” for this century(9). In his landmark paper, Iwashyna states:
“The emerging picture of ICU survivorship is deeply disturbing. In year after discharge, patients are ravaged. They cannot walk…their bodies refuse to function as they did before. Many intensivists have a file of heartbreaking letters from patients, grateful to be alive but desperate for help in getting their lives back.
Major national ICU trials groups emphasize with recent declining mortality rates in ICU patients that future trials need to focus on functional QoL as a primary endpoint, rather than mortality(1). Finally, in a recent survey of ICU survivors and family members, physical function was rated as most important outcome future ICU studies could address (over mortality) by both patients and family members(10)**. Thus, there remains a significant unmet need to develop new therapies to address the devastating impairments ICU survivors face and improve functional outcomes for the rapidly growing number of ICU survivors.
Severe muscle wasting, a hallmark of the development of ICU-acquired weakness and post-ICU functional disability is a common complication of critical illness (11*, 12). Impaired muscle protein homeostasis ultimately results in reduced muscle mass and muscle strength, which are both independent predictors of survival (13**, 14*). As many as 80% critical illness survivors will continue to exhibit exercise limitations, decreased physical quality of life (QoL), increased overall healthcare costs and higher utilization of health care services, after twelve months to even five years after ICU discharge (15, 16**). Effective interventions to address ICU-acquired weakness, or post-ICU syndrome (PICS), during and after ICU stay continue to be elusive (17**). The development of metabolically targeted interventions to ICU-AW have been challenging as there is significant heterogeneity in metabolic demands and energy requirements between ICU patients which are still poorly understood (17**). Thus, we believe it is imperative that targeted nutritional and metabolic interventions personalized to the highest risk metabolically-challenged and malnourished patients continue to be studied and developed. Undoubtedly, these nutritional and metabolic interventions need to be targeted to preserve and rebuild muscle mass to allow for functional recovery. Inevitably these nutritional and metabolic interventions must be teamed with structured, progressive, and objectively prescribed and measured exercise and physical therapy. This manuscript will discuss the latest innovations in:
Muscle mass evaluation;
Structured, progressive exercise and physical therapy;
Specific objective measurement technique to target and personalize these exercise interventions.
Effect of Critical Illness on Muscle Mass/Quality and Objective Evaluation of Muscle in ICU
A growing body of evidence suggests that the decline in muscle function and physical fitness both before, during and after critical illness is the result of not only a reduction in skeletal muscle mass but also a result of changes in muscle quality such as muscle composition and morphology (18-20*). Skeletal muscle quality is recognized as a marker of function in healthy individuals and critically ill patients. It is also an emerging descriptor of prognosis and is characterized by the accumulation of intra- and intermyocellular lipids which are associated with altered muscle function, insulin resistance, diabetes type 2 and obesity, as well as survival in critical illness (13, 21-23*). Qualifying lean tissue or muscle mass in clinical populations is of increasing importance due to the emerging associations between low muscle quality with low muscle mass/size and poor functional status after discharge ICU (16**). In regard to nutritional support and early mobilisation, research to date, has shown inconsistent results in terms of muscle wasting and functional outcome (13**). Therefore, there is a need for valid and reliable measures of skeletal muscle mass, quality and metabolic phenotype. This is important because distinguishing true muscle weakness from poor motivation or inability to complete the task is challenging and use of manual muscle testing in the early stages of critical illness is limited (24). Alterations in muscle composition, defined by a loss of muscle density and surface area measured by computed tomography (CT) in mechanically ventilated patients, are independently associated with higher 6-month mortality in several different critically ill populations (19, 25**, 26*). Intramuscular adipose tissue (IMAT) can also be derived from CT and MRI images and has been shown to correlate well with functional outcomes, physical fitness and mortality in the elderly population and patients undergoing major abdominal surgery (20, 23*, 27*, 28*). Muscle protein breakdown measured from biopsies of the vastus lateralis muscle, and by reduction in rectus femoris muscle cross-sectional area and compromised skeletal muscle bioenergetic status have been observed during the first week of critical illness (13**). This breakdown is more severe in patients with multiorgan failure (13**). With the advent of high-resolution NMR spectroscopy and MRI, muscle quality can be assessed by quantification of lipid components (IMAT) in the muscle tissue (27*). These techniques have been extensively reported but are complicated and not without risk to the critically ill patient (29)
Ultrasound has also been used to assess muscle composition during health and disease (30). It is cost-effective, non-invasive and sensitive to changes over time when compared to the other assessments such as muscle biopsy, CT or NMR/MRI (31*, 32*-33*).
Muscle wasting results in an increase of intramuscular adipose tissue (IMAT), fibrosis, myonecrosis and even effusion surrounding the fascicles (13**). Muscle with a high amount of intramuscular fat infiltration (IMAT) has a predominantly hyperechoic appearance, with only minor hypoechoic regions (34*).
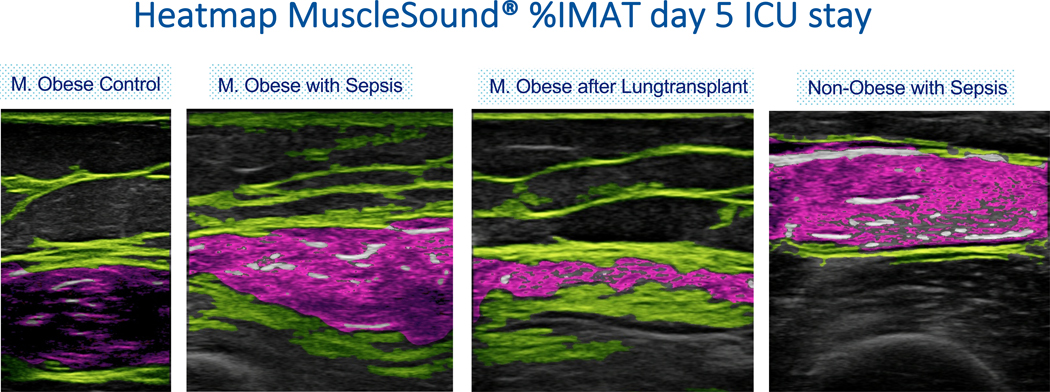
Equations intended to quantify and translate muscle echo Intensity into percent intramuscular adipose tissue (%IMAT) of the rectus femoris muscle were introduced by Young et al. through comparison of intramuscular adipose tissue determined from T1-weighted MRI image with ultrasound images of the rectus femoris muscle (35). More recently, these equations have been incorporated into an automated image analysis algorithm as part of MuscleSound® (Denver, Co, USA) Technology. MuscleSound® (Denver, Co, USA) image analysis uses proprietary software to identify muscle and fat boundaries, and analyze muscle composition using the principles and calibration equations and algorithms outlined above (35). The technology has been extensively used in healthy individuals, elite athletes and critically ill patients (see Figure 1), where temporal changes in muscle composition, including intramuscular glycogen content have been described and validated by muscle biopsies (36, 37)
Figure 1:
MuscleSound® echo-intensity heatmap of the rectus femoris muscle, showing different stage of muscle wasting in a clinical population.
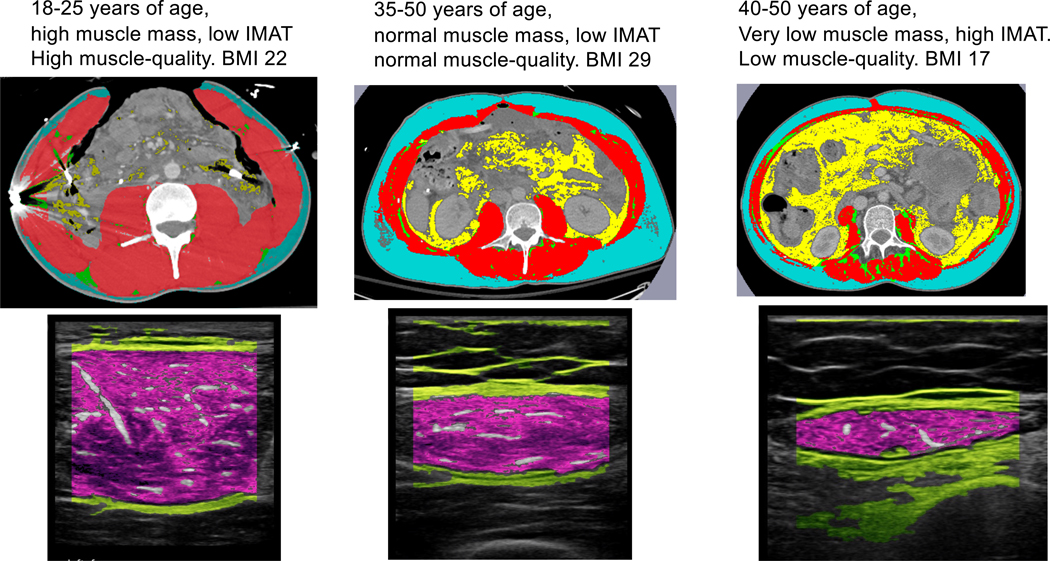
Increased IMAT combined with a decrease in muscle CSA (MCSA) has been described previously during critical care admission (13**). Puthucheary et al. found that intramuscular lipid accumulation, assessed by muscle biopsies, results in dysregulated lipid oxidation and reduced ATP bioavailability, which contributes to a compromised skeletal muscle bioenergetic status (13**). Thus, the accumulation of intramuscular lipids (IMAT) during ICU stay combined with the decrease of MCSA indicates a compromised skeletal muscle metabolic status and is synonymous with acute mitochondrial dysfunction and even perturbed regeneration (13**, 38). Figure 2 shows the comparison between a CT scan at the level of L3 (with segmentation analyses) and the MuscleSound® heatmap assessed from the rectus femoris muscle. IMAT derived from the MuscleSound IMAT algorithm compared to IMAT form segmented CT analyses, correlated well. Intramuscular fat infiltration (IMAT) is therefore not an ad-hoc process but shows the systemic nature of muscle metabolic dysfunction.
Figure 2:
Segmented CT analysis at the level of L3; blue is the subcutaneous adipose tissue (SAT) layer, red is muscle, green is intramuscular adipose tissue (IMAT) and yellow is visceral adipose tissue (VAT). MuscleSound® echo-intensity heatmap of the rectus femoris muscle.
Hypoxic and/or inflammatory stimuli, associated with critical illness, impairs muscle protein synthesis and replacement of the functional muscle tissue with IMAT due to reduced mitochondrial biogenesis; (39*). The systemic inflammatory response gives rise to skeletal muscle wasting within 24 hours upon admission ICU. Acute skeletal muscle wasting occurs early and rapidly defined as the reduction in mitochondrial beta-oxidation, mitochondrial biogenesis markers and intramuscular ATP content (13**).
Exercise Capacity in ICU Patients and Survivors:
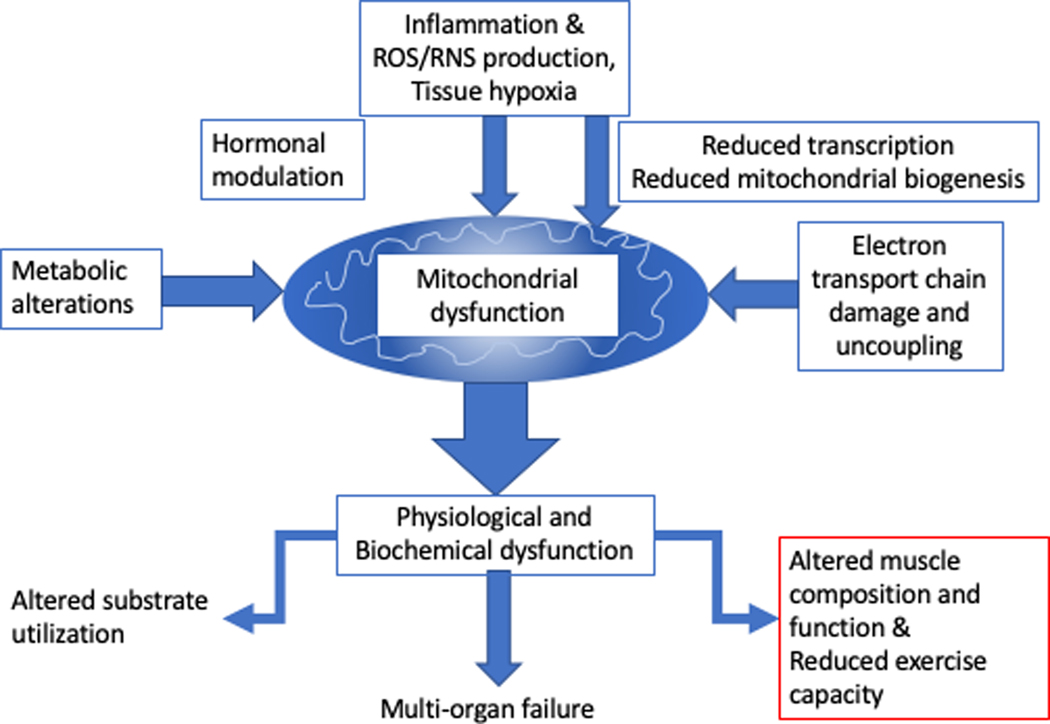
The underlying mechanisms of ICU-AW are key to understanding the role for formal measurement of exercise capacity in ICU patients and survivors, as well as the prescription of nutritional and exercise support both in and out of the intensive care environment. A significant component of the observed reduced exercise tolerance after critical illness is likely to arise from acquired systemic mitochondrial dysfunction (the degree of and recovery of which appears to predict survival from multi organ dysfunction) consequent to critical illness(40) (Figure 3). This acquired mitochondrial dysfunction urgently requires better description in translational and clinical models, as a potential target for assessment and intervention.
Figure 3:
Central role of mitochondrial dysfunction in impaired physical and muscle recovery in critical illness.
In the majority of individuals, aerobic deconditioning, especially at its most severe, is consequent to reduced mitochondrial capacity rather than a failure of oxygen delivery (40, 41). This is in contrast to the limitation seen in athletes (and the established dogma), where oxygen delivery is the limiting factor. This reduced mitochondrial capacity (coupled with dysfunctional responses to stressors such as infection or surgery consequent to established comorbidity) has important implications for individuals, predominant amongst these being altered metabolic fuel usage in response to exercise.
Cardiopulmonary Exercise Testing as a Tool to Assess and Guide Intensive Care Rehabilitation.
Cardiopulmonary exercise testing (CPET) is commonly used with established utility in the perioperative context to assess individual aerobic exercise capacity and increasingly as a tool to prescribe structured exercise prehabilitation with the goal of improving outcomes after major surgery (42). Above and beyond other measures of physical capacity, such as the 6-minute walk test, or questionnaires such as the Duke Activity Status Index, CPET provides multi-channel, objective, rich cardiopulmonary and metabolic data that both allows the calculation of peak oxygen uptake, and also the causes, including the underlying pathophysiology, of decreased exercise capacity.
Taking into account the increasing body of literature that supports the concept that multi-morbid deconditioned individuals are ‘primed’ to develop multi-organ pathophysiology, it is conceivable that CPET is in fact more of a test of mitochondrial function under physiological stress than of cardiopulmonary oxygen delivery capacity in most preoperative patients. This is the subject of ongoing research at Duke University in our ongoing and beyond.
Given the utility of Cardiopulmonary exercise testing in preoperative assessment, especially in objective assessment of metabolic capacity and the consequent prescription of exercise training protocols ‘matched’ to known exercise physiology, and also the potential utility of CPET, coupled with muscle quality and resting metabolic assessments, in the assessment of metabolic capacity for the guidance of nutritional support in the perioperative period, it is highly likely that CPET will become a useful tool for the assessment of the critical illness survivor, providing invaluable data to help guide nutritional and exercise interventions in the post-intensive care rehabilitative period (43**).
To date few studies have been undertaken where formal exercise testing has been used during or after critical illness. Early work in the Netherlands has confirmed apparent safety and feasibility of cardiopulmonary exercise testing on bed-based cycle ergometer in critically ill patients (43**). Similarly, there is a paucity of data available for formal CPET assessment in the post-intensive care period. In survivors of Acute Respiratory Syndrome, the majority of patients assessed 3 months after hospital discharge display reduced exercise capacity, that cannot be explained by persistent impairment of pulmonary function (44). Similarly, at around one month after discharge from hospital, general adult critical illness survivors demonstrate significant multifactorial exercise limitation, the underlying cause being described as general deconditioning (i.e. not ascribed to one organ system). In all studies, no significant adverse events were reported, despite the severe deconditioning of the patients assessed.
CPET as Part of an Integrated Pathway for Metabolism Support in Intensive Care
Critical illness is part of a health continuum. The physical condition of an individual, including their metabolic health (comprising aerobic fitness, metabolic fuel usage efficiency, body composition and nutritional status) and impacted/influenced by presence/absence of named co-morbidity prior to the development of critical illness predicts the likelihood of development of critical illness, the likely course of said illness and the chances of death or delayed/impaired recovery including ongoing health needs.
In major surgery, where rich preoperative assessment is feasible, it is recommended that thorough physiological and nutritional assessment guide preoperative optimization efforts. This is conceived of as being only part of the patient’s health journey through the perioperative period, where prehabilitation aims to improve functional resilience to the stress of surgical trauma, reducing the risk of functional decline below the threshold for dependence. Combined with muscle health and nutritional assessments, including metabolic cart measurements of caloric and metabolic substrate usage, an integrated optimization plan can be conceived of and initiated.
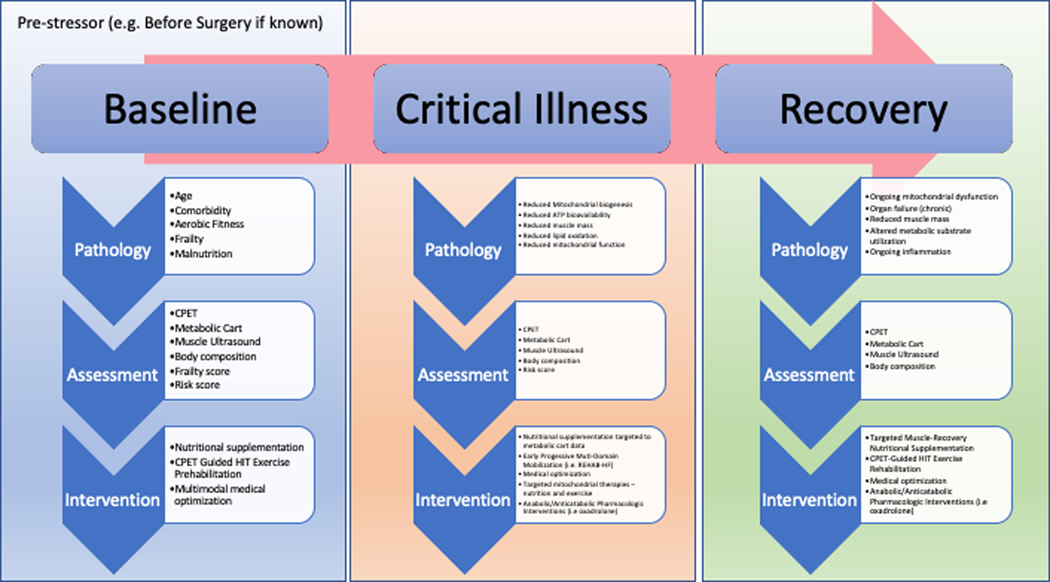
In general adult intensive care, the same approach can be taken with our critically ill patients where metabolic, nutritional and formal exercise capacity assessments can, and should form the cornerstone for ongoing objective assessment of and intervention in critical illness. These data will help assess stage of critical illness, recovery from illness and prescription of exercise intensity for effective training. Furthermore, the same assessments in the post intensive care period, just as conceived of in major surgery, can help guide ongoing interventions to create survivors and not victims from intensive care. Our proposed novel metabolic assessment and intervention pathways across all phases of critical illness to promote survivorship and optimal post-ICU muscle and physical recovery are summarized in Figure 4.
Figure 4:
Proposed novel metabolic assessment and intervention pathways across all phases of critical illness to promote survivorship and optimal post-ICU muscle and physical recovery. (Abbreviations- CPET- Cardiopulmonary Exercise Testing, HIT- High Intensity Training targeted to patients’ abilities by CPET testing results)
Key Role of Early Structured and Progressive Physical Therapy
Patients surviving critical illness are at risk for persistent functional mobility impairments that significantly impact their quality of life. Physical activity interventions that begin in the intensive care unit (ICU) are purported to mitigate the negative sequela of critical illness. For instance, the most recent systematic review with meta-analysis of 23 RCTs comprising 2,308 patients concluded that early intervention may be associated with decreased incidence of ICU-acquired muscle weakness, improved functional mobility capacity, and increased number of ventilator-free days and discharged-to-home rates. Unfortunately, rehabilitation did not significantly decrease ICU mortality, ICU length of stay and hospital length of stay and muscle strength were not significantly improved. The certainty of the evidence for these outcomes was “very low.” Data on activities of daily living, return to work, and delirium were not available in any of the trials. Rehabilitation of patients with sepsis might not decrease ICU mortality, but might improve QOL (45**) Unfortunately, most recent studies are demonstrating that current ICU rehabilitative strategies are failing to consistently improve outcomes (46**). Further, a majority of ICU patients are not being mobilized until day 9 or later and (8, 47, 48). A large multi-site point prevalence study showed that <20% of ICU patients were mobilized out of bed(49). More troubling, the current standard of practice of delayed ICU rehabilitation has shown limited benefit on ICU-AW and QoL outcomes Thus, the success of current ICU rehabilitation practice is tempered by the significant variations in frequency, duration, intensity, and volume of intervention and heterogeneity in outcome assessments and timing of those assessments across the RCTs. (45**, 50**) To address the latter limitation, the Physical Rehabilitation Core Outcomes In Critical illness (PRACTICE) study is using a modified Delphi consensus process with researcher, clinician, and patient/caregiver stakeholders to develop a core outcome set for trials of physical rehabilitation interventions delivered across the continuum of a patient’s recovery from the ICU until reintegration in the community following hospital discharge.(51**) At the present time though, implementation of standardized interventions in the ICU or that span ICU to hospital floor remains low in clinical practice.(51**)
An intervention design that addresses multiple domains necessary for independent functional mobility, that is individually tailored with targeted milestones for progression, and that extends through the hospital stay has yet to be fully explored. A potential model of interest is that used in the REHAB-HF trial (clinicaltrails.gov NCT02196038), which implemented exercise across domains of strength, balance, mobility, and endurance for HF patients with profound physical deficits paralleling those associated with critically illness.(52**) Another design option includes incorporation of assistive rehabilitation technology. Some of these technologies can be used to complement functional mobility interventions, especially during periods where full voluntary and active participation is not possible, as occurs with sedation or profound weakness. For instance, cycle ergometry is gaining more attention given its capacity to provide passive or active movement with or without functional electrical stimulation (FES). Currently, three major funded trials of cycle ergometry are of high interest. eStimCycle, conducted in Australia and the USA across 5 centers, compares cycle ergometry with FES and routine rehabilitation to routine rehabilitation alone; recruitment is complete and results are pending (clinicaltrials.gov NCT02214823).(53) Nutrition and Exercise in Critical Illness (NEXIS), conducted across four centers in the USA, compares cycle ergometry and amino acid supplementation with usual care; recruitment is in process (clinicaltrials.gov NCT03021902).(54*) CYCLE: A Randomized Clinical Trial of Early In-bed Cycling for Mechanically Ventilated Patients, conducted in Canada, USA, and Australia across 17 centers, compares cycle ergometry and routine rehabilitation care with routine rehabilitation care alone; recruitment is in process (clinicaltrials.gov NCT03471247).(55*) Valuable lessons will be learned from these multicenter and international trials.
Discussion
In short, as others have described in a recent elegant review of combining nutrition and exercise to optimize ICU recovery (56), the combination of nutrition and exercise is highly likely to be essential to optimize maintenance and recovery of muscle mass, strength, and physical function in critical illness survivors. Currently there are rapidly evolving technologies, many adapted from the domains of elite athletic performance and recovery that we believe provide unique opportunities for research and clinical programs in the ICU of tomorrow’s include techniques in common use by professional sports teams such as muscle-specific ultrasound for muscle mass/quality/glycogen measurement and cardiopulmonary exercise testing (CPET testing) that is routine now to guide training ability and goals even in amateur athletes. Why would the ICU patients we have invested so much of our time and energy as ICU providers (and a substantial fraction of many countries Gross Domestic Product) not deserve benefit from the study and use of these fundamental technologies? These technologies and exercise programs will allow optimal evaluation of muscle mass/quality; muscle function; aerobic and mitochondrial function; and optimal structured multi-domain progressive rehabilitative therapy to promote recovery of physical function in the ICU survivor. It is also essential we continue to evolve structure, multi-domain ICU rehabilitation and exercise programs that span the entire hospital stay of the ICU patient (such as that being studied in the REHAB-HF strategy described above). This is urgently needed for as stated in the recent key review of interventions for Post-ICU Syndrome (PICS) article (Approaches to Addressing Post–Intensive Care Syndrome among Intensive Care Unit Survivors by the Addressing Post Intensive Care Syndrome (APICS-01) study team) “Randomized controlled trials of physical rehabilitation interventions initiated several days after ICU admission have generally yielded no consistent evidence of benefit (46**).”
Conclusion
Thus, in conclusion, we must continue to look to other areas of medicine (i.e. heart failure, cancer) and to strategies employed by elite athletes for metabolic evaluation and rehabilitation if we hope to ultimately improve “ICU Survivorship”, address the impaired quality of life in ICU survivors and overcome “the defining challenge of critical care” for this century(9).
Key Points:
ICU survivors experience a high burden of muscle weakness, functional impairment and activity limitation, to address this challenge, in addition to optimal nutrition, we must learn to employ targeted metabolic/muscle assessment techniques and utilize structured, progressive ICU rehabilitative strategies
Severe muscle wasting, a hallmark of the development of ICU-acquired weakness and post-ICU functional disability is a common complication of critical illness and is caused by impaired muscle protein homeostasis ultimately results in reduced muscle mass and muscle strength, which are both independent predictors of survival.
New hand-held muscle-specific ultrasound technology allow for measurement of muscle mass, muscle quality, and muscle glycogen that will allow for objective evaluation of muscle changes during ICU and analysis of the effect of nutrition and exercise on muscle through hospital course.
Cardiopulmonary exercise testing (CPET) needs to become the standard for evaluation of metabolic/exercise physiology and exercise prescription in patients as new studes of CPET evaluation in acutely/chronically ill patients are showing exercise intolerance is largely due to persistent mitochondrial dysfunction and this is responseive to CPET-prescribed exercise training.
Recent data indicates we are poor at delivering effective, early ICU rehabilitation and that there is limited benefit of currently-employed later ICU rehabilitation on ICU-acquired weakness and QoL outcomes, thus we must evolve improved ICU-rehabilitation strategies to include progressive multi-disciplinarexercise that is carried out through entire ICU patient journey.
Acknowledgments
Conflict of Interest Disclosure:
PEW- Has received grant funding related to this work from NIH, Canadian Institutes of Health Research, Abbott, Baxter, Fresenius, Nutricia, and Takeda. PEW serves as a consultant to Abbott, Fresenius, Baxter, Nutricia, and Takeda for research related to nutrition in surgery and ICU care; received unrestricted gift donation for surgical and critical care nutrition research from Musclesound and Cosmed; received honoraria or travel expenses for CME lectures on improving nutrition care in surgery and critical care from Abbott, Baxter, Nutricia, and Fresenius. JW- receives grant funding support from the International Anesthesiology Research Society (IARS). JM- Receives research grant funding from Nutricia, MuscleSound, and Cosmed. AP- Has received grant funding related to this work from NIH, Canadian Institutes of Health Research, PCORI and American Physical Therapy Association.
ABBREVIATIONS:
- IMAT
Intramuscular adipose tissue
- %IMAT
percentage Intramuscular adipose tissue
- MT
muscle thickness
- MCSA
muscle cross-sectional area
- IMAT-R
Ratio %IMAT and MCSA
- CT
computed tomography
- NMR
nuclear magnetic resonance
- MRI
magnetic resonance imaging
- ARDS
acute respiratory distress syndrome
- MSK
musculoskeletal
REFERENCES:
- 1.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–16. [DOI] [PubMed] [Google Scholar]
- 2.Cochi SE, Kempker JA, Annangi S, Kramer MR, Martin GS. Mortality Trends of Acute Respiratory Distress Syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc. 2016;13(10):1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maca J, Jor O, Holub M, Sklienka P, Bursa F, Burda M, et al. Past and Present ARDS Mortality Rates: A Systematic Review. Respir Care. 2017;62(1):113–22. [DOI] [PubMed] [Google Scholar]
- 4.Herridge MS, Moss M, Hough CL, Hopkins RO, Rice TW, Bienvenu OJ, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–38. [DOI] [PubMed] [Google Scholar]
- 5.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–35. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RO, Suchyta MR, Kamdar BB, Darowski E, Jackson JC, Needham DM. Instrumental Activities of Daily Living after Critical Illness: A Systematic Review. Ann Am Thorac Soc. 2017;14(8):1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denehy L, Skinner EH, Edbrooke L, Haines K, Warrillow S, Hawthorne G, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17(4):R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153(3):204–5. [DOI] [PubMed] [Google Scholar]
- 10.**Dinglas VD, Chessare CM, Davis WE, Parker A, Friedman LA, Colantuoni E, et al. Perspectives of survivors, families and researchers on key outcomes for research in acute respiratory failure. Thorax. 2018;73(1):7–12.Key recent survey of ICU survivors and family members showing physical function was rated as most important outcome future ICU studies could address (over mortality) by both patients and family members
- 11.*Wollersheim T, Grunow JJ, Carbon NM, Haas K, Malleike J, Ramme SF, et al. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: an explorative trial. J Cachexia Sarcopenia Muscle. 2019;10(4):734–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batt J, Herridge M, Dos Santos C. Mechanism of ICU-acquired weakness: skeletal muscle loss in critical illness. Intensive Care Med. 2017;43(12):1844–6. [DOI] [PubMed] [Google Scholar]
- 13.**Puthucheary ZA, Astin R, McPhail MJW, Saeed S, Pasha Y, Bear DE, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73(10):926–35. [DOI] [PubMed] [Google Scholar]
- 14.*Nakanishi N, Oto J, Tsutsumi R, Iuchi M, Onodera M, Nishimura M. Upper and lower limb muscle atrophy in critically ill patients: an observational ultrasonography study. Intensive Care Med. 2018;44(2):263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNelly AS, Rawal J, Shrikrishna D, Hopkinson NS, Moxham J, Harridge SD, et al. An Exploratory Study of Long-Term Outcome Measures in Critical Illness Survivors: Construct Validity of Physical Activity, Frailty, and Health-Related Quality of Life Measures. Crit Care Med. 2016;44(6):e362–9. [DOI] [PubMed] [Google Scholar]
- 16.**Silveira L, Silva JMD, Tanaka C, Fu C. Decline in functional status after intensive care unit discharge is associated with ICU readmission: a prospective cohort study. Physiotherapy. 2019;105(3):321–7. [DOI] [PubMed] [Google Scholar]
- 17.**Bear DE, Parry SM, Puthucheary ZA. Can the critically ill patient generate sufficient energy to facilitate exercise in the ICU? Curr Opin Clin Nutr Metab Care. 2018;21(2):110–5. [DOI] [PubMed] [Google Scholar]
- 18.Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Front Physiol. 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Twisk JW, Oudemans-van Straaten HM, et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.*West MA, van Dijk DPJ, Gleadowe F, Reeves T, Primrose JN, Abu Hilal M, et al. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle. 2019;10(4):860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieber CC. Frailty - From concept to clinical practice. Exp Gerontol. 2017;87(Pt B):160–7. [DOI] [PubMed] [Google Scholar]
- 22.Kelley GA, Kelley KS. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp Gerontol. 2017;96:100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.*Czigany Z, Kramp W, Bednarsch J, van der Kroft G, Boecker J, Strnad P, et al. Myosteatosis to predict inferior perioperative outcome in patients undergoing orthotopic liver transplantation. Am J Transplant. 2020;20(2):493–503. [DOI] [PubMed] [Google Scholar]
- 24.Annetta MG, Pittiruti M, Silvestri D, Grieco DL, Maccaglia A, La Torre MF, et al. Ultrasound assessment of rectus femoris and anterior tibialis muscles in young trauma patients. Ann Intensive Care. 2017;7(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.**Dusseaux MM, Antoun S, Grigioni S, Beduneau G, Carpentier D, Girault C, et al. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS One. 2019;14(6):e0216991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.*Ng CC, Lee ZY, Chan WY, Jamaluddin MF, Tan LJ, Sitaram PN, et al. Low Muscularity as Assessed by Abdominal Computed Tomography on Intensive Care Unit Admission Is Associated With Mortality in a Critically Ill Asian Population. JPEN J Parenter Enteral Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 27.*Shenvi SD, Taber DJ, Hardie AD, Botstein JO, McGillicuddy JW. Assessment of magnetic resonance imaging derived fat fraction as a sensitive and reliable predictor of myosteatosis in liver transplant recipients. HPB (Oxford). 2020;22(1):102–8. [DOI] [PubMed] [Google Scholar]
- 28.*Zopfs D, Theurich S, Grosse Hokamp N, Knuever J, Gerecht L, Borggrefe J, et al. Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol. 2020;30(3):1701–8. [DOI] [PubMed] [Google Scholar]
- 29.Knight PH, Maheshwari N, Hussain J, Scholl M, Hughes M, Papadimos TJ, et al. Complications during intrahospital transport of critically ill patients: Focus on risk identification and prevention. Int J Crit Illn Inj Sci. 2015;5(4):256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milan S, HILL JC, Wischmeyer P 35th international symposium on intensive care and emergency medicine. Crit Care. 2015;19 Suppl 1(Suppl 1):P1-P578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.*Parry SM, Burtin C, Denehy L, Puthucheary ZA, Bear D. Ultrasound Evaluation of Quadriceps Muscle Dysfunction in Respiratory Disease. Cardiopulmonary Physical Therapy Journal. 2019;30(1):15–23. [Google Scholar]
- 32.*Hobson-Webb LD, Simmons Z. Ultrasound in the Diagnosis and Monitoring of Amyotrophic Lateral Sclerosis: A Review. Muscle Nerve. 2019;60(2):114–23. [DOI] [PubMed] [Google Scholar]
- 33.*Williams DGA, Molinger J, Wischmeyer PE. The malnourished surgery patient: a silent epidemic in perioperative outcomes? Curr Opin Anaesthesiol. 2019;32(3):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.*Rosen, Soliman K, B S. The Echogenic Appearance of the Diabetic Deltoid Muscle on Shoulder Ultrasound- Is This Simply from Adipose Tissue Infiltration, Can This Appearance Predict Type 2 Diabetes and be Used to Detect Pre-Diabetes?. High Value Care 4. 2019. [Google Scholar]
- 35.Young HJ, Jenkins NT, Zhao Q, McCully KK. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve. 2015;52(6):963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JC, Millan IS. Validation of musculoskeletal ultrasound to assess and quantify muscle glycogen content. A novel approach. Phys Sportsmed. 2014;42(3):45–52. [DOI] [PubMed] [Google Scholar]
- 37.Nieman DC, Shanely RA, Zwetsloot KA, Meaney MP, Farris GE. Ultrasonic assessment of exercise-induced change in skeletal muscle glycogen content. BMC Sports Sci Med Rehabil. 2015;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sciorati C, Clementi E, Manfredi AA, Rovere-Querini P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: cellular and molecular players. Cell Mol Life Sci. 2015;72(11):2135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.*Yang W, Hu P. Skeletal muscle regeneration is modulated by inflammation. J Orthop Translat. 2018;13:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Zwaard S, de Ruiter CJ, Noordhof DA, Sterrenburg R, Bloemers FW, de Koning JJ, et al. Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol (1985). 2016;121(3):636–45. [DOI] [PubMed] [Google Scholar]
- 41.Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, et al. Symmorphosis and skeletal muscle VO2 max : in vivo and in vitro measures reveal differing constraints in the exercise-trained and untrained human. J Physiol. 2016;594(6):1741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levett DZ, Grocott MP. Cardiopulmonary exercise testing, prehabilitation, and Enhanced Recovery After Surgery (ERAS). Can J Anaesth. 2015;62(2):131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.**Sommers J, Klooster E, Zoethout SB, van den Oever HLA, Nollet F, Tepaske R, et al. Feasibility of Exercise Testing in Patients Who Are Critically Ill: A Prospective, Observational Multicenter Study. Arch Phys Med Rehabil. 2019;100(2):239–46. [DOI] [PubMed] [Google Scholar]
- 44.Ong KC, Ng AW, Lee LS, Kaw G, Kwek SK, Leow MK, et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004;24(3):436–42. [DOI] [PubMed] [Google Scholar]
- 45.**Zhang L, Hu W, Cai Z, Liu J, Wu J, Deng Y, et al. Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS One. 2019;14(10):e0223185.Recent large systematic review and meta-analysis of early mobilization in ICU studies. Rehabilitation did not significantly decrease ICU mortality/length of stay and hospital length of stay and muscle strength were not significantly improved. The certainty of the evidence for these outcomes was “very low.” Data on activities of daily living, return to work, and delirium were not available in any of the trials. Rehabilitation of patients with sepsis might not decrease ICU mortality, but might improve QOL.
- 46.**Brown SM, Bose S, Banner-Goodspeed V, Beesley SJ, Dinglas VD, Hopkins RO, et al. Approaches to Addressing Post-Intensive Care Syndrome among Intensive Care Unit Survivors. A Narrative Review. Ann Am Thorac Soc. 2019;16(8):947–56.Key excellent recent review article on interventions to address PICS in ICU survivors. Data presented demonstrates recent studies of late ICU rehabilitation efforts are showing limited benefit of on ICU-acquired weakness and QoL outcomes. A “must-read” article in ICU recovery field.
- 47.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–505. [DOI] [PubMed] [Google Scholar]
- 49.Berney SC, Harrold M, Webb SA, Seppelt I, Patman S, Thomas PJ, et al. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15(4):260–5. [PubMed] [Google Scholar]
- 50.**de Queiroz RS, Saquetto MB, Martinez BP, Andrade EA, da Silva P, Gomes-Neto M. Evaluation of the description of active mobilisation protocols for mechanically ventilated patients in the intensive care unit: A systematic review of randomized controlled trials. Heart Lung. 2018;47(3):253–60.This article is the first to evaluate the integrality description of mobilization protocols in clinical trials to determine whether sufficient information is reported to allow replication in clinical and research settings.
- 51.**Connolly BA, Mortimore JL, Douiri A, Rose JW, Hart N, Berney SC. Low Levels of Physical Activity During Critical Illness and Weaning: The Evidence-Reality Gap. J Intensive Care Med. 2019;34(10):818–27.Despite purported benefits and feasibility of ICU physical rehabilitation, implementation in routine clinical practice, as assessed through behavioral mapping, yielded low physical activity behavior in patients on or weaning from mechanical ventilation. The results indicate that unit culture is important for optimizing physical activity opportunities.
- 52.**Pastva AM, Duncan PW, Reeves GR, Nelson MB, Whellan DJ, O’Connor CM, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials. 2018;64:118–27.This article presents a working model for maintaining intervention fidelity in physical intervention trials and illustrates how the intervention fidelity strategies align with the Treatment Fidelity Workgroup of the National Institutes of Health Behavior Change Consortium recommendations.
- 53.Parry SM, Berney S, Koopman R, Bryant A, El-Ansary D, Puthucheary Z, et al. Early rehabilitation in critical care (eRiCC): functional electrical stimulation with cycling protocol for a randomised controlled trial. BMJ Open. 2012;2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.*Heyland DK, Day A, Clarke GJ, Hough CT, Files DC, Mourtzakis M, et al. Nutrition and Exercise in Critical Illness Trial (NEXIS Trial): a protocol of a multicentred, randomised controlled trial of combined cycle ergometry and amino acid supplementation commenced early during critical illness. BMJ Open. 2019;9(7):e027893.This article describes the protocol for the first randomised controlled trial evaluating the combination of in-bed cycle ergometry exercise and protein supplementation started in the early phase of critical illness.
- 55.*Kho ME, Molloy AJ, Clarke FJ, Reid JC, Herridge MS, Karachi T, et al. Multicentre pilot randomised clinical trial of early in-bed cycle ergometry with ventilated patients. BMJ Open Respir Res. 2019;6(1):e000383.This article describes the pilot study methodology that informed the design of the largest to-date international multicenter trial of in-bed cycling, CYCLE RCT.
- 56.Heyland DK, Stapleton RD, Mourtzakis M, Hough CL, Morris P, Deutz NE, et al. Combining nutrition and exercise to optimize survival and recovery from critical illness: Conceptual and methodological issues. Clin Nutr. 2016;35(5):1196–206. [DOI] [PubMed] [Google Scholar]