Abstract
Reactivation of herpsviruses, mainly HSV, CMV and EBV, are frequent among critically ill patients. Although they are not immunocompromised from a classical point of view, these patients often present an alteration of their immune system favoring viral reactivation. Seropositive patients with sepsis and under mechanical ventilation are particularly at risk. Herpesviruses have a pulmonary tropism and can be responsible for non-resolving forms of acute respiratory distress syndrome with high mortality. However, the direct causality between herpesviruses reactivation and impaired outcomes among severely ill patients remains under debate.
Keywords: Acute respiratory distress syndrome (ARDS), Critically ill patients, Cytomegalovirus (CMV), Epstein Barr virus (EBV), Herpes simples virus (HSV), Herpesviruses, Immunocompetent patients, Immunoparalysis, Intensive care unit (ICU), Mechanical ventilation (MV), Mortality, Viral reactivation
Introduction
Herpesviruses family gathers more than 100 viruses in which 8 are strictly involved in human pathogenesis. Human herpesviruses are enveloped DNA viruses with a capsid. Herpes Simplex Virus 1 and 2 (HHV1/HSV1 and HHV2/HSV2), Varicella Zoster Virus (HHV3/VZV), Epstein Barr Virus (HHV4/EBV) and Cytomegalovirus (HHV5/CMV) are among the most known in human pathology. Herpesviruses are characterized by a primo infection often occurring during childhood and associated with aspecific signs then followed by a latency period. 50–85% (Pebody, 2004; Bradley et al., 2014) of immunocompetent adults are HSV seropositive and 60–80% are CMV seropositive (Gkrania-Klotsas et al., 2013). Reactivation may occur especially in case of immunosuppression and lead to life threatening infections. The pathogenicity of herpesviruses in immunocompromised patients such as bone marrow or solid organ-transplanted or HIV, is well known. Notably, HSV and CMV can be associated with severe community acquired pneumonia potentially evolving towards acute respiratory distress syndrome (ARDS). However, intensive care unit (ICU) patients, although not immunocompromised from a classical point of view, can experience herpesviruses reactivation ranging from 5% to 64% (Luyt et al., 2014) and 15% to 45% (Papazian et al., 2016) for HSV and CMV respectively, depending on the diagnostic technic used, and occurring at different time points during the ICU stay. HSV and CMV (and more recently EBV) (Fig. 1 ) reactivations among ICU patients have been extensively focused on. They appear to be more frequently retrieved in seropositive patients, or those with septic shock and prolonged mechanical ventilation (MV) (Jaber et al., 2005). Even if the debate is still opened, it has been frequently reported that herpesviruses reactivation can alter the prognosis of critically ill patients, although a causal link remains to be proven. HSV pulmonary reactivation has been described to be associated with a longer MV duration, ICU stay and mortality (Luyt et al., 2007). CMV reactivation is also associated with a higher mortality, MV duration and ICU length of stay (Li et al., 2018). The lungs being a usual site of latency for herpesviruses, reactivation in the respiratory tractus is frequent and herpesviruses are considered as an emerging cause of ventilator associated pneumonia (VAP) (Cantan et al., 2019). They might be responsible for direct epithelial injury, favor bacterial infections, enhance local inflammation and pulmonary fibrosis, and be involved in the pathogenesis or the prolongation of ARDS, which is associated with a high mortality (Bellani et al., 2016). Considering the abundance of the data on this question and the recent publication of randomized controlled trials, we aimed to summarize the evidence available on the role of the most frequently described herpesviruses in the ICU and especially in ARDS patients, decipher the pathophysiological ways implicated, the diagnostic methods and treatments and describe their impact on the outcomes.
Fig. 1.

(A) Herpes simplex virus 1: photography by electronic microscopy (credit: APHM, laboratory of virology). (B) CMV Photomicrograph (original magnification, 400; immunohistochemical marker for cytomegalovirus) shows positive intranuclear inclusion bodies (arrows) (credit: APHM, laboratory of virology). (C) Epstein Barr virus: photography by electronic microscopy (credit: APHM, laboratory of Virology).
HSV in ICU Patients
From Oral Reactivation to Bronchopneumonitis
In historical reports, the frequency of HSV detection in the throat of ICU patients reached 41% after surgery (Porteous et al., 1984). HSV was also isolated from the tracheobronchial secretions of a high percentage of patients, and from 30% to 71% of those with ARDS (Tuxen et al., 1982; Tuxen et al., 1987; Ong et al., 2004). A large prospective study showed that HSV was recovered from the upper and lower respiratory tract of 22% and 16% of ICU patients respectively (Bruynseels et al., 2003). When focusing on patients for whom a bronchoalveolar lavage (BAL) was performed, Linssen et al. (2008) found that HSV DNA was detected in 4.3% of samples in the community group, 15% in the non-ICU group and reached 32% of the ICU group. Patients older than 50 were more frequently concerned.
HSV reactivation occurs earlier than CMV in patients with sepsis admitted to the ICU, often during the first 10 days (Heininger et al., 2011). In many cases, HSV recovery from lower respiratory tract samples of non-immunocompromised ventilated patients corresponds to viral contamination from the mouth and/or throat but several studies have shown that histological aspects of tracheobronchitis (Tuxen et al., 1982) or bronchopneumonitis (Luyt et al., 2007) can be retrieved. In this cases, HSV is considered to be responsible for viral nosocomial pneumonia and possibly ARDS. HSV bronchopneumonitis is probably initiated by viral reactivation in the throat (secondary to critical illness and local microtrauma caused by endotracheal and gastric tubes, and oropharyngeal cavity suctioning), followed by contamination, colonization and infection of the bronchial tree and the lung. In a study on 201 non-immunocompromised patients under MV for at least 5 days (Luyt et al., 2007), HSV reactivation in the throat was diagnosed in 109 (54%) patients. It was asymptomatic in 56% of them, whereas it was associated with herpetic ulceration of the lip or gingivostomatitis in 48 (44%). Reactivation is the first step of viral VAP, followed by tracheal colonization, and lung involvement. The mechanism leading to reactivation is probably multifactorial, including an impaired function of the immune system frequently following bacterial sepsis and called “immunoparalysis,” microtrauma due to intubation and hormonal factors (Luyt et al., 2008; Hotchkiss et al., 2009; Luyt et al., 2007).
Clinical and Virological Diagnosis
Clinical symptoms of HSV reactivation with bronchopneumonitis are nonspecific, especially in ICU patients under MV. They are mainly fever, hypoxemia and purulent tracheal secretions, which can be easily confused with bacterial VAP. Oral herpetic lesions (lip ulceration or gingivostomatitis) are often associated and might be a warning sign. In ARDS patients, HSV bronchopneumonitis should be searched as the origin of ARDS especially when such signs are present or in unresolving ARDS without bacterial cause of VAP. Hemorrhagic aspect of the respiratory tractus under endoscopy can also be observed. The virological diagnosis is based on PCR in the throat and in respiratory samples. HSV detection can reflect either contamination (from mouth and/or throat for bronchial specimens) or local tracheobronchial virus excretion However, the positivity of such samples does not always mean bronchopneumonitis. Luyt et al. have described that HSV bronchopneumonitis is probable when clinical worsening, for example ARDS, is associated with positivity of lung HSV PCR and specific aspect on cytological examination of the cells in BAL or biopsy (Luyt et al., 2007). HSV-specific nuclear inclusion detection in cells recovered during BAL can diagnose parenchymal lung involvement. An easier way to diagnose HSV bronchopneumonitis could be virus-load assessment. This approach is based on the fact that the higher the virus load, the higher the incidence of HSV bronchopneumonitis. A threshold of 8 × 104 HSV copies/106 cells demonstrated a 81% sensitivity and 83% for diagnosing HSV bronchopneumonitis (Luyt et al., 2007). Linssen et al. reported that detection of more than 105 HSV-DNA copies/mL in lower respiratory material was associated with a significantly higher mortality (Linssen et al., 2008). In a recent study, a threshold of 105 copies/mL in BAL fluid or tracheal aspirates was considered as the cut-off between low and high load and associated with a probable invasive infection and patients prognosis (Schuierer et al., 2020).
How Does HSV Impact ICU/ARDS Patients Prognosis?
In one of the very first studies investigating the role of HSV in the ICU, Tuxen et al. (1987) showed that acyclovir was effective in preventing the high incidence of HSV in patients with ARDS but that it did not improve the severity of respiratory failure, the duration of ventilatory support or mortality. The authors concluded that routine prophylaxis of HSV was not recommended in ARDS patients. Bruynseels et al. (2003) found that the duration of stay in the ICU and in the hospital was significantly increased for patients with HSV reactivation in the throat, even when analyses were adjusted for disease severity. Mortality was also higher in those patients but this difference was due to disease severity.
Ong et al., in one of the largest studies available to date (Ong et al., 2004), detected active HSV replication in 27% of 393 ventilated ICU patients, which was associated with a nearly twofold increase in hospital mortality. Nevertheless, whether HSV replication in the lower respiratory tract has clinical consequences remains controversial.
When focusing specifically on HSV bronchopneumonitis patients, Luyt et al. (2007) found that they required longer MV, had more episodes of VAP, and a higher ICU length of stay. However, again, mortality was not different between patients with or without HSV bronchopneumonitis.
To better understand the proper negative role of HSV in ICU patients under MV, Luyt et al. (2019) designed an international randomized controlled trial (RCT) with a preemptive intravenous acyclovir administration. Patients under MV for at least 96 h who exhibited HSV oropharyngeal reactivation were enrolled. The main objective was the reduction of the duration of MV. Noteworthy, only 21 patients (9%) developed ARDS after randomization. Two-hundred and thirty-nine patients were enrolled and randomized. On day 60, there was no difference in the median number of ventilator-free days for acyclovir recipients and controls. Acyclovir was well-tolerated, without renal or neurologic adverse events, although the study was underpowered to assess major complications. Intriguingly, the authors reported a near significant decrease in mortality among patients randomized to acyclovir. On day 60, 26 (22%) acyclovir recipients and 39 (33%) controls had died (risk difference, 0.11, 95% CI − 0.004 to 0.22, P = .059). The hazard ratio for death within 60 days post-randomization for the acyclovir group vs. controls was 0.61 (95% CI 0.37–0.99, P = .047). Despite this trend towards lower day-60 mortality, the number of ventilator-free days was not different between groups, implying that survivors in the acyclovir group had a longer MV. This led the authors to suppose that the prolonged duration of MV in acyclovir survivors might be due to a higher number of patients with ExtraCorporeal Membrane Oxygenation (ECMO) support and a higher percentage of patients developing ARDS after randomization. One hypothesis is that acyclovir could improve the survival of mechanically ventilated patients who reactivate HSV at the cost of a prolonged MV duration. Very recently, respiratory secretions (BAL fluid or tracheal aspirates) of patients with VAP not responding to antibiotics were tested for HSV replication by quantitative real-time PCR. ICU survival times, clinical parameters, and radiographic findings were retrospectively compared between untreated and acyclovir treated patients with high (> 105 HSV copies/mL) and low (103–105 HSV copies/mL) viral load (Schuierer et al., 2020). Acyclovir improved median ICU survival and was associated with a significantly reduced hazard ratio for ICU death in high load patients only. Moreover, circulatory (norepinephrine doses) and pulmonary oxygenation function (median PaO2/FiO2 ratio) of high load patients improved significantly over the course of acyclovir treatment. The authors concluded on a putative causative role for HSV in this highly selected group of patients. The retrospective design of the study and the uncertain link between HSV positivity and VAP, in the absence of histopathologic evaluation, are the mains weaknesses of this study.
HSV Reactivation Under Veno-Venous (VV)-ECMO for Severe ARDS
In a specific cohort of 123 patients under VV-ECMO for ARDS, Hraiech et al. (2019) found that HSV reactivation in throat or BAL samples reached 54% of the patients. Patients were included only if viral reactivation occurred after ECMO implantation. HSV reactivation was associated with a longer duration of MV and ECMO as compared with non-reactivated patients. In multivariate analysis, HSV reactivation remained independently associated with a longer duration of MV and hospital length of stay suggesting a pejorative role of HSV in this very specific population. Whether ECMO triggers HSV reactivation remains to be documented.
Overall, several studies and meta-analysis (Coisel et al., 2012) suggest a negative impact of HSV reactivation in critically ill patients under MV and especially during ARDS, some studies showing a higher mortality, length of MV, ICU stay and also a higher rate of nosocomial infections (Table 1 ). The exact significance of HSV reactivation is still being debated, the main RCT evaluating a preemptive treatment failing to demonstrate an improvement on duration of MV but showed a trend towards a lower mortality. The debate being still unresolved, it seems interesting to screen HSV in upper/lower respiratory samples especially in patients with sepsis and prolonged MV or unresolved ARDS with virus-load determination when the technic is available. Acyclovir treatment might be proposed for patients with ARDS and HSV signs of bronchopneumonitis and/or high load of replication in respiratory samples.
Table 1.
Incidence, mortality and morbidity associated with HSV infection in non-immunocompromised ICU patients.
| Year/Reference | Study design | Inclusion criteria | Number of patients | HSV Detection method | Incidence of HSV reactivation | Mortality HSV +/HSV − (%) | Morbidity endpoints assessed |
|---|---|---|---|---|---|---|---|
| 1987 (Tuxen et al., 1987) | Double blind RCT | ARDS | 45 | Culture on respiratory secretions | 6% acyclovir 71% controla |
47 acyclovir 43 control |
DMV |
| 2003 (Bruynseels et al., 2003) | Prospective | Medico-surgical | 764 | Culture on throat or respiratory samples | 22% throat 16% respiratory samples |
33 vs.23b | ICU-LOSb/DMVb |
| 2003 (Cook et al., 2003) | Prospective | Surgical | 95 | Culture in blood and respiratory samples | 23% | 27 vs. 26 | ICU-LOS/DMV |
| 2004 (Ong et al., 2004) | Prospective | Medico-surgical under MV | 393 | PCR on respiratory samples | 27% | 41 vs. 24b | NA |
| 2007 (Engelmann et al., 2007) | Retrospective | Medico-surgical > 3 days ICU stay |
53 | Culture, PCR and indirect immunofluorescence on respiratory samples | 13% | 100 vs. 18b | DMVb |
| 2007 (Luyt et al., 2007) | Prospective | Medical MV > 5 days | 201 | Culture and PCR on respiratory samples | 54% | 48 vs. 42 | ICU-LOSb/DMVb/NIb |
| 2008 (Linssen et al., 2008) | Retrospective | VAP suspicion | 260 | PCR on BAL | 31% | 41 vs. 20b | NI |
| 2009 (De Vos et al., 2009) | Prospective | MV > 2 days | 105 | PCR on respiratory samples | 62% | 35 vs. 48 | DMVb/NIb |
| 2010 (Scheithauer et al., 2010) | Prospective, case control | Suspicion of pneumonia | 103 | PCR on respiratory samples | NA | 45 vs. 35 | ICU-LOSb/DMVb/NIb |
| 2010 (Smith et al., 2010) | Prospective | Patients under MV | 174 | PCR on respiratory samples | 66% | 33 vs. 32b | NA |
| 2011 (Bouza et al., 2011) | Prospective | VAP | 177 | Culture on respiratory samples | 13% | 77 vs. 57 | ICU-LOSb/DMVb/NIb |
| 2012 (Coisel et al., 2012) | Prospective | Pneumonia | 93 | PCR on BAL or IgM + | 24% | 42 vs. 20 | ICU-LOS/DMV/NI |
| 2012 (Costa et al., 2012) | Prospective | VAP suspicion | 237 | PCR on respiratory samples | 32% | 51 vs. 27b | MVb/ICU admissionb/Co-infectionb |
| 2019 (Hraiech et al., 2019) | Retrospective | Severe ARDS with VV ECMO > 2 days | 123 | PCR on throat or BAL | 49% | 48 vs. 59 | ICU-LOSb/DMVb/ECMODb |
| 2019 (Luyt et al., 2019) | Double blind RCT | MV > 96H, HSV reactivation | 239 | PCR on oropharyngeal swab | NA | 22 acyclovir vs. 33 placebo | ICU-LOS/DMV/NI |
| 2020 (Schuierer et al., 2020) | Retrospective | VAP not responding to antibiotics | 425 | PCR on respiratory samples | 30% | HR of death = 0.31, 95% (CI 0.11–0.92, P = .03) in high load patients with acyclovir treatment as compared with no treatment | Improved PaO2/FiO2, decreased norepinephrine doses over time in acyclovir treated patients with high load reactivationb |
BAL, broncho-alveolar lavage; DMV, duration of mechanical ventilation; ECMO, extracorporeal membrane oxygenation; ECMOD, ECMO duration; HR, hazard ratio; HSV, Herpes Simplex Virus; ICU-LOS, intensive care unit length of stay; NA, not available; NI, nosocomial infections; RCT, randomized controlled trial.
P < .05 between acyclovir treated patients and controls.
P < .05 between HSV positive and HSV negative patients.
Treatment
Acyclovir is proposed to treat HSV in ICU patients and has been shown to be safe in this indication (Luyt et al., 2019). A drug regimen of 5 mg/kg/8 h during 14–21 days is usually used in subjects with a normal renal function. Acute renal failure, hepatitis, hyperbilirubinemia and skin rash are the most commonly reported adverse events (Table 2 ).
Table 2.
Summary of the main antiviral treatments used for herpesviruses infections and their potential side-effects.
| Drug | Antiviral effect | Dose regimen and duration | Drug adjustment in case of renal replacement therapy | Side effects |
|---|---|---|---|---|
| Acyclovir | HSV1/HSV2/VZV | 5 mg/kg/8 h 14–21 days |
5 mg/kg/12 h (5 mg/kg/24 h if estimated CrCl < 25 mL/min) | Acute renal failure Hepatitis Hyperbilirubinemia Skin rash |
| Ganciclovir | CMV/HSV1/HSV2/VZV | 5 mg/kg/12 h 14–21 days |
2,5 mg/kg/12 h | Leuconeutropenia Thrombocytopenia Anemia Coma Seizure Hepatitis Hyperbilirubinemia Skin rash Acute renal failure |
| Foscarnet | CMV/HSV1/HSV2/VZV | Initial therapy: 60 mg/kg/8 h Maintenance treatment: 90–120 mg/kg/d Associated hydration |
35 mg/Kg/8 h (initial therapy) | Acute renal failure Hypocalcemia Paresthesia Nausea, vomiting Pancreatitis |
| Cidofovir | CMV | Initial therapy: 5 mg/kg/week for 2 weeks Maintenance treatment: 5 mg/kg/week every twice week |
Contraindication | Acute renal failure Fever, asthenia Nausea, vomiting Skin rash |
CMV, Cytomegalovirus; HSV1, Herpes simplex virus 1; HSV2, Herpes simplex virus 2; VZV, Varicella-zoster virus; CrCl, creatinine clearance.
CMV in ICU Patients
Mechanisms of Reactivation
The transition from latency to viral reactivation for CMV involves a certain degree of immunosuppression, even in patients with no previous immune dysfunction. Tumor necrosis factor (TNF) is probably involved in CMV reactivation after sepsis from a bacterial origin (Cook et al., 2006a; Hummel and Abecassis, 2002). It has been shown that TNF-α is able to directly stimulate immediate early (IE) CMV gene expression. Other mechanisms might also be involved in CMV reactivation.
The term “immunoparalysis” has been used to describe the abnormalities of immune system that has been reported in critically ill patients early during the ICU stay as a result of the underlying disease and/or the treatment (Hotchkiss et al., 2013). Studies of severely immunocompromised patients have suggested that T cell immunity is crucial in the control of CMV replication (Boeckh et al., 2015).
In particular, CMV replication was shown to be higher in patients with undetectable IFN-γ T cell responses than in patients with detectable responses (Clari et al., 2013). Other data suggest the crucial role of NK cells in keeping herpesviruses latent in humans (Biron et al., 1989). In a recent study done in ICU patients, the function of NK cells was altered regarding interferon-γ production just before the occurrence of reactivation (Chiche et al., 2012). Impaired natural killer cell function with reduced interferon-γ secretion precedes the occurrence of CMV reactivation among previously immunocompetent critically ill patients. This latter results suggest that in the context of global and major lymphopenia observed in ICU patients, dysfunction in NK cells may be involved in CMV reactivation. CMV is also involved in the impaired function of dendritic cells (Avdic et al., 2014).
Frequency of CMV Reactivation Among ICU Patients
CMV reactivation in non-immunocompromised ICU patients has been largely assessed during the last three decades (Domart et al., 1990; Limaye et al., 2008; Kalil and Florescu, 2009; Coisel et al., 2012) (Table 3 ). In a meta-analysis gathering studies with heterogeneous diagnostic methods, Kalil and Florescu (2009) found that overall, about 20% of ICU patients exhibit a CMV reactivation during their ICU stay. Of course, the serological status and the diagnostic methods are determining factors. CMV reactivation is diagnosed in approximately 33% of ICU seropositive patients suffering from a large variety of critical illnesses such as sepsis, cardiac failure, burns and trauma (Limaye et al., 2008). In another large cohort of medical patients mainly under MV and presenting a septic shock, 16% developed an active CMV infection as diagnosed by positive antigenemia and/or positive rapid viral culture in BAL (Chiche et al., 2012). Apart from CMV seropositivity, severity of the disease, sepsis and septic shock, and a length of ICU stay > 5 days are the main risks factors for CMV infection (Kalil and Florescu, 2009). In a match-controlled study, renal failure and steroid use were also described as risk factors (Jaber et al., 2005). CMV reactivation generally occurs later than HSV, around the 14th day of ICU stay when considering PCR in respiratory samples, and after the third week for blood samples PCR (Heininger et al., 2011).
Table 3.
Incidence, mortality and morbidity associated with CMV infection in non-immunocompromised ICU patients.
| Year/Reference | Study design | Inclusion criteria | Number of patients | CMV Detection method | Incidence of CMV reactivation (%) | Mortality CMV +/CMV −(%) | Morbidity endpoints assessed |
|---|---|---|---|---|---|---|---|
| 1990 (Domart et al., 1990) | Retrospective | Mediastinitis after cardiac surgery | 115 | Culture of blood and urine | 25 | 55 vs. 37 | Higher LOS in CMV + patients |
| 1998 (Kutza et al., 1998) | Prospective | Sepsis | 34 | Antigenemia + PCR in blood | 32 | 64 vs. 74 | NA |
| 1998 (Cook et al., 1998) | Retrospective | Sepsis | 142 | Culture in blood or BAL | 8 | 66 vs. 35a | ICU-LOS |
| 2001 (Heininger et al., 2001) | Prospective | SAPS II < 40, CMV seropositive | 56 | Culture and PCR in blood/tracheal secretions | 35 | 55 vs. 36 | ICU-LOSa |
| 2003 (Cook et al., 2003) | Prospective | ICU-LOS > 5 days | 104 | Culture in blood and tracheal secretions | 10 | 50 vs. 27 | ICU-LOSa/DMVa/NIa |
| 2005 (Jaber et al., 2005) | Retrospective, case control | Fever > 72 h without proven infection | 237 | Antigenemia | 17 | 50 vs. 28a | ICU-LOSa/DMVa/NIa |
| 2006 (von Müller et al., 2006) | Prospective | Septic shock | 25 | Antigenemia + culture in blood/tracheal secretions/urine | 32 | 63 vs. 35 | ICU-LOSa/DMVa/NIa |
| 2008 (Limaye et al., 2008) | Prospective | Burn, trauma and sepsis | 120 | PCR in blood | 33 | adjusted odds ratio 4.3 (95% CI, 1.6–11.9) for continued hospitalization or death by 30 days | |
| 2008 (Ziemann et al., 2008) | Retrospective | ICU-LOS > 14 days | 99 | PCR in blood | 35 | 29 vs. 11a | ICU-LOSa |
| 2009 (Chiche et al., 2009) | Prospective | MV > 2 days | 242 | AG ± BAL culture | 16 | 54 vs. 37a | ICU-LOSa/DMVa/NIa |
| 2011 (Bordes et al., 2011) | Prospective | Burns | 21 | PCR in blood | 71 | 33 vs. 20 | ICU-LOS/DMV |
| 2011 (Heininger et al., 2011) | Prospective | Sepsis | 86 | Culture or PCR in blood and tracheal secretions | 41 | 37 vs. 35 | H-LOSa/ICU-LOSa/DMVa |
| 2012 (Coisel et al., 2012) | Prospective | Pneumonia | 93 | Antigenemia + PCR in blood | 24 | 55 vs. 20a | ICU-LOSa/NI |
| 2014 (Bravo et al., 2014) | Prospective | Surgical ICU | 78 | PCR in blood and low respiratory tract and saliva | 46 | 56 vs. 36 | ICU LOSa/DMVa |
| 2015 (Frantzeskaki et al., 2015) | Prospective | MV CMV seropositive | 80 | PCR in blood | 14 | 45 vs. 27 | ICU-LOS/NI |
| 2016 (Cook et al., 2006b) | Prospective | ARDS MV > 4 days | 271 | PCR in blood | 27 | 31 vs. 15a | DMVa |
| 2017 (Forel et al., 2014) | Prospective | Septic shock and ICU LOS > 4 days | 399 | PCR in blood | 27 | 33 vs. 23a | NA |
| 2017 (Hraiech et al., 2017) | Open-label RCT | CMV seropositive > 24 h MV | 124 | PCR in blood, urine, throat, lung | 27 control 0.02 valganciclovirb 0.06 valacyclovirb |
15.9 control 41.2 valacyclovirb 21.7 valganciclovir |
Organ failure free-days, ICU LOS |
| 2017 (Cowley et al., 2017) | Double blind RCT | CMV seropositive > 24 h MV | 156 | PCR in blood, BAL and throat | 10/84 (12) ganciclovirb 28/72 (39) control |
15 placebo 12 Ganciclovir |
VFDa ICU-LOS/DMV/NI |
| 2019 (Hraiech et al., 2019) | Retrospective | Severe ARDS with VV ECMO > 2 days | 123 | PCR in blood or BAL or AG | 22 | 52 vs. 59 | ICU-LOSa/DMVa/ECMODa |
ARDS, acute respiratory distress syndrome; BAL, broncho-alveolar lavage; DMV, duration of mechanical ventilation; ECMO, extracorporeal membrane oxygenation; ECMOD, ECMO duration; HR, hazard ratio; HSV, Herpes Simplex Virus; ICU-LOS, intensive care unit length of stay; LOS, length of stay; NA, not available; NI, nosocomial infections; RCT, randomized controlled trial; VFD, ventilator-free days and alive at day 28.
P < .05 between CMV positive and CMV negative patients.
P < .05 as compared with control.
Clinical and Virological Diagnosis
As for HSV reactivation, CMV infection often gives non-specific signs. The typical figure is represented by a patient in the 2nd-3rd week of ICU stay with moderate fever, respiratory worsening with gas exchange impairment and chest radiograph modification, and negativity of bacterial samples, hepatic cytolysis or cholestasis. In this form, diagnosis is not easy and routine PCR monitoring could be useful, especially in seropositive patients at ICU admission (Papazian et al., 2016). The clinical picture is sometimes more obvious, including de novo or persistent ARDS, hepatitis, gastritis or colitis with diarrhea (Siciliano et al., 2014), cytopenia with hemophagocytosis in myelogram. It is fundamental to highlight that there is no radiological specificity and in particular, interstitial pneumonia as it is classically described in HIV patients is very uncommon (Fig. 2 ).
Fig. 2.

The radiological (chest radiographs and scans) patterns of HSV and CMV reactivation among ICU patients. (A) A 69 old man with suspicion of VAP with fever, gaz exchange worsening and chest radiograph infiltrates. BAL found no bacteria only positive CMV PCR. (B) A 62 years old man with history of bacterial pleuro-pneumonia and suspicion of VAP. CT scan found bilateral bronchiolar micronodules evocating pneumonia. Bacteriological culture of BAL performed without antibiotics was sterile. CMV PCR in blood and BAL was positive. (C) A 36 years old man with severe ARDS under VV-ECMO, fever and gaz exchange worsening. BAL culture found S. aureus and K. pneumonia but also throat, blood and BAL HSV reactivation and liver cytolysis.
The incidence of CMV reactivation also depends on the diagnosis method. Three technics have been used: viral cultures, antigenemia, and PCR. Culture-based techniques are considered outmoded because they are time-consuming and lack sensitivity. When analyzing only the studies that evaluated CMV infections by PCR/antigen in patients with positive CMV serology and ≥ 5 days in ICU, Kalil and Florescu (2009) found that the rate of active CMV infection increased to 36% as compared to 21% when including former studies based on culture diagnosis. The antigenemia assay is a technique based on direct detection of the CMV protein pp65 using monoclonal antibodies. It is sensitive, specific and quantitative. However, this technique is progressively replaced by PCR assays, given their high sensitivity and rapid turnover time. Quantitative PCR has been used to evaluate the severity of infection. Limaye et al. (2008) showed that a plasma CMV load > 1000 copies/mL occurred in 20% of seropositive patients. This population had a much higher risk for death or prolonged ICU stay by day-30 as compared to patients negative for CMV, whereas this risk was not so pronounced in patients with CMV reactivation at any level. At the moment, no specific threshold for the diagnosis can be proposed in either blood or respiratory samples. Some authors suggested that CMV PCR, when performed on respiratory samples, is a more sensitive technic than when performed in plasma (Heininger et al., 2011).
The Role of CMV in ICU/ARDS Patients Prognosis (Fig. 3)
Fig. 3.

The pathophysiological mechanisms by which CMV could alter the prognosis of ICU patients.
Several meta-analysis found that all-cause mortality was higher in ICU patients developing a CMV infection during their stay. In a meta-analysis on 13 studies with a total of 1258 patients, the mortality rate associated with active CMV infection was 1.93 times as high as that for patients without infection (Kalil and Florescu, 2009). A more recent meta-analysis (Li et al., 2018) on 18 studies involving 2398 immunocompetent patients admitted to the ICU reported that for CMV seropositive patients, the odds ratio for mortality in patients with CMV reactivation as compared with patients without CMV reactivation was 1.72. Patients with CMV infection required significantly longer MV and duration of ICU stay than patients without CMV infection. However, when analysis was limited to detection in blood, CMV infection without antiviral drug treatment or reactivation was not significantly associated with higher mortality. In patients with CMV infection and a VAP suspicion, mortality at day 60 was higher as compared with control patients. This difference remained significant after adjusting for age, SAPS II score on admission and SOFA score on the day of diagnosis (Coisel et al., 2012) . Furthermore, in 242 mechanically ventilated medical ICU patients (Chiche et al., 2009), active CMV infection was associated with an increased duration of MV in survivors presenting with an active CMV infection relative to controls. The number of ventilator free days and alive by day 60 was also dramatically reduced when patients developed a CMV infection. In the same study, the incidence of VAP as well as the occurrence of other nosocomial infections was higher in CMV positive patients. Two other studies reported an increased risk of nosocomial bacteremia during the ICU stay (Jaber et al., 2005; Coisel et al., 2012). Of course, the increased duration of MV might be a confounder. However, it is possible that CMV reactivation plays an immunosuppressive role, leading to an enhanced susceptibility towards bacterial infection. This has been suggested in a mice model of CMV reactivation triggered by ceacal-ligation and puncture (CLP), in which CMV positive mice developed abscessing form of staphylococcal pneumonia after exposure to Staphylococcus aureus whereas CMV negative mice had cleared bacteria from lungs within 5 days (Hraiech et al., 2017). Despite these results, two recently published studies failed to demonstrate an efficacy of CMV treatment. In a single-center, open-label, randomized, controlled clinical trial on CMV-seropositive patients undergoing MV for at least 24 h (Cowley et al., 2017), an anti-CMV prophylaxis with valacyclovir or low-dose valganciclovir reduced the rate of reactivation but the valacyclovir arm was halted prematurely because of higher mortality. Valganciclovir did not reduce mortality. The rate of reactivation in the control group was of 27%. This study shows the limit of the prophylactic treatment strategy in which ¾ of the patients are unnecessarily treated, with the risk of developing adverse events.
In a double-blind, randomized, placebo-controlled trial (Limaye et al., 2017), Limaye et al. included 156 seropositive mechanically ventilated patients (mainly sepsis), to assess change in IL-6 at day 14, according to treatment with IV ganciclovir or placebo, considering that ganciclovir would decrease the inflammation induced by CMV reactivation. The primary outcome was not different between groups. However, in prespecified exploratory analyses among the sepsis subset (88% of the enrolled cohorts), there were several improved outcomes in the ganciclovir arm, including a higher number of ventilator-free days, shorter duration of MV, and higher PaO2/FiO2 ratio among ventilated patients. In addition, a post hoc exploratory analysis among patients with sepsis who survived through day 28 showed a significantly shorter duration of MV in the ganciclovir arm (4 vs. 6.5 median days, P = .006).
CMV and ARDS
CMV reactivation and ARDS share tight links. CMV pneumonia has been described as a frequent cause of persistent ARDS. Papazian et al. unexpectedly found histological signs of CMV pneumonia in 25/86 ARDS patient’s surgical biopsies after more than 7 days of MV (Fig. 1). CMV was the only pathogen in most of time (Papazian et al., 1996). This work was the reason for performing a larger series of 100 open lung biopsies in ARDS in which 30 were positive for CMV infection (Papazian et al., 2007). Noteworthy, CVM and lung fibrosis were associated in four cases. It is probable that CMV enhances the progression of post-aggressive lung fibrosis because of its pro-inflammatory properties. This has been demonstrated in a mouse model of CLP inducing CMV reactivation. Three weeks after the surgical trigger, CMV reactivated mice had a much higher risk of lung fibrosis which was reversed in animals treated with ganciclovir (Cook et al., 2006b). In a large cohort of 399 patients with ARDS who required MV for more than 4 days, Ong et al. (2016) demonstrated that seropositive patients with CMV reactivation had both a longer duration of MV and higher mortality as compared to subjects without reactivation. The population-attributable fraction of ICU mortality due to CMV reactivation was estimated at 23% by day 30, meaning an absolute mortality difference of 4.4%. The authors concluded that CMV reactivation was independently associated with increased case fatality in immunocompetent ARDS patients who are CMV seropositive. In severe ARDS patients requiring VV-ECMO (Hraiech et al., 2019), CMV reactivation either in blood or BAL occurred in 40% of patients. CMV was most often combined with HSV reactivation. Patients with CMV reactivation had a prolonged duration of MV and ICU length of stay as compared with patients with no herpesviruses reactivation.
CMV Treatment
Pending the results of the ongoing and future trials, the use of a curative antiviral treatment should be considered when there is a CMV reactivation (positive antigenaemia and/or PCR) associated with clinical signs of infection (for example persistent ARDS) meaning CMV end-organ disease (Fig. 4 ) especially when risk factors are present. Pre-emptive treatment (CMV reactivation without clinical signs of infection) is currently under analyze and the result of a RCT should be soon known (NCT02152358). When CMV PCR is positive without any clinical signs, unless viral load is very high (greater than 10,000 copies/mL), trends in viral load may be more useful than a given value (Papazian et al., 2016; Forel et al., 2014). Ganciclovir, 5 mg/kg twice daily, during 14–21 days is the first line treatment when necessary. The main side-effects are hematological and renal toxicity. Foscarnet or cidofovir can be an alternative in case of occurrence of cytopenia but are associated with greater risk of renal failure (Table 2).
Fig. 4.
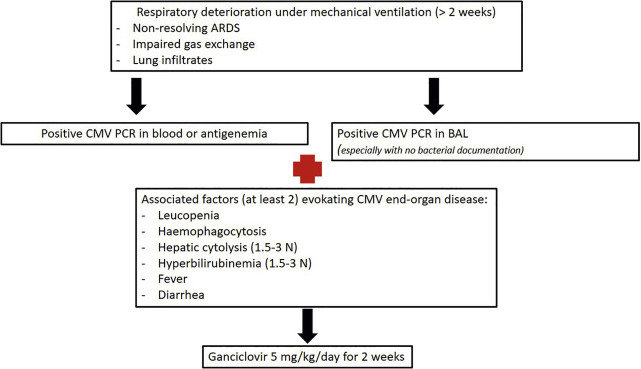
A propositional algorithm for the treatment of CMV reactivation with end-organ disease signs in ARDS patients.
HSV and CMV Co-reactivation
Co-infections of HSV/CMV have also been reported in as high as 27% of CMV reactivations (Coisel et al., 2012). In severe ARDS patients undergoing VV-ECMO, HSV/CMV co-reactivation occurred in 30% of cases and was associated with worse outcomes (Hraiech et al., 2019). Co-reactivation patients had a longer duration of MV and ICU stay as compared to HSV or CMV alone and non-reactivated patients. They also had a longer ECMO duration and hospital length of stay.
EBV and HHV-6
Besides HSV and CMV, other herpesviruses have been recently described in ICU patients. Their significance is not yet well understood.
EBV was found to be frequently retrieved in BAL and blood of ICU non-immunocompromised patients. In 329 patients with septic shock, Ong et al. described 157 (48%) EBV detection in the blood with an association with a higher mortality in reactivated patients (Ong et al., 2017). In a prospective observational study including 90 patients (Libert et al., 2015) with an ICU stay of ≥ 5 days, EBV was the most frequently reactivated herpesvirus (68%), before HSV and CMV. Co-reactivation with either HSV or CMV was frequent. An association between EBV reactivation and a higher mortality, length of MV and ICU stay as compared with non-EBV reactivated patients was found. Patients with two or more herpesviruses reactivation, whatever the subtype (EBV, CMV, or HSV), had longer duration of MV or ICU stay. In a series of 54 patients (Bonizzoli et al., 2016) admitted in ICU with a diagnosis of ARDS, without a known microbiological causative agent, EBV was detected in 23 of 54 patients (43%) in respiratory samples (BAL or throat sample), either as a single infection or as mixed infection (with HSV or CMV).
Overall, the role of EBV reactivation in ICU and specifically in ARDS patients is not yet clearly defined and a specific treatment against EBV premature.
It has also been reported that 49% of the patients with CMV reactivation also reactivated HHV-6 (Lopez Roa et al., 2015). The impact on outcome was synergistic between the two viruses. Indeed, the patients with co-reactivation of both HHV-6 and CMV had the greatest risk for death or continued hospitalization by day 30.
Discussion and Conclusion
Herpesviruses pathogenicity were first reported in transplant recipients, and they have been increasingly documented in critically ill patients since then. HSV and CMV reactivations are frequent in ICU patients and associated with mortality, prolonged MV and ICU stay in several observational studies and meta-analysis. An association with bacterial sepsis has also been reported, in animal and clinical studies. In ARDS patients, herpesviruses reactivation go hand in hand with an impaired prognosis and may prolong ECMO duration. However, a statistical association does not necessarily mean a causal link. Randomized controlled trials published to date have not permitted to know if herpesviruses are simple bystanders, witnesses of the severity of the disease or real pathogens (Schildermans and De Vlieger, 2020). In some cases, they are associated with a true disease; but sometimes, the relationship between viral reactivation and symptoms is not established. In these later cases, whereas a specific antiviral treatment may improve outcomes remains to be determined. To date, neither prophylactic acyclovir to prevent HSV reactivation nor prophylactic ganciclovir to prevent CMV reactivation can be recommended (Cantan et al., 2019). Preemptive treatment with acyclovir in patients with oropharyngeal HSV did not impact MV duration although the difference of mortality between groups was disturbing (Luyt et al., 2019). Moreover, two interventional studies have shown negative results and one was even stopped early because of higher mortality in patients who received antiviral treatment (Cowley et al., 2017; Limaye et al., 2017). The second study showed an increase in ventilator-free days at day 28 in patients with sepsis. Although this was a secondary endpoint in a subgroup, it warrants further research. Preemptive treatment of CMV reactivation with ganciclovir is under investigation (PTH [Preemptive Treatment for Herpesviridae] study, Clinical Trials no NCT02152358). Curative treatment of HSV bronchopneumonitis or CMV lung disease is based on expert opinions in patients with either cytological/histological proofs of lung involvement, high viral load, or specific clinical and biological patterns suggestive of CMV (Papazian et al., 2016; Cantan et al., 2019). Considering the pulmonary tropism of herpesviruses and the role of HSV in bronchopneumonitis and CMV in lung fibrosis, reactivation should be investigated in unresolving or worsening ARDS, rather by PCR in BAL.
In the light of what previous studies learned to us, some question persist and research perspectives should aim to:
-
-
Better scope the patients susceptible to have a reactivation and in whom it could be deleterious (seropositive, under prolonged VM, with sepsis and/or ARDS).
-
-
Better define reactivation from a virological point of view (blood, throat, lung samples? threshold of PCR? Use of quantitative real time PCR?)
-
-
Determine the place of tests assessing marker of upcoming reactivation (IFNγ production by CMV-specific T lymphocytes upon exposure to CMV-antigens) (Castón et al., 2016).
-
-
Assess the efficacy of curative treatment, in patients with reactivation and one or more signs evocating end organ disease, especially ARDS.
-
-
Precise the role of “emerging” herpesviruses such as EBV and HHV6.
References
- Avdic S., McSharry B.P., Slobedman B. Modulation of dendritic cell functions by viral IL-10 encoded by human cytomegalovirus. Frontiers in Microbiology. 2014;5:337. doi: 10.3389/fmicb.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Biron C.A., Byron K.S., Sullivan J.L. Severe herpesvirus infections in an adolescent without natural killer cells. The New England Journal of Medicine. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Boeckh M., Murphy W.J., Peggs K.S. Recent advances in cytomegalovirus: An update on pharmacologic and cellular therapies. Biology of Blood and Marrow Transplantation. 2015;21(1):24–29. doi: 10.1016/j.bbmt.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Bonizzoli M., Arvia R., di Valvasone S., Liotta F., Zakrzewska K., Azzi A., et al. Human herpesviruses respiratory infections in patients with acute respiratory distress (ARDS) Medical Microbiology and Immunology. 2016;205(4):371–379. doi: 10.1007/s00430-016-0456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordes J., Gaillard T., Maslin J., Esnault P., Goutorbe P., Brisou P. Cytomegalovirus infection monitored by quantitative real-time PCR in critically ill patients. Critical Care (London, England) 2011;15(2):412. doi: 10.1186/cc10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouza E., Giannella M., Torres M.V., Catalán P., Sánchez-Carrillo C., Hernandez R.I., et al. Herpes simplex virus: A marker of severity in bacterial ventilator-associated pneumonia. Journal of Critical Care. 2011;26(4) doi: 10.1016/j.jcrc.2010.10.008. 432.e1–e6. [DOI] [PubMed] [Google Scholar]
- Bradley H., Markowitz L.E., Gibson T., McQuillan G.M. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. The Journal of Infectious Diseases. 2014;209(3):325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- Bravo D., Clari M.A., Aguilar G., Belda J., Giménez E., Carbonell J.A., et al. Looking for biological factors to predict the risk of active cytomegalovirus infection in non-immunosuppressed critically ill patients. Journal of Medical Virology. 2014;86(5):827–833. doi: 10.1002/jmv.23838. [DOI] [PubMed] [Google Scholar]
- Bruynseels P., Jorens P.G., Demey H.E., Goossens H., Pattyn S.R., Elseviers M.M., et al. Herpes simplex virus in the respiratory tract of critical care patients: A prospective study. Lancet. 2003;362(9395):1536–1541. doi: 10.1016/S0140-6736(03)14740-X. [DOI] [PubMed] [Google Scholar]
- Cantan B., Luyt C.-E., Martin-Loeches I. Influenza infections and emergent viral infections in intensive care unit. Seminars in Respiratory and Critical Care Medicine. 2019;40(04):488–497. doi: 10.1055/s-0039-1693497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castón J.J., Cantisán S., González-Gasca F., Páez-Vega A., Abdel-Hadi H., Illescas S., et al. Interferon-γ production by CMV-specific CD8+ T lymphocytes provides protection against cytomegalovirus reactivation in critically ill patients. Intensive Care Medicine. 2016;42(1):46–53. doi: 10.1007/s00134-015-4077-6. [DOI] [PubMed] [Google Scholar]
- Chiche L., Forel J.-M., Roch A., Guervilly C., Pauly V., Allardet-Servent J., et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Critical Care Medicine. 2009;37(6):1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- Chiche L., Forel J.-M., Thomas G., Farnarier C., Cognet C., Guervilly C., et al. Interferon-γ production by natural killer cells and cytomegalovirus in critically ill patients. Critical Care Medicine. 2012;40(12):3162–3169. doi: 10.1097/CCM.0b013e318260c90e. [DOI] [PubMed] [Google Scholar]
- Clari M.A., Aguilar G., Benet I., Belda J., Giménez E., Bravo D., et al. Evaluation of cytomegalovirus (CMV)-specific T-cell immunity for the assessment of the risk of active CMV infection in non-immunosuppressed surgical and trauma intensive care unit patients. Journal of Medical Virology. 2013;85(10):1802–1810. doi: 10.1002/jmv.23621. [DOI] [PubMed] [Google Scholar]
- Coisel Y., Bousbia S., Forel J.-M., Hraiech S., Lascola B., Roch A., et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cook C.H., Yenchar J.K., Kraner T.O., Davies E.A., Ferguson R.M. Occult herpes family viruses may increase mortality in critically ill surgical patients. American Journal of Surgery. 1998;176(4):357–360. doi: 10.1016/s0002-9610(98)00205-0. [DOI] [PubMed] [Google Scholar]
- Cook C.H., Martin L.C., Yenchar J.K., Lahm M.C., McGuinness B., Davies E.A., et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Critical Care Medicine. 2003;31(7):1923–1929. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- Cook C.H., Trgovcich J., Zimmerman P.D., Zhang Y., Sedmak D.D. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. Journal of Virology. 2006;80(18):9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.H., Zhang Y., Sedmak D.D., Martin L.C., Jewell S., Ferguson R.M. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Critical Care Medicine. 2006;34(3):842–849. doi: 10.1097/01.ccm.0000201876.11059.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C., Sidoti F., Saldan A., Sinesi F., Balloco C., Simeone S., et al. Clinical impact of HSV-1 detection in the lower respiratory tract from hospitalized adult patients. Clinical Microbiology and Infection. 2012;18(8):E305–E307. doi: 10.1111/j.1469-0691.2012.03882.x. [DOI] [PubMed] [Google Scholar]
- Cowley N.J., Owen A., Shiels S.C., Millar J., Woolley R., Ives N., et al. Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically Ill patients: A randomized clinical trial. JAMA Internal Medicine. 2017;177(6):774–783. doi: 10.1001/jamainternmed.2017.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos N., Van Hoovels L., Vankeerberghen A., Van Vaerenbergh K., Boel A., Demeyer I., et al. Monitoring of herpes simplex virus in the lower respiratory tract of critically ill patients using real-time PCR: A prospective study. Clinical Microbiology and Infection. 2009;15(4):358–363. doi: 10.1111/j.1469-0691.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- Domart Y., Trouillet J.L., Fagon J.Y., Chastre J., Brun-Vezinet F., Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest. 1990;97(1):18–22. doi: 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- Engelmann I., Gottlieb J., Meier A., Sohr D., Ruhparwar A., Henke-Gendo C., et al. Clinical relevance of and risk factors for HSV-related tracheobronchitis or pneumonia: Results of an outbreak investigation. Critical Care (London, England) 2007;11(6):R119. doi: 10.1186/cc6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forel J.-M., Martin-Loeches I., Luyt C.-E. Treating HSV and CMV reactivations in critically ill patients who are not immunocompromised: Pro. Intensive Care Medicine. 2014;40(12):1945–1949. doi: 10.1007/s00134-014-3445-y. [DOI] [PubMed] [Google Scholar]
- Frantzeskaki F.G., Karampi E.-S., Kottaridi C., Alepaki M., Routsi C., Tzanela M., et al. Cytomegalovirus reactivation in a general, nonimmunosuppressed intensive care unit population: Incidence, risk factors, associations with organ dysfunction, and inflammatory biomarkers. Journal of Critical Care. 2015;30(2):276–281. doi: 10.1016/j.jcrc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Gkrania-Klotsas E., Langenberg C., Sharp S.J., Luben R., Khaw K.-T., Wareham N.J. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clinical Infectious Diseases. 2013;56(10):1421–1427. doi: 10.1093/cid/cit083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heininger A., Jahn G., Engel C., Notheisen T., Unertl K., Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Critical Care Medicine. 2001;29(3):541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Heininger A., Haeberle H., Fischer I., Beck R., Riessen R., Rohde F., et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Critical Care (London, England) 2011;15(2):R77. doi: 10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R.S., Coopersmith C.M., McDunn J.E., Ferguson T.A. The sepsis seesaw: Tilting toward immunosuppression. Nature Medicine. 2009;15(5):496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nature Reviews. Immunology. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraiech S., Bordes J., Mège J.L., de Lamballerie X., Charrel R., Bechah Y., et al. Cytomegalovirus reactivation enhances the virulence of Staphylococcus aureus pneumonia in a mouse model. Clinical Microbiology and Infection. 2017;23(1):38–45. doi: 10.1016/j.cmi.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Hraiech S., Bonnardel E., Guervilly C., Fabre C., Loundou A., Forel J.-M., et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Annals of Intensive Care. 2019;9(1):142. doi: 10.1186/s13613-019-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Abecassis M.M. A model for reactivation of CMV from latency. Journal of Clinical Virology. 2002;25(supplement 2):S123–S136. doi: 10.1016/s1386-6532(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Jaber S., Chanques G., Borry J., Souche B., Verdier R., Perrigault P.-F., et al. Cytomegalovirus infection in critically ill patients. Chest. 2005;127(1):233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- Kalil A.C., Florescu D.F. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Critical Care Medicine. 2009;37(8):2350–2358. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- Kutza A.S., Muhl E., Hackstein H., Kirchner H., Bein G. High incidence of active cytomegalovirus infection among septic patients. Clinical Infectious Diseases. 1998;26(5):1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- Li X., Huang Y., Xu Z., Zhang R., Liu X., Li Y., et al. Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: A systematic review and meta-analysis. BMC Infectious Diseases. 2018;18(1):289. doi: 10.1186/s12879-018-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert N., Bigaillon C., Chargari C., Bensalah M., Muller V., Merat S., et al. Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomedical Journal. 2015;38(1):70–76. doi: 10.4103/2319-4170.132905. [DOI] [PubMed] [Google Scholar]
- Limaye A.P., Kirby K.A., Rubenfeld G.D., Leisenring W.M., Bulger E.M., Neff M.J., et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A.P., Stapleton R.D., Peng L., Gunn S.R., Kimball L.E., Hyzy R., et al. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: A randomized clinical trial. JAMA. 2017;318(8):731–740. doi: 10.1001/jama.2017.10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linssen C.F.M., Jacobs J.A., Stelma F.F., van Mook W.N.K.A., Terporten P., Vink C., et al. Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Medicine. 2008;34(12):2202–2209. doi: 10.1007/s00134-008-1231-4. [DOI] [PubMed] [Google Scholar]
- Lopez Roa P., Hill J.A., Kirby K.A., Leisenring W.M., Huang M.-L., Santo T.K., et al. Coreactivation of human herpesvirus 6 and Cytomegalovirus is associated with worse clinical outcome in critically ill adults. Critical Care Medicine. 2015;43(7):1415–1422. doi: 10.1097/CCM.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt C.-E., Combes A., Deback C., Aubriot-Lorton M.-H., Nieszkowska A., Trouillet J.-L., et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. American Journal of Respiratory and Critical Care Medicine. 2007;175(9):935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- Luyt C.-E., Combes A., Nieszkowska A., Trouillet J.-L., Chastre J. Viral infections in the ICU. Current Opinion in Critical Care. 2008;14(5):605–608. doi: 10.1097/MCC.0b013e32830f1e12. [DOI] [PubMed] [Google Scholar]
- Luyt C.-E., Bréchot N., Chastre J. What role do viruses play in nosocomial pneumonia? Current Opinion in Infectious Diseases. 2014;27(2):194–199. doi: 10.1097/QCO.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Luyt C.-E., Forel J.-M., Hajage D., Jaber S., Cayot-Constantin S., Rimmelé T., et al. Acyclovir for mechanically ventilated patients with herpes simplex virus oropharyngeal reactivation: A randomized clinical trial. JAMA Internal Medicine. 2019;180(2):263–272. doi: 10.1001/jamainternmed.2019.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong G.M., Lowry K., Mahajan S., Wyatt D.E., Simpson C., O’Neill H.J., et al. Herpes simplex type 1 shedding is associated with reduced hospital survival in patients receiving assisted ventilation in a tertiary referral intensive care unit. Journal of Medical Virology. 2004;72(1):121–125. doi: 10.1002/jmv.10524. [DOI] [PubMed] [Google Scholar]
- Ong D.S.Y., Spitoni C., Klein Klouwenberg P.M.C., Verduyn Lunel F.M., Frencken J.F., Schultz M.J., et al. Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Medicine. 2016;42(3):333–341. doi: 10.1007/s00134-015-4071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D.S.Y., Bonten M.J.M., Spitoni C., Verduyn Lunel F.M., Frencken J.F., Horn J., et al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clinical Infectious Diseases. 2017;64(9):1204–1210. doi: 10.1093/cid/cix120. [DOI] [PubMed] [Google Scholar]
- Papazian L., Fraisse A., Garbe L., Zandotti C., Thomas P., Saux P., et al. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84(2):280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- Papazian L., Doddoli C., Chetaille B., Gernez Y., Thirion X., Roch A., et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Critical Care Medicine. 2007;35(3):755–762. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- Papazian L., Hraiech S., Lehingue S., Roch A., Chiche L., Wiramus S., et al. Cytomegalovirus reactivation in ICU patients. Intensive Care Medicine. 2016;42(1):28–37. doi: 10.1007/s00134-015-4066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebody R.G. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sexually Transmitted Infections. 2004;80(3):185–191. doi: 10.1136/sti.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous C., Bradley J.A., Hamilton D.N., Ledingham I.M., Clements G.B., Robinson C.G. Herpes simplex virus reactivation in surgical patients. Critical Care Medicine. 1984;12(8):626–628. doi: 10.1097/00003246-198408000-00003. [DOI] [PubMed] [Google Scholar]
- Scheithauer S., Manemann A.K., Krüger S., Häusler M., Krüttgen A., Lemmen S.W., et al. Impact of herpes simplex virus detection in respiratory specimens of patients with suspected viral pneumonia. Infection. 2010;38(5):401–405. doi: 10.1007/s15010-010-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildermans J., De Vlieger G. Cytomegalovirus: A troll in the ICU? Overview of the literature and perspectives for the future. Frontiers in Medicine. 2020;7:188. doi: 10.3389/fmed.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuierer L., Gebhard M., Ruf H.-G., Jaschinski U., Berghaus T.M., Wittmann M., et al. Impact of acyclovir use on survival of patients with ventilator-associated pneumonia and high load herpes simplex virus replication. Critical Care (London, England) 2020;24(1):12. doi: 10.1186/s13054-019-2701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano R.F., Castelli J.B., Randi B.A., Vieira R.D., Strabelli T.M.V. Cytomegalovirus colitis in immunocompetent critically ill patients. International Journal of Infectious Diseases. 2014;20:71–73. doi: 10.1016/j.ijid.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Conroy L.T., Pollock M., Ruddy J., Binning A., McCruden E.A.B. Detection of herpes viruses in respiratory secretions of patients undergoing artificial ventilation. Journal of Medical Virology. 2010;82(8):1406–1409. doi: 10.1002/jmv.21794. [DOI] [PubMed] [Google Scholar]
- Tuxen D.V., Cade J.F., McDonald M.I., Buchanan M.R., Clark R.J., Pain M.C. Herpes simplex virus from the lower respiratory tract in adult respiratory distress syndrome. The American Review of Respiratory Disease. 1982;126(3):416–419. doi: 10.1164/arrd.1982.126.3.416. [DOI] [PubMed] [Google Scholar]
- Tuxen D.V., Wilson J.W., Cade J.F. Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. The American Review of Respiratory Disease. 1987;136(2):402–405. doi: 10.1164/ajrccm/136.2.402. [DOI] [PubMed] [Google Scholar]
- von Müller L., Klemm A., Weiss M., Schneider M., Suger-Wiedeck H., Durmus N., et al. Active cytomegalovirus infection in patients with septic shock. Emerging Infectious Diseases. 2006;12(10):1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann M., Sedemund-Adib B., Reiland P., Schmucker P., Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Critical Care Medicine. 2008;36(12):3145–3150. doi: 10.1097/CCM.0b013e31818f3fc4. [DOI] [PubMed] [Google Scholar]


