In recent years, the complex interplay between genes and the environment has gained increased attention in Parkinson’s disease (PD), a progressive neurodegenerative disorder. Despite the identification of causal mutations in several genes (ie, LRRK2, GBA, SNCA, and others) and environmental or lifestyle factors linked to reduced risk (eg, smoking, caffeine) or increased risk (eg, dairy products, pesticides) for development of PD, our understanding of gene-environment interaction remains modest. Further exploration of how an individual’s environmental exposures (“exposome”) and their genome may intertwine to influence the development and progression of PD is thus warranted. This increased knowledge could have both mechanistic and clinical implications, leading to trials of disease-modifying and preventive strategies.
In this month’s issue of Movement Disorders, Luciano et al1 carried out a case-control study in individuals with LRRK2 gene mutations pathogenic for PD. The G2019S mutation, which is the predominant LRRK2 variant in this cohort, demonstrates variable age-dependent penetrance, with PD developing in 25% to 42% of carriers by the age of 80 years.2 This incomplete penetrance suggests that other factors play a role in LRRK2 mutation-driven PD. Recently, several case-control studies of symptomatic (“LRRK2-PD”) and asymptomatic (“LRRK2-non-PD”) LRRK2 mutation carriers have sought to identify potential markers of its penetrance. Iwaki et al3 evaluated a polygenic risk score in mutation carriers from several large research databases and found that a higher polygenic risk score was associated with greater odds of development of PD (odds ratio [OR], 1.34; 95% confidence interval [CI]: 1.09–1.64) per 1 standard deviation of increase from the cohort mean. Bakshi et al4 investigated urate in LRRK2-PD and LRRK2-non-PD from the Parkinson’s Progression Markers Initiative (PPMI) and LRRK2 Cohort Consortium (LCC) cohorts and discovered that the odds of having developed PD were approximately halved (OR, 0.46; 95% CI: 0.29–0.76) for each 2-mg/dL increment in plasma (or serum) urate. Caffeine, a well-studied, potential protectant in PD, has also been explored in mutation carriers, with both caffeine drinkers and those with higher levels of caffeine-linked plasma analytes observed to have lower odds of PD.5,6 Other studies of LRRK2 mutation carriers include targeted and untargeted metabolomics profiling showing a different metabolomic profile in LRRK2-PD and LRRK2-non-PD groups,7 and assessment of urinary phospholipids observing marginally higher levels of urinary 2,2′-di-18:1-bis(monoacylglycerol)phosphate in LRRK2-PD compared with LRRK2-non-PD subjects (P = 0.045). Notably, urinary phospholipid concentrations were greater in both the LRRK2-PD and LRRK2-non-PD groups compared with those without LRRK2 mutation, and thus they may not be a useful marker for LRRK2 resistance.8 Although yet to be investigated in humans, another potential indicator of LRRK2 PD resistance is 5′-deoxyadenosylcobalamin (AdoCbl), a physiologically active form of vitamin B12. In cellular and animal models of LRRK2 PD, AdoCbl was found to be a mixed-type allosteric modulator of LRRK2 kinase, reduced dopaminergic neurodegeneration, or dopamine deficits.9
Luciano et al1 evaluated the association between regular nonsteroidal anti-inflammatory drug (NSAID) use, defined as two or more pills per week for 6 months or more, and the presence of PD in 259 LRRK2-PD and 318 LRRK2-non-PD participants from the LCC and the PD-Genetic and Environmental Modifiers cohorts. Their retrospective case-control study found that regular NSAID use was associated with lower odds of having PD for any NSAID (OR, 0.34; 95% CI: 0.21–0.57), and separately for ibuprofen and aspirin (OR, 0.19 and 0.51, respectively). They concluded that regular NSAID use may be associated with reduced penetrance in LRRK2 mutation carriers. Although NSAID exposure has been previously identified as an inverse risk factor for idiopathic PD, this study is unique because it explored exposure in mutation carriers with and without PD and suggested a new marker of LRRK2 resistance. Study strengths included a large sample size of well-characterized mutation carriers (n = 577) and use of a validated questionnaire for NSAID exposure, the PD-Risk Factor Questionnaire. Their regression models accounted for important PD confounders, such as age, gender, and smoking status, and their analyses answered clinically relevant questions, such as specificity of association to NSAID type (ie, ibuprofen and aspirin), age group, and gender.
As noted by the authors, there were some limitations to their study. First of all, despite the inclusion of several potential confounders in their models, some covariates of established relevance were not accounted for, including caffeine exposure and serum urate levels. Because these two factors have also been identified as markers of LRRK2 penetrance,4-6 their absence from the regression models may have overestimated or underestimated an independent inverse association seen with NSAID exposure. Second, the reliance on retrospective, incomplete data precluded evaluation for an NSAID dose dependence, which, if present, may support biological relevance and inform the design of future NSAID interventional studies. In addition, as thoughtfully highlighted by the authors, the lack of information on the indication for NSAID use prevented them from assessing for confounding by indication. The inclusion of acetaminophen use as a non-NSAID that, like ibuprofen in particular, is also commonly used as an over-the-counter analgesic would have been helpful as a comparator to address confounding by analgesic indication. Similarly, the lack of corresponding data on idiopathic PD cases and controls (who do not carry a LRRK2 mutation) from the same cohorts prevented assessment of whether the inverse association between NSAIDs and PD subjects was greater in the presence of a LRRK2 mutation, which could offer insight into LRRK2 pathophysiology. Lastly, caution is required when interpreting the exposure-lagging analysis of 5 and 10 years, which was attempted to exclude reverse causation. Because premotor PD can occur up to 20 years before motor symptoms, some of the subjects may have been incorrectly classified as LRRK2-non-PD.
Most meta-analyses of NSAIDs and idiopathic PD have shown an inverse association10,11; however, not all of them have.12 This inconsistency in the literature is likely secondary to differences in study methodology, with some studies not differentiating between the NSAIDs13,14 or assessing only aspirin and nonaspirin NSAIDs.15,16 Many studies did not collect data on ibuprofen, an NSAID that has more consistently shown an inverse association with PD.10 In Luciano et al’s article,1 they found an inverse association for ibuprofen and aspirin. Consistent with the literature, they found lower odds of PD in ibuprofen consumers (OR, 0.19) compared with aspirin users (OR, 0.51). This NSAID differential effect may be because of their heterogeneous pharmacological properties. Although all NSAIDs inhibit the cyclooxygenase-2 enzyme, some have additional anti-inflammatory and potentially neuroprotective properties.17 Ibuprofen, unlike aspirin, is an agonist of the peroxisome proliferator-activated receptor-γ of the inhibitory transcription factor, which when activated results in the inhibition of inflammatory cytokines’ gene expression.18
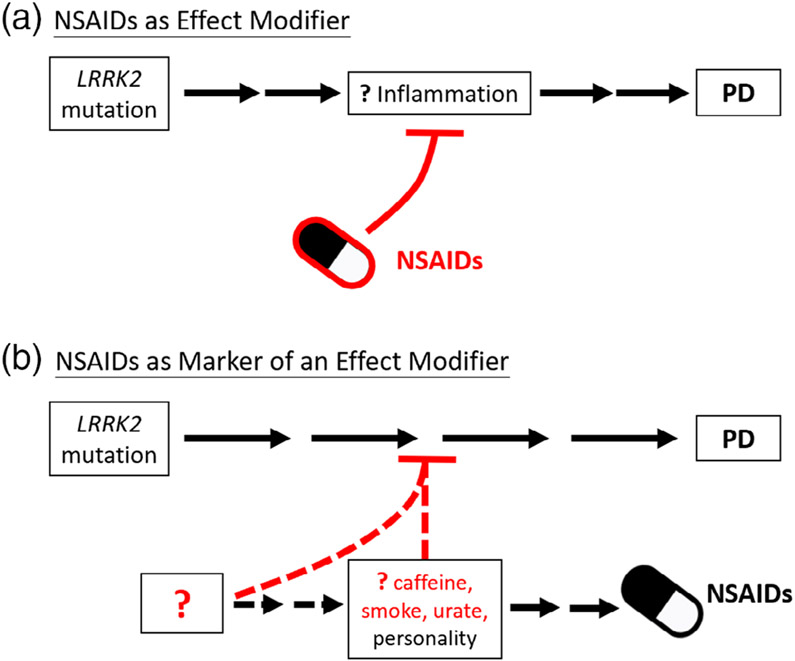
Neuroinflammation is thought to play a part in the pathogenesis of PD, which has been reported to feature activated microglia, upregulated cyclooxygenase-1 and −2 expression, and increased inflammatory cytokines and related molecules.17 In addition, polymorphisms in inflammatory cytokine genes (ie, tumor necrosis factor-α and interleukin-1β)19 and cell-surface human leukocyte antigen20 have been associated with increased PD risk. The role of LRRK2 in inflammation and immune system regulation is also being increasingly explored because it is expressed in the cells of the innate and adaptive immune system.21 Moreover, LRRK2 mRNA and protein levels are induced during proinflammatory states,22 and polymorphisms in the LRRK2 gene are associated with several other chronic inflammatory disorders, including Crohn’s disease23 and leprosy.24 Given this plausible mechanistic linkage between NSAIDs and LRRK2, Luciano et al’s1 findings of an inverse association of NSAIDs with LRRK2 PD suggest that if NSAIDs were truly protective against PD, then they may be particularly effective against LRRK2 PD. Indeed, the association was even greater in their LRRK2 study (RR, 0.42) than in other studies of idiopathic PD (pooled relative risk [RR], 0.90–0.95).11,12,25 Although replication of these results is warranted, ideally in a common cohort, they support consideration of NSAIDs as potential disease-modifying agents for LRRK2 PD. As schematized in Figure 1a, a LRRK2 mutation may contribute to an abnormal or excessive inflammatory response that in turn, if unchecked, results in PD. In this model, the inflammation and resultant neurodegeneration could be avoided or ameliorated by NSAIDs.
FIG. 1.
Alternative explanations for the inverse association between nonsteroidal anti-inflammatory drugs (NSAIDs) and LRRK2-driven Parkinson’s disease (PD), which may be based on a causal, protective role of NSAIDs (a) or one in which their use is an epiphenomenon (b). Question marks and dashed lines represent uncertain modifiers, mediators, or pathways within each model. Red color represents neuroprotective, inhibitory influences on pathogenicity of LRRK2 mutations on PD.
Alternatively, as depicted in Figure 1b, NSAID intake may be an epiphenomenon or a marker of an unknown determinant of reduced PD risk in mutations carriers, without actually influencing PD risk. NSAID exposure may be downstream of other well-recognized inverse risk factors and possible protectants, such as smoking, caffeine intake, or serum urate levels. Supporting smoking is the observation that smokers are more likely to report pain26 and to use analgesic drugs27 than never smokers, although the authors incorporated smoking history into their model, raising the possibility of an even stronger association without adjustment, if smoking history were less frequent in cases than controls. For serum urate, high levels are associated with gout, which may require NSAIDs for pain relief. In addition to caffeine often being found in analgesic formulations, its intake has also been associated with osteoarthritis,28,29 which is treated with NSAIDs. These are only a few alternative explanations for the observed inverse associations. This association may also be driven by an unknown protective influence that leads to a putative premorbid personality of PD,30 which may include reduced novelty seeking and stoicism that could also account for lower exposure to addictive (eg, nicotine, caffeine) and analgesic (eg, NSAID) substances.
Further exploration of NSAID use in LRRK2 mutation carriers in longitudinal cohorts would build on the cross-sectional study of Luciano et al1 to address whether exposure in fact predicts a lower rate of phenoconversion among healthy carriers or slower clinical progression among those with manifest LRRK2 PD. Fortunately, large prospectively characterized cohorts of LRRK2 mutation carriers are now being established and tracked long term. The PPMI genetic cohort and the online cohort Fox Insight, for example, have enrolled participants with and without a LRRK2 mutation and collected environmental data on NSAIDs, caffeine, smoking, and other relevant environmental and lifestyle factors using validated questionnaires. The identification of genetic or environmental modulators that reduce pathogenic mutation penetrance may prove invaluable. A better understanding of LRRK2 gene-environment interactions, in particular, may aid in the selection of modifiable risk factors that can be tested in preventative trials. However, prior to commencing such a trial, questions to be addressed include which NSAID to test, what dose to use, and for what duration. Despite wide, often over-the-counter use of NSAIDs, they are not without risks and currently should not be recommended as a disease-modifying therapy in LRRK2 mutation carriers. Nevertheless, associations of NSAIDs and other candidate protective factors with PD resistance among LRRK2 mutation carriers are encouraging and suggest that prevention trials targeting genetically at-risk individuals may not be too far off.
Acknowledgments
Funding agencies: This research was supported by Farmer Family Foundation Initiative for Parkinson’s Disease Research (M.A.S.), a Jane & Alan Batkin Research Fellowship (G.F.C.), The Edmond J. Safra Fellowship in Movement Disorders (G.F.C.), and National Institutes of Health (NIH) grant R01NS110879 (M.A.S.).
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report.
References
- 1.Luciano MS, Tanner CM, Meng C, et al. Non-steroidal anti-inflammatory use and LRRK2 Parkinson’s disease penetrance. Mov Disord 2020;35(10):1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AJ, Wang Y, Alcalay RN, et al. Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord 2017;32(10):1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwaki H, Blauwendraat C, Makarious MB, et al. Penetrance of Parkinson’s disease in LRRK2 p.G2019S carriers is modified by a polygenic risk score. Mov Disord 2020;35(5):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakshi R, Macklin EA, Logan R, et al. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann Neurol 2019; 85(4):593–599. [DOI] [PubMed] [Google Scholar]
- 5.Kumar PM, Paing SST, Li H, et al. Differential effect of caffeine intake in subjects with genetic susceptibility to Parkinson’s disease. Sci Rep 2015;5:15492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty GF, Maciuca R, Macklin EA, et al. Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: a metabolomic study. Neurology 2020. 10.1212/WNL.0000000000010863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen KK, Wang L, Aasly JO, et al. Metabolomic profiling in LRRK2-related Parkinson’s disease. PLoS One 2009;4(10):e7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcalay RN, Hsieh F, Tengstrand E, et al. Higher urine bis(Monoacylglycerol)phosphate levels in LRRK2 G2019S mutation carriers: implications for therapeutic development. Mov Disord 2020;35(1):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffner A, Li X, Gomez-Llorente Y, et al. Vitamin B12 modulates Parkinson’s disease LRRK2 kinase activity through allosteric regulation and confers neuroprotection. Cell Res 2019;29(4):313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011;76(10):863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 2010;74(12):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren L, Yi J, Yang J, Li P, Cheng X, Mao P. Nonsteroidal anti-inflammatory drugs use and risk of Parkinson disease: a dose-response meta-analysis. Medicine (Baltimore) 2018;97(37):e12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornebroek M, de Lau LML, Haag MDM, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology 2007;28(4):193–196. [DOI] [PubMed] [Google Scholar]
- 14.Powers KM, Kay DM, Factor SA, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov Disord 2008;23(1):88–95. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Zhang SM, Hernán MA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 2003;60(8):1059–1064. [DOI] [PubMed] [Google Scholar]
- 16.Hernán MA, Logroscino G, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology 2006;66(7):1097–1099. [DOI] [PubMed] [Google Scholar]
- 17.Asanuma M, Miyazaki I. Nonsteroidal anti-inflammatory drugs in experimental parkinsonian models and Parkinson’s disease. Curr Pharm Des 2008;14(14):1428–1434. [DOI] [PubMed] [Google Scholar]
- 18.Chaturvedi RK, Beal MF. PPAR: a therapeutic target in Parkinson’s disease. J Neurochem 2008;106(2):506–518. [DOI] [PubMed] [Google Scholar]
- 19.Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol 2007;64(6):836–840. [DOI] [PubMed] [Google Scholar]
- 20.Wissemann WT, Hill-Burns EM, Zabetian CP, et al. Association of Parkinson Disease with structural and regulatory variants in the HLA region. Am J Hum Genet 2013;93(5):984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakimi M, Selvanantham T, Swinton E, et al. Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm 2011;118(5):795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook DA, Kannarkat GT, Cintron AF, et al. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Park Dis 2017;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than thirty distinct susceptibility loci for Crohn’s disease. Nat Genet 2008;40(8):955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F-R, Huang W, Chen S-M, et al. Genomewide association study of leprosy. N Engl J Med 2009;361(27):2609–2618. [DOI] [PubMed] [Google Scholar]
- 25.Poly TN, Islam MMR, Yang H-C, Li Y-CJ. Non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease in the elderly population: a meta-analysis. Eur J Clin Pharmacol 2019;75(1): 99–108. [DOI] [PubMed] [Google Scholar]
- 26.John U, Hanke M, Meyer C, Völzke H, Baumeister SE, Alte D. Tobacco smoking in relation to pain in a national general population survey. Prev Med 2006;43(6):477–481. [DOI] [PubMed] [Google Scholar]
- 27.John U, Alte D, Hanke M, Meyer C, Völzke H, Schumann A. Tobacco smoking in relation to analgesic drug use in a national adult population sample. Drug Alcohol Depend 2006;85(1):49–55. [DOI] [PubMed] [Google Scholar]
- 28.Bang CH, Kim C, Kim J-H, Choi SJ, Song GG, Jung JH. Is knee osteoarthritis related to coffee drinking? A nationwide cross-sectional observational study. Clin Rheumatol 2019;38(3):817–825. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH. Investigating the possible causal association of coffee consumption with osteoarthritis risk using a Mendelian randomization analysis. Clin Rheumatol 2018;37(11):3133–3139. [DOI] [PubMed] [Google Scholar]
- 30.Menza MA, Forman NE, Goldstein HS, Golbe LI. Parkinson’s disease, personality, and dopamine. J Neuropsychiatry Clin Neurosci 1990;2(3):282–287. [DOI] [PubMed] [Google Scholar]