Abstract
Approximately 70% of breast cancer (BC) cases are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) BC. Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors have acted as star drugs for reversing endocrine therapy (ET) resistance and improving the prognosis of patients with HR+ advanced breast cancer (ABC) since they were initially approved. However, progression eventually occurs. In this review, we summarize the recent treatment strategies post CDK4/6 inhibitors: 1) CDK4/6 inhibitors plus exemestane and everolimus; 2) phosphoinositide-3-kinase (PI3K) inhibitor alpelisib plus fulvestrant for patients with PIK3CA mutation; 3) poly (ADP-ribose) polymerase (PARP) inhibitor for patients with germline PALB2 mutations, somatic BRCA1/2 mutations, or germline BRCA1/2 mutations; 4) exemestane and everolimus; and (5) chemotherapy. These strategies are all supported by evidence from clinical trials and retrospective studies. We also describe potential future treatment strategies post CDK4/6 inhibitors, such as the trophoblast cell surface antigen 2 (Trop-2) directed antibody–drug conjugate, cyclin-dependent kinase 7 (CDK7) inhibitors, and B-cell lymphoma-2 (BCL-2) inhibitors.
Keywords: breast cancer, CDK4/6 inhibitors, endocrine therapy resistance, subsequent therapy
Background
Breast cancer (BC) is the most common malignancy among women worldwide and seriously endangers the lives of patients, especially those with advanced breast cancer (ABC), in which the tumor metastasizes to other organs, such as the lungs, liver, brain, and bones.1–3 Surgery is less effective for ABC, and these patients have a poor prognosis.4–7 Approximately 70% of BC cases are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-), which are sensitive to endocrine therapy (ET).8,9 Since tamoxifen was approved in the 1970s, more and more patients have benefited from ET.10 The prognosis of patients with HR+ ABC has been significantly improved.11 With the approval of aromatase inhibitors (AIs; such as exemestane) and selective estrogen receptor downregulators (SERDs; such as fulvestrant and elacestrant), there are now more ET options for HR+ BC.12–15 However, patients eventually develop ET resistance.16–19
Since they were initially approved, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors (such as palbociclib, ribociclib and abemaciclib) have acted as star drugs for reversing ET resistance and improving the prognosis of patients with HR+ ABC.20–23 With good results from clinical trials, CDK4/6 inhibitors plus AIs or fulvestrant represent the standard first- or second-line therapy for HR+ ABC.24–35 Despite the efficacy of CDK4/6 inhibitors for HR+ ABC, progression eventually occurs.36–38
In this review, we summarize the recent treatment strategies for patients who have experienced progression post CDK4/6 inhibitors, based on evidence from clinical trials and retrospective studies, and describe the potential choices post CDK4/6 inhibitors.
Continue CDK4/6 Inhibitors and Add a Subsequent Line of Therapy
CDK4/6 Inhibitors Plus Mammalian Target of Rapamycin (mTOR) Inhibitor (Everolimus) and Steroidal AI (Exemestane)
At present, CDK4/6 inhibitors are combined with ET drugs. When progression occurs, CDK4/6 inhibitors can be continued with other ET drugs. The Phase II Triniti-1 trial was presented at the 2019 American Society of Clinical Oncology (ASCO) annual meeting and assessed the ongoing use of the CDK4/6 inhibitor ribociclib plus everolimus and exemestane post progression on CDK4/6 inhibitors.39 This regimen demonstrated a clinical benefit rate (CBR) at week 24 of 41%, and the median progression-free survival (mPFS) was 5.7 months in patients who had experienced progression post CDK4/6 inhibitors. The median overall survival (mOS) was not estimable at the data cutoff point. Subgroup analysis showed that the regimen had relatively poor efficacy in patients with estrogen receptor 1 (ESR1) mutation (6.9 vs 3.5 months; hazard ratio: 1.76, 95% confidence interval [CI]: 1.01–3.05).
CDK4/6 Inhibitors Plus Immunotherapy
In vitro research has shown that CDK4/6 inhibitors plus an anti-programmed cell death 1 ligand 1 (PD-L1) drug is a more effective regimen than either drug alone.40 Therefore, the phase II PACE trial is a randomized, open-label, multicenter trial assessing the utility of ongoing CDK4/6 inhibitors plus fulvestrant following progression on CDK4/6 inhibitors plus AIs.41 The patients in this trial were randomized into three groups: group A: fulvestrant monotherapy; group B: ongoing CDK4/6 inhibitor palbociclib plus fulvestrant; and Group C: anti-PD-L1 drug (avelumab) plus ongoing CDK4/6 inhibitor palbociclib and fulvestrant. We are looking forward to the trial results. The trial will indicate whether it is effective to continue the CDK4/6 inhibitor plus fulvestrant or plus an anti-PD-L1 drug and fulvestrant, among patients who developed resistance on CDK4/6 inhibitors plus AIs.
Change to a Regimen without CDK4/6 Inhibitors (Including ET-Based Regimens)
Phosphoinositide-3-Kinase (PI3K) Inhibitor (Alpelisib) Plus Fulvestrant
The PI3K pathway is frequently mutated in HR+ BC, which can lead to ET resistance.42,43 About 40% of PIK3CA mutations in HR+ BC lead to excessive PI3K pathway activation.44–47 PI3K inhibitors can inhibit the growth of estrogen-independent ER+ BC cells that exhibit PI3K pathway activation.48,49 Alpelisib (byl719) is a highly selective inhibitor of the PI3Kα subtype.50 The Phase III SOLAR-1 trial presented at the 2018 San Antonio Breast Cancer Symposium (SABCS)51,52 showed that the mPFS for patients with PI3KCA mutation was prolonged by alpelisib plus fulvestrant compared to placebo plus fulvestrant (11 vs 5.3 months, hazard ratio: 0.50–0.85, p=0.00065). For patients pretreated with CDK4/6 inhibitors, the mPFS of alpelisib plus fulvestrant was also prolonged compared to the control group (5.5 vs 1.8 months, hazard ratio: 0.48, 95% CI: 0.17–1.36), indicating that the regimen was effective among patients with PI3KCA mutation who were pretreated with CDK4/6 inhibitors. However, in this trial, only 20 patients had had PI3KCA mutation and had previously used CDK4/6 inhibitors, so the small sample size might have influenced the results. Next, the phase II BYLieve trial was presented at the 2020 ASCO annual meeting,53 and it assessed the efficacy of alpelisib plus fulvestrant or letrozole in patients with PIK3CA-mutated HR+, HER2- ABC post CDK4/6 inhibitors. Patients in Cohort A had developed resistance during treatment with CDK4/6 inhibitors plus AI and were treated with alpelisib plus fulvestrant. Their mPFS was 7.3 months, and 50.4% were alive without disease progression at 6 months. With a well-characterized safety profile and a sample size of 121 patients, the BYLieve trial supported the use of alpelisib plus fulvestrant for patients with PIK3CA-mutated HR+, HER2-, ABC post CDK4/6 inhibitors, confirming the SOLAR-1 results.
mTOR Inhibitor (Everolimus) Plus Steroidal AI (Exemestane)
In the phase III BOLERO-2 trial, exemestane plus everolimus significantly improved mPFS compared to exemestane plus placebo (11 vs 4.1 months).54,55 Subgroup analysis found that the more lines of treatment patients had received, the more benefits patients obtained from everolimus.56 However, none of the patients in this trial were previously treated with CDK4/6 inhibitors and there were no trials that directly assessed the efficacy of exemestane plus everolimus in patients post CDK4/6 inhibitors.
A retrospective study conducted in Portland showed that this regimen has the same effects on patients with HR+ ABC regardless of prior CDK4/6 inhibitor use (mPFS: 3.6 vs 4.2 months for prior CDK4/6 inhibitor use and no prior use, respectively, hazard ratio: 1.22, 95% CI: 0.65–2.28, p=0.538; mOS: 15.6 vs 11.3 months, respectively, hazard ratio: 0.70, 95% CI: 0.35–1.40, p=0.308).57 The study involved 43 patients, 17 who had received prior CDK4/6 inhibitors and 26 who had not. Patient characteristics, including other prior therapies and metastasis sites, were not significantly different. Thus, everolimus plus exemestane may be effective for HR+ ABC regardless of prior CDK4/6 inhibitor use.
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors
PARP inhibitors such as olaparib and talazoparib had good therapeutic effects on HER2- patients with germline BRCA mutation.58,59 However, the patients had not received prior CDK4/6 inhibitors. The phase II TBCRC 048 trial of olaparib monotherapy in metastatic BC patients with germline or somatic mutations in homologous recombination pathway genes was presented at the 2020 ASCO annual meeting.60 It showed that olaparib was effective for some patients post CDK4/6 inhibitors. In this trial, 93% of the HR+ and HER2- patients were previously treated with CDK4/6 inhibitors. Olaparib had significant effects on both patients with germline PALB2 mutations (objective response rate [ORR]: 82%, CBR at 18 weeks: 100%, mPFS: 13.3 months, 90% CI: 12 months to not reached) and patients with somatic BRCA1/2 mutations (ORR: 50%, CBR at 18 weeks: 67%, mPFS: 6.3 months, 90% CI: 4.4 months to not reached). This provides a new choice for patients with germline PALB2 mutations or somatic BRCA1/2 mutations post CDK4/6 inhibitor resistance.
The phase III EMBRACA trial compared the safety and efficacy of talazoparib monotherapy vs protocol-specific physician’s choice in patients with locally advanced BC with germline BRCA mutations.61 Prespecified subgroup analysis showed prolonged mPFS with talazoparib (9.4 vs 6.7 months, hazard ratio: 0.47, 95% CI: 0.32–0.71) for HR+/HER2- patients. However, there was no prespecified subgroup analysis of patients pretreated with CDK4/6 inhibitors. Talazoparib may be useful for patients pretreated with CDK4/6 inhibitors. Thus, PARP inhibitors may be tried for patients with germline BRCA1/2 mutations post CDK4/6 inhibitor resistance.60–62 All the above-mentioned clinical trials and retrospective studies are shown in Table 1.
Table 1.
Clinical Trials and Retrospective Studies Post CDK4/6 Inhibitors Mentioned
| Study ID | Phase | Population | Size | Intervention | Result |
|---|---|---|---|---|---|
| TRINITI-1 NCT02732119 |
I/II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors≥ 4 months | 95 | RIB+EVE+EXE | MPFS =5.7 months CBR at 24weeks:41% ORR:8.4% |
| PACE NCT03147287 |
II | ABC HR+ HER2- progression on AI+CDK4/6 inhibitors | 220 | Ful vs Ful+Pal vs Ful+Pal+Ave | NA |
| SOLAR-1 NCT02437318 |
III | HR+ HER2- ABC | 20 | Alpelisib+Ful vs Placebo +Ful |
MPFS:5.5 vs 1.8 months HR 0.48 (95% CI 0.17–1.36) |
| BYLieve NCT03056755 (cohort A) |
II | HR+ HER2- ABC with PIK3CA mutation progression on AI+CDK4/6 inhibitors | 121 | Alpelisib+Ful | Proportion of PFS patients at 6 months:50.4% MPFS:7.3 months |
| A retrospective study in Portland | NA | HR+ ABC with or without prior use of CDK4/6 inhibitors | 43 | EVE+EXE | MPFS: 3.6 vs 4.2 months, HR=1.22, 95% CI 0.65–2.28, p=0.538) MOS:15.6vs 11.3 months, HR=0.70, 95% CI 0.35–1.40, p=0.308) |
| TBCRC 048 NCT03344965 |
II | HR+ HER2- ABC germline or somatic mutations in homologous recombination pathway genes |
41 | Olaparib | Germline PALB2 mutations (ORR 82%, CBR of 18 weeks was 100%), somatic BRCA1/2 mutations (ORR 50%, CBR of 18 weeks was 67%) |
| EMBRACA NCT01945775 |
III | ABC with BRCA Mutation | ?/431 | Talazoparib vs chemotherapy | No prespecified subgroup for patients pretreated with CDK4/6 inhibitors |
Abbreviations: HR, hormone-receptor; HER2, human epidermal growth factor receptor 2; ABC, advanced breast cancer; CDK, Cyclin-dependent kinase; RIB, ribociclib; EVE, everolimus; EXE, exemestane; MPFS, median progression free survival; CBR, clinical benefit rate; ORR, objective response rate; AI, aromatase inhibitor; Ful, fulvestrant; Pal, palbociclib; Ave, avelumab; NA, not available; CI, confidence interval; MOS, median overall survival.
Other Targets
Trophoblast cell-surface antigen-2 (Trop-2) is expressed in epithelial cancers, including HR+ ABC, and it is associated with worse survival.63,64 A Trop-2 directed antibody–drug conjugate sacituzumab govitecan (IMMU-132) has shown benefit in HR+ ABC.65 This therapeutic agent is a potentially valuable for patients pretreated with CDK4/6 inhibitors. The phase III TROPICS-02 trial is currently investigating this agent.66
Cyclin-dependent kinase 7 (CDK7) inhibitors are emerging as promising BC drugs, being effective for HR+ BC in vitro and in vivo.67,68 A Phase I trial (NCT03363893) of patients pretreated with CDK4/6 inhibitors is ongoing.
B-cell lymphoma-2 (BCL2) is an estrogen-responsive gene that is overexpressed in approximately 80% of primary HR+ BC cases.69–71 Preclinical data (based on patient-derived xenograft models) indicate that BCL-2 inhibitors may be effective in HR+ BC.72 A phase I trial (NCT03584009) of patients pretreated with CDK4/6 inhibitors is ongoing.
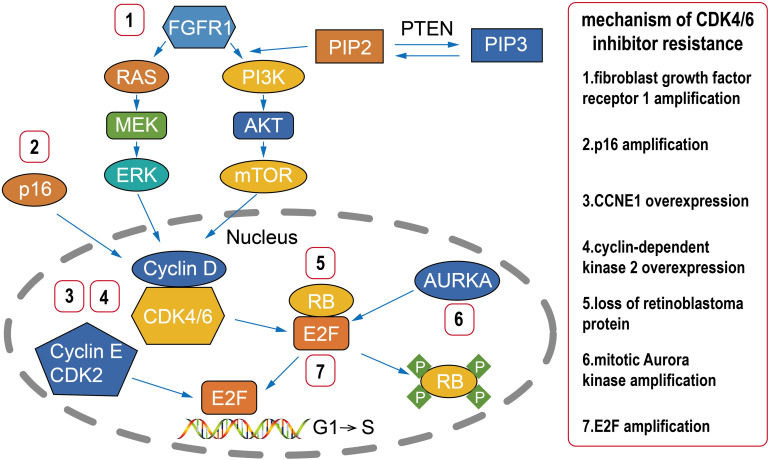
With the development of treatment and detection technologies, many other potential therapeutic targets have been found among patients with CDK4/6 inhibitor resistance (Figure 1).36,73 A better understanding of the mechanism of CDK4/6 inhibitor resistance may improve the rational selection of next-line therapy.37,38 Loss of retinoblastoma protein (RB), p16 amplification, CCNE1 overexpression, fibroblast growth factor receptor 1 (FGFR1) amplification, mitotic Aurora kinase (AURKA) amplification, E2F amplification, and cyclin-dependent kinase 2 (CDK2) overexpression have all been reported to be associated with CDK4/6 inhibitor resistance.73–83 We can use tissue or liquid biopsies to identify potential therapeutic targets in patients with CDK4/6 inhibitor resistance and provide individualized therapy based on the results.73 For example, if ESR1 mutation is found, we can use SERD drugs such as fulvestrant or elacestrant to deal with the ESR1 mutation.84,85 For patients with FGFR1 amplification, AURKA amplification, or CDK2 overexpression, FGFR1 inhibitors, AURKA inhibitors, and CDK2 inhibitors, respectively, could be used to treat patients who developed resistance during CDK4/6 inhibitor use.78–80 However, FGFR1 inhibitors, AURKA inhibitors, CDK2 inhibitors, and other new targeted drugs treating cancer associated with CDK4/6 inhibitor resistance are still in development for clinical trials (Table 2).86–88
Figure 1.
Mechanisms underlying CDK4/6 inhibitor resistance. Multiple factors involved in cell cycle regulation are associated with CDK4/6 inhibitor resistance, such as loss of RB, p16 amplification, CCNE1 overexpression, FGFR1 amplification, AURKA amplification, and E2F amplification.
Abbreviations: CDK, cyclin-dependent kinase; RB, retinoblastoma protein; AURKA, mitotic Aurora kinase; MEK, mitogen-activated ERK-activating kinase; mTOR, mammalian target of rapamycin; PIP2, phosphatidylinositol-4, 5-bisphosphate; PIP3, phosphatidylinositol-3,4,4-trisphosphate; PI3K, phosphoinositide-3-kinase; PTEN, phosphatase and tensin homolog; RAS, rat sarcoma; ERK, extracellular signal-regulated kinases; FGFR1, fibroblast growth factor receptor 1.
Table 2.
Other Clinical Trials Post CDK4/6 Inhibitors Globally
| Study ID | Phase | Population | Size | Intervention | Result |
|---|---|---|---|---|---|
| NCT04318223 | II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors+AI/TAM ± LHRHa |
168 | Ful+Pal | NA |
| SMILE study NCT04738292 |
II | ABC HR+ HER2- progression on AI+CDK4/6 inhibitors | 39 | Onapristone+Ful | NA |
| Veronica NCT03584009 |
II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors≥ 8 weeks | 103 | Venetoclax+Ful Vs Ful |
NA |
| TAKTIC NCT03959891 |
I | HR+ ABC with or without prior use of CDK4/6 inhibitors | 60 | Ipatasertib+Ful vs Ipatasertib+AI vs Ipatasertib+Ful+Pal |
NA |
| TATEN NCT04251169 |
II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | 46 | Pembrolizumab+ Paclitaxel | NA |
| MAINTAIN NCT02632045 |
II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors+AI | 132 | RIB+Ful vs Ful |
NA |
| EMERALD NCT03778931 |
III | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | 466 | Elacestrant vs Ful vs AI |
NA |
| FINER NCT04650581 |
III | ER+ HER2- ABC prior progression on a CDK4/6 inhibitors+AI | 250 | Ipatasertib+Ful vs Ful |
NA |
| NCT03955939 | I | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | 5 | LY3295668 Erbumine ±Endocrine therapy |
NA |
| NCT03803761 | II | ER+ HER2- ABC prior progression on a CDK4/6 inhibitors+AI | 66 | Copanlisib+Ful | NA |
| NCT04247126 | I | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | ?/80 | SY-5609+Ful | NA |
| NCT04553133 | I/II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | ?/144 | PF-07104091 vs PF-07104091 vs +Pal PF-07104091 +Pal+AI |
NA |
| NCT03519178 | I/II | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | ?/157 | PF-06873600 vs PF-06873600 + Endocrine Therapy |
NA |
| PALMIRA NCT03809988 |
II | HR+ HER2- ABC prior progression on Pal+AI/Ful | 198 | Pal+AI/Ful vs AI/Ful |
NA |
| NCT02738866 | II | HR+ HER2- ABC prior progression on Pal+AI | 100 | Pal+Ful | NA |
| TROPICS-02 NCT03901339 |
III | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | 400 | IMMU-132 vs chemotherapy |
NA |
| NCT04134884 | I | HR+ HER2- ABC prior progression on a CDK4/6 inhibitors | ?/32 | ASTX727+talazoparib | NA |
Abbreviations: HR, hormone-receptor; HER2, human epidermal growth factor receptor 2; ABC, advanced breast cancer; CDK, Cyclin-dependent kinase; TAM, tamoxifen; LHRHa, luteinizing Hormone Releasing Hormone analogues; RIB, ribociclib; ER, estrogen receptor; AI, aromatase inhibitor; Ful, fulvestrant; Pal, palbociclib; NA, not available; Onapristone, a progesterone antagonist; Venetoclax, BCL-2 inhibitor; Ipatasertib, AKT inhibitor; LY3295668 Erbumine, Aurora kinase A inhibitor; Copanlisib, PIK inhibitor; SY-5609, CDK7 inhibitor; PF-07104091, CDK2 inhibitor; PF-06873600, CDK2/4/6 inhibitor; IMMU-132, Trop-2 directed antibody–drug conjugate; ASTX727, cedazuridine+decitabine.
Chemotherapy
Chemotherapy is also a good choice for patients who develop resistance during CDK4/6 inhibitor use.89 With the approval of new and classic chemotherapeutics such as anthracyclines, taxanes, nanoparticle albumin-bound (nab)-paclitaxel, vinorelbine, capecitabine, platinum, and eribulin, which have all been shown to be effective for ABC in clinical trials, we now have more choices for first-line and later chemotherapy.90–97 There are three ongoing clinical trials (TATEN, TROPICS-02, NCT04134884) to assess the effect of chemotherapy post CDK4/6 inhibitors progression (Table 2). Due to its different mechanisms of action against BC, chemotherapy, a cytotoxic treatment, is effective for patients post CDK4/6 inhibitor resistance.
Conclusions
Since the approval of CDK4/6 inhibitors for patients with HR+ ABC, they have been accepted by global experts as they can reverse ET resistance and significantly improve the prognosis of these patients. As star drugs, CDK4/6 inhibitors have achieved amazing success by prolonging the PFS and OS of patients with HR+ ABC. Therefore, according to the National Comprehensive Cancer Network (NCCN) guidelines, CDK4/6 inhibitors have gradually been promoted from late- to first- and second-line treatment, indicating that they have good therapeutic effects.
However, in ABC, no matter how good CDK4/6 inhibitors are, resistance eventually occurs. How to deal with CDK4/6 inhibitor resistance will be a major BC research topic going forward. In this study, we have discussed Phase I, II and III clinical trials and retrospective studies post CDK4/6 inhibitors to try to answer this question. The current evidence supports the following conclusions regarding therapeutic strategies post CDK4/6 inhibitor use: (1) CDK4/6 inhibitors plus exemestane and everolimus have clinical benefits (mPFS: 5.7 months). However, for patients with ESR1 mutation, the effect is much lower than for patients with the wildtype gene. CDK4/6 inhibitors plus immunotherapeutic PD-L1 inhibitors were effective for HR+ BC in vitro, but there is not yet any obvious evidence from clinical trials. We are looking forward to the results of the PACE trial. (2) CDK4/6 inhibitor regimens can be replaced with a different regimen (including an ET-based regimen). For example, for patients with PIK3CA mutation, the PIK3 inhibitor alpelisib plus fulvestrant improves clinical outcomes (mPFS: 5.5–7.3 months according to the SOLAR-1 and BYLieve trials). Limited evidence suggests that everolimus plus exemestane is an effective post CDK4/6 inhibitors (mPFS: 3.6 months; mOS: 15.6 months). The phase II TBCRC 048 trial showed that for patients with germline PALB2 mutations or somatic BRCA1/2 mutations, olaparib can be used post CDK4/6 inhibitors. Olaparib or talazoparib can also be attempted in patients with germline BRCA1/2 mutations post CDK4/6 inhibitors. (3) When patients develop CDK4/6 inhibitor resistance, ET does not necessarily need to be continued, as chemotherapy can be started. As the earliest systemic treatment for BC, chemotherapy significantly improves prognosis. And with the approval of new and classic chemotherapeutics, we now have more choice for first-line or later chemotherapy strategies that are effective against CDK4/6 inhibitor resistance.
mTOR inhibitors and PIK3 inhibitors act as upstream signaling pathway of CDK4/6 inhibitors and are related to the causes of CDK4/6 inhibitor resistance. Thus, these drugs may be able to overcome CDK4/6 inhibitor resistance. PAPP inhibitors, chemotherapy, and other targeted drugs work in other pathways, causing cancer cell apoptosis in order to overcome CDK4/6 inhibitor resistance.
With a better understanding of BC, we can further our understanding of the mechanisms of CDK4/6 inhibitor resistance. Using tissue and liquid biopsies, we will be able to identify the mutations leading to the resistance and then use the mutations as targets and develop drugs to treat these mutations, which could provide individualized treatment options. For example, if the ESR1 mutation is found, we can use SERD drugs such as fulvestrant or elacestrant. Regarding other mutations, such as AURKA or FGFR2 mutations, the corresponding drugs are still in clinical trials. We believe that, in the near future, based on the mechanism of drug resistance, targeted treatment based on tissue or liquid biopsies could benefit patients. Future treatments will be more precise. With the development of effective targeted drugs, such as Trop-2 directed antibody–drug conjugate, CDK-7 inhibitor, BCL-2 inhibitor, we can delay or even eliminate CDK4/6 inhibitor resistance. Additionally, we can continue to use chemotherapy to overcome CDK4/6 inhibitor resistance.
Acknowledgments
The authors thank the members of their department for their research work.
Funding Statement
No funding support.
Abbreviations
BC, breast cancer; HR+, hormone receptor positive; Her2-, human epidermal growth factor receptor 2 negative; ABC, advanced breast cancer; ET, endocrine therapy; AIs, aromatase inhibitors; CDK, cyclin-dependent kinase; PARP, poly (ADP-ribose) polymerase; Trop-2, trophoblast cell surface antigen 2; CDK7, cyclin-dependent kinase 7; BCL-2, B-cell lymphoma-2; SERDs, selective estrogen receptor downregulators; CI, confidence interval; PD-L1, programmed cell death 1 ligand 1; PI3K, Phosphoinositide-3-kinase; mTOR, mammalian target of rapamycin; CBR, clinical benefit rate; PFS, progression-free survival; OS, overall survival; ESR1, estrogen receptor 1; SABCS, San Antonio Breast Cancer Symposium; ASCO, American Society of Clinical Oncology; NCCN: National Comprehensive Cancer Network; RB, retinoblastoma protein; AURKA, mitotic Aurora kinase; MEK, mitogen-activated ERK-activating kinase; PIP2, phosphatidylinositol-4, 5-bisphosphate; PIP3, phosphatidylinositol-3,4,4-trisphosphate; PI3K, phosphoinositide-3-kinase; PTEN, phosphatase and tensin homolog; RAS, rat sarcoma; ERK, extracellular signal-regulated kinase; FGFR1, fibroblast growth factor receptor 1; HR, hormone receptor; RIB, ribociclib; EVE, everolimus; EXE, exemestane; mPFS, median progression-free survival; mOS, median overall survival; ORR, objective response rate; Ful, fulvestrant; Pal, palbociclib; Ave, avelumab; NA, not available. TAM, tamoxifen; LHRHa, luteinizing Hormone Releasing Hormone analogues; ER, estrogen receptor.
Data Sharing Statement
Not applicable.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Author Contributions
Both authors made substantial contributions to review conception and design, acquisition of data, and interpretation of data. Chao Li drafted the manuscript and Xujun Li revised it critically for important intellectual content. Both authors agreed to submit to the journal. Both authors gave final approval for the version to be published and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Globocan. Breast cancer fact sheet; 2018. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf. Accessed November16, 2020.
- 2.Institute NC. Cancer Stat facts: female breast cancer. 2019; Available from: https://seer.cancer.gov/statfacts/html/breast.html. Accessed November16, 2020.
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.MacNeill F, Karakatsanis A Over surgery in breast cancer. Breast. 2017:284–289. doi: 10.1016/j.breast.2016.10.023. Epub 2016 Nov 25. PMID: 27894703. [DOI] [PubMed] [Google Scholar]
- 5.Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2 PMID: 31548545. [DOI] [PubMed] [Google Scholar]
- 6.Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16(13):1380–1388. doi: 10.1016/S1470-2045(15)00135-7. Epub 2015 Sep 9. PMID: 26363985. [DOI] [PubMed] [Google Scholar]
- 7.Soran A, Ozmen V, Ozbas S, et al. Randomized trial comparing resection of primary tumor with no surgery in stage iv breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25(11):3141–3149. PMID: 29777404. doi: 10.1245/s10434-018-6494-6 [DOI] [PubMed] [Google Scholar]
- 8.Serra F, Lapidari P, Quaquarini E, Tagliaferri B, Sottotetti F, Palumbo R. Palbociclib in metastatic breast cancer: current evidence and real-life data. Drugs Context. 2019;8:212579. doi: 10.7573/.dic.212579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan LR, Schein PS, Woolley PV, et al. Therapeutic use of tamoxifen in advanced breast cancer: correlation with biochemical parameters. Cancer Treat Rep. 1976;60:1437–1443. [PubMed] [Google Scholar]
- 11.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi: 10.2174/1871520616666160502122724 PMID: 27137076. [DOI] [PubMed] [Google Scholar]
- 12.Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547 [DOI] [PubMed] [Google Scholar]
- 13.Nagaraj G, Ma C. Revisiting the estrogen receptor pathway and its role in endocrine therapy for postmenopausal women with estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2015;150:231–242. doi: 10.1007/s10549-015-3316-4 [DOI] [PubMed] [Google Scholar]
- 14.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–1291. doi: 10.1093/jnci/djj357 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wang Z, Shao Z. Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2- negative advanced breast cancer: a review. Cancer Med. 2019;8:1943–1957. doi: 10.1002/cam4.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiavon G, Smith IE. Endocrine therapy for advanced/metastatic breast cancer. Hematol Oncol Clin North Am. 2013;27(4):715–36, viii. PMID: 23915741. doi: 10.1016/j.hoc.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Dowsett M. Endocrine resistance in advanced breast cancer. Acta Oncol. 1996;35(Suppl 5):91–95. doi: 10.3109/02841869609083979 PMID: 9142976. [DOI] [PubMed] [Google Scholar]
- 18.Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34(3):427–438.e6. doi: 10.1016/j.ccell.2018.08.008. PMID: 30205045; PMCID: PMC6327853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanker AB, Sudhan DR, Arteaga CL Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496–513. doi: 10.1016/j.ccell.2020.03.009. PMID: 32289273; PMCID: PMC7169993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, Phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. PMID: 26947331. doi: 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 21.Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. PMID: 28580882. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 22.Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-Negative breast cancer that progressed on endocrine therapy-MONARCH 2: a Randomized Clinical Trial. JAMA Oncol. 2019;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782. PMID: 31563959; PMCID: PMC6777264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. PMID: 29860922. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 24.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155 PMID: 29718092. [DOI] [PubMed] [Google Scholar]
- 25.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. PMID: 29804902. doi: 10.1016/S1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 26.Rossi V, Berchialla P, Giannarelli D, et al. Should all patients with HR-positive HER2-Negative metastatic breast cancer receive CDK 4/6 inhibitor as first-line based therapy? A network meta-analysis of data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers (Basel). 2019;11(11):1661. doi: 10.3390/cancers11111661. PMID: 31717791; PMCID: PMC6896062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2019;30(11):1842. doi: 10.1093/annonc/mdz215. PMID: 31407010; PMCID: PMC6927326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat. 2018;168(1):127–134. doi: 10.1007/s10549-017-4518-8. PMID: 29164421. PMCID: PMC5847028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson-Lomba O, Dalal AA, Ayyagari R, et al. Systematic literature review of clinical trials of endocrine therapies for premenopausal women with metastatic HR+ HER2- breast cancer. Breast J. 2019;25(5):880–888. PMID: 31290203. doi: 10.1111/tbj.13345 [DOI] [PubMed] [Google Scholar]
- 30.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. PMID: 28968163. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 31.Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi: 10.1038/s41523-018-0097-z. PMID: 30675515. PMCID: PMC6336880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised Phase 2 study. Lancet Oncol. 2015;(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. PMID: 25524798. [DOI] [PubMed] [Google Scholar]
- 33.Finn RS, Crown JP, Ettl J, et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016;18(1):67. doi: 10.1186/s13058-016-0721-5. PMID: 27349747; PMCID: PMC4924326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. PMID: 30632023. PMCID: PMC6438948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rugo HS, Finn RS, Gelmon K, et al. Progression-free survival outcome is independent of objective response in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with palbociclib plus letrozole compared with letrozole: analysis from PALOMA-2. Clin Breast Cancer. 2020;20(2):e173–e180. PMID: 31836434. doi: 10.1016/j.clbc.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 36.Pandey K, An HJ, Kim SK, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int J Cancer. 2019;145(5):1179–1188. doi: 10.1002/ijc.32020. PMID: 30478914. PMCID: PMC6767051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCartney A, Migliaccio I, Bonechi M, et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front Oncol. 2019;9:666. doi: 10.3389/fonc.2019.00666. PMID: 31396487; PMCID: PMC6664013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(8):2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. PMID: 27020857. PMCID: PMC5426059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardia A, Hurvitz SA, DeMichele A. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): efficacy, safety, and biomarker results. J Clin Oncol. 2019;(suppl; abstr 1016):37. doi: 10.1200/JCO.2019.37.15_suppl.1016 [DOI] [Google Scholar]
- 40.Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465. PMID: 28813415. PMCID: PMC5570667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erica Mayer E. Palbociclib after CDK and endocrine therapy (PACE). Available from: Palbociclib After CDK and Endocrine Therapy (PACE) - Full Text View. ClinicalTrials.gov. Accessed November16, 2020.
- 42.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29(33):4452–4461. doi: 10.1200/JCO.2010.34.4879. PMID: 22010023. PMCID: PMC3221526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosch A, Li Z, Bergamaschi A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7(283):283ra51. doi: 10.1126/scitranslmed.aaa4442.PMID: 25877889; PMCID: PMC4433148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer IA, Abramson VG, Formisano L, et al. A Phase Ib study of alpelisib (BYL719), a pi3kα-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23(1):26–34. doi: 10.1158/1078-0432.CCR-16-0134. PMID: 27126994. PMCID: PMC5085926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sai J, Owens P, Novitskiy SV, et al. PI3K inhibition reduces mammary tumor growth and facilitates antitumor immunity and anti-PD1 responses. Clin Cancer Res. 2017;23(13):3371–3384. doi: 10.1158/1078-0432.CCR-16-2142. PMID: 28003307. PMCID: PMC5479746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loi S, Haibe-Kains B, Majjaj S, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci U S A. 2010;107(22):10208–10213. doi: 10.1073/pnas.0907011107. PMID: 20479250. PMCID: PMC2890442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller TW, Hennessy BT, González-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. PMID: 20530877. PMCID: PMC2898598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowder RJ, Phommaly C, Tao Y, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69(9):3955–3962. doi: 10.1158/0008-5472.CAN-08-4450. PMID: 19366795. PMCID: PMC2811393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller TW, Balko JM, Fox EM. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1(4):338–351. doi: 10.1158/2159-8290.CD-11-0101. PMID: 22049316. PMCID: PMC3204388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croessmann S, Sheehan JH, Lee KM, et al. PIK3CA C2 domain deletions hyperactivate phosphoinositide 3-kinase (pi3k), generate oncogene dependence, and are exquisitely sensitive to PI3Kα inhibitors. Clin Cancer Res. 2018;24(6):1426–1435. doi: 10.1158/1078-0432.CCR-17-2141. PMID: 29284706. PMCID: PMC5856622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.André F, Ciruelos E, Rubovszky G, et al; SOLAR-1 Study Group. Alpelisib for PIK3CA-Mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904 PMID: 31091374. [DOI] [PubMed] [Google Scholar]
- 52.Rugo HS, André F, Yamashita T, et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Ann Oncol. 2020;31(8):1001–1010. PMID: 32416251. doi: 10.1016/j.annonc.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 53.Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): bYLieve study results. J Clin Oncol. 2020;38(suppl; abstr 1006). doi: 10.1200/JCO.2020.38.15_suppl.1006 [DOI] [Google Scholar]
- 54.Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–884. doi: 10.1007/s12325-013-0060-1. PMID: 24158787. PMCID: PMC3898123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann Oncol. 2014;25(12):2357–2362. doi: 10.1093/annonc/mdu456. PMID: 25231953. PMCID: PMC6267855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol. 2016;34(5):419–426. doi: 10.1200/JCO.2014.60.1971. PMID: 26503204. PMCID: PMC5070556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook MM, Rabadi LA, Kaempf AJ, Saraceni MM, Savin MA, Mitri ZI. Everolimus plus exemestane treatment in metastatic hormone receptor-positive breast cancer patients previously treated with CDK4/6 inhibitor therapy. Oncologist. 2020;26:101–106. doi: 10.1002/onco.13609 PMID: 33230905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. PMID: 28578601. doi: 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 59.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. PMID: 30110579. doi: 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020:JCO2002151. PMID: 33119476. doi: 10.1200/JCO.20.02151 [DOI] [PubMed] [Google Scholar]
- 61.Rugo HS, Ettl J, Hurvitz SA, et al. Outcomes in clinically relevant patient subgroups from the EMBRACA Study: talazoparib vs physician’s choice standard-of-care chemotherapy. JNCI Cancer Spectr. 2019;4(1):pkz085. doi: 10.1093/jncics/pkz085. PMID: 32337496; PMCID: PMC7050154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turk AA, Wisinski KB. PARP inhibitors in breast cancer: bringing synthetic lethality to the bedside. Cancer. 2018;124(12):2498–2506. doi: 10.1002/cncr.31307. PMID: 29660759. PMCID: PMC5990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambrogi F, Fornili M, Boracchi P, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9(5):e96993. doi: 10.1371/journal.pone.0096993.; PMID: 24824621; PMCID: PMC4019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vidula N, Yau C, Rugo HS. Trop2 gene expression (Trop2e) in primary breast cancer (BC): correlations with clinical and tumor characteristics. J Clin Oncol. 2017;35([abstract]):1075. doi: 10.1200/JCO.2017.35.15_suppl.1075 [DOI] [Google Scholar]
- 65.Kalinsky K, Diamond JR, Vahdat LT, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31(12):1709–1718. PMID: 32946924. doi: 10.1016/j.annonc.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 66.Rugo HS, Bardia A, Tolaney SM, et al. TROPiCS-02: a Phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer. Future Oncol. 2020;16(12):705–715. PMID: 32223649. doi: 10.2217/fon-2020-0163 [DOI] [PubMed] [Google Scholar]
- 67.McDermott MSJ, Sharko AC, Munie J, et al. CDK7 inhibition is effective in all the subtypes of breast cancer: determinants of response and synergy with EGFR inhibition. Cells. 2020;9(3):638. doi: 10.3390/cells9030638. PMID: 32155786; PMCID: PMC7140476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attia YM, Shouman SA, Salama SA, et al. Blockade of CDK7 reverses endocrine therapy resistance in breast cancer. Int J Mol Sci. 2020;21(8):2974. doi: 10.3390/ijms21082974. PMID: 32340192; PMCID: PMC7215326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20:2890–2901. doi: 10.1128/MCB.20.8.2890-2901.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merino D, Lok SW, Visvader JE, Lindeman GJ. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene. 2016;35:1877–1887. doi: 10.1038/onc.2015.287 [DOI] [PubMed] [Google Scholar]
- 71.Oakes SR, Vaillant F, Lim E, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012;109:2766–2771. doi: 10.1073/pnas.1104778108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaillant F, Merino D, Lee L, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24:120–129. [DOI] [PubMed] [Google Scholar]
- 73.Roberto M, Astone A, Botticelli A, et al. CDK4/6 inhibitor treatments in patients with hormone receptor positive, Her2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers (Basel). 2021;13(2):332. doi: 10.3390/cancers13020332. PMID: 33477469; PMCID: PMC7830463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36(16):2255–2264. doi: 10.1038/onc.2016.379. PMID: 27748766. PMCID: PMC5393973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Zhong X, Wan S, et al. p16(INK4a) expression in retinoblastoma: a marker of differentiation grade. Diagn Pathol. 2014;9:180. doi: 10.1186/s13000-014-0180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dean JL, McClendon AK, Hickey TE, et al. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle. 2012;11(14):2756–2761. doi: 10.4161/.cc.21195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guarducci C, Bonechi M, Benelli M, et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4:38. doi: 10.1038/s41523-018-0092-4. PMID: 30511015. PMCID: PMC6261939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10(1):1373. doi: 10.1038/s41467-019-09068-2. PMID: 30914635; PMCID: PMC6435685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drago JZ, Formisano L, Juric D, et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR+) breast cancer. Clin Cancer Res. 2019;25(21):6443–6451. doi: 10.1158/1078-0432.CCR-19-0138. PMID: 31371343. PMCID: PMC6825550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong X, Du J, Parsons SH, et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2019;9(2):248–263. PMID: 30373917. doi: 10.1158/2159-8290.CD-18-0469 [DOI] [PubMed] [Google Scholar]
- 81.Teh JLF, Cheng PF, Purwin TJ, et al. In Vivo E2F reporting reveals efficacious schedules of MEK1/2-CDK4/6 targeting and mTOR-S6 resistance mechanisms. Cancer Discov. 2018;8(5):568–581. doi: 10.1158/2159-8290.CD-17-0699. PMID: 29496664. PMCID: PMC6858088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 Inhibitors via the hippo pathway. Cancer Cell. 2018;34(6):893–905.e8. doi: 10.1016/j.ccell.2018.11.006. PMID: 30537512; PMCID: PMC6294301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Leeuw R, McNair C, Schiewer MJ, et al. MAPK reliance via acquired CDK4/6 inhibitor resistance in cancer. Clin Cancer Res. 2018;24(17):4201–4214. doi: 10.1158/1078-0432.CCR-18-0410. PMID: 29739788. PMCID: PMC6125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579. doi: 10.1038/ncomms11579. PMID: 27174596. PMCID: PMC4869259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dustin D, Gu G, Fuqua SAW. ESR1 mutations in breast cancer. Cancer. 2019;125(21):3714–3728. doi: 10.1002/cncr.32345. PMID: 31318440. PMCID: PMC6788940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Assoro AB, Liu T, Quatraro C, et al. The mitotic kinase Aurora–a promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERα(+) breast cancer cells. Oncogene. 2014;33(5):599–610. doi: 10.1038/onc.2012.628. PMID: 23334326. PMCID: PMC4058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Opyrchal M, Salisbury JL, Zhang S, et al. Aurora-A mitotic kinase induces endocrine resistance through down-regulation of ERα expression in initially ERα+ breast cancer cells. PLoS One. 2014;9(5):e96995. doi: 10.1371/journal.pone.0096995. PMID: 24816249; PMCID: PMC4016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turner N, Pearson A, Sharpe R, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70(5):2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. PMID: 20179196. PMCID: PMC2832818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogata R, Kishino E, Saitoh W, Koike Y, Kurebayashi J. Resistance to cyclin-dependent kinase (CDK) 4/6 inhibitors confers cross-resistance to other CDK inhibitors but not to chemotherapeutic agents in breast cancer cells. Breast Cancer. 2020. PMID: 32860163. doi: 10.1007/s12282-020-01150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robert NJ, Vogel CL, Henderson IC, et al. The role of the liposomal anthracyclines and other systemic therapies in the management of advanced breast cancer. Semin Oncol. 2004;31(6Suppl 13):106–146. doi: 10.1053/j.seminoncol.2004.09.018 PMID: 15717740. [DOI] [PubMed] [Google Scholar]
- 91.Mauri D, Kamposioras K, Tsali L, et al. Overall survival benefit for weekly vs. three-weekly taxanes regimens in advanced breast cancer: a meta-analysis. Cancer Treat Rev. 2010;36(1):69–74. PMID: 19945225. doi: 10.1016/j.ctrv.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 92.Petrelli F, Di Cosimo S, Lonati V, Barni S. Vinorelbine with capecitabine, an evergreen doublet for advanced breast cancer: a systematic literature review and pooled-analysis of Phase II-III studies. Clin Breast Cancer. 2016;16(5):327–334. PMID: 27282844. doi: 10.1016/j.clbc.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 93.Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33(6):594–601. doi: 10.1200/JCO.2013.52.4892. PMID: 25605862. PMCID: PMC4463422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jerusalem G, De Boer RH, Hurvitz S, et al. Everolimus plus exemestane vs everolimus or capecitabine monotherapy for estrogen receptor-positive, HER2-Negative Advanced Breast Cancer: the BOLERO-6 Randomized Clinical Trial. JAMA Oncol. 2018;4(10):1367–1374. doi: 10.1001/jamaoncol.2018.2262. PMID: 29862411; PMCID: PMC6233772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao EY, Shen Y, Pleasance E, et al. Homologous recombination deficiency and platinum-based therapy outcomes in advanced breast cancer. Clin Cancer Res. 2017;23(24):7521–7530. doi: 10.1158/1078-0432.CCR-17-1941 PMID: 29246904. [DOI] [PubMed] [Google Scholar]
- 96.Petrelli F, Barni S, Bregni G, de Braud F, Di Cosimo S. Platinum salts in advanced breast cancer: a systematic review and meta-analysis of randomized clinical trials. Breast Cancer Res Treat. 2016;160(3):425–437. PMID: 27770282. doi: 10.1007/s10549-016-4025-3 [DOI] [PubMed] [Google Scholar]
- 97.Garrone O, Miraglio E, Vandone AM, Vanella P, Lingua D, Merlano MC Eribulin in advanced breast cancer: safety, efficacy and new perspectives. Future Oncol. 2017;(30):2759–2769. doi: 10.2217/fon-2017-0283. PMID: 29219017. [DOI] [PubMed] [Google Scholar]