Abstract
The recent scientific research has provided clinicians with the tools for substantially upgrading the standard of care in the field of bronchial asthma. Nevertheless, satisfactory asthma control still remains an unmet need worldwide. Identifying the major determinants of poor control in different asthma severity levels represents the first step towards the improvement of the overall patients’ management. The present review aims to provide an overview of the main unmet needs in asthma control and of the potential tools for overcoming the issue. Implementing a personalized medicine approach is essential, not only in terms of pharmacological treatments, biologic drugs or sophisticated biomarkers. In fact, exploring the complex profile of each patient, from his inflammation phenotype to his preferences and expectations, may help in filling the gap between the big potential of currently available treatments and the overall unsatisfactory asthma control. Telemedicine and e-health technologies may provide a strategy to both optimize disease assessment on a regular basis and enhance patients’ empowerment in managing their asthma. Increasing patients’ awareness as well as the physicians’ knowledge about asthma phenotypes and treatment options besides corticosteroid probably represent the key and more difficult goals of all the players involved in asthma management at every level.
Keywords: asthma, asthma control, severe asthma, mild to moderate asthma, telemedicine, personalized medicine
Uncontrolled Asthma: Still a Challenge
Asthma prevalence in the general population ranges from 1 to 18%.1–3 In the European Union, it affects 8.2% of the adult population and 9.4% of children.4,5 In the last few decades, the knowledge about both pathophysiological mechanisms/phenotypes and therapeutic options has significantly increased. In particular, the introduction of biologic drugs for severe asthma paved the way to a true revolution in the field of asthma management, by potentially allowing a precision medicine approach.6
Despite the availability of different treatments that have been proven to be effective in most patients, if regularly taken, satisfactory asthma control still remains an unmet need worldwide.7,8 It represents a major limitation, in fact the burden of poorly controlled asthma is quite relevant in terms of both direct (health care services, medications) and indirect (sickness absence from work, disability, other) costs.1,2,9 Unsatisfactory asthma control has been described in patients affected by different GINA-based asthma severity levels,10 which on the other hand conditions the relevance and the clinical implications of poor control on asthma outcomes and individuals’ quality of life. Also, different factors can be responsible for inadequate control according to the asthma severity levels, such as a true difficult to treat disease in the case of GINA step 5 asthma or poor adherence in mild-to-moderate asthma.6,11 However, identifying the major determinants of unsatisfactory asthma control is essential in order to implement tailored strategies aimed at improving the overall patients’ management. The present review aims to highlight the main unmet needs and the potential determinants of poor asthma control by describing the peculiar role and relevance of common limitations to the achievement of optimal disease management in the context of each one of the different asthma severity levels. Through the same approach, potential tools for overcoming the issue, including telemedicine and the new e-health technologies, are explored.
Mild-to-Moderate Asthma – Reasons for Poor Control
According to robust evidence in the literature, the clinical manifestations of mild-to-moderate asthma can be optimally controlled through a number of appropriate treatment options.10 Controlled asthma means minimal or no symptoms during the day and night, no asthma attacks, no emergency visits, minimal need for reliever medications, no limitations on daily activities, nearly normal lung function and minimal or no side effects from medication.12 Once trigger factors, such as allergens, have been minimized or when possible removed, clinical control can be achieved by reducing chronic inflammation of the airways by controller therapy, mainly including inhaled corticosteroids. However, in the real-life experience, asthma control is still lower than expected13,14 and the disease burden heavily impacts both on the patient’s quality of life and on health care costs.15 Many factors accounting for that can be identified.
The main issue related to the overall unsatisfactory control in mild-to-moderate asthma is the patient’s adherence to prescribed inhaled therapies.16 It is well known that low adherence is a widely spread problem among patients affected by chronic disease.17 In asthmatic patients this attitude is even more relevant, when considering that patients often perceive the disease in terms of episodic symptoms more than chronic illness.
When talking about a patient’s lack of adherence, we have to consider both intentional and unintentional issues.17–19 The intentional non-adherence is the consequence of an active and rational decision following the patient’s evaluation of the advantages and disadvantages of the prescribed treatment. Of course, the decision-making process is highly influenced by the overall information at each individual's disposal.19
When the decision of stopping the prescribed treatment or altering doses has to do with the patient’s poor awareness and low perception of disease severity, unintentional non-adherence is occurring.19 It leads on one hand to irregular ICS/LABA intake, and on the other hand generates concerns about regular therapy. The less patients are aware of the consequence of the disease and of the importance of a stable therapy, the more they will develop concerns or doubts about the true need for regular drug intake and, even more relevant, they will be afraid of possible adverse events related to that. The unintentional non-adherence also results from practical barriers to treatment, such as language barriers, forgetfulness and inadequate understanding of the instructions.20,21 Under this category, a main problem is incorrect inhalation technique. As a matter of fact, many patients are not able to correctly use their device.21 Many video-tutorials are nowadays available, still they are often not sufficient, and the right inhalation technique must be reviewed at every ambulatory visit.10,22 Due to the existence of many different types of inhaler, it is the physician’s duty to consider which would be the best one for every patient through a personalized evaluation. When sustainable, avoiding or reducing the number of inhalation devices is helpful; in fact, simplified therapeutic regimens may contribute to improve the overall adherence.20,21
When bad asthma control is reported by an asthmatic patient regularly assuming the prescribed therapies, asthma diagnosis deserves to be confirmed and/or asthma severity reassessed, especially if the diagnosis was primarily made in a different setting, such as the primary care one, where many times not all available instruments for a correct assessment are available.10,23 The co-existence of comorbidities and/or the patient’s habits that interfere with asthma treatment represents a further determinant of unsatisfactory control.
For example, rhinitis is a well-known and frequent asthma comorbidity that requires to be specifically addressed. Treating rhinitis in patients affected by both rhinitis and asthma significantly improves asthma control.10,24 Another important aspect to evaluate is the patient’s smoking habit, which is a well-known risk factor for poor clinical and functional outcomes in asthma due to enhanced neutrophilic inflammation, augmented oxidative stress in the airways and increased leukotriene local production.25,26
Not only patients or difficult to treat asthma phenotypes may hamper the achievement of disease control. Poor physicians’ adherence to asthma guidelines is a known “risk factor” for suboptimal disease management, especially when talking about general practitioners.27 Allergists and respiratory medicine specialists seem to do better and to achieve better patient outcomes.27–29 However, still very recently a survey investigating self-reported guideline agreement and adherence among a sample of allergists and pulmonologists reported an adherence rate below 50% regarding specific guideline recommendations, namely providing the patients with an asthma action plan and regularly assessing the inhaler technique.30 On one hand it is indubitable that general recommendations need to be tailored to the specific context each physician operates; in on the other hand, the results of the above-mentioned survey for sure identify room for improvement in the light of better asthma management.
Mild-to-Moderate Asthma – Unmet Needs and Potential Strategies
The main unmet need in mild-to-moderate asthma management seems to be unsatisfactory adherence to the treatment.16 As major implications, asthma attacks requiring Emergency Room (ER) access and, even worse, asthma deaths have been described mostly in mild-to-moderate asthmatics.11,31–34 A recent case series of asthma deaths highlighted the as needed use of SABA and only intermittent ICS or ICS/LABA courses as a common feature shared by all the described patients.11 Similarly, poor adherence rate to the prescribed drugs, lack of regular follow-up visits, unsatisfactory disease awareness and consequently inadequate knowledge about self-management of an asthma attack have been identified by many studies as the determinants associated with ER admissions due to asthma major exacerbations in the adult population.31–34
Together with ERs, community pharmacies also represent a first-line health care service, which seems to be more easily accessed by asthmatics when compared to respiratory medicine specialists or general practitioners.1,35,36 Patients easily refer to the pharmacy to get reliever medications or inhaled drugs in general and bypass the medical follow-up visit. However, a recent large study including community pharmacies confirmed that non-optimal asthma control was more frequent among patients with mild-to-moderate asthma and that low treatment adherence was the only determinant of poorly controlled asthma.36
The above-mentioned information suggests that first-line health care services, namely ERs and community pharmacies, in addition to GPs and specialists, should be involved in detecting asthmatic patients at risk of asthma exacerbations due to inadequate adherence to pharmacological treatment and follow-up programmes. In fact, a recent study reported that among patients admitted to the ER for an asthma attack, most were classified with white to yellow codes, according to the triage severity scoring system;31 this suggests that those admissions were related to poorly controlled and not to truly severe asthma, and were probably preventable by simply optimizing treatment adherence and by providing patients with a personalized asthma action plan. Under that perspective, providing the patients with a scheduled appointment with an asthma-expert specialist within the same hospital before the ER discharge may facilitate their engagement. Regarding community pharmacies, several studies have explored their potential in supporting and implementing the doctors’ initiatives.35 As they are much more accessible for the patient compared with outpatient clinics, they can be involved in different activities including asthma control assessment, educational interventions or counselling.1,35,36
Active involvement of patients in the management of their disease plays also a pivotal role. The selection of the most appropriate treatment is for sure a doctor’s decision, based on complex clinical evaluations. However, sharing the treatment approach and the reasons behind it with the patients may enhance their disease awareness.37,38 When possible under a clinical perspective, discussing with the patients about different options in terms of device or treatment schedule and exploring their preferences and expectations may increase their engagement in asthma management.39 In any case, a tailored asthma action plan, including prevention and management of asthma exacerbation, should always be shared with the patients.10
Finally, the patient’s adherence may be improved by working on feelings or thoughts that lead them to therapy discontinuation or to a low adherence. For this purpose, it is reasonable not to use only classical outcome measures to evaluate disease control, but also to decide with the patient which are the most important outcomes influencing his/her quality of life.40 By defining common goals, collaboration replaces “prescriptions”, and patients can experience positive effects of therapies in aspects of their lives they consider the most important.
Severe Asthma – Reasons for Poor Control
According to the international ERS/ATS guidelines, severe asthma can be defined as a condition requiring GINA 4–5 level of medications to be controlled or which remains uncontrolled despite that treatment (ERS/ATS).41 Therefore, in that condition the achievement of optimal control can be considered intrinsically more difficult than at the lower levels of severity by definition. However, more easily treatable determinants may contribute to the disease control complexity besides severity. In fact, unsatisfactory treatment adherence has been described in severe asthmatics as well, and may be responsible for poor control even if at a lesser extent in comparison with mild/moderate asthma.11,42 Patients with severe asthma who underuse ICS complain of significantly more frequent exacerbations, despite a regular biologic treatment.43 Besides the constant administration of the therapy, the correct inhalation technique has to be regularly assessed in any asthmatic, including patients with severe asthma. It is part of a personalized medicine approach identifying the optimal inhaler device and easiest treatment schedule for every patient. In fact, when sustainable, simplifying regimens are associated with improved adherence.20,21
Under a pathophysiological perspective, symptoms in severe asthma are the results of different determinants, besides bronchial inflammation and hyper-reactivity, including comorbidities, and psychological and behavioural factors.41 For that reason, especially in the case that severe asthma remains difficult to control, reassessing the asthma diagnosis is essential in order to define the pathophysiological relevance of conditions other than asthma and to classify them as asthma comorbidities or concomitant diseases interfering with the disease control. The identification and management of those treatable traits may provide great improvement to the asthma control.41 The most frequent extra-respiratory comorbidities are atopy, chronic rhino-sinusitis, sleep apnoea, gastroesophageal reflux and obesity, whereas smoking habits, psychological disorders such as depression or anxiety are the most common psychosocial traits.44 Vocal cord dysfunction affects one out of four patients with asthma45 and its prevalence is even higher in severe asthma;46 if not properly treated with speech and language therapy it may be responsible for pharmacological over-treatment. The same problem may be due to dysfunctional breathing, which can be successfully cured with physiotherapy. Weight loss in obese patients with behavioural interventions, such as diet and physical exercise, may positively impact on respiratory symptoms. Smoking is responsible for reduced steroid response, decline of lung function and bronchial hyperactivity; thus its cessation has to be strongly encouraged. Other treatable traits, such as rhinitis and gastroesophageal reflux, have a slight role in the control of the disease.41,44 The identification of different inflammatory phenotypes of severe asthma as T2 high eosinophilic or T2 low, neutrophilic or pauci-granulocytic has led to a more personalized therapy, based on the use of different monoclonal antibodies.
T2 high inflammation is mainly driven by IL-5, IL-4 and IL-13, which orchestrate a complex immune cascade including eosinophils as the main cellular player. That scenario offers a number of potential therapeutic targets, which have been largely explored and pharmacological research is still ongoing.47 IL-17, IL-6 and IL-23, together with airway smooth-muscle or neural dysfunction, drive the heterogeneous inflammatory patterns within the T2 low phenotypes, including neutrophilic and paucigranulocytic asthma. Basic research is still ongoing to better understand underlying mechanisms and identify new therapeutic targets for T2 low phenotypes.48 Defining the prevalence of the two above-mentioned phenotypes is not easy;6 according to a recent analysis of the UK Severe Asthma Registry, around 50% of severe asthmatic patients could be categorized as Th2 high whilst less than 10% could be labelled as T2 low.49 However, a variable stability of the phenotypes has been reported over time, thus suggesting the need for a regular assessment.50 Moreover, the currently available biologics are only effective in eosinophilic or allergic asthma, whereas no options exist in non-eosinophilic asthma.44 Furthermore, the different biology of neutrophils, which are widely involved in the immune response and other biologic processes, suggests that a treatment strategy based on anti-IL-5 or anti-IgE mechanisms, leading to a strong reduction or elimination of eosinophils or IgE, may be not optimal in every patient.44,48 Currently, although several pathways may be potentially targeted in T2 low asthma, no specific treatments are available. Moreover, concomitant treatable traits in the same patient with eosinophilic asthma may enhance the inflammation with a specific different inflammatory pattern.48,50 In fact, smoking, sleep apnoea and obesity are characterized by neutrophilic inflammation, which can overlap a pre-existing eosinophilic inflammation. Therefore, the efficacy of an anti-eosinophilic treatment might be hampered by a concomitant neutrophilic inflammation.44 One more criticism in eosinophilic asthma is that the choice of the monoclonal antibodies is based, besides the clinical features, on unspecific biomarkers, including blood eosinophilic count, exhaled nitric oxide and serum total IgE.51 In fact, an intra-individual variability of blood eosinophils over time has to be taken into account as well as the impact on these biomarkers of several factors such as smoking, diet and concomitant treatments as inhaled and oral steroids.52 On the opposite, once a treatment option has been identified, a regular assessment of biomarkers is helpful in monitoring the treatment effect as well as the patients’ adherence to the treatment itself. The evaluation of blood eosinophil count, fractional exhaled nitric oxide and sputum where available is advised during the treatment course; their trend, together with lung function, exacerbation rate and OCS use, is part of a composite of outcomes contributing to the definition of the clinical response after a 4 to 6 month biologic treatment trial period.53,54
In T2 low asthma, the proportion of neutrophils greater than 60% in induced sputum are helpful in the diagnostic work-up but their role in driving the therapeutic management of patients is controversial.55 IL-6 in serum seems to be suggestive of airway remodelling in obesity-related and other phenotypes of neutrophilic asthma.48 However, the substantial lack of specific, easy to assess and clinically useful biomarkers contributes to the difficult management of the patients affected by T2 asthma.
One more crucial issue in severe asthma is the overuse of OCS and the subsequent risk of side effects, which have to be carefully assessed particularly in patients on regular treatment.56 Hypertension, osteoporosis and bone fracture, cataract and glaucoma, diabetes, respiratory infections, reduced growth velocity in children, and hypothalamic–pituitary–adrenal axis suppression represent systemic corticosteroids-related major adverse events described in severe asthmatics.57 Similar effects have been reported in patients treated with high doses of inhaled steroids for a prolonged time.58 This OCS overuse might be somehow plausible in non-eosinophilic asthma; in fact, the poor response to steroid therapy may lead to increase the dosage and then to overtreatment.44 On the opposite it seems unjustifiable in eosinophilic asthma, given the proved steroid sparing effect of anti-IgE and anti-IL-5 treatments. As mentioned above, the risks of side effects are not negligible also in patients receiving high dosage of inhaled steroids, given the systemic absorption of those drugs.59 In terms of worldwide perspective, the sustainability of biologics for severe asthma due to their high costs sustain a huge socioeconomic problem. The consequence is the lack of their availability particularly in underdeveloped countries and the unfeasibility to offer the same effective treatment in any country, despite the economic income.60
Severe Asthma – Unmet Needs and Potential Strategies
Targeting specific steps of the immune-inflammatory cascade through highly selective drugs represents a true revolution in the field of severe asthma management, and brings with it the potential of achieving optimal disease control in every severe asthma inflammatory phenotype.51 On the other hand, although paradoxical, some biologics-related issues represent a major unmet need. Patient selection is still challenging mainly for two reasons: low/non-responder cases more or less to all the available biologics have been described, despite when prescription criteria were fully matched; and a not negligible proportion of patients are eligible for more than one drug.51,61 On the other hand, no one of the easily accessible biomarkers is up to now able to specifically predict the response to one biologic drug.62 Another major challenge is related to the management of Th2 low inflammation phenotypes. Two main limitations hamper the achievement of optimal control in that subpopulation: the typical poor responsiveness to steroids, whether systemic or inhaled, is associated with frequent, difficult to treat exacerbations; and the lack of biologics, at least among the marketed ones, able to selectively address specific targets of Th2 low inflammation.48,51
Most of the ongoing scientific research in the field of severe asthma is focused on the above-mentioned issues and will probably provide new tools and treatment options in the next few years.
In the meantime, a careful and extensive assessment of our patients besides blood eosinophils and bronchial inflammation, including comorbidities and concomitant diseases, may help in identifying different therapeutic targets and in selecting the most effective biologic drug. In fact, clinical trials and post-hoc analysis on one side, and the increasing real-life evidenceoin the other, are providing more and more data about the impact of each treatment option on extra-bronchial targets.51,63,64
Furthermore, accessibility to the currently available biologic drugs deserves to be enhanced. It has been recently highlighted that, with few exceptions, the monoclonal antibodies market is limited to the developed and high-income countries.60 High costs for sure represent an issue, and the availability of generic drugs in the field of biologics may provide a chance. On the other hand, poor awareness and expertise regarding severe asthma, as well as the lack of proper tools to assess and manage it, potentially limit the routine use of biologics in low and middle-income countries besides their costs. However, a difficult identification of severe asthmatic patients characterizes developed and high-income countries too.6 In fact, the emersion of severe asthma requires specific knowledge of the disease’s complexity, which cannot rely on questionnaires or patient reported outcomes only. Patients, GPs and different specialists should be involved in large-scale awareness campaigns in order to spread the knowledge about severe asthma hallmarks and treatment options besides steroids.
Technology and Telemedicine: A Step Forward in Optimizing Patients’ Management
E-health is defined as:
An emerging field at the intersection of medical informatics, public health and business, referring to health services and information delivered or enhanced through the Internet and related technologies.65
In the last years a number of different tools have been developed and marketed, including digital apps, telemedicine, mobile health, virtual health care teams, electronic health records, medication trackers and clinical decision support systems.66
E-health technologies and telemedicine may substantially contribute to two main aspects connected with the management of asthmatic patients. On one side, they facilitate regular patient assessment even in the presence of a difficult in-person relationship between doctor and patient. It may happen for geographical or work reasons, or in the case of limitations or delay in the access to specialist care.66,67 Under this perspective, the COVID-19 pandemic has impressively impacted on the health care resources setting, and the restricted hospital admissions have prompted the spread of telemedicine in several chronic conditions, including bronchial asthma.68 In the case of severe asthma, besides pandemic restrictions, patients undergoing at home a self-administered biologic drug need regular tele-monitoring.69 However, telemedicine may be applicable for the management of any asthma severity level. In fact, besides the home spirometry and digital visits, the regular therapy intake can be quite easily monitored.70 The recent development of inhaler trackers monitoring the real-time usage of inhaled drugs may represent a step forward in achieving a better adherence to therapy. Depending on the electronic device, they can register only the number and the time of the inhalations or may also provide information about the inhalation technique. They can also be matched with applications that collect data and send reminders and feedback on ICS and SABA use.70,71 The inhalation technique may be also improved by dedicated video-tutorials.
The other aspect of asthma management that can be substantially implemented by e-health technologies and telemedicine has to do with patients’ empowering, which is essential for achieving optimal asthma control. In fact, different tools including digital apps providing warning notifications on the disease control and reminder messages about the treatment intake may contribute to develop disease awareness and improve correct self-management.66,70,71 Of course, a regular health care professional is essential for the selection of patients to be addressed to such tools and for the proper use of e-health technologies.
The telemedicine-based approach offers many tools and resources, but its wide potential has been explored by a limited number of studies so far. Brown and Odenthal confirmed the efficacy of telemedicine in asthma management in improving asthma control scores and FEV%;72 furthermore, the patients’ evaluation was similar for telemedicine and in-person visits.73 Another Danish study investigated an interactive Internet-based asthma-monitoring tool and demonstrated an improvement in asthma symptoms, drug adherence, lung function and airway hyper-responsiveness.74 A recent study carried out among inner-city children with low income explored the effectiveness of a telemedicine model based on lessons from a network of asthma specialists (allergist or pulmonologist and psychologist) provided through a Propeller Health web platform.75 The study highlighted a significant improvement in Composite Asthma Severity Index scores and drug adherence, and a reduced use of health care resources. A randomized trial compared the use and efficacy of an interactive smartphone-based personalized asthma action plan (AAP) with paper-based AAP among teens, which represents a well-known at-risk age group in terms of poor asthma outcomes. Within the intervention group, patients accessed their AAP smartphone more times per week than in the paper AAP group. The findings suggest that smartphone AAPs are a feasible method to improve self-management and asthma outcomes in this paediatric and young-adult population.76
However, some issues related to telemedicine have to be highlighted. A study by Huckvale et al77 found that nearly half of the apps recommended for acute asthma management strategies were not evidence-based and only a small amount of apps offered information consistent with international medical guidelines, making their use potentially harmful for patients. Moreover, the use of the technology may be easier for youngsters and adults, but might be an obstacle for older ages. One more drawback of telemedicine is its economical sustainability. The individual and social costs of technology implicate the need for significant investment in infrastructure in order to implement telemedicine programs. Moreover, high-speed Internet capacity has an additional cost, especially in those places that do not have the bandwidth to support high-quality video conferencing necessary for satisfactory telemedicine visits.70
E-Health medicine offers an unexpectedly wide number of technological resources and opens new horizons for the future health services.68,70 However, telemedicine has to be considered as an integration of the current management of medicine and not a potential replacement. In fact, the availability of telemedicine visits may greatly support the patients’ follow-up management, but at the same time is not able to provide the same empathetic environment as in a traditional medical setting, which is the cornerstone of a positive relationship between doctor and patient and has a pivotal role in developing patients’ disease awareness.40 Moreover, the belief that telemedicine is for everyone is far from the reality. In fact, digital medicine is now mainly targeted for youngsters and adults and is less suitable for elderly ages. Furthermore, accessibility to the Internet is far from being achieved for every country in the world and therefore the development of e-health medicine may take into account the risk of potential differences in the management of the diseases all over the world.
Conclusions
The recent scientific research has provided clinicians with the tools for substantially upgrading the standard of care in the field of bronchial asthma. It means reducing steroid-related morbidity, especially for severe asthma, and most importantly ameliorating patients’ quality of life. The current challenge is filling the gap between that big potential and its implementation in daily clinical practice, or in other words making concrete for every patient the personalized medicine approach.
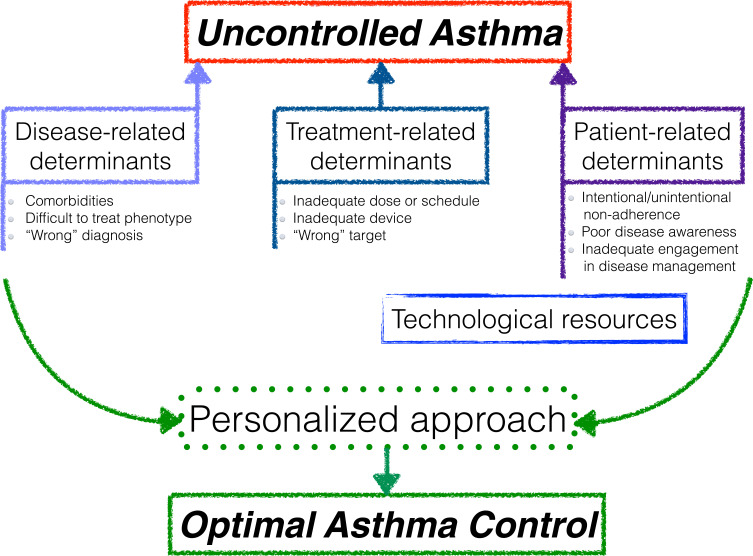
Identifying the major determinants of poor control represents the first step towards the improvement of the overall patients’ management (Figure 1). Precision medicine is much more than pharmacological treatments, biologic drugs or sophisticated biomarkers; in fact, a tailored approach entails exploring the complex profile of each patient, from his inflammation phenotype to his preferences and expectations. Telemedicine and e-health technologies may provide a strategy to both optimize disease assessment on a regular basis and enhance patients’ empowerment in managing their asthma. Increasing patients’ awareness as well as the physicians’ knowledge about asthma phenotypes and treatment options besides corticosteroids probably represent the key and more difficult goals of all the players involved in asthma management at every level.
Figure 1.
Overview of major determinants of poor control and potential intervention strategies.
Funding Statement
No funding to declare.
Disclosure
The authors reported no conflicts of interest for this work.
References
- 1.Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62(6):496–502. doi: 10.1136/thx.2006.064709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Bousquet PJ, Godard P, et al. The public health implications of asthma. Bull WHO. 2005;83(7):548–554. [PMC free article] [PubMed] [Google Scholar]
- 3.Wijnant SRA, Lahousse L, De Buyzere ML, et al. Prevalence of asthma and COPD and blood eosinophil count in a middle-aged Belgian population. J Clin Med. 2019;8(8):1122. doi: 10.3390/jcm8081122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eurostat. Respiratory disease statistics. Available from: http://ec.europa.eu/eurostat/statistics-explained/index.php/Respiratory_diseases_statistics. Accessed January, 2021.
- 5.Selroos O, Kupczyk M, Kuna P, et al. National and regional asthma programmes in Europe. Eur Respir Rev. 2015;24(137):474–483. doi: 10.1183/16000617.00008114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caminati M, Senna G. Uncontrolled severe asthma: starting from the unmet needs. Curr Med Res Opin. 2019;35(2):175–177. doi: 10.1080/03007995.2018.1528218 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO programmes and projects. Chronic respiratory diseases. Asthma. Available from: http://www.who.int/respiratory/asthma/en/. Accessed January, 2021.
- 8.World Health Organization. WHO projections of mortality and burden of the disease 2004–2030. Available from: http://www.who.int/healthinfo/global/burden_disease/Dth6_2030.xls. Accessed January, 2021.
- 9.Australian Centre for Asthma Monitoring. Health Care Expenditure and the Burden of Disease Due to Asthma in Australia. Canberra: Australian Institute of Health and Welfare; 2005:43. [Google Scholar]
- 10.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Available from: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf. Accessed December, 2020.
- 11.Vianello A, Caminati M, Crivellaro M, et al. Fatal asthma; is it still an epidemic? World Allergy Organ J. 2016;9(1):42. doi: 10.1186/s40413-016-0129-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horne R, Price D, Cleland J, et al. Can asthma control be improved by understanding the patient’s perspective? BMC Pulm Med. 2007;7:8. doi: 10.1186/1471-2466-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabe KF, Vermeire PA, Soriano JB, et al. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–807. doi: 10.1183/09031936.00.16580200 [DOI] [PubMed] [Google Scholar]
- 14.Van Ganse E, Boissel JP, Gormand F, et al. Level of control and hospital contacts in persistent asthma. J Asthma. 2001;38(8):637–643. doi: 10.1081/JAS-100107541 [DOI] [PubMed] [Google Scholar]
- 15.Van Ganse E, Laforest L, Pietri G, et al. Persistent asthma: disease control, resource utilisation and direct costs. Eur Respir J. 2002;20(2):260–267. doi: 10.1183/09031936.02.02542001 [DOI] [PubMed] [Google Scholar]
- 16.Busse W, Fang J, Marvel J, et al. Uncontrolled asthma across GINA treatment steps 2 − 5 in a large US patient cohort. J Asthma. 2021;12:1–15. doi: 10.1080/02770903.2021.1897834 [DOI] [PubMed] [Google Scholar]
- 17.Senna G, Caminati M, Lockey RF. Allergen immunotherapy adherence in the real world: how bad is it and how can it be improved? Curr Treat Options Allergy. 2015;2(1):39–53. doi: 10.1007/s40521-014-0037-6 [DOI] [Google Scholar]
- 18.Hardtstock F, Maywald U, Timmermann H, et al. Extent of non-adherence and non-persistence in asthma patients: analysis of a large claims data set. J Asthma. 2021;20:1–11. doi: 10.1080/02770903.2021.1871738 [DOI] [PubMed] [Google Scholar]
- 19.Wroe A. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25(4):355–372. doi: 10.1023/A:1015866415552 [DOI] [PubMed] [Google Scholar]
- 20.George M. Adherence in asthma and COPD: new strategies for an old problem. Respir Care. 2018;63(6):818–831. doi: 10.4187/respcare.05905 [DOI] [PubMed] [Google Scholar]
- 21.Lavorini F, Janson C, Braido F, et al. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther Adv Respir Dis. 2019;13:1753466619884532. doi: 10.1177/1753466619884532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aydın MR, Aydemir Y, Aydın A, et al. The effect of video presentation showed in the outpatient clinic waiting area on the success of inhaler device use in chronic respiratory diseases. Heart Lung. 2021;50(2):323–328. doi: 10.1016/j.hrtlng.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 23.Magnoni MS, Caminati M, Senna G, et al. Asthma under/misdiagnosis in primary care setting: an observational community-based study in Italy. Clin Mol Allergy. 2015;13(1):26. doi: 10.1186/s12948-015-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi: 10.1038/s41572-020-00227-0 [DOI] [PubMed] [Google Scholar]
- 25.Tiotiu A, Ioan I, Wirth N, et al. The impact of tobacco smoking on adult asthma outcomes. Int J Environ Res Public Health. 2021;18(3):992. doi: 10.3390/ijerph18030992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulet LP, Boulay ME, Coxson HO, et al. Asthma with irreversible airway obstruction in smokers and nonsmokers: links between airway inflammation and structural changes. Respiration. 2020;8:1–11. [DOI] [PubMed] [Google Scholar]
- 27.Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med. 1999;14(8):499–511. doi: 10.1046/j.1525-1497.1999.08168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Marco R, Cazzoletti L, Cerveri I, et al; ISAYA Study Group. Are the asthma guideline goals achieved in daily practice? A population-based study on treatment adequacy and the control of asthma. Int Arch Allergy Immunol. 138;2005:225–234. doi: 10.1159/000088723 [DOI] [PubMed] [Google Scholar]
- 29.Braido F, Baiardini I, Alleri P, et al. Asthma management in a specialist setting: results of an Italian respirtory society survey. Pulm Pharmacol Ther. 2017;44:83–87. doi: 10.1016/j.pupt.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 30.Cloutier M, Akinbami L, Salo P, et al. Use of national asthma guidelines by allergists and pulmonologists: a national survey. J Allergy Clin Immunol Pract. 2020;8(9):3011–3020.e2. doi: 10.1016/j.jaip.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caminati M, Vianello A, Ricci G, et al. Trends and determinants of emergency room admissions for asthma: a retrospective evaluation in Northeast Italy. World Allergy Organ J. 2019;12(7):100046. doi: 10.1016/j.waojou.2019.100046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suruki RY, Daugherty JB, Boudiaf N, et al. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi: 10.1186/s12890-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Jahdali H, Anwar A, Al-Harbi A. Factors associated with patient visits to the emergency department for asthma therapy. BMC Pulm Med. 2012;12:80. doi: 10.1186/1471-2466-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pola-Bibian B, Dominguez-Ortega J, Vilà-Nadal G. Predictors of asthma relapse in patients who attended an emergency department. Allergy Asthma Proc. 2018;39(4):292–298. doi: 10.2500/aap.2018.39.4130 [DOI] [PubMed] [Google Scholar]
- 35.Senna G, Caminati M, Bovo C, et al. The role of the pharmacy in the management of bronchial asthma: a literature-based evaluation. Ann Allergy Asthma Immunol. 2017;118(2):161–165. doi: 10.1016/j.anai.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 36.Caminati M, Cegolon L, Bacchini M, et al. The potential role of local pharmacies to assess asthma control: an Italian cross-sectional study. BMC Public Health. 2021;21(1):19. doi: 10.1186/s12889-020-10080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedenrud T, Jakobsson A, El Malla H, et al. “I did not know it was so important to take it the whole time” - self-reported barriers to medical treatment among individuals with asthma. BMC Pulm Med. 2019;19(1):175. doi: 10.1186/s12890-019-0934-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baiardini I, Novakova S, Mihaicuta S, et al. Adherence to treatment in allergic respiratory diseases. Expert Rev Respir Med. 2019;13(1):53–62. doi: 10.1080/17476348.2019.1554438 [DOI] [PubMed] [Google Scholar]
- 39.De Keyser HH, Ramsey R, Federico MJ. They just don’t take their medicines: reframing medication adherence in asthma from frustration to opportunity. Pediatr Pulmonol. 2020;55(3):818–825. doi: 10.1002/ppul.24643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruffydd-Jones K, Hansen K. Working for better asthma control: how can we improve the dialogue between patients and healthcare professionals? Adv Ther. 2020;37(1):1–9. doi: 10.1007/s12325-019-01131-0 [DOI] [PubMed] [Google Scholar]
- 41.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 42.Caminati M, Vianello A, Andretta M, et al. Low adherence to inhaled corticosteroids/long-acting beta(2)-agonists and biologic treatment in severe asthmatics. ERJ Open Res. 2020;6(2):00017–2020. doi: 10.1183/23120541.00017-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.d’Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. doi: 10.1183/13993003.02259-2019 [DOI] [PubMed] [Google Scholar]
- 44.Hinks TSC, Levine SJ, Brusselle GG. Treatment options in type-2 low asthma. Eur Respir J. 2021;57(1):2000528. doi: 10.1183/13993003.00528-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, An J, Won H, et al. Prevalence and impact of comorbid laryngeal dysfunction in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2020;145(4):1165–1173. doi: 10.1016/j.jaci.2019.12.906 [DOI] [PubMed] [Google Scholar]
- 46.Vertigan AE, Kapela SL, Gibson PG. Laryngeal dysfunction in severe asthma: a Cross-Sectional Observational Study. J Allergy Clin Immunol Pract. 2021;9(2):897–905. doi: 10.1016/j.jaip.2020.09.034 [DOI] [PubMed] [Google Scholar]
- 47.Caminati M, Pham DL, Bagnasco D, et al. Type 2 immunity in asthma. World Allergy Organ J. 2018;11(1):13. doi: 10.1186/s40413-018-0192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ntontsi P, Samitas K, Zervas E, et al. Severe asthma: what is new in the new millennium. Curr Opin Allergy Clin Immunol. 2020;20(2):202–207. doi: 10.1097/ACI.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 49.Jackson D, Busby J, Pfeffer P, et al. UK severe asthma registry. Characterisation of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2021;76(3):220–227. doi: 10.1136/thoraxjnl-2020-215168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kupczyk M, Dahlén B, Sterk PJ, et al. BIOAIR investigators stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014;69(9):1198–1204. doi: 10.1111/all.12445 [DOI] [PubMed] [Google Scholar]
- 51.Caminati M, Bagnasco D, Rosenwasser LJ, et al. Biologics for the treatments of allergic conditions: severe asthma. Immunol Allergy Clin North Am. 2020;40(4):549–564. doi: 10.1016/j.iac.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 52.Hartl S, Breyer MK, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020;55(5):1901874. doi: 10.1183/13993003.01874-2019 [DOI] [PubMed] [Google Scholar]
- 53.Pepper A, Hanania N, Humbert M, et al. How to assess effectiveness of biologics for asthma and what steps to take when there is not benefit. J Allergy Clin Immunol Pract. 2021;9(3):1081–1088. doi: 10.1016/j.jaip.2020.10.048 [DOI] [PubMed] [Google Scholar]
- 54.Ulrik C, Lange P, Hilbergc O. Fractional exhaled nitric oxide as a determinant for the clinical course of asthma: a systematic review. Eur Clin Respir J. 2021;8(1):1891725. doi: 10.1080/20018525.2021.1891725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diamant Z, Vijverberg S, Alving K, et al. Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy. 2019;74(10):1835–1851. doi: 10.1111/all.13806 [DOI] [PubMed] [Google Scholar]
- 56.Tran T, King E, Sarkar Nan C, et al. Oral corticosteroid prescription for France, Germany, Italy and UK. Eur Res J. 2020;55(6):1902363. doi: 10.1183/13993003.02363-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lefebvre P, Duh M, Lafeuille M, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin. 2017;33(1):57–65. doi: 10.1080/03007995.2016.1233101 [DOI] [PubMed] [Google Scholar]
- 58.Rogliani P, Ritondo B, Puxeddu E, et al. Experimental glucocorticoid receptor agonists for the treatment of asthma: a systematic review. J Exp Pharmacol. 2020;12:233–254. doi: 10.2147/JEP.S237480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maijers I, Kearns N, Harper J, et al. Oral steroid-sparing effect of high-dose inhaled corticosteroids in asthma. Eur Respir J. 2020;55(1):1901147. doi: 10.1183/13993003.01147-2019 [DOI] [PubMed] [Google Scholar]
- 60.Caminati M, Morais-Almeida M, Bleecker E, et al. Biologics and global burden of asthma: a worldwide portrait and a call for action. World Allergy Organ J. 2021;14(2):100502. doi: 10.1016/j.waojou.2020.100502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menzella F, Bertolini F, Biava M, et al. Severe refractory asthma: current treatment options and ongoing research. Drugs Context. 2018;7:212561. doi: 10.7573/dic.212561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narendra D, Blixt J, Hanania NA. Immunological biomarkers in severe asthma. Semin Immunol. 2019;46:101332. doi: 10.1016/j.smim.2019.101332 [DOI] [PubMed] [Google Scholar]
- 63.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 64.Olivieri B, Tinazzi E, Caminati M, et al. Biologics for the treatment of allergic conditions: eosinophil disorders. Immunol Allergy Clin North Am. 2020;40(4):649–665. doi: 10.1016/j.iac.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 65.Oh H, Rizo C, Enkin M, Jadad A, Powell J, Pagliari C. What is eHealth (3): a systematic review of published definitions. J Med Internet Res. 2005;7(1). doi: 10.2196/jmir.7.1.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonini M. Electronic health (e-health): emerging role in asthma. Curr Opin Pulm Med. 2017;23(1):21–26. doi: 10.1097/MCP.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 67.Berlinski A, Chervinskiy SK, Simmons AL, et al. Delivery of high-quality pediatrics pirometry in rural communities: a novel use for telemedicine. J Allergy Clin Immunol Pract. 2018;6(3):1042–1044. doi: 10.1016/j.jaip.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 68.Persaud YK, Portnoy JM. Ten rules for implementation of a telemedicine program to care for patients with asthma. J Allergy Clin Immunol Pract. 2021;9(1):13–21. doi: 10.1016/j.jaip.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snoswell CL, Rahja M, Lalor AF. A systematic review and meta-analysis of change in health-related quality of life for interactive telehealth interventions for patients with asthma. Value Health. 2021;24(2):291–302. doi: 10.1016/j.jval.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 70.Poowuttikul P, Seth D. New concepts and technological resources in patient education and asthma self-management. Clin Rev Allergy Immunol. 2020;59(1):19–37. doi: 10.1007/s12016-020-08782-w [DOI] [PubMed] [Google Scholar]
- 71.Nguyen E, Miao B, Pugliese N, et al. Systematic review of mHealth applications that interface with inhaler sensors in asthma. J Allergy Clin Immunol Pract. 2021;9(2):SS2213–8. doi: 10.1016/j.jaip.2020.08.049 [DOI] [PubMed] [Google Scholar]
- 72.Brown W, Odenthal D. The uses of telemedicine to improve asthma control. J Allergy Clin Immunol Pract. 2015;3(2):300–301. doi: 10.1016/j.jaip.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 73.Portnoy JM, Waller M, De Lurgio S, et al. Telemedicine is as effective as in-person visits for patients with asthma. Ann Allergy Asthma Immunol. 2016;117(3):241–245. doi: 10.1016/j.anai.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen LM, Phanareth K, Nolte H, et al. Internet-based monitoring of asthma: a long-term, randomized clinical study of 300 asthmatic subjects. J Allergy Clin Immunol. 2005;115(6):1137–1142. doi: 10.1016/j.jaci.2005.03.030 [DOI] [PubMed] [Google Scholar]
- 75.Lin NY, Ramsey RR, Miller JL, et al. Telehealth delivery of adherence and medication management system improves outcomes in inner-city children with asthma. Pediatr Pulmonol. 2020;55(4):858–865. doi: 10.1002/ppul.24623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosnaim GS, Stempel DA, Gonzalez C, et al. The impact of patient self-monitoring via electronic medication monitor and mobile app plus remote clinician feedback on adherence to inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2020:S2213-2198(20)31220–4. [DOI] [PubMed] [Google Scholar]
- 77.Huckvale K, Morrison C, Ouyang J, et al. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med. 2015;13(1):1–15. doi: 10.1186/s12916-015-0303-x [DOI] [PMC free article] [PubMed] [Google Scholar]