Abstract
Brain metastases are the most common type of brain tumours, harbouring an immune microenvironment that can in principle be targeted via immunotherapy. Elucidating some of the immunological intricacies of brain metastases has opened a therapeutic window to explore the potential of immune checkpoint inhibitors in this globally lethal disease. Multiple lines of evidence suggest that tumour cells hijack the immune regulatory mechanisms in the brain for the benefit of their own survival and progression. Nonetheless, the role of the immune checkpoint in the complex interplays between cancers cells and T cells and in conferring resistance to therapy remains under investigation. Meanwhile, early phase trials with immune checkpoint inhibitors have reported clinical benefit in patients with brain metastases from melanoma and non-small cell lung cancer. In this review, we explore the workings of the immune system in the brain, the immunology of brain metastases, and the current status of immune checkpoint inhibitors in the treatment of brain metastases.
Keywords: Brain metastasis, immunotherapy, neuro-oncology, PD-1, PD-L1
Fares et al. explore the emerging principles of brain immunology, the intricacies of the immune microenvironment of brain metastases, and the efficacy of immune checkpoint inhibitors in the treatment of secondary tumours in the brain.
Introduction
Brain metastases are known to possess extraordinary intratumoural heterogeneity and special features that allow them to impede immunotherapy. Brain immunology, specifically, is governed by a set of doctrines that are different from the immune system elsewhere.1 The blood–brain barrier plays an important role in dictating the entry of immune agents into the brain, leading to an immunosuppressive environment within the brain parenchyma.2 As such, metastatic tumours are offered a safe haven in the brain, which makes them a major threat and an important target for therapeutic advancement.
In general, the brain boasts an immunosuppressive environment that does not permit the trafficking of immune cells. Discoveries in the past decade have shown that a special lymphatic system exists to drain from the dura mater of the brain to the peripheral lymph nodes.3–7 It was further emphasized that endothelial, epithelial and glial brain barriers possess varied accessibility to different immune-cell subtypes.8 New evidence continues to reveal a role for immune cells in the tumour microenvironment.1 These results have debunked the widely held belief that the brain is an immune-privileged organ, which is free from immune activity. In fact, antigens derived from the brain are capable of inducing an immune reaction in cervical lymph nodes.6,7,9,10 In addition, this heterogeneous blood–tumour barrier permeability in the setting of brain metastasis can facilitate the infiltration of multiple immune cells from the peripheral circulation.11,12
As our acumen on the immunology of brain metastases continues to grow, we learn that the CNS is home to a variety of antigen-presenting cells (APCs) such as microglia, dendritic cells and macrophages, as well as astrocytes.8,13 T cells are now known to roam freely in the brain. Yet, this does not take away from the uniqueness of the brain immune system, as it continues to be more limited than the immune system in peripheral organs.
Brain metastatic cells are famous for their ability to manipulate immune responses.14 While this ability might be helpful in limiting destructive inflammation, it can limit the access of T cells among others. This helps brain metastases escape antitumour immune responses,15,16 promoting metastatic survival and chemoresistance. The large populations of microglia and monocytes in the brain further contribute to decreasing cell-mediated immunity,17–19 leaving metastatic progression unchecked.
Reports in the literature continue to emphasize that the factors that contribute to immunosuppression at the primary tumour site are similar to those that contribute to it in the brain, highlighting the role of regulatory T cells and the expression of immune checkpoint programmed cell death 1–ligand 1 (PD-L1),20,21 which is associated with T cell futility.16,22 Nevertheless, recent trials with immune checkpoint inhibitors have shown survival and antitumour benefit in patients with melanoma brain metastases.23
With the brain as a common site of residence for metastatic cancerous and noncancerous cells, interactions between these cellular components seem inevitable. In this review, we explore the intricacies of the immune microenvironment of brain metastases and the efficacy of immune checkpoint inhibitors in the treatment of secondary tumours in the brain.
The immunology of brain metastases
Brain metastases exhibit one of the most diverse immune cell landscapes with substantial infiltration of T cells and neutrophils. In addition, a variety of immune agents, namely microglia and macrophages, lymphocytes and astrocytes complete the immune microenvironment of metastatic tumour cells.
Tumour-associated myeloid cells and microglia
Cells of myeloid origin are characterized by high plasticity and the ability to assimilate signals from cytokines, chemokines and growth factors. Their activation can promote cellular invasion, angiogenesis, metastasis and immune suppression.24,25 It has been estimated that cells of myeloid origin comprise up to 32.7% of intratumoural cells in brain metastases.26 Nevertheless, recent studies show that while tumour-associated myeloid cells and microglia (TAMs) can be significantly increased in gliomas compared with non-tumour tissue, lymphocytes are more prevalent in brain metastases.27
Myeloid cells arise from two distinct sources, including the periphery (bone marrow-derived macrophages; CD49D+) or the yolk sac (microglia; CD49D−).28–30 They tend to accumulate in settings of higher brain tumour grade and engage in significant bidirectional crosstalk with the tumour cells.31–33
Microglia are a central component of the brain immune microenvironment.34 Preclinical observations proposed a role for microglia in the extravasation stage of the metastatic process.35 In the case of inflammation, myeloid cells are recruited from circulating monocytes.36 The immune role of microglia involves the presentation of antigens, cytotoxic activity via nitric oxide and superoxide, and phagocytic capacity.37 In brain metastatic settings, microglia show restricted upregulation of interleukin (IL)-6 that exerts immunosuppressive effects on T cells and mediates resistance to immune checkpoint blockade.38 In addition, microglia limit the expression of TREM1 receptors, which modulate pro-inflammatory responses during neuroinflammation.39,40 Microglia-released chemokines, such as C-X-C motif chemokine 5 (CXCL5) and CXCL8, are increased in the setting of brain metastases; these chemokines recruit immunosuppressed neutrophils into the metastatic niche.27
Microglia express a variety of proteins that play conflicting roles in immune induction and suppression. The expression of high mobility group box 1 protein (HMGB1) facilitates antigen presentation and activation of the adaptive immune system. However, TAMs can also express immunosuppressive proteins like PD-L1,41,42 usually triggered by inflammation and/or necrosis.43 These biological properties allow TAMs to regulate and preserve the immune balance, preventing any swelling and, ultimately, damage within the confined area of the skull. Nevertheless, metastatic tumour cells hijack the immunosuppressive ability of TAMs to evade the immune system and promote their own survival.44 TAMs also secrete nitric oxide and drain the tumour microenvironment in the brain of amino acids that are essential for the activation of cytotoxic T cells, which leads to the blockade of immune signalling pathways, such as IL-2 signalling, an important stimulatory pathway for the immune system.44,45 Moreover, the production of reactive oxygen species leads to the degradation of proteins, lipids and nucleic acids, pushing T cells to commit to apoptosis.44 The coexistence of reactive oxygen species and nitric oxide within the same microenvironment further leads to the formation of peroxynitrites, leading to the nitrosylation of T-cell receptors. This disturbs the mechanism of interaction of T cells with tumour cells and may contribute to metastatic immune resistance.45
Microglia have also been shown to express neurotrophin (NT)-3 to regulate immune cellular activation. NT-3, similar to other neurotrophins, plays a major role in stimulating and controlling neurogenesis.46 In metastatic settings, NT-3-expressing microglia are hijacked to assist in the formation of brain metastases.47,48 In addition, the secretion of IL-10 and transforming growth factor beta (TGFβ) by myeloid cells and microglia induces the activity of M2 TAMs and regulatory T cells, which are known to suppress immune responses.45 The role of microglia extends, in some cases, to guiding the invasion of metastatic cells into the brain.44,49 Imaging studies have shown that a wall of microglial cells is situated at the border between the metastatic cells and the brain parenchyma.50
Macrophages are generally known to modulate between polarizing states: M1 and M2. While the M1 state is pro-inflammatory, inducing a Th1 response against foreign pathogens and cancer cells, the M2 state contributes to immunosuppression and cellular repair. Although understanding of macrophage polarization remains limited in the setting of brain metastasis, TAMs generally presume an M2 composition, causing immunosuppression and assisting tumour cells in evading immunity.44 Nevertheless, polarization of macrophages differs between different regions of the brain. In parenchymal metastases, the release of cytokines such as lymphotoxin β and the increase in NF-κB1 activity demonstrate a direct involvement in the M2 polarization of macrophages.51 Subsequently, this results in the secretion of growth factors, remodelling of the extracellular matrix (ECM), and angiogenesis.52 The release of TGFβ, matrix metalloproteinases (MMP) 2, MMP9, cathelicidin, cathepsins and scavenger receptor class A (SRA) have been reported to play a major role in pro-metastatic ECM remodelling, whereas the secretion of vascular endothelial growth factor A (VEGFA) and platelet-derived growth factor (PDGF) contribute to angiogenic formations.25,52
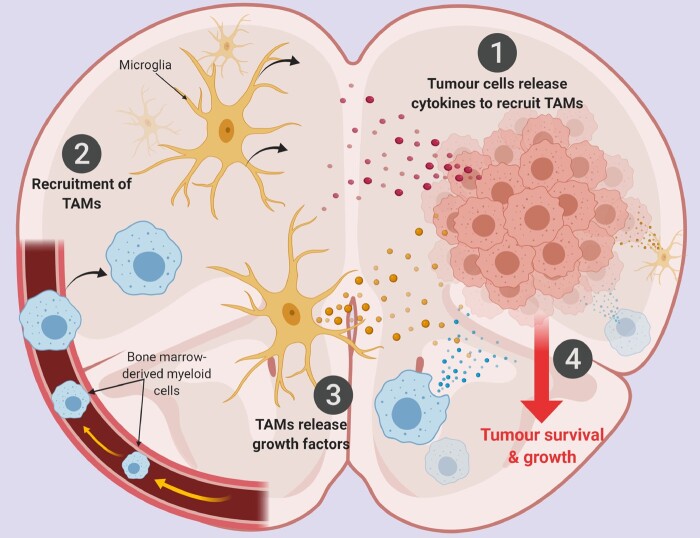
Upon settling in the brain parenchyma, metastatic tumour cells release factors to recruit TAMs to the tumour microenvironment (Fig. 1). Factors like VEGFA, chemokine ligand (CCL) 2, CCL5, CCL9, CCL18 and colony stimulating factor 1 (CSF1) have been reported to be released in such settings.25,52,53 One of the therapeutic strategies used to target TAMs is the colony stimulating factor 1 receptor (CSF1R) inhibitors that inhibit the release of pro-survival factors by the TAMs. Inhibition of CSF1R either depletes or depolarizes TAMs.31,32
Figure 1.
The interplay between metastatic tumour cells and TAMs in the brain microenvironment. (1) Upon entry into the brain parenchyma, tumour cells release cytokines and chemokines, such as CSF1, GM-CSF, MCP-1, HGF, SDF-1 and CX3CL1, to recruit myeloid cells from the periphery and brain-resident microglia into the tumour niche. (2) Myeloid cells derived from the bone marrow and the yolk sac enter the brain and microglia migrate towards the tumour cells. (3) TAMs release growth factors, such as EFG, IL-6, TGF-β, IL-1β and other proteases. (4) This promotes tumour survival and growth. Created with BioRender.com.
Unlike microglia, myeloid cells are poor APCs. They express isoforms of human leukocyte antigen (HLA)-G and HLA-E, in addition to the major histocompatibility complex (MHC) type I molecules that prevent the lysis of natural killer (NK) cells and T cells.25 Nonetheless, their immunosuppressive ability is illustrated through the co-expression of inhibitory molecules, such as PD-L1 and PD-L2, and the release of inhibitory chemokines, such as IL-10, TGFβ, CCL5, CCL20 and CCL22, which prevent the activation of the adaptive immune response in various cancers.25,44,54
T cells and NK cells
Analysis of brain metastases confirmed a significantly higher proportion of lymphocytes, with melanoma brain metastases exhibiting the most lymphocytic infiltrates and CD8+ T cells predominating other lymphocytic fractions.27 Yet, the level of inflammation around metastatic tumours is different from one patient to another and between cancer types, as the concentration of T cells around brain metastatic cells can vary from nil to very high.55,56 The discrepancy in CD8+ T-cell density can be associated with the time at which brain metastases occurred.57 Another explanation for this variation can be seen in the protein expression of metastatic cells. For example, high PD-L1 expression eases immune evasion and allows the metastatic cell to escape checkpoint by T cells (Fig. 2).56 Moreover, expression of forkhead box p3 (FOXP3) as well as programmed cell death protein 1 (PD-1) by regulatory T cells favours immune suppression in the metastatic tumour microenvironment.56,58
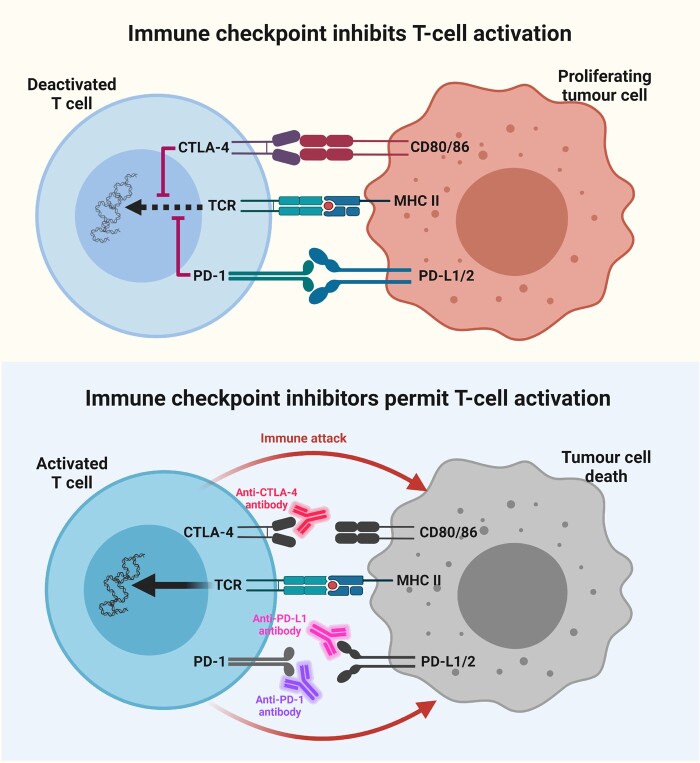
Figure 2.
T-cell regulation through an immune checkpoint. Top: The expression of CTLA4 and PD-1 on T cells serves as an immune checkpoint through which tumour cells can bind and deactivate T cells. Suppression of effector T-cell responses provides metastatic cancer cells with the opportunity to proliferate. Bottom: Immune checkpoint inhibitors, such as anti-CTLA4, anti-PD-L1 and anti-PD-1 antibodies, prevent tumour cells from applying the brakes to T-cell activation, which subsequently leads to T-cell activation, immune attack and tumour cell death. Created with BioRender.com.
In terms of clinical outcomes and survival, it has been demonstrated that patients with increased immune responses and cytotoxic T-cell infiltration of metastatic tumours show better prognoses.58–60 Melanoma brain metastases exhibited higher T-cell infiltration than breast cancer brain metastases.61 This may be the reason behind the success of some immunotherapeutic regimens in improving survival in patients with metastatic melanoma to the brain.62,63 Furthermore, the infiltration of brain metastases with high levels of effector CD3+, cytotoxic CD8+ or memory CD45RO+ T cells has been shown to improve survival.61 In addition, increased T-cell trafficking into the brain metastatic areas decreases the integrity of white matter tracts, providing a method to identify immunologically active microenvironments in the brain using diffusion tensor MRI.64
The blood–brain barrier also plays a role in limiting the infiltration of cytotoxic T cells. Angiogenesis and neovascularization are key hallmarks of metastasis.65 Nonetheless, the magnitude of angiogenic potential differs between cancer types.
Aside from expressing inhibitory proteins and receptors, such as cytotoxic T-lymphocyte associated protein 4 (CTLA4), PD-1, lymphocyte-activation gene 3 (LAG3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), inducible T-cell costimulator (ICOS), and T cell immunoreceptor with Ig and ITIM domains (TGIT), regulatory T cells can also secrete large volumes of immunosuppressive cytokines. IL-10, IL-35 and TGFβ are reported to be released by regulatory T cells in the tumour microenvironment.66 Moreover, regulatory T cells have been documented to drain the tumour microenvironment of immune-stimulatory cytokines, like IL-2, deactivating the antitumour Th1 immune response. More recently, it was shown that T-cell receptors that exhibit pan-cancer cell recognition via MHC class I-related proteins lacked detectable reactivity in melanoma. Providing the T cells with the MC.7.G5 T-cell receptors rendered them capable of killing autologous melanoma.67
NK cells also partake in the intratumoural immune response in the brain tumour microenvironment.68 They have been shown to be present in metastatic brain tumours, gliomas and craniopharyngiomas.69,70 Like cytotoxic T cells, NK cell functioning is often diminished in patients with brain tumours due to the release of anti-inflammatory molecules, such as TGFβ, by tumour cells. In addition, tumours that secrete inflammatory mediators like cyclooxygenases (COX) and prostaglandin E2 suppress NK cell antitumour activity.71
Neutrophils
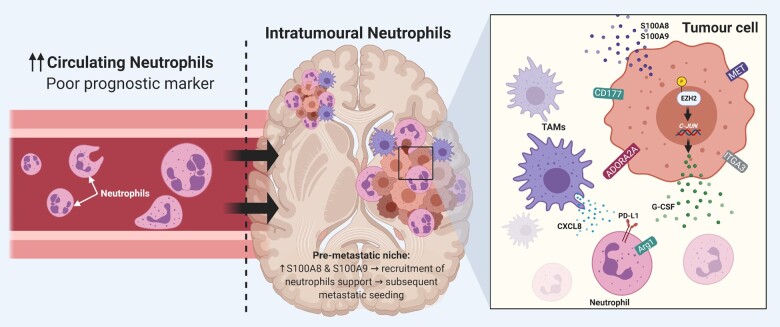
Despite being the most abundant circulating immune cells, the function of neutrophils in the immune microenvironment of brain metastases remains unclear. Yet, recent advances have elucidated some of the mechanisms that govern neutrophil recruitment and activation in the brain metastatic microenvironment. Neutrophils can be recruited to the brain microenvironment through the secretion of CXCL8 chemoattractant by TAMs. The upregulation of ITGA3 and CXCL17 by brain metastatic cells further increases neutrophil abundance at the scene.27 The overexpression of the adenosine receptor ADORA2A suppresses the pro-inflammatory phenotype of neutrophils.27,72 Moreover, overexpression of CD177 in brain metastatic cells affects neutrophil migration and activation and suppresses T-cell proliferation.27 In addition, the upregulation of MET is associated with the recruitment of immunosuppressive neutrophils to the immune microenvironment in brain metastases.27,73
Recently, it has been shown that brain metastatic cells overexpress an epigenetic modifying protein, enhancer of zeste homolog 2 (EZH2), to stimulate signalling pathways that suppress recruited neutrophils.74 In the setting of brain metastases, EZH2 phosphorylation changes its function from being a methyltransferase to a transcription factor that increases c-JUN expression.74 This leads to the secretion of pro-tumorigenic inflammatory cytokines, including G-CSF, which recruits Arg1+ and PD-L1+ immunosuppressive neutrophils into the brain to drive metastasis outgrowth.74 Blocking the influx of this subset of neutrophils by G-CSF-blocking antibodies or immune checkpoint blockade therapies combined with Src inhibitors impeded brain metastasis in multiple mouse models.74 Furthermore, neutrophils can play a role in priming the brain metastatic niche. It has been shown that S100 calcium-binding protein A (S100A)-8 and S100A9 are upregulated in the pre-metastatic niche in the brain, leading to recruitment of neutrophils, which support subsequent metastatic seeding and colonization (Fig. 3).75
Figure 3.
Role of neutrophils in brain metastases. Left: In the setting of brain metastases, a high neutrophil-to-lymphocyte ratio is associated with poor prognostic outcomes. Middle: In the tumour microenvironment, neutrophils are recruited through increased release of S100A8 and S100A9 proteins by tumour cells. Right: The increased expression of CD117, ADORA2A, ITGA3 and MET by brain metastatic cells leads to neutrophilic suppression in the immune microenvironment. The phosphorylation of EZH2 protein increases the expression of c-JUN, which leads to the release of G-CSF. G-CSF, in turn, leads to the recruitment of neutrophils that overexpress PD-L1 and Arg1. Furthermore, tumour-associated myeloid cells and microglia release chemokines, such as CXCL8, to suppress neutrophils in the tumour microenvironment. Created with BioRender.com.
Neutrophils have a prognostic value in the setting of brain metastases. A high ratio of neutrophils to lymphocytes in the peripheral blood is associated with reduced survival time, even after surgical resection of brain metastases.76 Furthermore, in patients treated with stereotactic radiosurgery, the post-treatment neutrophil-to-lymphocyte ratio was associated with poor overall survival.77 Nevertheless, in patients with non-small cell lung cancer (NSCLC) who harbour EGFR mutations, a neutrophil-to-lymphocyte ratio ≤2.99 was associated with prolonged survival.78 Therefore, the neutrophil-to-lymphocyte ratio could serve as a useful prognostic biomarker in specific patients with brain metastases.
Astrocytes
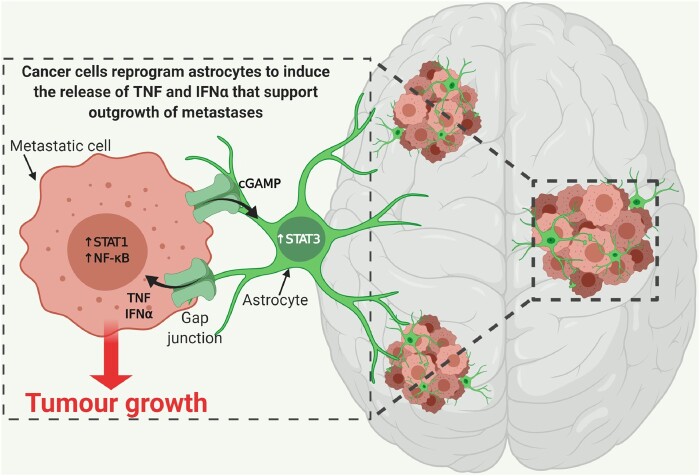
Astrocytes are some of the most abundant cell types that are unique to the CNS and play important roles in mediating tissue-specific communication in the brain. Therefore, it is likely that metastases that preferentially grow within the brain must find ways to adapt and favourably interact with these unfamiliar cellular players.8 Brain metastatic cells are known to express high levels of IL-1β upon interaction with astrocytes. This leads to the activation of the Notch signalling pathway, which increases stemness of cancer stem cells and drives their growth in the tumour microenvironment.79 Moreover, it was shown that cancer cells within established brain metastases form functional gap junctions with astrocytes in the adjacent microenvironment, thereby creating a conduit for bidirectional communication to support outgrowth.80,81 Through these gap junctions, cancer cells reprogram astrocytes by providing cGAMP to induce a pro-inflammatory program, characterized by the production of interferon alpha (IFNα) and tumour necrosis factor alpha (TNFα).80,81 In turn, these cytokines support outgrowth of metastases by activating signal transducer and activator of transcription (STAT) 1 and nuclear factor-κB (NF-κB) signalling within cancer cells (Fig. 4). By interfering with the formation of gap junctions through pharmacological regimens, this heterotypic signalling loop can be blocked, thus mitigating brain metastasis outgrowth.81 In addition, astrocytes possess the capability to release factors that can remodel the ECM, such as MMPs and heparanase, which contribute to the invasion of metastatic cells across the blood–brain barrier and facilitate subsequent metastatic colonization.82,83
Figure 4.
Interaction of astrocytes with brain metastatic cancer cells. Metastatic cancer cells release cGAMP to reprogram astrocyte behaviour and increase STAT3 signalling. In turn, astrocytes form gap junctions with metastatic cancer cells in the brain to exchange stimulatory signals, such as TNF and IFNα, which activate STAT1 and NF-κB pro-tumorigenic signalling. Created with BioRender.com.
Brain metastatic cells induce and maintain the co-option of a pro-metastatic program driven by STAT3 in a subpopulation of reactive astrocytes surrounding metastatic lesions.84 These reactive astrocytes benefit metastatic cells by their modulatory effect on the innate and acquired immune system. Blocking STAT3 signalling in reactive astrocytes reduces experimental brain metastasis from different primary tumour sources, even at advanced stages of colonization.84,85 A safe and orally bioavailable treatment that inhibits STAT3 exhibits significant antitumour effects in patients with advanced systemic disease that included brain metastasis.84
Immune checkpoint in brain metastases
From an evolutionary perspective, cancer cells have developed various features to escape immune surveillance. PD-L1 is an immune-checkpoint molecule that regulates immune homeostasis and prevents autoimmunity.86 In pathological conditions, such as pancreatic cancer, the expression of PD-L1 shields the tumour cells from the activation of cytotoxic T cells.87 Unlike other immune-surveillance molecules like CTLA4, the expression of PD-1 is not limited to T cells, but occurs in B cells as well.88 This highlights the wider range of functions exhibited by PD-L1.89
It is unclear how metastatic brain cells develop mechanisms to overcome the effect of T cells. Heavy reliance on breaking the interaction between T cells and metastatic brain cancer cells and the ablation of specific metabolic pathways result in activation of pro-survival mechanisms in the cancer cells, presenting a potential therapeutic avenue against lethal advancement. The PD-1 receptor was first identified on thymocytes in response to a pro-apoptotic stimulus. Further studies linked the function of this receptor to immunological tolerance. Like CTLA4, PD-1 belongs to the CD28 family and is known for its inhibitory effect. Upon antigenic stimulation, the tail region of PD-1 is phosphorylated, leading to the recruitment and activation of SHP-2.90 This is followed by complex formation with SRC-based family receptors, resulting in the activation of ERK and RAS signalling pathways in primary brain tumours.91 The exact mechanism by which this occurs is still unclear but it seems to be related to the ability of PD-1 to inhibit the production of IFN-induced nitric oxide,92 suppress glucose metabolism, and inhibit arginine and tryptophan amino acids.93
Most recently it was shown that therapeutic reduction of PD-1 expression leads to cytoprotective autophagy induction in tumour cells, thereby increasing their survival. Two PD-1 receptor ligands are known: PD-L1 and PD-L2 belonging to the B7 family.94,95 PD-1, PD-L1 and PD-L2 were observed to be differentially expressed between primary and metastatic tumours.95 Further histopathological examination of these immune checkpoints in metastatic lesions revealed that their expression is correlated with shorter overall survival.95
The inhibition of PD-1 and CTLA4 on T cells in brain metastases seems to also depend on the status of the systemic disease. In melanoma brain metastases, the efficacy of intracranial immune checkpoint inhibition was observed only when an extracranial tumour was present.96,97 Extracranial presence further increased the trafficking of CD8+ T cells and TAMs to brain tumours, in response to immune checkpoint therapy. Moreover, tumour-induced peripheral immunosuppression promotes brain metastases in patients with NSCLC. Increased expression of PD-L1 on peripheral TAMs decreases T-cell activation and trafficking, leading to poor outcomes.98
Potential mechanisms that limit immune responses to checkpoint inhibitors also include brain access and vascular permeability. Despite being leaky to a variable extent, blood vessels in brain tumour settings are less permeable than in extracranial tumours.2,11 Anti-PD-1 and anti-CTLA4 blocking antibodies have limited access to intracranial tumours and thus their ability to reduce PD-1 and CTLA4 inhibition of T cells is diminished. Nevertheless, effective immune responses against brain metastatic cells can be dependent on the production of antigen-specific T cells as a result of PD-1 blockade or CTLA4 inhibition in the extracranial tumour site.97,99 Moreover, tumour antigens of brain metastases may reach draining lymph nodes, leading to the induction of T-cell priming and the release of antigen-specific T cells from checkpoint inhibition in the extracranial tumour-draining lymph nodes.97
Brain metastatic cells can be genetically and phenotypically different from their primary tumour origin. Genomic characterization of brain metastases revealed that in 53% of cases, clinically informative alterations in brain metastases were not detected in the matched primary tumour sample.100 Distal extracranial and regional lymph node metastases were highly divergent from brain metastases, whereby detected alterations associated with sensitivity to PI3K/AKT/mTOR, CDK and HER2/EGFR inhibitors in the brain metastases.100 These differences between primary and metastatic tumour cells further include alterations in immune responses, inflammation-related pathways, NF-kB1 activity and cytokine profiles.51 For example, lymphotoxin β expression was directly correlated with M2 polarization of parenchymal macrophages in the setting of brain metastases.51
The expression of immune checkpoint molecules in the immune microenvironment of brain metastases can affect the immune modulatory response against tumour cells. Studies exploring PD-1 expression on T cells in the setting of brain metastases showed wide discrepancies in results, with PD-1 expression noted in 3.1–68% of brain metastatic samples.21,101,102 PD-L1 expression was detected in 25–28% of immune cells in the setting of brain metastatic cells.101,103,104 On tumour cells, PD-L1 expression ranged from 22% to 75% in brain metastatic samples.21,101–107
Therapeutic advancements against metastasis are particularly successful when targeting multiple pathways of the metastatic process. In the clinical setting, therapeutic efficacy and significant prolongation of survival were observed with interventions that simulate anticancer responses mediated by T cells. Ongoing preclinical studies demonstrate survival benefit in models of melanoma, lung and breast cancers upon blockade of interactions between T cells and cancer cells.108 Nonetheless, the mechanism of interplay remains wide open as clinical translation of such therapies has only been able to demonstrate benefit in melanoma brain metastasis.23
Clinical outcomes with immune checkpoint inhibitors
Anti-CTLA4 therapy
Ipilimumab is a fully human monoclonal antibody against CTLA4, which plays a pivotal role in downregulating the production of cytotoxic T cells.109 The effect of ipilimumab was first noted intracranially in a phase 3 trial in patients with metastatic melanoma, in which an improvement in survival was seen.110 This was subsequently followed by a phase 2 trial that focused on patients with melanoma brain metastases; however, ipilimumab only showed activity in small and non-symptomatic brain lesions.111,112 Patients who were initially asymptomatic from a neurological standpoint had a 24% intracranial response rate, whereas symptomatic patients on corticosteroids had a 10% response rate.111 Overall survival rate amongst patients with melanoma brain metastases at 1 year was ∼20% across studies.111–113 Moreover, baseline metabolic tumour volume measured on 18F-fluorodeoxyglucose PET/CT appears to be a strong independent prognostic factor in melanoma patients treated with ipilimumab.114 This technique could be used to personalize immunotherapy in future clinical studies.
Anti-PD-1/PD-L1 therapy
The PD-1/PD-L1 pathway may be a promising target for immune checkpoint inhibition. Anti-PD-1/PD-L1 antibodies such as nivolumab and pembrolizumab have been approved for advanced melanoma, kidney cancer and NSCLC. A phase 2 study with pembrolizumab on patients with brain metastatic melanoma and NSCLC observed partial responses in 25% of the patients with melanoma and 44% of the patients in the NSCLC arm, with a median overall survival of 7.7 months.115–117 Patients with melanoma brain metastases treated with nivolumab or pembrolizumab showed a median overall survival of 9.9 months.118 Moreover, a phase 2 trial with pembrolizumab in melanoma brain metastases induced a median progression-free survival and an overall survival of 2 and 17 months, respectively; 48% of patients were alive after 2 years.119 In patients with NSCLC brain metastases, nivolumab achieved an overall response rate of 17%.120 In addition, the use of the anti-PD-L1 antibody atezolizumab in patients with brain metastases has shown an overall survival benefit of 16 months.121 Atezolizumab also increased progression-free survival when compared with docetaxel.122 Nevertheless, overall survival was similar when comparing patients with NSCLC who were treated with nivolumab alone versus docetaxel (Table 1).123
Table 1.
Clinical findings for immune checkpoint inhibitors as monotherapy or in combination in brain metastases
| Strategy | Study type | Selected patients | Primary tumour | Results | Reference |
|---|---|---|---|---|---|
| Ipilimumab | Phase 3 | 82 | Melanoma | Ipilimumab improved overall survival by 3–4 months. Adverse events were reversible with appropriate treatment. | Hodi et al.110 |
| Ipilimumab | Retrospective | 165 | Melanoma | Overall survival rate at 1 year was 20%. Treatment was relatively safe. | Heller et al.113 |
| Ipilimumab | Phase 2 | 72 | Melanoma | Ipilimumab had 24% intracranial response rate when metastases were small and asymptomatic. The drug had no unexpected toxicity. | Margolin et al.111 |
| Pembrolizumab | Phase 2 | 10 | NSCLC | Partial responses were recorded in four patients. No grade ≥ 3 adverse events. | Goldberg et al.116 |
| Pembrolizumab | Phase 2 | 17 | Melanoma, lung cancer | Partial responses were observed in three patients. Grade 3 liver function abnormalities were noted in one patient. | Kluger et al.115 |
| Pembrolizumab | Phase 2 | 36 | Melanoma, NSCLC | Response was achieved in 4 (22%) of 18 patients with melanoma and 6 (33%) of 18 patients with NSCLC. | Goldberg et al.117 |
| Nivolumab | Retrospective | 46 | NSCLC | At time of last assessment, 33% of patients had no evidence of CNS progression (stable/decreased brain lesions). | Goldman et al.123 |
| Nivolumab or pembrolizumab | Retrospective | 66 | Melanoma | Response rate was 21% and disease control rate was 56%. Median overall survival was 9.9 months. Patients requiring corticosteroids had shorter overall survival (4.8 versus 13.1 months). | Parakh et al.118 |
| Atezolizumab | Phase 3 | 85 | NSCLC | Median overall survival for patients treated with atezolizumab was 20.1 months versus 11.9 months for patients treated with docetaxel. | Rittmeyer et al.121 |
| Nivolumab and corticosteroid | Case report | 1 | NSCLC | Nivolumab was effective in shrinking symptomatic brain metastases in a patient with first-line chemotherapy failure. | Pluchart et al.124 |
| Nivolumab and ipilimumab | Phase 2 | 94 | Melanoma | At 14 months, the rate of intracranial clinical benefit was 57%. The rate of complete response was 26%, the rate of partial response was 30%, and the rate of stable disease for at least 6 months was 2%. | Tawbi et al.23 |
| Nivolumab and ipilimumab | Phase 2 | 35 | Melanoma | At 17 months, intracranial responses were achieved by 16 patients (46%). Intracranial complete responses occurred in six patients (17%). | Long et al.125 |
| Pembrolizumab | Phase 2 | 23 | Melanoma | Six patients (26%) had a response. Median progression-free and overall survival were 2 and 17 months, respectively. Most responders had higher pretreatment tumour CD8 cell density and PD-L1 expression. | Kluger et al.119 |
| Nivolumab | Retrospective | 409 | NSCLC | Disease control rate was 39%: four patients had a complete response, 64 a partial response and 96 showed stable disease. The median overall survival was 8.6 months. | Crino et al.120 |
| Atezolizumab | Phase 3 | 123 | NSCLC | In patients with asymptomatic metastases, median overall survival was longer with atezolizumab than with docetaxel (16.0 versus 11.9 months). | Gadgeel et al.122 |
| Nivolumab and ipilimumab | Phase 2 | 119 | Melanoma | In asymptomatic patients, the clinical benefit rate was 58.4%. In symptomatic patients, the clinical benefit rate was 22.2%. | Tawbi et al.126 |
More information can be found in the Supplementary material.
Often, patients with brain metastases are given corticosteroids to decrease symptoms of tumour-related inflammation. While patients with melanoma brain metastases who received ipilimumab and corticosteroid treatment showed worse outcomes,111 patients with NSCLC brain metastases do not seem to show the same with nivolumab. In a case report, the effect of nivolumab was obtained despite concomitant high-dose corticosteroid therapy.124 Partial shrinkage of the brain tumour was detected with no unexpected adverse events.124 More studies are needed to ascertain the reason why patients taking corticosteroids had worse outcomes with ipilimumab. It is possible that these patients’ tumours had unfavourable prognostic features at baseline, or that corticosteroid use counteracted the effectiveness of the checkpoint inhibitor.
Combination of immunotherapies
Combination immunotherapy can have better therapeutic effects than monotherapy. Using high-dimensional single-cell profiling, combination therapy with anti-CTLA4 plus anti-PD-1 elicited cellular responses that are partially distinct from those induced by either monotherapy.127 The combination mediated a switch from expansion of phenotypically exhausted CD8 T cells to expansion of activated effector CD8 T cells.127 Recently, the combination of ipilimumab and nivolumab showed the best benefit with an estimated 12-month survival reaching 81.5% in patients with melanoma brain metastases.23 A recent update of the study reported an intracranial objective response rate of 54%; the 18-month survival rate was 75%.126 In a subgroup of patients with symptomatic brain metastases, the 6-month overall survival rate reached 66% at a median follow-up of 5.2 months.126 In a similar trial with a median follow up of 17 months, ipilimumab and nivolumab demonstrated intracranial responses in 16 of 35 patients (46%), whereas only 5 of 25 patients (20%) who received nivolumab, and one of 16 patients (6%) who received ipilimumab showed similar responses.125 The combination therapy further saw six patients (17%) achieving a complete intracranial response, whereas only three patients (12%) who received nivolumab had a similar response.125
Immunotherapy and radiation
Stereotactic radiosurgery or whole-brain radiation therapy with or without surgery has been the mainstay for the treatment of patients with symptomatic brain metastases.128 As various studies point out, the effects of radiotherapy on metastatic tissue combine DNA damage,129,130 possible release of damage-associated molecular patterns or stress molecules,131 and the expression of MHC-I molecules for effective antigen presentation.132,133 However, overall, radiation fails to produce a durable anticancer effect. Simultaneously, developing immune checkpoints by cancer cells and the suppressive microenvironment contribute to tumourigenicity.134–136
Several studies have shown that the combination of stereotactic radiation and ipilimumab is safe in the management of melanoma brain metastases.137–140 In addition, the combination of nivolumab and stereotactic radiation improved overall survival and progression-free survival as compared with historical controls treated with radiation alone.140 A meta-analysis of patients with brain metastases from melanoma, NSCLC and renal cell carcinoma showed that the use of immune checkpoint inhibitors alongside stereotactic radiation (median 20 Gy in one fraction) improved 1-year overall survival to 65%, as compared to 52% in cases treated with monotherapy.141 Another study also showed an improved median overall survival of 24.7 months compared to 12.9 months with radiation therapy alone (Table 2).154 Radiation leads to the upregulation of PD-L1 on cancer cells, which sensitizes them to anti-PD-L1 therapy.155,156 In addition, it increases blood–brain barrier permeability allowing better penetration of immunotherapy into the brain. Nevertheless, the high doses of steroids administered with whole-brain radiation therapy to decrease intracranial oedema pose a problem due to their immunosuppressive effects.111 This has caused many centres to move away from using steroids routinely for all patients with brain metastases.142
Table 2.
Clinical findings for combinatory approaches with immune checkpoint inhibitors and radiotherapy in brain metastases
| Strategy | Study type | Selected patients | Primary tumour | Results | Reference |
|---|---|---|---|---|---|
| Ipilimumab and WBRT | Retrospective | 27 | Melanoma | Median survival was 21.3 months. The Diagnosis-Specific Graded Prognostic Assessment score was the most significant predictor of overall survival. | Knisely et al.138 |
| Ipilimumab and SRS | Retrospective | 25 | Melanoma | Ipilimumab neither increased toxicity nor improved intracerebral disease control in patients with limited brain metastases who received SRS. | Mathew et al.139 |
| Ipilimumab and SRS or WBRT | Retrospective | 33 | Melanoma | Patients had an overall median survival of 18.3 months. Ipilimumab was associated with a significantly reduced risk of death in patients with melanoma brain metastases who underwent radiotherapy. | Silk et al.137 |
| Ipilimumab with WBRT or SRS | Case series | 16 | Melanoma | Favourable systemic response was more likely when ipilimumab was administered within 3 months of radiation. | Schoenfeld et al.142 |
| Ipilimumab and SRS | Retrospective | 46 | Melanoma | Patients treated with SRS during or before ipilimumab had better overall survival and less regional recurrence than those treated with SRS after ipilimumab (1-year survival: 65% versus 56% versus 40%) | Kiess et al.143 |
| Nivolumab and SRS | Retrospective | 26 | Melanoma | Eight patients (11%) had a ≥ 20% increase in tumour volume. Median overall survival was 12 months. | Ahmed et al.140 |
| SRS with ipilimumab or nivolumab/pembrolizumab | Retrospective | 33 | Melanoma | Concurrent use of immunotherapy and SRS resulted in a greater reduction in lesion volume. Reduction in lesion volume was greater for nivolumab/pembrolizumab than ipilimumab. | Qian et al.144 |
| SRS with ipilimumab, nivolumab and/or pembrolizumab | Retrospective | 48 | Melanoma | One-year intracranial control rates for SRS + checkpoint inhibitors ranged from 60% (local) to 85% (distal). SRS + checkpoint inhibitors significantly reduced tumour recurrence compared with SRS alone. | Acharya et al.145 |
| SRS with ipilimumab or pembrolizumab | Retrospective | 18 | Melanoma | Concurrent checkpoint inhibitors and SRS significantly improved progression-free survival and increased lesion regression compared with SRS alone. | Yusuf et al.146 |
| Ipilimumab and WBRT or SRS | Phase 1 | 16 | Melanoma | Ipilimumab + WBRT led to progression-free survival of 2.5 months and overall survival of 8 months. Ipilimumab + SRS led to progression-free survival of 2.5 months and overall survival was not reached. | Williams et al.147 |
| Ipilimumab and SRS | Retrospective | 25 | Melanoma | Median overall survival was 35.8 months and local control was 94.8%. Regional brain control and time to progression were significantly improved when SRS was delivered ≤30 days after ipilimumab. | Skrepnik et al.148 |
| Ipilimumab and SRS | Retrospective | 20 | Melanoma | Use of ipilimumab within 4 months of SRS was safe. This treatment regimen was not associated with improved outcomes. | Patel et al.149 |
| SRS with ipilimumab, BRAF inhibitor and/or pembrolizumab | Retrospective | 179 | Melanoma | SRS with checkpoint inhibitors and BRAF inhibitor was highly protective. Overall survival was best in BRAF-wild-type patients receiving SRS + pembrolizumab (12.26 months), and in BRAF-mutated patients receiving SRS + BRAF inhibitors + pembrolizumab (14.82 months). | Gaudy- Marqueste et al.150 |
| SRS and ipilimumab | Retrospective | 46 | Melanoma | The effect of SRS and ipilimumab on local recurrence-free duration seemed greater when SRS was performed before or during ipilimumab treatments. The timing of immunotherapy and SRS may affect local recurrence-free duration and post- SRS oedema. | Cohen-Inbar et al.151 |
| SRS with ipilimumab or pembrolizumab | Retrospective | 39 | Melanoma | Median survival with SRS + ipilimumab was 7.5 months, and 20.4 months with SRS + pembrolizumab. Median brain control was 7.5 months with SRS + ipilimumab, and 12.7 months with SRS + pembrolizumab. | Choong et al.152 |
| Nivolumab and SRS | Retrospective | 17 | NSCLC | The 6-month rate of distant brain control for patients treated with SRS during or prior to nivolumab therapy was 57% compared to 0% among patients who received nivolumab before SRS. | Ahmed et al.153 |
| SRS with ipilimumab, nivolumab and/or pembrolizumab | Retrospective | 79 | Melanoma, NSCLC, renal cell carcinoma | Median overall survival was 24.7 months. SRS with concurrent checkpoint inhibitors was associated with improved survival compared with SRS alone and non-concurrent SRS and checkpoint inhibitors. | Chen et al.154 |
SRS = stereotactic radiosurgery; WBRT = whole brain radiation therapy. More information can be found in the Supplementary material.
Immunotherapy and other therapies
Chemotherapy may indeed potentiate the effect of immune checkpoint inhibitors by increasing T-cell responses and disrupting the activity of TAMs.157 Nevertheless, its exact mechanism of action in the setting of brain metastasis remains unknown. In NSCLC, pembrolizumab in addition to chemotherapy has become the standard-of-care therapy.158 Clinical trials focusing on patients with brain metastases who are receiving immunotherapy in addition to targeted therapy are lacking.159 Although, in some trials, patients with untreated, asymptomatic and small (maximum diameter 15 mm) brain metastases are allowed, the percentage of these patients and their outcomes are not reported.158 Secondary analyses focusing on small subgroups of patients with brain metastases report conflicting results. In small cell lung cancer (SCLC) brain metastases, adding atezolizumab to chemotherapy did not increase overall survival160; however, adding durvalumab to chemotherapy showed survival benefit in similar patients with brain metastases.161 Combining ipilimumab with fotemustine (an alkylating agent used in the treatment of metastatic melanoma) yielded an overall survival of 27.8% at the 3-year interval (Table 3).162–164
Table 3.
Clinical findings for combinatory approaches with immune checkpoint inhibitors and targeted therapy in brain metastases
| Strategy | Study type | Selected patients | Primary tumour | Results | Reference |
|---|---|---|---|---|---|
| Ipilimumab + fotemustine | Phase 2 | 20 | Melanoma | Ten patients (50%) achieved disease control | Di Giacomo et al.162 |
| Ipilimumab + fotemustine | Phase 2– follow-up | 20 | Melanoma | Overall survival and 3-year survival rate were 12.7 months and 27.8%, respectively. Increase from baseline to week 12 in ‘memory’ but not in ‘naïve’ T cells conferred better survival. The neutrophil-to-lymphocyte ratio correlated with significantly better survival at early time points | Di Giacomo et al.163 |
| Atezolizumab + carboplatin + etoposide | Phase 1/3 | 22 | SCLC | Adding atezolizumab to carboplatin and etoposide produced a significant improvement in overall survival and progression-free survival in first line extensive-stage SCLC | Mok et al.160 |
| Durvalumab + etoposide | Phase 3 | 28 | SCLC | At median follow-up of 14.2 months, disease progression was noted in 17 of 28 patients (61%) | Paz-Ares et al.161 |
More information can be found in the Supplementary material.
Targeted therapy in addition to immune checkpoint blockade can further enhance therapeutic effects. Inhibition of the MAPK pathway increases the efficacy of dendritic cells, T cells and NK cells, and decreases the activity of tumour-associated fibroblasts and TAMs in brain tumours.165–167 The addition of Toll-like receptor 1/2 (TLR1/2) ligand to anti-CTLA4 antibody in a mouse model of melanoma enhanced the antitumour efficacy of anti-CTLA4 by increasing Fcγ receptor IV expression in macrophages, which in turn increased the depletion of tumour-infiltrating regulatory T cells.168 These findings are likely extensible to other checkpoint antibodies in cancer patients. In melanoma, trials are now ongoing with combinations of PD-L1 or CTLA4 inhibitors with BRAF and/or MEK inhibitors. Unfortunately, no trials specifically report or exclusively focus on patients with brain metastases.
Angiogenesis in the brain can affect immune function. Vascular abnormalities are a hallmark of brain metastases and facilitate immune evasion. Elevated levels of proangiogenic factors, such as VEGF and angiopoietin 2, play a major role in this process.169 Nevertheless, angiogenesis can be a double-edged sword. While the formation of new vasculature may increase inflammation in the tumour immune microenvironment and the trafficking of effector T cells into the scene, the abnormal vasculature can limit the movement of immune cells. VEGF alters the expression of adhesion molecules on endothelial cells and immune cells, limiting proper adhesion and subsequent passage into the tumour microenvironment.2 As such, the inhibitors of angiogenesis may normalize the vasculature and increase T-cell trafficking to the location of metastases, switching the immunosuppressive tumour microenvironment to an immunosupportive one and increasing the therapeutic potential of immune checkpoint inhibitors, as can be seen in malignant gliomas.170 Furthermore, the combination of both regimens can decrease the collection of inflammatory molecules and oedema around the tumour site and subsequently reduce the need for steroidal therapy. Moreover, bevacizumab reduces TAMs that express S100A9, which reduces immunosuppression and restores anti-tumour immunity.171 Bevacizumab in addition to atezolizumab and chemotherapy is a first-line therapeutic option in patients with advanced NSCLC.172 Interim analyses of a phase 2 trial with pembrolizumab and bevacizumab for melanoma and NSCLC brain metastases showed that 12 of 20 patients (60%) exhibited an objective tumour response; seven complete responses and five partial responses.173 Unfortunately, patients with brain metastases continue to be excluded from such trials.
Surgery and immunotherapy
The role of neurosurgery and its effect on immunotherapeutic efficacy remains unclear. Nevertheless, early data show that the two modalities can potentiate therapeutic effects and improve patient outcomes. Advances in neurosurgery have significantly improved the safety of surgical resection of brain metastases.174,175 Surgical resection can improve patient performance and decrease the use of steroids. In a small study of 12 patients with melanoma brain metastases whose treatment regimens involved craniotomies and ipilimumab, nine patients had better performance status after surgery.176 Furthermore, the sequence and timing of neurosurgery and immunotherapy contributes to overall outcomes. A clinical study of 142 patients with melanoma brain metastases showed that immunotherapy-naïve patients who underwent surgical resection prior to immune checkpoint blockade had longer median overall survival (22.7 months) than patients treated with immunotherapy alone (10.8 months) or immunotherapy followed by surgery (9.4 months).177 In glioma, early clinical trials show improvement in survival with surgery and PD-1 blockade.178,179
Discussion
Patients with brain metastases continue to be excluded from clinical trials due to their dismal outcomes and poor prognoses.98 Many prospective and retrospective immunotherapeutic studies exclude patients with brain metastases despite increasing data that point to potential efficacy against intracranial metastases.180 In a multivariate analysis, brain metastases were not associated with poorer survival in patients treated with immune checkpoint inhibitors in NSCLC.181 Stable patients with brain metastases without baseline corticosteroids and a good diagnosis-specific graded prognostic assessment (DS-GPA) classification have the best prognosis.181 Combining different immune checkpoint inhibitors together or with radiation, chemotherapy, targeted therapy, antiangiogenic therapy and/or neurosurgery seems to potentiate their effect in the setting of brain metastases. Therefore, randomized controlled trials for patients with brain metastases are needed to fully understand the exact clinical benefit of immunotherapy as monotherapy or in combination. In addition, the exact biological mechanism that governs the function of immune checkpoint inhibitors in the immunosuppressive environment of the brain needs to be determined.
Immunotherapy has not yet been brought to the brain metastasis space in patients with breast cancer. Despite clinical benefit being achieved in patients with brain metastases from melanoma and NSCLC,23,117 breast cancer remains out of bounds. Preclinical studies in metastatic breast cancer have illustrated a lower immune content in brain metastases.182 The development of immune strategies to understand and alter the complex brain immune microenvironment is needed. Few clinical trials are exploring this avenue (Table 4). One trial is studying the effect of atezolizumab in combination with HER2-targeting agents in HER2-positive breast cancer, and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer (NCT02605915). Another trial is exploring the combination of atezolizumab with stereotactic radiotherapy in patients with brain metastases from triple-negative breast cancer (NCT03483012). Radiotherapy can be an important tool to induce the immunogenicity of ‘cold’ tumours, such as breast cancer brain metastases. Doses and fractionation that trigger inflammatory responses in combination with immune checkpoint inhibitors can trigger the effector immune cells in the brain tumour microenvironment.186 Radiation has the ability to upregulate MHC-I expression on tumour cell surfaces and to allow antigen presentation of tumour-specific peptides for recognition by cytotoxic T cells.187 Furthermore, DNA damage induced by radiation can lead to the production of new antigens that can be recognized by the immune system.188 In addition, radiation activates the stimulator of interferon genes (STING) pathway, which plays a central role in anti-tumour immunity.189,190 Nevertheless, radiotherapy can also lead to pro-tumorigenic effects. DNA damage induced by radiotherapy may increase tumour resistance and aggressiveness. In addition, brain metastatic cancer cells can employ the STING pathway and produce factors that activate the STAT1 and NF-κB pathways to support tumour growth and resistance.81 As such, more research is warranted to acquire a comprehensive biological insight into the immune mechanisms induced by radiotherapy. In clinical trials, the combination of radiotherapy and immunotherapy shows signs of promising intracranial response but marked improvement in overall survival is yet to be reported. Tumour-instigated immunosuppression remains dominant over attempts to instigate anti-tumour immunities. Thus, modalities that aim to deplete the immune microenvironment of TAMs and inhibit the activity of suppressive cytokines in the immune milieu can lead to better activation of effector T cells.191,192
Table 4.
Ongoing clinical trials using immune checkpoint blockade for brain metastases
| NCTID | Strategy | Phase | Start date | Number enrolled | Primary tumour | Primary outcome | Notes and preliminary results |
|---|---|---|---|---|---|---|---|
| NCT02460068 | Fotemustine versus fotemustine + ipilimumab versus ipilimumab + nivolumab | 3 | 2012 | 168 | Melanoma | Overall survival | Recruitment status unknown |
| NCT02097732 | Ipilimumab + SRS | 2 | 2014 | 4 | Melanoma | Intracranial control rate | Active, not recruiting patients |
| NCT02696993 | Nivolumab + SRS ± ipilimumab | 1/2 | 2016 | 88 | NSCLC | Dose-limiting toxicity, progression-free survival | Concurrent SRS with nivolumab/ipilimumab is safe with durable tumour responses183 |
| NCT02681549 | Pembrolizumab + bevacizumab | 2 | 2016 | 53 | NSCLC, melanoma | Objective response rate | 12 of 20 patients had a response: 7 complete and 5 partial173 |
| NCT02858869 | Pembrolizumab + SRS | 1 | 2016 | 30 | NSCLC, melanoma | Dose-limiting toxicity | The 6-month local control (94.0%), progression-free survival (50%), and overall survival (77.4%) were higher than for monotherapy alone, with no added toxicity184 |
| NCT02716948 | SRS + nivolumab | 1 | 2016 | 90 | Melanoma | Incidence of adverse events | Recruiting patients |
| NCT03325166 | Pembrolizumab + ferumoxytol | 2 | 2017 | 20 | NSCLC | Sensitivity and specificity of relative cerebral blood volume | Recruiting patients |
| NCT02978404 | SRS + nivolumab | 2 | 2017 | 26 | NSCLC, SCLC, melanoma, renal cell carcinoma | Progression-free survival | In 3 of 8 patients, there was 1 partial response and 2 cases of stable disease. SRS + nivolumab is safe with no acute toxicity185 |
| NCT03175432 | Bevacizumab + atezolizumab ± cobimetinib | 2 | 2017 | 60 | Melanoma | Objective response rate, safety profile | Recruiting patients |
| NCT03563729 | Steroid + pembrolizumab or ipilimumab + nivolumab | 2 | 2018 | 80 | Melanoma | Progression-free and overall survival | Recruiting patients |
| NCT03728465 | Ipilimumab + nivolumab | 2 | 2018 | 68 | Melanoma | Intracranial control rate | Recruiting patients |
| NCT03340129 | Ipilimumab + nivolumab with SRS | 2 | 2019 | 218 | Melanoma | Neurological cause of death | Recruiting patients |
| NCT04047602 | Checkpoint inhibitor + SRS | Prospective | 2019 | 42 | Any solid tumour | Symptomatic radiation necrosis rate | Recruiting patients |
| NCT04021420 | Sonocloud + nivolumab | 1 | 2019 | 21 | Melanoma | Most successful dose | Recruiting patients |
| NCT04461418 | Checkpoint inhibitors + steroids | 2 | 2020 | 20 | Any solid tumour | Objective response rate | Not yet recruiting |
| NCT03955198 | SRS + durvalumab | 2 | 2020 | 100 | NSCLC | Progression-free survival | Recruiting patients |
| NCT04434560 | Neoadjuvant nivolumab versus ipilimumab | 2 | 2020 | 40 | Solid tumour | Proliferation of circulating T cells | Recruiting patients |
| NCT04187872 | LITT + pembrolizumab | 1 | 2020 | 15 | Solid tumour | Immune effect | Recruiting patients |
| NCT04348747 | Anti-HER2/3 dendritic cell vaccine + pembrolizumab | 2 | 2020 | 23 | Breast cancer | Objective response rate | Not yet recruiting patients |
SRS = stereotactic radiosurgery; WBRT = whole brain radiation therapy. More information can be found in the Supplementary material.
There is a dire need for predictive biomarkers and prognostic scales that permit prospective evaluation of patients on immune checkpoint inhibitors.193 Diagnostic clinical trials in patients with brain metastases are scarce and suffer from poor trial design and follow-up.194 The characterization of circulating tumour cells and cell-free DNA can help reveal the molecular foundations of brain metastases and offers the chance to observe possible changes in response to immunotherapy. The integration of machine learning in the assessment and classification of patients with brain metastases seems inevitable to identify tumour-infiltrated diagnostic regions and assess the response of metastatic lesions faster and more accurately.195 In addition, future studies should continue to explore the genetic, phenotypic and immune regulatory differences between primary tumours and brain metastases.
There are many opportunities to improve the efficacy of immune checkpoint inhibitors in light of reported observations. The timing and sequence of administration of immunotherapy alongside other regimens can be crucial to the overall clinical outcome. In addition, the receptor expression patterns on T cells differ at different anatomical locations.96 Therefore, enhancing organ-specific T-cell trafficking to the brain is an important goal to achieve. Furthermore, NK cells have been shown to be increased in response to immune checkpoint inhibitors; however, the mechanism of action and ways to potentiate this response remain to be elucidated.
The role of B cells in enhancing the response to immune checkpoint blockade remains unclear. Cell depletion studies reveal that NK cells and CD8+ T cells are required for intracranial anti-PD-1/anti-CTLA4 efficacy.96 Nevertheless, new studies show that the incidence of B cells in human tumours, such as melanoma and sarcomas, is associated with better response to immunotherapy.196–198 In primary brain tumours, it was revealed that TAMs can transfer functional PD-L1 via microvesicles to confer on regulatory B cells the ability to suppress CD8+ T-cell activation.199 Similar mechanisms may be occurring in the setting of brain metastases. In glioma, a B cell-based vaccine, with CD40 agonism and IFNγ stimulation, migrates to key secondary lymphoid organs and is proficient at antigen cross-presentation, which promotes both the survival and the functionality of CD8+ T cells. Combining this vaccine with radiation and PD-L1 blockade conferred tumour eradication in 80% of treated tumour-bearing animals.200 As such, designing strategic immune therapies that can address B cell responses in brain metastases is essential for more effective treatments.
Brain metastatic cancer cells arising from primary tumours have the propensity and the necessary metabolic properties to colonize the brain. The brain is the organ with the highest energy demand and the ability to rewire its metabolism in response to varying stressors.201 It poses metabolic challenges to a colonizing cancer cell, ranging from fuel and oxygen availability to oxidative stress. As such, brain metastases display a remarkable metabolic flexibility by utilizing acetate, glutamine and branched-chain amino acids as alternative sources of fuel.202 Brain metastases upregulate the synthesis of acetyl-CoA synthetase enzyme 2, which allows them to fuel the tricarboxylic acid cycle and convert acetate to acetyl-CoA for energy metabolism.203 Furthermore, brain metastases oxidize branched-chain amino acids and glutamine to survive and proliferate in the absence of glucose.204 In addition, brain metastases can produce glucose by upregulating fructose-1,6-bisphosphatase 2 for gluconeogenesis.204 Comparing the molecular profiles and gene expression patterns of melanoma brain metastases with patient-matched extracranial metastases identified significant immunosuppression and enrichment of oxidative phosphorylation in brain metastases, which was confirmed by direct metabolic profiling and [U-13C]-glucose tracing.15 Increased oxidative phosphorylation leads to oxygen deprivation and hypoxia in the immune microenvironment, hampering immune cell function. Effector T cells suffer from metabolic insufficiency and dysfunction due to nutrient depletion.205 Though oxygen metabolism is less vital for T-cell effector function, oxygen consumption is necessary for memory formation and T-cell proliferation.206 As such, the inhibition of oxidative phosphorylation in the immune microenvironment of brain metastases can help lift the immunosuppression and lead to better immune activity against brain metastatic cells. Metabolic interventions in combination with immune checkpoint inhibitors can further enhance the therapeutic effect against brain metastases.
Delivery of immune therapies and the trafficking of cytotoxic T cells to the brain are hampered by the presence of the blood–brain barrier. Overcoming this barrier is essential to achieve therapeutic benefit akin to what is achieved in extracranial disease. A permeable blood–brain barrier may facilitate the presentation of tumour-associated antigens and enhance the effects of immune cells in the tumour microenvironment. Therefore, strategies that involve physical disruption of the blood–brain barrier can improve antigen presentation, enable immune checkpoint inhibitor transmigration, and increase immune cell trafficking into the immune microenvironment to further sensitize brain metastases to immunotherapies. In recent years, a number of strategies have been explored to hijack barriers posed by the blood–brain barrier. Recent preclinical developments with focused ultrasound allow the delivery of therapeutic agents to the brain that were once deemed undeliverable.207 In a clinical trial on patients with recurrent glioblastoma, pulsed ultrasound in combination with systemically injected microbubbles showed that the approach is safe and well tolerated.208 Through focused ultrasound sonication, engineered NK cells against the HER2 receptor were capable of crossing the blood–brain barrier in the setting of breast cancer brain metastases.209 The use of whole-brain radiation therapy has shown conflicting results in regard to its capability of enhancing the delivery of therapeutic molecules across the blood–brain barrier. In a self-controlled pilot study, whole-brain radiation therapy failed to boost intracerebral gefitinib concentration in patients with brain metastatic NSCLC who were treated with the drug for 14 days.210 Yet, the permeability of gefitinib after treatment for 30 days increased in accordance with the escalated dose of whole-brain radiation therapy in NSCLC brain metastases.211 Intravital microscopy and computational modelling show that a single, low dose of radiation therapy can induce transient, dynamic and localized vascular bursting, as well as enlarge blood vessel volume. Increased permeability can facilitate extravasation of immune checkpoint inhibitors from blood vessels in tumours.212 Nevertheless, the downfalls of whole-brain radiation therapy are its side effects. Cerebral oedema may be induced or worsened after the initiation of whole-brain radiation therapy. As a result, whole-brain radiation therapy is usually preceded by corticosteroid therapy, which may affect the efficacy of immune checkpoint blockade.213 Therefore, finding the right balance between the safety profile and the therapeutic benefits of interventions, such as focused ultrasound and whole-brain radiation therapy, is vital in assessing ways to enhance the penetration of immune checkpoint inhibitors into the tumour microenvironment of brain metastases. Moreover, the translation of some of these preclinical modalities to the brain metastatic clinical setting is necessary for proper evaluation of efficacy and outcomes.
Exploring new models for the induction of immune checkpoint blockade is necessary to potentiate the effects of immunotherapy. Preclinically, nanotherapy in conjunction with photothermal induction and immune checkpoint blockade has been shown to be effective in brain tumours.214 Targeted nanoscale immunoconjugates presented on a natural biopolymer scaffold, poly(β-l-malic acid), with covalently attached anti-CTLA4 or anti-PD-1 antibodies for systemic delivery across the blood–brain barrier and activation of local brain anti-tumour immune response resulted in an increase in CD8+ T cells, NK cells and macrophages along with a decrease in regulatory T cells in the tumour microenvironment of gliomas.215 It also significantly increased survival when compared to animals treated with single checkpoint inhibitor-bearing nanoscale immunoconjugates or free anti-CTLA4 or anti-PD-1 antibodies.215 Moreover, therapeutic targeting of PD-L1 on TAMs using nano-immunotherapy synergizes with radiation in primary brain tumours.216 Similar strategies can be used against brain metastases. Furthermore, preclinical data demonstrate that neural stem cells can deliver systemic agents that target brain metastases from melanoma and breast cancer.217,218 In addition, delivering apoptosis-promoting genes to the immunosuppressive TAMs in brain metastases can be done through haematopoietic stem cells.26 Thus, utilizing the inherent ability of stem cells to cross the blood–brain barrier and rocket towards tumours can help in increasing immune targeting of brain metastases. Moreover, oncolytic viruses have shown promise in targeting primary brain tumours and potentiating the immune response against tumour cells.219 The clinical delivery of oncolytic viruses to brain metastases has yet to be done. Preclinical data show that intravenous delivery of an oncolytic Orthoreovirus can immunologically prime brain metastases against PD-1 blockade.220 In addition, the utilization of CAR T cells to target HER2-positive breast cancer brain metastases offers a new immunotherapeutic medium for exploration.221
The rise of cancer neuroscience as a field opens a window to explore how the immune microenvironment interacts with its neural counterpart in the setting of brain metastases. Given how astrocytes affect the immune response towards tumour cells, it is rational to believe that the nervous tissue contributes in one way or another to the immunogenicity of brain metastases. Recent discoveries reveal that excitatory neurotransmission enables NMDAR signalling in brain metastases.95 Breast cancer cells that have metastasized to the brain upregulate neurotransmitter receptor expression and extend perisynaptic processes to receive neuronal activity-dependent neurotransmitter signals that trigger a receptor-mediated signalling cascade, induce inward currents in the malignant cells, and drive growth of breast cancer brain metastases.95 In gliomas, glutamatergic synaptic input and electrochemical communication drives brain tumour progression.222,223 Dissecting the neural–immune–cancer interactions is necessary to obtain a comprehensive picture of how brain metastases progress through immune checkpoint evasion. In fact, these interactions may be contributing to the effect of immunotherapies in the brain.
Conclusion
The brain continues to be a special organ in terms of immune regulation, with the blood–brain barrier being a major contributor to this immune state. The immunosuppressive role of the tumour microenvironment in the setting of brain metastases emphasizes the need for new therapeutic strategies that can reverse immunosuppression. These strategies should aim to favour the M1 state of macrophages or help in the recruitment of cytotoxic T cells. They can also target immunosuppressive elements like regulatory T cells and TAMs. So far, immunotherapy has failed to show significant benefit in breast cancer brain metastases (unlike in metastatic melanoma and NSCLC). As such, approaches that aim to flip cold brain metastatic tumours to hot ones should be emphasized and the potential risk of inducing autoimmunity should be evaluated. It is also important to consider that immunosuppression is a protective mechanism that keeps the brain safe from excessive inflammation and subsequent oedema that may harm the sensitive structures of the brain, leading to damage. Understanding the biological underpinnings and detailed mechanisms that are implicated in immunosuppression of brain metastases is vital to design novel immune interventions that are safe, efficacious and can elicit an enduring response. Embracing combination therapy with immune checkpoint inhibitors seems to yield better clinical outcomes and survival benefit. As such, strategies that are based on the biological mechanisms of these inhibitors should be adopted. Developing appropriate preclinical models that recapitulate the immune microenvironment in the setting of brain metastases is a necessity for the advancement of novel therapies that can be translated to clinical practice.
Funding
This work was supported by NIH grants R35CA197725 (M.S.L.), R01NS87990 (M.S.L.) and R01NS093903 (M.S.L.), and by the Ministry of Science and Higher Education of the Russian Federation grant №075-15-2020-926 (I.U.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
- MHC
major histocompatibility complex
- NSCLC
non-small cell lung cancer
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death 1-ligand 1
- SCLC
small cell lung cancer
- TAMs
tumour-associated myeloid cells and microglia
References
- 1. Sampson JH, Gunn MD, Fecci PE, et al. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fares J, Kanojia D, Rashidi A, et al. Genes that mediate metastasis across the blood-brain barrier. Trends Cancer. 2020;6(8):660-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu X, Deng Q, Ma L, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020;30(3):229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Absinta M, Ha S-K, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:e29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Da Mesquita S, Fu Z, Kipnis J.. The meningeal lymphatic system: A new player in neurophysiology. Neuron. 2018;100(2):375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quail DF, Joyce JA.. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326-341. [28292436] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song E, Mao T, Dong H, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louveau A, Herz J, Alme MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21(10):1380-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osswald M, Blaes J, Liao Y, et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016;22(24):6078-6087. [DOI] [PubMed] [Google Scholar]
- 13. Quail DF, Joyce JA.. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farber SH, Tsvankin V, Narloch JL, et al. Embracing rejection: Immunologic trends in brain metastasis. Oncoimmunology. 2016;5(7):e1172153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer GM, Jalali A, Kircher DA, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019;9(5):628-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badie B, Schartner JM.. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46(4):957-961; discussion 961-962. [DOI] [PubMed] [Google Scholar]
- 18. Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parney IF, Waldron JS, Parsa AT.. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110(3):572-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugihara AQ, Rolle CE, Lesniak MS.. Regulatory T cells actively infiltrate metastatic brain tumors. Int J Oncol. 2009;34(6):1533-1540. [DOI] [PubMed] [Google Scholar]
- 21. Berghoff AS, Ricken G, Widhalm G, et al. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015;66(2):289-299. [DOI] [PubMed] [Google Scholar]
- 22. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noy R, Pollard JW.. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41(1):49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andreou T, Rippaus N, Wronski K, et al. Hematopoietic stem cell gene therapy for brain metastases using myeloid cell-specific gene promoters. J Natl Cancer Inst. 2020;112(6):617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klemm F, Maas RR, Bowman RL, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643-1660.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowman RL, Klemm F, Akkari L, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17(9):2445-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coniglio SJ, Eugenin E, Dobrenis K, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schulz M, Salamero-Boix A, Niesel K, et al. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front Immunol. 2019;10:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Q, Barres BA.. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225-242. [DOI] [PubMed] [Google Scholar]
- 35. Engelhardt B, Vajkoczy P, Weller RO.. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123-131. [DOI] [PubMed] [Google Scholar]
- 36. Ajami B, Bennett JL, Krieger C, et al. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142-1149. [DOI] [PubMed] [Google Scholar]
- 37. Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58-69. [PMC free article] [PubMed] [Google Scholar]
- 38. Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011-5022. [DOI] [PubMed] [Google Scholar]
- 39. Liu Q, Johnson EM, Lam RK, et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol. 2019;20(8):1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu P, Zhang X, Liu Q, et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10(8):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bradbury MW, Westrop RJ.. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poggi A, Musso A, Dapino I, et al. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunol Lett. 2014;159(1-2):55-72. [DOI] [PubMed] [Google Scholar]
- 43. Bunt SK, Sinha P, Clements VK, et al. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176(1):284-290. [DOI] [PubMed] [Google Scholar]
- 44. Leibold AT, Monaco GN, Dey M.. The role of the immune system in brain metastasis. Curr Neurobiol. 2019;10(2):33-48. [PMC free article] [PubMed] [Google Scholar]
- 45. Parker KH, Beury DW, Ostrand-Rosenberg S.. Myeloid-derived suppressor cells: Critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fares J, Bou Diab Z, Nabha S, et al. Neurogenesis in the adult hippocampus: History, regulation, and prospective roles. Int J Neurosci. 2019;129(6):598-611. [DOI] [PubMed] [Google Scholar]
- 47. Brantley EC, Guo L, Zhang C, et al. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol. 2010;3(6):380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Louie E, Chen XF, Coomes A, et al. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene. 2013;32(35):4064-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pukrop T, Dehghani F, Chuang H-N, et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58(12):1477-1489. [DOI] [PubMed] [Google Scholar]
- 50. Amit M, Laider-Trejo L, Shalom V, et al. Characterization of the melanoma brain metastatic niche in mice and humans. Cancer Med. 2013;2(2):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rippaus N, Taggart D, Williams J, et al. Metastatic site-specific polarization of macrophages in intracranial breast cancer metastases. Oncotarget. 2016;7(27):41473-41487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402-1410. [DOI] [PubMed] [Google Scholar]
- 53. Laoui D, Movahedi K, Van Overmeire E, et al. Tumor-associated macrophages in breast cancer: Distinct subsets, distinct functions. Int J Dev Biol. 2011;55(7-9):861-867. [DOI] [PubMed] [Google Scholar]
- 54. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942-949. [DOI] [PubMed] [Google Scholar]
- 55. Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berghoff AS, Venur VA, Preusser M, et al. Immune checkpoint inhibitors in brain metastases: From biology to treatment. Am Soc Clin Oncol Educ Book. 2016;35:e116-e122. [DOI] [PubMed] [Google Scholar]
- 57. Zhou J, Gong Z, Jia Q, et al. Programmed death ligand 1 expression and CD8+ tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498(4):751-757. [DOI] [PubMed] [Google Scholar]
- 58. Tao H, Mimura Y, Aoe K, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75(1):95-101. [DOI] [PubMed] [Google Scholar]
- 59. Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clemente CG, Mihm MC Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303-1310. [DOI] [PubMed] [Google Scholar]
- 61. Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5(1):e1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zakaria R, Platt-Higgins A, Rathi N, et al. T-cell densities in brain metastases are associated with patient survival times and diffusion tensor MRI changes. Cancer Res. 2018;78(3):610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fares J, Fares MY, Khachfe HH, et al. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chaudhary B, Elkord E.. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines (Basel). 2016;4(3):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Crowther MD, Dolton G, Legut M, et al. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol. 2020;21:178-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fares J, Fares MY, Fares Y.. Natural killer cells in the brain tumor microenvironment: Defining a new era in neuro-oncology. Surg Neurol Int. 2019;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stevens A, Kloter I, Roggendorf W.. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer. 1988;61(4):738-743. [DOI] [PubMed] [Google Scholar]
- 70. Yang I, Han SJ, Sughrue ME, et al. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: Evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011;115(3):505-511. [DOI] [PubMed] [Google Scholar]
- 71. Bottcher JP, Bonavita E, Chakravarty P, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022-1037.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barletta KE, Ley K, Mehrad B.. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Glodde N, Bald T, van den Boorn-Konijnenberg D, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47(4):789-802.e9. [DOI] [PubMed] [Google Scholar]
- 74. Zhang L, Yao J, Wei Y, et al. Blocking immunosuppressive neutrophils deters pY696-EZH2-driven brain metastases. Sci Transl Med. 2020;12(545):eaaz5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Y, Kosaka A, Ikeura M, et al. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro Oncol. 2013;15(7):891-903. [23595625] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mitsuya K, Nakasu Y, Kurakane T, et al. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of worse survival after resection in patients with brain metastasis. J Neurosurg. 2017;127(2):433-437. [DOI] [PubMed] [Google Scholar]
- 77. Chowdhary M, Switchenko JM, Press RH, et al. Post-treatment neutrophil-to-lymphocyte ratio predicts for overall survival in brain metastases treated with stereotactic radiosurgery. J Neurooncol. 2018;139(3):689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li H, Wang W, Yang X, et al. The clinical prognostic value of the neutrophil-to-lymphocyte ratio in brain metastases from non-small cell lung cancer-harboring EGFR mutations. Cancer Manag Res. 2020;12:5659-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xing F, Kobayashi A, Okuda H, et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5(3):384-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen Q, Boire A, Jin X, et al. Corrigendum: Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2017;544(7648):124. [DOI] [PubMed] [Google Scholar]
- 81. Chen Q, Boire A, Jin X, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marchetti D, Li J, Shen R.. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000;60(17):4767-4770. [PubMed] [Google Scholar]
- 83. Shumakovich MA, Mencio CP, Siglin JS, et al. Astrocytes from the brain microenvironment alter migration and morphology of metastatic breast cancer cells. FASEB J. 2017;31:5049-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Priego N, Zhu L, Monteiro C, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24(7):1024-1035. [DOI] [PubMed] [Google Scholar]
- 85. Priego N, Zhu L, Monteiro C, et al. Author Correction: STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24(9):1481. [DOI] [PubMed] [Google Scholar]
- 86. Balar AV, Weber JS.. PD-1 and PD-L1 antibodies in cancer: Current status and future directions. Cancer Immunol Immunother. 2017;66(5):551-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hu ZI, Hellmann MD, Wolchok JD, et al. Acquired resistance to immunotherapy in MMR-D pancreatic cancer. J Immunother Cancer. 2018;6(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shimada T, Hoshino Y, Tsunemi T, et al. Neuromyelitis optica spectrum disorder after treatment with pembrolizumab. Mult Scler Relat Disord. 2019;37:101447. [DOI] [PubMed] [Google Scholar]
- 89. Du Four S, Maenhout SK, De Pierre K, et al. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology. 2015;4(4):e998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Salmond RJ, Alexander DR.. SHP2 forecast for the immune system: Fog gradually clearing. Trends Immunol. 2006;27(3):154-160. [16458607] [DOI] [PubMed] [Google Scholar]
- 91. Qiu XY, Hu DX, Chen WQ, et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5 Pt A):1754-1769. [DOI] [PubMed] [Google Scholar]
- 92. Feng J, Yang H, Zhang Y, et al. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. 2017;36(42):5829-5839. [DOI] [PubMed] [Google Scholar]
- 93. Robainas M, Otano R, Bueno S, Ait-Oudhia S.. Understanding the role of PD-L1/PD1 pathway blockade and autophagy in cancer therapy. Onco Targets Ther. 2017;10:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dong Y, Sun Q, Zhang X.. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8(2):2171-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zeng Q, Michael IP, Zhang P, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573(7775):526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Taggart D, Andreou T, Scott KJ, et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci USA. 2018;115(7):E1540-E1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lorger M, Andreou T, Fife C, et al. Immune checkpoint blockade—How does it work in brain metastases? Front Mol Neurosci. 2019;12:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fares J, Kanojia D, Cordero A, et al. Current state of clinical trials in breast cancer brain metastases. Neurooncol Pract. 2019;6(5):392-401. [31555454] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wei SC, Duffy CR, Allison JP.. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069-1086. [DOI] [PubMed] [Google Scholar]
- 100. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Berghoff AS, Ricken G, Wilhelm D, et al. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J Neurooncol. 2016;130(1):19-29. [DOI] [PubMed] [Google Scholar]
- 102. Harter PN, Bernatz S, Scholz A, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015;6(38):40836-40849. [26517811] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Teglasi V, Reiniger L, Fabian K, et al. Evaluating the significance of density, localization, and PD-1/PD-L1 immunopositivity of mononuclear cells in the clinical course of lung adenocarcinoma patients with brain metastasis. Neuro Oncol. 2017;19(8):1058-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kluger HM, Zito CR, Barr ML, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015;21(13):3052-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ogiya R, Niikura N, Kumaki N, et al. Comparison of immune microenvironments between primary tumors and brain metastases in patients with breast cancer. Oncotarget. 2017;8(61):103671-103681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Takamori S, Toyokawa G, Okamoto I, et al. Discrepancy in programmed cell death-ligand 1 between primary and metastatic non-small cell lung cancer. Anticancer Res. 2017;37(8):4223-4228. [DOI] [PubMed] [Google Scholar]
- 108. Fares J, Fares MY, Fares Y.. Immune checkpoint inhibitors: Advances and impact in neuro-oncology. Surg Neurol Int. 2019;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Weber J. Review: Anti-CTLA-4 antibody ipilimumab: Case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864-872. [DOI] [PubMed] [Google Scholar]
- 110. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465. [DOI] [PubMed] [Google Scholar]
- 112. Margolin K. Ipilimumab in a Phase II trial of melanoma patients with brain metastases. Oncoimmunology. 2012;1(7):1197-1199. [23170278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Heller KN, Pavlick AC, Hodi FS, et al. Safety and survival analysis of ipilimumab therapy in patients with stable asymptomatic brain metastases. J Clin Oncol. 2011;29(15_suppl):8581-8581. [Google Scholar]
- 114. Ito K, Schöder H, Teng R, et al. Prognostic value of baseline metabolic tumor volume measured on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging. 2019;46(4):930-939. [30488098] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kluger HM, Goldberg SB, Sznol M, et al. Safety and activity of pembrolizumab in melanoma patients with untreated brain metastases. J Clin Oncol. 2015;33(15_suppl):9009-9009. [Google Scholar]
- 116. Goldberg SB, Gettinger SN, Mahajan A, et al. Activity and safety of pembrolizumab in patients with metastatic non-small cell lung cancer with untreated brain metastases. J Clin Oncol. 2015;33(15_suppl):8035-8035. [Google Scholar]
- 117. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Parakh S, Park JJ, Mendis S, et al. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer. 2017;116(12):1558-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol. 2019;37(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Crino L, Bronte G, Bidoli P, et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer. 2019;129:35-40. [DOI] [PubMed] [Google Scholar]
- 121. Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gadgeel SM, Lukas RV, Goldschmidt J, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer. 2019;128:105-112. [DOI] [PubMed] [Google Scholar]
- 123. Goldman JW, Crino L, Vokes EE, et al. Nivolumab (nivo) in patients (pts) with advanced (adv) NSCLC and central nervous system (CNS) metastases (mets). J Clin Oncol. 2016;34(15_suppl):9038-9038. [Google Scholar]
- 124. Pluchart H, Pinsolle J, Cohen J, et al. Partial response of pulmonary adenocarcinoma with symptomatic brain metastasis to nivolumab plus high-dose oral corticosteroid: A case report. J Med Case Rep. 2017;11(1):183. [28679408] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681. [DOI] [PubMed] [Google Scholar]
- 126. Tawbi HA-H, Forsyth PAJ, Hodi FS, et al. Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204). J Clin Oncol. 2019;37(15_suppl):9501. [Google Scholar]
- 127. Wei SC, Anang N-A, Sharma R, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci USA. 2019;116(45):22699-22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yao Z, Zheng Z, Ke W, et al. Prognostic nomogram for bladder cancer with brain metastases: A National Cancer Database analysis. J Transl Med. 2019;17(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Woditschka S, Evans L, Duchnowska R, et al. DNA double-strand break repair genes and oxidative damage in brain metastasis of breast cancer. J Natl Cancer Inst. 2014;106(7):dju145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Seol HJ, Yoo HY, Jin J, et al. The expression of DNA damage checkpoint proteins and prognostic implication in metastatic brain tumors. Oncol Res. 2011;19(8-9):381-390. [DOI] [PubMed] [Google Scholar]
- 131. Schmid TE, Multhoff G.. Radiation-induced stress proteins—The role of heat shock proteins (HSP) in anti- tumor responses. Curr Med Chem. 2012;19(12):1765-1770. [DOI] [PubMed] [Google Scholar]
- 132. Rudqvist NP, Pilones KA, Lhuillier C, et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol Res. 2018;6(2):139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Berghoff AS, Lassmann H, Preusser M, et al. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin Exp Metastasis. 2013;30(1):69-81. [DOI] [PubMed] [Google Scholar]
- 134. Du Four S, Maenhout SK, Niclou SP, et al. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res. 2016;6(11):2514-2531. [PMC free article] [PubMed] [Google Scholar]
- 135. You H, Baluszek S, Kaminska B.. Immune microenvironment of brain metastases-are microglia and other brain macrophages little helpers? Front Immunol. 2019;10:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li YD, Lamano JB, Lamano JB, et al. Tumor-induced peripheral immunosuppression promotes brain metastasis in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2019;68(9):1501-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906. [24403263] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23(3):191-195. [DOI] [PubMed] [Google Scholar]
- 140. Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27(3):434-441. [DOI] [PubMed] [Google Scholar]
- 141. Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol. 2019;130:104-112. [DOI] [PubMed] [Google Scholar]
- 142. Schoenfeld JD, Mahadevan A, Floyd SR, et al. Ipilmumab and cranial radiation in metastatic melanoma patients: A case series and review. J Immunother Cancer. 2015;3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368-375. [25754629] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122(19):3051-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Acharya S, Mahmood M, Mullen D, et al. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol. 2017;2(4):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yusuf MB, Amsbaugh MJ, Burton E, et al. Peri-SRS administration of immune checkpoint therapy for melanoma metastatic to the brain: Investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg. 2017;100:632-640.e4. [DOI] [PubMed] [Google Scholar]
- 147. Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;99(1):22-30. [DOI] [PubMed] [Google Scholar]
- 148. Skrepnik T, Sundararajan S, Cui H, et al. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology. 2017;6(3):e1283461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol. 2017;40(5):444-450. [DOI] [PubMed] [Google Scholar]
- 150. Gaudy-Marqueste C, Dussouil AS, Carron R, et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer. 2017;84:44-54. [DOI] [PubMed] [Google Scholar]
- 151. Cohen-Inbar O, Shih HH, Xu Z, et al. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg. 2017;127(5):1007-1014. [DOI] [PubMed] [Google Scholar]
- 152. Choong ES, Lo S, Drummond M, et al. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer. 2017;75:169-178. [DOI] [PubMed] [Google Scholar]
- 153. Ahmed KA, Kim S, Arrington J, et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol. 2017;133(2):331-338. [DOI] [PubMed] [Google Scholar]
- 154. Chen L, Douglass J, Kleinberg L, et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(4):916-925. [DOI] [PubMed] [Google Scholar]
- 155. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458-5468. [DOI] [PubMed] [Google Scholar]
- 156. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Emens LA, Middleton G.. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Powell S, Abreu DR, Langer C, et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) in NSCLC with brain metastases: Pooled analysis of KEYNOTE-021, 189, and 407. Ann Oncol. 2019;30:v606-v607. [DOI] [PubMed] [Google Scholar]
- 159. Fares J, Kanojia D, Rashidi A, et al. Landscape of combination therapy trials in breast cancer brain metastasis. Int J Cancer. 2020;147:1939-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mok TSK, Reck M, Horn L, et al. IMpower133: Primary efficacy and safety + CNS-related adverse events in a phase I/III study of first-line (1L) atezolizumab + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann Oncol. 2018;29:ix173. [Google Scholar]
- 161. Paz-Ares L, Dvorkin M, Chen Y, et al. ; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939. [DOI] [PubMed] [Google Scholar]
- 162. Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): An open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13(9):879-886. [DOI] [PubMed] [Google Scholar]
- 163. Di Giacomo AM, Ascierto PA, Queirolo P, et al. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Ann Oncol. 2015;26(4):798-803. [DOI] [PubMed] [Google Scholar]
- 164. Lyle M, Long GV.. The role of systemic therapies in the management of melanoma brain metastases. Curr Opin Oncol. 2014;26(2):222-229. [DOI] [PubMed] [Google Scholar]
- 165. Callahan MK, Masters G, Pratilas CA, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol Res. 2014;2(1):70-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Callahan M, Masters G, Katz J, et al. The immunological impact of the RAF inhibitor BMS908662: Preclinical and early clinical experience in combination with CTLA-4 blockade. J Clin Oncol. 2012;30(15_suppl):2521-2521. [Google Scholar]
- 167. Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sharma N, Vacher J, Allison JP.. TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion. Proc Natl Acad Sci USA. 2019;116(21):10453-10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wang Y, Huang N, Li H, et al. Promoting oligodendroglial-oriented differentiation of glioma stem cell: A repurposing of quetiapine for the treatment of malignant glioma. Oncotarget. 2017;8(23):37511-37524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Feng P-H, Chen K-Y, Huang Y-C, et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2018;13(7):958-967. [DOI] [PubMed] [Google Scholar]
- 172. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. [DOI] [PubMed] [Google Scholar]
- 173. Kluger HM, Forsyth P, Khushalani N, et al. RBTT-07. A phase 2 trial of pembrolizumab and bevacizumab in melanoma brain met patients. Neuro Oncol. 2019;21(Suppl_6):vi220. [Google Scholar]
- 174. Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044-1055; discussion 1055-6. [DOI] [PubMed] [Google Scholar]
- 175. Liu Q, Tong X, Wang J.. Management of brain metastases: History and the present. Chin Neurosurg J. 2019;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jones PS, Cahill DP, Brastianos PK, et al. Ipilimumab and craniotomy in patients with melanoma and brain metastases: A case series. Neurosurg Focus. 2015;38(3):E5. [DOI] [PubMed] [Google Scholar]
- 177. Alvarez-Breckenridge C, Giobbie-Hurder A, Gill CM, et al. Upfront surgical resection of melanoma brain metastases provides a bridge toward immunotherapy-mediated systemic control. Oncologist. 2019;24(5):671-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470-476. [DOI] [PubMed] [Google Scholar]
- 179. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Aquilanti E, Brastianos PK.. Immune checkpoint inhibitors for brain metastases: A primer for neurosurgeons. Neurosurgery. 2020;87:E281-E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hendriks LEL, Henon C, Auclin E, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol. 2019;14(7):1244-1254. [DOI] [PubMed] [Google Scholar]
- 182. Sammons S, Van Swearingen AED, Anders CK.. The promise of immunotherapy for breast cancer brain metastases. Curr Breast Cancer Rep. 2019;11(4):241-247. [Google Scholar]
- 183. Li J, Wang Y, Tang C, et al. Concurrent nivolumab and ipilimumab with brain stereotactic radiosurgery for brain metastases from non-small cell lung cancer: A phase I trial. J Clin Oncol. 2020;38(15_suppl):2531-2531. [Google Scholar]
- 184. Khan MK, Nasti T, Yushak ML, et al. Interim results of prospective pilot phase II trial of concurrent anti-PD-1 and stereotactic radiosurgery (SRS) for melanoma and NSCLC patients with brain metastases (NCT02858869). J Clin Oncol. 2020;38(15_suppl):e22002. [Google Scholar]
- 185. Alameddine R, Wong P, Masucci L, et al. MA08. 11 early safety data of a phase I/II combining nivolumab and stereotactic brain radiosurgery for treatment of brain metastases in patients with NSCLC. J Thorac Oncol. 2018;13(10):S385. [Google Scholar]
- 186. Brown JS, Sundar R, Lopez J.. Combining DNA damaging therapeutics with immunotherapy: More haste, less speed. Br J Cancer. 2018;118(3):312-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lauss M, Donia M, Harbst K, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017;8(1):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Barber GN. STING: Infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Chen Q, Sun L, Chen ZJ.. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142-1149. [DOI] [PubMed] [Google Scholar]
- 191. Zhu L, Narloch JL, Onkar S, et al. Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. J Immunother Cancer. 2019;7(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sevenich L. Turning "Cold" Into "Hot" tumors-opportunities and challenges for radio-immunotherapy against primary and metastatic brain cancers. Front Oncol. 2019;9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fares J, , Cordero A, , Kanojia D, et al. The Network of Cytokines in Brain Metastases. Cancers. 2021;13(1): 33466236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Fares J, Kanojia D, Rashidi A, et al. Diagnostic clinical trials in breast cancer brain metastases: Barriers and innovations. Clin Breast Cancer. 2019;19(6):383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hollon TC, Pandian B, Adapa AR, et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat Med. 2020;26(1):52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556-560. [DOI] [PubMed] [Google Scholar]
- 197. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561-565. [DOI] [PubMed] [Google Scholar]
- 199. Lee-Chang C, Rashidi A, Miska J, et al. Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol Res. 2019;7(12):1928-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lee-Chang C, Miska J, Hou D, et al. Activation of 4-1BBL+ B cells with CD40 agonism and IFNγ elicits potent immunity against glioblastoma. J Experiment Med. 2021;218(1):doi:10.1084/jem.20200913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Magistretti PJ, Allaman I.. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883-901. [DOI] [PubMed] [Google Scholar]
- 202. Schild T, Low V, Blenis J, et al. Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer Cell. 2018;33(3):347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Mashimo T, Pichumani K, Vemireddy V, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159(7):1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Chen J, Lee H-J, Wu X, et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015;75(3):554-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Scharping NE, Menk AV, Moreci RS, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. van der Windt GJW, Everts B, Chang C-H, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhang DY, Dmello C, Chen L, et al. Ultrasound-mediated delivery of paclitaxel for glioma: A comparative study of distribution, toxicity, and efficacy of albumin-bound versus Cremophor formulations. Clin Cancer Res. 2020;26(2):477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Carpentier A, Canney M, Vignot A, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8(343):343re2. [DOI] [PubMed] [Google Scholar]
- 209. Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73(6):1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Fang L, Sun X, Song Y, et al. Whole-brain radiation fails to boost intracerebral gefitinib concentration in patients with brain metastatic non-small cell lung cancer: A self-controlled, pilot study. Cancer Chemother Pharmacol. 2015;76(4):873-877. [DOI] [PubMed] [Google Scholar]
- 211. Zeng Y-D, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget. 2015;6(10):8366-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Miller MA, Chandra R, Cuccarese MF, et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci Transl Med. 2017;9(392):doi:10.1126/scitranslmed.aal0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Rogers LR. Neurologic complications of cancer, 2nd ed. Contemporary Neurology Series . Neuro Oncol. 2009;11(1):96-97. [Google Scholar]
- 214. Liu Y, Chongsathidkiet P, Crawford BM, et al. Plasmonic gold nanostar-mediated photothermal immunotherapy for brain tumor ablation and immunologic memory. Immunotherapy. 2019;11(15):1293-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Galstyan A, Markman JL, Shatalova ES, et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10(1):3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Zhang P, Miska J, Lee-Chang C, et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc Natl Acad Sci USA. 2019;116(47):23714-23723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Aboody KS, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8(2):119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kanojia D, Balyasnikova IV, Morshed RA, et al. Neural stem cells secreting anti-HER2 antibody improve survival in a preclinical model of HER2 overexpressing breast cancer brain metastases. Stem Cells. 2015;33(10):2985-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Desjardins A, Gromeier M, Herndon JE 2nd, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Samson A, Scott KJ, Taggart D, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med. 2018;10(422). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Priceman SJ, Tilakawardane D, Jeang B, et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin Cancer Res. 2018;24(1):95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532-538. [DOI] [PubMed] [Google Scholar]
- 223. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.