Abstract
A close interaction between gut immune responses and distant organ-specific autoimmunity including the CNS in multiple sclerosis has been established in recent years. This so-called gut–CNS axis can be shaped by dietary factors, either directly or via indirect modulation of the gut microbiome and its metabolites. Here, we report that dietary supplementation with conjugated linoleic acid, a mixture of linoleic acid isomers, ameliorates CNS autoimmunity in a spontaneous mouse model of multiple sclerosis, accompanied by an attenuation of intestinal barrier dysfunction and inflammation as well as an increase in intestinal myeloid-derived suppressor-like cells. Protective effects of dietary supplementation with conjugated linoleic acid were not abrogated upon microbiota eradication, indicating that the microbiome is dispensable for these conjugated linoleic acid-mediated effects. Instead, we observed a range of direct anti-inflammatory effects of conjugated linoleic acid on murine myeloid cells including an enhanced IL10 production and the capacity to suppress T-cell proliferation. Finally, in a human pilot study in patients with multiple sclerosis (n = 15, under first-line disease-modifying treatment), dietary conjugated linoleic acid-supplementation for 6 months significantly enhanced the anti-inflammatory profiles as well as functional signatures of circulating myeloid cells. Together, our results identify conjugated linoleic acid as a potent modulator of the gut–CNS axis by targeting myeloid cells in the intestine, which in turn control encephalitogenic T-cell responses.
Keywords: conjugated linoleic acid, multiple sclerosis, gut–CNS axis, dietary supplementation, intestinal inflammation
Fleck et al. report that dietary supplementation with conjugated linoleic acid elicits anti-inflammatory and suppressive myeloid cell responses in the intestine, resulting in amelioration of CNS autoimmunity in mice and a beneficial modulation of myeloid cell responses in patients with relapsing-remitting multiple sclerosis.
Introduction
Multiple sclerosis is a chronic inflammatory disease of the CNS affecting more than 2.3 million individuals worldwide. The aetiology of multiple sclerosis largely remains enigmatic and seems to involve multiple factors, including both genetic and environmental influences. Mainly on the basis of epidemiological studies, several environmental factors have been implicated in disease pathogenesis, such as low vitamin D status, certain viral infections (e.g. Epstein-Barr virus), childhood obesity, and dietary habits such as a so-called Western diet characterized by high levels of saturated fatty acids, carbohydrates, sodium chloride and low fibre intake.1–4 On the other hand, several nutrients have been associated with CNS autoimmune disease amelioration, including polyunsaturated fatty acids, vitamin D supplementation, probiotics and microbial metabolites, such as propionic acid.4–8
Albeit the concept of dietary modulation of autoimmunity itself is not new, its mechanistic basis has been reshaped in recent years due to a growing understanding of the close and reciprocal interaction between nutritional factors, the gut microbiome and the intestinal immune system.9–13 Here, the nutritional composition has a direct impact on gut microbiome composition, which shapes local intestinal immune responses and in turn affects even distant (auto)immune responses. One prominent example highlighting the key role of this gut–CNS axis in the context of CNS autoimmunity is the absence of CNS inflammation in a spontaneous model of experimental autoimmune encephalomyelitis (EAE) under germ-free conditions. This suggests that the protective effect is due to the absence of certain commensal bacteria.14 Notably, several dietary factors have already been shown to shape the gut microbiome, thereby modulating experimental CNS autoimmunity.15,16 By contrast, it has been demonstrated that the gut microbiota from patients with multiple sclerosis facilitates the development of EAE in mice, suggesting that microbiome alterations may indeed be linked to disease (re)occurrence.17,18
However, in spite of these conceptual advances in our understanding of the gut–CNS axis and its influence on CNS autoimmunity, so far it has not been convincingly demonstrated that targeted modulation of nutritional factors in humans shapes ongoing CNS autoimmunity. Several smaller clinical trials dealing with specific dietary interventions in multiple sclerosis have been performed, but with inconclusive results.8,19 This is most likely due to serious constraints especially in study designs caused by lack of proper control groups, small patient numbers, imprecise inclusion criteria, and lack of MRI-based end points.8,19 Additionally, several studies have described alterations in the gut microbiome composition in multiple sclerosis cohorts; however, the results of the individual studies were highly variable and to date no causal link between microbiome alterations and disease manifestation has been established in humans.20–22 Furthermore, some dietary interventions yielded diverging effects in mice and humans, such as changes to dietary salt intake.16,23 Although of conceptual importance, other approaches might not be feasible in patients, such as dietary deprivation of the essential amino acid tryptophan, which impaired encephalitogenic T-cell responses via modulation of the gut microbiome in an experimental model.15
One intervention with considerable potential for disease modification in the context of autoimmune diseases is dietary supplementation with conjugated linoleic acid (CLA), a naturally occurring fatty acid in meat and dairy products of ruminants. CLA has been shown to confer protection in various animal models of inflammatory bowel disease24–26 and to beneficially modulate immune responses in Crohn’s disease patients.27,28 The latter effect is particularly interesting in light of the acknowledged role of the gut microbiome in shaping local as well as peripheral inflammatory responses in these diseases.27–31 Mechanistically, these anti-inflammatory effects of CLA and its metabolites have been at least partly linked to activation of the nuclear receptors PPARα, PPARγ and PPARδ in immune cells.32 Furthermore, in atherosclerosis, the anti-inflammatory capacity of CLA supplementation is mediated by the induction of the IL10 signalling pathway in monocytes.33
In the present study, we therefore aimed to evaluate the potential of dietary CLA supplementation to modulate the disease outcome in a spontaneous mouse model of CNS autoimmunity. Moreover, we performed a proof-of-concept study in patients with relapsing-remitting multiple sclerosis (RRMS), who received dietary supplementation with CLA for 6 months.
Materials and methods
Mice
All strains were bred on a C57BL/6 background. Double transgenic opticospinal encephalomyelitis (OSE) animals (×TCRMOG)34 were generated by crossbreeding transgenic mice carrying either myelin oligodendrocyte glycoprotein (MOG)-specific B cell receptor (IgHMOG knock-in, previously known as Th mouse)35 or MOG35–55-specific T-cell receptor (TCRMOG knock-in, previously known as 2D2 mouse),36 originally purchased from Jackson Laboratory. C57BL/6J mice for in vitro set-ups were purchased from Janvier Labs or Charles River. All mice were maintained in individually ventilated cages at the local animal facility of the Westfälische Wilhelms-Universität Münster and were used for experiments up to the age of 14 weeks independent of their sex or body weight. All animal studies were performed according to the guidelines of the animal ethics committee (German Animal Welfare Act) and were approved by the governmental authorities Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (LANUV), Recklinghausen].
Control and conjugated linoleic acid-rich chow
OSE mating pairs and offspring were fed as indicated in Fig. 1A with either C1000 control chow (Altromin Spezialfutter) or CLA-rich chow (processed by Altromin Spezialfutter; 0.9% CLA; isomers cis-9, trans-11 and trans-10, cis-12), which is based on C1000 chow where 11.1 g sunflower oil was exchanged with Tonalin® TG80 (supplied by BASF/BTC-Europe GmbH) per 1 kg chow. To prevent oxidation of CLA the chow was vacuum-packed for long-term storage and kept under a nitrogen atmosphere for daily usage.
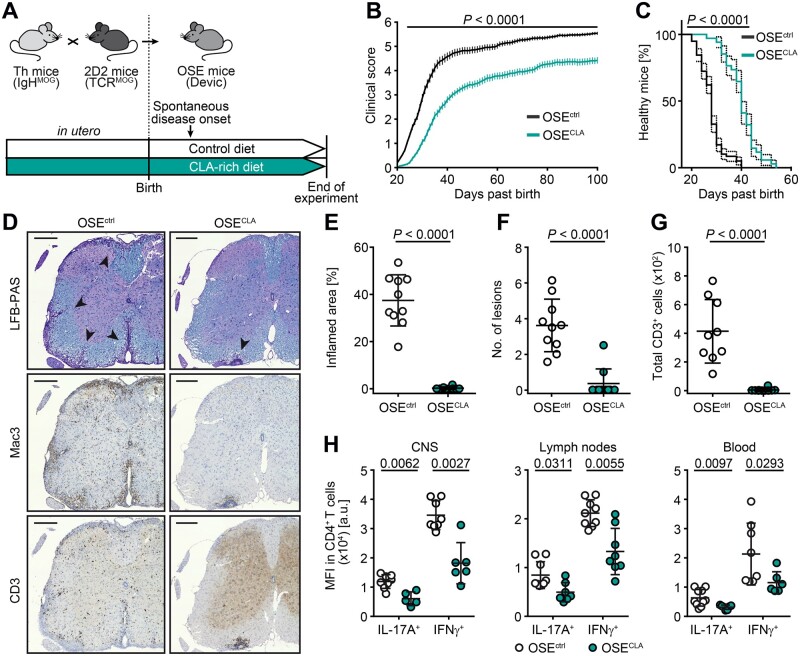
Figure 1.
Dietary supplementation with CLA ameliorates spontaneous CNS autoimmunity. (A–E) Breeding pairs and OSE offspring were fed with CLA-enriched or control (C1000) chow ad libitum. (B and C) Clinical scores are shown over 100 days from OSEctrl (n = 58) and OSECLA mice (n = 34). (B) Disease courses are illustrated as mean group score over time ± SEM. (C) Disease onset (score ≥ 1) is shown as percentage of healthy mice. (D–G) Immune cell infiltration within spinal cords of OSEctrl (n ≥ 9) and OSECLA mice (n = 10) was assessed via histology. (D) Representative spinal cord stainings, percentages of inflamed area of total white matter marked by (E) Mac3+ infiltrates, (F) total counts of Mac3+ lesions, and (G) CD3+ cells are shown. (H) CD4+ T cells derived from the CNS, inguinal lymph nodes or blood of OSEctrl (n ≥ 8) and OSECLA mice (n ≥ 5) were restimulated for cytokine analysis. Dot plots depict representative mean fluorescence intensity (MFI) of cytokine expression shown as mean ± SD, whereas each dot represents one individual mouse. Statistics: (B) two-way ANOVA with Bonferroni correction; (C) log-ranked Mantel-Cox test (dotted lines indicate 95% confidence interval); (E–H) Mann-Whitney U-test. Scale bars = 200 µm.
Treatment with antibiotics
After weaning, OSE mice were treated with antibiotics [1 mg/ml ampicillin (aniMedica), 1 mg/ml colistin (aniMedica), 5 mg/ml streptomycin (Sigma-Aldrich)] over a period of 3 days via drinking water.
Disease scoring
Beginning at 20 days of age, mice were clinically scored on a daily basis until Day 100 past birth. Disease severity was determined using a scale from 0 to 8 as described in Klotz et al.37: 0, healthy; 1, slight waddling; 2, weak unilateral paresis of hind limbs; 3, severe unilateral or weak bilateral paresis of hind limbs; 4, moderate bilateral paresis of hind limbs; 5, complete paralysis of hind limbs; 6, plegia of hind limbs; 7, quadriplegia; and 8, death. In accordance with animal welfare requirements, mice were sacrificed when a score of 6 was reached to minimize the extent of burden.
Histology and immunohistochemistry
Mice were sacrificed under deep anaesthesia by intracardiac perfusion with PBS followed by perfusion with 4% (w/v) paraformaldehyde (PFA) dissolved in PBS. Organs were removed and fixed in 4% PFA overnight; except the small intestine, which was flushed with PBS, opened longitudinally, and rolled up starting with the distal part before fixation (Swiss-rolling technique). Prior to embedding in paraffin, the spinal cord was cut into 7–10 transverse segments (3-mm thick) and coronal brain cuts were made. Sections (3 µm) were stained by haematoxylin and eosin and Luxol fast blue including periodic acid-Schiff (LFB-PAS). Immunohistochemistry was performed using a biotin-streptavidin peroxidase technique (Dako) and an automated immunostainer (AutostainerLink 48, Dako). Sections were pretreated with citrate buffer (pH 6 or 9) in a steamer. The primary antibodies were specific to Mac3 (clone M3/84, also known as CD107b or LAMP-2; BD Pharmingen) or CD3 (MCA, Serotec). 3,3′-diaminobenzidine (Dako) was used as a colour substrate and sections were mounted with Eukitt® mounting medium (O. Kindler GmbH) after dehydration. Images were acquired with the microscope AxioObserver (Carl Zeiss) or BioRevo BZ-9000 (Keyence) using the software AxioVision or BZ-II Analyzer (Keyence).
Histopathological scoring of intestinal tissue slices
Scoring of haematoxylin and eosin-stained small intestinal tissue slices was performed in a blinded fashion adapted from previous publications.15,38 Epithelial changes and intestinal architecture, i.e. hyperplasia and mucosal follicle formation, and the extent of inflammatory cell infiltration in the lamina propria or submucosa were rated from 0 (none) to 5 (extensive pathological changes).
Isolation of murine mononuclear cells
Mononuclear cells were isolated from CNS, blood, bone marrow, and inguinal lymph nodes as previously described.39 After the removal of Peyer’s patches, lymphocytes were isolated from the intestinal lamina propria according to the manufacturer’s instructions for the Lamina Propria Dissociation Kit (Miltenyi). For flow cytometric measurement of intracellular cytokines, isolated immune cells were stimulated with phorbol myristate acetate (PMA; 5 ng/ml, Sigma-Aldrich), ionomycin (200 ng/ml, Cayman Chemical), and GolgiPlugTM (1 μl/ml, BD Pharmingen) for 4 h at 37°C.
Flow cytometry of murine immune cells
All antibodies, if not otherwise stated were obtained from BioLegend. Surface marker staining using antibodies against murine CD3 (clone: 17A2), CD4 (clone: GK1.5), CD11b (clone: M1/70), CD45 (clone: 30-F11), Ly6G (clone: 1A8), IL17A (clone: TC11-18H10.1) and IFNγ (clone: XMG1.2) was performed as previously described.40 Intracellular staining was performed with BD Cytofix/CytoperTM Fixation/Permeabilization Solution Kit (BD Bioscience) according to the manufacturer’s protocol. The acquisition was performed with the flow cytometer Gallios (Beckman Coulter). Results were analysed with FlowJo software (BD Life Science). To estimate a population’s mean fluorescence intensity (MFI), its geometric mean was used.
Immunoglobulin M ELISA of faecal lysates
To quantify the amount of total protein and concentration of immunoglobulin M (IgM) in faecal lysates, faeces of OSE mice were collected on consecutive days and stored at −20°C prior to extraction. Faecal samples were dissolved in 500 µl PBS containing 0.1% Tween. After centrifugation, total protein content was measured in the supernatant by the colourimetric DC Protein Assay Kit (Bio-Rad) with bovine serum albumin as standard. The concentration of IgM in faecal lysates was assessed with the Ready-Set-Go! ELISA Kit (Invitrogen eBioscience) according to the manufacturer’s instructions.
Microbiome composition analysis
Faecal samples were collected from the caecum of OSE mice and ∼200 µg of faeces were used for DNA extraction via ZymoBIOMICSTM DNA Miniprep Kit (Zymo Research). The 16S rDNA gene variable region V3 of the extracted faecal DNA was amplified and sequenced with the MiSeq Reagent Kit v2 (Illumina) at the Institute for Hygiene, Münster. The Illumina reads obtained were analysed with the mother software package (v1.40.5)41 following the standard operating procedure for 16S rDNA gene sequence data analysis.42 In brief, read pairs were assembled into contigs, and sequences with ambiguous bases (NS) and/or inadequate assembling were removed. After merging duplicate reads, unique sequences were aligned to the SILVA reference database (SSU_Ref database v.132).43 Chimeric and erroneously amplified sequences were removed from the analysis. Bacterial sequences were then classified into operational taxonomic units; the relative abundance and Bray-Curtis distance-based principal coordinates analysis were visualized with phyloseq (v1.20.0) R package.44
CLA-treatment of murine myeloid cells in vitro
For in vitro experiments, murine myeloid cells were treated without and with CLA-mix containing 50 µM cis-9, trans-11 CLA-isomer and 50 µM trans-10, cis-12 CLA-isomer (Sigma-Aldrich).
RT2-PCR-Profiler PCR Array
CD11b+ cells were isolated from spleens of C57BL/6 mice and incubated in the presence or absence of CLA-mix for 24 h. RNA was isolated using the RNeasy® Micro Kit (Qiagen) and cDNA synthesis was performed from 500 ng total RNA using RT2 FirstStrand Kit (Qiagen). The RT2 Profiler PCR Array Mouse Inflammatory Response and Autoimmunity (#PAMM-077Z; Qiagen) was conducted according to the manufacturer’s protocol.
Analysis of myeloid cell responses in vitro
Bone marrow-derived macrophages (BMMs) were generated as previously described45 and treated with CLA-mix during a differentiation period of 7 days. Cytokine concentrations were measured in the supernatant of mature BMMs after 72 h (IFNγ and CCL2) and 96 h (IL10) using Ready-Set-Go! ELISA Kits (eBioscience) according to the manufacturer’s instructions. For analysis of intracellular glutathione levels, BMMs were stained with monochlorobimane (mBCI, 50 μM; Invitrogen) at 37°C for 30 min. Intracellular reactive oxygen species production was measured in living BMMs using dihydroethidium fluorescence measurements according to a protocol outlined in Kovac et al.46 and images were subsequently analysed using MetaFluor® Fluorescence Ratio Imaging Software (Molecular Devices, LLC). To measure the metabolic activity, mature BMMs were harvested, seeded to poly-d-lysine-coated Seahorse plates, and incubated overnight at 37°C. As described in Klotz et al.47 oxygen consumption rate was determined with a Seahorse XFp Extracellular Flux Analyzer (Agilent Technologies) and data were analysed with Wave Software (Agilent Technologies).
Suppression assay
The suppression assay was performed according to Hucke et al.48 Briefly, splenic T cells were isolated by nylon wool enrichment, labelled with 5 μM eFluorTM 670 (eBioscience), and subsequently co-cultured with BMMs (ratio 2:1) in the presence of 200 ng/ml soluble anti-CD3 antibody (eBioscience, Clone: 145-2C11). After 72 h, CD4+ T-cell proliferation was assessed by flow cytometric evaluation of MFI of eFluorTM 670.
Study approval
Blood sampling of treatment-naïve RRMS patients (diagnosed according to the current McDonald criteria) was approved by the local ethics committee of the Westfälische Wilhelms-Universität Münster (2010-262-f-S). The study protocol for the proof-of-concept study CLAiMS was approved by the ethics committee of the Ärztekammer Westfalen-Lippe and the Westfälische Wilhelms-Universität Münster (2016-053-f-S, approved on 05.04.2016). All participants provided written informed consent for the planned procedures prior to entry into the study, in accordance with the Declaration of Helsinki.
Blood sampling and generation of peripheral blood mononuclear cells
Around 40 ml blood was obtained from treatment-naïve RRMS patients or patients participating in the proof-of-concept trial CLAiMS via vein puncture and collected into S-Monovette® syringes containing EDTA (Sarstedt). Peripheral blood mononuclear cells (PBMCs) were isolated immediately from the fresh blood by LymphoprepTM (Stemcell Technologies) density gradient centrifugation as described in Klotz et al.47
CLAiMS proof-of-concept trial
Patients were included in the clinical study at the Department of Neurology with the Institute of Translational Neurology at University Hospital Münster according to the inclusion and exclusion criteria listed in Supplementary Table 1. The study cohort consisted of 15 RRMS patients meeting the current McDonald 2010 criteria. Only patients without relapses within the last 3 months and a stable first-line immunomodulatory medication for at least 3 months were included. Study subjects were predominantly female (73.3%), aged on average 36.7 years, and had a mean baseline EDSS score of 0.8. Demographics for all patients from our clinical trial including baseline disease characteristics are described in Supplementary Table 2. Daily dietary supplementation with 2.1 g CLA was administered orally via capsules. To validate the results of the study, the participants’ adherence to study medication was monitored with intake diaries, which were counterchecked against the returned emptied blister packages. Based on these data, the actual number of capsules ingested was captured and compared to the prescribed number of CLA capsules according to the study protocol to calculate the patients’ compliance rate. For data analysis, all patients were included.
Flow cytometry of human peripheral blood mononuclear cells
PBMCs were analysed by multicolour flow cytometry and monocyte subsets were identified using the following markers as defined by Mukherjee et al.49 classical: HLA-DR+ LinX (CD3, CD19, CD66b, CD56)− CD14+ CD16−; intermediate: HLA-DR+ LinX (CD3, CD19, CD66b, CD56)− CD14+ CD16+ and non-classical: HLA-DR+ LinX (CD3, CD19, CD66b, CD56)− CD14dim CD16+. PBMC were stained with the following antibodies together with True Stain Monocyte BlockerTM (BioLegend) according to the manufacturer’s instructions. All antibodies, if not otherwise stated were obtained from BioLegend. Surface marker staining using antibodies against human CD68 (clone: Y1/82A), CD14 (clone: M5E2), CD3 (clone: HIT3a), CD19 (clone: HIB19), CD56 (clone: HCD56), CD66b (clone: G10F5), HLA-DR (clone: L243), CD16 (clone: 3G8, Beckman Coulter) was performed as described previously. For intracellular staining of IFNγ (clone: 4S.B3), S100A9 (Calgranulin B, clone: 27 E10, ImmunoTools), IL1b (clone: H1b-98), IL6 (clone: MQ2-13A5) and MCP-1 (CCL2, clone: 2H5), PBMC were either left unstimulated or stimulated with 100 ng/ml lipopolysaccharide from Escherichia coli O127: B8 (Sigma-Aldrich, Cat. No: L4516) for 2 h. For this staining, the Transcription Factor Staining Buffer Set (eBioscience) was used according to the manufacturer’s instructions. Dead cells were discriminated using Zombie NIRTM (BioLegend). Flow cytometric measurement was performed with Navios or CytoFLEX (both Beckman Coulter). Data were analysed using Kaluza software (Beckman Coulter). In addition, unbiased data analysis was performed by a dimensionality reduction approach, as described previously.50 Flow cytometry single-cell data were processed by Barnes-Hut stochastic neighbour embedding (known as bh-SNE) dimensionality reduction in t-distributed stochastic neighbour embedding-based visualization (viSNE), an implementation in MATLAB. The phenograph algorithm was applied to identify local phenotypic similarities.51
Metabolic assays of human monocytes
CD14+ monocytes were isolated from frozen PBMCs of RRMS patients in the CLAiMS trial. Overnight resting and oxygen consumption rate measurement were performed as described above for murine cells, but assessment was perfomed with the Seahorse XFe96 Extracellular Flux Analyzer (Agilent Technologies).
Gene expression analysis with nanoString
CD14+ monocytes were isolated from frozen PBMCs of either naive RRMS patients or patients in the CLAiMS trial, whereas only monocytes from naive RRMS patients were incubated in the presence or absence of CLA-mix (75 µM per isomer) for 24 h. RNA was isolated using RNeasy® Plus Micro Kit (Qiagen) according to the manufacturer’s instructions. Measurement of the nCounter® Human Myeloid Innate Immunity Panel was performed with the nCounter® FLEX Dx (NanoString Technologies) at the Division of Translational Pathology, Münster. Quality control, normalization, and differential expression analysis were performed with nSolver Analysis Software 4.0 (Advanced Analysis Tool). Undetectable genes were excluded from further analysis. Adjustment of P-values was performed according to the Benjamini-Yekutieli correction.
Statistical analysis
GraphPad Prism 6 software and R version 3.6.2 were used to perform statistical analysis and visualize the results. All results illustrated in dot plots are shown as the mean ± standard deviation unless stated otherwise. Box-and-whisker plots display the median, interquartile interval of 95% (box) as well as minimum and maximum (whiskers). Unpaired, two-sided Mann-Whitney U-tests were used as non-parametric tests to compare two groups. Paired, two-sided Student’s t-tests were used for in vitro experiments and the CLAiMS study. For the statistical calculation of disease courses, two-way ANOVA with Bonferroni correction was performed and for disease onset the log-ranked Mantel-Cox test with 95% confidence intervals. P-values < 0.05 were considered to be statistically significant.
Data availability
Data and full code of all scripts are available upon reasonable request from the corresponding author. There is no restriction on the availability of materials described in the study.
Results
Dietary CLA supplementation ameliorates disease course in OSE mice
To assess the influence of dietary CLA supplementation on the onset and disease course of spontaneous CNS autoimmunity, we used a double transgenic mouse model, where mice harbour MOG-specific T-cell receptor (TCR) transgenic T cells as well as MOG-specific immunoglobulin heavy-chain transgenic B cells on a C57BL/6 background [×IgHMOG, for simplicity referred to as OSE (opticospinal encephalomyelitis) mice; Fig. 1A and Supplementary Fig. 1A]. Because of the spontaneous occurrence of disease and independence from any exogenous triggers, this model is particularly suitable for analysis of modifying factors of disease induction.34,52 Strikingly, continuous dietary supplementation with CLA starting at the time of conception (Fig. 1A), resulted in a profoundly ameliorated disease course in mice that received CLA-enriched chow (OSECLA mice, n = 34) over the whole observation period of 100 days, when compared to mice fed with control chow (OSEctrl mice, n = 58) (P < 0.0001; Fig. 1B). Furthermore, OSECLA mice exhibited a significant delay in disease onset (median onset on Day 30 in OSECLA mice as compared to Day 24 in OSEctrl mice; Fig. 1C). This disease amelioration was accompanied by a highly significant reduction in immune cell infiltrates within the spinal cords of OSECLA mice as illustrated by detailed immune cell quantification of histological specimens from OSECLA mice compared to OSEctrl mice (Fig. 1D–G). Analysis of peripheral as well as CNS-located pro-inflammatory CD4+ T-cell responses in OSECLA mice compared to age-matched OSEctrl mice in the early phase of the disease (i.e. 6 weeks after birth) by flow cytometry revealed that IL17A, as well as IFNγ production, were significantly diminished in OSECLA mice (Fig. 1H). Together, our data illustrate that continuous dietary supplementation with CLA ameliorates the disease course of spontaneous CNS autoimmunity and inhibits both peripheral and CNS-specific pro-inflammatory CD4+ T-cell responses.
Dietary CLA supplementation decreases intestinal inflammation in context of CNS autoimmunity
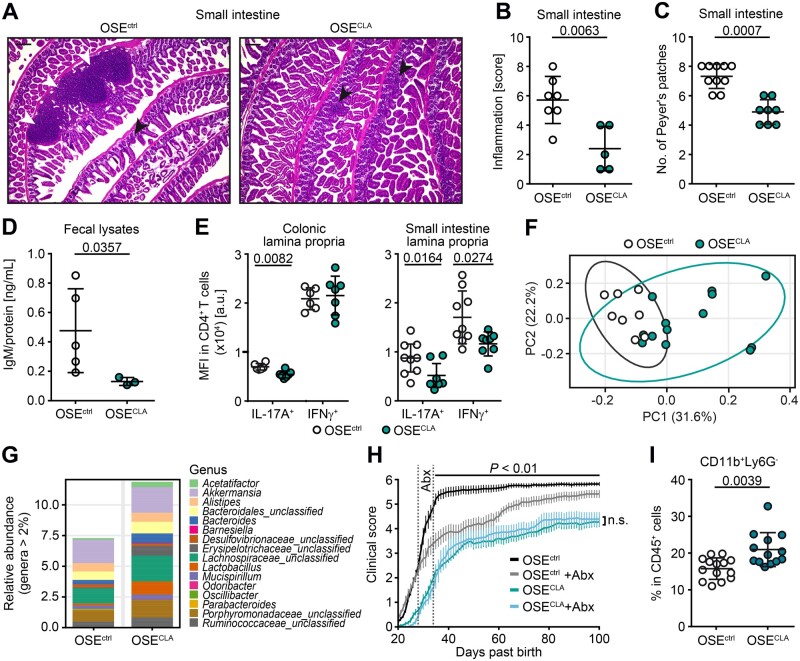
As dietary modifications have a profound impact on the intestinal immune system, and since organ-specific autoimmune diseases including CNS autoimmunity are affected by intestinal immune responses,14 we next evaluated the impact of dietary CLA supplementation on the gut. As described in other mouse models of CNS autoimmunity,14,53 diseased OSEctrl mice displayed notable immune cell infiltrations in the intestinal lamina propria; however, this was diminished in OSECLA mice (Fig. 2A). Quantification of intestinal inflammation (Fig. 2B and Supplementary Fig. 1B) and Peyer’s patches (Fig. 2C) in the small intestine revealed a significant reduction in OSECLA mice. Enhanced immune cell infiltration is often associated with altered intestinal barrier function, which is accompanied by enhanced levels of the surrogate marker IgM in the faeces.54 Indeed, intestinal barrier dysfunction was significantly reduced in OSECLA mice compared to OSEctrl mice at the age of 5–10 weeks (Fig. 2D). Additionally, we observed reduced pro-inflammatory T-cell responses as illustrated by flow cytometric analysis of IL17A and IFNγ expression by CD4+ T cells isolated from the lamina propria of OSECLA versus OSEctrl mice (Fig. 2E). Analysis of the gut microbiome in CLA-supplemented mice revealed profound changes in overall microbiome composition, as illustrated by principal component analysis and relative abundance of genera (Fig. 2F and G). To investigate whether CLA-mediated effects on EAE disease course depend on the gut microbiome, we temporarily eradicated the gut microbiome by short-term antibiotic treatment of OSECLA and OSEctrl mice for 3 days after weaning (Days 28–34 after birth).55,56 As expected, ablation of the microbiome by short-term antibiotic treatment alone resulted in a temporary disease amelioration in OSEctrl mice55; however, treatment with antibiotics did not abrogate beneficial CLA-mediated effects on EAE disease course (Fig. 2H). In line with the emerging role of myeloid-derived suppressor cells (MDSCs) for control of chronic intestinal inflammation, we evaluated frequencies of MDSC-like cells (CD11b+ Ly6G−) and observed a significant increase in the intestinal lamina propria of OSECLA compared to OSEctrl (Fig. 2I and Supplementary Fig. 1C).57
Figure 2.
CLA supplementation reduces intestinal inflammation in OSE mice. (A) Haematoxylin and eosin (HE)-stained small intestine tissue slices of representative OSEctrl and OSECLA mice (black arrows = mucosal inflammatory cell infiltrates; white arrows = submucosal inflammation/expanding lymphoid follicles). (B) Histopathological intestinal inflammation score of the small intestine was assessed for OSEctrl (n = 7) and OSECLA mice (n = 5). (C) Total counts of Peyer’s patches in the small intestine of OSEctrl (n = 10) and OSECLA mice (n = 8) as well as (D) concentrations of faecal immunoglobulin M (IgM) normalized to total protein amount in OSEctrl (n = 5) and OSECLA mice (n = 3) are shown. (E) Representative MFI of cytokine expression in intestinal CD4+ T cells is illustrated for age-matched OSEctrl (n ≥ 6) and OSECLA mice (n ≥ 7). (F and G) Analysis of the gut microbiome of OSEctrl (n = 8) and OSECLA mice (n = 13) is shown as (F) PCA and (G) bar graph of the relative abundance at the genus level. (H) Short-term treatment with antibiotics (Abx) has been applied to OSEctrl + Abx (n = 9) and OSECLA +Abx mice (n = 9). The clinical scores of these mice and their non-antibiotic-treated controls (OSEctrln = 17; OSECLAn = 15) are illustrated as mean group scores over time ± SEM. (I) Frequency of colonic MDSC-like cells (CD11b+ Ly6G−) in OSEctrl (n = 14) versus OSECLA mice (n = 13) was determined via flow cytometry. Scatter dot plots depict mean ± SD, whereas each dot represents one individual mouse. Statistics: (H) two-way ANOVA with Bonferroni correction; (B–E and I) Mann-Whitney U-test. Scale bars = 200 µm; n.s. = not significant.
Taken together, our data illustrate that dietary supplementation with CLA modulates intestinal immune responses with a downmodulation of pro-inflammatory myeloid cells and a concomitant increase in MDSC-like cells. We therefore wondered whether intestinal myeloid cells might be a key player in mediating the beneficial CLA effect on systemic immune responses.
CLA induces anti-inflammatory and suppressive phenotype in myeloid cells in vitro
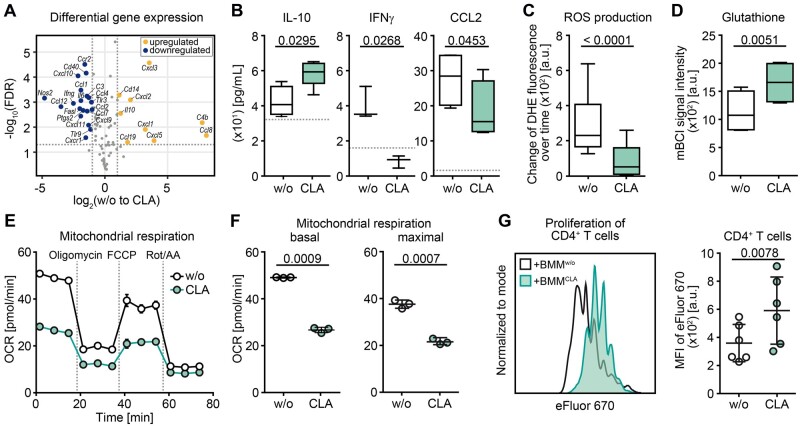
Based on these findings we evaluated whether CLA might directly enhance anti-inflammatory and suppressive properties of myeloid cells. First, we determined CLA-induced changes in pro- and anti-inflammatory gene expression patterns in unstimulated isolated murine splenic myeloid cells by RT2 profiler array and observed differential gene expression upon CLA exposure (50 µM cis-9, trans-11 and 50 µM trans-10, cis-12-isomer) as depicted in Fig. 3A. Downregulated genes comprised pro-inflammatory markers, such as Nos2 (iNos), Ccr2 (Cd192), Ifng and Il6, whereas significantly upregulated genes included the anti-inflammatory cytokine Il10 (Fig. 3A). Accordingly, quantification of key cytokines and chemokines in supernatants of CLA-exposed unstimulated bone-marrow-derived myeloid cells (BMM) revealed that IL10 secretion was significantly enhanced upon CLA exposure, whereas secretion of IFNγ and CCL2 (MCP-1) was significantly reduced (Fig. 3B). Moreover, CLA altered the oxidative capacities of myeloid cells, as the production rate of reactive oxygen species was significantly reduced by CLA treatment, whereas levels of the reactive oxygen species scavenger glutathione were increased (Fig. 3C and D). Analysis of the mitochondrial respiratory capacity of myeloid cells by real-time measurement of the oxygen consumption rate revealed a highly significant reduction of both basal as well as maximal respiration by CLA (Fig. 3E and F). Overall, these data indicate that CLA inhibited a range of pro-inflammatory functions of myeloid cells and enhanced the production of the anti-inflammatory cytokine IL10, which all represent common features of MDSC-like cells.57
Figure 3.
CLA treatment enhances anti-inflammatory and suppressive features of myeloid cells in vitro. (A) Differential expression analysis of immune response-related genes via RT2 Profiler array is illustrated as a volcano plot of the false discovery rate (FDR) corrected P-values against fold change of untreated versus CLA-treated (50 µM of each isomer = CLA-mix) splenic CD11b+ (n = 4; blue = downregulated; yellow = upregulated). (B–G) BMMs were differentiated without or with 50 µM CLA-mix over 7 days. At least three independent experiments were performed for each readout. (B) Concentrations of secreted cytokines in cell supernatants were assessed by ELISA. Box plots display the median, interquartile interval of 95% (box) as well as minimum and maximum (whiskers) of pooled data (n ≥ 3 per group). Dotted lines indicate the detection limit. Characterization of (C) reactive oxygen species-production rate (DHE = dihydroethidium, n = 6) and (D) glutathione levels (mBCI = monochloromobimane, n = 4) in BMMs are shown. (E and F) Real-time measurement of oxygen consumption rate (OCR) in BMMs is shown as (E) graph of one example oxygen consumption rate measurement as well as (F) dot plots of basal and maximal respiration of one example experiment out of three independent experiments with in total six mice, whereas one dot represents one technical replicate. (G) Suppression assay with anti-CD3-activated splenic CD4+ T cells and differentiated BMMs was performed with or without CLA treatment (ratio 2:1). Left: Example MFI profile of eFluorTM 670 in CD4+ T cells of one experiment is shown. Right: Dot plot illustrates pooled data of MFI of CD4+ T cells, whereas each dot represents the mean of three technical replicates from BMMs generated from one individual mouse. Dot plots depict mean ± SD. Statistics: paired two-tailed Student’s t-test.
We therefore set out to determine whether CLA-exposed myeloid cells might function as bona fide MDSCs and evaluated their capacity to control the proliferation of activated pro-inflammatory CD4+ T cells. Indeed, bone marrow-derived myeloid cells exposed to CLA during differentiation for 7 days significantly limited proliferation of stimulated CD4+ T cells in a classical suppression assay (Fig. 3G). Taken together, we were able to demonstrate that CLA exerts direct effects on myeloid cells, limiting their pro-inflammatory capacities and promoting suppressive functions of these cells.
Effects of dietary CLA supplementation on human myeloid cells in RRMS: proof of concept
Based on our data generated in the murine system, we next aimed to address whether CLA might also exert anti-inflammatory effects on human myeloid cells. In a first step, we evaluated the direct effects of CLA in vitro on transcriptional signatures of CD14+ monocytes derived from untreated RRMS patients by NanoString-based transcriptional profiling. As depicted in Fig. 4A in a heat map, the analysis revealed a striking change in transcriptional signatures of CLA-exposed patient-derived monocytes as compared to their untreated counterparts. In particular, CLA exposure elicited a transcriptional signature known to be associated with a myeloid suppressive phenotype, i.e. downregulation of genes involved in T-cell activation, antigen presentation, interferon- and TNF-signalling as well as genes for pro-inflammatory cytokines and chemokines [namely CCL3 (MIP1A), CXCL10 (IP-10), IL1B, IL18], whereas anti-inflammatory genes [IL4I1 (FIG1), MMP9, PPARG] were upregulated.57–60 Furthermore, differential expression was observed in genes known to be related to proliferation or development of MDSC-like cells, such as CEBPB, FABP4, IRF4, IRF8, and JAK/STAT-signalling related genes57–61 (Supplementary Fig. 2 and Supplementary Table 3).
Figure 4.
Dietary CLA supplementation in multiple sclerosis patients alters blood myeloid cell composition by enhancing anti-inflammatory and suppressive subsets and functional properties. (A) CD14+ monocytes were isolated from frozen PBMC of RRMS patients and cultured in the presence or absence of 75 µM CLA-mix for 24 h (n = 3). Transcriptional signatures were analysed using the nCounter® Myeloid Innate Immunity Gene Expression Panel (NanoString) and significant differentially expressed genes (P-value < 0.05 and adjusted P-value < 0.5) are illustrated as a heat map. (B) Study design of proof-of-concept trial CLAiMS is shown. (C–G) Frozen PBMC of study subjects at baseline (BL) and 6 months (6M) after CLA supplementation as depicted in B, were stained with monocyte-specific antibodies and analysed by multi-colour flow cytometry. (C) Percentages of CD14+/dim CD16+ (intermediate and non-classical) and CD14+ CD16− (classical) monocytes were evaluated by manual gating (n = 15). (D) Unbiased cluster analysis of monocyte panels is shown as bh-SNE plot of unstimulated monocytes (left) and LPS-stimulated monocytes (right) (n = 12) comparing baseline and 6 months of CLA supplementation. Significantly upregulated clusters are depicted in orange and downregulated clusters are displayed in blue. Clusters with a high (continuous line) and low (dotted line) cytokine expression profile (CCL2, IFNγ, IL1β, IL6, IL8) are highlighted. (E) MFI of pro-inflammatory cytokines in unstimulated CD14+ monocytes, and (F) MFI of CD68 and S100A9 expression in CD14+ CD16− (classical) monocytes were evaluated by manual gating (n = 12). (G) MFI of S100A9 expression in HLA-−CD14+ cells (MDSC-like phenotype) (n = 12) is shown. (H) Transcriptional signatures of CD14+ monocytes isolated at baseline and after 6 months of CLA supplementation were analysed by the nCounter® Myeloid Innate Immunity Gene Expression Panel (NanoString) and illustrated as a heat map (n = 12, P-value < 0.05). (I) Oxygen consumption rate of CD14+ monocytes isolated at BL and after 6M of CLA supplementation is shown for basal and maximal respiration (n ≥ 10). Dot plots depict mean ± SD. Each dot represents one individual patient at baseline or 6 months; in E–G only, dots belonging to the same patient are connected by a line. Statistics: paired two-tailed Student’s t-test; reg. = regulated.
This encouraged us to perform a small open-label proof-of-concept study in 15 stable RRMS patients to investigate the immunomodulatory effects of standardized dietary CLA supplementation as an add-on to their first-line disease-modifying treatment for 6 months (study design in Fig. 4B). Supplementation with CLA was well tolerated without any significant side effects, and the compliance rate was above 93% (Supplementary Table 4). Multi-parameter flow cytometry analysis of the patients’ PBMCs revealed a clear impact of CLA supplementation on myeloid cell subsets. Specifically, we found a downmodulation of CD14+ CD16+ non-classical as well as intermediate monocytes at 6 months of CLA supplementation as compared to baseline and a reciprocal increase in CD14+ CD16− classical monocytes (Fig. 4C). A detailed analysis of monocyte signatures by an unbiased single-cell dimensionality reduction approach confirmed that inflammatory cytokine-producing monocyte clusters were already significantly downregulated in an unstimulated state after 6 months of CLA supplementation (Fig. 4D). Upon monocyte stimulation, we found significantly upregulated clusters lacking pro-inflammatory signatures on CLA supplementation (Fig. 4D). Notably, evaluation of cytokine and chemokine production of unstimulated CD14+ monocytes by conventional gating revealed a significant downregulation of several key pro-inflammatory mediators on CLA supplementation, including IFNγ, IL1β, IL6 and CCL2 (Fig. 4E). Furthermore, the pro-inflammatory marker CD68 was significantly downregulated on CD14+ monocyte subsets upon CLA supplementation,62 whereas S100A9, which is associated with MDSC differentiation, was upregulated (Fig. 4F and Supplementary Fig. 3).63 Interestingly, a subset of MDSC-like cells (characterized by HLA-DRlow/– CD14+) also showed a highly significant increase in the expression of S100A9 after 6 months of supplementation with CLA (Fig. 4G). In line with our flow cytometry data, analysis of transcriptional changes in isolated CD14+ monocytes via NanoString-based transcriptional profiling revealed significant changes upon 6 months of CLA supplementation (Fig. 4H and Supplementary Table 2). In particular, dietary CLA supplementation also led to a reduction of IL1B, IL18 and HLA-DR expression. Finally, on a functional level, these CD14+ monocytes exhibited significant alterations in their metabolic properties, characterized by a significant downregulation of mitochondrial respiratory activity both for basal and for maximal respiration (Fig. 4I).
Taken together, dietary CLA supplementation in RRMS patients results in broad changes within the monocyte compartment with a downmodulation of pro-inflammatory subsets and functional properties, accompanied by an increase in anti-inflammatory subsets and functions.
Discussion
Here, we report that dietary supplementation with CLA exerts strong protective effects in a spontaneous mouse model of CNS autoimmunity. This was accompanied by a reduction of intestinal inflammation and a notable shift within the intestinal myeloid cell population with a decrease in mature macrophages and a concomitant increase in myeloid suppressor-like cells, suggesting a key role of local innate immune regulation for CLA-mediated beneficial effects on CNS autoimmunity.
In recent years, the relevance of local immune modulation within the intestine for shaping a variety of autoimmune diseases, including CNS autoimmunity, has been highlighted by experimental studies, some of which addressed the gut–CNS axis.14,55,64 Thus, it has been shown that germ-free mice harbour a strongly impaired intestinal immune system and are resistant to the development of CNS autoimmunity, and that CNS autoimmunity is exacerbated upon restitution of the intestinal microbiota.14 Different mechanisms and immune cell players have been implicated in this gut-mediated shaping of systemic immune responses: First, dietary components can mediate direct effects on intestinal immune cells, such as the wheat amylase trypsin inhibitors, proteins that activate lamina propria myeloid cells via toll-like receptor 4 and promote intestinal as well as distant inflammatory responses.65–68 Second, dietary factors can shape gut microbiome composition and the microbiota themselves can trigger either pro- or anti-inflammatory effects, mainly via the production of immune-active metabolites.15 Moreover, enhanced dietary salt depleted the gut commensal Lactobacillus murinus, thereby favouring pro-inflammatory Th17 cells and enhancing CNS autoimmunity.16 Another recent example is dietary tryptophan restriction, which alters the metabolic properties of the gut microbiota and in turn restricts encephalitogenic T-cell responses.15 In contrast to these studies, our data suggest that CLA does not primarily act via modulation of the gut microbiome or the resulting metabolome, as short-term broad antibiotic eradication of the gut microbiome ameliorated the disease course in OSEctrl mice as described,55,56 but did not abolish CLA-mediated effects on the disease course. Instead, our in vitro data rather suggest that CLA may directly modulate intestinal myeloid cells, as they illustrate that CLA restricts a range of pro-inflammatory responses of myeloid cells and enhances key anti-inflammatory functions, including the production of IL10 and their capacity to limit CD4+ T-cell proliferation. However, it should be noted that CLA supplementation in our mouse model was started in utero by feeding the mothers. In light of the central role of the gut microbiota, especially during development of the immune system, an indirect modulation of intestinal myeloid cells by the microbiota early in life cannot be excluded by our approach.69
Notably, some of the observed alterations of myeloid cells in vitro are hallmarks of MDSCs, and accordingly, we observed a significant increase in MDSC-like cells in the intestine of OSECLA mice, which suggests that dietary CLA supplementation might at least partly act via induction of myeloid cells with suppressive properties. Moreover, there was no significant difference in the frequencies of MSDC-like cells in the bone marrow of OSECLA mice (Supplementary Fig. 1C), which suggests an increased recruitment of MDSC-like cells to the intestinal lamina propria rather than an enhanced production of MDSC-like cells from haematopoietic progenitor cells. It has been shown that targeted induction of MDSC-like cells alleviates intestinal70 and rheumatoid inflammation,71,72 as well as experimental autoimmune myasthenia gravis.73 In the context of CNS autoimmunity, the role of MDSCs is less clear; however, they can limit CNS inflammation by the promotion of lymphocyte apoptosis,74 control of T-cell responses,75 as well as by preventing B-cell accumulation within the CNS.76 These studies pointed towards a beneficial role of MDSCs for modulation of autoimmunity; however, they did not investigate MDSC induction via dietary modulation. In the field of cancer research, where the role of MDSCs for suppression of anti-tumour immune responses has been well-established, dietary effects on MDSC frequencies or function have already been described. A high salt diet, for example, inhibits tumour growth by blocking MDSC functions in several tumour models.77 In contrast, a high-fat diet enhanced MDSC accumulation, thereby promoting tumour progression and metastasis formation.78
Dietary CLA supplementation has already been shown to exert beneficial effects in the context of experimental inflammation-induced colorectal cancer79 and inflammatory bowel disease.24,28,80 Mechanistically, this has been linked to CLA-mediated activation of the nuclear receptor PPARγ both in colonic epithelial cells and in macrophages.24,28,81 In a model of atherosclerosis and of inflamed white adipose tissue, dietary CLA supplementation promoted anti-inflammatory properties in myeloid cells,33,82,83 especially via enrichment of anti-inflammatory macrophages. All these studies are well in line with our findings: First, they underline that dietary CLA supplementation ameliorates intestinal inflammation, and we now extend these findings by providing evidence that this mechanism might be relevant for the control of CNS autoimmunity. This is of particular interest since intestinal barrier disruption and inflammation have already been linked to experimental CNS autoimmunity and more recently to multiple sclerosis.84,85 Our results support the concept of altered intestinal immune responses as a contributing factor for the development of CNS autoimmunity and emphasize the close interrelation of these two distant sites of immune responses. Second, they provide further evidence that dietary CLA supplementation can promote the anti-inflammatory properties of myeloid cells in various organs. Finally, beneficial effects of CLA supplementation have already been observed in a pilot trial in patients suffering from Crohn’s disease,27 demonstrating the general feasibility of CLA supplementation in humans and the translation of protective murine findings in the context of human chronic inflammatory diseases. In light of this pilot study, we decided to perform a proof-of-concept trial of dietary CLA supplementation in 15 patients with RRMS to evaluate whether we can replicate some of our key immunological findings from our preclinical dataset in humans. Notably, we observed changes within the peripheral blood myeloid cell compartment with a relative increase in anti-inflammatory subsets and a decrease in pro-inflammatory cell subsets. Furthermore, the cytokine profile of monocytes from CLA-treated patients was substantially altered, and CLA supplementation also modulated the metabolic properties of monocytes by interfering with mitochondrial respiration, suggesting that CLA interferes with the metabolic reprogramming necessary for full activation as has been shown for other anti-inflammatory approaches.86–88 For obvious ethical reasons, we could not investigate intestinal immune cell subset composition in our patients, but it is tempting to speculate that the intestine/gut plays a crucial role in the anti-inflammatory priming of these myeloid cells as can be concluded from our preclinical data.
Although the precise site of action of this myeloid cell-mediated modulation of adaptive immune responses is not clear, several previous studies support the hypothesis of a peripheral immune regulation by these cells,48,89 which is in line with our results from animal experiments. While we did not observe significant changes in CNS-located myeloid cell subset composition within the CNS of OSECLA mice (Supplementary Fig. 1D), others showed that anti-inflammatory myeloid cells may control pro-inflammatory immune responses within the CNS.76,90
The size of our pilot trial precludes any reliable conclusions with regard to the clinical efficacy of dietary CLA supplementation in multiple sclerosis patients, and this definitely needs to be explored in a larger randomized and placebo-controlled trial, ideally using a classical MRI-based end point as an established surrogate marker of disease activity in RRMS. In our opinion, the performance of such a trial is feasible, in particular considering our pilot data demonstrating high tolerability and treatment adherence over 6 months. Furthermore, we did not observe any clinical relapses in those patients adhering to the combination of CLA supplementation and first-line immune modulatory treatment of multiple sclerosis. Especially in light of the still unsolved problems of treatment adherence as well as side-effects of immunomodulatory treatment, complementary treatment via targeted dietary modification represents a highly attractive therapeutic approach in the multiple sclerosis treatment landscape and putatively also in other autoimmune diseases with a key role of the intestinal immune system in immune (dys)regulation, such as systemic lupus erythematosus, inflammatory bowel disease and rheumatoid arthritis.91,92 In other clinical trials employing higher doses of CLA (above 4 g per day) in the field of obesity, type 2 diabetes or atherosclerosis, metabolic side-effects such as liver steatosis, elevation of liver enzymes, and increases in triglyceride levels have been described, which were not observed in our cohort.93–96 This may be due to the lower CLA concentrations used in our trial, but this should be monitored in follow-up trials.
Taken together, our study provides evidence that dietary CLA supplementation is a promising novel strategy for the control of CNS autoimmunity as a complementary approach to conventional treatment. Future studies, especially with regard to targeted dietary intervention in RRMS patients, are warranted to characterize the therapeutic potential of this novel approach in humans.
Supplementary Material
Acknowledgements
We thank Annika Engbers, Andrea Pabst, Claudia Kemming, Elke Hoffmann, Luzia Buchholz, Janine Meyer and Maj Lisa Frankenberg for excellent technical support. Furthermore, we thank BASF/BTC-Europe GmbH for supporting us with Tonalin™ TG80, and Angelos Sagredos, who provided the CLA for dietary supplementation in humans.
Funding
This study was supported by the German Research Foundation (DFG) grant number CRC SFB TR-128 A08 to L.K. and D.S., CRC SFB TR-128 A09 to H.W. and C.C.G., and CRC SFB TR-128 Z02 to H.W. and T.K., Innovative Medical Research (IMF, Medical Faculty, University of Münster) grant number I-HU121212 to S.H. and L.K. as well as HE111812 to L.K. M.F.P. and P.R. received support from the DFG SFB 1182 C2 and the RU 5042 P3. Additionally, this study was supported by grant Kl3/010/19 from the Interdisciplinary Centre for Clinical Research (IZKF, Medical Faculty, University of Münster) to L.K. F.T. and M.Ha. were supported by the ‘Medizinerkolleg’ Münster scholarship provided by the Medical Faculty Münster.
Competing interests
The authors report that BASF/BTC-Europe GmbH had no influence on data interpretation. A.K.F. and C.J. received travel support from Novartis. M.E. received speaker honoraria and travel support from Sanofi Genzyme. M.L. received travel support from Biogen. S.K. received research funding support from Biogen and honoraria from Sanofi, Esai, and Genzyme. M.T. received research funding from the German Cancer Aid (Deutsche Krebshilfe), German Research Foundation (DFG), Innovative Medical Research program (Medical Faculty, University of Münster), Wilhelm Sander-Stiftung, Maria Möller Stiftung, and NanoString. He received compensation for serving on scientific advisory boards for Bristol-Myers Squib and Novartis. He received speaker honoraria and/or travel support from Novartis and GlaxoSmithKline. D.S. receives research support from the German Research Foundation (DFG) and the European Union. C.C.G. received research funding from the German Research Foundation (DFG single grant GR3946-3/1, CRC SFB TR-128 A09), the European Union (Horizon 2020 ReSToRe), the Interdisciplinary Center for Clinical Studies (IZKF), Biogen and Novartis. She received speaker honoraria from MyLan, Bayer Health Care and Genzyme and travel expenses for attending meetings from Biogen, Euroimmun, Genzyme, MyLan, Novartis Pharma GmbH and Bayer Health Care. T.K. received research funding from the German Research Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Münster, National MS Society, European Leukodystrophy Association, Progressive MS Alliance, European Commission (H2020-MSCA-ITN-2018) and Novartis. She received compensation for serving on scientific advisory boards (Frequency Therapeutics, Inc.) and speaker honoraria from Novartis. H.W. received honoraria for acting as a member of Scientific Advisory Boards for Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG and Sanofi-Aventis as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, TEVA and WebMD Global. He is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis and the Swiss Multiple Sclerosis Society. His research is funded by the BMBF, DFG, Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, IZKF Münster and RE Children’s Foundation, Biogen, GlaxoSmithKline GmbH, Roche Pharma AG and Sanofi-Genzyme. L.K. received compensation for serving on Scientific Advisory Boards for Alexion, Genzyme, Janssen, Merck Serono, Novartis and Roche. She received speaker honoraria and travel support from Bayer, Biogen, Genzyme, Grifols, Merck Serono, Novartis, Roche, Santhera and Teva. She receives research support from the German Research Foundation, the IZKF Münster, IMF Münster, Biogen, Novartis and Merck Serono.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- BMM =
bone marrow-derived macrophage
- CLA =
conjugated linoleic acid
- MDSC =
myeloid-derived suppressor cell
- MFI =
mean fluorescence intensity
- OSE =
opticospinal encephalomyelitis
- PBMC =
peripheral blood mononuclear cells
- RRMS =
relapsing-remitting multiple sclerosis
References
- 1. Amato MP, Derfuss T, Hemmer B, et al. Environmental modifiable risk factors for multiple sclerosis: report from the 2016 ECTRIMS focused workshop. Mult Scler J. 2018;24:590-603. [DOI] [PubMed] [Google Scholar]
- 2. Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014;71:306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler J. 2013;19:1323-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riccio P, Rossano R.. Diet, gut microbiota, and vitamins D + A in multiple sclerosis. Neurotherapeutics. 2017;15:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haghikia A, Jörg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817-829. [DOI] [PubMed] [Google Scholar]
- 6. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, et al. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide A expression. J immunol. 2010;185:4101-4108. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz K, Barthelmes J, Stolz L, Beyer S, Diehl O, Tegeder I. ‘ Disease modifying nutricals’ for multiple sclerosis. Pharmacol Ther. 2015;148:85-113. [DOI] [PubMed] [Google Scholar]
- 8. Wergeland S, Torkildsen Ø, Bø L, Myhr KM. Polyunsaturated fatty acids in multiple sclerosis therapy. Acta Neurol Scand. 2012;126:70-75. [DOI] [PubMed] [Google Scholar]
- 9. Bienenstock J, Kunze W, Forsythe P.. Microbiota and the gut–brain axis. Nutr Rev. 2015;73:28-31. [DOI] [PubMed] [Google Scholar]
- 10. De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleck AK, Schuppan D, Wiendl H, Klotz L. Gut-CNS-axis as possibility to modulate inflammatory disease activity-implications for multiple sclerosis. Int J Mol Sci. 2017;18:1526.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fung TC, Olson CA, Hsiao EY.. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Illiano P, Brambilla R, Parolini C.. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020;287:833-855. [DOI] [PubMed] [Google Scholar]
- 14. Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538-541. [DOI] [PubMed] [Google Scholar]
- 15. Sonner JK, Keil M, Falk-Paulsen M, et al. Dietary tryptophan links encephalogenicity of autoreactive T-cells with gut microbial ecology. Nat Commun. 2019;10:4877.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilck N, Matus MG, Kearney SM, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. 2017;114:10719-10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T-cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114:10713-10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta LR, Dworkin RH, Schwid SR.. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat Rev Neurol. 2009;5:82-92. [DOI] [PubMed] [Google Scholar]
- 20. Cekanaviciute E, Pröbstel AK, Thomann A, et al. Multiple sclerosis-associated changes in the composition and immune functions of spore-forming bacteria. mSystems. 2018;3:e00083-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ochoa-Repáraz J, Kirby TO, Kasper LH.. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8:a029017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ventura RE, Iizumi T, Battaglia T, et al. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci Rep. 2019;9:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fitzgerald KC, Munger KL, Hartung H-P, et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol. 2017;82:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bassaganya-Riera J, Reynolds K, Martino-Catt S, et al. Activation of PPAR γ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777-791. [DOI] [PubMed] [Google Scholar]
- 25. Butz DE, Li G, Huebner SM, Cook ME. A mechanistic approach to understanding conjugated linoleic acid’s role in inflammation using murine models of rheumatoid arthritis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R669-676. [DOI] [PubMed] [Google Scholar]
- 26. Yang M, Pariza MW, Cook ME.. Dietary conjugated linoleic acid protects against end stage disease of systemic lupus erythematosus in the nzb/w f1 mouse. Immunopharmacol Immunotoxicol. 2000;22:433-449. [DOI] [PubMed] [Google Scholar]
- 27. Bassaganya-Riera J, Hontecillas R, Horne WT, et al. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn’s disease. Clin Nutr. 2012;31:721-727. [DOI] [PubMed] [Google Scholar]
- 28. Bassaganya-Riera J, Viladomiu M, Pedragosa M, et al. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS One. 2012;7:e31238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glassner KL, Abraham BP, Quigley EMM.. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16-27. [DOI] [PubMed] [Google Scholar]
- 30. Jang YJ, Kim W-K, Han DH, Lee K, Ko G.. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10:696-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weng YJ, Gan HY, Li X, et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J Dig Dis. 2019;20:447-459. [DOI] [PubMed] [Google Scholar]
- 32. Viladomiu M, Hontecillas R, Bassaganya-Riera J.. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol. 2016;785:87-95. [DOI] [PubMed] [Google Scholar]
- 33. McCarthy C, Duffy MM, Mooney D, et al. IL-10 mediates the immunoregulatory response in conjugated linoleic acid-induced regression of atherosclerosis. FASEB J. 2013;27:499-510. [DOI] [PubMed] [Google Scholar]
- 34. Krishnamoorthy G, Lassmann H, Wekerle H, Holz A.. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T-cell/B cell cooperation. J Clin Invest. 2006;116:2385-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Litzenburger T, Fässler R, Bauer J, et al. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med. 1998;188:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein–specific T-cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klotz L, Kuzmanov I, Hucke S, et al. B7-H1 shapes T-cell–mediated brain endothelial cell dysfunction and regional encephalitogenicity in spontaneous CNS autoimmunity. Proc Natl Acad Sci USA. 2016;113:E6182-E6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Erben U, Loddenkemper C, Doerfel K, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7:4557-4576. [PMC free article] [PubMed] [Google Scholar]
- 39. Hucke S, Floßdorf J, Grützke B, et al. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator-activated receptor γ. Brain. 2012;135:1586-1605. [DOI] [PubMed] [Google Scholar]
- 40. Klotz L, Burgdorf S, Dani I, et al. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T-cell–intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537-7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590-D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McMurdie PJ, Holmes S.. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klotz L, Dani I, Edenhofer F, et al. Peroxisome proliferator-activated receptor γ control of dendritic cell function sontributes to development of CD4+ T-cell anergy. J Immunol. 2007;178:2122-2131. [DOI] [PubMed] [Google Scholar]
- 46. Kovac S, Domijan AM, Walker MC, Abramov AY. Seizure activity results in calcium- and mitochondriaindependent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 2014;5:e1442-e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klotz L, Eschborn M, Lindner M, et al. Teriflunomide treatment for multiple sclerosis modulates T-cell mitochondrial respiration with affinity-dependent effects. Sci Transl Med. 2019;11:eaao5563.. [DOI] [PubMed] [Google Scholar]
- 48. Hucke S, Herold M, Liebmann M, et al. The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an IL-10-dependent fashion. Acta Neuropathol. 2016;132:413-431. [DOI] [PubMed] [Google Scholar]
- 49. Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lohmann L, Janoschka C, Schulte-Mecklenbeck A, et al. Immune cell profiling during switching from natalizumab to fingolimod reveals differential effects on systemic immune-regulatory networks and on trafficking of non-T-cell populations into the cerebrospinal fluid—results from the ToFingo successor study. Front Immunol. 2018;9:1560.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levine JH, Simonds EF, Bendall SC, et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162:184-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bettelli E, Baeten D, Jäger A, et al. Myelin oligodendrocyte glycoprotein–specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duc D, Vigne S, Bernier-Latmani J, et al. Disrupting myelin-specific Th17 cell gut homing confers protection in an adoptive transfer experimental autoimmune encephalomyelitis. Cell Rep. 2019;29:378-390. [DOI] [PubMed] [Google Scholar]
- 54. Yadav SK, Boppana S, Ito N, et al. Gut dysbiosis breaks immunological tolerance toward the central nervous system during young adulthood. Proc Natl Acad Sci USA. 2017;114:E9318-E9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041-6050. [DOI] [PubMed] [Google Scholar]
- 56. Stanisavljević S, Čepić A, Bojić S, et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Sci Rep. 2019;9:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veglia F, Perego M, Gabrilovich D.. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee S-E, Lim J-Y, Kim TW, et al. Matrix metalloproteinase-9 in monocytic myeloid-derived suppressor cells correlate with early infections and clinical outcomes in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:32-42. [DOI] [PubMed] [Google Scholar]
- 59. Ostrand-Rosenberg S, Fenselau C.. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol. 2018;200:422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yue Y, Huang W, Liang J, et al. IL4I1 is a novel regulator of M2 macrophage polarization that can inhibit T-cell activation via L-tryptophan and arginine depletion and IL-10 production. PLoS One. 2015;10:e0142979.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lamas Bervejillo M, Bonanata J, Franchini GR, et al. A FABP4-PPARγ signaling axis regulates human monocyte responses to electrophilic fatty acid nitroalkenes. Redox Biol. 2020;29:101376.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2017;97:4-13. [DOI] [PubMed] [Google Scholar]
- 63. Dai J, Kumbhare A, Youssef D, McCall CE, El Gazzar M. Intracellular S100A9 promotes myeloid-derived suppressor cells during late sepsis. Front Immunol. 2017;8:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yadav SK, Ito N, Mindur JE, Mathay M, Dhib-Jalbut S, Ito K. Dysregulation of immune response to enteric bacteria triggers the development of spontaneous experimental autoimmune encephalomyelitis. J Immunol. 2016;196:153. [Google Scholar]
- 65. Zevallos VF, Raker V, Tenzer S, et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology. 2017;152:1100-1113. [DOI] [PubMed] [Google Scholar]
- 66. Zevallos VF, Raker VK, Maxeiner J, Scholtes P, Steinbrink K, Schuppan D. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur J Nutr. 2019;58:1507-1514. [DOI] [PubMed] [Google Scholar]
- 67. Ashfaq-Khan M, Aslam M, Qureshi MA, et al. Dietary wheat amylase trypsin inhibitors promote features of murine non-alcoholic fatty liver disease. Sci Rep. 2019;9:17463.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bellinghausen I, Weigmann B, Zevallos V, et al. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice. J Allergy Clin Immunol. 2019;143:201-12.e4. [DOI] [PubMed] [Google Scholar]
- 69. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713-722. [DOI] [PubMed] [Google Scholar]
- 70. Zhou J, Huang S, Wang Z, et al. Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Nat Commun. 2019;10:2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fujii W, Ashihara E, Hirai H, et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol. 2013;191:1073-1081. [DOI] [PubMed] [Google Scholar]
- 72. Park M-J, Lee S-H, Kim E-K, et al. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci Rep. 2018;8:3753.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Y, Tu Z, Qian S, et al. Myeloid-derived suppressor cells as a potential therapy for experimental autoimmune myasthenia gravis. J Immunol. 2014;193:2127-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moliné-Velázquez V, Cuervo H, Vila-del Sol V, Ortega MC, Clemente D, de Castro F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011;21:678-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alabanza LM, Esmon NL, Esmon CT, Bynoe MS. Inhibition of endogenous activated protein C attenuates experimental autoimmune encephalomyelitis by inducing myeloid-derived suppressor cells. J Immunol. 2013;191:3764-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Knier B, Hiltensperger M, Sie C, et al. Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Nat Immunol. 2018;19:1341-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Willebrand R, Hamad I, Van Zeebroeck L, et al. High salt inhibits tumor growth by enhancing anti-tumor immunity. Front Immunol. 2019;10:1141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Clements VK, Long T, Long R, Figley C, Smith DMC, Ostrand-Rosenberg S. Frontline Science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. 2018;103:395-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARγ. J Nutr. 2010;140:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bassaganya-Riera J, Hontecillas R.. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2010;13:569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505-531. [DOI] [PubMed] [Google Scholar]
- 82. Kanter J, Goodspeed L, Wang S, et al. 10,12 conjugated linoleic acid-driven weight loss is protective against atherosclerosis in mice and is associated with alternative macrophage enrichment in perivascular adipose tissue. Nutrients. 2018;10:1416.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pini M, Touch S, Poirier H, et al. Adipose tissue adaptive response to trans-10,cis-12-conjugated linoleic acid engages alternatively activated M2 macrophages. FASEB J. 2016;30:241-251. [DOI] [PubMed] [Google Scholar]
- 84. Buscarinu MC, Romano S, Mechelli R, et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics. 2018;15:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nouri M, Bredberg A, Weström B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T-cells. PLoS One. 2014;9:e106335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chakhtoura M, Chain RW, Sato PY, et al. Ethyl pyruvate modulates murine dendritic cell activation and survival through their immunometabolism. Front Immunol. 2019;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duroux-Richard I, Roubert C, Ammari M, et al. MiR-125b controls monocyte adaptation to inflammation through mitochondrial metabolism and dynamics. Blood. 2016;128:3125-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grunwell JR, Yeligar SM, Stephenson S, et al. TGF-β1 suppresses the type I IFN response and induces mitochondrial dysfunction in alveolar macrophages. J Immunol. 2018;200:2115-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cantoni C, Cignarella F, Ghezzi L, et al. Mir-223 regulates the number and function of myeloid-derived suppressor cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2017;133:61-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moliné-Velázquez V, Ortega MC, Vila del Sol V, Melero-Jerez C, de Castro F, Clemente D. The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid-derived suppressor cell fate and viability. Neurobiol Dis. 2014;67:149-164. [DOI] [PubMed] [Google Scholar]
- 91. Brown EM, Kenny DJ, Xavier RJ.. Gut microbiota regulation of T-cells during inflammation and autoimmunity. Annu Rev Immunol. 2019;37:599-624. [DOI] [PubMed] [Google Scholar]
- 92. Pan F, Tang W, Zhou Z, Gilkeson G, Lang R, Jiang W. Intestinal macrophages in mucosal immunity and their role in systemic lupus erythematosus disease. Lupus. 2018;27:1898-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Benjamin S, Prakasan P, Sreedharan S, Wright AD, Spener F. Pros and cons of CLA consumption: an insight from clinical evidences. Nutr Metab (Lond). 2015;12:4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.EFSA. Scientific opinion on the safety of “conjugated linoleic acid (CLA)-rich oil” (Tonalin® TG 80) as a novel food ingredient. EFSA J. 2010;8:1600. [Google Scholar]
- 95. den Hartigh LJ. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients. 2019;11:370.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Song H-J, Grant I, Rotondo D, Mohede I, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr. 2005;59:508-517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and full code of all scripts are available upon reasonable request from the corresponding author. There is no restriction on the availability of materials described in the study.