Dilated cardiomyopathy (DCM), characterized by dilation and dysfunction of one or both ventricles [1], is the most prevalent form of cardiomyopathy. 30–50% of DCM cases are genetically determined [2, 3]. Genetic variants over a number of proteins that affect cardiomyocyte function are an important cause of DCM. Up until recently, mutations in the Ryanodine receptor 2 (RYR2) gene have been shown to be involved, especially in catecholaminergic polymorphic ventricular tachycardia (CPVT) [4], and arrhythmogenic cardiomyopathy [3]. Herein, is reported on a family with a novel early truncation in RYR2 associated with an autosomal-dominant form of DCM.
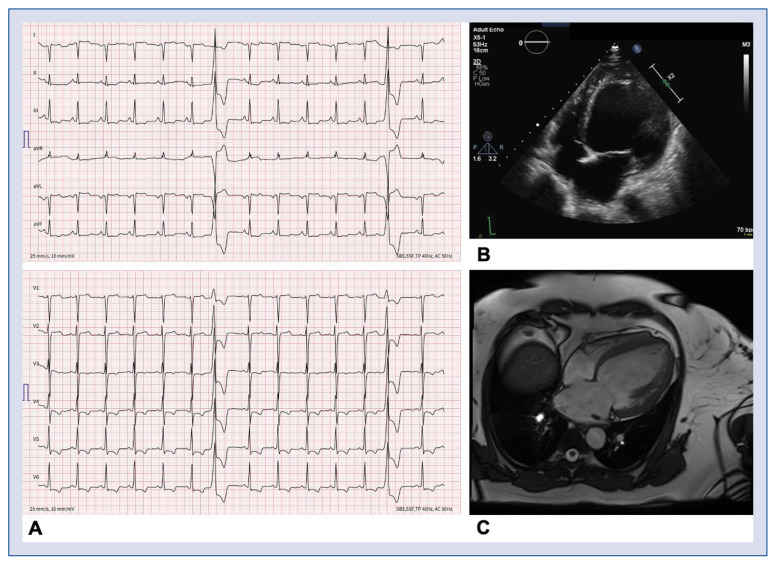
A 51-year-old woman of Caucasian descent (Case 1) was admitted to the documented hospital for decompensated heart failure (New York Heart Association [NYHA] stage IV). Her 12-lead electrocardiogram (ECG) showed sinus rhythm with T-wave inversions (TWI) in leads II, III, aVF and V3–V6 (Fig. 1A). The transthoracic echocardiography (TTE) showed a heavily dilated left ventricle (LV) (LV end diastolic volume index [LVEDVi] 129 mL/m2) with a decreased LV ejection fraction (LVEF) of < 15%, in the presence of diffuse hypokinesia without evidence of an LV thrombus (Fig. 1B). The left atrium (LA) was moderately dilated (LA volume index [LAVI] 45 mL/m2). Magnetic resonance imaging (MRI) showed no evidence of fibrosis or fatty infiltration, but confirmed DCM (Fig. 1C) with normal right ventricle (RV) dimensions and function. Medical therapy for heart failure was started and optimized including lisinopril (5 mg bid), later changed to valsartan (50 mg tid), bisoprolol (5 mg bid), spironolactone (25 mg qd), and torasemide (10 mg qd). 48 h Holter-ECG only showed a very low premature ventricular complex (PVC) burden (0.3%) and no tachyarrhythmia, while two exercise stress tests revealed no PVCs or other forms of ventricular tachyarrhythmia. Nevertheless, despite being on optimal guideline-directed medical therapy at 4 month follow-up, a markedly decreased LVEF on TTE (22%) necessitated the implantation of a subcutaneous implantable cardioverter-defibrillator (ICD) for primary prevention. The family history indicated a familial autosomaldominant form of DCM: the mother of the index case was transplanted for heart failure due to DCM at the age of 45 years, while the mother’s brother died of heart failure due to DCM at the age of 70. No genetic tests or tissue were available. During cascade screening, one of the daughters (Case 2) of the index patient, a 26-year-old woman, was found to have a slightly dilated LV (LVEDVi 66 mL/m2) with a normal LVEF (57%). Her 12-lead ECG showed normal sinus rhythm and normal de-/repolarization. A 48 h Holter-ECG showed a low PVC burden (0.06%) and no tachyarrhythmia. Further investigations revealed late gadolinium enhancement (LGE) on cardiac MRI, specifically in the LV apical and septal areas, as well as a transmural scar in the inferior LV.
Figure 1.
Diagnostic work-up in the index patient. A. 12-lead electrocardiogram showing sinus rhythm with T wave inversions in II, III, aVF and V3–V6, and two premature ventricular complexes originating from the anterobasal left ventricle (LV); B. Transthoracic echocardiogram showing a heavily dilated LV (LV end-diastolic volume index: 129 mL/m2); C. Cardiac magnetic resonance imaging, confirming dilated cardiomyopathy without fibrosis or fatty infiltration.
The genetic test performed in the index patient (Case 1; performed using next generation sequencing technology — Illumina’s Trusight Cardio sequencing panel, covering 176 genes) resulted in a previously unreported variant in Exon 4 of the RYR2 gene (heterozygous c.294G>A; p.Trp98Ter), which is considered likely pathogenic (class IV) following the 2015 American College of Medical Genetics criteria [5], since it is an early truncating variant and leads to a significantly shortened and dysfunctional protein product. The same variant was identified through Sanger Sequencing in the phenotypically affected daughter (Case 2), while it was not identified in the two other healthy siblings. Recently, evidence has been emerging about genes encoding sarcoplasmatic reticulum (SR) proteins as putative for DCM [3].
The cardiac RYR2 is an important calcium (Ca2+) release channel of the SR and plays an essential role in excitation-contraction coupling in cardiomyocytes [6]. RYR2 dysfunction causes an abnormal Ca2+ leakage from the SR, which can generate delayed afterdepolarizations, which in turn can lead to ventricular arrhythmias [7]. RYR2 variants altering the termination of Ca2+ release seem to lead to a cardiomyopathic phenotype, which is usually associated with mutations in sarcomeric proteins. Specifically, DCM-associated sarcomeric variants tend to decrease the myofilament Ca2+ sensitivity and thus increase cytosolic Ca2+ transients. The abnormal cytosolic Ca2+ transient resulting from altered myofilament Ca2+ sensitivity is thought to trigger cardiac remodeling (via Ca2+/calmodulin-dependent signaling pathways, the calcineurin/NFAT pathways, or apoptotic signaling) that can lead to DCM [8]. Moreover, in dystrophic cardiomyopathy, the RYR hypersensitivity for Ca2+ due to redox modifications is not only responsible for excessive stress responses, but also changes the signal transduction linking L-type Ca2+ channels to RYRs during excitation-contraction coupling [9]. This connection between abnormal RYR function and dystrophic cardiomyopathy further underlines a putative role for abnormal RYR function not only in arrhythmogenesis, but also in cardiomyopathies.
Genetic variants in the RYR2 gene are frequently autosomal dominant and usually associated with CPVT, but radical variants such as truncating variants, have also been described in the setting of DCM. There are various studies, where RYR2 variants have been recognized as causative in a small number of patients with DCM. In 2007, Bhuiyan et al. [10] reported two families with a deletion in exon 3 of the RYR2 gene, displaying a phenotype of CPVT in some family members and a DCM phenotype with LV dysfunction in other family members. Ohno et al. [6] linked large deletions in exon 3 of the RYR2 gene to LV non-compaction cardiomyopathy in two unrelated probands and their affected family members. This is in line with the present findings: the variant in this family leads to a stop codon which generates an early truncation (exon 4 out of 105 exons), leading to a dysfunctional protein product. Together with the, albeit limited, co-segregation shown in the reported family, this confirms a putative pathogenic role of the current reported truncating heterozygous RYR2 genetic variant (c.294 294G>A; p.Trp98Ter) in the setting of familial DCM.
Acknowledgements
We thank Robert Manka, MD, Department of Radiology and Cardiology, University Heart Center, Zurich, Switzerland, for performing and interpreting the cardiac MRI scans. The Zurich ARVC Program is supported by generous grants from the Georg and Bertha Schwyzer-Winniker Foundation, the Baugarten Foundation, Swiss National Science Foundation, Swiss Heart Foundation and Wild Foundation.
We thank Felix Tanner, MD, Division of Echocardiography, University Heart Center, Zurich, Switzerland, for performing and interpreting the Echocardiography.
Footnotes
Conflict of interest: Dr. Steffel has received consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Bayer, Berlin Chemie/Menarini, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, WebMD, and Zoll. He reports ownership of CorXL. Dr. Steffel has received grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic. Dr. Saguner and Dr. Breitenstein have received educational grants from Boston Scientific. Dr. Flammers reports fees from Alnylam, Amgen, AstraZeneca, Bayer, Fresenius, Imedos Systems, Medtronic, Novartis, Pfizer, Roche, Vifor, and Zoll, unrelated to this article. The other authors report no conflicts of interest related to this work.
References
- 1.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 2.McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res. 2017;121(7):722–730. doi: 10.1161/CIRCRESAHA.117.309711. [DOI] [PubMed] [Google Scholar]
- 3.McNally EM, Mestroni L. Dilated cardiomyopathy: genetic determinants and mechanisms. Circ Res. 2017;121(7):731–748. doi: 10.1161/CIRCRESAHA.116.309396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerrone M, Colombi B, Santoro M, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96(10):e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 5.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno S, Omura M, Kawamura M, et al. Exon 3 deletion of RYR2 encoding cardiac ryanodine receptor is associated with left ventricular non-compaction. Europace. 2014;16(11):1646–1654. doi: 10.1093/europace/eut382. [DOI] [PubMed] [Google Scholar]
- 7.Meissner G. The structural basis of ryanodine receptor ion channel function. J Gen Physiol. 2017;149(12):1065–1089. doi: 10.1085/jgp.201711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y, Tian X, Wang R, et al. Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ Res. 2012;110(7):968–977. doi: 10.1161/CIRCRESAHA.111.256560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullrich ND, Fanchaouy M, Gusev K, et al. Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;297(6):H1992–H2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhuiyan ZA, van den Berg MP, van Tintelen JP, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116(14):1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]