Abstract
Background
ST2 is a circulating biomarker that is well established for predicting outcome in heart failure (HF). This is the first study to look at ST2 concentrations in optimally treated patients with stable but significant left ventricular systolic dysfunction (LVSD) compared to patients with severe aortic stenosis (AS).
Methods
Two cohorts were retrospectively studied: 94 patients undergoing transcatheter aortic valve implantation for severe AS (63 with normal ejection fraction [EF] and 31 with reduced EF), and 50 patients with severe LVSD from non-valvular causes. ST2 pre-procedural samples were taken, and repeated again at 3 and 6 months. Patients were followed-up for 2 years. Data was analyzed using SPSS software.
Results
Baseline concentrations of soluble ST2 did not differ significantly between the HF group and AS group with normal EF (EF ≥ 50%). However, in the AS group with a low EF (EF < 50%) ST2 concentrations were significantly higher that the HF group (p = 0.009). New York Heart Association class IV HF, baseline N-terminal pro-B-type natriuretic peptide and gender were all independent predictors of soluble ST2 (sST2) baseline concentrations.
Conclusions
Raised ST2 concentrations in the context of severe AS may be a marker for subclinical or clinical left ventricular dysfunction. More research is required to assess its use for assessment of prognosis and response to treatment.
Keywords: ST2, biomarkers, aortic stenosis, transcatheter aortic valve implantation, heart failure
Introduction
ST2 is a circulating biomarker of the interleukin (IL) 1 gene family that binds with the IL-33 ligand. This exists either in a transmembrane receptor (ST2L) which binds to circulating IL-33 and reduces tissue fibrosis, or a soluble receptor (sST2) which blocks this beneficial effect by binding itself to IL-33. Elevated plasma concentrations of ST2 are powerful predictors of death, pump failure and arrhythmias [1]. It is now a well validated biomarker for predicting outcome in heart failure (HF) [2–4]. It may also be useful in the serial monitoring of patients with HF [5].
ST2 is thought to be produced in the peripheral vasculature [6] in response to increased biomechanical stress on the myocardium [7]. ST2 may also be involved with remodeling in left ventricular hypertrophy (LVH), and it is thought to be a marker of cardiac fibrosis. However, its precise pathophysiological roles in HF and LVH remain unclear. Since severe aortic stenosis (AS) and established HF are associated with biomechanical stress on the myocardium and patients with severe AS additionally have microvascular ischemia, under examination was whether the ST2 concentrations in patients with severe AS were similar to those in patients with established HF who were on optimal medical therapy.
Methods
Ethical approval of the protocol and for collection of samples to measure and analyze biomarker concentrations were obtained from the London (Dulwich) and East Midlands National Research Ethics Service Committee.
A total of 144 patients were retrospectively studied, comprising 50 patients with severe left ventricular (LV) systolic dysfunction (LVSD) due to non-valvular causes and 94 patients undergoing transcatheter aortic valve implantation (TAVI) for severe AS. Between October 2011 and October 2012, 50 patients were recruited with chronic HF in the New York Heart Association (NYHA) classes I–III and LV ejection fraction (EF) ≤ 40% from the HF clinics at Kings College Hospital, London. All patients were on optimum tolerated HF medications, comprising of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, mineralocorticoid receptor antagonists and diuretics. The target doses were determined according to current guidelines [8]. Exclusion criteria included a cardiovascular admission or change in HF medication within 4 weeks of recruitment, a planned cardiovascular admission, significant renal impairment (estimated glomerular filtration rate [eGFR] < 20 mL/min/1.73 m2), or the inability or unwillingness to consent. This HF cohort has been described previously [5].
Additionally, 97 patients were studied who had undergone TAVI from March 2009 to May 2012 for severe AS. 3 patients were excluded for not having blood samples taken for biomarker analysis; therefore, 94 patients comprised the AS group. Patients who had TAVI for “valve in valve” replacement for aortic regurgitation were also excluded. The details of investigations and outcomes were obtained from electronic databases and patient records. ST2 samples were drawn prior to the interventional procedure.
The blood samples were obtained by venepuncture after the patients had rested for 20 min in a semi-recumbent position and collected in tubes containing ethylenediaminetraacetic acid. The serum ST2 was measured by enzyme linked immunosorbent assay (R&D Systems Europe, Ltd, Abingdon, UK). The ST2 assay contains an NS0-expressed recombinant human ST2 and has been shown to accurately quantify the recombinant factor. The intra-assay precision was 5.6%, 4.4% and 4.5% and the inter-assay precision was 7.1%, 5.4% and 6.3% at 5.4, 12.6 and 20.6 μg/L, respectively. The limit of detection was 0.005 μg/L and the reference range is 6.74–20.4 μg/L [9].
The LV dimensions, function and mass were derived from the transthoracic echocardiographic evaluations. The TAVI procedures were performed by experienced cardiologists using Sapien 3 and Sapien XT valves (Edwards Life Sciences, Irvine, CA, USA). Serial ST2 concentrations were measured between 3 and 6 months from baseline. At the end of a 2 year follow-up, the primary end-point was all-cause mortality.
Statistical analysis
Statistical analyses were performed using the IBM® SPSS® package version 22 (IBM Corporation, Armonk, NY, USA). Normally distributed data were expressed as mean and standard deviation and the groups were compared using the Student t-test. Non-normally distributed data were expressed as medians plus interquartile range and were compared using the Kruskall-Wallis and Mann-Whitney-U tests. Categorical data were summarized using numbers and percentages and the groups were compared with respect to these data using the χ2 test. Stepwise multiple linear regression was used to identify the predictors of baseline sST2 concentration using a probability (F) of 0.05 for entry into and 0.10 for removal from the model. Survival analysis was performed on all-cause mortality and survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. A value of p < 0.05 considered statistically significant.
Results
The baseline characteristics of patients are shown in Table 1. A total of 144 patients with an average age of 78 years were included in the analysis, of whom 89 were male and 55 were female. Of these patients, 113 (78.4%) had an LVEF ≥ 50%, and 31 (21.6%) had an LVEF < 50%. When 40% was used as the cut-off value for EF, 132 (91.7%) patients had an EF ≥ 40% and 12 (8.3%) had an EF < 40%. Using 40% as the cut off for EF had little impact on the final statistical analysis (data not shown). Twenty-four out of 144 (16.7%) patients had renal dysfunction, which was defined as an eGFR < 45 mL/min/1.73 m2. Of the 50 patients who comprised the HF group, 24 had ischemic cardiomyopathy and 26 had non-ischemic cardiomyopathy.
Table 1.
Baseline characteristics of study population.
| Variable | HF (n = 50) | AS, EF > 50% (n = 63) | AS, EF < 50% (n = 31) | Total (n = 144) | P |
|---|---|---|---|---|---|
| Age [years]* | 68.5 (21) | 85.0 (8) | 86.0 (7) | 81.00 (15) | < 0.001 |
| Male | 82.0% | 54.0% | 45.2% | 61.8% | 0.001 |
| Diabetes mellitus | 16.0% | 22.2% | 25.8% | 20.8% | 0.536 |
| Hypertension | 50.0% | 52.4% | 51.6% | 51.4% | 0.968 |
| Dyslipidemia | 56.0% | 28.6% | 16.1% | 35.4% | < 0.001 |
| NYHA class: | < 0.001 | ||||
| I | 10.0% | 1.6% | 0.0% | 4.2% | |
| II | 70.0% | 25.4% | 12.9% | 38.2% | |
| III | 20.0% | 71.4% | 74.2% | 54.2% | |
| IV | 0.0% | 1.6% | 12.9% | 3.5% | |
| Systolic BP [mmHg]** | 116.88 (19.4) | 132.89 (22.1) | 125.90 (25.2) | 125.83 (22.9) | 0.001 |
| Diastolic BP [mmHg]** | 68.12 (10.7) | 68.73 (11.9) | 68.61 (14.5) | 68.49 (12.0) | 0.963 |
| Heart rate [bpm]* | 66.00 (18) | 74.00 (17) | 71.00 (16) | 70.00 (20) | 0.026 |
| ICD (not CRT-D) | 14.0 | 0.0 | 0.0 | 4.9 | 0.001 |
| PPM | 8.0 | 14.3 | 12.9 | 11.8 | 0.576 |
| CRT | 28.0 | 0.0 | 6.5 | 11.1 | < 0.001 |
| Sinus rhythm | 76.0 | 65.1 | 54.8 | 66.7 | 0.039 |
| Baseline QRS [ms]* | 118.50 (68) | 96.00 (35) | 119.00 (52) | 110.00 (49) | < 0.001 |
| eGFR [mL/min/1.73 m2]* | 62.0 (26) | 64.0 (26) | 51.0 (33) | 61.50 (28) | 0.253 |
| Hemoglobin [g/L]** | 137.2 (14.2) | 122.38 (16.0) | 124.32 (16.2) | 127.94 (16.8) | < 0.001 |
| LV mass [g]* | 248.5 (93.0) | 253.3 (76.9) | 280.6 (103.0) | 198.76 (147) | 0.03 |
| Baseline EF [%]* | 31.50 (11) | 59.00 (8) | 42.00 (12) | 45.00 (23) | < 0.001 |
| Baseline NT-proBNP [ng/L]* | 300.00 (1201) | 1756.00 (3353) | 5263.00 (11907) | 1446.00 (3862) | < 0.001 |
| Na+ [mmol/L]* | 138.50 (5) | 138.0 (4) | 137.00 (5) | 138.00 (4) | 0.099 |
The data presented are the medians and the interquartile ranges (*) or the means and standard deviations (**). AS — aortic stenosis; BP — blood pressure; CRT — cardiac resynchronization therapy; CRT-D — cardiac resynchronization therapy-defibrillator; eGFR — estimated glomerular filtration rate; HF — heart failure; EF — ejection fraction; ICD — implantable cardioverter defibrillator; LV — left ventricular; Na — sodium; NT-proBNP — N-terminal pro-B-type natriuretic peptide; NYHA — New York Heart Association; PPM — permanent pacemaker
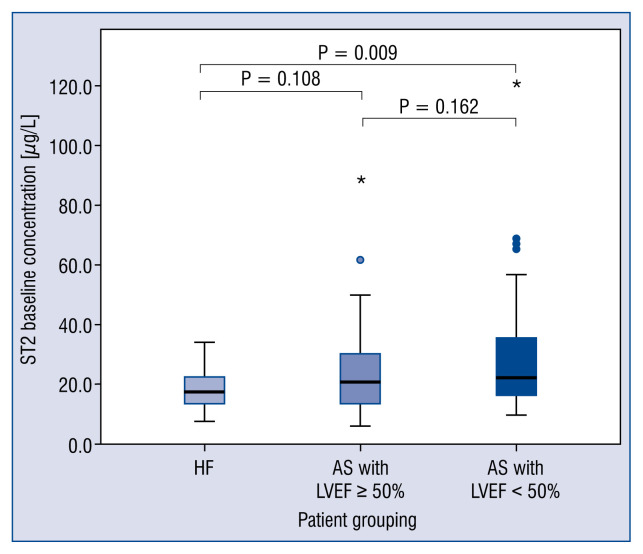
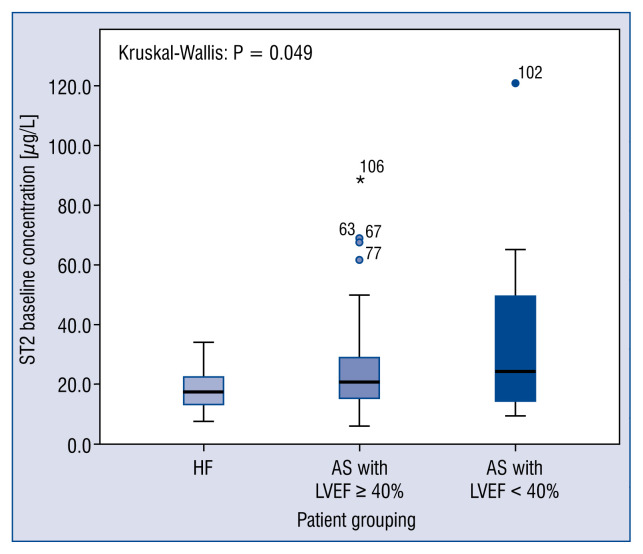
Baseline concentrations of sST2 did not differ significantly between the HF group and those in the AS group who had EFs ≥ 50%. The baseline concentrations of sST2 were significantly higher for patients in the AS group who had low EFs (< 50%) compared with those in the HF group (p = 0.009) (Fig. 1), a finding that did not change appreciably when the analysis was repeated using an EF cut-off value of 40% (Fig. 2). The latter analysis was performed in an attempt to standardize the definition of LV systolic function, because the HF cohort had an EF cut-off value of 40%.
Figure 1.
Box plots of the cohort median ST2 concentrations using a left ventricular ejection fraction (LVEF) cut-off value of < 50%. The Kruskal-Wallis test indicated that the difference between the patients with heart failure (HF) and those with aortic stenosis (AS) and a low ejection fraction with respect to ST2 concentration was significant (*p = 0.009).
Figure 2.
Box plots of the cohort baseline ST2 concentrations using a left ventricular systolic dysfunction (LVSD) cut-off value of < 40%. The Kruskal-Wallis test indicated that the difference between the patients with heart failure (HF) and those with aortic stenosis (AS) and LVSD with respect to the ST2 concentration was significant (p = 0.049); LVEF — left ventricular ejection fraction; *p = 0.049.
A multivariate linear regression analysis was performed to determine the factors that predict the baseline sST2 concentration using a stepwise method and the following variables: baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration, cohort (AS with EF < 50% and AS with EF > 50%), age, eGFR, gender, hypertension, diabetes, hypercholesterolemia, baseline QRS, LV mass, baseline EF, baseline end-diastolic volume, left atrial volume, and NYHA class (I–IV). NYHA class IV HF, baseline NT-proBNP level, and gender were identified as independent predictors of baseline sST2 concentrations (Table 2).
Table 2.
Stepwise multivariate linear regression analysis of the baseline sST2 concentration.
| Non-standardized coefficient | Standardized coefficient | T-statistic | P value | ||
|---|---|---|---|---|---|
|
|
|
||||
| Beta | SE[A1] | Beta | |||
| NYHA IV | 34.6 | 8.3 | 0.41 | 4.12 | < 0.001 |
| Male gender | 11.7 | 3.7 | 0.31 | 3.13 | 0.003 |
| NT-proBNP [ng/L] | 0.001 | 0.0 | 0.40 | 4.01 | < 0.001 |
NT-proBNP — N-terminal pro–B-type natriuretic peptide; NYHA — New York Heart Association; SE — standard error
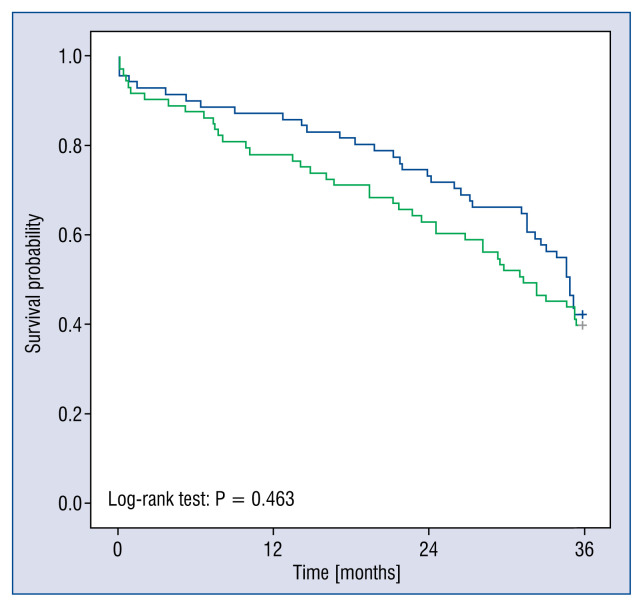
Figure 3 presents the Kaplan-Meier survival curves. The log-rank test did not determine a significant difference between the cohorts that had baseline ST2 concentrations that were either above or below the median concentration (p = 0.463) (Fig. 3).
Figure 3.
Kaplan-Meier plots for the cohorts split at the median ST2 concentration. The green line is the high ST2 concentration, and the blue line is the low ST2 concentration.
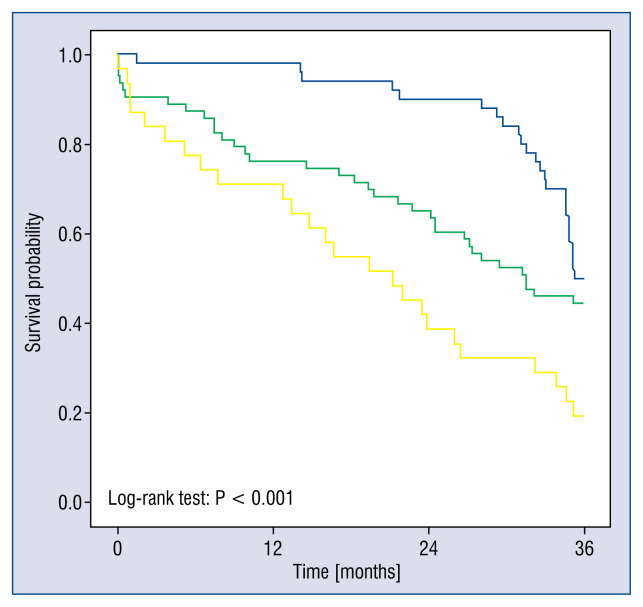
Significant differences were evident between the HF group and the AS subgroup with an EF > 50% and between the HF group and the AS subgroup with EF < 50% with respect to all-cause mortality (log-rank test: p < 0.001) (Fig. 4). These differences persisted when EF was changed to a cut-off value of 40% and when 30-day mortality was excluded from the analysis (data not shown). This is consistent with current knowledge from previous studies [10].
Figure 4.
Kaplan-Meier plots of survival probability of the cohorts using a left ventricular systolic dysfunction (LVSD) cut-off value of left ventricular ejection fraction (LVEF) < 50%. The blue line represents the heart failure group, the green line represents the aortic stenosis (AS) group without LVSD, and the yellow line represents the AS group with LVSD. The scale of the x-axis is in days up to 3 years.
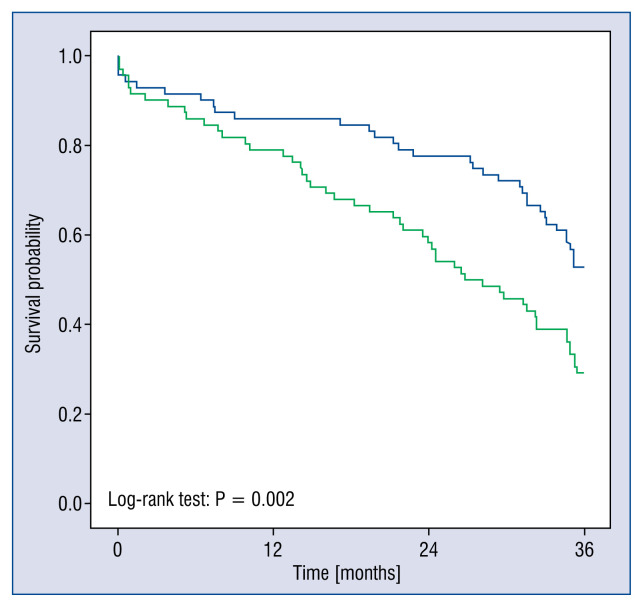
A comparison of survival according to the baseline NT-proBNP levels that were dichotomized at the median concentration, showed a significant difference with respect to prediction of death (log-rank; p = 0.002) (Fig. 5).
Figure 5.
Kaplan-Meier plots of the survival probability of the patient population according to baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP). This was split at the NT-proBNP median concentration, up to the time at which the data were censored, which was 3 years. The blue line represents patients with NT-proBNP concentration above median and the green line represent the patients with NT-proBNP concentration below median.
The receiver operating curve analysis revealed an area under the curve (AUC) of 0.580 for all-cause mortality at 2 years. The serial ST2 concentrations determined from 3 to 6 months from baseline showed a change of +0.88 μg/L in the HF group and a change of +0.47 μg/L in patients with AS who had undergone TAVI, a difference that was not significant. The AUC for all-cause mortality at 2 years improved to 0.602 when the analysis included the 3–6-month ST2 concentrations.
Discussion
ST2 has been extensively studied and validated as a biomarker for HF [4, 11] but it is much less well described in association with severe AS. Bartunek et al. [6] demonstrated that ST2 concentrations in the coronary sinus in AS were non-significantly different to controls. Therefore, ST2 production is likely to be extra-cardiac, even though its actions directly affect the myocardium. The presence of biomechanical stress is believed to initiate production of ST2 in the vascular endothelium, which in turn binds to IL-33 receptors to inhibit or promote fibrogenesis [6, 7].
Findings from one small study found elevated ST2 concentrations in stenotic but non-regurgitant aortic valve leaflet tissue [12]. This may reflect the difference in pathological changes that the aortic valve undergoes. Although, it is unlikely that the degree of ST2 expression within valve tissue would affect serum concentrations significantly, this has yet to be formally tested.
The findings from the present study indicate that the ST2 concentrations were similarly elevated in patients with HF and in those with severe AS. However, the ST2 concentrations were significantly higher in patients with severe AS and LVSD, which concurs with a poorer prognosis in this group of patients [13]. This result was unexpected, because LVH from severe AS is not usually associated with the same levels of myocardial fibrosis as those seen in patients with severe LVSD.
The presence of raised ST2 concentrations may be an early warning sign for subclinical LVSD or decompensated AS in patients with AS. While it is yet to be determined whether measuring ST2 concentrations could help clinicians decide when to intervene, the findings from one study have demonstrated that raised ST2 concentrations may predict a poorer prognosis in asymptomatic patients with severe AS [14].
ST2 has emerged as marker of inflammation, fibrosis, cardiac stress and remodeling. In many studies ST2 has been shown to be a BNP independent predictor of cardiac and all-cause mortality. ST2 reflects the dynamic changes in a failing heart and thus parallel the myocardial remodeling and risk of cardiac events. Its value is potentially more significant in patients with normal systolic LV function but with LVH.
Sample size in the current study was small and not sufficiently powered to determine whether ST2 concentration would be a good predictor of mortality in AS. However, 3-year outcomes for patients in the HF cohort and AS with an LVEF > 50% were similar. ST2 concentrations were also similar. The 3-year outcome was significantly worse for AS patients with impaired LV systolic function, and the baseline ST2 concentrations were significantly higher. A repeat of the analysis using a cut-off value of 40% for the LVSD generated similar results. The ST2 concentrations appeared to be independent of LV mass, which was an echocardiographically derived value, and were independent of diabetes, eGFR, QRS duration, EF and age.
ST2 is thought to be a marker of fibrosis, but not hypertrophy; hence, ST2 may be a useful marker for fibrotic disease or the lack of response to aortic valve intervention in AS and HF [5]. This should be investigated in a future prospective study as levels of fibrosis were not measured in this study. Raised ST2 concentrations, however, did not appear to predict group outcomes in the current study. This was surprising and may reflect the relatively small sample size in this study. In the future, there may be a role for combining ST2 and NT-proBNP levels to provide clinicians with additional information for the management of cardiovascular disease [15].
ST2 has emerged as marker of inflammation, fibrosis, cardiac stress and remodeling. In many studies ST2 has been shown to be BNP independent predictor of cardiac and all-cause mortality. ST2 reflects dynamic changes in a failing heart and thus, parallel the myocardial remodeling and risk of cardiac events. Its value is potentially more significant in HF preserved EF and patients with LVH.
Undertaking serial measurements of the ST2 concentrations did not appear to show significant differences in concentrations, but analysis of the data revealed that ST2 concentration changed from −99 to 29 μg/L in patients who had undergone TAVI, which indicates a large variation within a small population. While the results may have been influenced by statistical outliers, many patients did not show significant changes in their ST2 concentrations post-TAVI.
Limitations of the study
One limitation of the current study was the small sample size of the HF cohort, which was a consequence of strict inclusion criteria for the study. Furthermore, this study was an observational cohort study and its results are, therefore, subject to undetermined confounding factors; and hence, should be interpreted with caution. It is acknowledged that average ages of the HF and AS cohorts were very different. However, since TAVI is a life-extending and, sometimes, life-saving procedure, it was believed that a 3-year follow-up duration was a reasonable life expectancy post-TAVI for patients selected to undergo the procedure. It was also recognized that this study did not include a healthy control group. However, these data are hypothesis generating and they will support a much larger prospective study that is designed to determine the effects of changing ST2 levels on the outcomes in this population.
Conclusions
The serum ST2 concentrations are elevated to similar levels in patients with severe LVSD and in the population of patients with severe AS who underwent TAVI. The concentrations were highest in patients with severe LVSD and severe AS. This is the first study to compare ST2 concentrations in optimally treated patients with stable but significant LV dysfunction with those in patients with severe AS. Raised ST2 concentrations from increased biomechanical stress on the myocardium in the context of severe AS may be a marker for subclinical or clinical LV dysfunction.
Ongoing biomarker research in this area should not focus solely on replacing NT-proBNP or even troponin, but rather on investigating early pathophysiological changes in the myocardium, which may lead to future therapeutic targets. It is tempting to speculate that early detection of disease progression using novel biomarkers or significant changes in interval testing may be of future clinical relevance, but larger prospective studies will be required to address these gaps in knowledge.
Acknowledgements
This work was funded by King’s College Hospital R&D Grant, and was supported by the Department of Health via a National Institute for Health Research Biomedical Research Centre award to Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust
Footnotes
Conflict of interest: None declared
References
- 1.Januzzi JL, Mebazaa A, Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am J Cardiol. 2015;115(7 Suppl):26B–31B. doi: 10.1016/j.amjcard.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Zhang R, Zhang Y, An T, et al. Prognostic value of sST2 and galectin-3 for death relative to renal function in patients hospitalized for heart failure. Biomark Med. 2015;9(5):433–441. doi: 10.2217/bmm.15.12. [DOI] [PubMed] [Google Scholar]
- 3.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Januzzi JL, Pascual-Figal D, Daniels LB. ST2 testing for chronic heart failure therapy monitoring: the International ST2 Consensus Panel. Am J Cardiol. 2015;115(7 Suppl):70B–75B. doi: 10.1016/j.amjcard.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 5.Piper SE, Sherwood RA, Amin-Youssef GF, et al. Serial soluble ST2 for the monitoring of pharmacologically optimised chronic stable heart failure. Int J Cardiol. 2015;178:284–291. doi: 10.1016/j.ijcard.2014.11.097. [DOI] [PubMed] [Google Scholar]
- 6.Bartunek J, Delrue L, Van Durme F, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008;52(25):2166–2174. doi: 10.1016/j.jacc.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimpo M, Morrow DA, Weinberg EO, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 9.R & D Systems. Human ST2/IL-33 R Quantikine ELISA Kit. [Accessed 03/11/2018]. https://www.rndsystems.com/products/human-st2-il-33-rquantikine-elisa-kit_dst200 .
- 10.Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J. 1988;9(Suppl E):57–64. doi: 10.1093/eurheartj/9.suppl_e.57. [DOI] [PubMed] [Google Scholar]
- 11.Bayes-Genis A, Januzzi JL, Gaggin HK, et al. ST2 pathogenetic profile in ambulatory heart failure patients. J Card Fail. 2015;21(4):355–361. doi: 10.1016/j.cardfail.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Sawada H, Naito Y, Hirotani S, et al. Expression of interleukin-33 and ST2 in nonrheumatic aortic valve stenosis. Int J Cardiol. 2013;168(1):529–531. doi: 10.1016/j.ijcard.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 13.Carabello BA. Is it ever too late to operate on the patient with valvular heart disease? J Am Coll Cardiol. 2004;44(2):376–383. doi: 10.1016/j.jacc.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 14.Lancellotti P, Dulgheru R, Magne J, et al. Elevated plasma soluble ST2 is associated with heart failure symptoms and outcome in aortic stenosis. PLoS One. 2015;10(9):e0138940. doi: 10.1371/journal.pone.0138940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim N, Januzzi JL. The potential role of natriuretic peptides and other biomarkers in heart failure diagnosis, prognosis and management. Expert Rev Cardiovasc Ther. 2015;13(9):1017–1030. doi: 10.1586/14779072.2015.1071664. [DOI] [PubMed] [Google Scholar]