Abstract
Background:
Cardiac resynchronization therapy (CRT) reduces mortality and heart failure hospitalizations in patients with mild heart failure.
Objective:
To estimate the cost-effectiveness of adding CRT to an implantable cardioverter-defibrillator (CRT-D) compared with implantable cardioverter-defibrillator (ICD) alone among patients with left ventricular systolic dysfunction, prolonged intraventricular conduction, and mild heart failure.
Design:
Markov decision model.
Data Sources:
Clinical trials, clinical registries, claims data from Centers for Medicare & Medicaid Services, and Centers for Disease Control and Prevention life tables.
Target Population:
Patients aged 65 years or older with a left ventricular ejection fraction (LVEF) of 30% or less, QRS duration of 120 milliseconds or more, and New York Heart Association (NYHA) class I or II symptoms.
Time Horizon:
Lifetime.
Perspective:
Societal.
Intervention:
CRT-D or ICD alone.
Outcome Measures:
Life-years, quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs).
Results of Base-Case Analysis:
Use of CRT-D increased life expectancy (9.8 years versus 8.8 years), QALYs (8.6 years versus 7.6 years), and costs ($286 500 versus $228 600), yielding a cost per QALY gained of $61 700.
Results of Sensitivity Analyses:
The cost-effectiveness of CRT-D was most dependent on the degree of mortality reduction: When the risk ratio for death was 0.95, the ICER increased to $119 600 per QALY. More expensive CRT-D devices, shorter CRT-D battery life, and older age also made the cost-effectiveness of CRT-D less favorable.
Limitations:
The estimated mortality reduction for CRT-D was largely based on a single trial. Data on patients with NYHA class I symptoms were limited. The cost-effectiveness of CRT-D in patients with NYHA class I symptoms remains uncertain.
Conclusion:
In patients with an LVEF of 30% or less, QRS duration of 120 milliseconds or more, and NYHA class II symptoms, CRT-D appears to be economically attractive relative to ICD alone when a reduction in mortality is expected.
Primary Funding Source:
National Institutes of Health, University of Copenhagen, U.S. Department of Veterans Affairs.
Cardiac resynchronization therapy (CRT) aims to restore ventricular synchrony by simultaneously pacing the right and left ventricles. Previous studies have shown that CRT improves clinical outcomes (1–3) and is cost-effective (4–7) among patients who have moderate to severe heart failure, reduced systolic function, and ventricular conduction delay. Current clinical guidelines thus recommend CRT in addition to an implantable cardioverter-defibrillator (ICD) in this population (8, 9).
The potential value of adding CRT to an ICD is less clear for patients with mild heart failure. Recent clinical trials have shown that CRT also improves outcomes in patients with mild heart failure (10–14), but the cost-effectiveness of CRT in these less symptomatic patients has yet to be established. The only previous studies of this question were limited by being trial-based and having only a short-term follow-up (15) or by substantial assumptions regarding projected mortality (16).
Most patients with left ventricular systolic dysfunction receive an ICD because it reduces mortality (17–19), is cost-effective (8, 20), and is recommended by clinical guidelines (8, 9). Consequently, the most clinically relevant question about CRT is not whether the device is cost-effective compared with medical therapy alone but whether adding CRT capability to ICD (CRT-D) is cost-effective. Furthermore, because the role of CRT is well-established among patients with New York Heart Association (NYHA) class III or IV heart failure, we assessed the cost-effectiveness of CRT-D for patients with NYHA class I or II heart failure who had reduced systolic function (left ventricular ejection fraction ≤30%), and QRS duration of at least 120 milliseconds.
Methods
Study Design
We developed a decision-analytic model to estimate the lifetime costs and benefits of CRT-D versus ICD in patients with mild heart failure. We discounted costs and benefits at an annual 3% rate (21) and performed the analysis from a societal perspective. We expressed outcomes in terms of 2014 U.S. dollars, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). Four of the authors performed independent literature searches in PubMed, focused on publications relevant to our study population, to ascertain the values used to populate the model assumptions.
Decision-Analytic Model
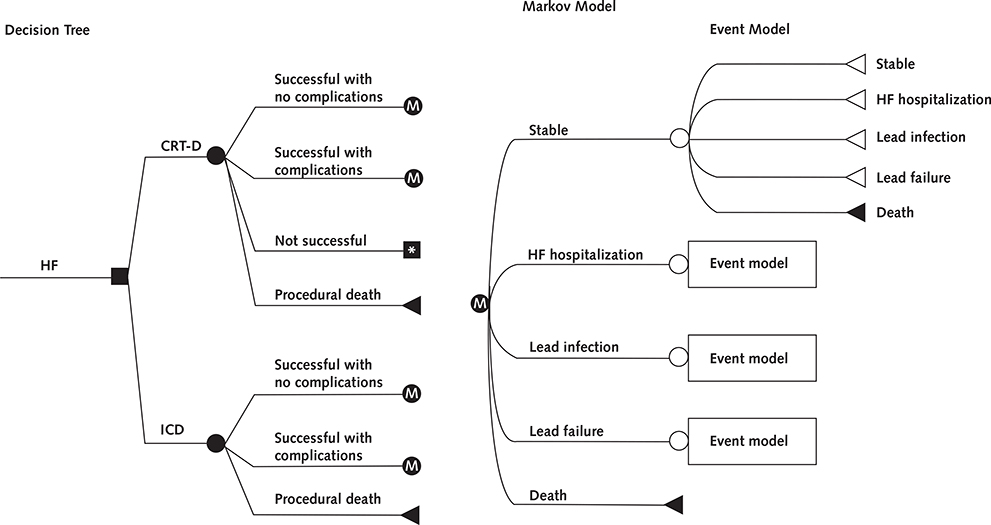
We used a 2-component model: a decision tree to assess the costs and outcomes of initial implantation of a CRT-D or ICD device, followed by a state-transition Markov model to assess long-term outcomes (Figure 1). Device implantation could be successful without complications, be successful with complications, be unsuccessful, or result in procedural death. Patients who had successful device implantation entered the Markov model, which tracked a hypothetical cohort of 65-year-old patients with mild heart failure (NYHA class I or II symptoms) until death or age 100 years. Given that the most common reason for failed CRT-D implantation is an inability to pass the coronary sinus lead, we assumed that patients with an unsuccessful CRT-D implantation received an ICD. During each 30-day cycle, patients could remain stable, become hospitalized for heart failure, experience lead infection or lead failure, or die. We constructed the model by using TreeAge Pro 2013 (TreeAge Software). The model is available upon request.
Figure 1.
Decision tree and Markov model.
The square represents the choice between the implantation of an ICD or CRT-D, whereas the circles represent chance nodes. Patients who receive a device can have a successful implantation with or without complication or die during the procedure. Patients assigned to receive CRT-D can further have an unsuccessful implantation, in which case they will receive an ICD. Patients surviving implantation enter the Markov model (assigned “M”), which represents the clinical events that can occur during each 30-day period until the patient dies. CRT-D = cardiac resynchronization therapy combined with an ICD; HF = heart failure; ICD = implantable cardioverter-defibrillator.
* Remains in CRT-D cohort but receives ICD with same outcomes/risks as “ICD successful with no complications” group.
Mortality
Because the risk for death from general (noncardiac) causes increases with age, we separated the risk for death into general population and excess cardiac components (see Appendix for detailed description of model inputs, available at www.annals.org). We based our general population mortality on the age- and sex-specific risks for death from the Centers for Disease Control and Prevention life tables (22), and we used the risk for cardiovascular death from the ICD group of RAFT (Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (12) to estimate excess cardiac mortality. We held the excess cardiac mortality constant while allowing the general population mortality to rise with age; cardiac death therefore comprised a gradually decreasing fraction of the overall risk for death. Given that the age-specific risks for death differ for men and women, we first studied male and female cohorts separately. To estimate the expected value for the overall population, we then weighted these results according to the populations enrolled in RAFT and the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (79% male patients) (11, 12).
Efficacy
We modeled the efficacy of CRT-D on the basis of reductions in the risk for heart failure hospitalization and death, assuming that CRT-D reduced only cardiac (as opposed to all-cause) mortality. We based the magnitude of the benefits on the results of a recent meta-analysis of trials that compared CRT-D primarily with ICD (Appendix Table 1, available at www.annals.org), using only the estimates reported for patients with NYHA class I or II symptoms (0.8 in the base-case analysis) (13).
We determined the risk for initial heart failure hospitalization from the results of MADIT-CRT (11) and RAFT (only patients with NYHA class II symptoms) (12). We accounted for the increased risk for heart failure rehospitalization, as well as the increased risk for death within 30 days of the initial hospitalization, by using previously reported data (23). We used the duration of hospitalization for heart failure from the mean length of stay for fiscal year 2014 reported by the Centers for Medicare & Medicaid Services (CMS) for diagnosis-related group (DRG) 292 (Table 1).
Table 1.
Base-Case Model Parameters
| Parameter | CRT-D | ICD | References |
|---|---|---|---|
| Patient age, y | 65 | 65 | 11, 12 |
| Device implantation, % | |||
| Procedural success | 94 | 100 | 13 |
| Implant complication* | 5 | 3 | 24 |
| Procedural death | 0.3 | 0.2 | 24 |
| Efficacy | |||
| Mortality (risk ratio, CRT-D vs. ICD)† | 0.80 | - | 13 |
| Hospitalization (risk ratio, CRT-D vs. ICD) | 0.69 | - | 12, 13 |
| Mortality | |||
| Baseline riskfor cardiac death‡ | 0.0037 | 0.0037 | 12 |
| Device complications | |||
| Lead failure‡ | 0.0022 | 0.0015 | 25 |
| Lead infection‡ | 0.0007 | 0.0007 | 25–28 |
| Death from lead failure‡ | 0.013 | 0.013 | 29 |
| Death from lead infection‡ | 0.05 | 0.05 | 27, 30 |
| Length of lead failure hospitalization, d | 2 | 2 | Expert opinion |
| Length of lead infection hospitalization, d | 5 | 5 | CMS |
| Battery life, y | 4 | 5 | 31 |
| Heart failure events | |||
| Baseline hospitalization‡ | 0.006 | 0.008 | 11, 12 |
| Subsequent hospitalization‡ | 0.06 | 0.06 | 23 |
| Death afterheart failure hospitalization‡ | 0.05 | 0.05 | 23 |
| Length of heart failure hospitalization, d | 5 | 5 | CMS |
| Device costs, $ | |||
| Implantation without complication | 40 892 | 33 323 | CMS |
| Implantation with complication | 49 418 | 41 850 | CMS |
| Failed CRT-D implantation | 33 892 | - | CMS |
| Lead failure | 16 425 | 16 361 | CMS |
| Lead infection | 49 149 | 40 812 | CMS |
| Generator change | 34 902 | 27 884 | CMS |
| Heart failure costs, $ | |||
| Heart failure hospitalization | 6227 | 6227 | CMS |
| Heart failure background costs | 1298/mo | 1298/mo | CMS, 32, 33 |
| Utilities | |||
| Baseline | 0.884 | 0.874 | 15 |
| Decrement for age75yorolder | Baseline × 0.931 | Baseline × 0.931 | 34 |
| Decrement forheart failure hospitalization | Baseline × 0.828for7 d§, followed by 0 for 5 d | Baseline × 0.828 for 7 d§, followed by 0 for 5 d | 19, 35 |
| Decrement for lead failure | 0 for 2 d | 0 for 2 d | 19 |
| Decrement for lead infection | 0 for 5 d | 0 for 5 d | 19 |
CMS = Centers for Medicare & Medicaid Services;CRT-D = cardiac resynchronization therapy combined with an implantable cardioverter-defibrillator; ICD = implantable cardioverter-defibrillator.
Defined as pneumothorax, hematoma, and complications related to the coronary sinus.
Corresponding to a derived risk ratio (applied to cardiac death only) of 0.69.
Indicates unit in monthly probability terms.
Corresponding to a drop to New York Heart Association class III symptoms.
Adverse Events
We obtained the probabilities of complications related to device implantation from systematic reviews of the clinical trials evaluating ICD and CRT devices (13, 24) and the probabilities of lead failure, lead infection, and resultant death based on published registry data (25–30). We obtained the length of hospitalization associated with a lead infection from the mean length of stay for DRG 872 from CMS fiscal year 2014 (Table 1).
Costs
We included physician, anesthesia, and hospital costs related to device implantation, device extraction, and device reimplantation by using the CMS Physician Fee Schedule Final Rule for physician and anesthesia costs from fiscal year 2014 (see the Appendix for detailed description of model inputs), and we estimated the amount of anesthesia time required for each implantation on the basis of expert opinion. For hospital costs (CMS Final Rule for fiscal year 2014), we included labor, nonlabor, and capital costs with the appropriate DRG multiplier. The incremental cost of a CRT-D device was $7000, based on expert opinion and literature estimates (15). We estimated the expected battery lives of CRT-D and ICD devices according to previously reported data (31). We accounted for both physician and hospital costs associated with subsequent hospitalizations. For both cohorts, we estimated costs associated with routine heart failure care by including outpatient visits, prescribed drugs, and laboratory expenses (32), and we assumed device interrogation costs to be equivalent between the 2 groups. These costs were superimposed on age-specific background health care costs (Table 1) (33).
Utilities
On the basis of EuroQol-5D index scores, we used baseline utility values of 0.884 for patients with CRT-D and 0.874 for patients with ICD to reflect both the quality-of-life decrement associated with mild heart failure in the stable state and the ability of CRT to alleviate heart failure symptoms (15). To account for the decrease in quality of life associated with aging, we multiplied the baseline utility by a factor of 0.931 for individuals aged 75 years and older, based on previously reported age-specific utilities (see the Appendix for detailed description of model inputs) (34).
For heart failure hospitalization and device-related complications, we assessed quality-of-life tolls (drop to zero) for the duration of time spent in the hospital (19). For heart failure hospitalizations, we further accounted for a reduction in quality of life to the level of NYHA class III symptoms for a specific period before hospitalization (assumed to be 7 days in our base-case analysis) by multiplying the baseline utility by a factor of 0.828 (Table 1) (35) for a short-term morbidity deduction of 1.2 quality-adjusted days of life.
Model Validity
We assessed the internal and predictive validity of the model. We compared the survival curves predicted by the model for each cohort to those reported by the MADIT-CRT and RAFT trials (11, 12). In addition, we compared the modeled survival of the ICD cohort to long-term follow-up data available from the MADIT-II ICD primary prevention trial (36).
Sensitivity and Uncertainty Analyses
We performed 1- and 2-way deterministic sensitivity analyses to ascertain key drivers influencing the cost-effectiveness of CRT-D. We altered the proportion of deaths attributable to cardiovascular causes (and, simultaneously, the relative risk reduction in mortality from CRT-D versus ICD). We also conducted an exploratory analysis to evaluate the effects of varying the mortality risk ratio in a cohort consisting only of patients with NYHA class I symptoms, using higher baseline utility values (see the Appendix for detailed description of model inputs) and assuming that CRT-D had no benefit on the rate of heart failure hospitalization. Finally, we performed probabilistic sensitivity analyses to account for the influence of simultaneous changes in correlated and uncertain model inputs on the cost-effectiveness of CRT-D. We randomly sampled model inputs from distributions based on their expected values and uncertainty distributions derived from a review of the literature and clinical judgment (Appendix Table 2, available at www.annals.org) and ran 10 000 independent simulations to estimate the probability that CRT-D would meet a particular willingness-to-pay threshold (37).
Role of the Funding Source
None of the funding sources had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Validity of the Model
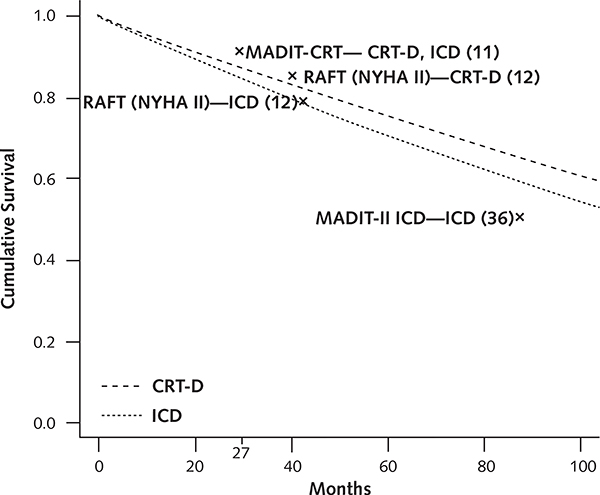
The model’s mortality estimates for both the ICD and CRT-D cohorts fell between the values reported by the MADIT-CRT and RAFT investigators (Appendix Figure 1, available at www.annals.org). At 40 months, our cohort had 117 deaths in the CRT-D group and 150 deaths in the ICD group (compared with 110 and 154, respectively, in RAFT), along with 123 heart failure hospitalizations in the CRT-D group and 174 in the ICD group (compared with 115 and 159, respectively, in RAFT) (Appendix Table 3, available at www.annals.org). In addition, the number of model-predicted clinical events was within the range reported by the studies. These findings indicate that the model appeared to reasonably reflect the study outcomes of RAFT (which excluded patients with NYHA class I symptoms) but overestimated event rates compared with the MADIT-CRT trial, which comprised patients with NYHA class I and II symptoms.
Base-Case Analysis
We found that CRT-D increased life expectancy (9.8 years versus 8.8 years) and QALYs (8.6 years versus 7.6 years) compared with implantation of an ICD alone (Table 2). Further, CRT-D reduced the number of heart failure hospitalizations per person (0.9 versus 1.1). The shorter battery life of CRT-D devices, combined with the prolonged survival, resulted in more generator changes per patient in this cohort (3.1 versus 2.2). Adverse events related to the devices were rare, with the number of lead failures and lead infections similar between the 2 groups.
Table 2.
Health and Economic Outcomes of CRT-D versus ICD
| Variable | CRT-D | ICD |
|---|---|---|
| Adverse events | ||
| Heartfailure hospitalizations per patient | 0.9 | 1.1 |
| Generator changes per patient | 3.1 | 2.2 |
| Lead failures per patient | 0.3 | 0.2 |
| Lead infections per patient | 0.1 | 0.1 |
| LYs | 9.8 | 8.8 |
| QALYs | 8.6 | 7.6 |
| Lifetime costs, $ | 286 500 | 228 600 |
| Incremental cost perLY and QALY gained versus ICD, $ |
||
| Cost/LY gained | 59 500/LY | - |
| Cost/QALY gained | 61 700/QALY | - |
CRT-D = cardiac resynchronization therapy combined with an implantable cardioverter-defibrillator; ICD = implantable-cardioverter defibrillator; LY = life-year; QALY = quality-adjusted life-year.
Overall, CRT-D increased average lifetime health care expenditures by $77 000, most of which was due to the higher cost of the initial device implantation ($7600 including physician and anesthesia costs), generator changes ($47 500), and the cost of background heart failure care over the longer period of survival ($23 300); these increased expenditures were counterbalanced by $1400 in savings from fewer heart failure hospitalizations.
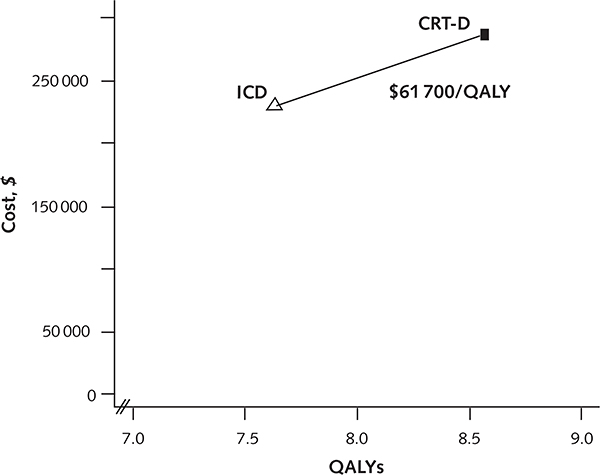
The improved health outcomes and higher cost of CRT-D relative to ICD alone yielded an ICER of $59 500 per life-year gained and $61 700 per QALY gained (Table 2; cost-effectiveness frontier as shown in Appendix Figure 2, available at www.annals.org).
Sensitivity Analyses
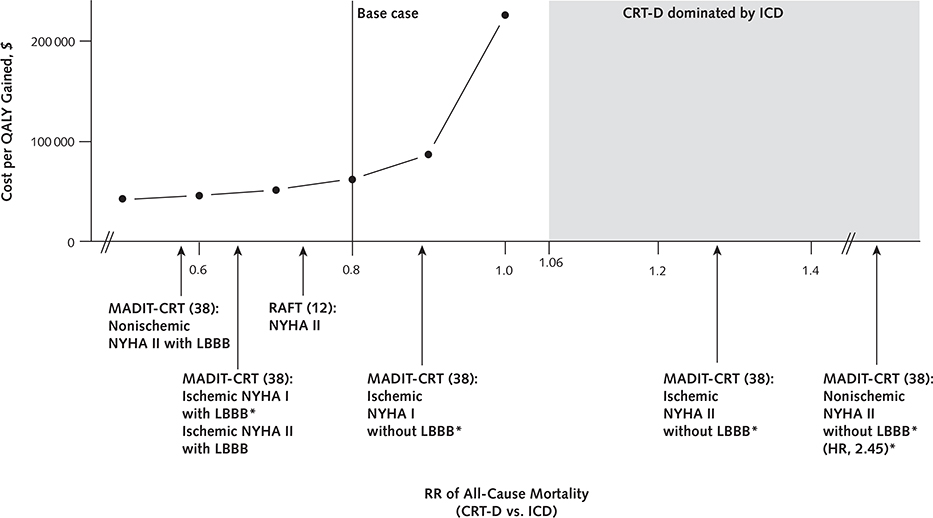
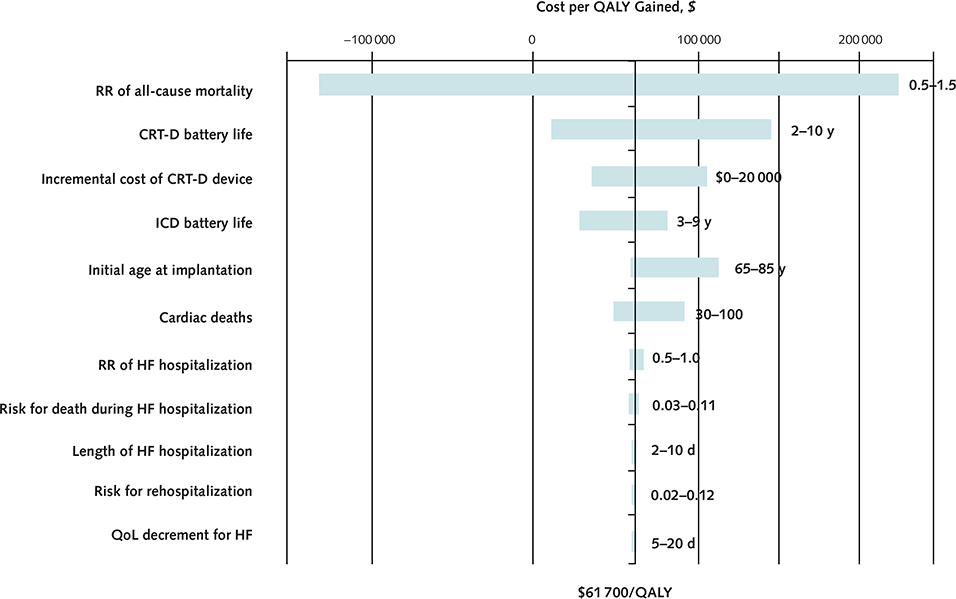
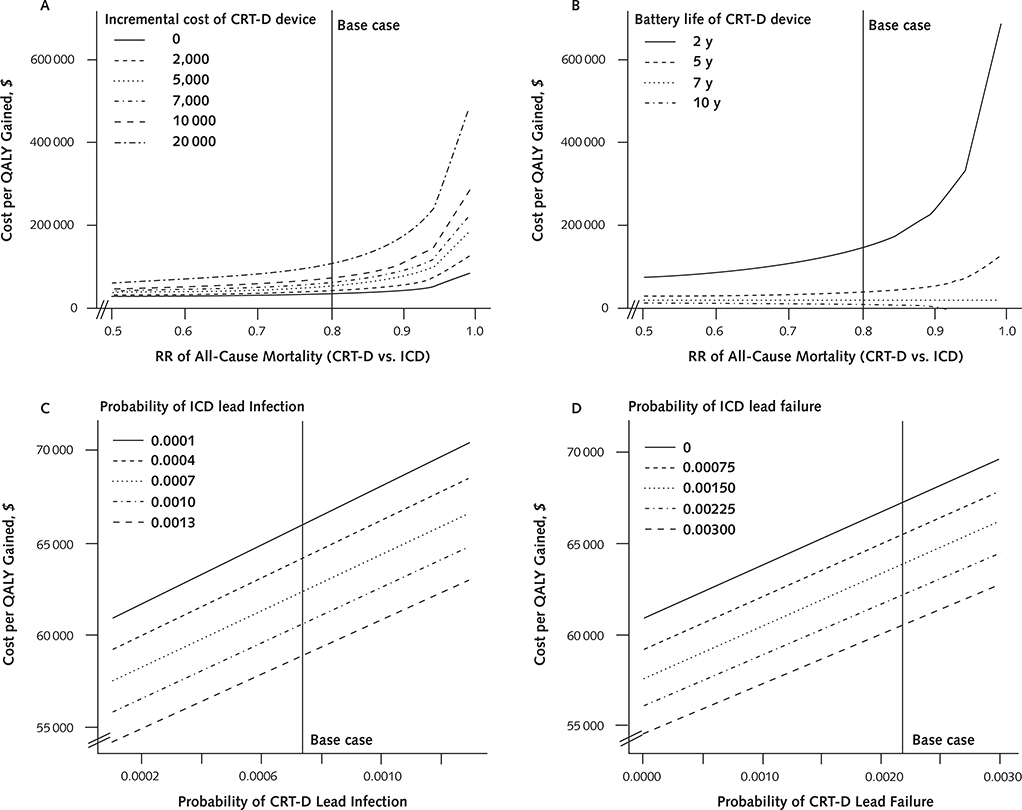
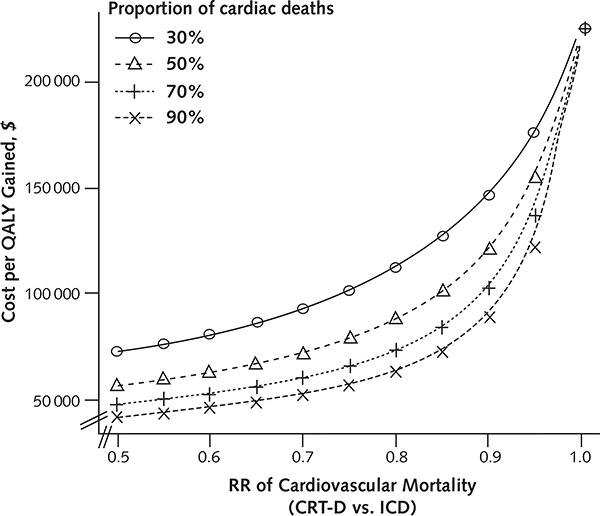
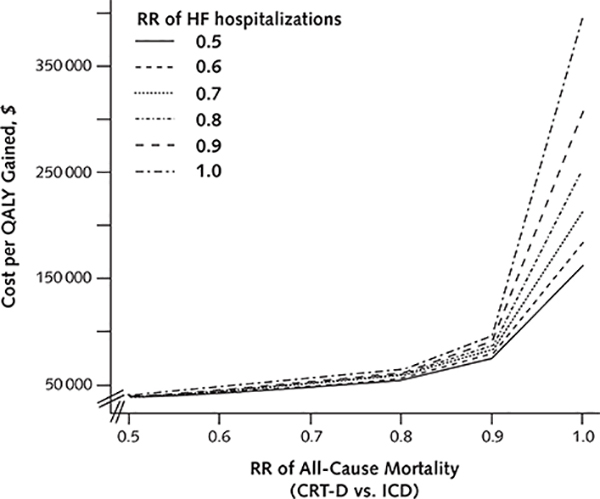
The magnitude of mortality benefit associated with CRT-D was the key determinant of its cost-effectiveness (Appendix Figure 3, available at www.annals.org). Specifically, CRT-D became less cost-effective as the survival difference between the 2 groups narrowed (Figure 2; Appendix Figure 4 [panels A and B], available at www.annals.org). At a risk ratio of 0.93 for all-cause mortality, CRT-D cost $100 000 per QALY gained; at a risk ratio of 0.97, CRT-D cost $150 000 per QALY gained. At risk ratios exceeding 1.06, ICD dominated CRT-D.
Figure 2.
Effect of changes in mortality risk ratio on model outcome.
Arrows indicate the risk ratios of different patient subgroups. The shaded region demarcates the risk ratio range over which ICD dominates CRT-D. CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; LBBB = left bundle branch block; MADIT-CRT = Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy; NYHA = New York Heart Association class; QALY = quality-adjusted life-year; RAFT = Resynchronization-Defibrillation for Ambulatory Heart Failure Trial; RR = risk ratio.
* Subgroups in which there is a non–statistically significant change in relative risk.
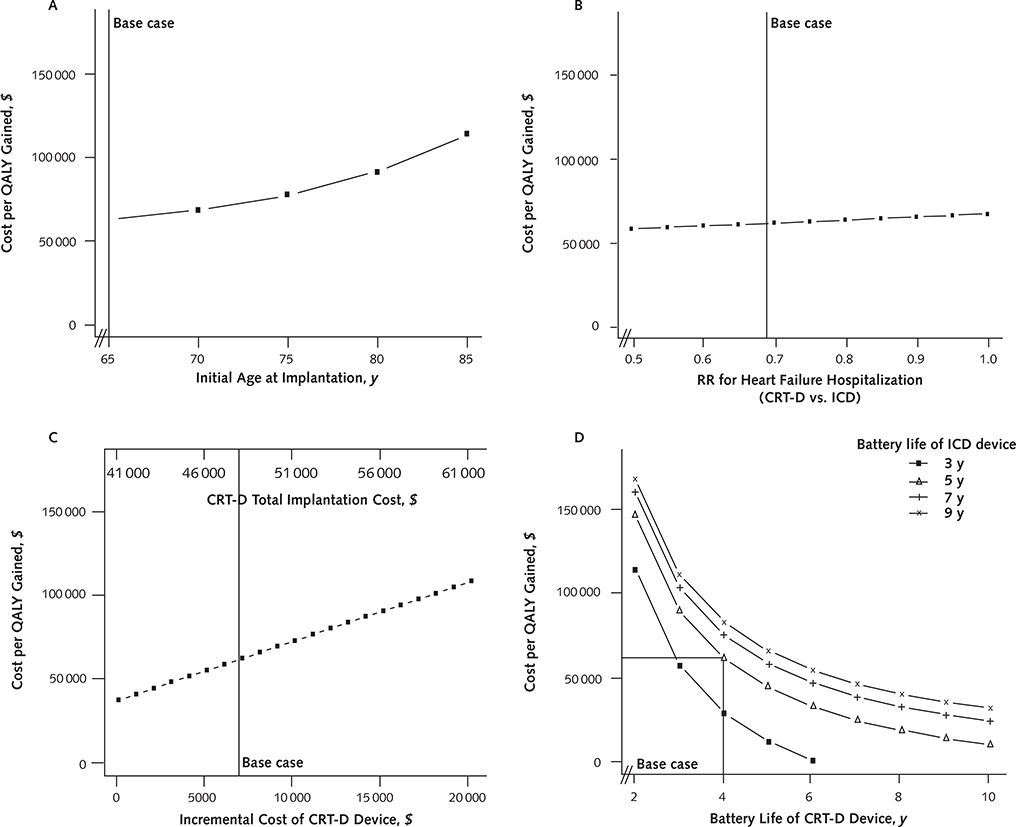
In older patients, CRT-D was less cost-effective. At an initial implantation age of 85 years, it cost $113 100 per QALY gained (Figure 3, A).
Figure 3.
Sensitivity analyses.
The influence on the incremental cost-effectiveness ratio on varying ages at initial device implantation (A), RRs for heart failure hospitalization (B), incremental costs of CRT-D versus ICD (C), and battery lives of devices (D). CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; QALY = quality-adjusted life-year; RR = risk ratio.
The extent to which CRT-D reduced the risk for heart failure hospitalizations did not materially affect the cost-effectiveness of the device: varying the risk ratio from 0.5 to 1.0 increased the cost of CRT-D per QALY gained from $58 800 to $68 400 (Figure 3, B).
We examined the effect of device cost on the cost-effectiveness of CRT-D to account for the fact that device prices may vary by model. If the additional cost of the CRT-D device beyond that of the ICD exceeded $18 000, CRT-D cost more than $100 000 per QALY gained (Figure 3, C).
The battery life of CRT-D was also an important factor: CRT-D became less cost-effective when its battery life was considerably shorter than that of ICD (Figure 3, D). Relative to an assumed average ICD battery life of 5 years, the cost of CRT-D approached $100 000 per QALY gained when CRT-D battery life decreased to less than 3 years. Conversely, at an ICD battery life of 3 years, CRT-D became cost-saving when CRT-D battery life exceeded 6 years.
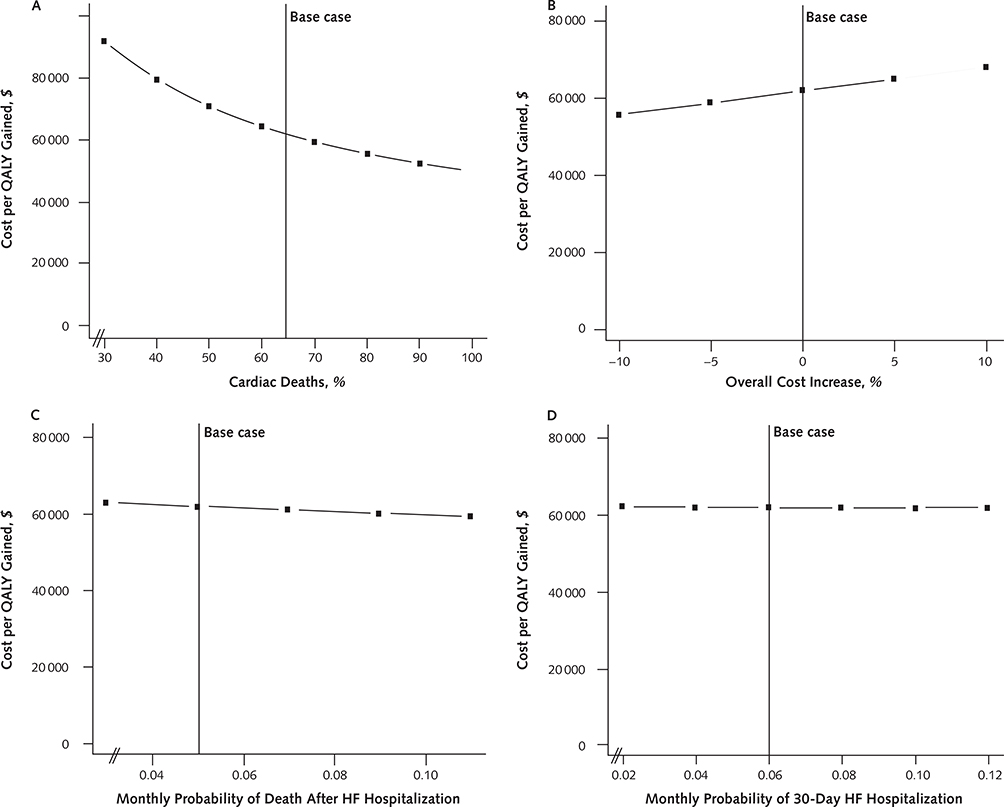
As the proportion of deaths attributable to cardiovascular causes increased, CRT-D became more cost-effective. When 85% of deaths were cardiovascular, the relative risk reduction in mortality from CRT-D versus ICD improved to 0.74 (from 0.80) and the cost-effectiveness of CRT-D decreased to $53 900 per QALY. When we performed analysis on a cohort of patients with a mortality rate paralleling that of MADIT-CRT, the cost of CRT-D was $83 600 per QALY (Appendix Figures 5 [panel A] and 6, available at www.annals.org).
The cost-effectiveness of CRT-D did not change substantially with rates of device complications (Appendix Figure 4, C and D), variations in all costs associated with both treatment strategies (Appendix Figure 5, B), risk for death after heart failure hospitalization (Appendix Figure 5, C), risk for 30-day rehospitalization (Appendix Figure 5, D), or the duration of utility drop resulting from heart failure rehospitalization (Appendix Figure 3). Furthermore, variations in baseline utilities or the magnitude of utility decrement associated with hospitalization or worsening heart failure did not affect the cost-effectiveness of CRT-D.
In our exploratory analysis of a cohort consisting only of patients with NYHA class I, mortality risk ratios of 0.91 and 0.95 were required for CRT-D to cost less than $100 000 and $150 000 per QALY, respectively (Appendix Figure 7, available at www.annals.org).
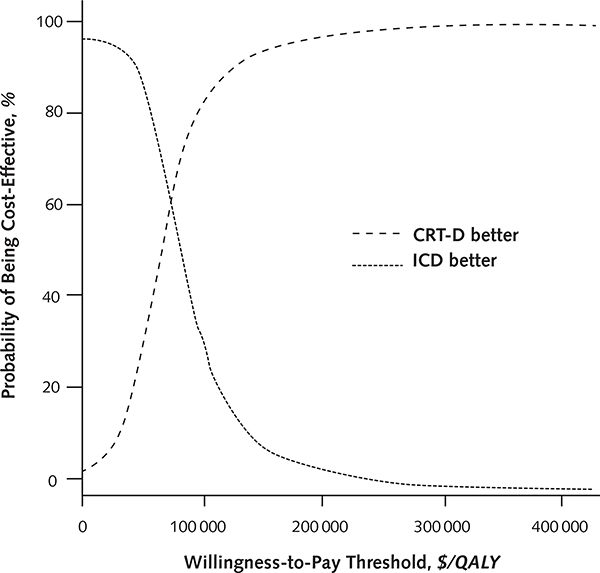
In probabilistic sensitivity analysis, CRT-D was preferred in 31% of simulations at a willingness-to-pay threshold of $50 000, in 79% of simulations at a threshold of $100 000, and in 93% at a threshold of $150 000 (Appendix Figure 8, available at www.annals.org).
Discussion
In patients with mildly symptomatic heart failure, reduced left ventricular function, and prolonged QRS duration, implantation of a CRT-D appears to be economically attractive compared with an ICD alone, with an ICER of $61 700 per QALY gained. This finding depends on CRT-D providing a mortality benefit and is thus most applicable to patients with NYHA class II symptoms and QRS duration greater than 150 milliseconds or left bundle branch block (LBBB). Given that such a survival benefit has not yet been established in patients with NYHA class I symptoms, the cost-effectiveness of CRT-D in this setting remains uncertain.
The cost per QALY gained of CRT-D increased sharply when the reduction in the risk for death was assumed to be less, underscoring the importance of proper patient selection for cardiac resynchronization. Both RAFT and MADIT-CRT found CRT-D to be more beneficial in patients with QRS duration greater than 150 milliseconds, and long-term follow-up data from MADIT-CRT suggest that the mortality benefit of CRT-D may be limited to patients with LBBB (38). These findings parallel the CRT guidelines for patients with NYHA class II symptoms put forth by the major societies, with the strongest recommendation being for patients with an LBBB greater than 150 milliseconds and the weakest for patients with a non-LBBB greater than 150 milliseconds (9).
In addition, MADIT-CRT demonstrated a nonsignificant trend toward mortality benefit in patients with ischemic cardiomyopathy, LBBB, and NYHA class I symptoms, indicating that QRS width and morphology may also help identify a subset of patients with NYHA class I symptoms in whom CRT-D is most clinically efficacious and cost-effective. Similarly, CRT-D may be more cost-effective in patients with nonischemic cardiomyopathy because such patients are potentially more likely to derive physiologic benefit from this intervention (39). Conversely, the degree of systolic dysfunction may not be a useful criterion by which patients are stratified, as a post hoc analysis of MADIT-CRT demonstrated that the magnitude of benefit conferred by CRT-D was independent of baseline left ventricular ejection fraction (40).
The incremental cost-effectiveness of CRT-D in our study is similar to that of the ICD ($50 000 to $70 000 per QALY gained) (19), which is the other established device therapy for heart failure.
A recent comparative effectiveness evaluation of CRT-D and ICD supports the clinical benefit of CRT-D seen in randomized trials. Using propensity-score matching in a real-world, contemporary patient population, Masoudi and colleagues found that CRT-D reduced all-cause mortality and cardiovascular readmission to a degree similar to that reported in randomized trials (41). Furthermore, they concluded that the reduction in heart failure hospitalization associated with CRT-D was most evident in patients with LBBB, again suggesting that LBBB may be a potential characteristic to help identify patients in whom CRT-D may be most cost-effective.
The results of the remaining sensitivity analyses were intuitive. The cost-effectiveness of CRT-D was attenuated as it became more expensive relative to ICD. Similarly, more durable CRT-D batteries resulted in fewer generator changes and lower cost, thus favoring CRT-D. The decreased life expectancy of very elderly individuals reduced the magnitude of the mortality benefit of CRT-D, thus making it more expensive in this population. Finally, because we confined the CRT-D mortality benefit to reduce only death from cardiac causes, CRT-D became more cost-effective with an increase in the proportion of cardiac deaths.
It may seem surprising that the model results were not influenced by the changes in the risk reduction for heart failure hospitalization attributable to CRT-D, rate of heart failure rehospitalization, risk for death during a heart failure hospitalization, or duration of utility decrease resulting from heart failure rehospitalization. These findings probably reflect the overall severity of illness in this patient population; in patients with mild failure, these events are too rare to exert any meaningful difference on the overall outcome.
Unlike previous studies evaluating the cost-effectiveness of CRT-D in mild heart failure (15, 16), our study evaluated outcomes based on a lifetime perspective and used values from published trial data (as opposed to estimates based on projected survival functions) to model mortality.
To account for the increasing risk for noncardiac causes of death over time, we elected to hold the absolute risk for cardiac death constant. We based this value on the proportion of cardiac deaths reported in RAFT, which is lower than that reported in other trials of patients with mild heart failure (42). We did not augment the magnitude of the mortality benefit of CRT-D over time to account for patients progressing to more advanced stages of heart failure, although these patients are likely to derive more benefit from cardiac resynchronization (1–3). We used such a strategy because we believed it to be a conservative one that minimizes the risk of overstating the clinical efficacy and cost-effectiveness of CRT-D.
The point estimate that we used to quantify the benefit of CRT-D was heavily influenced by the results of RAFT; in contrast, the primary results of MADIT-CRT did not find a mortality benefit associated with CRT-D. This may have been due to the lower mortality rate of the MADIT-CRT population—which included patients with NYHA class I and II symptoms—as well as the shorter duration of follow-up of MADIT-CRT (29 months versus 40 months), as the survival curves between the CRT-D and ICD treatment groups in RAFT began to separate after 2 years (12). Furthermore, the survival benefit associated with cardiac resynchronization in lower-risk patients is estimated to accelerate over time (43), and as mentioned previously, 67-month follow-up data from MADIT-CRT did find a mortality benefit associated with CRT-D in patients with LBBB (38).
We modeled our cohort on the basis of the demographic characteristics of the RAFT and MADIT-CRT participants, who were predominantly male; our findings may thus not completely apply to women. However, the CRT-related clinical benefit may be greater among women (potentially because their cardiomyopathy is more likely to be nonischemic in nature) (44), suggesting that the cost-effectiveness of CRT-D may be more favorable in women than in men.
We did not evaluate the cost-effectiveness of CRT-D versus CRT only. However, the relevance of this question is unclear because the most recent treatment guidelines recommend the implantation of an ICD in the types of patients included in our study cohort (8, 20). Finally, we used Medicare costs in our analysis, and thus our findings may not be generalizable to other countries.
In conclusion, among patients with mildly symptomatic heart failure, left ventricular systolic dysfunction, and prolonged QRS duration, the addition of CRT to ICD may be cost-effective relative to ICD alone, with an ICER of $61 700 per QALY. This result depends on a mortality reduction from CRT-D and is thus most applicable to patients with NYHA class II symptoms who have QRS duration of 150 milliseconds or greater or LBBB.
EDITORS’ NOTES.
Context
Although cardiac resynchronization therapy (CRT) improves outcomes for patients with mild heart failure, it is not known whether it is cost-effective.
Contribution
In this analysis, CRT in addition to the implantation of an implantable-defibrillator (ICD) was cost-effective.
Caution
Available data did not allow evaluation of cost-effectiveness in patients with asymptomatic (New York Heart Association class I) heart failure.
Implication
CRT with ICD appears to be cost-effective in patients with mild heart failure.
Acknowledgments
Grant Support: Dr. Woo was supported by a KL2 award from the National Mentored Career Development Award of the Stanford Clinical and Translational Science Award (National Institutes of Health [NIH] KL2 TR 001083). Ms. Strandberg was supported by an NIH/National Library of Medicine Research Training Award (5 T15 LM007033–31). Dr. Schmiegelow was supported by a grant from the University of Copenhagen, Denmark. Dr. Owens was supported by the U.S. Department of Veterans Affairs. Dr. Goldhaber-Fiebert was supported in part by an NIH/National Institute on Aging Career Development Award (NIH K01 AG037593).
Disclosures: Dr. Woo reports grants from National Institutes of Health during the conduct of this study. Drs. Hlatky and Owens report personal fees from Zoll LifeVest outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14–1804.
Appendix: Detailed Description of Model Inputs
Mortality
We based the proportion of cardiac deaths in our cohort (65% in the base-case analysis) on the proportion of cardiac deaths in RAFT (12) because it was the only trial of the benefit of CRT-D in mild heart failure that reported the risk for cardiac death separately. To account for the fact that patients with heart failure have an increased risk for death when compared with the general population, we used the risk for death from the Centers for Disease Control and Prevention death tables to approximate the risk for noncardiac death in our cohort. We then estimated total mortality by summing the risk for cardiac death in the ICD group of RAFT with the respective Centers for Disease Control and Prevention risk for death and varied the proportions of cardiac death in our sensitivity analyses.
Risk Ratio
Given that most trials reported their results in terms of all-cause mortality, we translated the reported risk ratio for all-cause mortality into a derived risk ratio for cardiac mortality—assuming that CRT-D had no benefit on noncardiac mortality—on the basis of the following equation: risk ratio for all-cause mortality = (proportion of cardiac death × derived risk ratio) + (proportion of noncardiac death × 1). For the base case, we assumed a risk ratio for all-cause mortality of 0.8 (13) and cardiac death proportion of 65% (12), yielding a derived risk ratio of 0.69. We then applied this derived risk ratio to the risk for cardiac death for patients receiving a CRT-D device.
Implantation Complications
We used the rates of implantation-related complications reported in a systematic review of randomized, controlled trials by van Rees and colleagues (24). For ICD, we used the rates for nonthoracotomy ICD implantation and calculated the overall implantation complication rate as the sum of pneumothorax (0.9%) and hematoma and bleeding at the implant site (2.2%): 3.1% in total. For CRT-D implantation, the complication rate was calculated as the sum of pneumothorax (0.9%), complications related to the coronary sinus (2.0%), and hematoma and bleeding at the implant site (2.4%): 5.3% in total.
Costs
We considered physician, anesthesia, and hospital costs related to procedures and hospitalizations. We calculated physician procedure costs by using the CMS Physician Fee Schedule Final Rule for fiscal year 2014. On the basis of this, we multiplied a conversion factor of 35.8228 by the sum of the physician work, practice expense, and malpractice relative value units (RVUs) for the appropriate Current Procedural Terminology (CPT) codes (33208, 33225, 33249, 33263, 33229, 33264, 33220, 33215, 33226, 33235, 33224, 33217). For each hospitalization, we calculated physician inpatient costs by multiplying the conversion factor by the sum of the RVUs for 1 admit day (CPT 99222), 1 discharge day (CPT 99239), and the balance of the hospital length of stay by the RVUs for CPT 99232.
We derived anesthesia costs associated with procedures via a conversion factor of 22.6765, base rate of 7, and CPT code 00534. We calculated the total cost per procedure by adding the base rate to the number of 15-minute increments involved in the procedure (for example, 8 for a 120-minute procedure), and then multiplying this sum by the conversion factor. The estimated times per procedure were based on expert opinion: ICD implantation (60 minutes), CRT-D implantation (120 minutes), generator change (30 minutes), lead revision (30 minutes), ICD system replacement (120 minutes), and CRT-D system replacement (240 minutes).
We also based total hospital costs on the CMS Final Rule for fiscal year 2014. We calculated the total hospital cost by multiplying the sum of the labor, nonlabor, and capital cost rates ($3316.23, $2032.53, and $425.49, respectively), by the appropriate DRG multiplier (DRG codes 226, 227, 245, 260, 261, 262, 265, 872).
Utilities
We assumed a baseline utility of 0.884 for the CRT-D group and 0.874 for the ICD group, which were data from the MADIT-CRT trial (mean age, 64 years [SD, 11]) (15). To account for age-related decrement in quality of life in individuals aged 75 years or older, we took the ratio of utilities Nyman and colleagues (34) reported for this age group (0.755) to that reported for 65- to 74-year-olds (0.811) and multiplied this ratio by the baseline utility.
To calculate the utility associated with a decrease to NYHA class III symptoms, we took the ratio of the NYHA class III utility (0.673) to the average of the NYHA class I and NYHA class II utilities (0.813) reported by Göhler and colleagues (35). We then multiplied this ratio by the baseline utility.
For our NYHA class I cohort analysis, we compared the baseline MADIT-CRT utility for the ICD group (0.874) to a weighted average of the NYHA class I and NYHA class II utilities reported by Göhler and colleagues (0.784, weighted according to the percentage of patients with NYHA class I symptoms enrolled in MADIT-CRT). We multiplied the ratio of these 2 values (1.081) by Göhler and colleagues’ NYHA class I utility (0.855), resulting in a baseline value of 0.924 for the ICD group. For the CRT-D group, we multiplied the ICD utility by the ratio between the CRT-D and ICD groups reported in MADIT-CRT (1.011), yielding a baseline value of 0.935 for the CRT-D group.
Appendix Table 1.
Randomized, Controlled Trials Evaluating the Efficacy of CRT-D versus ICD in Patients With Mild Heart Failure
| Study, Year (Reference) | Treatment Comparison | Patients, n | NYHA class | LVEF, % | QRS Duration, ms | Deaths, n | Months | HR for Mortality (95% CI) | HR for Heart Failure Hospitalization (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Al-Majed et al, 2011 (meta-analysis) (13) | CRT vs. usual care | 4054* | I/II | ≤40 | NA | 407 | 0.80 (0.67–0.96) | 0.69 (0.59–0.80) | |
| RAFT, 2010(12) | CRT-D vs. ICD | 138 | II | ≤30 | ≥120 | 264 | 40 | 0.73 (0.61–0.88) | 0.70 (0.55–0.85) |
| MADIT-CRT, 2009 (11) | CRT-D vs. ICD | 1820 | I/II | ≤30 | ≥120 | 127 | 29 | 1.00 (0.69–1.44) | 0.59 (0.47–0.74) |
| REVERSE, 2008 (10, 13) | CRT-on vs. -off | 610 | I/II | ≤40 | ≥130 | 12 | 12 | 1.37 (0.37–4.99) | 0.52 (0.26–1.01) |
| MIRACLE ICD II, 2004(13, 45) | CRT-D vs. ICD | 186 | II | ≤35 | ≥130 | 4 | 6 | 1.19 (0.17–8.26) | NA |
CRT-D = cardiac resynchronization therapy combined with an ICD; HR = hazard ratio; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; MADIT-CRT = Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy; MIRACLE ICD II = Multicenter InSync Randomized Clinical Evaluation ICD II; NA = not available; NYHA = New York Heart Association; RAFT = Resynchronization-Defibrillation for Ambulatory Heart Failure Trial; REVERSE = REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction.
Number of patients in analysis of all-cause mortality. The analysis of heart failure hospitalization included 3863 patients.
Appendix Table 2.
Parameter Distributions for Probabilistic Sensitivity Analysis
| Variable | Mean (95% CI) | Type | Defining Parameters | References |
|---|---|---|---|---|
| Device implantation | ||||
| CRT-D procedural failure (%) | 6.0 (5.5–6.5) | Beta | α=502.4, β=7871.6 | 13 |
| CRT-D implant complication (%) | 5.0 (4.6–5.4) | Beta | α=480.6, β=9131.4 | 24 |
| ICD implant complication (%) | 3.0 (2.4–3.7) | Beta | α=83.9, β=2714.1 | 24 |
| CRT-D procedural death (%) | 0.30(0.15–0.50) | Beta | α=11.6, β=3863.4 | 24 |
| ICD procedural death (%) | 0.20(0.07–0.40) | Beta | α=6.0, β=3010.0 | 24 |
| Efficacy | ||||
| Mortality (risk ratio, CRT-D vs ICD) | 0.80(0.67–0.96) | Log normal | μ=-0.228, SE=0.102 | 13 |
| Hospitalization (risk ratio, CRT-D vs ICD) | 0.69(0.59–0.80) | Log normal | μ=-0.374, SE=0.081 | 12, 13 |
| Device complications | ||||
| CRT-D lead failure* | 0.0022 (0.0020–0.0024) | Beta | α=411.9, β=186818.8 | 25 |
| ICD lead failure* | 0.0015(0.0014–0.0017) | Beta | α=421.3, β=280424.7 | 25 |
| Lead infection* | 0.0007 (0.006–0.008) | Beta | α=184.3, β=263099.7 | 25–28 |
| Death from lead failure* | 0.013(0.009–0.018) | Beta | α=34.2, β=2593.8 | 29 |
| Death from lead infection* | 0.05 (0.03–0.08) | Beta | α=11.4, β=216.6 | 27, 30 |
| Length of lead failure hospitalization (days) | 2.0 (0.0–5.9) | Log normal | μ=0.347, SE=0.833 | Expert opinion |
| Length of lead infection hospitalization (days) | 5.0 (4.8–5.2) | Log normal | μ=1.609, SE=0.020 | CMS |
| CRT-D battery life (years) | 4.0 (1.9–5.9) | Log normal | μ=1.326, SE=0.263 | 31 |
| ICD battery life (years) | 5.0 (2.2–8.0) | Log normal | μ=1.588, SE=0.287 | 31 |
| Heart failure events | ||||
| Baseline hospitalization* | 0.008(0.004–0.013) | Beta | α=11.7, β=1449.3 | 11, 12 |
| Subsequent hospitalization* | 0.060 (0.055–0.065) | Beta | α=609.1,0=9541.9 | 23 |
| Death following heart failure hospitalization* | 0.050(0.046–0.054) | Beta | α=507.6, β=9643.5 | 23 |
| Length of heart failure hospitalization (days) | 5 (5–5) | Log normal | μ=1.609, SE=0 | CMS |
| Device costs, $ | ||||
| CRT-D implantation without complication | 40 892 (38 936–42 848) | Normal | SE=997.76 | CMS |
| ICD implantation without complication | 33 323(31 729–34 917) | Normal | SE=813.08 | CMS |
| CRT-D implantation with complication | 49 418(47 161–51 675) | Normal | SE=1151.44 | CMS |
| ICD implantation with complication | 41 850(39 939–43 761) | Normal | SE=975.11 | CMS |
| Failed CRT-D implantation | 33 892 (32 271–35 513) | Normal | SE=827.00 | CMS |
| CRT-D lead failure | 16 425(15 163–17 686) | Normal | SE=643.62 | CMS |
| ICD lead failure | 16 361 (15 104–17 618) | Normal | SE=641.13 | CMS |
| CRT-D lead infection | 49 149(47 685–50 613) | Normal | SE=747.06 | CMS |
| ICD lead infection | 40 812 (39 596–42 027) | Normal | SE=620.34 | CMS |
| CRT-D generator change | 34 902 (31 872–37 932) | Normal | SE=1546.16 | CMS |
| ICD generator change | 27 884(25 463–30 305) | Normal | SE=1235.26 | CMS |
| Heart failure costs | ||||
| Heart failure hospitalization | 6227 (6041–6413) | Normal | SE=94.80 | CMS |
| Heart failure background costs | 1298/month (1256–1340) | Normal | SE=21.68 | CMS, 32, 33 |
| Utilities | ||||
| CRT-D baseline utility | 0.884 (0.860–0.906) | Beta | α=661.2 β=86.8 | 15 |
| ICD baseline utility | 0.874(0.844–0.902) | Beta | α=439.6, β=63.4 | 15 |
| Decrement prior to heart failure hospitalization | 0.828 (0.809–0.846) | Beta | α=1347.7 β=280.3 | 35 |
| Duration of decrement prior to heart failure hospitalization | 7 days (0–21) | Log normal | μ=1.599, SE=0.832 | Expert opinion |
CMS = Centers for Medicare & Medicaid Services; CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator.
Indicates unit in monthly probability terms.
Appendix Table 3.
Comparison of Model Results With Trial Data
| Outcome | Events/Patients, n/n (%) |
|||||
|---|---|---|---|---|---|---|
| Model Results |
RAFT Results (12) |
MADIT-CRT Results (11) |
||||
| CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | |
| Heart failure (29 mo) | 124/1089 (11.3) | 140/731 (19.1) | - | - | 151/1089(13.9) | 167/731 (22.8) |
| Death (29 mo) | 128/1089 (11.8) | 108/731 (14.8) | - | - | 74/1089(6.8) | 53/731 (7.3) |
| Heart failure hospitalization (40 mo) | 123/708 (17.3) | 174/730 (23.8) | 115/708(16.2) | 159/730 (21.8) | - | - |
| Death (40 mo) | 117/708 (16.5) | 150/730 (20.5) | 110/708(15.5) | 154/730 (21.1) | - | - |
CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; MADIT-CRT = Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy; RAFT = Resynchronization-Defibrillation for Ambulatory Heart Failure Trial.
Appendix Figure 1.
Survival curves for model cohorts.
The dashed line represents the survival curve for patients receiving CRT-D, and the dotted line the survival in patients receiving an ICD (in the current study cohort). In MADIT-CRT, 92.7% of patients receiving an ICD and 93.2% of those receiving CRT-D were alive after a mean follow-up of 29 months. In RAFT, 78.9% of patients receiving an ICD and 84.5% of those receiving CRT-D were alive after a mean follow-up of 40 months. Model survival curves are in essence Kaplan–Meier curves, assuming sampling without replacement. However, the survival estimates from the published studies are based on the mean follow-up time and the total number of deaths and thus assume sampling with replacement. CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; MADIT-II = Multicenter Automatic Defibrillator Implantation Trial II; MADIT-CRT = Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy; RAFT = Resynchronization-Defibrillation for Ambulatory Heart Failure Trial.
Appendix Figure 2.
Cost-effectiveness frontier.
CRT-D = cardiac resynchronization therapy combined with an implantable cardioverter-defibrillator; ICD = implantable cardioverter-defibrillator; QALY = quality-adjusted life-year.
Appendix Figure 3.
Cost of QALYs gained, by changes in variables in a one-way sensitivity analysis.
The bars show the change in the QALY gained of CRT-D with changes in the variables included in one-way sensitivity analysis. CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; HF = heart failure; QALY = quality-adjusted life-year; QoL = quality of life; RR = risk ratio.
Appendix Figure 4.
Additional sensitivity analyses.
The influence on the incremental cost-effectiveness ratio of varying mortality RRs and incremental device costs of CRT-D (A), mortality RRs and CRT-D battery lives (B), rates of lead infection (C), and rates of lead failure (D). The solid vertical line represents the base case. CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; QALY = quality-adjusted life-year; RR = risk ratio.
Appendix Figure 5.
Additional sensitivity analyses.
The influence on the incremental cost-effectiveness ratio of varying proportions of cardiac deaths (A), changes in overall cost levels (B), risks for death after HF hospitalization (C), and risks for 30-day repeat HF hospitalization (D). The solid vertical line represents the base case. HF = heart failure; QALY = quality-adjusted life-year.
Appendix Figure 6.
Additional sensitivity analyses.
The influence on the incremental cost-effectiveness ratio of varying proportions of cardiac deaths and RRs for cardiac death. CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; QALY = quality-adjusted life-year; RR = risk ratio.
Appendix Figure 7.
Effect of changes in mortality RR on model outcome only in patients with New York Heart Association class I symptoms.
CRT-D = cardiac resynchronization therapy combined with an ICD; HF = heart failure; ICD = implantable cardioverter-defibrillator; QALY = quality-adjusted life-year; RR = risk ratio.
Appendix Figure 8.
Probabilistic sensitivity analysis.
The curves illustrate the proportion of samples in which CRT-D or ICD is the preferred strategy at a given willingness-to-pay threshold. CRT-D = cardiac resynchronization therapy combined with an ICD; ICD = implantable cardioverter-defibrillator; QALY = quality-adjusted life-year.
Footnotes
Reproducible Research Statement: Study protocol and data set: Not available. Statistical code: Available from Ms. Strandberg.
Current author addresses and author contributions are available at www.annals.org.
References
- 1.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. ; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. ; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005; 352:1539–49. [DOI] [PubMed] [Google Scholar]
- 3.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–14. [DOI] [PubMed] [Google Scholar]
- 4.Nichol G, Kaul P, Huszti E, Bridges JF. Cost-effectiveness of cardiac resynchronization therapy in patients with symptomatic heart failure. Ann Intern Med. 2004;141:343–51. [DOI] [PubMed] [Google Scholar]
- 5.Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, et al. ; CARE-HF investigators. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J. 2005;26:2681–8. [DOI] [PubMed] [Google Scholar]
- 6.Feldman AM, de Lissovoy G, Bristow MR, Saxon LA, De Marco T, Kass DA, et al. Cost effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46: 2311–21. [DOI] [PubMed] [Google Scholar]
- 7.Yao G, Freemantle N, Calvert MJ, Bryan S, Daubert JC, Cleland JG. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28:42–51. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ; ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 9.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. ; American College of Cardiology Foundation. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013; 61:e6–75. doi: 10.1016/j.jacc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C; REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52: 1834–43. doi: 10.1016/j.jacc.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. ; MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009; 361:1329–38. doi: 10.1056/NEJMoa0906431 [DOI] [PubMed] [Google Scholar]
- 12.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. ; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540 [DOI] [PubMed] [Google Scholar]
- 13.Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med. 2011;154:401–12. doi: 10.7326/0003-4819-154-6-201103150-00313 [DOI] [PubMed] [Google Scholar]
- 14.Santangeli P, Di Biase L, Pelargonio G, Dello Russo A, Casella M, Bartoletti S, et al. Cardiac resynchronization therapy in patients with mild heart failure: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2011;32:125–35. doi: 10.1007/s10840-011-9584-y [DOI] [PubMed] [Google Scholar]
- 15.Noyes K, Veazie P, Hall WJ, Zhao H, Buttaccio A, Thevenet-Morrison K, et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol. 2013; 24:66–74. doi: 10.1111/j.1540-8167.2012.02413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linde C, Mealing S, Hawkins N, Eaton J, Brown B, Daubert JC; REVERSE study group. Cost-effectiveness of cardiac resynchronization therapy in patients with asymptomatic to mild heart failure: insights from the European cohort of the REVERSE (Resynchronization Reverses remodeling in Systolic Left Ventricular Dysfunction). Eur Heart J. 2011;32:1631–9. doi: 10.1093/eurheartj/ehq408 [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 18.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 19.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. [DOI] [PubMed] [Google Scholar]
- 20.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61:1318–68. doi: 10.1016/j.jacc.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 21.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 22.Arias E United States life tables, 2008. Natl Vital Stat Rep. 2012; 61:1–63. [PubMed] [Google Scholar]
- 23.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56:362–8. doi: 10.1016/j.jacc.2010.02.053 [DOI] [PubMed] [Google Scholar]
- 24.van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ, van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol.2011;58:995–1000. doi: 10.1016/j.jacc.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 25.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, et al. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–65. doi: 10.1016/j.hrthm.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 26.Uslan DZ, Sohail MR, St Sauver JL, Friedman PA, Hayes DL, Stoner SM, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–75. [DOI] [PubMed] [Google Scholar]
- 27.Margey R, McCann H, Blake G, Keelan E, Galvin J, Lynch M, et al. Contemporary management of and outcomes from cardiac device related infections. Europace. 2010;12:64–70. doi: 10.1093/europace/eup362 [DOI] [PubMed] [Google Scholar]
- 28.Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32: 991–8. doi: 10.1093/eurheartj/ehq497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng A, Wang Y, Curtis JP, Varosy PD. Acute lead dislodgements and in-hospital mortality in patients enrolled in the national cardiovascular data registry implantable cardioverter defibrillator registry. J Am Coll Cardiol. 2010;56:1651–6. doi: 10.1016/j.jacc.2010.06.037 [DOI] [PubMed] [Google Scholar]
- 30.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–9. [DOI] [PubMed] [Google Scholar]
- 31.Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL, et al. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013; 6:488–97. doi: 10.1161/CIRCOUTCOMES.111.000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banka G, Heidenreich PA, Fonarow GC. Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. J Am Coll Cardiol. 2013;61:1440–6. doi: 10.1016/j.jacc.2012.12.022 [DOI] [PubMed] [Google Scholar]
- 33.Hartman M, Catlin A, Lassman D, Cylus J, Heffler S. U.S. Health spending by age, selected years through 2004. Health Aff (Millwood). 2008;27:w1–w12. [DOI] [PubMed] [Google Scholar]
- 34.Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, Sullivan PW. Quality-of-life weights for the US population: self-reported health status and priority health conditions, by demographic characteristics. Med Care. 2007;45:618–28. [DOI] [PubMed] [Google Scholar]
- 35.Göhler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, et al. Utility estimates for decision-analytic modeling in chronic heart failure—health states based on New York Heart Association classes and number of rehospitalizations. Value Health. 2009; 12:185–7. doi: 10.1111/j.1524-4733.2008.00425.x [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, et al. ; Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265–71. doi: 10.1161/CIRCULATIONAHA.110.940148 [DOI] [PubMed] [Google Scholar]
- 37.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–77. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg I, Kutyifa V, Moss AJ. Survival with cardiac-resynchronization therapy [Letter]. N Engl J Med. 2014;371:477–8. doi: 10.1056/NEJMc1407182 [DOI] [PubMed] [Google Scholar]
- 39.Rickard J, Kumbhani DJ, Popovic Z, Verhaert D, Manne M, Sraow D, et al. Characterization of super-response to cardiac resynchronization therapy. Heart Rhythm. 2010;7:885–9. doi: 10.1016/j.hrthm.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Kutyifa V, Kloppe A, Zareba W, Solomon SD, McNitt S, Polonsky S, et al. The influence of left ventricular ejection fraction on the effectiveness of cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2013;61:936–44. doi: 10.1016/j.jacc.2012.11.051 [DOI] [PubMed] [Google Scholar]
- 41.Masoudi FA, Mi X, Curtis LH, Peterson PN, Curtis JP, Fonarow GC, et al. Comparative effectiveness of cardiac resynchronization therapy with an implantable cardioverter-defibrillator versus defibrillator therapy alone: a cohort study. Ann Intern Med. 2014;160:603–11. doi: 10.7326/M13-1879 [DOI] [PubMed] [Google Scholar]
- 42.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. ; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364:11–21. doi: 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 43.Finegold JA, Raphael CE, Levy WC, Whinnett Z, Francis DP. Quantification of survival gain from cardiac resynchronization therapy: nonlinear growth with time, and greater gain in low-risk patients, make raw trial data an underestimate of real-world behavior. J Am Coll Cardiol. 2013;62:2406–13. doi: 10.1016/j.jacc.2013.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, et al. ; MADIT-CRT Executive Committee. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol. 2012;59:2366–73. doi: 10.1016/j.jacc.2012.01.065 [DOI] [PubMed] [Google Scholar]
Web-Only Reference
- 45.Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA, et al. ; Multicenter InSync ICD II Study Group. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–8. [DOI] [PubMed] [Google Scholar]