Abstract
Ocimum basilicum essential oil (EO) was evaluated for its biological effects on M. domestica. Characterization of O. basilicum EO revealed the presence of methyl chavicol (70.93%), linalool (9.34%), epi-α-cadinol (3.69 %), methyl eugenol (2.48%), γ-cadinene (1.67%), 1,8-cineole (1.30%) and (E)-β-ocimene (1.11%). The basil EO and its constituents methyl chavicol and linalool elicited a neuronal response in female adults of M. domestica. Adult female flies showed reduced preference to food source laced with basil EO and methyl chavicol. Substrates treated with EO and methyl chavicol at 0.25% resulted in an oviposition deterrence of over 80%. A large ovicidal effect was found for O. basilicum EO (EC50 9.74 mg/dm3) followed by methyl chavicol (EC50 10.67 mg/dm3) and linalool (EC50 13.57 mg/dm3). Adults exposed to EO (LD50 10.01 μg/adult) were more susceptible to contact toxicity than to methyl chavicol and linalool (LD50 13.62 μg/adult and LD50 43.12 μg/adult respectively). EO and its constituents methyl chavicol and linalool also induced the detoxifying enzymes Carboxyl esterase (Car E) and Glutathione S – transferases (GST).
Keywords: Basil oil, Secondary metabolites, Housefly, Electrophysiology, Detoxifying enzymes, Ovipositional deterrence
Introduction
The cosmopolitan housefly, Musca domestica L. (Diptera: Muscidae), is synanotrophic and lives in close association with humans and livestock causing annoyance, food spoilage and pathogen transmission (causing enteric disease, typhoid, and shigellosis) (Sasaki et al. 2000; Kumar et al. 2012; Khamesipour et al. 2018; Moon 2019). The transmission of microbes is facilitated through fecal, body, and regurgitation routes (Fotedar 2001; Meerberg et al. 2007; Wanaratana et al. 2013). The ability of houseflies to acquire avian influenza H9N2 may aid in spreading spreading the virus to humans and poultry (Salamatian et al. 2020). Reports from Wuhan confirmed 2–10% of COVID-19 patients had diarrhoea and abdominal pain, a symptom that could have occurred due to faecal-oral transmission (Wang et al. 2020). In such a situation, vectoral role of flies carrying virus from faecal visits to human settlements cannot be ruled out (Dehghani and Kassiri 2020).
M. domestica management in human settlements, animal and poultry sheds mainly involves the application of insecticides or raising the birds on feed premixed with growth regulators (Macovei et al. 2008; Ghosh and Zurek 2015). The excessive dependence on chemical insecticides leads to the development of insecticide resistance in flies to pyrethroids, organophosphates, spinosad, indoxacarb and spiromesifen (Khan et al. 2015; Alam et al. 2020) and also build-up of xenobiotics that are toxic to humans and nontarget animals. Alternatively, botanical-based insecticides pose minimal safety risks and can be used in tandem with biocontrol agents (Kaufman et al. 2010; Khan et al. 2015; Scott 2017; Saeed et al. 2018; Senthil-Nathan 2020; Shi et al. 2020). This necessitates the development of “green” insecticides and repellents with diverse modes of action (Pavela and Benelli 2016; Benelli and Pavela 2018; Pavela et al. 2019; Isman 2020) that can mitigate the problems posed by synthetic insecticides to humans and the environment (Isman 2017; Pandiyan et al. 2019; Ikbal and Pavela 2019). The essential oils (EO) are an alternative to synthetic insecticides as they possess potential biological effect on insect pests with low mammalian toxicity (Koul et al. 2008; Chellappandian et al. 2018; Vasantha-Srinivasan et al. 2018). Several studies have exhibited the larvicidal, ovicidal, adulticidal, repellence, and ovipositional deterrence of essential oils on M. domestica which can be exploited for its management (Pavela and Benelli 2016; Khater and Geden 2019).
Sweet basil, Ocimum basilicum, belongs to the Lamiaceae family and is a herbaceous and perennial plant grown in Asia, Africa, Central and South America (Simon et al. 1999). O. basilicum EO is widely used in the preparation of food, cosmetics and medicine (da silva Moura et al. 2020). The major constituents of O. basilicum EO, methyl chavicol and linalool exhibit antioxidant, anesthetic, anti-inflammatory and antimicrobial properties (Radulovic et al. 2013; Varga et al. 2017). In addition, they are reported to be toxic (Rice and Coats 1994; Palacios et al. 2009; Tarelli et al. 2009; Gallardo et al. 2015; Tian 2017) and insect repellent (Delille 2007; Tian 2017).
The reports on basil EO and its constituents, linalool and methyl chavicol were limited to fumigant and contact toxicity to adults and larval stages of M. domestica (Palacios et al. 2009; Tian 2017). In our studies, we focused on the electrophysiological and olfactory response of O. basilicum EO and its major constituents to adult females of M. domestica. Furthermore, we investigated the ovicidal, adulticidal and the activity of detoxification enzyme in adult exposed to O. basilicum EO, linalool and methyl chavicol.
Materials and methods
House fly rearing
House fly, M. domestica adults were collected using a sweep net from suburbs of Bengaluru, India. The collected adult flies were kept in 30×30×30 cm cage (aluminium frame fitted with acrylic sheets). The flies were fed with diluted honey solution (10%) in water. An oviposition substrate (250 gm) prepared by mixing wheat bran + milk powder + egg yolk powder (10: 2: 1) with water was placed in the cage to facilitate the mated flies to lay their eggs. The larvae on hatching continued to feed on wheat bran medium. When the larvae in the medium reached third instar, ragi (Elusine coracona) husk was added to the dry larval medium to facilitate pupation. The fly rearing unit was maintained at 28 ± 2 °C, RH 65 ± 5%. The bio stages (eggs 0–3 h old and 2–3 days old adult females) of the flies collected from the rearing chamber were used in experiments.
Extraction and characterization of essential oil
Leaves of O. basilicum (300 g) were extracted by hydro-distillation in a Clevenger type apparatus to obtain EO. The leaves along with 500 ml of water were loaded into a round bottom flask placed over a heating mantle. The contents in the flask were heated to 100 °C. The oil along with water collected in the receiver tube was phase separated using a separating funnel. The collected essential oil was dried by passing over anhydrous sodium sulphate to remove the moisture trace and stored in amber vials at 4 °C until use.
The O. basilicum oil was characterized using GC-MS (Agilent GC- 7890A (G3440A) and MS- G3171A 5975) as suggested by Ravindran et al. (2019). One μl of 0.01% essential oil diluted in dichloromethane was introduced by microsyringe into injector port attached to HP-5 MS Phenylmethylsilox capillary column (30 m × 250 μm i.d. × 0.25 μm film thickness, Agilent Technologies, USA) through a glass liner. The oven and column temperature were maintained at 40 °C for 1 min and then raised at the rate of 20 °C per minute to 280 °C. The temperature during the post run was held at 300 °C for 10 min. The temperature in injector and detector was maintained at 250 °C. The total run was for 23 min. In MS, the ion source temperature was set at 250 °C. The flow rate of the carrier gas Helium was maintained at 1 ml per minute. An electron ionization (EI) mode with 70 eV ionization energy was used for the GC–MS identification. Identification of the components in EO was done by considering the relative peak percent area, retention time and mass fragmentation pattern using the NIST library. Major constituents in oil were verified by co injecting the compounds.
Chemicals
Methyl chavicol and linalool used for bioassays were purchased from Sigma Aldrich. HPLC grade dichloromethane and acetone were purchased from Merck. Dimethyl-2,2-dichlorovinyl phosphate (DDVP) and Imidacloprid (PESTANAL®) analytical standard were purchased from Sigma Aldrich. Eserine and Fast Blue RR salt were procured from Fluka (Sigma Aldrich). α-Napthyl acetate (α-NA), 2,4-dinitrochlorobenzene (CDNB), Coomassie brilliant blue G-250, pyrogallol, guaiacol, and H2O2 were purchased from Shanghai Chemical Industry Co., Ltd, China.
Electroantennography (EAG)
The response of M. domestica adult female antennae (3 days old) to sweet basil EO, methyl chavicol, linalool and neem oil was recorded using an electroantennographic system (Syntech). The dual electrode probe was used for mounting the antennal prep. The head of the adult female fly was decapitated and mounted on one electrode and the proximal tip funiculus to another electrode using a conductive gel (Spectra 360 Parker, Orange, New Jersey). The clean air (activated charcoal filtered) was continuously flushed over the antennae. The EO and its constituent’s methyl chavicol, linalool and neem oil were diluted in HPLC grade dicholoromethane at concentration of 1 μg μL-1. Dichloromethane alone was used as a control. One μl of the aliquot amounting to 1 μg of the test compound placed on Whatman filter paper strips (Advantec 5C (110 mm) Japan of 2 cm length and 4 mm diameter) was dried for 5 min in fume hood, and then it was inserted into the Pasteur pipettes. This setup was connected to stimulus controller (CS 05 Syntech) by Tygon silicone tube. The first puff was blown off after 30 seconds of loading filter paper. After sixty seconds, the antennae were exposed to vapour phase of the stimulus through pipette placed 15 mm upstream from the antennae that had continuous air stream (pulse time 0.5 seconds, continuous flow 25 ml/s, pulse flow 21 ml/s) as suggested by Venugopal and Subaharan (2019). Between the stimulus puffs, a time delay of 20 s was maintained. The antennal responses were recorded through a high impedance probe that was in turn connected to amplifier (IDAC-4, Syntech), and the signals were recorded with EAG software (Syntech). Responses were expressed as a summated response of neurons, sorted according to shape and amplitude, emitted during 1 s after the onset of the stimulation. The control stimulus was at the beginning, middle and end of each session. EO and its constituents and neem oil were tested on six fly antennae with four replications of per stimuli per antennae in randomized manner.
Y olfactometer assay
The olfactory response of 2–3 days old adult females to sweet basil EO its constituent methyl chavicol, linalool and neem oil was evaluated using a glass Y-tube olfactometer having a main arm of 17 cm and choice arms of 17 cm with an inner diameter of 4 cm. Atmospheric air pumped using an air sampler was allowed to flow out through activated charcoal cartridge with a flow rate of 0.5 L/min. The purified air was then let into the arms of the Y-tube with steady flow rate. Whatman No. 1 filter paper stripes (3 cm length and 0.5 cm width) were treated with 200 μL of 20% sugar solution and dried for 30 min. To this 10 μL of 100 ppm of odorants diluted in dichloromethane was loaded, and the paper strips were allowed to dry in room temperature for 10 min to permit the solvent to evaporate. The paper strips prepared as mentioned above without the odorants was used as control. Both treated and control paper strips were inserted into an odour tube that was connected between the air flow tube and the Y-tube arm. The entire Y-tube setup was placed inclined at 20°. Pairwise comparison was made between odorants and control. The adult female flies were starved for 4 h with water satiation prior to test. A total of 100 female flies (N = 100) were introduced into the main arm. The choice made by the flies in an arm was considered if they crossed the halfway mark made in the arm in 3 min of start of the test. Those flies that failed to participate in the test were considered non-respondents. The odorants were switched between the arms to avoid position effect. The test was conducted at room temperature of 25 ± 2 °C under red light with slight modification as suggested by Ravindran et al. (2019).
Oviposition repellence
Gravid M. domestica females (10 Nos.) housed in cage (30 × 30 × 30 cm) made of aluminium frame having acrylic sheets were exposed to 2 g of oviposition substrate (wheat bran + milk powder + egg yolk powder (10: 2: 1) treated with EO, methyl chavicol, linalool and neem oil dissolved in acetone so as to achieve 0.05, 0.15 and 0.25% concentrations. Oviposition substrate treated with acetone alone was maintained as control. In both the cases, the solvent was allowed to evaporate from the oviposition substrate prior to their placement in treated and control dish that were placed at diagonal ends in the floor of the cage. Four replications were maintained (One cage per replicate). The number of eggs laid were counted after 24 h. The percent effective repellency (ER%) and Oviposition Activity Index (OAI) for flies exposed to EO, linalool, methyl chavicol and neem oil were calculated.
Ovicidal effect
Freshly laid M. domestica eggs (0–3 h) were collected using a soft paint brush. Twenty eggs of M. domestica were placed in the base of the 50-ml plastic sample container (Tarsons) having screw cap lid. Sweet basil EO, methyl chavicol and linalool of varied concentration ranging from 0.5 to 79 mg/dm3 were applied to filter paper attached to the base of the lid. Acetone alone was maintained as control and DDVP was maintained as positive control. This setup was sealed tightly using a parafilm and placed in incubator at 28 ± 2 °C and RH 65 ± 5%. The number of hatched and unhatched eggs were counted after 48 h. The experiments were replicated for five times.
Contact toxicity
Topical bioassay
Acute toxicity of EO, methyl chavicol and linalool to female M. domestica was assessed by topical application. Acetone was used as a solvent to prepare varying concentration of EO, methyl chavicol and linalool. Acetone alone was maintained as negative control and imidacloprid was used as positive control. Preliminary range finding test was done to fix the doses. Two-day-old adult female flies were immobilized using CO2. The flies were transferred to flat surface, and 1 μl of the test compounds were placed on the pronota using a microapplicator. The treated adults were transferred to an insect breeding dish (Himedia) having ventilation in the lid. The adults had access to 10% honey solution soaked in absorbent cotton. Four replications were maintained per treatment. Mortality was assessed 24 h after the treatment. Those flies that ceased to move its appendages in mild pin prick were considered dead (Subaharan et al. 2021).
Enzyme assays
The surviving flies after treatment with LC50 doses of EO, methyl chavicol and linalool (9,98, 13.62 and 43.13 μg/adult, respectively) were used for the extraction of whole-body homogenate. Acetone-treated adults were used as control. The flies were homogenised in ice cold 50-mM phosphate buffer (pH 7.4). Homogenates were centrifuged at 4 oC for 20 min at 10,000 rpm. The supernatant was subjected to protein estimation using Bradford’s method (Bradford 1976) and used as an enzyme source for the estimation of carboxylesterase (CarE) and glutathione S-transferase (GST) activities.
Glutathione S-transferase (GST) activity was determined as described by Habig et al. (1974) and Zhang et al. (2007). The components in reaction mixture include 30 μl of enzyme solution, 10 μl each of reduced glutathione (GSH) and 1-chloro-2,4-dinitrobenzene (CDNB) (dissolved in ethanol and prepared with 50 mM po4 buffer, PH 7.4) and 950 μl phosphate buffer. Control mixture had 10 μl each of GSH and1-chloro-2,4-dinitrobenzene and 950 or 980 μl of phosphate buffer. The rate of change in absorbance at 340 nm was measured for 5 min in 96-well microplate reader and converted to specific activity using extinction coefficient of 9.6 mM-1 after necessary path length correction.
The activity of carboxylesterases (Car E) was determined using the method suggested by Li et al. (2007). The reaction mixture consisting of 15 μl of enzyme homogenate made up to 500 ul with sodium phosphate buffer (50 mM, pH 7.4) and 800 μl α- naphthyl acetate (dissolved in acetone, prepared in PO4 buffer) was incubated in dark condition for 20 min. The control mix had 800 μl of substrate and 500 μl of phosphate buffer (50 mM, pH 7.4). Formation of blue colour on addition of 200 μl of staining solution indicated the production of α-napthol. The absorbance was measured at 595 nm using microplate reader (iMark, BioRad). Enzyme activity in the sample was calibrated using α-napthol standard curve.
For both the enzyme estimation, three biological replicates were maintained per treatment. The specific activity of the enzyme was expressed as nmol/min/mg protein.
Statistical analysis
The egg and adult mortality data were subjected to probit analysis to determine median lethal dose LD50 and the corresponding 95 % CI values and chi-square test were calculated. In the case of Y-tube olfactory assay, the binomial test was used to compare the orientation of the flies to the arms with odorant and control. Pooled EAG response of antennae to EO and its constituents, variations in effective repellence (ER), OAI and variation in enzyme activity was compared using one-way analysis of variance (ANOVA) followed by Tukey’s Post hoc test (P < 0.05) using SPSS.
Oviposition Activity Index (OAI) was calculated using the formula.
where NT is the total number of eggs on the treated substrate and NC the total number of eggs on the control substrate (Cheah et al. 2013). For OAI values ≤ −0.3, the EO was considered as repellent (Kramer and Mulla 1979).
The percent effective repellency (ER%) for EO, linalool, methyl chavicol and neem oil was calculated using the following formula:
ER% = NC−NT/ NC × 100 as suggested by Siriporn and Mayura (2012)
Results
GC-MS analysis for Ocimum basilicum EO
The hydrodistillation of O. basilicum yielded an essential oil content of 0.5 ml/100 g of herbage. The extracted O. basilicum EO was characterized by gas chromatography coupled to mass spectroscopy. The compounds were identified by the reported retention index. The chemical formula, molecular weight, and their chemical structures are presented in Table 1 and Fig, 1. The major components present in O. basilicum EO were methyl chavicol (70.93%), linalool (9.34%), epi-α-cadinol (3.69 %), methyl eugenol (2.48%), γ-cadinene (1.67%), 1,8-cineole (1.30%) and (E)-β-ocimene (1.11%).
Fig. 1.
Structures of chemical constituents in O. basilicum essential oil
Table 1.
Chemical composition of the O. basilicum essential oil
| No | Component | Chemical formulae | Molecular weight g/mol |
Reported RRI | Experimental RRI | Percentage Composition |
|---|---|---|---|---|---|---|
| 1 | β-Pinene | C10H16 | 136 | 974 | 978 | 0.12 |
| 2 | Myrcene | C10H16 | 136 | 988 | 991 | 0.48 |
| 3 | Limonene | C10H16 | 136 | 1024 | 1028 | 0.14 |
| 4 | 1,8-Cineole | C10H18O | 154 | 1026 | 1032 | 1.3 |
| 5 | (E)-β-ocimene | C10H16 | 136 | 1044 | 1047 | 1.11 |
| 6 | Linalool | C10H18O | 154 | 1095 | 1101 | 9.34 |
| 7 | Camphor | C10H16O | 152 | 1141 | 1140 | 1.44 |
| 8 | Methyl chavicol | C10H12O | 148 | 1195 | 1208 | 70.93 |
| 9 | p-Anisaldehyde | C8H8O2 | 137 | 1247 | 1257 | 0.28 |
| 10 | Bornyl acetate | C12H20O2 | 196 | 1287 | 1288 | 0.1 |
| 11 | β-Bourbonene | C15H24 | 204 | 1387 | 1387 | 0.09 |
| 12 | β-Elemene | C15H24 | 204 | 1389 | 1394 | 0.62 |
| 13 | Methyl eugenol | C11H14O2 | 178 | 1403 | 1408 | 2.48 |
| 14 | β-Caryophyllene | C15H24 | 204 | 1417 | 1422 | 0.45 |
| 15 | α-Guaiene | C15H24 | 204 | 1437 | 1441 | 0.29 |
| 16 | α-Humulene | C15H24 | 204 | 1452 | 1456 | 0.24 |
| 17 | Germacrene D | C15H24 | 204 | 1484 | 1484 | 0.16 |
| 18 | Bicyclogermacrene | C15H24 | 204 | 1500 | 1499 | 0.35 |
| 19 | α-Bulnesene | C15H24 | 204 | 1509 | 1508 | 0.63 |
| 20 | γ-Cadinene | C15H24 | 204 | 1513 | 1517 | 1.67 |
| 21 | trans-Calamenene | C15H22 | 202 | 1521 | 1526 | 0.12 |
| 22 | (E)-Nerolidol | C15H26O | 222 | 1561 | 1566 | 0.11 |
| 23 | 4-Methoxycinnamaldehyde | C10H10O2 | 162 | 1562 | 1571 | 0.67 |
| 24 | Spathulenol | C15H24O | 220 | 1577 | 1582 | 0.91 |
| 25 | Caryophyllene oxide | C15H24O | 220 | 1582 | 1587 | 0.5 |
| 26 | Humulene epoxide II | C15H24O | 220 | 1608 | 1613 | 0.25 |
| 27 | 1,10-di-epi-Cubenol | C15H26O | 222 | 1618 | 1619 | 0.54 |
| 28 | Epi-α-cadinol | C15H26O | 222 | 1638 | 1646 | 3.69 |
| 29 | α-Eudesmol | C15H26O | 222 | 1652 | 1655 | 0.12 |
| 30 | α-Cadinol | C15H26O | 222 | 1652 | 1659 | 0.18 |
| Total | 99.31 |
Electroantennograph (EAG) response
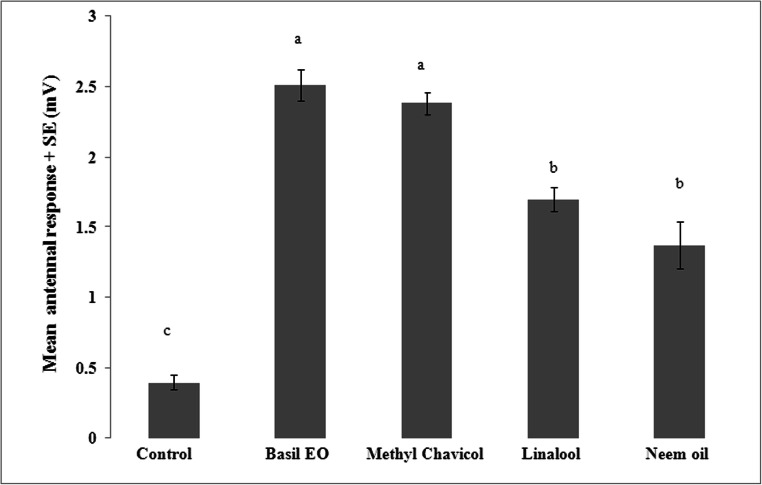
The sweet basil EO and its constituents methyl chavicol, linalool and positive control neem oil at 1 μg treatment caused the neuronal response in female adults (Fig. 2) (t = 2.71; P < 0.05). The mean EAG response to air was less than 0.5 mV. Sweet basil EO and its major constituent methyl chavicol caused highest mean antennal response of 2.51and 2.38 mV, respectively, and were at par. The response of female adults to linalool (1.69 mV) was lower than the methyl chavicol but higher than neem oil (1.37 mV).
Fig. 2.
Mean (+ SE) antennal EAG responses of female M. domestica to the assay performed using 1 μL of 1000 ppm of sweet basil EO, methyl chavicol, linalool and neem oil. Bars having same letter do not differ significantly at P < 0.05 (ANOVA followed by Tukey test)
Olfactometer bioassay
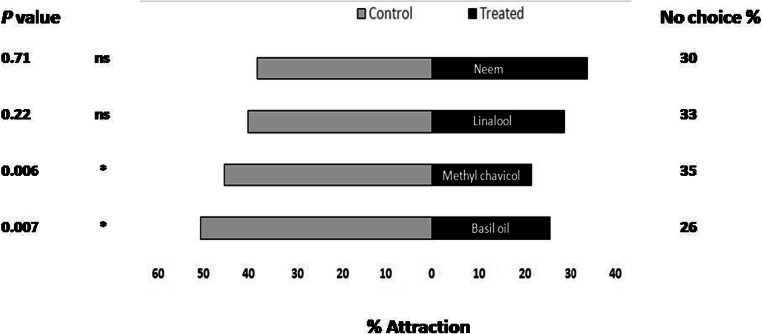
There was significant reduction in adult female flies selecting the arm having food source with sweet basil EO (P < 0.05) and methyl chavicol (P < 0.05) as compared with arm having food source alone (Control). However, there was no significant difference between the flies choosing the arms having food source with linalool (P = 0.22) and neem oil (P = 0.7) and control having food source alone (Fig. 3).
Fig. 3.
Behavioural response of adult female M. domestica in a two choice Y- olfactometer (Per cent repelled n = 100). Starved adult female flies were offered a choice between treated arm (Food source with sweet basil EO and its constituents’ methyl chavicol, linalool and neem oil) and control arm (Food source alone). Both bars represent the per cent flies orienting to treated and control arm. The non-responding adult female flies were presented in right hand side (No choice %). Asterisks show a preference that was significantly different (binomial test) from a 50:50 distribution: * = P < 0.05. The flies that failed to respond were excluded from the statistical analysis
Ovipositional repellence
Effective repellence (ER%) and oviposition activity index (OAI) of O. basilicum EO and its constituent’s methyl chavicol and linalool at three concentration (0.05, 0.15 and 0.25%) was determined. The ER% and OAI for EO and its constituents was concentration dependant and increased with concentration. Among the samples tested, higher oviposition deterrence to M. domestica adults was observed in EO (89.52+1.00), methyl chavicol (80.12+ 2.40) and linalool (78.99+2.93) at a dose of 0.25 % which was superior to neem oil 0.25%. EO and methyl chavicol at 0.15 % were at par in causing oviposition deterrence to M. domestica adults. At a lower concentration of 0.05% the EO, methyl chavicol and linalool caused a lowest deterrence ranging from 27.83 - 35.86 % (F 11,36 = 50.07 P < 0.001). Across the concentration, neem oil caused lower oviposition deterrence.
O. basilicum EO, methyl chavicol, linalool and neem oil caused significant difference on OAI. O. basilicum EO, methyl chavicol at 0.25% caused an OAI −0.81 + 0.01 and −0.67 + 0.03, respectively. All the samples tested at 0.15 and 0.05 % recorded OAI value below −0.5. An OAI value from −0.5 to −1 is an indication of good ovipositional repellence effect. In the case of neem oil, all the doses tested had OAI less than −0.5 (F 11,36 = 53.87 P < 0.001) (Table 2).
Table 2.
Effective repellence of O. basilicum EO, methyl chavicol, linalool and neem oil against M. domestica
| Test sample | Concentration (%) | Effective Repellency (ER) | OAI |
|---|---|---|---|
| O. basilicum EO | 0.05 | 35.86 + 0.88e | −0.21+ 0d |
| 0.15 | 71.44 + 4.24bc | −0.50 + .02c | |
| 0.25 | 89.52 + 1.00a | −0.81 + 0.01a | |
| Methyl chavicol | 0.05 | 33.27 + 0.95e | −0.19 + 0.0d |
| 0.15 | 59.81 + 4.36cd | −0.43 + 0.04c | |
| 0.25 | 80.12 + 2.40ab | −0.67 + 0.03ab | |
| Linalool | 0.05 | 31.28 + 2.07e | −0.18 + 0.01d |
| 0.15 | 56.54 + 3.75d | −0.39 + 0.03c | |
| 0.25 | 78.99 + 2.93ab | −0.65 + 0.03b | |
| Neem oil | 0.05 | 27.83 + 3.57e | −0.16 + 0.02d |
| 0.15 | 53.95 + 1.70d | −0.37+ 0.01c | |
| 0.25 | 62.31 + 4.18cd | −0.45 + 0.04c |
The data are given as Mean ± SE. *Denote significant different at P <0. 05 compared with the control.
Means followed by same alphabet in a column do not differ significantly by Tukeys test P < 0.05.
Ovicidal effect
O. basilicum EO and its constituent methyl chavicol and linalool were evaluated for ovicidal activity. O. basilicum EO had 5.8-fold higher ovicidal activity (EC50 9.74 mg/dm3) on M. domestica eggs than linalool (EC50 13.57 mg/dm3). Among the constituents, methyl chavicol elicited 1.27-fold higher toxicity than linalool (EC50 10.67 mg/dm3). The ovicidal activity of DDVP was superior to (EC50 390.37 mg/dm3) EO and its constituents (Table 3).
Table 3.
Ovicidal activity of O. basilicum EO, methyl chavicol and linalool on M. domestica eggs
| Test sample | Concentration (mg/dm3) | 48 h% mortality + SE | EC50 (mg/dm3) |
95% CL | df | Chi-square | P value |
|---|---|---|---|---|---|---|---|
| O. basilicum EO | 45.59 | 100 + 0 | |||||
| 30 | 86 + 2.91 | 9.74 | 7.36–12.75 | 5 | 10.79 | 0.058 | |
| 22.79 | 68 + 2.54 | ||||||
| 11.4 | 51 + 4.30 | ||||||
| 5.7 | 28 + 2.54 | ||||||
| 0.3 | 16 + 1.87 | ||||||
| 0.15 | 6 + 1.0 | ||||||
| Methyl chavicol | 45.59 | 99 + 0.00 | 10.67 | 8.70–13.01 | 5 | 8.83 | 0.116 |
| 30 | 85 + 1.58 | ||||||
| 22.79 | 72 + 2.54 | ||||||
| 11.39 | 48 + 2.54 | ||||||
| 5.6 | 29 + 2.91 | ||||||
| 2.8 | 8 + 1.22 | ||||||
| 1.5 | 3 + 1.22 | ||||||
| Linalool | 79.33 | 100 + 0.00 | 13.57 | 11.39–15.93 | 6 | 10.58 | 0.102 |
| 63.47 | 94 + 2.44 | ||||||
| 39.66 | 84 + 4.30 | ||||||
| 23.8 | 74 + 1.87 | ||||||
| 15.86 | 63 + 2.54 | ||||||
| 11.9 | 44 + 2.91 | ||||||
| 7.93 | 20 + 2.23 | ||||||
| 3.96 | 12 + 2.54 | ||||||
| DDVP | 1.2 | 95 + 2.73 | 0.15 | 0.13–0.18 | 5 | 1.43 | 0.92 |
| 0.64 | 84 + 2.44 | ||||||
| 0.32 | 69 + 4.30 | ||||||
| 0.16 | 51 + 2.91 | ||||||
| 0.08 | 28 + 2.54 | ||||||
| 0.04 | 15 + 1.58 | ||||||
| 0.02 | 8 + 1.22 |
Each value represents the mean of five replicates, and each set-up had 20 individuals (n = 100).
95% CL=confidence interval at 95% confidence level
Topical bioassay
A toxicity assay carried out showed that O. basilicum EO topical application resulted in higher mortality over methyl chavicol and linalool to adult stages. Adults exposed to EO (LD50 10.01 μg/adult) were more susceptible than those exposed to methyl chavicol and linalool (LD50 13.62 μg/adult and LD50 43.12 μg/adult, respectively). Imidacloprid was more toxic (1.41 μg/adult) than EO and its constituents (Table 4).
Table 4.
Acute toxicity of O. basilicum EO, methyl chavicol and linalool on M. domestica Topical application
| Test sample | Stage | Period (Hours) | LD50 * | 95% CL | df | Chi-square | P value |
|---|---|---|---|---|---|---|---|
| O.basilicum EO | Adult | 24 | 10.01 | 9.19–10.94 | 6 | 11.83 | 0.066 |
| Methyl chavicol | Adult | 24 | 13.62 | 10.76–16.78 | 4 | 9.19 | 0.05 |
| Linalool | Adult | 24 | 43.12 | 23.80–64.39 | 2 | 5.93 | 0.05 |
| Imidacloprid | Adult | 24 | 1.41 | 1.19–1.68 | 5 | 2.8 | 0.718 |
*(μg/adult)
Enzyme assay
The effect LD50 dose of O. basilicum EO, methyl chavicol and linalool on M. domestica adult detoxifying enzymes carboxyl esterase and GST is reported in the Table 5.
Table 5.
Activities of carboxylesterase and glutathione S-transferase in M. domestica adult
| Treatment | GST a, b |
ER | Car E a, b |
ER |
|---|---|---|---|---|
| Control | 15.58 + 0.49 e | 1.35 + 0.04e | ||
| O. basilicum EO LD 50 | 71.83 + 1.92 a | 4.61 | 2.21+ 0.00b | 1.63 |
| Methyl chavicol LD 50 | 48.16 + 2.76 b | 3.09 | 1.53 + 0.03 de | 1.13 |
| Linalool LD50 | 13.86 + 0.37 e | 0.88 | 2.84 + 0.07 a | 2.10 |
aMeans within a column followed by the same letter is not significantly different (One-way ANOVA)
bUnit of enzymes: GST - Glutathione S-transferase and Car E - Carboxylesterase = n moles/min/mg protein/min
The enzyme activities were expressed as enzyme ratio (ER, mean activity of enzyme in different treatments/mean activity of enzyme in control group)
Adults exposed to linalool at LD50 value exhibited inhibition of GST, but it was on par with control. However, LD50 values of EO and methyl chavicol caused induction of GST levels with EO having a higher ER (4.61) than methyl chavicol (3.09) (F 6,14 = 224.49 P < .005). Topical application of EO, methyl chavicol and linalool induced the enzymes Car E in adults. In the case of Car E levels in adults, those exposed to methyl chavicol at LD50 had Car E levels at par with control samples. Linalool had higher level of induction of Car E in adults than O. basilicum EO (F 6,14 = 224.49 P < .005).
Discussion
Indiscriminate use of chemicals has led to development of insecticide resistance and negative effects to consumers. There is a growing demand for environmentally safe pesticides to manage M. domestica. Essential oils (EO) derived from plant parts contain bioactive compounds that can be used in isolation or in combination for managing pests of agriculture, medical and veterinary importance (Isman 2006; Pavela 2011; Pavela and Benelli 2016; Senthil-Nathan 2020; Khater and Geden 2019). The biodegradable nature and safety of these botanically-derived chemicals to nontarget organisms provides an alternative to synthetic insecticides (Poorjavad et al. 2014; Chellappandian et al. 2019). The potency of essential oil and its constituents against M. domestica has been reported earlier (Sinthusiri and Soonwera 2014; Benelli et al. 2018). The insecticidal, ovicidal, deterrence/repellence and growth regulating effect of O. basilicum crude extracts and EO against M. domestica was reported earlier (Pavela 2008a; El Zayyat et al. 2015; Chowdhary et al. 2018).
Chemical characterization
In our study, sweet basil EO contained methyl chavicol (70.93 %) and linalool (9.34%) as major constituents. This agrees with earlier reports mentioning methyl chavicol, linalool, β-elemene and camphor as major constituents of basil oil (Sonmezdag et al. 2018). The proportion of the constituents in basil oil varies according to the plant chemotype (Telci et al. 2006; Ogendo et al. 2008), such as methyl chavicol, linalool, methyl eugenol and methyl cinnamate types (Lawrence 1998). The EO of O. basilicum in our study contained thirty components, accounting for 99.31% of the total composition. As the EO used in our study had a higher load of methyl chavicol (70.93%), it could be considered a methyl chavicol chemotype as proposed by Varga et al. (2017).
In our study, the EAG assay revealed that the neuronal response of the antennae of adult female M. domestica was higher to sweet basil EO and methyl chavicol followed by linalool and neem oil. As EAG response is linked to insect olfaction (Ghabbari et al. 2018) higher amplitude of response trace to EO and methyl chavicol may be due to higher number of receptors in antennal neuron to these stimuli that may influence the fly’s behaviour. Basil oil when presented at 100 ng produced a mean antennal response of 0.99 mv in the coconut rhinoceros beetle, Oryctes rhinoceros (Ravindran et al. 2019). White and Hobson (1993) reported that methyl chavicol elicited an EAG response in the mountain pine beetle. Methyl chavicol was reported as a antiaggregant to bark beetles. When methyl chavicol was mixed with attractive bait, it reduced the attraction of Western pine beetle, Dendroctonus brevicomis and mountain pine beetle, D.ponderosae by 60 and 68%, respectively (Hobson Kenneth 1995). Screening of the odorants for antennal response using EAG provides a lead to select the compounds for behavioural assays (Cosse et al. 1995; Zito et al. 2013; Ruschioni et al. 2015) as the EAG active compounds in the essential oils could elicit attractive or repulsive behavioural response (Meza et al. 2020)
Compounds that result in a physiological response in antennae need not be behaviorally active, hence an olfactory assay must be performed to establish the behavioral response (Ravindran et al. 2019). In the Y-tube olfactory assay, the adults of M. domestica preferred the control arm containing a food source alone over the arm having food source laced with sweet basil EO and methyl chavicol. This aversive response of M. domestica adults to EO and methyl chavicol may be due to its ability to perceive the odorants in antennae. The peripheral response of antennal neurons to EO and methyl chavicol in EAG provides a physiological basis for the aversive olfactory mediated behaviour in Y- tube assay. The essential oils of Ocimum gratissimum, Thymus serpyllum, Myristica fragrans and Curcuma amada caused 100% repellence to housefly for a duration of 5 h. (Singh and Singh 1991). Vanillin, p-menthane-3,8-diol (PMD) and neem oil at 5% caused significant repellence to M. domestica adults (Khater and Geden 2019). Combinations of EOs, viz., Chrysopogon zizanioides, Cinnamomum zeylanicum, and Lavandula angustifolia along with sunflower oil caused repellence to the blow fly Lucilia sericata (Khater and Geden 2019). Methyl chavicol, a constituent in Tagetes filifolia and O. selloi, was also found to be repellent to Aedes aegypti (Diptera) (Gleiser et al. 2011) and Anopheles braziliensis (Diptera) (Padilha de Paula et al. 2003). Sadeh et al. (2019) reported that rosemary variety 11 having 0.6–0.9 % methyl chavicol elicited repellency to whitefly, Bemesia tabaci. Hobson Kenneth (1995) further reported that methyl chavicol reduced the aggregation of Dendroctonus beetles. Repellents prevent the orientation/alighting of houseflies on treated surfaces and can therefore reduce the spread of diseases in locations where the housefly is high (Haselton et al. 2015). Identifying repellents for the house fly is of paramount importance because of its negative impacts on agriculture and human health (Malik et al. 2007). Assessing the repellents of bioactive compounds to flies in small space using olfactometers is difficult to arrive at a conclusion. The behavioural response involving cage studies will facilitate to confirm the response in Y-tube olfactory assays (Khater and Geden 2019).
Cages having oviposition substrate treated with O. basilicum EO and its components methyl chavicol and linalool at 0.25% showed highest ovipositional repellence against M. domestica with values ranging from 79 to 89 % and an OAI of −0.65 to −0.67. Essential oil of O. gratissimum at 2% also caused 100% repellent activity against M. domestica (Singh and Singh 1991). The repellence of essential oil against M. domestica has impact on the population build up (Maganga et al. 1996). The toxic effects of O. basilicum, EO and its constituent’s methyl chavicol and linalool to larval, pupal and adult stages of M. domestica has been documented (Pavela 2008b; Tarelli et al. 2009; Scalerandi et al. 2018). Identifying the essential oil with ovicidal activity can contain the pest buildup (Hong et al. 2018). Eggs of M. domestica are easier to target than motile larval and adult stages. O. basilicum EO had 5.8-fold higher ovicidal activity on M. domestica eggs than its constituent’s linalool or methyl chavicol. Eggs of mosquito vectors, Aedes aegypti, Anopheles dirus and Culex quinquefasciatus exposed to O. basilicum oil had extremely low hatch rates (EC50 < 1.9%) (Siriporn and Mayura 2012) O. basilicum oil at 1.0 ml/ 38.5 ml of air caused 100% mortality of Callosobruchus chinensis (L.) 3 days after the exposure period (Abd El-Salam 2010), and linalool also showed ovicidal activity against the eggs of insecticide resistant lice BR-HL females (Yang et al. 2009). Transgenic Nicotiana tabacum producing higher levels of linalool were not preferred for oviposition by Helicoverpa armigera as linalool was reported to cause repellence (McCallum et al. 2011).
We observed that topical application of O. basilicum EO was more toxic than methyl chavicol and linalool to adult stages of M. domestica. Increased toxicity in EO may be due to the combined effect of the compounds in natural oil as reported by Cheng et al. (2009). In contrast, methyl chavicol (11.01 ppm) and linalool (35.17 ppm) in isolation had higher larvicidal activity than Clausena anisate EO as a whole (LC50 119.59 ppm) (Govindarajan 2010). Chrysopogon zizanioides, Cinnamomum zeylanicum, and Lavandula angustifolia (0.6%) caused 100% mortality of adult M. domestica by topical and fumigant exposure (Khater and Geden 2019). The lipophilicity coupled with low molecular weight of EOs enable them to show various modes of action on insects (Pavela and Benelli 2016). The structure and functional group of terpenoids facilitates their entry into the insect cuticle and attach to the target site to bring in desirable bioaction (Rice and Coats 1994).
Resistance of insects to xenobiotics and phytochemicals is due to metabolic detoxification mediated by the action of enzymes like, glutathione S-transferase (GST) and esterase (Castaneda et al. 2010; Waliwitiya et al. 2012). GST is involved in the metabolism of endogenous compounds and acts by conjugation to make them water soluble and less toxic (Yu 2004). Esterases act by binding, sequestering, and detoxifying toxic chemicals (Hegeto et al. 2015)
Our investigations reveal that GST and Car E activity in adult stage of M. domestica increased on exposure to O. basilicum EO and methyl chavicol and linalool. M. domestica adults exposed to EO at LD 50 values induced a higher level of GST as compared with control and constituents. Treating with LD50 doses of linalool caused significant increase in Car E levels in adults. This agrees with earlier studies that C. pipiens larvae exposed O. basilicum EO at LC50 values stimulated GST activity. The increase in degrading enzymes may be due to increase in active compounds in the insect body (Zibaee and Bandani 2010). Previous reports state that plant-derived products having a mixture of compounds that including triterpenoids and phenols inhibit the activity of GST and Car E (Tak et al. 2017; Yang et al. 2018). An increase in the degrading enzymes Car E and GST on exposure of insects to natural products have been reported earlier (Kumrungsee et al. 2014). Larvae of mosquitoes, C.quinquefasciatus and A. stephensi exposed to Lantana camera root and Anacardium occidentale exhibited a rise in GST activity. This scenario of rise in enzymes does not limit to insecticide resistance alone, and this could be due to degrading enzymes generated by reactive oxygen species (ROS) (Vontas et al. 2001). Induction of detoxifying enzymes due to exposure to EO may be due to synergy caused by the constituents in EO (Miresmailli et al. 2006; Isman et al. 2008; Jiang et al. 2009; Senthil-Nathan 2013). Increase in degrading enzyme levels is clear indication that EO and its constituents possess cidal effect in M. domestica and the fall out of which is the possibilities of resistance development, which needs further investigation.
The work in this study has demonstrated the bioactive potential of O. basilicum EO and its constituents, methyl chavicol and linalool in toxicity to eggs and adult stage. The electrophysiological and behavioral response of adult female flies to EO and its constituents by inducing repellence and ovipositional deterrence in adults can be used to deter fly populations from infesting human settlements and animal sheds. O. basilicum EO as whole induces a higher ovicidal, adulticidal and repellency to flies, and these traits make it fit for integrated pest management (IPM) of M. domestica. Further studies are needed to develop a formulation of O. basilicum EO for sustained spatiotemporal release rates as there is a market demand for plant-derived parts for managing the pests like M. domestica.
Acknowledgements
Support provided by ICAR – NBAIR and facilities provided by Chairperson, DOS in Zoology, University of Mysore is acknowledged. Suggestions provided by Dr. Shannon Olsson, NCBS TIFR in fine tuning the manuscript, are acknowledged.
Authors’ contribution
Rajendran Senthoorraja: Investigation, Data generation. Kesavan Subaharan: Conceptualization, Writing - Original Draft, Data Curation. Sowmya Manjunath: Investigation on biochemical aspects. Muthu Gounder Mohan: Resources. Vppalayam Shanmugam Pragadheesh: Investigation, Validation. Nandagopal Bakthavatsalam: Writing - Review & Editing. Sekarappa Basavarajappa: Writing - Review & Editing. Sengottayan Senthil-Nathan: Writing - Original Draft and editing.
All authors read and approved the final manuscript.
Funding
The work was supported by ICAR – NBAIR as institutional project. The first author was supported as JRF by Department of Biotechnology, Govt. of India vide: BT/PR10174/NNT/28/716/2013. Facilities provided by Chairperson, DOS in Zoology, University of Mysore, is acknowledged.
Data availability
Data is available by request to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
Authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rajendran Senthoorraja and Kesavan Subaharan contributed equally to this work.
References
- Abd El-Salam AME. Toxic and deterrent effects of two volatile oils against cowpea weevil, Callosobruchus chinensis (Coleoptera: Bruchidae) Arch Phytopathol Plant Protect. 2010;43(16):1596–1607. doi: 10.1080/03235400802677735. [DOI] [Google Scholar]
- Alam M, Shah RM, Shad SA, Binyameen M. Fitness cost, realized heritability and stability of resistance to spiromesifen in house fly, Musca domestica L. (Diptera: Muscidae) Pestic Biochem Physiol. 2020;168:104648. doi: 10.1016/j.pestbp.2020.104648. [DOI] [PubMed] [Google Scholar]
- Benelli G, Pavela R. Beyond mosquitoes-essential oil toxicity and repellency against bloodsucking insects. Ind Crop Prod. 2018;117:382–392. doi: 10.1016/j.indcrop.2018.02.072. [DOI] [Google Scholar]
- Benelli G, Pavela R, Giordani C, Casettari L, Curzi G, Cappellacci L, Petrelli R, Maggi F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind Crop Prod. 2018;112:668–680. doi: 10.1016/j.indcrop.2017.12.062. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castaneda LE, Figueroa CC, Nespolo RF. Do insect pests perform better on highly defended plants? costs and benefits of induced detoxification defenses in the aphid Sitobion avenae. J Evol Biol. 2010;23(11):2474–2483. doi: 10.1111/j.1420-9101.2010.02112.x. [DOI] [PubMed] [Google Scholar]
- Cheah SX, Tay JW, Chan LK, Jaal Z. Larvicidal, oviposition, and ovicidal effects of Artemisia annua (Asterales: Asteraceae) against Aedes aegypti, Anopheles sinensis, and Culex quinquefasciatus (Diptera: Culicidae) Parasitol Res. 2013;112(9):3275–3282. doi: 10.1007/s00436-013-3506-0. [DOI] [PubMed] [Google Scholar]
- Chellappandian M, Vasantha-Srinivasan P, Senthil-Nathan S, Karthi S, Thanigaivel A, Ponsankar A, Kalaivani K, Hunter WB. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ Int. 2018;113:214–230. doi: 10.1016/j.envint.2017.12.038. [DOI] [PubMed] [Google Scholar]
- Chellappandian M, Senthil-Nathan S, Vasantha-Srinivasan P, Karthi S, Thanigaivel A, Kalaivani K, Sivanesh H, Stanley-Raja V, Chanthini KM, Shyam-Sundar N. Target and non-target botanical pesticides effect of Trichodesma indicum (Linn) R. Br. and their chemical derivatives against the dengue vector, Aedes aegypti L. Environ Sci Pollut Res. 2019;26(16):16303–16315. doi: 10.1007/s11356-019-04870-3. [DOI] [PubMed] [Google Scholar]
- Cheng SS, Chang HT, Lin CY, Chen PS, Huang CG, Chen WJ, Chang ST. Insecticidal activities of leaf and twig essential oils from Clausena excavata against Aedes aegypti and Aedes albopictus larvae. Pest Manag Sci. 2009;65(3):339–343. doi: 10.1002/ps.1693. [DOI] [PubMed] [Google Scholar]
- Chowdhary K, Kumar A, Sharma S, Pathak R, Jangir M. Ocimum sp. source of biorational pesticides. Ind Crop Prod. 2018;122:686–701. doi: 10.1016/j.indcrop.2018.05.068. [DOI] [Google Scholar]
- Cosse AA, Todd JL, Millar JG, Martinez LA, Baker TC. Electroantennographic and coupled gas chromatographic-electroantennographic responses of the Mediterranean fruit fly, Ceratitis capitata, to male-produced volatiles and mango odor. J Chem Ecol. 1995;21:1823–1836. doi: 10.1007/BF02033679. [DOI] [PubMed] [Google Scholar]
- da Silva Moura EDS, D'Antonino Faroni LR, Fernandes Heleno FF, Aparecida Zinato Rodrigues AAZ, Figueiredo Prates LH, Lopes Ribeiro de Queiroz ME. Optimal extraction of Ocimum basilicum essential oil by association of ultrasound and hydrodistillation and its potential as a biopesticide against a major stored grains pest. Molecules. 2020;25(12):2781. doi: 10.3390/molecules25122781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani R, Kassiri H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease 2019 (COVID-19) Arch Clin Infect Dis Online ahead of Print. 2020;15(COVID-19):e102863. doi: 10.5812/archcid.102863. [DOI] [Google Scholar]
- Delille L. Medicinal Plants in Algeria. AIgeria: BERTI; 2007. [Google Scholar]
- El Zayyat EA, Soliman MI, Elleboudy NA, Ofaa SE. Musca domestica laboratory susceptibility to three ethnobotanical culinary plants. Environ Sci Pollut Res. 2015;22(20):15844–15852. doi: 10.1007/s11356-015-4796-9. [DOI] [PubMed] [Google Scholar]
- Fotedar R. Vector potential of houseflies (Musca domestica) in transmission of Vibrio cholerae in India. Acta Trop. 2001;78(1):31–34. doi: 10.1016/S0001-706X(00)00162-5. [DOI] [PubMed] [Google Scholar]
- Gallardo A, Picollo MI, Mougabure-Cueto G. Lethal activity of individual and mixed monoterpenoids of geranium essential oil on Musca domestica. Parasitol Res. 2015;114(3):1229–1232. doi: 10.1007/s00436-015-4315-4. [DOI] [PubMed] [Google Scholar]
- Ghabbari M, Guarino S, Caleca V, Saiano F, Sinacori M, Baser N, Mediouni-Ben Jemaa J, Lo Verde G. Behavior-modifying and insecticidal effects of plant extracts on adults of Ceratitis capitata (Wiedemann) (Diptera Tephritidae) J Pest Sci. 2018;91:907–917. doi: 10.1007/s10340-018-0952-6. [DOI] [Google Scholar]
- Ghosh A, Zurek L. Fresh steam-flaked corn in Cattle feedlots is an important site for fecal coliform contamination by house flies. J Food Prot. 2015;78(3):567–572. doi: 10.4315/0362-028X.JFP-14-429. [DOI] [PubMed] [Google Scholar]
- Gleiser RM, Bonino MA, Zygadlo JA. Repellence of essential oils of aromatic plants growing in Argentina against Aedes aegypti (Diptera: Culicidae) Parasitol Res. 2011;108:69–78. doi: 10.1007/s00436-010-2042-4. [DOI] [PubMed] [Google Scholar]
- Govindarajan M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac J Trop Med. 2010;3(11):874–877. doi: 10.1016/s1995-7645(10)60210-6. [DOI] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-Transferases -The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- Haselton AT, Acevedo A, Kuruvilla J, Werner E, Kiernan E, Dhar P. Repellency of α-pinene against the house fly, Musca domestica. Phytochem. 2015;117:469–475. doi: 10.1016/j.phytochem.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Hegeto LA, Ronqui L, Lapenta AS, Albuquerque FA. Identification and functional characterization of esterases in Euschistus heros (Hemiptera, Pentatomidae) and their relationship with thiamethoxam and lambda-cyhalothrin. Genet Mol Res. 2015;14(3):11079–11088. doi: 10.4238/2015.September.22.1. [DOI] [PubMed] [Google Scholar]
- Hobson Kenneth R (1995) Host compounds as semiochemicals for bark beetles. In: Salom Scott M, Hobson Kenneth R (eds) Application of semiochemicals for management of bark beetle infestations: proceedings of an Informal Conference, USDA Forest Service General Technical Report INT-GRT-318, pp 48–51
- Hong T, Perumalsamy H, Jang K, Na E, Ahn Y. Ovicidal and larvicidal activity and possible mode of action of phenylpropanoids and ketone identified in Syzygium aromaticum bud against Bradysia procera. Pestic Biochem Physiol. 2018;145:29–38. doi: 10.1016/j.pestbp.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Ikbal C, Pavela R. Essential oils as active ingredients of botanical insecticides against aphids. J Pest Sci. 2019;92:971–986. doi: 10.1007/s10340-019-01089-6. [DOI] [Google Scholar]
- Isman MB. Botanical Insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51(1):45–46. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Isman MB. Bridging the gap: moving botanical insecticides from the laboratory to the farm. Ind Crop Prod. 2017;110:10–14. doi: 10.1016/j.indcrop.2017.07.012. [DOI] [Google Scholar]
- Isman MB. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu Rev Entomol. 2020;65(1):233–249. doi: 10.1146/annurev-ento-011019-025010. [DOI] [PubMed] [Google Scholar]
- Isman MB, Wilson JA, Bradbury R. Insecticidal activities of commercial rosemary oils (Rosmarinus officinalis) against larvae of Pseudaletia unipuncta and Trichoplusia ni in relation to their chemical compositions. Pharm Biol. 2008;46(2):82–87. doi: 10.1080/13880200701734661. [DOI] [Google Scholar]
- Jiang Z, Akhtar Y, Bradbury R, Zhang X, Isman MB. Comparative toxicity of essential oils of Litsea pungens and Litsea cubeba and blends of their major constituents against the cabbage looper, Trichoplusia ni. J Agric Food Chem. 2009;57(11):4833–4837. doi: 10.1021/jf900274r. [DOI] [PubMed] [Google Scholar]
- Kaufman PE, Nunez SC, Mann RS, Christopher GJ, Scharf ME. Nicotinoid and pyrethroid insecticide resistance in houseflies (Diptera: Muscidae) collected from Florida dairies. Pest Manag Sci. 2010;66(3):290–294. doi: 10.1002/ps.1872. [DOI] [PubMed] [Google Scholar]
- Khamesipour F, Lankarani KB, Honarvar B, Kwenti TE. A systematic review of human pathogens carried by the housefly (Musca domestica L.) BMC Public Health. 2018;18(1):1049. doi: 10.1186/s12889-018-5934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HAA, Akram W, Arshad M, Haffez F. Toxicity and resistance of field collected Musca domestica (Diptera: Muscidae) against insect growth regulator insecticides. Parasitol Res. 2015;115(4):1385–1390. doi: 10.1007/s00436-015-4872-6. [DOI] [PubMed] [Google Scholar]
- Khater HF, Geden CJ. Efficacy and repellency of some essential oils and their blends against larval and adult house flies, Musca domestica L. (Diptera: Muscidae) J Vector Ecol. 2019;44(2):256–263. doi: 10.1111/jvec.12357. [DOI] [PubMed] [Google Scholar]
- Koul O, Walia S, Dhaliwal GS. Essential oils as green pesticides: Potential and constraints. Biopestic Int. 2008;4:63–84. [Google Scholar]
- Kramer WL, Mulla S. Oviposition attractants and repellents of mosquitoes: Oviposition responses of Culex mosquitoes to organic infusions. Environ Entomol. 1979;8(6):1111–1117. doi: 10.1093/ee/8.6.1111. [DOI] [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Insecticidal evaluation of essential oils of Citrus sinensis L. (Myrtales: Myrtaceae) against housefly, Musca domestica L. (Diptera: Muscidae) Parasitol Res. 2012;110(5):1929–1936. doi: 10.1007/s00436-011-2719-3. [DOI] [PubMed] [Google Scholar]
- Kumrungsee N, Pluempanupat W, Koul O, Bullangpoti V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J Pest Sci. 2014;87(4):721–729. doi: 10.1007/s10340-014-0602-6. [DOI] [Google Scholar]
- Lawrence BM. A world perspective. Proceedings of the 10th international congress of essential oils, fragrances and flavors, Washington, DC, USA. USA: Amsterdam: Elsevier Science Publisher BV; 1998. p. 161. [Google Scholar]
- Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52(1):231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- Macovei L, Miles B, Zureki L. Potential of houseflies to contaminate ready-to-eat food with antibiotic-resistant enterococci. J Food Prot. 2008;71(2):435–439. doi: 10.4315/0362-028X-71.2.435. [DOI] [PubMed] [Google Scholar]
- Maganga ME, Gries G, Gries R. Repellency of various oils and pine oil constituents to house flies (Diptera: Muscidae) Environ Entomol. 1996;25(5):1182–1187. doi: 10.1093/ee/25.5.1182. [DOI] [Google Scholar]
- Malik A, Singh N, Satya S. House fly (Musca domestica): A review of control strategies for a challenging pest. J Environ Sci Health B. 2007;42(4):453–469. doi: 10.1080/03601230701316481. [DOI] [PubMed] [Google Scholar]
- McCallum EJ, Cunningham JP, Lucker J, Zalucki MP, De Voss JJ, Botella JR. Increased plant volatile production affects oviposition, but not larval development, in the moth Helicoverpa armigera. J Exp Biol. 2011;214:3672–3677. doi: 10.1242/jeb.059923. [DOI] [PubMed] [Google Scholar]
- Meerberg BG, Vermeer HM, Kijlstra A. Controlling of pathogen transmission by flies on organic pig farms – A Review. Outlook Agric. 2007;36(3):193–197. doi: 10.5367/000000007781891432. [DOI] [Google Scholar]
- Meza FC, Roberts JM, Sobhy IS, Okumu FO, Tripet F, Bruce TJA. Behavioural and electrophysiological responses of female Anopheles gambiae mosquitoes to volatiles from a mango bait. J Chem Ecol. 2020;46:387–396. doi: 10.1007/s10886-020-01172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miresmailli S, Bradbury R, Isman MB. Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag Sci. 2006;62(4):366–371. doi: 10.1002/ps.1157. [DOI] [PubMed] [Google Scholar]
- Moon RD (2019) Muscid flies (Muscidae). In: Gary Mullen R, Lance Durden A (eds) Medical and veterinary entomology. Academic Press, London, pp 345–368
- Ogendo JO, Kostyukovsky M, Ravid U, Matasyoh JC, Deng AL, Omolo EO, Kariuki ST, Shaaya E. Bioactivity of Ocimum gratissimum L. Oil and two of its constituents against five insect pests attacking stored food products. J Stored Prod Res. 2008;44(4):328–334. doi: 10.1016/j.jspr.2008.02.009. [DOI] [Google Scholar]
- Padilha de Paula J, Gomes-Carneiro MR, Paumgartten FJ. Chemical composition, toxicity and mosquito repellency of Ocimum selloi oil. J Ethnopharmacol. 2003;88(2-3):253–260. doi: 10.1016/s0378-8741(03)00233-2. [DOI] [PubMed] [Google Scholar]
- Palacios SM, Bertoni A, Rossi Y, Santander R, Urzua A. Insecticidal activity of essential oils from native medicinal plants of Central Argentina against the housefly, Musca domestica (L.) Parasitol Res. 2009;106(1):207–212. doi: 10.1007/s00436-009-1651-2. [DOI] [PubMed] [Google Scholar]
- Pandiyan GN, Mathew N, Munusamy S. Larvicidal activity of selected essential oil in synergized combinations against Aedes aegypti. Ecotoxicol Environ Saf. 2019;174:549–556. doi: 10.1016/j.ecoenv.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Pavela R. Acute and synergistic effects of some monoterpenoid essential oil compounds on the House Fly (Musca domestica L.) J Essent Oil Bear Plants. 2008;11(5):451–459. doi: 10.1080/0972060X.2008.10643653. [DOI] [Google Scholar]
- Pavela R. Insecticidal properties of several essential oils on the house Fly (Musca domestica L.) Phytother Res. 2008;22(2):274–278. doi: 10.1002/ptr.2300. [DOI] [PubMed] [Google Scholar]
- Pavela R. Insecticidal properties of phenols on Culex quinquefasciatus Say and Musca domestica L. Parasitol Res. 2011;109(6):1547–1553. doi: 10.1007/s00436-011-2395-3. [DOI] [PubMed] [Google Scholar]
- Pavela R, Benelli G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21(12):1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Pavela R, Maggi F, Iannarelli R, Benelli G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019;193:236–271. doi: 10.1016/j.actatropica.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Poorjavad N, Goldansaz SH, Dadpour H, Khajehali J. Effect of Ferula assafoetida essential oil on some biological and behavioral traits of Trichogramma embryophagum and T. evanescens. Biol Control. 2014;59:403–413. doi: 10.1007/s10526-014-9583-x. [DOI] [Google Scholar]
- Radulovic NS, Blagojevic PD, Miltojevic AB. α-Linalool marker compound of forged/synthetic sweet basil (Ocimum basilicum L.) essential oils. J Sci Food Agric. 2013;93(13):3292–3303. doi: 10.1002/jsfa.6175. [DOI] [PubMed] [Google Scholar]
- Ravindran P, Subaharan K, Vibina V, Chandran KP, Prathibha PS, Sujithra M. Essential oil in management of coconut rhinoceros beetle Oryctes rhinoceros L. Indian J Entomol. 2019;81(3):603–608. doi: 10.5958/0974-8172.2019.00136.6. [DOI] [Google Scholar]
- Rice PJ, Coats JR. Insecticidal properties of several monoterpenoid to the housefly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae), and Southern Corn rootworn (Coleoptera: Chrysomelidae) J Econ Entomol. 1994;87(5):1172–1179. doi: 10.1093/jee/87.5.1172. [DOI] [PubMed] [Google Scholar]
- Ruschioni S, Riolo P, Verdolini E, Peri E, Guarino S, Colazza S, Romani R, Isidoro N. Fine Structure of Antennal Sensilla of Paysandisia archon and Electrophysiological Responses to Volatile Compounds Associated with Host Palms. PLoS One. 2015;10(4):e0124607. doi: 10.1371/journal.pone.0124607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh D, Nitzan N, Shachter A, Ghanim M, Dudai N. Rosemary–Whitefly Interaction: A continuum of repellency and volatile combinations. J Econ Entomol. 2019;112(2):616–624. doi: 10.1093/jee/toy375. [DOI] [PubMed] [Google Scholar]
- Saeed R, Abbas N, Razaq M, Mahmood Z, Naveed M, Ur Rehman HM. Field evolved resistance to pyrethroids, neonicotinoids and biopesticides in Dysdercus koenigii (Hemiptera: Pyrrhocoridae) from Punjab, Pakistan. Chemosphere. 2018;213:149–155. doi: 10.1016/j.chemosphere.2018.09.042. [DOI] [PubMed] [Google Scholar]
- Salamatian I, Moshaverinia A, Razmyar J, Ghaemi M. In vitro acquisition and retention of low-pathogenic avian influenza H9N2 by Musca domestica (Diptera: Muscidae) J Med Entomol. 2020;57(2):563–567. doi: 10.1093/jme/tjz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kobayashi M, Agui N. Epidemiological potential of excretion and regurgitation by Musca domestica (Diptera: Muscidae) in the dissemination of Escherichia coli O157: H7 to Food. J Med Entomol. 2000;37(6):945–949. doi: 10.1603/0022-2585-37.6.945. [DOI] [PubMed] [Google Scholar]
- Scalerandi E, Flores GA, Palacio M, Defago MT, Carpinella MC, Valladares G, Bertoni A, Palacios SM. Understanding Synergistic Toxicity of Terpenes as Insecticides: Contribution of Metabolic Detoxification in Musca domestica L. Front Plant Sci. 2018;9:1579. doi: 10.3389/fpls.2018.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JG. Evolution of resistance to pyrethroid insecticides in Musca domestica L. Pest Manag Sci. 2017;73(4):716–722. doi: 10.1002/ps.4328. [DOI] [PubMed] [Google Scholar]
- Senthil-Nathan S. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front Physiol. 2013;4:359. doi: 10.3389/fphys.2013.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil-Nathan S. A review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front Physiol. 2020;10:1591. doi: 10.3389/fphys.2019.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhang L, Mi J, Gao X. Role transformation of fecundity and viability: The leading cause of fitness costs associated with beta-cypermethrin resistance in Musca domestica. PLoS One. 2020;15(1):e0228268. doi: 10.1371/journal.pone.0228268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JE, Morales MR, Phippen WB, Vieira RF, Hao Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. In: Janick J, editor. Perspectives on New crops and new uses. VA: Alexandria: ASHS Press; 1999. pp. 499–505. [Google Scholar]
- Singh D, Singh AK. Repellent and insecticidal properties of essential oils against housefly, Musca domestica L. Int J Trop Insect Sci. 1991;12(04):487–491. doi: 10.1017/s1742758400011401. [DOI] [Google Scholar]
- Sinthusiri J, Soonwera M. Oviposition deterrent and ovicidal activities of seven herbal essential oils against female adults of housefly, Musca domestica L. Parasitol Res. 2014;113(8):3015–3022. doi: 10.1007/s00436-014-3964-z. [DOI] [PubMed] [Google Scholar]
- Siriporn P, Mayura S. The effects of herbal essential oils on the oviposition deterrent and ovicidal activities of Aedes aegypti (Linn.), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (Say) Trop Biomed. 2012;29(1):138–150. [PubMed] [Google Scholar]
- Sonmezdag AS, Amanpour A, Kelebek H, Selli S. The most aroma-active compounds in shade-dried aerial parts of basil obtained from Iran and Turkey. Ind Crop Prod. 2018;124:692–698. doi: 10.1016/j.indcrop.2018.08.053. [DOI] [Google Scholar]
- Subaharan K, Senthoorraja R, Manjunath S, Thimmegowda GG, Pragadheesh VS, Bakthavatsalam N, Mohan MG, Senthil-Nathan S, David KJ, Basavarajappa S, Ballal C. Toxicity, behavioural and biochemical effect of Piper betle L. essential oil and its constituents against housefly, Musca domestica L. Pestic Biochem Physiol. 2021;174:104804. doi: 10.1016/j.pestbp.2021.104804. [DOI] [PubMed] [Google Scholar]
- Tak JH, Jovel E, Isman MB. Effects of rosemary, thyme and lemongrass oils and their major constituents on detoxifying enzyme activity and insecticidal activity in Trichoplusia ni. Pestic Biochem Physiol. 2017;140:9–16. doi: 10.1016/j.pestbp.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Tarelli G, Zerba EN, Alzogaray RA. Toxicity to vapor exposure and topical application of essential oils and monoterpenes on Musca domestica (Diptera: Muscidae) J Econ Entomol. 2009;102(3):1383–1388. doi: 10.1603/029.102.0367. [DOI] [PubMed] [Google Scholar]
- Telci I, Bayram E, Yılmaz G, Avcı B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.) Biochem Syst Ecol. 2006;34(6):489–497. doi: 10.1016/j.bse.2006.01.009. [DOI] [Google Scholar]
- Tian Y (2017) Toxicity and repellency of essential oils to the house fly (Musca domestica L.). Master of Science Thesis, Auburn University, Alabama
- Varga F, Carovic-Stanko K, Ristic M, Grdisa M, Liber Z, Satovic Z. Morphological and biochemical intraspecific characterization of Ocimum basilicum L. Ind Crop Prod. 2017;109:611–618. doi: 10.1016/j.indcrop.2017.09.018. [DOI] [Google Scholar]
- Vasantha-Srinivasan P, Thanigaivel A, Edwin ES, Ponsankar A, Senthil-Nathan S, Selin-Rani S, Kalaivani K, Hunter WB, Duraipandiyan V, Al-Dhabi NA. Toxicological effects of chemical constituents from Piper against the environmental burden Aedes aegypti Liston and their impact on non-target toxicity evaluation against biomonitoring aquatic insects. Environ Sci Pollut Res Int. 2018;25(11):10434–10446. doi: 10.1007/s11356-017-9714-x. [DOI] [PubMed] [Google Scholar]
- Venugopal V, Subaharan K. Electrophysiological and behavioral response of red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Dryophthoridae) to fermented coconut sap neera. J Plant Crop. 2019;47(2):82–89. doi: 10.25081/jpc.2019.v47.i2.5767. [DOI] [Google Scholar]
- Vontas JG, Small GJ, Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem J. 2001;357(1):65–72. doi: 10.1042/bj3570065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waliwitiya R, Nicholson RA, Kennedy CJ, Lowenberger CA. The synergistic effects of insecticidal essential oils and piperonylbutoxide on biotransformational enzyme activities in Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2012;49(3):614–623. doi: 10.1603/ME10272. [DOI] [PubMed] [Google Scholar]
- Wanaratana S, Amonsin A, Chaisingh A, Panyim S, Sasipreeyajan J, Pakpinyo S. Experimental assessment of houseflies as vectors in avian influenza subtype H5N1 transmission in chickens. Avian Dis. 2013;57(2):266–272. doi: 10.1637/10347-090412-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PR, Hobson KR. Stereospecific antennal response by the red turpentine beetle, Dendroctonus valens to chiral monoterpenes from ponderosa pine resin. J Chem Ecol. 1993;19(10):2193–2202. doi: 10.1007/BF00979657. [DOI] [PubMed] [Google Scholar]
- Yang YC, Lee SH, Clark JM, Ahn YJ. Ovicidal and adulticidal activities of Origanum majorana essential oil constituents against insecticide-susceptible and pyrethroid/malathion-resistant Pediculus humanus capitis (Anoplura: Pediculidae) J Agric Food Chem. 2009;57(6):2282–2287. doi: 10.1021/jf803738z. [DOI] [PubMed] [Google Scholar]
- Yang H, Piao X, Zhang L, Song S, Xu Y. Ginsenosides from the stems and leaves of Panax ginseng show antifeedant activity against Plutella xylostella (Linnaeus) Ind Crop Prod. 2018;124:412–417. doi: 10.1016/j.indcrop.2018.07.054. [DOI] [Google Scholar]
- Yu SJ. Induction of detoxification enzymes by triazine herbicides in the fall armyworm, Spodoptera frugiperda. Pestic Biochem Physiol. 2004;80(2):113–122. doi: 10.1016/j.pestbp.2004.06.005. [DOI] [Google Scholar]
- Zhang L, Gao X, Liang P. Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae) Pestic Biochem Physiol. 2007;89(1):65–72. doi: 10.1016/j.pestbp.2007.03.001. [DOI] [Google Scholar]
- Zibaee A, Bandani A. A study on the toxicity of a medicinal plant, Artemisia annua L. (Asteracea) extracts to the sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae) J Plant Prot Res. 2010;50(1):79–85. doi: 10.2478/v10045-010-0014-4. [DOI] [Google Scholar]
- Zito P, Guarino S, Peri E, Sajeva M, Colazza S. Electrophysiological and behavioural responses of the housefly to “sweet” volatiles of the flowers of Caralluma europaea (Guss.) N.E. Br. Arthropod-Plant Interact. 2013;7:485–489. doi: 10.1007/s11829-013-9270-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available by request to the corresponding author.