Abstract
Background
Heavy menstrual bleeding (HMB) is often the first bleeding symptom for female individuals with inherited bleeding disorders. Guidelines recommend performing the hemostatic evaluation at HMB presentation. Von Willebrand factor (VWF) levels increase with stress, making it unclear if VWF studies during acute bleeding are beneficial in diagnosing von Willebrand disease (VWD).
Objectives
To determine the utility of testing for VWD during acute HMB.
Patients/Methods
This retrospective cohort study evaluated VWF levels of individuals presenting to the emergency department (ED) with HMB from January 1, 2017, to December 31, 2018, after prospective implementation of a clinical practice guideline recommending hemostatic evaluation in the ED. We compared VWF and factor VIII (FVIII) levels between acute presentation and follow‐up visit after bleeding resolution. We compared the diagnostic accuracy of initial and follow‐up labs.
Results
During the study period, 221 individuals were seen in the ED for acute HMB, and 39 had VWD testing at both time points. Median FVIII and VWF levels were higher during acute bleeding than at follow‐up. The difference in VWF levels between visits was negligible when initial FVIII value was normal. Overall incidence of VWD was 7.5%; 69% of those with VWD had low VWF levels during acute HMB.
Conclusion
VWD testing during acute HMB detects the majority of individuals with VWD but also leads to elevated levels of VWF, potentially limiting at the accuracy of diagnostic labs during acute bleeding episodes. Delayed testing until resolution of anemia and active bleeding may provide more accurate diagnostic evaluation for VWD.
Keywords: adolescent, blood coagulation tests, female, hemostatics, menorrhagia, Von Willebrand disease, von Willebrand factor
Essentials.
Von Willebrand factor (VWF) levels increase with physiologic stress.
Bleeding disorder evaluation is recommended during episodes of acute heavy menstrual bleeding (HMB).
VWF levels are significantly elevated during acute HMB episodes, limiting diagnostic utility.
During acute bleeding, VWF levels are close to baseline when factor VIII level is normal.
1. INTRODUCTION
Heavy menstrual bleeding (HMB) is common in adolescent girls; almost 40% report HMB. HMB is defined as “excessive menstrual blood loss that interferes with the woman’s physical, emotional, social and material quality of life (QOL) and can occur alone or in combination with other symptoms.” 1 Excessive menstrual blood loss is quantified as blood loss >80 mL per menstrual cycle, as this amount leads to iron deficiency anemia (IDA). IDA can have devastating effects on cognition, leading to decreased scholastic performance, mathematic abilities, attention, and concentration. 2 , 3 , 4 , 5 , 6 There is evidence that QOL is compromised in individuals with HMB. 7
While it is widely accepted that the pathophysiology of HMB differs between adolescents and older women, the recommended diagnostic evaluation of adolescents does not differ from older women in international guidelines or the American College of Obstetrics and Gynecology (ACOG) Committee Opinion. 3 , 8 Adolescents commonly have dysfunction of the hypothalamic‐pituitary‐ovarian axis while older women more commonly have anatomic or systemic pathology. 9 , 10 , 11
HMB is often the presenting sign of bleeding disorders and must be considered for all women. 12 , 13 The majority of women with a bleeding disorder report a history of HMB. 2 , 4 Inherited bleeding disorders are relatively common in women with HMB, with incidence estimates ranging from 10% to 62% 14 , 15 , 16 , 17 , 18 ; a retrospective study found inherited bleeding disorders in 33% of adolescent girls with HMB referred for subspecialty hematology care, which likely demonstrates a more accurate estimation. 14 Most commonly, von Willebrand disease (VWD) (5%‐36%) and platelet function defects (2%‐22%) are described, while clotting factor deficiencies (8%‐9%) are reported less frequently. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
The diagnosis of VWD in the setting of acute HMB is challenging. Physiologic and psychological stress both cause elevations in von Willebrand factor (VWF), and both types of stress are present in adolescents with HMB. 27 , 28 , 29 This stress response makes the reliability of VWD testing during acute bleeding unclear.
A clinical pathway was created at our institution to improve the diagnosis of bleeding disorders and standardize management. All adolescents presenting to the emergency department (ED) or inpatient unit with acute HMB were screened according to a modified Claire Philipp screening tool (Figure 1); if an individual screened positive or had active bleeding, the pathway encouraged up‐front laboratory evaluation based on international expert panel recommendations, and recommended treatment based on symptoms and hemoglobin. 3 The creation of a clinical pathway puts us in a unique place to evaluate adolescent girls presenting with acute HMB treated in a standardized process. This study aims to characterize the ability to detect inherited bleeding disorders in adolescents presenting with acute HMB (defined per screening tool: Figure 1), and quantify the accuracy of VWD testing during acute bleeding.
FIGURE 1.
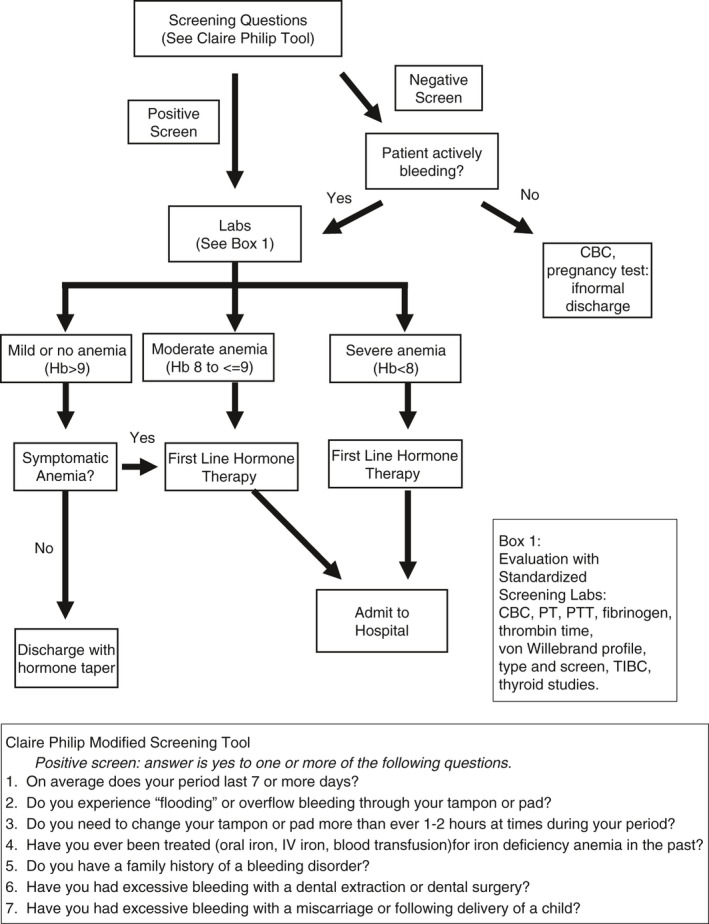
Children’s Healthcare of Atlanta Emergency Department Acute HMB Clinical Practice Guideline. CBC, complete blood count; HMB, heavy menastrual bleeding; PT, prothrombin time; PTT, partial thromboplastin time; TIBC, total iron‐binding capacity
2. PATIENTS AND METHODS
2.1. Study design and participants
This retrospective cohort study evaluates the results of VWD testing during acute bleeding and follow‐up. While the analysis is retrospective, the clinical pathway was prospectively designed with the intention of determining the effectiveness of VWD labs at the time of acute HMB. Subjects were identified using International Classification of Diseases, Ninth Revision and Tenth Revision codes for HMB (N93.9, N92.1, N93.8, N92.0, N92.6, N92.2, N92.4) for ED or inpatient encounters between January 1, 2017, and December 31, 2018. Inclusion criteria included postmenarchal status, and either actively bleeding on presentation or a positive screen on the modified Claire Philipp screening tool (Figure 1). Subjects included had VWD studies (factor VIII [FVIII], VWF antigen (VWF:Ag) and VWF ristocetin cofactor assay [VWF:RCo]) obtained both during an acute HMB episode and during a subsequent visit to Children’s Healthcare of Atlanta (CHOA). Subjects were excluded for a previously diagnosed bleeding disorder, previous testing for bleeding disorders, anticoagulant use, or active oncologic or rheumatologic condition. Subjects with a positive pregnancy test or within 3 months postpartum were excluded. There were no age‐based exclusions. For subjects with two or more encounters within the study period, data were collected from the earliest encounter. Approval was obtained from the CHOA Institutional Review Board with a waiver of informed consent and assent.
2.2. Data collection
A REDCap database was designed to capture demographic, clinical, diagnostic, and follow‐up information. Data were manually extracted from the electronic medical record. Laboratory values from the encounter were collected; if duplicate laboratory tests were obtained, the initial value was recorded. Repeat values were used only if the test was repeated within 6 hours due to concern for an erroneous result. Follow‐up visits were assessed by chart review in the CHOA system through April 30, 2019; the first set of VWF labs obtained after discharge were recorded.
2.3. Laboratory assay and intervention
All coagulation studies were performed locally. VWF:Ag and FVIII assays were performed at the CHOA laboratory using a STA‐R Evolution analyzer (Stago, North York, Toronto, ON, Canada). The FVIII assay uses the STA‐Deficient VIII, and the kit for the VWF:Ag is STA‐Liatest VWF:Ag. The VWF:RCo assay was performed at Emory University in the coagulation laboratory using the BCS XP system (Siemens, Marburg, Germany) using BC von Willebrand reagent.
2.4. Statistical analysis
Descriptive statistics were performed to evaluate patient characteristics. Statistics were derived using frequency and percentages for categorical variables and median with interquartile range for continuous variables. Between‐group differences for means were calculated with paired t tests, and differences in medians were calculated with Mann‐Whitney and Wilcoxon rank‐sum tests. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS AND DISCUSSION
3.1. Patient demographics
Over the 2‐year study period, 221 adolescent girls received care in the ED or inpatient unit for acute HMB; of these, 127 girls had VWD studies during their ED visit, and 39 of those individuals had VWD studies at an outpatient visit after bleeding resolution (Table 1). The cohort was primarily Black (44%) and non‐Hispanic (85%) girls, with a median age at presentation of 13.4 years (interquartile range, 12.4‐15.3). The most frequent reported symptom of HMB was bleeding lasting >7 days (87.2%), followed by changing sanitary pad more frequently than every 2 hours (33.3%) and flooding (33.3%). Median hemoglobin was 7.8 g/dL, and 87% had labs consistent with iron deficiency. The high prevalence of iron deficiency supports the hypothesis that the majority of adolescents were experiencing the physiologic stress of iron deficiency or iron deficiency anemia. Eighty‐one percent of the subjects were admitted to the hospital for HMB (admission criteria: <9 g/dL or symptomatic anemia), and 20.5% of the study population had a repeat ED visit within 30 days due to a concern related to HMB or anemia. Adolescents who had repeat labs in the clinic had a slightly lower median age (13.5 years vs 14.4 years) and a lower presenting hemoglobin (7.8 g/dL vs 9.5 g/dL) but had no difference in bleeding symptoms as demonstrated by the Claire Philip screening tool than those who did not follow up.
TABLE 1.
Characteristics of subjects who had diagnostic VWD labs drawn during acute hospital presentation and at outpatient follow‐up visit
| Characteristic |
Subjects n = 39 |
|---|---|
| Age at presentation, y | 13.5 (12.4‐15.3) |
| Race | |
| Black or African American | 17 (43.6) |
| White | 15 (38.5) |
| Asian | 1 (2.6) |
| American Indian or Alaskan Native | 1 (2.6) |
| Multiracial | 1 (2.6) |
| Declined/Not reported | 4 (10.3) |
| Ethnicity | |
| Hispanic | 6 (15.4) |
| BMI, kg/m2 | 23.6 (20.8–29.4) |
| Modified Philipp screening tool | |
| Bleeding for >7 d | 34 (87.2) |
| Flooding/overflow bleeding | 13 (33.3) |
| Changing feminine hygiene product every 1 to 2 hours. | 13 (33.3) |
| Treated for iron deficiency in the past | 8 (20.5) |
| Family history of bleeding disorder | 0 |
| Excessive bleeding with dental procedure | 1 (5.1) |
| Excessive bleeding with miscarriage/childbirth | 0 |
| Not documented | 2 (5.1) |
| Laboratory values | |
| Hemoglobin, g/dL | 7.75 (6.10‐9.10) |
| Ferritin, ng/mL | 5.18 (2.59‐13.9) |
| Normal or abnormal findings on Philipp screening tool | 2 (1‐3) |
| Disposition | |
| Admit to hospital | 30 (76.9) |
| Return emergency department visit within 30 d | 8 (20.5) |
| Outpatient follow‐up | |
| Hematology | 21 (53.8) |
| Gynecology | 24 (61.5) |
| Time to follow‐up, d | |
| Hematology | 41 (1‐322) a |
| Gynecology | 32 (6‐384) a |
Median (IQR) or n (%).
Abbreviations: BMI, body mass index; IQR, interquartile range; VWD, von Willebrand disease.
Median (range).
3.2. Von Willebrand studies at presentation and follow‐up
VWF:Ag, VWF:RCo, and FVIII were significantly higher at ED presentation than at follow‐up (Figure 2). The median FVIII at presentation was 245.5% (95% confidence interval [CI], 196‐278) versus 137.5% (95% CI, 126‐181) at follow‐up (P = .002). Median VWF:Ag was 129% with (95% CI, 111‐158) versus 83% (95% CI, 72‐101) at follow‐up (P = .006), and the median VWF:RCo was 106% (95% CI, 82‐139) at presentation versus 63.5% (95% CI, 58‐77) at follow‐up (P = .002). Given the degree of anemia present in the majority of subjects, physiologic stress likely contributed to the elevated levels at the time of acute HMB. The median individual change from ED presentation to follow‐up was −62.4% for FVIII, −27% for VWF:Ag, and −23.5% for VWF:RCo. Both at a population level and individual level, all values in the VWD profile showed significant elevation at the time of acute HMB.
FIGURE 2.
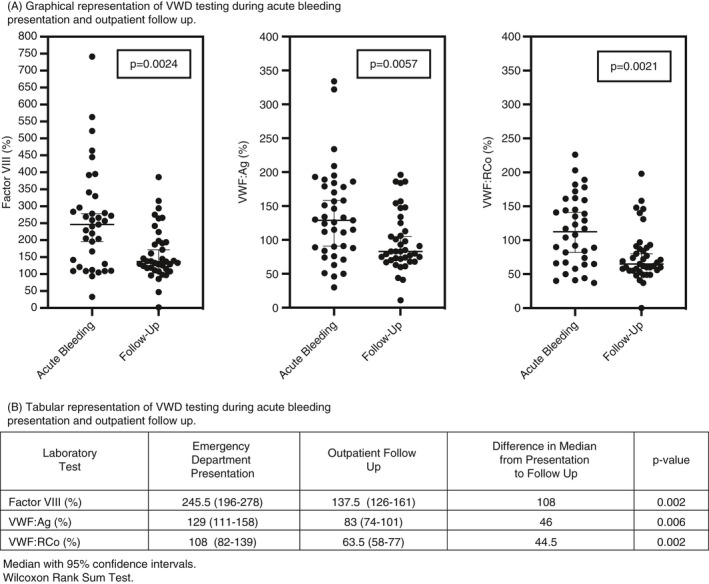
Von Willebrand disease (VWD) diagnostic laboratory studies at time of emergency department presentation with acute bleeding and outpatient follow‐up. (A) Graphical representation of VWD testing during acute bleeding presentation and outpatient follow‐up. (B) Tabular representation of VWD testing during acute bleeding presentation and outpatient follow‐up. VWD, von Willebrand disease; VWF:Ag, von Willebrand factor antigen; VWF:RCo, von Willebrand factor ristocetin cofactor
Of the 127 individuals who had VWD studies done during their hospital encounter, 16 were found to have VWD or low VWF. Of the 16, 11 were identified on the basis of labs during their initial presentation (68.8%), and 5 were diagnosed from labs at a follow‐up visit.
Although the current position statement from ACOG recommends VWD testing at the time of presentation, our study demonstrated that these levels are elevated during acute HMB. Knowing the effect of stress on VWD studies, it is debatable if this testing should be done up front or delayed until HMB and anemia have resolved. Although the majority of subjects with VWD were detected on their initial labs, the stress response affected initial values. Delaying the evaluation until symptom resolution would limit the effect of stress on diagnostic labs. Since treatment of HMB is rarely altered with a diagnosis of VWD, the acute care would not be compromised.
Poor outpatient follow‐up limits the effectiveness of delayed diagnostic evaluation. In the overall cohort, only 16.7% attended a hematology clinic, and 24.0% attended a gynecology clinic. By delaying diagnostic evaluation to the outpatient setting, several individuals whose VWD was identified on initial labs may not have followed up. The perception that a bleeding disorder had been ruled out potentially decreased follow‐up. Several providers and parents reported this incorrect perception due to normal initial screening labs. Delayed testing may encourage outpatient follow‐up.
Although our study is the first to look at VWD studies at presentation for acute HMB and upon resolution of bleeding, there is not yet sufficient evidence to make a clear recommendation for up‐front versus delayed testing. While up‐front testing detected almost 70% of VWD diagnoses and likely decreased the time from symptom onset to diagnosis, it puts a burden on the ED to follow up labs and provides false reassurance when labs are normal.
3.3. Stratification by FVIII levels
Since FVIII, like VWF, increases with stress, we stratified individuals by FVIII to help determine if a normal FVIII level could identify subjects who were at their steady state rather than a state of stress (Figure 3). For those with elevated FVIII at presentation (defined at >150%), the median change in VWF levels between ED presentation and follow‐up was a decrease by 35% for both VWF:Ag and VWF:RCo (P=.017 and P=.012, respectively). The change in VWF:Ag and VWF:RCo was 2% and −1%, respectively, in subjects with normal FVIII on their labs sent from the ED visit. These findings indicate that clinicians should consider the FVIII value when determining if VWF levels are indicative of a true steady state for the individual versus a stress response. It appears that normal VWF:Ag and VWF:RCo levels in the setting of a normal FVIII level are reassuring against a diagnosis of VWD.
FIGURE 3.
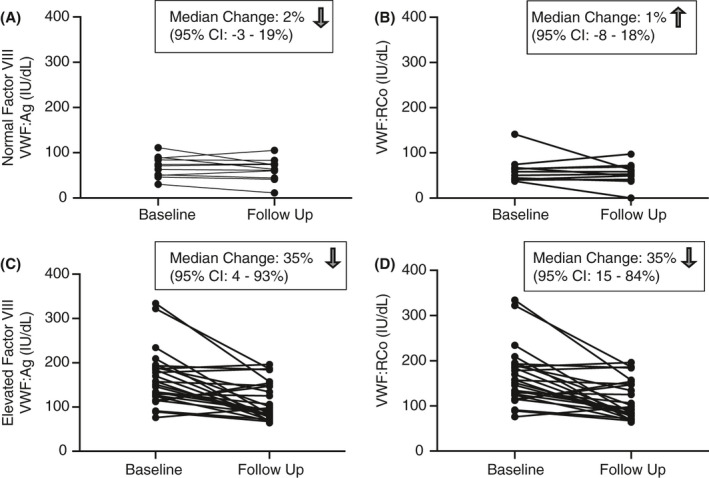
Change in (A, C) von Willebrand factor antigen (VWF:Ag) and (B, D) von Willebrand factor ristocetin cofactor assay (VWF:RCo) from hospital presentation to outpatient follow‐up, stratified by factor VIII (FVIII) level. Normal FVIII defined as <150%
3.4. Von Willebrand cutoff of 100% (IU/dL) in acutely bleeding population
Given the active stress response in our population, we evaluated a recently published threshold in which initial VWF:Ag or VWF:RCo over 100% (or IU/dL) had a high negative predictive value (NPV) against VWD in pediatric patients undergoing evaluation for bleeding disorders. 30 We wanted to determine if this cutoff would have a high NPV despite the stress‐related elevations in FVIII and VWF. In our cohort, of those with a baseline VWF:Ag or VWF:RCo >100, there was a 93.2% (95% CI, 74.9%‐99.1%) and 95.0% (95% CI, 75.1%‐99.9%) NPV for VWD, respectively.
While the NPV was slightly lower in our cohort, overall it remained high, indicating that this threshold may be valid in the setting of acute bleeding. The poor follow‐up limits the accuracy of the NPV, as it is possible that cases of VWD were missed on the basis of their ED labs being in the normal range. We need more uniform data to more carefully elucidate the utility of this screening cutoff in an acutely bleeding population.
4. CONCLUSIONS
In the setting of acute HMB, diagnostic studies for VWD are elevated from an individual’s baseline, likely due to the physiologic stress of anemia. This is the first study to examine the results of VWD diagnostic laboratory tests in the acute HMB population. While guidelines recommend testing for VWD at the time of acute HMB, our study demonstrates that this may lead to false elevation in VWF levels which can mask a diagnosis of VWD and can provide false reassurance. Despite the demonstrated elevation of VWF during acute bleeding, the majority of adolescents with low VWF and VWD were identified during their acute bleeding episode, elucidating the need for further studies to help determine the optimal timing for testing in this population.
AUTHOR CONTRIBUTIONS
MCB and RFS designed the study. MCB conducted statistical analysis and abstracted data with MHW. RF, KW, MK, KC, and RFS designed the clinical practice guideline. All authors reviewed and approved the manuscript.
RELATIONSHIP DISCLOSURE
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by the Georgia Clinical and Translational Science Alliance Postdoctoral Research Training Award (TL1TR002382‐01 and UL1TR002378‐01) and the National Hemophilia Foundation Takeda Clinical Fellowship Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Hemophilia Foundation, or Takeda.
Handling Editor: Dr Neil Zakai.
Contributor Information
Megan C. Brown, Email: Megan.Brown@choa.org, @MeganBrownMD.
Michael H. White, @mhughwhite.
Robert F. Sidonio, Jr, @nashgreenie.
REFERENCES
- 1. (NICE) NCCfWsaCsH . Heavy menstrual bleeding clinical guideline: RCOG Press; 2007. [PubMed]
- 2. Zia A, Journeycake JM, Jain S, Sarode R. Comprehensive evaluation of bleeding disorders in adolescents with heavy menstrual bleeding. Blood. 2016;128(22):876. [Google Scholar]
- 3. James AH, Kouides PA, Abdul‐Kadir R et al. Evaluation and management of acute menorrhagia in women with and without underlying bleeding disorders: consensus from an international expert panel. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):124‐134. [DOI] [PubMed] [Google Scholar]
- 4. Kadir RA, Economides DL, Sabin CA, Pollard D, Lee CA. Assessment of menstrual blood loss and gynaecological problems in patients with inherited bleeding disorders. Haemophilia. 1999;5(1):40‐48. [DOI] [PubMed] [Google Scholar]
- 5. Karlsson TS, Marions LB, Edlund MG. Heavy menstrual bleeding significantly affects quality of life. Acta Obstet Gynecol Scand. 2014;93(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 6. Von Mackensen S. Quality of life in women with bleeding disorders. Haemophilia. 2011;17(suppl 1):33‐37. [DOI] [PubMed] [Google Scholar]
- 7. Gokyildiz S, Aslan E, Beji NK, Mecdi M. The effects of menorrhagia on women’s quality of life: a case‐control study. ISRN Obstet Gynecol. 2013;2013:918179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Screening and Management of Bleeding Disorders in Adolescents With Heavy Menstrual Bleeding: ACOG COMMITTEE OPINION SUMMARY, Number 785. Obstet Gynecol. 2019;134(3):658‐659. [DOI] [PubMed] [Google Scholar]
- 9. American College of O, Gynecologists . ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive‐aged women. Obstet Gynecol. 2013;121(4):891‐896. [DOI] [PubMed] [Google Scholar]
- 10. Powers JM, Stanek JR, Srivaths L, Haamid FW, O'Brien SH. Hematologic considerations and management of adolescent girls with heavy menstrual bleeding and anemia in US children’s hospitals. J Pediatr Adolesc Gynecol. 2018;31(5):446‐450. [DOI] [PubMed] [Google Scholar]
- 11. Sweet MG, Schmidt‐Dalton TA, Weiss PM, Madsen KP. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85(1):35‐43. [PubMed] [Google Scholar]
- 12. O’Brien SH. Evaluation and management of heavy menstrual bleeding in adolescents: the role of the hematologist. Blood. 2018;2018(1):390‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sriprasert I, Pakrashi T, Kimble T, Archer DF. Heavy menstrual bleeding diagnosis and medical management. Contracept Reprod Med. 2017;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zia A, Jain S, Kouides P et al. Bleeding disorders in adolescents with heavy menstrual bleeding in a multicentre prospective US cohort. Haematologica. 2020;105(7):1969‐1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351(9101):485‐489. [DOI] [PubMed] [Google Scholar]
- 16. Dilley A, Drews C, Miller C et al. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001;97(4):630‐636. [DOI] [PubMed] [Google Scholar]
- 17. Philipp CS, Dilley A, Miller CH et al. Platelet functional defects in women with unexplained menorrhagia. J Thromb Haemost. 2003;1(3):477‐484. [DOI] [PubMed] [Google Scholar]
- 18. Edlund M, Blomback M, von Schoultz B, Andersson O. On the value of menorrhagia as a predictor for coagulation disorders. Am J Hematol. 1996;53(4):234‐238. [DOI] [PubMed] [Google Scholar]
- 19. Ahuja SP, Hertweck SP. Overview of bleeding disorders in adolescent females with menorrhagia. J Pediatr Adolesc Gynecol. 2010;23(6 suppl):S15‐21. [DOI] [PubMed] [Google Scholar]
- 20. James AH. Bleeding disorders in adolescents. Obstet Gynecol Clin North Am. 2009;36(1):153‐162. [DOI] [PubMed] [Google Scholar]
- 21. Claessens EA, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139(3):277‐280. [DOI] [PubMed] [Google Scholar]
- 22. Oral E, Cagdas A, Gezer A, Kaleli S, Aydin Y, Ocer F. Hematological abnormalities in adolescent menorrhagia. Arch Gynecol Obstet. 2002;266(2):72‐74. [DOI] [PubMed] [Google Scholar]
- 23. Bevan JA, Maloney KW, Hillery CA, Gill JC, Montgomery RR, Scott JP. Bleeding disorders: a common cause of menorrhagia in adolescents. J Pediatr. 2001;138(6):856‐861. [DOI] [PubMed] [Google Scholar]
- 24. Philipp CS, Faiz A, Dowling N et al. Age and the prevalence of bleeding disorders in women with menorrhagia. Obstet Gynecol. 2005;105(1):61‐66. [DOI] [PubMed] [Google Scholar]
- 25. Mikhail S, Varadarajan R, Kouides P. The prevalence of disorders of haemostasis in adolescents with menorrhagia referred to a haemophilia treatment centre. Haemophilia. 2007;13(5):627‐632. [DOI] [PubMed] [Google Scholar]
- 26. Chi C, Pollard D, Tuddenham EG, Kadir RA. Menorrhagia in adolescents with inherited bleeding disorders. J Pediatr Adolesc Gynecol. 2010;23(4):215‐222. [DOI] [PubMed] [Google Scholar]
- 27. Nichols WL, Hultin MB, James AH et al. von Willebrand disease (VWD): evidence‐based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171‐232. [DOI] [PubMed] [Google Scholar]
- 28. van Loon JE, Sonneveld MA, Praet SF, de Maat MP, Leebeek FW. Performance related factors are the main determinants of the von Willebrand factor response to exhaustive physical exercise. PLoS One. 2014;9(3):e91687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zgraggen L, Fischer JE, Mischler K, Preckel D, Kudielka BM, von Kanel R. Relationship between hemoconcentration and blood coagulation responses to acute mental stress. Thromb Res. 2005;115(3):175‐183. [DOI] [PubMed] [Google Scholar]
- 30. Doshi BS, Rogers RS, Whitworth HB et al. Utility of repeat testing in the evaluation for von Willebrand disease in pediatric patients. J Thromb Haemost. 2019;17(11):1838‐1847. [DOI] [PubMed] [Google Scholar]


