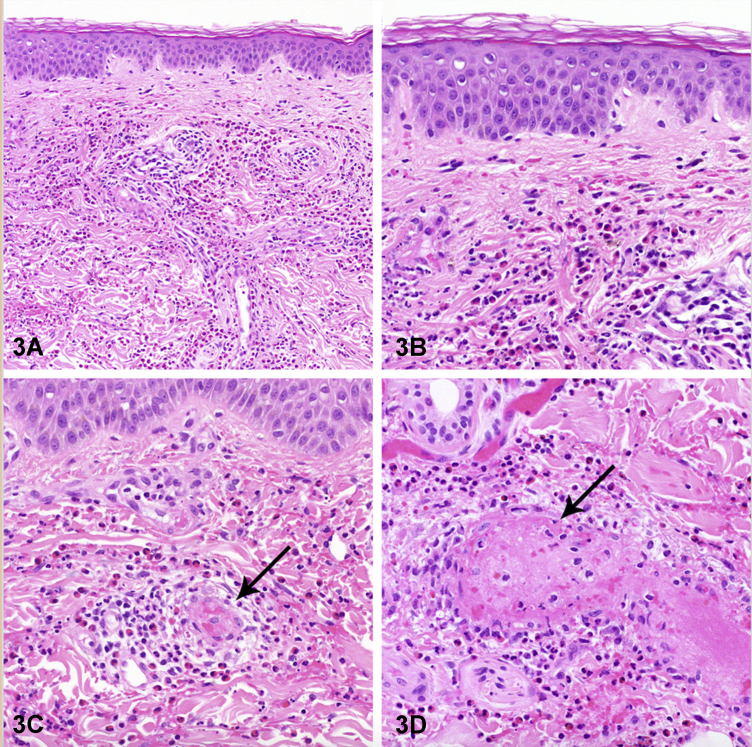
A 56-year-old man with no past medical history presented with dry cough and dyspnea. He was given antibiotics and bronchodilators which did not improve his symptoms. Three years after the initial symptoms began, he developed persistent hypereosinophilia with an absolute eosinophil count as high as 2250 cells/uL. Prednisone (40 mg daily) was initially helpful, but his symptoms returned once the drug was tapered. Subsequently, he developed new myalgias, arthralgias, joint swelling, hives, and polycyclic purpuric plaques with hemorrhagic zones and erythema on his trunk and extremities (Figs 1 and 2). A skin biopsy of the rash revealed necrotizing vasculitis with eosinophils (Fig 3). Bone marrow biopsy showed marked eosinophilia with immature eosinophils.
Fig 1.

Fig 2.

Fig 3.
Question 1: What is the most likely diagnosis?
-
A.
Microscopic Polyangiitis
-
B.
Hypereosinophilic syndrome (HES)
-
C.
Bullous pemphigoid
-
D.
Strongyloidiasis
-
E.
Drug rash with eosinophilia and systemic symptoms (DRESS)
Answers:
-
A.
Microscopic Polyangiitis – Incorrect. Microscopic polyangiits often presents as cough accompanied by skin changes. Necrotizing vasculitis and palpable purpura are also common manifestations. However, microscopic polyangiitis does not typically present with hypereosinophilia. Eosinophilic granulomatosis with polyangiitis would be more consistent with the presentation described. Antineutrophil cytoplasmic antibodies testing can exclude these diagnoses, and was found to be negative in this patient.
-
B.
HES – Correct. HES is characterized by persistent absolute eosinophil count of >1500 cells/uL as well as the involvement of at least two organ systems (in this case, bone marrow and skin) with no evidence of parasites, allergies, or other causes of elevated eosinophil count.1 Over half of patients with HES will have skin involvement, but this is often non-specific.2 Histopathology is also variable, but most commonly shows superficial, dermal, interstitial, or perivascular eosinophilic infiltration.2 The polycyclic purpuric plaques as seen with this patient are unusual.
-
C.
Bullous pemphigoid – Incorrect. Bullous pemphigoid is an autoimmune disease that classically causes tense, fluid-filled blisters. It is not often associated with dyspnea, arthralgias, or cough. Subepidermal blistering is the most common histological feature, and necrotizing vasculitis with eosinophils or bone marrow involvement are unlikely.
-
D.
Strongyloidiasis – Incorrect. Dry cough, skin changes, and peripheral eosinophilia are consistent with strongyloides infection. However, the rash in strongyloidiasis is typically raised, pruritic, serpiginous streaks on the thighs and trunk, localized urticaria, or angioedema. Skin biopsy is uncommonly performed, but often shows larvae in the dermis.
-
E.
DRESS – Incorrect. DRESS most commonly occurs 2-8 weeks after a new medication as a morbilliform eruption. Eosinophilia is common, and approximately 30% of people develop dyspnea or dry cough. Histopathology is nonspecific, but most often shows interface dermatitis with basal vacuolization and can show vascular damage as in this patient. However, DRESS often has a more acute, severe disease course associated with a new medication which does not fit this patient's clinical course.
Question 2: What additional testing is not recommended to further assess this patient's eosinophilia?
-
A.
Testing for parasites
-
B.
Tryptase
-
C.
Evaluation of urine eosinophils
-
D.
Assessment of medication list
-
E.
Chest imaging
Answers:
-
A.
Testing for parasites – Incorrect. Testing for serum strongyloides antibody and stool ova and parasites are important for ruling out parasitic infection as a cause of hypereosinophilia, especially for patients with travel or residence in endemic countries or certain occupations, like slaughterhouse workers. This patient had negative testing.
-
B.
Tryptase – Incorrect. Elevated tryptase could point to systemic macrocytosis as the cause of hypereosinophilia. Systemic mastocytosis often presents with thrombocytopenia, splenomegaly, urticaria, flushing, or anaphylaxis. This patient had normal tryptase levels.
-
C.
Urine eosinophils – Correct. Urine eosinophil evaluation has no benefit in evaluating peripheral eosinophilia. Urine eosinophils are most commonly seen in acute interstitial nephritis, glomerulonephritis, chronic kidney disease, and pyelonephritis.
-
D.
Assessment of medication list – Incorrect. A careful assessment of the history of current medications, herbal supplements, and over the counter medications is essential. Common causes of eosinophilia include non-steroidal anti-inflammatory drugs, aspirin, penicillins, ranitidine, allopurinol, and phenytoin. The patient was not on any of these mediations and did not start any new medications.
-
E.
Chest imaging – Incorrect. Chest imaging and testing for antineutrophil cytoplasmic antibodies is important for patients with respiratory symptoms, such as this patient, to assess for eosinophilic granulomatosis with polyangiitis.
Question 3: Which additional finding supports a diagnosis of HES?
-
A.
Bone marrow biopsy with a percentage of eosinophils <20% of all nucleated cells
-
B.
Presence of Philadelphia chromosome
-
C.
FIPL1-PDGFRA fusion
-
D.
B-cell receptor (BCR) clonal rearrangement
-
E.
Elevation of IL-2
Answers:
-
A.
Bone marrow biopsy with a percentage of eosinophils <20% of all nucleated cells – Incorrect. Eosinophils >20% of all nucleated cells on bone marrow section is most consistent with HES.1 This patient had bone marrow biopsy evidence of extensive involvement of eosinophils.
-
B.
Presence of Philadelphia chromosome – Incorrect. A Philadelphia chromosome is caused by the translocation of a piece of chromosome 9 and 22 resulting in a BCR-ABL gene that is commonly seen in chronic myeloid leukemia (CML). Eosinophilia is a common component of CML.3 All potential causes of eosinophilia, including CML, must be excluded before a diagnosis of HES is made.
-
C.
FIPL1-PDGFRA fusion – Correct. Fusion of FIPL1-PDGFRA is caused by a deletion on chromosome 4q12, which causes constitutive tyrosine kinase activation. This fusion is implicated in 10-14% of patients with HES.4 This patient had fluorescence in situ hybridization testing for PDGFR fusions in hypereosinophilia and a 95-gene next-generation sequencing panel that showed a wild-type PDGFRA as well as wild-type c-KIT and JAK2 (other molecular variants causing hypereosinophilia).4 These tests were negative.
-
D.
BCR clonal rearrangement – Incorrect. T-cell receptor variants have been implicated in HES, but not BCR rearrangements.4 The lymphatic variant of HES is driven by T-cell receptor rearrangements and often has predominant skin involvement.4 This patient had evidence of clonal peripheral blood T-cell receptor gene rearrangement, but the marrow T-cell receptor gene rearrangements and T-cell flow cytometry were negative.
-
E.
Elevation of IL-2 – Incorrect. IL-5 is most specific for eosinophil differentiation, not IL-2. Abnormal IL-5 producing T cells and IL-5 production by peripheral blood mononuclear cells have been implicated in patients with HES which causes elevated levels of IL-5.4 Other type II cytokines like IL-13, IL-9, and IL-10 have also been associated with eosinophils.
Conflicts of interest
None disclosed.
Acknowledgments
We would like to thank Dr Inga-Marie Schaefer, MD for providing the histology images used in this case.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Klion A.D. How I treat hypereosinophilic syndromes. Blood. 2015;126(9):1069–1077. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazmierowski J.A., Chusid M.J., Parrillo J.E., Fauci A.S., Wolff S.M. Dermatologic manifestations of the hypereosinophilic syndrome. Arch Dermatol. 1978;114(4):531–535. [PubMed] [Google Scholar]
- 3.Reiter A., Gotlib J. Myeloid neoplasms with eosinophilia. Blood. 2017;129(6):704–714. doi: 10.1182/blood-2016-10-695973. [DOI] [PubMed] [Google Scholar]
- 4.Havelange V., Demoulin J.B. Review of current classification, molecular alterations, and tyrosine kinase inhibitor therapies in myeloproliferative disorders with hypereosinophilia. J Blood Med. 2013;4:111–121. doi: 10.2147/JBM.S33142. [DOI] [PMC free article] [PubMed] [Google Scholar]