Abstract
Liquid biopsy recently gained widespread attention as a noninvasive alternative/complementary technique to tissue biopsy in patients with cancer. As technological advances have improved both feasibility and turnaround time, liquid biopsy has expanded tumor molecular analysis with acknowledgement of both spatial and temporal heterogeneity, overcoming many limitations of traditional tissue biopsy. Because of its diagnostic, prognostic, and predictive value, liquid biopsy has been extensively studied also in metastatic colorectal cancer. Indeed, as personalized medicine establishes its role in cancer treatment, genetic biomarkers unveiling the emergence of early resistance are needed. Among the wide variety of tumor analytes amenable to collection, circulating DNA and circulating tumor cells are the most adopted approaches, and both carry clinical relevance in colorectal cancer. However, few studies focused on comparing feasibility between these two approaches. In this review, we discuss the potential implications of liquid biopsy in metastatic colorectal cancer, assessing the advantages and drawbacks of circulating DNA and circulating tumor cells, and highlighting the most relevant trials for clinical practice.
Key Points
| Circulating tumor cells and circulating tumor DNA have been extensively studied in metastatic colorectal cancer with regard to their diagnostic, prognostic, and predictive impact; however, data of direct comparison between these two techniques are lacking. |
| Circulating tumor DNA yields higher sensitivity and suitability than circulating tumor cells, thus being the main plasma biomarker employed in clinical trials and closer to reach clinical practice for metastatic colorectal cancer. However, circulating tumor cells boast the unique potential to serve as a platform for ex vivo culture and xenografting. |
| Randomized clinical trials are needed to establish how to integrate liquid biopsy to improve the prognosis of patients affected by metastatic colorectal cancer. |
Introduction
Colorectal cancer (CRC) is one of the most common and most lethal cancers, representing 10.2% of new cases and 9.2% of cancer-related deaths, with a 5-year relative overall survival (OS) ranging from 90% in localized disease to 14% in metastatic CRC (mCRC) [1, 2]. In current clinical practice, therapeutic choices are driven by tumor biopsy-derived molecular profiles, contemplating the status of RAS and BRAF genes, and the DNA mismatch repair (MMR) system. Implementation of next-generation sequencing (NGS) broadened the molecular analysis with the aim of expanding targeted therapies also in mCRC [3, 4]. In this scenario, liquid biopsy (LB) gained widespread attention as a noninvasive alternative/complementary technique to tissue biopsy. The term LB refers to the analysis of tumor-derived biomarkers, most frequently in the blood, including circulating DNA and circulating tumor cells (CTCs) [5]. Liquid biopsy has been identified as a diagnostic, prognostic, and predictive biomarker, allowing early cancer detection, molecular profiling, estimation of relapse risk, selection of anticancer drugs, monitoring of treatment response, and identification of drug resistance mechanisms [6]. Advantages over tissue biopsy, including minimal invasiveness and fast turnaround, allow feasibility of close repeated testing and extensive molecular characterization by depicting both spatial heterogeneity (intra-tumoral and between different tumor sites) and temporal heterogeneity (mainly caused by anticancer treatments over time) [7, 8]. In particular, mCRC was proven highly heterogenous, with continuous clonal evolution especially under the selective pressure of anti-cancer agents. Thus, routine monitoring of real-time tumor-associated genomic changes may appoint LB as a mainstay for treatment selection for the continuum of care in mCRC; furthermore, spatial omni-comprehensiveness of LB may overcome tissue biopsy as a more accurate tool for high-burden tumors [9–11]. In this review, we discuss the potential implications of LB in mCRC focusing on comparison between CTCs and circulating DNA.
Definition and Techniques Adopted for LB
CTCs
First discovered through LB in the late nineteenth century, CTCs are intact cancer cells originating from both the primary tumor and metastases, allowing an understanding of cancer spreading and metastasis [12]. Circulating tumor cells present with different proportions in various tumors, usually with a low concentration due to a short half-life (1–10 cells per 10 mL of blood as compared to 107–108 leukocytes) [13]. Heterogeneity hampers the definition of both sensitive and specific markers for collection. Circulating tumor cells are mainly separated by means of certain physical (i.e., filtration) and biological properties (i.e., cell surface expression), generally requiring an enrichment step (to maximize collection) and a detection step [14]. To date, the CellSearch system is the only US Food and Drug Administration-approved platform for the clinical detection of CTCs in patients with cancer, demonstrating a reproducible CTC count and prognostic value also in mCRC. It uses positive enrichment through epithelial cell adhesion molecule antibodies and then detection by immunostaining for cytokeratins and DAPI as markers for intact epithelial cells, together with absent CD45 as an exclusion marker labeling leukocytes [15]. In CRC, CTCs were detectable through CellSearch with a 36.2% sensitivity, correlating with clinical staging (up to 60.7% in mCRC) [16]. As an alternative, the AdnaTest integrates epithelial cell adhesion molecule-based enrichment with detection of cancer-specific genes through polymerase chain reaction (PCR), overcoming the expression loss of surface proteins due to epithelial-mesenchymal transition [17, 18]. Moreover, the combination of the CellSearch assay with the AdnaTest further improved the CTC detection rate in mCRC (CellSearch positivity rate 33%, AdnaTest 30%, combined 50%) [19]. The advent of microfluidic platforms such as the ‘CTC-chip’ further increased detection rates, efficiently collecting viable CRC-CTCs in microposts coated with a mixture of antibodies, under laminar flow [20]. When comparing CellSearch with IsoFlux, a microfluidic system with immunomagnetic implementation, the CRC-CTC yield was eight times higher with the latter [21]. Enrichment through leukapheresis could also substantially increase the CRC-CTC detection when combined with CellSearch [22]. The EPISPOT test and photoacoustic flow cytometry, the latter allowing in vivo CTC detection through the skin, are the most cutting-edge technologies in development [23, 24]. Overall, technological advances have led to dozens of new platforms with implemented capture efficiency, throughput rates, purity, and capability of molecular analysis [25, 26].
Circulating DNA
Circulating free DNA (cfDNA) was first detected in the mid-Twentieth century, consisting of fragments of non-encapsulated DNA in the bloodstream of healthy individuals [27]. In the late 1970s, comparable circulating tumor DNA (ctDNA) from malignant cells was discovered in patients with cancer [28]. As a result of increased cell turnover, patients with cancer usually have higher cfDNA levels than healthy subjects, with a variable ctDNA-to-cfDNA ratio of 0.01–90% depending on the tumor type, biological behavior, and cancer stage [29]. Among various malignancies, mCRC harbors the highest ctDNA amount [30]. Apart from genetic alterations, epigenetic modifications can be captured on ctDNA, most commonly in the form of DNA methylation. Difficulties in ctDNA detection include low-frequency aberrations and non-neoplastic age-dependent alterations in common driver genes. Hence, ctDNA detection requires highly sensitive and specific approaches [31]. Targeted methods such as quantitative and digital PCR (dPCR) mainly differ in their limit of ctDNA detection, reaching rates of 0.1% and 0.005%, respectively [32, 33]. Further high-resolution PCR-based technologies such as BEAMing, ARMS, and UltraSEEK further enhance the sensitivity to 0.001% and allele specificity up to a single base difference with the detection of multiple minor variants in a single reaction [34–36]. While PCR remains confined to the analysis of few loci, NGS provides simultaneous targeted characterization of multiple genomic alterations focusing on regions of interest for mutations, but also copy number variations and chromosomal rearrangements [37, 38]. Untargeted whole genome sequencing further expands the genomic analysis, with potential applicability to extensive clonal tracking due to tumor progression or treatments, at the expense of higher costs and lower sensitivity especially for low-frequency ctDNA. However, fragment size analysis and selective sequencing of specific fragment sizes boosted ctDNA detection for whole genome sequencing in plasma samples from patients with mCRC [39]. Furthermore, a new whole genome sequencing method named Plasma-Seq overcame this issue by providing sequencing in less than 48 h [40, 41]. Finally, circulating methylated DNA (cmDNA) is similarly evaluated through targeted or untargeted interrogation. Apart from conventional direct bisulfite sequencing or pyrosequencing, novel methods such as methylation-specific PRC and methyl-BEAMing were found capable of sensitive detection of cmDNA [42, 43]. A summary of circulating DNA and CTCs with regard to advantages and drawbacks of both methods is available in Fig. 1, while the main features with regard to mCRC are listed in Table 1.
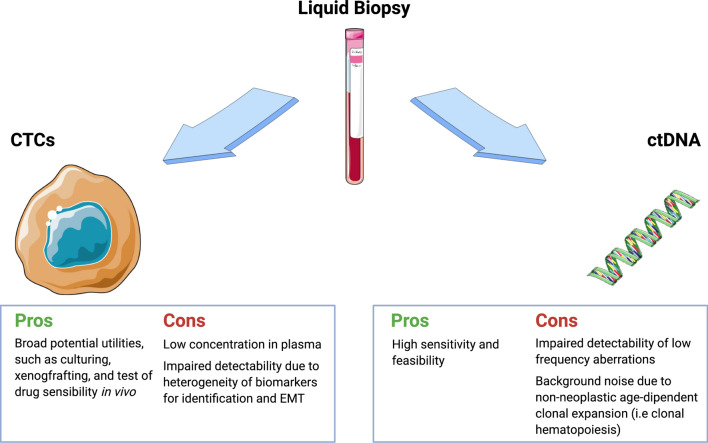
Fig. 1.
Advantages and disadvantages of circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in metastatic colorectal cancer (mCRC). Collection of CTCs is mainly impaired by their low abundance in plasma (median 2 CTCs/7.5 mL of peripheral blood in mCRC). Furthermore, high heterogeneity of cell surface expression and loss of epithelial markers (due to epithelial-mesenchymal transition [EMT]) also contribute to complexity in CTC isolation; their use allows cell culturing and xenografting, hence bridging the clinical and preclinical scenarios. On the contrary, ctDNA is easily detectable in peripheral blood and its feasibility allows intensive clonal tracking and monitoring of emerging resistance mechanisms during active treatment in mCRC; however, a low frequency of certain genomic aberrations and interference of non-neoplastic clonal expansion may compromise sensitivity and specificity. Created with smart.servier.com
Table 1.
Main features of CTCs and ctDNA focusing on mCRC. Created with smart.servier.com
CTCs
|
ctDNA
|
|
|---|---|---|
| Origin | Viable and apoptotic cells | Mainly apoptotic cells |
| Components | DNA, RNA, proteins, metabolites | DNA |
| Suitable analyses | Genomics (mutations, copy number alterations, epigenetic alterations, fusion genes); transcriptomics (mRNA, including splice variants); proteomics; single-cell level analysis | Mutations, copy number alterations, epigenetic alterations, fusion genes |
| Culturing and xenografting | Yes | No |
| Sensitivity and specificity | Low sensitivity due to low abundance in plasma (especially for mCRC), heterogeneity of biomarkers for identification and EMT; around 50% sensitivity when combining CellSearch assay and the AdnaTest; variable specificity according to detection methods | High sensitivity due to large abundance in plasma (especially for mCRC); detection of mutant alleles with a fractional abundance up to 0.001% with dPCR; improved sensitivity and specificity with emerging tumor-informed techniques; impaired specificity due to background noise from non-neoplastic age-dependent alterations (i.e., clonal hematopoiesis) |
| Applications in the continuum of care for patients with mCRC | Prognosis, prediction of treatment response, molecular profiling, clonal evolution tracking and early identification of resistance mechanisms, treatment response monitoring, early detection of recurrence and MRD, in vivo tests of drug sensitivity | Prognosis, prediction of treatment response, molecular profiling, clonal evolution tracking and early identification of resistance mechanisms, treatment response monitoring, early detection of recurrence, and MRD |
CTCs circulating tumor cells, ctDNA circulating tumor DNA, dPCR digital polymerase chain reaction, EMT epithelial-mesenchymal transition, mCRC metastatic colorectal cancer, MRD minimal residual disease, mRNA messenger RNA
Exosomes
Exosomes are small extracellularly secreted vesicles (50–150 nm), containing evaluable biomarkers such as nucleic acids [44]. Given the preservation of RNA from RNAase enzymatic activity, exosomes may provide a source of messenger RNA and microRNA (miRNA), the latter being small non-coding RNA molecules implied in RNA silencing and carcinogenesis [45, 46]. Exosomes can be isolated from peripheral blood using biophysical properties such as centrifugation or precipitation, and immunoaffinity capture. After extraction procedures, targeted or untargeted methods are employed for RNA detection [47]. Despite limited research in this field for CRC, serum exosomal miRNA such as miRNA21, miRNA23, miRNA19a, and miRNA92a were identified as the most increased in this disease, some of them correlating with tumor prognosis, recurrence, and sensitivity to anti-cancer agents [48–52]. However, an in-depth analysis of exosomes in mCRC is beyond the scope of this review.
Prognostic and Predictive Value of LB
As treatment options expanded in mCRC, imaging studies remain the main discriminating factor for progression to anticancer therapy. Blood biomarkers are needed to predict tumor response and determine a priori reliable prognostic groups of patients with mCRC.
CTCs
Several studies demonstrated that CTC levels are associated with shorter survival in mCRC, as they positively correlate with tumor burden and aggressiveness. Cohen and colleagues proved feasibility of CTC isolation in 50 patients with mCRC, with 77% presenting with ≥ 1 CTC/7.5 mL of blood and a median yield of 2 CTCs/7.5 mL, dramatically less than other malignancies [53, 54]. In a following study on 430 patients with mCRC, ≥ 3 CTCs/7.5 mL was associated with shorter progression-free survival (PFS) and OS (PFS 4.5 vs 7.9 months, p = 0.0002; OS 9.4 vs 18.5 months, p < 0.0001). Conversion of baseline CTC count under cytotoxic chemotherapy from ≥ 3 to <3 within 5 weeks of treatment was associated with longer PFS and OS (PFS 6.2 vs 1.6 months; p = 0.02; OS 11.0 vs 3.7 months, p = 0.0002) [55]. The combination of CTCs and carcinoembryonic antigen (CEA) was capable of finer stratification in patients with baseline CEA ≥ 25 ng/mL, as those with < 3 baseline CTCs had longer OS (OS 20.8 vs 11.7 months, p = 0.001) and, among patients with a similar CEA reduction after treatment, the three CTC cut-off at 6–12 weeks distinguished two opposite prognostic groups (OS 19.8 vs 5 months, p = 0.0001) [56]. These results were corroborated by several studies, including ancillary studies of the CAIRO2 and the MACRO TTD trials, and a meta-analysis of 2388 patients with CRC [57–59]. Association of the CTC count with poorer PFS and OS was also confirmed through a meta-analysis of 264 patients with resectable colorectal liver metastases (CRLM) [OS hazard ratio (HR) 2.47, p < 0.0001; PFS HR 2.07, p < 0.0001] [60]. High-CTC levels were related with non-resectability and impaired survival after metastasectomy, suggesting CTCs as a tool for decision making before liver resection. Furthermore, patients with ≥ 2 CTCs/7.5 mL experienced reduced PFS and OS when analyzing all patients and resectable patients only (p < 0.001) [61]. Given the proven prognostic value, some authors suggest that CTCs could help identify those patients benefiting the most from treatment intensification, avoiding high-toxicity regimens in low-CTC mCRC [62]. High-quality, well-designed, large-scale, multicenter prospective interventional studies are needed to assess if CTC-based therapeutic decisions could improve prognosis in mCRC. To date, the main effort to employ CTCs as a prognostic tool for treatment intensification or de-escalation in patients with mCRC comes from the VISNU-1 and VISNU-2 trials. In the phase III VISNU-1 trial, patients with mCRC with three or more baseline CTCs/7.5 mL (poor prognostic factor) were randomized to receive first-line bevacizumab with either FOLFOXIRI or FOLFOX, with superior PFS for the triplet arm [63]. Conversely, low-risk (< 3 CTCs/7.5 mL) patients with RAS wild-type (WT) mCRC were treated with doublet FOLFIRI with bevacizumab or cetuximab in the phase II VISNU-2 trial, to assess the influence of BRAF and PI3K mutational status on treatment efficacy [64]. Because a high-CTC count was established as a negative prognostic factor, this non-invasive biomarker may guide treatment selection in the mCRC first-line setting [65].
Circulating DNA
As with CTCs, circulating DNA was found to be prognostic in mCRC. Spindler and colleagues measured cfDNA in 55 patients with mCRC, achieving a wide range distribution (22–3922 ng/mL blood) and a mean value of 1157 ng/mL, with cfDNA levels > 1000 ng/mL correlating with shorter survival (p = 0.02) [29]. Similarly, a retrospective post hoc study of the NORDIC-VII trial found that high cfDNA levels before chemotherapy were associated with an impaired outcome (OS HR 1.83, p < 0.001), then confirmed by a meta-analysis of 1076 patients with mCRC (OS HR = 2.39, p < 0.0001) [66, 67]. A retrospective analysis of the CORRECT trial showed that clinical benefit from regorafenib was associated with KRAS and PIK3CA WT ctDNA status (KRAS WT vs mutant-pinteraction 0.74; PIK3CA WT vs mutant-pinteraction 0.85) as well as low cfDNA concentration (low vs high cfDNA-pinteraction 0.601) [68]. Furthermore, combined analysis of cfDNA and ctDNA added a supplementary prognostic impact [69]. Early changes in ctDNA levels were suggested as a biomarker for treatment response and outcomes in patients with mCRC. In a study of 29 patients with mCRC assessing ctDNA levels at baseline and 8 weeks after chemotherapy, ctDNA halved the predicted radiological response and resulted in longer PFS and OS (PFS HR 0.33, p = 0.04; OS HR 0.30, p = 0.08) [70]. Similarly, serial ctDNA analysis was able to identify a subsequent increase in ctDNA in initial responders to cytotoxic agents, effectively anticipating tumor progression as proven by shorter PFS and OS [71]. These results are consistent with those from the 82 patients with mCRC in the PLACOL study, monitoring ctDNA or hypermethylated alleles by dPCR [72]. The prognostic role of ctDNA was confirmed also in 41 patients with CRLM, where a decreased ctDNA/b-globin ratio after liver surgery predicted longer disease-free survival (366 vs 102 days, p < 0.001) [73]. In another cohort of 18 patients with CRLM, ctDNA was more sensitive than CEA in detecting minimal residual disease (ctDNA 100% vs CEA 56%, p = 0.008) and predicting postoperative recurrence (p = 0.003) [74]. In the PREDATOR trial, sensitivity for minimal residual disease was improved by personalized tumor-informed ctDNA detection, assessing plasma mutations according to erstwhile tumor tissue profiling in 113 oligometastatic patients with CRC (HR 4.59, p < 0.001) [75]. Finally, cmDNA due to epigenetic changes was employed to circumvent the absence of cancer-specific mutations in cfDNA. In a study assessing five methylated loci, 87% of 182 patients with mCRC showed positivity in one or more loci, dynamically correlating with overall response rate and PFS in those receiving anticancer therapy [76]. Amatu et al. confirmed baseline and dynamic cmDNA as prognostic and predictive in patients with mCRC treated with regorafenib [77]. Validation of circulating DNA in prospective clinical trials is warranted. The ongoing trial NCT03844620 is currently evaluating ctDNA capability to serve as a guide for precocious interruption of regorafenib or TAS-102 independently from radiological progression [78].
Molecular Profiling and Monitoring of Resistance Mechanisms Through LB
In the era of personalized medicine, the administration of targeted therapies is still relatively impeded by difficulties in tissue biopsy, which is not as feasible as necessary for extended molecular characterization and assessment of evolving molecular targets. LB is currently under investigation as a tool for genomic profiling in mCRC. Figure 2 shows a potential algorithm for LB as a guide for treatment selection and monitoring according to clonal tracking in patients with mCRC receiving active treatment.
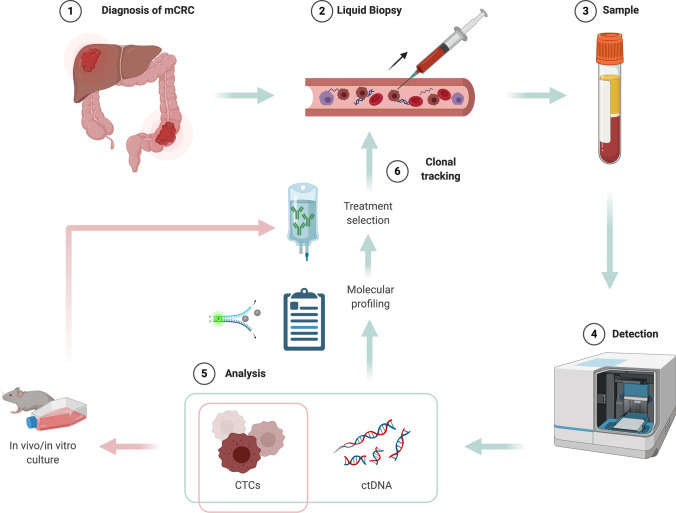
Fig. 2.
Clonal tracking with liquid biopsy (LB) in patients with metastatic colorectal cancer (mCRC) receiving active treatment. After mCRC diagnosis (1), the collection of blood samples for LB (2,3) allows the detection of circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) (4). Their analysis provides useful information for molecular profiling and treatment selection; additionally, CTCs can be employed for culturing and xenografting, thus adding relevant data of tumor sensitivity to the selected treatment (5). Given the high feasibility of LB, new blood samples are then collected during active treatment, to monitor the emergence of resistance through clonal tracking (6). Created with BioRender.com
CTCs
Cayrefourcq and colleagues were the first to report the successful establishment of a stable mCRC-derived CTC line. However, only one long-term CTC line was achieved from 71 unique blood samples (1.4%). Noteworthy, this required a CTC count over 300/10 mL, available only for two samples. Despite the low success rate of CTC line generation, culturing allowed NGS-based genomic analysis, followed by single-cell transcriptome, proteome, and secretome analysis, and xenotransplantation. Importantly, KRAS and BRAF concordance was proven between primary tumor, metastases, cell line, and xenografts [79]. Moreover, CTC genotyping allowed the identification of tissue-undetected mutations and secondary mechanisms of resistance to anti-epidermal growth factor receptor (EGFR) drugs [80, 81]. Altogether, the rarity of CTCs and strain from CTC line establishment are the major drawbacks in this setting [82].
Circulating DNA
The tumor mutational landscape is reflected by ctDNA, and high concordance with tissue analysis (> 80%) has been reported regarding main genomic alterations in mCRC [10, 30, 70]. The ongoing COLOMATE (NCT03765736) and TARGET trials are screening ctDNA genetic alterations in mCRC for enrolment onto targeted therapy-based/early-phase clinical trials [83]. Here, follows the main fields of interest for ctDNA profiling in mCRC.
Resistance to Anti-EGFR MoAb
Mutant status of RAS or BRAF is known to confer primary resistance to anti-EGFR monoclonal antibodies (MoAb) [84, 85]. With the advent of LB, many studies focused on the detection of RAS ctDNA in patients with mCRC, proving > 90% concordance with tissue analysis [10, 86–89]. The absence of liver metastases and primary tumor resection were the main clinical factors associated with unsuccessful ctDNA detection in the AGEO RASANC prospective study [89]. In the CAPRI-GOIM trial, first-line cetuximab-based therapy yielded consistent PFS and OS dichotomy between patients with RAS MT and WT mCRC, when retrospectively assessed by both LB and tissue biopsy [90]. Discordant cases were attributed to high spatial heterogeneity, as tissue biopsy cannot fully reflect clonality in mCRC. Indeed, a higher frequency of ctDNA KRAS, NRAS, and BRAF mutations were found as compared with tissue (59% vs 44%, 11.8% vs 8.8%, and 14.4% vs 7.2%, respectively) [91]. Even if previously sensitive to an anti-EGFR MoAb, most RAS WT mCRC eventually acquire secondary resistance. Siravegna et al. tracked clonal evolution through ctDNA during treatment with anti-EGFR MoAb and found emerging alterations in RAS, ERBB2, FLT3, EGFR, and other genes [10]. A prospective LB-based trial monitored patients with KRAS WT mCRC receiving first-line FOLFIRI-cetuximab and found that persistent RAS, BRAF, and PIK3CA WT circulating status correlated with prolonged response, while an increase in mutant cfDNA predicted acquired resistance [92]. Besides, LB drastically anticipated radiographic progression as ctDNA KRAS MT alleles were detectable > 10 months beforehand [11]. Siena et al. reported results from a prospective analysis in patients with mCRC, confirming that serial plasma biopsies are more inclusive than tissue biopsies for evaluating RAS acquired mutations under selective pressure of anti-EGFR treatment. In a series of 15 patients with paired tumor tissue biopsy and plasma samples, BEAMing on LB showed a far higher RAS MT emergence rate than tissue analysis by BEAMing and NGS (57.1% vs 7.1% and 9.5%, respectively, p = 0.008), though without an impact on PFS. However, only RAS mutations were investigated by LB in this study, leaving aside other known molecular mechanisms of resistance [93]. A similar prospective investigation expanded the analysis to ERBB2 and MET amplifications, with high heterogeneity [94]. Eventually, LB could help detect those preexisting tissue biopsy-concealed resistant subclones proliferating and expanding under positive pressure of anti-EGFR treatments, until conferring resistance to therapy [95].
Resistance to HER2-Targeted Therapies
Several actionable genomic alterations are emerging as resistance mechanisms and potential therapeutic targets in mCRC. Approximately 5% of advanced KRAS WT mCRC were found harboring ERBB2 amplification/HER2 overexpression (HER2+). Recently, ERBB2 amplification was found responsible for both primary and secondary resistance to anti-EGFR MoAb [96]. In this population of mCRC, anti-HER2 dual blockade regimens were successfully tested in the HERACLES (trastuzumab-lapatinib) and MyPathway (trastuzumab-pertuzumab) trials with a similar ORR of around 30%, and the most recent MOUNTAINEER (trastuzumab-tucatinib) with a 55% ORR. Data from the DESTINY-CRC01 trial using the antibody drug-conjugate trastuzumab-deruxtecan have been released at ASCO 2020 with promising results also in anti-HER2 pretreated mCRC [97–100]. Liquid biopsy was recently explored as a method of assessment for HER2 status in a cohort of 18 patients with cetuximab-resistant mCRC, with 4/18 (22%) classified as ctDNA HER2+, concordantly with tissue samples at re-biopsy [101]. In a larger cohort of 344 patients with advanced gastrointestinal tumors (74% of which mCRC), ctDNA analysis found 5% had ctDNA HER2+ [102]. Siravegna and coworkers assessed concordance between LB and tissue analysis in a retrospective study from the HERACLES trial, with ctDNA correctly identifying 96.6% of samples as HER2+ and predicting benefit from HER2-targeted therapy [103]. Furthermore, an NGS analysis on ctDNA identified primary resistance mechanisms, undetectable with tissue biopsy, revealing that interventional LB could prevent ineffective employment of anti-HER2 treatment in > 85% of cases [104].
Other Biomarkers for Targeted Therapy
Around 10% of CRC harbor BRAF mutations, mostly V600E, conferring poor prognosis and representing an unmet medical need. The BEACON trial recently established BRAF as a therapeutic target in mCRC [105]. In an earlier study of 85 tissue-selected BRAF V600E MT mCRC, cfDNA BEAMing analysis revealed that 83.5% had detectable-BRAF mutations in plasma at baseline, dynamically predicting treatment response to anti-BRAF therapy [106]. Immunotherapy is a well-established therapeutic option for MMR-deficient mCRC [3, 4]. While few studies reported >98% accuracy of ctDNA MMR-deficient noninvasive identification, an ongoing prospective trial (NCT03594448) is investigating serial LB as a detection method for MMR-deficient mCRC [107, 108].
LB-Driven Retreatment Strategies with Anti-EGFR MoAb
Within the continuum of care, retreatment with anti-EGFR MoAb is a valid therapeutic strategy, especially in the way of a rechallenge after a wash-out period from previous progression [109, 110]. Liquid biopsy is the most suitable approach to determine restored sensitivity to anti-EGFR MoAb before a rechallenge. The CRICKET trial, a proof-of-concept study of third-line rechallenge with cetuximab-irinotecan, prospectively collected ctDNA in 28 patients with tissue-proven RAS/BRAF WT mCRC, revealing that only patients with RAS WT ctDNA at the time of the rechallenge were achieving partial response and positive correlation with PFS (PFS 4.0 vs 1.9 months, p = 0.03) [111]. These results, together with the post-hoc analyses of JACCRO CC-08, JACCRO CC-09, and E-Rechallenge trials, suggest that ctDNA could guide the selection of patients benefiting from an anti-EGFR rechallenge, by excluding those with detectable resistant clones [112, 113]. The ongoing biomarker-driven CHRONOS trial (NCT03227926) will strengthen these findings by adopting ctDNA analysis as an inclusion criterion. This proof-of-concept prospective study enrolls patients with RAS/BRAF WT mCRC and prior sensitivity to anti-EGFR treatment, who then developed secondary resistance and were treated with one or more following lines of therapy. Candidates are only eligible for panitumumab rechallenge when absence/> 50% decrease in ctDNA RAS fractional mutation is proven after progression on first-line anti-EGFR, together with the absence of ctDNA EGFR ectodomain mutations. Similarly, the phase II NCT03087071 trial involves LB for RAS, BRAF, and EGFR ectodomains before rechallenge with panitumumab: patients without any RAS and BRAF mutations receive panitumumab as a single agent, whereas others receive a combination of panitumumab and trametinib. Martinelli and colleagues recently announced a proof-of-concept LB-driven prospective study of sequential treatments in patients with RAS/BRAF WT mCRC (CAPRI 2-GOIM trial), where treatment choices will be defined by ctDNA results. According to the study design, LB is collected after first-line anti-EGFR treatment to determine whether progression is on account of secondary resistance to anti-EGFR or other independent mechanisms, offering two sequence strategies: a continuum treatment with cetuximab beyond first line with a chemotherapy regimen switch in patients with ctDNA RAS WT; or a second-line treatment with FOLFOX-bevacizumab in patients with ctDNA RAS MT mCRC and then, if the patient reverts back to ctDNA RAS WT, rechallenge with cetuximab-irinotecan in the third line or, conversely, a standard-of-care third-line treatment in the case of persistent ctDNA RAS MT [114]. Finally, ctDNA could guide the correct sequence of anti-EGFR MoAb. When secondary resistance is due to certain mutations of EGFR ectodomain identified as S492R, K467, or R451C mutants, the tumor ceases to be sensitive to cetuximab but not to panitumumab, thus allowing switching of anti-EGFR moAb [115]. According to a recent study, up to 16% of S492R EGFR mutations were detected in 239 post-cetuximab plasma samples of patients with mCRC [116].
Direct Comparison between CTCs and Circulating DNA
Bettegowda and colleagues used dPCR to identify rearrangements in CTC-DNA and ctDNA of nine patients with CRC, of which five had mCRC. In 100% of cases, ctDNA could be measured, whereas CTC-DNA was detectable in three out of five (60%), those with the most abundant ctDNA levels. The average number of ctDNA fragments was > 50-fold higher than analogous levels of CTCs [30]. Similarly, Germano et al. performed a parallel evaluation of CTCs and ctDNA in a cohort of 20 patients with mCRC. A 100% ctDNA detectability was assessed by droplet dPCR (ddPCR), whilst CTCs were isolated in 37% of patients, with >1 CTC/10 mL of blood in only two patients, again in those with a higher amount of ctDNA (p < 0.005). Tissue-LB concordance for ctDNA RAS, BRAF, and ERBB2 was 85%, while CTCs were too limited for this analysis [117]. In the prospective PRODIGE-14 trial, levels of CTCs and KRAS ctDNA were investigated in a cohort of 153 patients with potentially resectable CRLM treated with first-line triplet (FOLFIRINOX) or doublet chemotherapy associated with targeted therapy. Assessment through CellSearch at inclusion showed ≥ 1 CTC/7.5 mL in 42% of patients, while 91% of 46 tissue-genotyped KRAS MT patients were confirmed by ddPCR. The proportion of patients with ≥ 3/7.5 mL CTCs decreased during chemotherapy from 19% at baseline to 3% after 4 weeks and 0% before liver surgery; likewise, ctDNA lowered from 91 to 63% and then 19%. Detectable KRAS ctDNA after 4 weeks was associated with a lower R0/R1 surgical outcome (p = 0.01) and, among patients with R0/R1 resection, detectable ctDNA levels before surgery conferred shorter OS (p < 0.001). In multivariate analysis, ≥ 3 CTCs was an independent prognostic factor for OS both at baseline and after 4 weeks of therapy [118]. Onidani and coworkers improved CTC detection using an epithelial cell adhesion molecule-independent approach in a cohort of 20 patients with gastrointestinal cancer, among whom 11 had mCRC (55%), with a median 14.5 CTCs/mL. A combination NGS analysis of genomic profiles obtained from CTCs and ctDNA showed low concordance, suggesting high tumor heterogeneity. An additional cohort of seven patients with mCRC undergoing anti-EGFR treatment was studied for the appearance of resistance genetic alterations at the time of progression, with 100% ctDNA and 57% CTC-DNA detection [81]. Table 2 summarizes and directly compares the sensitivity for ctDNA and CTCs (or CTC-DNA) reported by these investigations of mCRC, showing higher detection rates for ctDNA.
Table 2.
Comparison of sensitivity for ctDNA and CTCs in mCRC
| Study | Detectable ctDNA (%) | Detectable CTCs or CTC-DNA |
|---|---|---|
|
Bettegowda et al. [30] 5 patients with mCRC |
100 | 60 |
|
Germano et al. [117] 20 patients with mCRC |
100 | 37 |
|
Bidard et al. [118] 46 patients with mCRC |
91 | 42 |
|
Onidani et al. [81] 7 patients with mCRC |
100 | 57 |
CTCs circulating tumor cells, CTC-DNA DNA extracted from circulating tumor cells, ctDNA circulating tumor DNA, mCRC metastatic colorectal cancer
Conclusions
Recent advances in terms of feasibility in the clinic and a faster turnaround time, together with molecular-implemented assessment of both spatial and temporal cancer heterogeneity, made LB a valid noninvasive candidate to challenge traditional tissue biopsy. Circulating tumor cells and circulating DNA are the tumor components most widely studied with this approach, carrying diagnostic, prognostic, and predictive information also in mCRC. Ongoing trials assessing their role in mCRC are reported in Tables 3 and 4. Remarkable future applications of LB could be envisioned all along the continuum of care of patients with mCRC (Fig. 3). In the first-line treatment setting, LB could expedite molecular profiling for the administration of targeted anticancer agents (i.e., anti-EGFR drugs) [86, 119, 120]. Further, prognostically proven ctDNA and CTC levels could became additional decision criteria for de-escalation/intensification of upfront backbone chemotherapy and modulation of post-operative treatment in the oligometastatic setting, even if the ideal cut-off in this regard is missing [29, 55, 60, 63, 75]. In later lines of therapy, ctDNA could inform treatment sequence and drug selection, by identification of the best timing for anti-EGFR retreatment, based on RAS and EGFR clonal tracking, or identification of mechanisms of resistance for upfront or sequential enhanced targeting [112]. Molecular selection for clinical trials has already been taking advantage of LB owing to its increased feasibility and comprehensiveness for screening criteria [83, 120, 121]. As a future prospective, “molecular” progression will complement radiological evaluation, while computational mathematics already proved capable of predicting time-to-treatment failure in patients with mCRC through clonal tracking forecast [78, 122]. Finally, although not a focus of the present review, LB may have a role as a screening tool in CRC and is currently under intense investigation aiming to guide decisions about adjuvant treatment for patients with stage II–III surgically resected CRC [123, 124]. In conclusion, LB represents a major opportunity for better tailoring the whole patient care in mCRC, with ctDNA especially demonstrating, from initial pioneering studies in this tumor type [11], an ever-increasing acceleration toward multiple clinical applications.
Table 3.
Trials prospectively assessing the role of liquid biopsy in mCRC
| Study | Study population | Liquid biopsy analysis | Primary endpoints | |
|---|---|---|---|---|
| Observational studies | ||||
| COLOMATE (NCT03765736) | Locally advanced and/or mCRC | ctDNA | To facilitate accrual to molecularly assigned therapies; to facilitate clinically annotated genomic analyses | |
| PERMED01 (NCT02342158) | Locally advanced and metastatic malignancies | ctDNA and CTCs | To facilitate accrual to molecularly assigned therapies | |
| TARGET | Locally advanced and metastatic malignancies | ctDNA | To match patients with a broad range of advanced cancers to early-phase clinical trials based on ctDNA analysis | |
| NCT03594448 | MSI mCRC | ctDNA | To test concordance between MSI status in ctDNA and primary tumor tissues; to correlate MSI ctDNA changes with response to therapy | |
| RASINTRO (NCT03259009) | mCRC under treatment with anti-EGFR MoAb rechallenge | ctDNA | To correlate ctDNA RAS MT with PFS of anti-EGFR rechallenge | |
| OPTIMAL-II (NCT03750175) | mCRC under treatment with anti-EGFR MoAb | ctDNA |
Feasibility and reliability of cfDNA-based selection of KRAS, NRAS, and BRAF WT Patients with mCRC who will benefit from anti-EGFR MoAb and cfDNA analysis during therapy and at time of progression |
|
| NCT03401957 | mCRC treated with cetuximab-based infusional 5-fluorouracil regimen as first line | ctDNA | To observe the percentage and the time to onset of RAS MT | |
| PERSEIDA (NCT02792478) | RAS WT mCRC in first-line treatment | ctDNA | To evaluate the appearance of new RAS MT at disease progression and prior to radiological documentation | |
| PREDATOR (ESMO 2020, Loupakis et al) | Oligometastatic CRC treated with curative intent | ctDNA | To evaluate PFS according to post-operative ctDNA status | |
| Interventional studies | ||||
| Study and phase of research | Study population | Liquid biopsy analysis | Intervention | Primary endpoints |
|
Phase Ib/II |
Resected mCRC with detectable ctDNA following resection of all known liver metastases (cohort D) | ctDNA | M7824 (anti-PD-L1/TGF-beta-RII fusion protein) for 6 doses after resection and completion of standard-of-care therapy | ctDNA clearance |
|
CHRONOS (NCT03227926) Phase II |
RAS/BRAF WT mCRC previously sensitive to anti-EGFR MoAb and then progressed, after ≥1 following line of therapy, with >50% drop in RAS mutational load at serial ctDNA analyses or absent RAS MT/EGFR ectodomain MT ctDNA | ctDNA | Rechallenge of third-line panitumumab monotherapy | ORR |
|
Phase II |
RAS/BRAF WT mCRC with clinical benefit and then progression to anti-EGFR MoAb | ctDNA | Rechallenge of panitumumab monotherapy in patients with EGFR ectodomain MT ctDNA or without neither EGFR ectodomain nor KRAS/NRAS/BRAF MT ctDNA; panitumumab and trametinib in patients with KRAS/NRAS/BRAF MT ctDNA | ORR |
| CAPRI 2-GOIM | RAS/BRAF WT anti-EGFR refractory mCRC | ctDNA | Continuum of treatment with cetuximab if RAS WT ctDNA; second-line FOLFOX and bevacizumab if RAS MT ctDNA and then third-line rechallenge with cetuximab and irinotecan if back to RAS WT ctDNA or standard-of-care therapy if persistent RAS MT ctDNA | ORR |
|
Phase II |
Pretreated mCRC | ctDNA | Comparison of either regorafenib or TAS-102 continuation based on early changes in ctDNA analysis or according to standard of care | Early change in ctDNA as a predictor of radiographic progression; comparison of adverse events between ctDNA and standard-of-care arms |
|
MoLiMoR (NCT04554836) Phase II |
RAS MT mCRC | ctDNA | Comparison of FOLFIRI vs FOLFIRI plus intermittent addition of cetuximab when ctDNA analysis demonstrates conversion to RAS WT | PFS |
|
VISNÙ-1 (NCT01640405) Phase III |
Previously untreated patients with mCRC with ≥3 CTCs/7.5 mL | CTCs | Comparison of first-line FOLFOX plus bevacizumab vs FOLFOXIRI plus bevacizumab | PFS |
|
VISNÙ-2 (NCT01640444) Phase II |
Previously untreated patients with KRAS WT mCRC with <3 CTCs/7.5 mL | CTCs | Comparison of first-line FOLFIRI plus bevacizumab vs FOLFIRI plus cetuximab based on BRAF and PI3K mutational status | PFS |
CTCs circulating tumor cells, ctDNA circulating tumor DNA, EGFR epidermal growth factor receptor, MoAb monoclonal antibodies, MSI microsatellite instability, MT mutated, ORR overall response rate, PD-L1 programmed death-ligand 1, PFS progression-free survival, TGF transforming growth factor, WT wild-type
Table 4.
Ongoing trials retrospectively assessing the role of liquid biopsy in mCRC
| Study and phase of research | Study population | Liquid biopsy analysis | Intervention | Primary endpoint | Role of liquid biopsy analysis |
|---|---|---|---|---|---|
|
CAVE (NCT04561336) Phase II |
Pretreated RAS WT mCRC | ctDNA | Combination of avelumab plus cetuximab | OS | To compare OS and PFS in ctDNA RAS/BRAF WT and MT populations |
|
CR-SEQUENCE (NCT03635021) Phase III |
Untreated RAS WT left-sided mCRC | ctDNA | FOLFOX plus panitumumab followed by FOLFIRI plus bevacizumab vs FOLFOX plus bevacizumab followed by FOLFIRI plus panitumumab | PFS | To state the clinical impact of clonal dynamics of ctDNA |
|
KISIMA-01 (NCT04046445) Phase Ib |
Pretreated mCRC progressed to standard-of-care therapies | ctDNA | ATP128, with or without BI 754091 | Safety and tolerability of ATB128 and BI754091 | To detect early signal of relapse through ctDNA in patients treated with ATP128 and BI 754091 before and after liver surgery |
|
IMPROVE (NCT04425239) Phase II |
Untreated RAS/BRAF WT mCRC | ctDNA | Continuous vs intermittent panitumumab and FOLFIRI in first line | PFS | To evaluate potential biomarkers of primary and secondary resistance analyzing ctDNA |
|
iRE-C (NCT04108481) Phase I–II |
Liver predominant mCRC | ctDNA | Yttrium-90 radioembolization (Y90-RE) in combination with durvalumab | Safety and tolerability of Yttrium-90 radioembolization combined with durvalumab and ORR | To determine changes in the expression profile and in ctDNA levels after treatment |
|
Phase II |
Left-sided RAS WT mCRC | ctDNA | FOLFOXIRI plus panitumumab | ORR | To evaluate velocity of response through ctDNA clearance |
|
Phase I–II |
RAS MT mCRC | ctDNA | Onvansertib in combination with FOLFIRI and bevacizumab | Safety and tolerability of onvansertib plus FOLFIRI plus bevacizumab and ORR | To evaluate reduction of RAS MT in ctDNA |
|
AIO–KRK-01160 (NCT04034459) Phase II |
BRAF MT mCRC | Not specified | FOLFOXIRI plus bevacizumab vs FOLFOXIRI plus cetuximab in first line | ORR | To investigate molecular biomarkers for the prediction of sensitivity and secondary resistance of cetuximab |
|
Phase I–II |
FOLFIRI-resistant mCRC | ctDNA | Combination of SCO-101 with FOLFIRI rechallenge | Safety and tolerability of SCO-101 and ORR | To evaluate the change in ctDNA from baseline until first computed tomography scan |
ctDNA circulating tumor DNA, mCRC metastatic colorectal cancer, MT mutated, ORR overall response rate, OS overall survival, PFS progression-free survival, WT wild-type
Fig. 3.
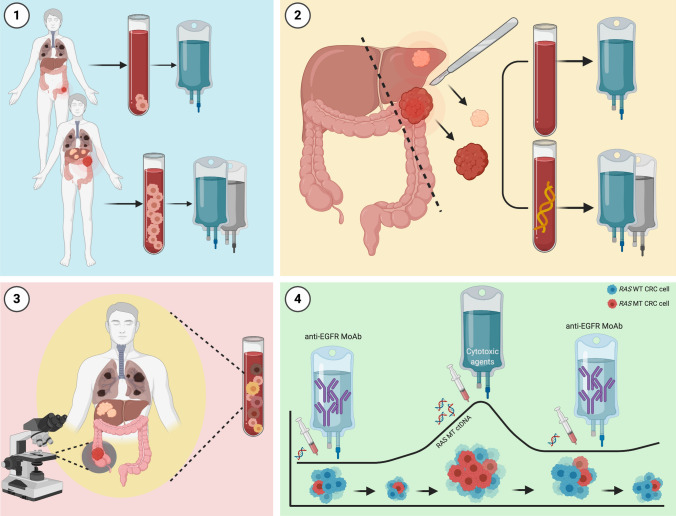
Overview of potential clinical applicability for circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in the continuum of care for patients with metastatic colorectal cancer (mCRC). Circulating tumor cells and ctDNA yield potential clinical applicability in treatment guidance for patients with mCRC: (1) the CTC count may guide treatment selection, with regard to therapy intensification and de-escalation; (2) after surgical resection for oligometastatic colorectal cancer (CRC), post-surgical treatment strategies may be modulated according to minimal residual disease (MRD), as determined by ctDNA levels in peripheral blood; (3) ctDNA may refine molecular profiling in mCRC by capturing tumor molecular heterogeneity, which is otherwise unachievable by single-tissue biopsy exploring just one site of the disease; and (4) ctDNA allows clonal tracking and real-time monitoring of emerging resistance, guiding treatment selection and rechallenge with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAb). MT mutant. Created with BioRender.com
Acknowledgments
We thank Fondazione Oncologia Niguarda Onlus for support and inspiration to write this article.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Declarations
Funding
The authors are supported by Fondazione Oncologia Niguarda Onlus.
Conflicts of Interest/Competing Interests
Salvatore Siena is an advisory board member for Amgen, Bayer, BMS, CheckmAb, Clovis, Daiichi-Sankyo, Merck, Roche-Genentech, and Seattle Genetics. Andrea Sartore-Bianchi is an advisory board member for Amgen, Bayer, Sanofi, and Servier. Giorgio Patelli, Caterina Vaghi, Federica Tosi, Gianluca Mauri, Alessio Amatu, Daniela Massihnia, Silvia Ghezzi, Erica Bonazzina, Katia Bencardino, and Giulio Cerea have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Availability of data and material
Not applicable.
Authors’ contributions
GP, CV, FT, and ASB performed the literature search and were major contributors in devising and writing the manuscript. GM, AA, DM, SG, EB, KB, and GC contributed by identifying ongoing trials and provided a critical revision of the draft. ASB and SS provided the concept and design of the review, critical revision of the draft, and final approval of the version to be submitted. All authors read and approved the final manuscript.
Code availability
Not applicable.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.SEER. Cancer of the colon and rectum: cancer stat facts. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed 14 Apr 2020.
- 3.National Comprehensive Cancer Network. Colon cancer (version 4.2020). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 19 Jun 2020.
- 4.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 5.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 6.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 7.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 9.McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:827. doi: 10.1038/nm0715-827b. [DOI] [PubMed] [Google Scholar]
- 11.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashworth TR. A case of cancer in which cells similar to those in the tumours where seen in the blood after death. Aust Med J. 1869;14:146–47. [Google Scholar]
- 13.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 14.Normanno N, Cervantes A, Ciardiello F, De Luca A, Pinto C. The liquid biopsy in the management of colorectal cancer patients: current applications and future scenarios. Cancer Treat Rev. 2018;70:1–8. doi: 10.1016/j.ctrv.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the Cell Search system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 16.Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–938. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 17.Lim SH-S, Becker TM, Chua W, Ng WL, de Souza P, Spring KJ. Circulating tumour cells and the epithelial mesenchymal transition in colorectal cancer. J Clin Pathol. 2014;67:848–853. doi: 10.1136/jclinpath-2014-202499. [DOI] [PubMed] [Google Scholar]
- 18.Zieglschmid V, Hollmann C, Mannel J, Albert W, Jaeschke-Melli S, Eckstein B, et al. Tumor-associated gene expression in disseminated tumor cells correlates with disease progression and tumor stage in colorectal cancer. Anticancer Res. 2007;27:1823–1832. [PubMed] [Google Scholar]
- 19.Gorges TM, Stein A, Quidde J, Hauch S, Röck K, Riethdorf S, et al. Improved detection of circulating tumor cells in metastatic colorectal cancer by the combination of the Cell Search® System and the AdnaTest®. PLoS ONE. 2016;11:e0155126. doi: 10.1371/journal.pone.0155126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabezas-Camarero S, De la Orden-García V, Veganzones-de-Castro S, Mediero-Valeros B, Fuentes-Ferrer ME, Sánchez Ruiz AC, et al. Performance of two immunoafinity-based methods for CTC detection and molecular characterization in advanced colorectal cancer. J Clin Oncol. 2018;36:e15648. [Google Scholar]
- 22.Fischer JC, Niederacher D, Topp SA, Honisch E, Schumacher S, Schmitz N, et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci USA. 2013;110:16580–16585. doi: 10.1073/pnas.1313594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69–76. doi: 10.1007/978-3-642-28160-0_6. [DOI] [PubMed] [Google Scholar]
- 24.Galanzha EI, Zharov VP. Circulating tumor cell detection and capture by photoacoustic flow cytometry in vivo and ex vivo. Cancers. 2013;5:1691–1738. doi: 10.3390/cancers5041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10:374–394. doi: 10.1016/j.molonc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 27.Mandel P. Metais P [Not available] C R Seances Soc Biol Fil. 1948;142:241–243. [PubMed] [Google Scholar]
- 28.Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 29.Schwarzenbach H, Stoehlmacher J, Pantel K, Goekkurt E. Detection and monitoring of cell-free DNA in blood of patients with colorectal cancer. Ann NY Acad Sci. 2008;1137:190–196. doi: 10.1196/annals.1448.025. [DOI] [PubMed] [Google Scholar]
- 30.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra4. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitzer E, Perakis S, Geigl JB, Speicher MR. The potential of liquid biopsies for the early detection of cancer. NPJ Precis Oncol. 2017;1:36. doi: 10.1038/s41698-017-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanmamed MF, Fernández-Landázuri S, Rodríguez C, Zárate R, Lozano MD, Zubiri L, et al. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015;61:297–304. doi: 10.1373/clinchem.2014.230235. [DOI] [PubMed] [Google Scholar]
- 34.Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3:551–559. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 35.Little S. Amplification-refractory mutation system (ARMS) analysis of point mutations. Curr Protoc Hum Genet. 2001;Chapter 9:Unit 9.8. [DOI] [PubMed]
- 36.Mosko MJ, Nakorchevsky AA, Flores E, Metzler H, Ehrich M, van den Boom DJ, et al. Ultrasensitive detection of multiplexed somatic mutations using MALDI-TOF mass spectrometry. J Mol Diagn. 2016;18:23–31. doi: 10.1016/j.jmoldx.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Newman AM, Bratman SV, To J, Wynne JF, Eclov NCW, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20:71–88. doi: 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 39.Mouliere F, Chandrananda D, Piskorz AM, Moore EK, Morris J, Ahlborn LB, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. 2018;10:eaat4921. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leary RJ, Sausen M, Kinde I, Papadopoulos N, Carpten JD, Craig D, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4:162ra54. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heitzer E, Ulz P, Belic J, Gutschi S, Quehenberger F, Fischereder K, et al. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med. 2013;5:1–16. doi: 10.1186/gm434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Chen W, Papadopoulos N, Goodman S, Bjerregaard NC, Laurberg S, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol. 2009;27:858–863. doi: 10.1038/nbt.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniūnaitė K, Jarmalaitė S, Kriukienė E. Epigenomic technologies for deciphering circulating tumor DNA. Curr Opin Biotechnol. 2019;55:23–29. doi: 10.1016/j.copbio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho J, Oliveira C. Extracellular vesicles: powerful markers of cancer evolution. Front Immunol. 2015;5:685. doi: 10.3389/fimmu.2014.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balacescu O, Sur D, Cainap C, Visan S, Cruceriu D, Manzat-Saplacan R, et al. The impact of miRNA in colorectal cancer progression and its liver metastases. Int J Mol Sci. 2018;19:3711. doi: 10.3390/ijms19123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE. 2014;9:e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113:275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosseini M, Khatamianfar S, Hassanian SM, Nedaeinia R, Shafiee M, Maftouh M, et al. Exosome-encapsulated microRNAs as potential circulating biomarkers in colon cancer. Curr Pharm Des. 2017;23:1705–1709. doi: 10.2174/1381612822666161201144634. [DOI] [PubMed] [Google Scholar]
- 51.Peng Z-Y, Gu R-H, Yan B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J Cell Biochem. 2019;120(2):1457–1463. doi: 10.1002/jcb.27291. [DOI] [PubMed] [Google Scholar]
- 52.Ulivi P, Canale M, Passardi A, Marisi G, Valgiusti M, Frassineti GL, et al. Circulating plasma levels of miR-20b, miR-29b and miR-155 as predictors of bevacizumab efficacy in patients with metastatic colorectal cancer. Int J Mol Sci. 2018;19:307. doi: 10.3390/ijms19010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen SJ, Alpaugh RK, Gross S, O’Hara SM, Smirnov DA, Terstappen LWMM, et al. Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6:125–132. doi: 10.3816/CCC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- 54.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci USA. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013;24:420–428. doi: 10.1093/annonc/mds336. [DOI] [PubMed] [Google Scholar]
- 57.Tol J, Koopman M, Miller MC, Tibbe A, Cats A, Creemers GJM, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21:1006–1012. doi: 10.1093/annonc/mdp463. [DOI] [PubMed] [Google Scholar]
- 58.Sastre J, Maestro ML, Gómez-España A, Rivera F, Valladares M, Massuti B, et al. Circulating tumor cell count is a prognostic factor in metastatic colorectal cancer patients receiving first-line chemotherapy plus bevacizumab: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. Oncologist. 2012;17:947–955. doi: 10.1634/theoncologist.2012-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang X, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer. 2014;14:976. doi: 10.1186/1471-2407-14-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156–2165. doi: 10.1245/s10434-013-2907-8. [DOI] [PubMed] [Google Scholar]
- 61.Seeberg LT, Waage A, Brunborg C, Hugenschmidt H, Renolen A, Stav I, et al. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg. 2015;261:164–171. doi: 10.1097/SLA.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 62.Krebs MG, Renehan AG, Backen A, Gollins S, Chau I, Hasan J, et al. Circulating tumor cell enumeration in a phase II trial of a four-drug regimen in advanced colorectal cancer. Clin Colorectal Cancer. 2015;14(115–22):e2. doi: 10.1016/j.clcc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Aranda E, Viéitez JM, Gómez-España A, Gil Calle S, Salud-Salvia A, Graña B, et al. FOLFOXIRI plus bevacizumab versus FOLFOX plus bevacizumab for patients with metastatic colorectal cancer and ≥3 circulating tumour cells: the randomised phase III VISNÚ-1 trial. ESMO Open. 2020;5:e000944. doi: 10.1136/esmoopen-2020-000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aranda E, Garcia-Alfonso P, Vieitez JM, Ortiz MJ, López-López C, Reina-Zoilo JJ, et al. Randomized phase II study on the influence of BRAF and PIK3CA mutations on the efficacy of FOLFIRI plus bevacizumab (Bev) or cetuximab (Cet), as first line therapy of patients (pts) with RAS wild-type metastatic colorectal carcinoma (mCRC) and <3 baseline circulating tumor cells (bCTCs) J Clin Oncol. 2019;37:3549. [Google Scholar]
- 65.Sastre J, de la Orden V, Martínez A, Bando I, Balbín M, Bellosillo B, et al. Association between baseline circulating tumor cells, molecular tumor profiling, and clinical characteristics in a large cohort of chemo-naïve metastatic colorectal cancer patients prospectively collected. Clin Colorectal Cancer. 2020;19:e110–e116. doi: 10.1016/j.clcc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Hamfjord J, Guren TK, Dajani O, Johansen JS, Glimelius B, Sorbye H, et al. Total circulating cell-free DNA as a prognostic biomarker in metastatic colorectal cancer before first-line oxaliplatin-based chemotherapy. Ann Oncol. 2019;30:1088–1095. doi: 10.1093/annonc/mdz139. [DOI] [PubMed] [Google Scholar]
- 67.Spindler K-LG, Boysen AK, Pallisgård N, Johansen JS, Tabernero J, Sørensen MM, et al. Cell-free DNA in metastatic colorectal cancer: a systematic review and meta-analysis. Oncologist. 2017;22:1049–1055. doi: 10.1634/theoncologist.2016-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabernero J, Lenz H-J, Siena S, Sobrero A, Falcone A, Ychou M, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937–948. doi: 10.1016/S1470-2045(15)00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spindler K-LG, Appelt AL, Pallisgaard N, Andersen RF, Brandslund I, Jakobsen A. Cell-free DNA in healthy individuals, noncancerous disease and strong prognostic value in colorectal cancer. Int J Cancer. 2014;135:2984–2291. doi: 10.1002/ijc.28946. [DOI] [PubMed] [Google Scholar]
- 70.Osumi H, Shinozaki E, Yamaguchi K, Zembutsu H. Early change in circulating tumor DNA as a potential predictor of response to chemotherapy in patients with metastatic colorectal cancer. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-53711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyskjær I, Kronborg CS, Rasmussen MH, Sørensen BS, Demuth C, Rosenkilde M, et al. Correlation between early dynamics in circulating tumour DNA and outcome from FOLFIRI treatment in metastatic colorectal cancer. Nature. 2019;9:11542. doi: 10.1038/s41598-019-47708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garlan F, Laurent-Puig P, Sefrioui D, Siauve N, Didelot A, Sarafan-Vasseur N, et al. Early evaluation of circulating tumor DNA as marker of therapeutic efficacy in metastatic colorectal cancer patients (PLACOL Study) Clin Cancer Res. 2017;23:5416–5425. doi: 10.1158/1078-0432.CCR-16-3155. [DOI] [PubMed] [Google Scholar]
- 73.Iwai T, Yamada T, Takahashi G, Takeda K, Koizumi M, Shinji S, et al. Circulating cell-free long DNA fragments predict post-hepatectomy recurrence of colorectal liver metastases. Eur J Surg Oncol. 2020;46:108–114. doi: 10.1016/j.ejso.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loupakis F, Derouazi M, Murgioni S, Rizzato MD, Sharma S, Renner D, et al. 405MO Personalized circulating tumour DNA assay for the detection of minimal residual disease in CRC patients after resection of metastases. Ann Oncol. 2020;31:S413. [Google Scholar]
- 76.Barault L, Amatu A, Siravegna G, Ponzetti A, Moran S, Cassingena A, et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut. 2018;67:1995–2005. doi: 10.1136/gutjnl-2016-313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amatu A, Schirripa M, Tosi F, Lonardi S, Bencardino K, Bonazzina E, et al. High circulating methylated DNA is a negative predictive and prognostic marker in metastatic colorectal cancer patients treated with regorafenib. Front Oncol. 2019;9:622. doi: 10.3389/fonc.2019.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raghav KPS, Wang XS, Xiao L, Dasari A, Morris VK, Johnson B, et al. A randomized study evaluating tailoring of advanced/metastatic colorectal cancer (mCRC) therapy using circulating cell-free tumor DNA (ctDNA) (TACT-D) J Clin Oncol. 2020;38:TPS77. [Google Scholar]
- 79.Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015;75:892–901. doi: 10.1158/0008-5472.CAN-14-2613. [DOI] [PubMed] [Google Scholar]
- 80.Takeda K, Yamada T, Takahashi G, Iwai T, Ueda K, Kuriyama S, et al. Analysis of colorectal cancer-related mutations by liquid biopsy: Utility of circulating cell-free DNA and circulating tumor cells. Cancer Sci. 2019;110:3497–3509. doi: 10.1111/cas.14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Onidani K, Shoji H, Kakizaki T, Yoshimoto S, Okaya S, Miura N, et al. Monitoring of cancer patients via next-generation sequencing of patient-derived circulating tumor cells and tumor DNA. Cancer Sci. 2019;110:2590–2599. doi: 10.1111/cas.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alberter B, Klein CA, Polzer B. Single-cell analysis of CTCs with diagnostic precision: opportunities and challenges for personalized medicine. Expert Rev Mol Diagn. 2016;16:25–38. doi: 10.1586/14737159.2016.1121099. [DOI] [PubMed] [Google Scholar]
- 83.Rothwell DG, Ayub M, Cook N, Thistlethwaite F, Carter L, Dean E, et al. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat Med. 2019;25:738–743. doi: 10.1038/s41591-019-0380-z. [DOI] [PubMed] [Google Scholar]
- 84.Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2016;8:3980–4000. doi: 10.18632/oncotarget.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.García-Foncillas J, Alba E, Aranda E, Díaz-Rubio E, López-López R, Tabernero J, et al. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann Oncol. 2017;28:2943–2949. doi: 10.1093/annonc/mdx501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017;28:1294–1301. doi: 10.1093/annonc/mdx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmiegel W, Scott RJ, Dooley S, Lewis W, Meldrum CJ, Pockney P, et al. Blood-based detection of RAS mutations to guide anti-EGFR therapy in colorectal cancer patients: concordance of results from circulating tumor DNA and tissue-based RAS testing. Mol Oncol. 2017;11:208–219. doi: 10.1002/1878-0261.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bachet JB, Bouché O, Taieb J, Dubreuil O, Garcia ML, Meurisse A, et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: the AGEO RASANC prospective multicenter study. Ann Oncol. 2018;29:1211–1219. doi: 10.1093/annonc/mdy061. [DOI] [PubMed] [Google Scholar]
- 90.Normanno N, Esposito Abate R, Lambiase M, Forgione L, Cardone C, Iannaccone A, et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann Oncol. 2018;29:112–118. doi: 10.1093/annonc/mdx417. [DOI] [PubMed] [Google Scholar]
- 91.Thierry AR, El Messaoudi S, Mollevi C, Raoul JL, Guimbaud R, Pezet D, et al. Clinical utility of circulating DNA analysis for rapid detection of actionable mutations to select metastatic colorectal patients for anti-EGFR treatment. Ann Oncol. 2017;28:2149–2159. doi: 10.1093/annonc/mdx330. [DOI] [PubMed] [Google Scholar]
- 92.Toledo RA, Cubillo A, Vega E, Garralda E, Alvarez R, de la Varga LU, et al. Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab. Oncotarget. 2017;8:35289–35300. doi: 10.18632/oncotarget.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siena S, Sartore-Bianchi A, Garcia-Carbonero R, Karthaus M, Smith D, Tabernero J, et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann Oncol. 2018;29:119–126. doi: 10.1093/annonc/mdx504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, et al. Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23:2414–2422. doi: 10.1158/1078-0432.CCR-16-1863. [DOI] [PubMed] [Google Scholar]
- 95.Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4:1269–1280. doi: 10.1158/2159-8290.CD-14-0462. [DOI] [PubMed] [Google Scholar]
- 96.Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29:1108–1119. doi: 10.1093/annonc/mdy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 98.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strickler JH, Zemla T, Ou F-S, Cercek A, Wu C, Sanchez FA, et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Ann Oncol. 2019;30:v200. [Google Scholar]
- 100.Siena S, Di Bartolomeo M, Raghav KPS, Masuishi T, Loupakis F, Kawakami H, et al. A phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): DESTINY-CRC01. J Clin Oncol. 2020;38(15_Suppl):4000. [Google Scholar]
- 101.Takegawa N, Yonesaka K, Sakai K, Ueda H, Watanabe S, Nonagase Y, et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget. 2016;7:3453–3460. doi: 10.18632/oncotarget.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schrock AB, Pavlick D, Klempner SJ, Chung JH, Forcier B, Welsh A, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with advanced cancers of the gastrointestinal tract or anus. Clin Cancer Res. 2018;24:1881–1890. doi: 10.1158/1078-0432.CCR-17-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siravegna G, Sartore-Bianchi A, Nagy RJ, Raghav K, Odegaard JI, Lanman RB, et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin Cancer Res. 2019;25:3046–3053. doi: 10.1158/1078-0432.CCR-18-3389. [DOI] [PubMed] [Google Scholar]
- 104.Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell. 2018;34(148–62):e7. doi: 10.1016/j.ccell.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 106.Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willis J, Lefterova MI, Artyomenko A, Kasi PM, Nakamura Y, Mody K, et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin Cancer Res. 2019;25:7035–7045. doi: 10.1158/1078-0432.CCR-19-1324. [DOI] [PubMed] [Google Scholar]
- 108.Silveira AB, Bidard F-C, Kasperek A, Melaabi S, Tanguy M-L, Rodrigues M, et al. High-accuracy determination of microsatellite instability compatible with liquid biopsies. Clin Chem. 2020;66:606–613. doi: 10.1093/clinchem/hvaa013. [DOI] [PubMed] [Google Scholar]
- 109.Parseghian CM, Loree JM, Morris VK, Liu X, Clifton KK, Napolitano S, et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann Oncol. 2019;30:243–249. doi: 10.1093/annonc/mdy509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mauri G, Pizzutilo EG, Amatu A, Bencardino K, Palmeri L, Bonazzina EF, et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: systematic review of different strategies. Cancer Treat Rev. 2019;73:41–53. doi: 10.1016/j.ctrv.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 111.Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, et al. RAS mutations in circulating tumor DNA (ctDNA) and clinical outcomes of rechallenge treatments with anti-EGFR antibodies in patients with metastatic colorectal cancer (mCRC) Ann Oncol. 2019;30:iv114. doi: 10.1200/PO.20.00109. [DOI] [PubMed] [Google Scholar]
- 113.Nakamura M. Phase II study of cetuximab rechallenge in patients with RAS wild-type metastatic colorectal cancer: E-Rechallenge Trial. Ann Oncol. 2019;30:vi116. [Google Scholar]
- 114.Martinelli E, Ciardiello D, Martini G, Troiani T, Cardone C, Vitiello PP, et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives. Ann Oncol. 2020;31:30–40. doi: 10.1016/j.annonc.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 115.Arena S, Bellosillo B, Siravegna G, Martínez A, Cañadas I, Lazzari L, et al. Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin Cancer Res. 2015;21:2157–2166. doi: 10.1158/1078-0432.CCR-14-2821. [DOI] [PubMed] [Google Scholar]
- 116.Newhall K, Price T, Peeters M, Kim TW, Li J, Cascinu S, et al. Frequency of S492R mutations in the epidermal growth factor receptor: analysis of plasma DNA from metastatic colorectal cancer patients treated with panitumumab or cetuximab monotherapy. Ann Oncol. 2014;25:ii109. doi: 10.1080/15384047.2020.1798695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Germano G, Mauri G, Siravegna G, Dive C, Pierce J, Di Nicolantonio F, et al. Parallel evaluation of circulating tumor DNA and circulating tumor cells in metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:80–83. doi: 10.1016/j.clcc.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 118.Bidard F-C, Kiavue N, Ychou M, Cabel L, Stern M-H, Madic J, et al. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: a prospective ancillary study to the Unicancer Prodige-14 Trial. Cells. 2019;8(6):516. doi: 10.3390/cells8060516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tack V, Ligtenberg MJL, Tembuyser L, Normanno N, Vander Borght S, van Krieken HJ, et al. External quality assessment unravels interlaboratory differences in quality of RAS testing for anti-EGFR therapy in colorectal cancer. Oncologist. 2015;20:257–262. doi: 10.1634/theoncologist.2014-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70–83. doi: 10.1016/j.ejca.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 121.Arnold D, Prager GW, Quintela A, Stein A, Moreno Vera S, Mounedji N, et al. Beyond second-line therapy in patients with metastatic colorectal cancer: a systematic review. Ann Oncol. 2018;29:835–856. doi: 10.1093/annonc/mdy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khan KH, Cunningham D, Werner B, Vlachogiannis G, Spiteri I, Heide T, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov. 2018;8:1270–1285. doi: 10.1158/2159-8290.CD-17-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lonardi S, Montagut C, Pietrantonio F, Elez E, Sartore-Bianchi A, Tarazona N, et al. The PEGASUS trial: post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients. J Clin Oncol. 2020;38:TPS4124. [Google Scholar]