Abstract
Objectives:
The cardio-protective capacity of high-density lipoprotein cholesterol (HDL-C) post-menopause has been challenged. HDL subclasses, lipid contents and function might be better predictors of cardiovascular risk than HDL-C. Changes in these measures have not been characterized over the menopause transition (MT) with respect to timing relative to the final menstrual period (FMP).
Approach and Results:
471 women with HDL particle subclasses [nuclear magnetic resonance spectroscopy total, large, medium and small HDL particles (HDL-P) and HDL size], HDL lipid content [HDL phospholipids (HDL-PL) and triglycerides (HDL-Tg)] and HDL function [cholesterol efflux capacity (HDL-CEC)] measured for a maximum of 5 time points across the MT were included. HDL-C and total HDL-P increased across the MT. Within the 1 to 2 years bracketing the FMP, large HDL-P and HDL size declined while small HDL-P and HDL-Tg increased. Although overall HDL-CEC increased across the MT, HDL-CEC per HDL-P declined. Higher concentrations of total, large and medium HDL-P, and greater HDL size were associated with greater HDL-CEC, while of small HDL-P were associated with lower HDL-CEC. Associations of large HDL-P and HDL size with HDL-CEC varied significantly across the MT such that higher large HDL-P concentrations and greater HDL size were associated with lower HDL-CEC within the 1-2 years around the FMP.
Conclusions:
Although HDL-C increased over the MT, HDL subclasses and lipid content showed adverse changes. While overall HDL-CEC increased, HDL-CEC per HDL particle declined, consistent with reduced function per particle. Large HDL-P may become less efficient in promoting HDL-CEC during the MT.
Keywords: Lipoproteins, climacteric, last menstrual period, efflux capacity, high-density lipoproteins
Graphical Abstract
Introduction
As women traverse menopause, their low-density lipoprotein cholesterol (LDL-C) increases (1), whereas the direction of changes in high-density lipoprotein cholesterol (HDL-C) varies. Some studies reported a significant reduction (2), others reported an increase around menopause (1,3,4) and some showed no changes in HDL-C (5).
Despite the strong evidence showing that higher HDL-C is cardio-protective (6), the results from Mendelian randomization studies (7, 8) and the failure of several HDL-C raising agents to reduce cardiovascular disease (CVD) risk in statin-treated individuals (9) cast doubt on a causal cardio-protective association of HDL-C. This conundrum is especially pertinent to women who experience a higher risk of CVD (10) when reaching midlife and an unclear pattern of HDL-C change over the menopause transition (MT). Mounting studies in midlife women report higher HDL-C as a CVD risk marker (11–13). As women traverse menopause, it appears that the direction of the association between HDL-C and CVD risk switches from protective to harmful (13–15). Using data from the Study of Women’s Health Across the Nation (SWAN), increases in HDL-C levels over the MT were associated with atherosclerotic progression (14). This finding was consistent with recent data from an independent sample of postmenopausal women from the Multi-Ethnic Study of Atherosclerosis (MESA), where higher HDL-C was associated with higher risk of carotid plaque presence (15).
One interpretation of this pattern of associations in midlife women is that higher HDL-C levels may be a marker of HDL dysfunctionality rather than a true indicator of CVD risk. For instance, an impairment in HDL receptors, found to play a role in regulating the reverse cholesterol transport (16), transporting cholesterol from peripheral tissues to the liver for biliary excretion, could lead to increase CVD risk while simultaneously increasing HDL-C (17). Collectively, these findings underscore the complex nature of HDL (16) and suggest potential alterations over ovarian aging in other HDL metrics that are not captured by the static measurement of HDL-C.
Novel metrics of HDL particle subclasses, lipid content and function have shown strong associations with CVD risk independent of HDL-C promising a better determination of HDL clinical utility as a biomarker of CVD risk and a target of novel therapies (18–22). The best recognized anti-atherogenic function of HDL particles (HDL-P) is their ability to promote reverse cholesterol transport by accepting cholesterol from lipid-laden macrophages as determined by the HDL cholesterol efflux capacity (HDL-CEC) assay (23). Independent of HDL-C, impaired HDL-CEC has been associated with the incidence of coronary artery disease (CAD) (21) and with more carotid plaque (22). Other potentially key metrics of HDL are the concentrations of various HDL-P subclasses, which may vary in their abilities to promote the cholesterol efflux from macrophages (23,24), and thus could better reflect the HDL cardio-protective function than HDL-C (23). HDL subclasses can provide information on CVD risk that is independent of HDL-C (18,19,25). Moreover, in addition to variation in their size, HDL subclasses vary in their lipid content [phospholipids (PL) and triglycerides (Tg)] (26), which could impact their ability to promote the cholesterol efflux from macrophages. Enrichment of HDL-P with Tg and depletion of PL can impair HDL-CEC (27,28). Lower HDL phospholipids (HDL-PL) and higher HDL triglycerides (HDL-Tg) contents are associated with extent and presence of CAD (22,25,26). Smaller HDL-P are enriched with Tg in myocardial infarction patients (26). Therefore, HDL content of PL and Tg provides additional characterizations of HDL that may contribute to the cholesterol efflux process and CVD risk (22,23).
The MT is an extraordinary phase of life when women experience unique biological changes in hormone levels, body composition and an acceleration of subclinical CVD (29). It is critical to fully understand the nature of the biological changes in HDL that accompany ovarian aging. Limited data exist on the relationship between HDL subclasses and menopause with inconsistent findings (2,30–34). The majority of these studies were cross-sectional, and therefore were limited in the ability to evaluate the dynamic changes in HDL subclasses as related to the MT and the time elapsed since the final menstrual period (FMP). Importantly, very limited studies evaluated HDL function, as measured by HDL-CEC over the MT (35). The relationships between different sizes of HDL-P, their PL and Tg contents, and their ability to promote cholesterol efflux from macrophages have not been characterized in women traversing menopause.
We aimed to characterize changes in HDL subclasses, HDL lipid content (HDL-PL, and HDL-Tg) and function (HDL-CEC) over the MT, as well as associations of HDL subclasses and lipid content with HDL function. We additionally aimed to assess whether timing relative to the FMP modifies these associations.
Methods
The authors declare that all supporting data are available within the article [and its online supplementary files]. SWAN provides access to public use datasets that include data from SWAN screening, baseline, and follow-up visits (https://agingresearchbiobank.nia.nih.gov/, http://www.swanstudy.org/swan-research/data-access/). To preserve participant confidentiality, some, but not all, of the data are contained in the public use datasets. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center (swanaccess@edc.pitt.edu).
Study participants:
SWAN is an ongoing, multi-ethnic, community-based, longitudinal study of the MT. The design of the SWAN study has been described before (36). In brief, 3,302 women (age: 42- 52 years) were recruited between 1996 and 1997 at 7 different sites across the United States. Eligibility criteria for SWAN recruitment included having an intact uterus and at least one ovary, not being pregnant or lactating at recruitment time, and having at least one menstrual period within the last 3 months prior to recruitment, with no use of hormone therapy (HT).
The SWAN HDL study, an ancillary study to SWAN, aims to characterize the changes in HDL subclasses, lipid content and function measures over ovarian aging, and how these changes interact to impact the HDL cardio-protective capacity in women as they cross through the MT. Five-hundred fifty-eight (n=558) women from SWAN were selected to be part of SWAN HDL based on having at least one visit before and two visits after the FMP with available stored blood specimens (a total of 1,461 samples). HDL metrics were measured on stored samples 2-5 times over the MT for each participant (coincident with SWAN visit 1, follow-up visits 3-9, and visit 12). A total of 87 women did not have an observed FMP date and thus were excluded leaving 471 women with a maximum of 1,288 observations overtime for the current analysis.
Written informed consent was provided by all participants prior to SWAN enrollment, and study protocols were approved by the institutional review boards at each SWAN site.
Cholesterol efflux capacity and HDL lipid content:
HDL-CEC, HDL-PL and HDL-Tg were all measured at the Rader laboratory at the University of Pennsylvania. HDL-CEC was measured using the efflux of fluorescence-labeled cholesterol as has been described previously (20). Briefly, J774 mouse macrophage cells were plated and labeled with 2 μCi/mL of 3H cholesterol overnight. The cells were then incubated in the presence of 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP (cAMP), an up regulator of ATP-binding cassette transporter-1 (ABCA1) for 6 hours. Proteins containing apolipoprotein B (ApoB) were removed from plasma by polyethylene glycol precipitation. Cells were then incubated with the equivalent of 1% ApoB–depleted serum or plasma for 2 hours at a temperature of 37°C. Each medium was then collected and passed through a 0.22 μM filter to to remove cell debris and radioactivity determined by liquid scintillation counting. Media without serum were used as baseline controls. Isopropanol extraction was then used to quantify radioactive cholesterol that has been incorporated into cellular lipids. The quantity of radioactive cellular cholesterol was determined after isopropanol extraction. Percent cholesterol efflux capacity was calculated by the following formula: [(cpm of 3H cholesterol in the media - cpm of 3H cholesterol in serum free media) / (cpm of 3H cholesterol in the cells + cpm of 3H cholesterol in the media)] × 100. The intra and inter assay coefficients of variation were 3.7% and 10.1% respectively. To measure HDL-PL and HDL-Tg, HDL was first isolated from serum by phosphotungstic acid precipitation (FujiFilm Wako Pure Chemical Corporation). HDL-PL and HDL-Tg were then measured according to manufacturer’s protocol (Wako: 433-36201 and Roche: 20767107322, respectively) using the Roche Cobas C311 clinical analyzer. The inter-assay coefficients of variation were 3.5% and 3.9% for HDL-PL and HDL-Tg respectively.
Nuclear Magnetic Resonance (NMR) Spectroscopy:
HDL subclasses and size were measured at LabCorp (Morrisville, NC, USA) by the NMR Spectroscopy LipoProfile-3 algorithm (37), using the Vantera Clinical Analyzer, which is an automated, 400 MHz NMR spectroscopy platform. Each lipoprotein subclass produces unique NMR signals that are specific in frequency and shape. The amplitude of the signal is proportional to the number of particles that are releasing the signal. To obtain the amplitude of each subpopulation of subclasses, the line shape of the signal envelope was modeled as a sum of all lipoprotein signals. The areas of different subpopulations were multiplied by conversion factors to quantify the concentrations, which were then grouped into small, medium or large HDL-P. The total HDL-P concentration was obtained by adding up the concentrations of all subclasses. The average size of HDL-P was calculated by adding the diameter of each subclass multiplied by its relative mass percentage from NMR signal amplitude. HDL subclasses were grouped into large (9.4-14 nm), medium (8.2-9.4 nm) and small (7.3-8.2 nm) HDL-P. The intra- and inter-assay coefficients of variation for HDL-P concentrations and size ranged from 0.6% to 3.7% (intra-assay) and 1.5% to 4.0% (inter-assay).
Study covariates:
Race/ethnicity and education were self-reported at the baseline SWAN visit. Age, measured body mass index (BMI) (Kg/m2), and menopause status were collected at every visit.
Fasting triglycerides and total cholesterol were evaluated by the Hitachi 747-200 clinical analyzer at the Medical Research Laboratories (Highland Heights, KY) (SWAN baseline visit – visit 7) or by the ADVIA assay at the University of Michigan (SWAN visits 9 and 12). University of Michigan results were calibrated so that results are comparable to those produced by the Medical Research Laboratories. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald equation when triglycerides were <400mg/dL (38). Complement C3 was performed on the Alfa-Wasserman ACE analyzer using the K-ASSAY (KAMIYA BIOMEDICAL COMPANY) complement C3 reagent.
Menopausal status was based on the frequency and pattern of the menstrual bleeding within the past 12 months prior to each visit. In this analysis, menopausal status was categorized as either premenopausal/early perimenopausal (no changes in menstrual bleeding or at least one menstrual bleed within the last three months with some changes in the intervals of cycle), late perimenopause (no menstrual bleed within the last 3 months but at least one cycle within the last 12 months), postmenopausal (no menstrual cycle within the last 12 months, either due to natural menopause or surgical menopause by bilateral salpingo-oophorectomy), or unknown due to HT use. Observations at which menopause status were unknown due to hysterectomy were excluded from longitudinal analysis. The FMP was defined as the date of the last menstrual period reported in the visit immediately prior to the first visit that a woman was classified as postmenopausal. Ever use of HT was also self-reported at every SWAN visit.
Statistical analysis:
Abundance of HDL subclasses was calculated as the percentage of each HDL subclass concentration relative to the total HDL-P concentration. Linear mixed effect models were used to separately estimate and compare the geometric means of each HDL subclass abundance by menopausal status overtime adjusted for multiple comparisons. The linear trend for menopausal stages (from pre- to post-) was assessed by treating menopausal status as a continuous variable in linear mixed effect models. LOESS plots were used to assess whether changes in each HDL metric relative to the time of the FMP were linear. Inflection points were identified from LOESS plots. Piecewise linear mixed-effects models were then used to estimate the yearly changes in each LOESS-identified time segments for each HDL measure. For simple presentation, the identified segments (i.e., between successive inflection points) were labeled as premenopause, perimenopause or postmenopause stage for analytes with 3-4 identified segments and as pre-/peri-menopause or postmenopuase for analytes with 2 identified segments. To assess associations of HDL subclasses and lipid content metrics with HDL function, linear mixed effects models with repeated measures of HDL-CEC as a function of HDL subclasses and lipid content measures (separate model for each analyte) were created. To assess whether the tested longitudinal association of each HDL subclasses and lipid content measures with HDL-CEC varied by time relative to the FMP, an indicator variable of time relative to the FMP based on inflection points identified for HDL-CEC measure was created as follows: >0.5 years before FMP (premenopause), ≤0.5 years before to ≤1.5 years after the FMP (perimenopause), and >1.5 years after FMP (postmenopause). An interaction term for this indicator variable with each HDL subclasses and lipid content metric was included in the model to test effect modification by time relative to the FMP.
Results
At the first available visit, participants were mainly White (51.8%), not yet postmenopausal (89.2%) with only 9.8% having ever used HT, Table 1. Summary statistics of CVD risk factors, lipids and HDL metrics are presented in Table 1.
Table 1.
Characteristics at SWAN HDL Baseline
| Variable | N=471 |
|---|---|
| Age, years, mean (SD) | 50.19(2.7) |
| Race, n(%) | |
| White | 244(51.8%) |
| Black | 130(27.6%) |
| Chinese | 52(11.0%) |
| Hispanic | 3(0.6%) |
| Japanese | 42(8.9%) |
| Education, n(%) | |
| High School or less | 83 (17.7%) |
| Some College/College | 249 (53.1) |
| Post-Graduate | 137(29.2%) |
| Menopausal Status, n(%) | |
| Pre/Early Peri-menopausal | 374(79.4%) |
| Late Peri-menopausal | 37(7.9%) |
| Postmenopausal | 51(10.8%) |
| Unknown due to HT use | 9 (1.9%) |
| Ever Use Hormone, n(%) | 46 (9.8%) |
| Use any of antilipemic, antidiabetic or antihypertensive medication, n(%) | 75 (15.9%) |
| Time relative to FMP, years, median (Q1, Q3) | −2.08(−3.54, −0.78) |
| BMI, kg/m2, median (Q1, Q3) | 26.54(22.95, 31.71) |
| C3, mg/dl, mean (SD) | 125.01(29.24) |
| Total Cholesterol, mg/dL, mean (SD) | 193.93(34.06) |
| LDL-C, mg/dL, mean (SD) | 112.40(31.03) |
| HDL-C, mg/dL, mean (SD) | 59.32(14.22) |
| Triglycerides, mg/dL, median (Q1, Q3) | 112.55(78.69) |
| apoA1, mg/dl, mean (SD) | 163.63 (26.16) |
| Total HDL-P, umol/L, mean (SD) | 34.67(5.97) |
| Large HDL-P, umol/L, mean (SD) | 8.49(3.59) |
| Medium HDL-P, umol/L, mean (SD) | 11.19(6.19) |
| Small HDL-P, umol/L, mean (SD) | 14.99(7.03) |
| HDL Size, nm, mean (SD) | 9.54(0.54) |
| HDL-PL, mg/dL, mean (SD) | 54.15(10.26) |
| HDL-Tg, mg/dL, median (Q1, Q3) | 17.00(14.00, 21.00) |
| HDL-CEC, %, mean(SD) | 3.95(0.67) |
apoA1: apolipoprotein A1; BMI: body mass index; C3: complement protein C3; E2: estradiol; FMP: final menstrual period; HDL-C: high-density lipoprotein cholesterol; HDL-CEC: high-density lipoprotein cholesterol efflux capacity; HDL-P: high-density lipoprotein particles; HDL-PL: high-density lipoprotein phospholipids; HDL-Tg: high-density lipoprotein triglycerides; LDL-C: low-density lipoprotein cholesterol.
Abundance of HDL subclasses by menopausal stage
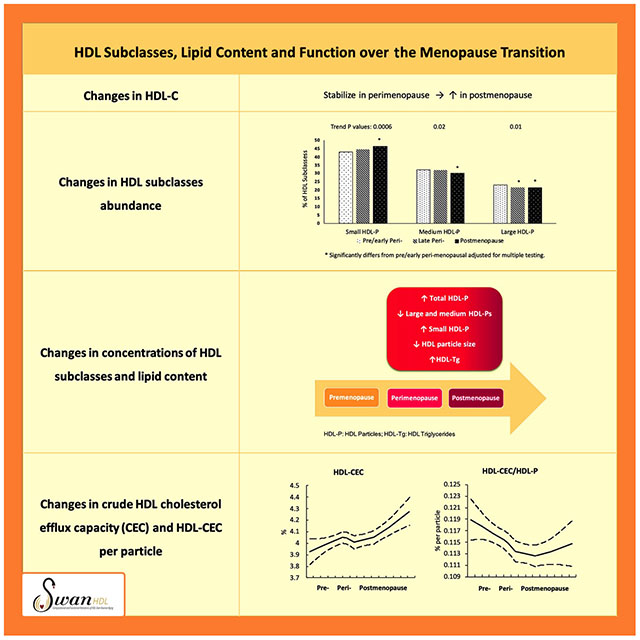
The small HDL-P was the most abundant HDL subclass whereas the large HDL-P was the least abundant subclass at each stage of the MT. As women progressed from one stage to another, the percentage of small HDL-P increased while that of large HDL-P decreased, Figure 1.
Figure 1. Abundance of HDL particle subclasses relative to total HDL-P by menopausal stage over the menopausal transition.
At all stages of the menopause transition, small HDL-P were the most abundance particles. As women traverse menopause, the abundance of small HDL-P relative to total HDL-P increased while of large HDL-P decreased (Sample size: 468 women with 1268 observations).
* Significantly differs from pre/early peri-menopausal adjusted for multiple testing
HDL-P: high-density lipoprotein particles
Changes in HDL metrics relative to the FMP during the premenopause, perimenopause and postmenopause stages
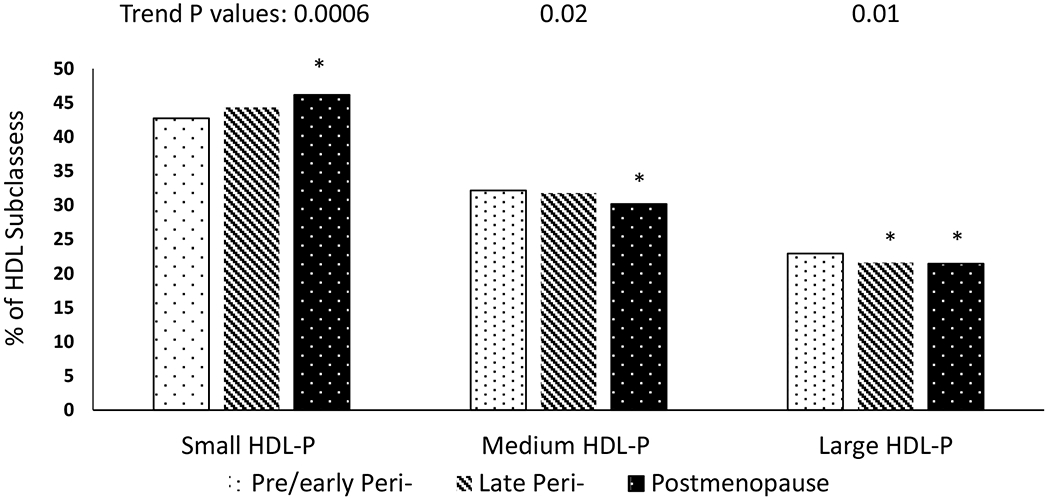
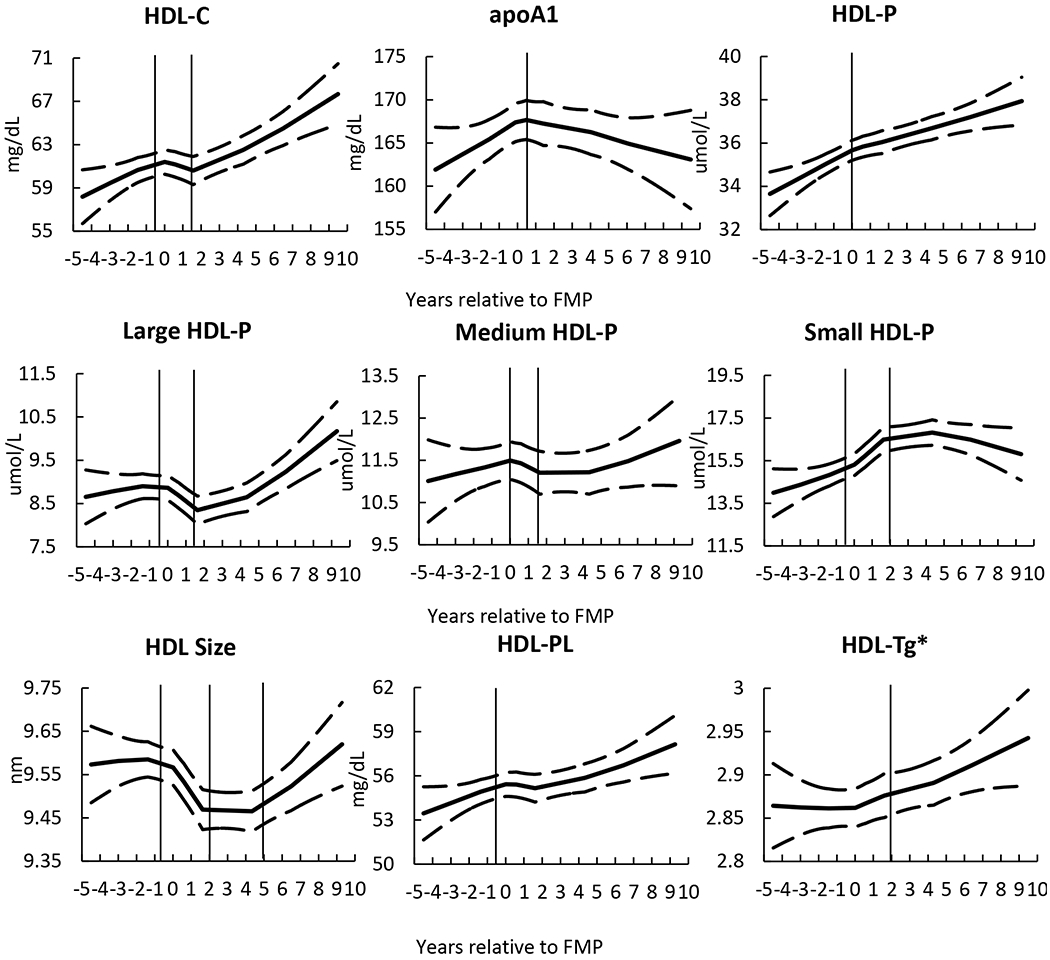
Overall, HDL-C and total HDL-P increased across the MT, while apoA1 started to decline during the perimenopause stage. During this stage, large HDL-P and HDL size declined while small HDL-P and HDL-Tg increased, Figure 2 and Table 2. Although HDL-CEC showed an overall increase over the MT, HDL-CEC per total HDL-P declined, Figure 3 and Table 2. Results were additionally adjusted for time varying smoking status, BMI or use of any antilipemic, antidiabetic or antihypertension and findings were similar (data not shown). Level of each HDL metric at the beginning of each stage is presented in Table 2.
Figure 2. Trajectories of HDL conventional and composition metrics over the menopause transition.
Although HDL-C increased over the MT, measures of HDL subclasses and lipid content showed changes consistent with major remodeling effects of HDL- Vertical lines represent identified inflection points demarcating 3 stages (for metrics with 3 segments): pre-, peri-, and postmenopause for 2 stages (for metrics with 2 segments): pre-/peri-, and postmenopause. Solid line is the locally weighted smoothing (LOESS) results and dash lines are upper and lower 95% confident interval. The number of observations (Sample size: 1,197 to 1,217 observations, varied by HDL metrics) was restricted to 5 years before FMP and 10 years after FMP to reduce the variability due to limited sample size at two ends.
* HDL-Tg is log transformed
apoA1: apolipoprotein A1; FMP: final menstrual period; HDL-C: high-density lipoprotein cholesterol; HDL-P: high-density lipoprotein particles; HDL-PL: high-density lipoprotein phospholipids; HDL-Tg: high-density lipoprotein triglycerides.
Table 2.
Level and annual changes of HDL metrics by time relative to the final menstrual period
| HDL metric | Premenopause stage* | Perimenopause stage* | Postmenopause stage* | |||
|---|---|---|---|---|---|---|
| At stage start | During stage | At stage start | During stage | At stage start | During stage | |
| Mean (SD) | Annual change(SE) † | Mean (SD) | Annual change(SE) † | Mean (SD) | Annual change(SE) † | |
| HDL-C, mg/dL | 60.37(14.44) | 0.92 (0.24) | 62.04(15.75) | 0.08(0.41) | 62.75(15.56) | 0.41(0.12) |
| Large HDL-P NMR, umol/L | 8.9(3.54) | 0.20(0.06) § | 9.0(3.91) | −0.31(0.10) | 8.75(4.02) | 0.12(0.03) § |
| Medium HDL-P NMR, umol/L | 11.34(6.05) | 0.07(0.12) | 11.73(6.02) | 0.14(0.33) | 11.28(5.69) | −0.11(0.07) |
| Small HDL-P NMR, umol/L | 14.25(6.78) | 0.05(0.14) § | 15.92(6.96) | 0.82(0.22) | 16.71(6.8) | 0.04(0.08) § |
| HDL size NMR †, nm | 9.6(0.52) | 0.02(0.01) § | 9.56(0.55) | −0.07(0.01) | 9.32(0.54) | 0.01(0.01) § |
| HDL-CEC % | 4(0.69) | 0.04(0.01) | 4.07(0.65) | −0.0003(0.02) | 4.08(0.67) | 0.01(0.01) |
| HDL metric | Pre-/Perimenopause stage* | Postmenopause stage* | ||||
| Mean (SD) | Annual change(SE) † | Mean (SD) | Annual change(SE) † | |||
| apoA1, mg/dl | 163.52(26.06) | 1.52(0.39) ‡ | 167(31.36) | −0.97(0.21) | ||
| Total HDL-P NMR , umol/L | 34.58(5.7) | 0.48(0.09) ‡ | 36.36(6.37) | 0.18(0.04) | ||
| HDL-PL, mg/dL | 54.53(10.26) | 0.59(0.16) ‡ | 55.81(11.46) | 0.12(0.06) | ||
| HDL-Tg (log transformed), mg/dl | 18.16(5.75) | 0.005(0.003) | 19.02(5.79) | 0.01(0.003) | ||
| CEC% / Total HDL-P NMR | 0.12(0.02) | −0.001(0.0003) | 0.11(0.02) | −0.0002(0.0002) | ||
Menopause stages were identified based on the following cut points of years relative to the FMP for each HDL metric as identified by LOESS plots: HDL metrics with 3-4 segments [HDL-C: −0.5, 1.5; Large HDL-P: −0.5, 1.5; Medium HDL-P: 0, 1.5; Small HDL-P: −0.5, 2; HDL size: −0.5, 2, 5; HDL-CEC%: −0.5, 1.5]; HDL metrics with 2 segments [apoA1: 0.5; Total HDL-P: 0; HDL-PL: −0.5; HDL-Tg: 2; CEC% / Total HDL-P: 1.5]. Bold indicates significantly differ from zero at P ≤0.05.
Models adjusted for age at FMP, study site and race/ethnic group. Annual change in the fourth segment for HDL size was not significant and was not significantly differ from annual change in segment 3
Significantly differs from postmenopause stage
Significantly differ from perimenopause stage
apoA1: apolipoprotein A1; FMP: final menstrual period; HDL-C: high-density lipoprotein cholesterol; HDL-CEC: high-density lipoprotein cholesterol efflux capacity; HDL-P: high-density lipoprotein particles; HDL-PL: high-density lipoprotein phospholipids; HDL-Tg: high-density lipoprotein triglycerides.
Figure 3. Trajectories of HDL function metrics over the menopause transition.
While overall HDL-CEC increased over the MT, the HDL-CEC per HDL particle declined, consistent with reduced function- Vertical lines represent identified inflection points demarcating 3 stages: pre-, peri-, and postmenopause. Solid line is the locally weighted smoothing (LOESS) results and dash lines are upper and lower 95% confident interval. The number of observations (Sample size: 1,197 to 1,217 observations, varied by HDL metrics) was restricted to 5 years before FMP and 10 years after FMP to reduce the variability due to limited sample size at two ends.
HDL-CEC: high-density lipoprotein cholesterol efflux capacity; HDL-P: high-density lipoprotein particles
Longitudinal associations of HDL conventional and composition metrics with HDL function
In unadjusted and adjusted analyses, higher concentrations of HDL-C and apoA1 were associated with greater HDL-CEC over the MT. Moreover, higher concentrations of total, large and medium HDL-P and greater HDL size were associated with greater HDL-CEC while higher concentrations of small HDL-P were associated with lower HDL-CEC, Table 3.
Table 3.
Longitudinal associations of HDL conventional and composition metrics with HDL-CEC%
| HDL metrics | HDL-CEC% | |||
|---|---|---|---|---|
| Unadjusted | Adjusted* | |||
| β(SE) | P value | β(SE) | P value | |
| HDL-C, mg/dL | 0.027(0.0010) | <.0001 | 0.027(0.001) | <.0001 |
| apoA1, mg/dl | 0.012(0.0005) | <.0001 | 0.011 (0.0006) | <.0001 |
| Total HDL-P , umol/L | 0.044(0.002) | <.0001 | 0.040(0.003) | <.0001 |
| Large HDL-P, umol/L | 0.088(0.004) | <.0001 | 0.093(0.005) | <.0001 |
| Medium HDL-P, umol/L | 0.023(0.002) | <.0001 | 0.021(0.003) | <.0001 |
| Small HDL-P, umol/L | −0.005(0.002) | 0.0239 | −0.007(0.002) | 0.005 |
| HDL size, nm | 0.403(0.033) | <.0001 | 0.437(0.037) | <.0001 |
| HDL-PL, mg/dL | 0.040(0.001) | <.0001 | 0.040(0.001) | <.0001 |
| HDL-Tg, mg/dL † | 0.480(0.052) | <.0001 | 0.524(0.055) | <.0001 |
Adjusted for study site, race/ethnicity, segment indicator, time varying age, body mass index, and C3
Log transformed
apoA1: apolipoprotein A1; HDL-C: high-density lipoprotein cholesterol; HDL-CEC: high-density lipoprotein cholesterol efflux capacity; HDL-P: high-density lipoprotein particles; HDL-PL: high-density lipoprotein phospholipids; HDL-Tg: high-density lipoprotein triglycerides.
Effect modification of time relative to FMP on associations of HDL conventional and composition metrics with HDL-CEC
Adjusted associations of large HDL-P and HDL size with HDL-CEC varied by menopause stage as defined by time relative to the FMP; higher large HDL-P concentrations, and greater HDL size were associated with lower HDL-CEC during perimenopause stage compared to other stages, Figure 4 and supplemental Table I. Similar pattern was seen for associations of apoA1 and HDL-CEC by menopausal stage, supplemental Table I.
Figure 4. Associations of Large HDL-P (Panel A) and HDL size (Panel B) with HDL-CEC by menopausal stage.
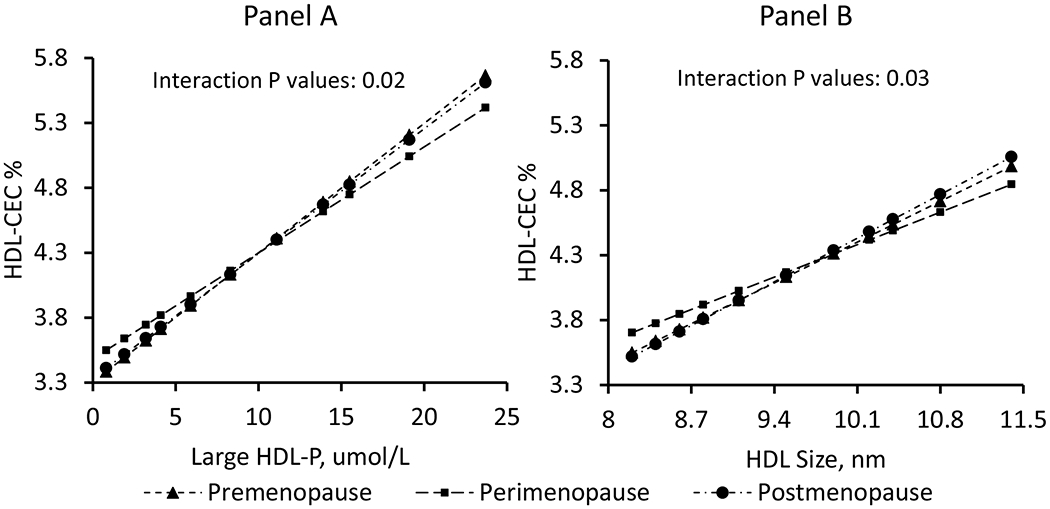
Higher concentrations of large HDL-P (Panel A) and greater HDL size (Panel B) were associated with lower HDL-CEC within the 1 to 2 years around the FMP (perimenopause stage) compared to other stages, suggesting inefficiency in the large HDL-P over the MT (Sample size: 469 women with 1,246 observations).
HDL-CEC: high-density lipoprotein cholesterol efflux capacity; HDL-P: high-density lipoprotein particles ; MT: menopause transition
Discussion
The current study provides strong evidence of a disconnection between the widely used measure HDL-C and novel measures of HDL subclasses, lipid content, and function in women traversing menopause. Although HDL-C increased over the MT, other metrics of HDL showed adverse changes supporting the concept that HDL becomes dysfunctional over the MT. Within the 1 to 2 years bracketing the FMP (perimenopause stage), large HDL-P and HDL size declined while small HDL-P and HDL-Tg increased. Although the absolute measure of HDL-CEC increased over the MT, HDL-CEC normalized to HDL-P, declined sharply indicating that the ability of HDL-P to promote the cholesterol efflux capacity from macrophages is compromised over the MT. Interestingly, higher concentrations of large HDL-P and greater HDL size were associated with lower HDL-CEC within the 1 to 2 years around the FMP compared to other stages, suggesting inefficiency in the large HDL-P over the MT.
HDL-C over the MT:
Earlier analysis of a larger sample of SWAN women over a shorter time across the FMP reported increases in HDL-C around the FMP that leveled off thereafter (1). The current analysis from a smaller subgroup of SWAN women over a longer time across the FMP showed HDL-C to slightly decline around the FMP but increase afterward. This overall increase in HDL-C is consistent with a recent analysis of 672 natural postmenopausal women showing an overall increase in HDL-C trajectory over ages from 53 to 69 years (6). Other studies, mainly cross-sectional, indicated that postmenopausal women have lower levels of HDL-C than premenopausal women (2,3). Yet, a recent meta-analysis of 66 studies of 114,655 midlife women did not demonstrate any difference in HDL-C concentration by menopausal status (39). An obvious explanation of the inconsistent literature on HDL-C changes as related to the MT is the differences in study designs and methodologies used in defining menopause. However, another less acknowledged, but plausible, explanation is that the widely used HDL-C measure is a static measure of only one component of the HDL molecule. As such, HDL-C may not adequately represent the complex nature of HDL composition and function that could be changing during the MT and hence lacks uniformity when evaluating women at different stages of the MT across studies.
HDL subclasses over the MT:
Few studies assessed the relationship between HDL subclasses and menopause. Some studies reported lower levels of large [HDL2-C] (30) and higher levels of small [HDL3-C] subclasses in post- as compared with premenopausal women (2,31,32) while other studies suggested the reverse (33, 34). These inconsistent findings could be related to the cross-sectional study design and the different methods used to measure HDL subclasses which did not directly quantify the concentrations or sizes of large and small particles. We are not aware of any other study that prospectively assessed changes in HDL subclasses relative to the FMP. The Framingham Offspring study, although cross-sectional in nature and not specifically focused on menopause (40), measured HDL subclasses using NMR as in our study. Women had a two-fold higher concentration of large HDL-P than men. Additionally, the observed sex differences in HDL-P size decreased with age. The Framingham Offspring study suggested a slight decline in large HDL-P with age and an increase in medium and small HDL-P mainly around the age of 50 in women, a common age of menopause. Our findings of HDL subclasses changes over the MT are thus consistent with these reported findings from the Framingham Offspring study.
HDL-CEC over the MT:
The association between HDL-CEC and menopause has not been comprehensively assessed before. In a smaller analysis among 46 SWAN women with HDL-CEC measured both before and after menopause, we reported a significant increase in HDL-CEC (35). Our current findings are consistent with our previous preliminary analysis (35) confirming HDL-CEC increases over the MT. Our study additionally shows that when HDL-CEC was normalized to HDL-P, a significant decline in HDL-CEC per particle was observed underscoring the complex nature of HDL function that needs to be assessed along with metrics of HDL subclasses. Our findings suggest that the ability of HDL-P to promote cholesterol efflux is compromised during the MT and the large HDL-P might play a role. We found that greater concentrations of large HDL-P and larger HDL size were associated with less HDL-CEC during the time bracketing 1 to 2 years around the FMP (perimenpause), compared to other time segments, suggesting large particles become less efficient in moving cholesterol from macrophages during the MT. Interestingly, the large HDL subclass is more vulnerable to oxidative modification compared to the small dense subclass (41).
The MT accompanied by hormonal and metabolic changes that could impact HDL remodeling and function:
Transitioning through menopause might contribute to dynamic changes in HDL metabolism and function via estradiol reduction (29). Decline in estradiol enhances the lipolytic enzymes activity, which plays critical roles in converting HDL from large to small particles (42). Additionally, estradiol promotes the cholesterol efflux capacity from vascular smooth muscle cells (43) and reduces cholesteryl ester accumulation in human monocyte-derived macrophages obtained from CHD patients (44). Hormonal and metabolic alterations over ovarian aging could trigger risk factor accumulation, which, in turn, could lead to chronic inflammation (45) found to modify HDL subclasses distribution and lipid content (25) and impact HDL function (46, 47). The MT is associated with changes in body fat deposition, making women more vulnerable to insulin resistance and metabolic syndrome (29); all associated with increased oxidative stress and more inflammation (48) and thus contributing to changes in HDL metabolism over ovarian aging (49).
Strengths and limitations:
The detailed, prospectively collected, clinical data from the SWAN study on the MT and time related to the FMP along with a comprehensive panel of novel, well-developed, metrics of HDL subclasses, lipid content, and function in a multi-racial/ethnic sample of women traversing menopause are major strengths. Limitations include the lack of measures of HDL proteomics and lipidomics as well as measures of HDL antioxidant capacity and paraoxonase-1. Future studies should comprehensively assess how the reported changes in HDL metrics over the MT could impact risk of CVD in postmenopausal women.
Clinical implications:
Regardless of the current uncertainty of the clinical utility of HDL-C, HDL-C is still a critical component in risk prediction models (50) irrespective of menopausal status. Our results call for a re-evaluation of the contribution of HDL-C to risk prediction in postmenopausal women as well as considering other measures of HDL that found to better reflect the complex nature of HDL and predict CVD risk beyond HDL-C. We are facing a challenging emergent question about the utility of HDL in clinical practice, pharmaceutical and epidemiological research. This question is especially relevant to midlife and older women as evident by accumulating findings casting doubt on HDL-C risk prediction. The rich recent literature on HDL underlines the extreme complexity of HDL molecules and the importance of considering many aspects of HDL composition, structure and function to better understand the underlying biology. Our results provide new insights on whether clinicians should be concerned about HDL in older women.
Conclusions:
Although HDL-C increased over the MT, measures of HDL subclasses and lipid content showed adverse changes. While HDL-CEC increased, HDL-CEC per particle declined. Large HDL-P may become less efficient in promoting HDL-CEC during the MT. Our results support efforts to identify novel targets for HDL-based therapies and better HDL biomarkers of CVD risk in women.
Supplementary Material
Highlights.
High-density lipoprotein cholesterol (HDL-C) is one of the factors used in CVD risk prediction equations.
Changes in HDL-C level in women traversing menopause have not been consistent.
As women traverse menopause, they experience an increase in their HDL-C level that is accompanied by adverse changes in other novel measures of HDL.
There is a clear disconnection between HDL-C and measures of HDL subclasses, lipid content and function in women during the menopause transition.
It is critical to re-evaluate HDL-C as a factor used in risk prediction equation in midlife women.
Acknowledgements:
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - Present; Dan McConnell 2011 – 2013; MaryFran Sowers 2000 – 2011
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
We thank Issam Abushaban for designing the graphical abstract of this article.
Funding: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
The SWAN Repository (U01AG017719).
The Study of Women’s Health Across the Nation (SWAN) HDL ancillary study has grant support from National Institute on Aging (NIA) AG058690.
The content of this abstract is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Abbreviations
- apoA1
Apolipoprotein A1
- BMI
Body mass index
- C3
Complement protein C3
- FMP
the final menstrual period
- HDL-C
high-density lipoprotein cholesterol
- HDL-CEC
cholesterol efflux capacity
- HDL-P
HDL particles
- HDL-PL
HDL phospholipids
- HDL-Tg
HDL triglycerides
- MT
the menopause transition
Footnotes
Disclosures: None
References
- 1.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, McNamara JR, Fruchart JC, et al. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res. 1996;37:1886–1896. [PubMed] [Google Scholar]
- 3.Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. Am J Epidemiol. 2009;169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Keeffe LM, Kuh D, Fraser A, Howe LD, Lawlor D, Hardy R. Age at period cessation and trajectories of cardiovascular risk factors across mid and later life. Heart 2020;106:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukami K, Koike K, Hirota K, Yoshikawa H, Miyake A. Perimenopausal changes in serum lipids and lipoproteins: a 7-year longitudinal study. Maturitas.1995;22:193–197. [DOI] [PubMed] [Google Scholar]
- 6.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986;256:2835–2838. [PubMed] [Google Scholar]
- 7.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson TG, Sanderson E, Palmer TM, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020. 23;17:e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenson RS. The High-Density Lipoprotein Puzzle: Why Classic Epidemiology, Genetic Epidemiology, and Clinical Trials Conflict? Arterioscler Thromb Vasc Biol 2016;36:777–782. [DOI] [PubMed] [Google Scholar]
- 10.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 11.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis 2007;195:e191–196. [DOI] [PubMed] [Google Scholar]
- 12.Keidar S, Bogner I, Gamliel-Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high-density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. J Clin Lipidol 2009;3:345–350. [DOI] [PubMed] [Google Scholar]
- 13.Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women’s Health Across the Nation Heart women. Menopause 2011;18:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol. 2016;10:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Khoudary SR, Ceponiene I, Samargandy S, et al. HDL (High-Density Lipoprotein) Metrics and Atherosclerotic Risk in Women. Arterioscler Thromb Vasc Biol 2018;38:2236–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol 2010;21(3):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016. 11;351:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012;60:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora S, Glynn RJ, Ridker PM. HDL cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 2013;128:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. New England Journal of Medicine 2011;364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohatgi A, Khera A, Berry JD, et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N Engl J Med 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwala AP, Rodrigues A, Risman M, et al. High-Density Lipoprotein (HDL) Phospholipid Content and Cholesterol Efflux Capacity Are Reduced in Patients With Very High HDL Cholesterol and Coronary Disease. Arterioscler Thromb Vasc Biol 2015;35:1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arteriosclerosis Thrombosis and Vascular Biology 2010; 30:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du XM, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res 2015;116:1133–1142. [DOI] [PubMed] [Google Scholar]
- 25.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res 2013;54:2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papathanasiou A, Kostara C, Cung MT, et al. Analysis of the composition of plasma lipoproteins in patients with extensive coronary heart disease using 1H NMR spectroscopy. Hellenic J Cardiol 2008;49:72–78. [PubMed] [Google Scholar]
- 27.Nakanishi S, Vikstedt R, Söderlund S, et al. Serum, but not monocyte macrophage foam cells derived from low HDL-C subjects, displays reduced cholesterol efflux capacity. J Lipid Res 2009;50:183–1892. [DOI] [PubMed] [Google Scholar]
- 28.Greene DJ, Skeggs JW, Morton RE. Elevated triglyceride content diminishes the capacity of high density lipoprotein to deliver cholesteryl esters via the scavenger receptor class B type I (SR-BI). J Biol Chem 2001;276:4804–4811. [DOI] [PubMed] [Google Scholar]
- 29.El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause 2019;26:1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascarenhas-Melo F, Sereno J, Teixeira-Lemos E, et al. Markers of increased cardiovascular risk in postmenopausal women: focus on oxidized-LDL and HDL subpopulations. Dis Markers 2013;35:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med 1994;154:2349–2355. [PubMed] [Google Scholar]
- 32.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis 1993;98:83–90. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostis P, Stevenson JC, Crook D, Johnston DG, Godsland IF. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015;81:62–68. [DOI] [PubMed] [Google Scholar]
- 34.Swiger KJ, Martin SS, Blaha MJ, et al. Narrowing sex differences in lipoprotein cholesterol subclasses following mid-life: the very large database of lipids (VLDL-10B). J Am Heart Assoc 2014;3:e000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Khoudary SR, Hutchins PM, Matthews KA, et al. Cholesterol Efflux Capacity and Subclasses of HDL Particles in Healthy Women Transitioning Through Menopause. J Clin Endocrinol Metab 2016;101(9):3419–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowers M, Crawford S, Sternfeld B et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, editors. Menopause: Biology and Pathology. New York: Academic Press, 2000:175–188. [Google Scholar]
- 37.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847–870. [DOI] [PubMed] [Google Scholar]
- 38.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 39.Ambikairajah A, Walsh E, Cherbuin N. Lipid profile differences during menopause: a review with meta-analysis. Menopause 2019;26:1327–1333. [DOI] [PubMed] [Google Scholar]
- 40.Freedman DS, Otvos JD, Jeyarajah EJ, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem 2004;50:1189–1200. [DOI] [PubMed] [Google Scholar]
- 41.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med 2011;17:594–603. [DOI] [PubMed] [Google Scholar]
- 42.Berg GA, Siseles N, González AI, Ortiz OC, Tempone A, Wikinski RW. Higher values of hepatic lipase activity in postmenopause: relationship with atherogenic intermediate density and low density lipoproteins. Menopause 2001;8:51–57. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Liu Y, Zhu L, et al. 17β-estradiol promotes cholesterol efflux from vascular smooth muscle cells through a liver X receptor α-dependent pathway. Int J Mol Med 2014;33:550–558. [DOI] [PubMed] [Google Scholar]
- 44.Corcoran MP, Lichtenstein AH, Meydani M, Dillard A, Schaefer EJ, Lamon-Fava S. The effect of 17{beta}-estradiol on cholesterol content in human macrophages is influenced by the lipoprotein milieu. J Mol Endocrinol 2011;47:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansell BJ, Fonarow GC, Fogelman AM. High-density lipoprotein: is it always atheroprotective? Curr Atheroscler Rep 2006;8:405–411. [DOI] [PubMed] [Google Scholar]
- 47.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res 2004;95:764–772. [DOI] [PubMed] [Google Scholar]
- 48.Gaspard U. Hyperinsulinaemia, a key factor of the metabolic syndrome in postmenopausal women. Maturitas 2009;62:362–365. [DOI] [PubMed] [Google Scholar]
- 49.Rosenson RS, Brewer HB Jr, Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 2016;13:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959; Erratum in: J Am Coll Cardiol 2014;63:3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


