Abstract
Membrane phospholipid metabolism forms lysophospholipids, which possess unique biochemical and biophysical properties that influence membrane structure and dynamics. However, lysophospholipids also function as ligands for G protein-coupled receptors that influence embryonic development, postnatal physiology and disease. The two most well-studied species - lysophosphatidic acid (LPA) and sphingosine 1-phophosphate (S1P) are particularly relevant to vascular development, physiology and cardiovascular diseases. This review summarizes the role of LPA and S1P in vascular developmental processes, endothelial cell biology and their roles in cardiovascular disease processes. In addition, we also point out the apparent connections between lysophospholipid biology and the Wnt pathway, an evolutionarily-conserved fundamental developmental signaling system. The discovery that components of the lysophospholipid signaling system are key genetic determinants of cardiovascular disease has warranted current and future research in this field. As pharmacological approaches to modulate lysophospholipid signaling have entered the clinical sphere, new findings in this field promises to influence novel therapeutic strategies in cardiovascular diseases.
Graphical Abstract
A. Introduction
The endothelium is a dynamic and heterogeneous organ system that responds and adapts to various stimuli during embryonic development and postnatal homeostasis. During early development of the vascular system, endothelial cells (EC) undergo vascular network formation in response to hypoxic stimuli and cooperate with other developmental events in organogenesis. Arteries, capillaries, and veins, each with their repertoire of constituent cell types, undergo vessel-specific EC differentiation. Moreover, the vasculature of each organ system exhibits heterogeneity in structure and function, a phenomenon referred to as organotypic EC specialization. Even within the same vessel segment or vascular bed there is local heterogeneity in EC structure, gene expression and function. This allows for plasticity, adaptability and resilience of the vascular system to the changing environments that the organism encounters, thus providing optimal responses for vascular barrier function, tone (contraction/relaxation), inflammation and resolution, thrombus formation, directional blood flow, and the transport of molecules and cells between blood and tissues 1, 2.
The diversity of endothelial subtypes is in part determined by a multitude of cell surface receptors that respond to local and/or systemic factors. Well-characterized EC receptors include receptor tyrosine kinases such as the angiopoietin (Ang) receptor TIE2, vascular endothelial growth factor (VEGF) receptors VEGFR1-3, as well as a variety of G protein-coupled receptors (GPCRs). GPCR expression in individual ECs varies depending on the vascular bed, vessel type, flow and developmental context 3-6. Such GPCRs respond to a variety of ligands including small peptides (endothelins, bradykinin, neuropeptides, apelin), morphogenetic factors (Wnt proteins), proteases (thrombin, trypsin), extracellular matrix (ECM), chemokines, 17β-estradiol, mechanical forces, metabolites, protons (pH), and bioactive lipids (lysolipids, eicosanoids, etc.). In this review, we focus on lysolipid GPCRs, in particular those that respond to metabolites of the membrane phospholipids - sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA), as central orchestrators of vascular development and tractable therapeutic targets in cardiovascular, autoimmune and pathogen-induced diseases.
B. Lysolipids in the vascular system
S1P and LPA metabolism
S1P and LPA are the two well-characterized bioactive lysolipid species. These molecules have hydrophobic backbones and polar phosphate head groups, which makes them impermeant to cellular membranes. S1P is less water soluble than LPA, thus necessitating carrier proteins (termed chaperones) in extracellular compartments 7, 8. S1P circulates at concentrations of ~0.7 to 1 μM in the chaperone-associated form. Circulating LPA concentrations are much lower (estimated to be in the ten to hundreds of nM) (reviewed in 7, 8). The majority of circulating S1P (~65%) is bound to the Apolipoprotein M (ApoM) on high density lipoprotein (HDL) particles, while the remainder is associated with albumin 9, 10. In contrast, ~65% of LPA is associated with albumin while the remainder is predominantly HDL-bound 11, 12. Thus, HDL and albumin are the primary chaperones for both S1P and LPA. Notably, murine development and survival proceeds in the absence of both ApoM and albumin, whereas in such situations S1P associates with other macromolecules in vivo, such as ApoA4 13.
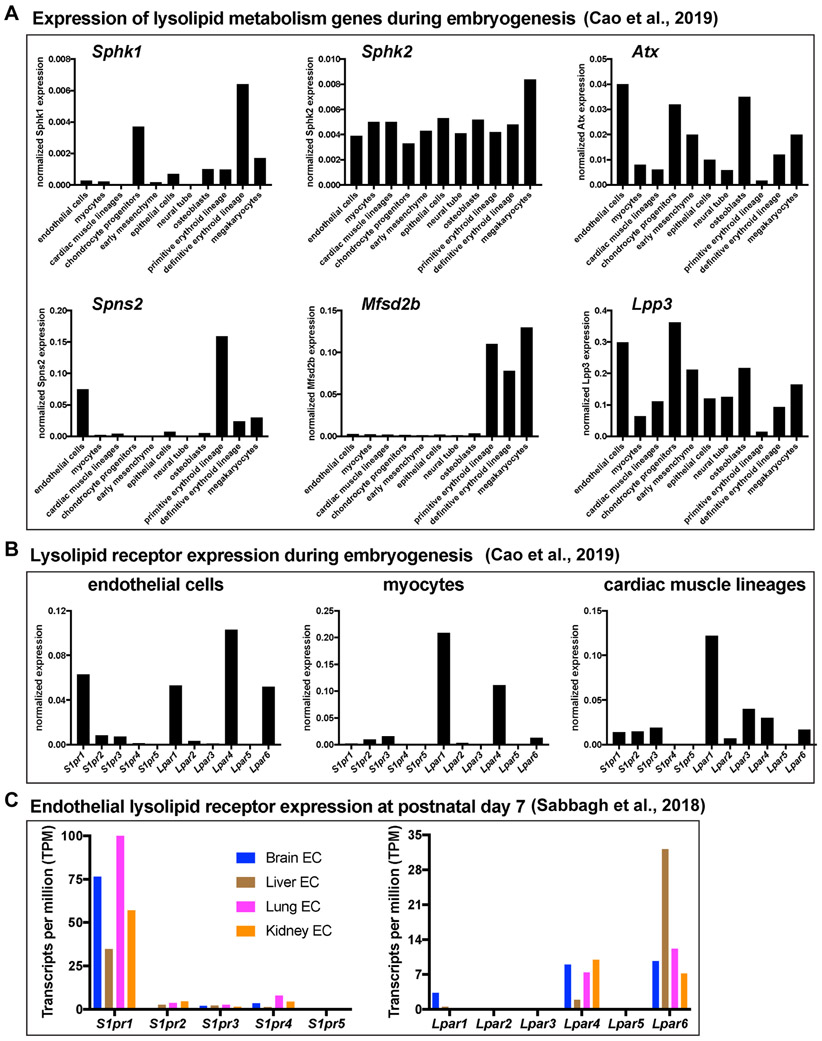
Albumin-bound S1P and LPA are short-lived in circulation (t1/2 < 20 min) 11, 14, 15 and their concentrations in blood are determined by substrate availability and the activities of metabolic enzymes 8. Two sphingosine kinase enzymes, SPHK1 and SPHK2, phosphorylate intracellular sphingosine to generate S1P 16, even though a fraction of secreted SPHK1 is present in plasma 17. Erythrocyte-mediated S1P export to blood by the major facilitator family transporter 2b (MFSD2B) accounts for the majority of circulatory chaperone-bound S1P 18. Meanwhile, S1P synthesized in blood and lymphatic vessel endothelial cells is transported to blood or lymph by another transporter - spinster 2 (SPNS2) 19-22. Endothelial SPNS2-mediated S1P transport accounts for ~10% of plasma S1P 23 and at least 80% of lymph S1P 21, 24, 25. Thus, S1P in lymph is primarily generated in lymphatic endothelium, whereas S1P in blood is primarily derived from red blood cells and, to a lesser extent, endothelial cells. Restricted expression of Spns2, Sphk1, and Mfsd2b in endothelial and/or erythroid cell types has been corroborated by single cell RNA sequencing from mouse embryos (Figure 1).
Figure 1. Expression of lysolipid metabolic and signaling genes during embryogenesis and in postnatal endothelium.
(A-B) Expression of genes encoding lysolipid metabolic enzymes or transporters (Sphk1, Sphk2, Lpp3, Mfsd2b, Spns2) (A) or receptors (S1pr1-5, Lpar1-6) (B) in selected cell types and embryonic structures. Single-cell (sc)RNA-seq data are from a publicly available database (https://oncoscape.v3.sttrcancer.org/atlas.gs.washington.edu.mouse.rna/landing) provided by the authors 210. (C) Expression of S1P and LPA receptors from RNA-seq of freshly-isolated EC from postnatal day 7 mouse brain, liver, lung, or kidney 6.
LPA synthesis is regulated by autotaxin (ATX), a secreted phospholipase D that removes the choline moiety from lysophosphatidylcholine to generate LPA 26. Though lysophosphatidic acids are derived from phospholipids of variable chain lengths, the most abundant circulating species in mammals is 18:1 oleoyl-LPA 26. A significant portion of circulating LPA is synthesized in blood upon ATX-catalyzed hydrolysis of circulating lysophospholipids, which are present in lipoproteins (LDL and VLDL) and other carriers 26. During embryonic development, ATX is widely expressed and exhibits particularly high expression in osteoblasts, chondrocyte progenitors, endothelium, early mesenchyme, and megakaryocytes (Figure 1).
The influence of diet and microbiome composition on sphingolipid levels and liver health is an active area of research. Dairy products and eggs are enriched with S1P precursors such as sphingomyelin 27. However, hydrolytic enzymes in the small intestine and colon, including sphingomyelinase, ceramidase, and glucoceramidase catabolize most ingested sphingolipids to free fatty acids and sphingosine. Nonetheless, sphingolipid-enriched diets have been shown to improve liver health, reduce lipid accumulation in tissue as well as circulating cholesterol in rodent models 28-30, as well as inhibit atherosclerosis 31. Bacteroides, the major genus in the human gut after weaning 32, expresses serine palmitoyltransferase enzymes that participate in de novo sphingolipid synthesis 33. Notably, bacterial-derived sphingolipids transfer to host gut epithelium and hepatic portal vein tissue 33. Links between gut microbiota, sphingolipids, dyslipidemia, and cardiovascular health warrant further investigation.
Vascular lysolipid receptors
S1P and LPA are high-affinity ligands that bind their respective receptors with apparent nanomolar dissociation constants 34-36. There are six known LPA receptors (LPAR1-6) 37 and five known S1P receptors (S1PR1-5) 38. Single-cell RNA-sequencing of mice at embryonic day (E)9.5 to E13.5 corroborated previous studies 3, 39-41 demonstrating that S1pr1 is abundantly expressed in ECs (Figure 1). S1pr2-5 are expressed at relatively low levels in ECs both during embryogenesis and postnatally (Figure 1). Among the S1PR-deficient mice that have been generated, only S1pr1-knockout (KO) animals die embryonically 41 (see Table 1).
Table 1.
Vascular phenotypes observed in mice with altered lysolipid signaling
| Mouse strain | allele description |
lethality | yolk sac | embryo proper |
developing retina vasculature |
Reference PMID |
|---|---|---|---|---|---|---|
| S1P receptors | ||||||
| S1pr1-KO | null allele | E14.5 | lacks blood, edematous | hemorrhage, pericardial cavity edema | - | 11032855 |
| EC S1pr1-KO | Tie2-Cre | E14.5 | lacks blood, edematous | hemorrhage, enlarged pericardial cavity | - | 12869509 |
| EC S1pr1-KO | Cdh5-CreERT2 | - | - | - | hypersprouting | 22975328 22975327 |
| EC S1pr1-Tg over-expression | Cdh5-CreERT2 | - | - | - | hyposprouting | 22975328 |
| S1pr2-KO | null allele | - | - | grossly normal | - | 15138255 |
| S1pr3-KO | null allele | - | - | grossly normal | - | 15138255 |
| S1pr1,2-KO | null alleles | E12.5 | - | hemorrhage, poor vascular networks | - | 15138255 |
| S1pr1,2,3-KO | null alleles | E11.5 | - | hemorrhage, poor vascular networks | - | 15138255 |
| S1pr1,2,3-KO | Rosa-CreERT2 | - | - | - | hypersprouting | 32059774 |
| EC S1pr1,2,3-KO | Cdh5-CreERT2 | - | - | - | hypersprouting | 32059774 |
| LPA receptors | ||||||
| Lpar1-KO | null allele | - | - | grossly normal | - | 14697676 |
| Lpar2-KO | null allele | - | - | grossly normal | - | 12215548 |
| Lpar3-KO | null allele | - | - | grossly normal | - | 15875025 |
| Lpar4-KO | null allele | E10.5-E18.5 (35%) | poor vascular network | hemorrhage | - | 20713964 31335323 |
| Lpar5-KO | null allele | - | - | grossly normal | - | 23039190 |
| Lpar6-KO | null allele | - | - | grossly normal | hyposprouting | 30804442 31335323 |
| Lpar4,6-KO | null alleles | E11.5 | poor vascular network, large vessels absent | poor vascular networks, pericardial effusion, developmental delay, axial turning defect | - | 31335323 |
| EC Lpar4,6-KO | Cdh5-CreERT2 | - | - | - | hyposprouting | 31335323 |
| S1P or LPA metabolic enzymes | ||||||
| Sphk1-KO | null allele | - | - | grossly normal | - | 15459201 |
| Sphk2-KO | null allele | - | - | grossly normal | - | 16314531 |
| Sphk1,2-KO | null alleles | E13.5 | - | hemorrhage | - | 16314531 |
| RBC Sphk1,2-DKO | EpoR-Cre | E13.5 | hypersprouting | hemorrhage | - | 25250575 |
| Atx-KO | null allele | E10.5 | poor vascular network, large vessels absent | poor cranial and cardiac vascular networks, neural tube defects, axial turning defect | - | 16829511 16782887 |
| EC Atx-KO | Tie2-Cre | - | grossly normal | grossly normal | grossly normal | Thesis link |
| Atx-Tg over-expression | CAG-Cre | E10.5 | poor vascular network, large vessels absent | developmental delay, neural tube defects | - | 25992708 |
| Atx-Tg over-expression | CAG-CreERT2 | - | - | hyposprouting | 25992708 | |
| Lpp3-KO | null allele | E6.5 (30%), E10.5 (70%) | poor vascular network, large vessels absent | axis duplication, hemorrhage | - | 12925589 |
| EC Lpp3-KO | Tie2-Cre | E8.5-E13.5 | poor vascular network | hemorrhage, cranial vascular defects, malformed branchial arch arteries, endocardial defects, trabeculation defects | - | 27125875 24504738 |
| GPCR signaling components | ||||||
| EC Rac1-KO | Tie2-Cre | E9.5 | poor vascular network, large vessels absent | numerous vascular abnormalities, branchial arch arteries absent | - | 18245172 |
| EC Rac1-KO | Cdh5-CreERT2 | - | - | hemorrhage | EC dense at vascular front, hypovascular nascent vascular network | 26872874 |
| EC RhoA-KO | Tie2-Cre | - | grossly normal | grossly normal | - | 31406143 |
| EC RhoA-KO | Cdh5-CreERT2 | - | - | - | grossly normal | 31406143 |
| EC Cdc42-KO | Tie2-Cre | E9.5 | poor vascular network, large vessels absent | hemorrhage, defective vascular lumens, EC polarity defects | - | 26253403 |
| EC Cdc42-KO | Cdh5-CreERT2 | inducible mid-gestation | - | hemorrhage, defective vascular lumens, EC polarity defects | EC dense vascular network, reduced tip cell filopodia | 26253403 |
| EC Gα13-KO | Tie2-Cre | E9.5-E11.5 | poor vascular network, large vessels absent | hemorrhage, developmental delay, pericardial dilatation, cranial vascular defects | - | 15919816 |
| Rock1,2-KO | null alleles | E8.5-E9.5 | poor vascular network, large vessels absent | axial turning defect, developmental delay | - | 21895889 |
Lpar1, Lpar4, and Lpar6 are expressed in embryonic cardiovascular cells including endothelial, smooth muscle, and cardiomyocyte lineages (Figure 1). Among these cell types, Lpar2 and Lpar5 are not significantly expressed and Lpar3 expression is limited to cardiac muscle lineages. While mice lacking any single LPAR can survive to term, only Lpar4−/− mice exhibit partially penetrant embryonic lethality as a result of defective vasculature 7, 42. This phenotype is fully penetrant and more severe in Lpar4−/−Lpar6−/− double-KO mice 43, suggesting that these receptors have redundant functions during embryogenesis.
S1P and LPA regulate vascular development
Discrete functions of S1P and LPA receptors are attributable in part to their unique distribution, ligand availability, heterotrimeric G protein coupling, and activation of Rho family small GTPases (reviewed in 7, 36). Endothelial S1PR1 expression and signaling through Gi/Rac is restricted to perfused, S1P-containing vessels and is essential for vascular stabilization and inhibition of VEGF-induced hypersprouting 23, 39, 40, 44, 45 (Figure 2). In the absence of endothelial S1P signaling, most, if not all, developing vascular networks exhibit excessive sprouts, branches and hemorrhagic areas that are incompatible with life after embryonic day (E)14.5. Developing capillaries and veins in S1PR-deficient retinas fail to express specialized components of neurovasculature and instead up-regulate migration- and tip cell-associated genes, such as Esm1, Angpt2, and Apln 45. These findings suggest that S1PR signaling is needed for vascular stability, patterning and organotypic specialization during organ development through pathways conserved from tight coevolution which integrate signaling fitness, mechanical and metabolic inputs46.
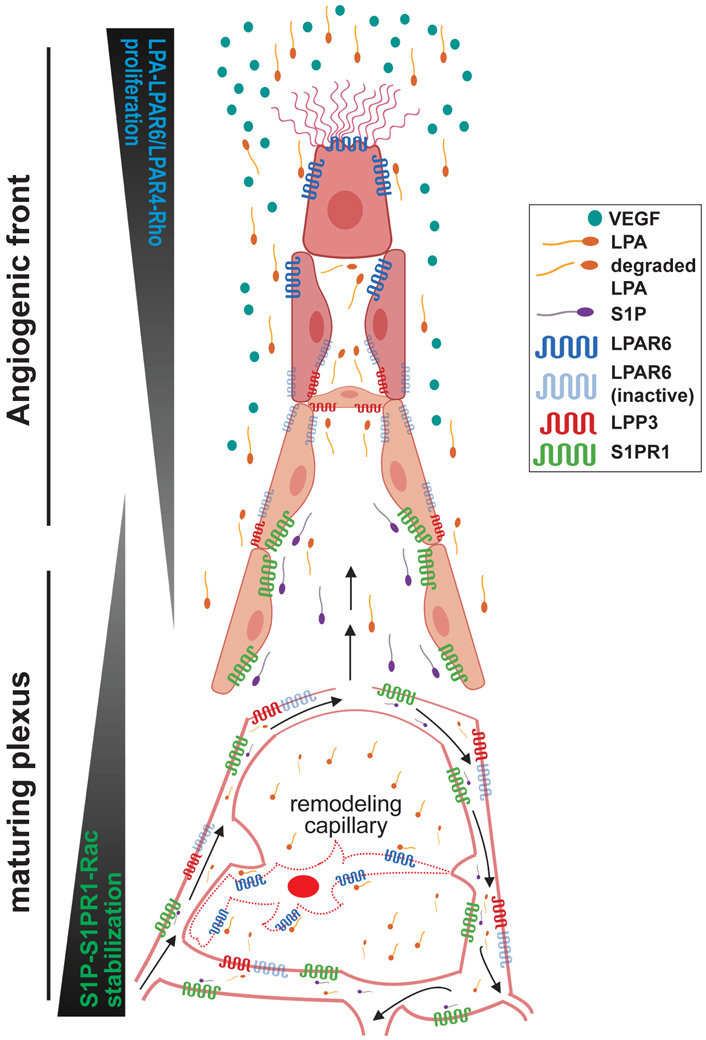
Figure 2. Schema of LPA and S1P signaling during retinal sprouting angiogenesis.
The angiogenic front of a developing vascular plexus, composed of ~6-10 “rows” of endothelial cells (EC), is surrounded by high levels of VEGF 1. Here, LPA activates EC LPAR6 (and possibly LPAR4), promoting proliferation and migration downstream of Gα12/13 and Rho family GTPases in cooperation with VEGF receptor signaling. S1PR1 expression is relatively low in these EC.
Cell-cell contacts are stronger in more mature vascular regions and are speculated to be enriched with active LPP3, which degrades LPA and limits EC LPAR signaling. Meanwhile, as these maturing vascular regions acquire S1P from flowing blood (black arrows), EC express high levels of S1PR1. S1P-S1PR1 signaling contributes to vascular stability (e.g. stabilization of adherens junctions) and permits organotypic vascular specialization in the retina. These S1PR1-mediated events are likely downstream of RAC1 activation.
Growing vascular networks undergo remodeling to optimize tissue perfusion (black arrows). We speculate that poorly perfused EC are “selected” for pruning in part because they become deficient in S1P-S1PR1 signaling. Resultant vascular instability decreases cell-cell adhesions and reduces LPP3 activity, thereby creating micro-domains of LPA that activate LPAR6 on remodeling EC to promote incorporation into well-perfused capillaries.
Unlike S1P/S1PR1 signaling, which is generally perfusion-dependent for receptor expression and ligand availability, LPA appears to be more constitutively available and particularly important for LPAR signaling in ECs of non-perfused vascular sprouts 43, 47, 48, 49 (Figure 2). LPAR4 and LPAR6-mediated activation of Gα12/13 / Rho GTPase is required for endothelial proliferation, vascular branching and network expansion 43, 47, 50. These findings suggest that S1P signaling is engaged in ECs undergoing integration within the endothelium, maturation and stabilization, whereas LPA signaling occurs in ECs undergoing migration or proliferation. In addition, LPA signaling appears to be involved in vessels undergoing regression 48.
Accumulating evidence demonstrates that the integral membrane protein lipid phosphate phosphatase 3 (LPP3) inhibits endothelial LPAR signaling specifically at cell membrane regions that participate in cell-cell contact 43, 47, 50-53, thereby restricting LPAR signaling to non-contact sites 50. During vascular development, ECs deficient in cell-cell contacts are found primarily in blind-ended sprouts 54 and remodeling vessels 55 (Figure 2). LPP3, a lipid phosphate phosphatase with an extracellular mode of action, is likely an essential cell-autonomous regulator of EC LPAR signaling because EC-specific LPP3-KO embryos die by E11.5 with severe vascular defects in extraembryonic vasculature and in the embryo proper 51, 56. Some of these defects may be a consequence of LPP3 loss of function destabilizing β-catenin which has been demonstrated to vary with cell density57. Since ß-catenin is a key intracellular regulator of adherens junctions and Wnt signaling pathways, this nexus may be the point at which lysolipid and Wnt signaling pathways intersect.
S1P signaling in endothelial cells
In human umbilical vein endothelial cells (HUVECs), S1P binding to S1PR1 induces Ca2+ mobilization, activation of the GTPase Rac, actin polymerization of the cortical cytoskeleton, and adherens junction assembly 58, 59. Concomitantly, S1PR1 inhibits adenylyl cyclase activity by coupling to Gαi 58, thus reducing intracellular cAMP levels. Other downstream targets of S1PR1 activation include phosphoinositide 3-kinase (PI3K)/Akt activation, phospholipase C (PLC) activation and increased phosphorylated Src (p-Src) 36, 60.
Vascular S1P signaling results in pleiotropic yet protective endothelial cell changes. S1PR1-mediated actin rearrangement and adherens junction assembly increases endothelial barrier function in vitro and is likely a key mechanism by which S1PR1 enhances vascular integrity and perfusion in lung, trachea, and retina tissues 39, 45, 61-65. In the postnatal brain, endothelial S1PR1 limits leakage of small (< 3 kDa) molecules 66 and also functions in neural progenitor and glial cells to inhibit postnatal hemorrhage in germinal matrix 67. In large arteries, the S1PR1/Gαi/Akt axis regulates blood pressure and vascular tone via activation of endothelial nitric oxide synthase, which produces NO to induce vasorelaxation 65, 68-70. During embryonic development, the S1PR1/Rac signaling axis is essential as either S1P deficiency, EC-specific S1PR1 deficiency, or EC-specific Rac1 deficiency results in severe vascular defects and embryonic lethality 16, 39, 40, 44, 45, 71, 72.
S1P-deficient embryos lacking sphingosine kinases (Sphk1−/−Sphk2−/−) exhibit severe hemorrhage, dilated blood vessels, and do not survive after E13.5 16. Similarly, global or EC-specific S1pr1 deletion results in severe hemorrhage, disruption of adherens junctions, and hyper-branching of distal vascular beds (e.g. brain and retinal vessels) and major proximal arteries (e.g. dorsal aorta), which is not compatible with life after E14.5 39, 40, 44, 45. These animals also exhibit widespread vascular hypersprouting, which is characterized by increased tip cell frequency and filopodia density in developing vasculature consistent with a failure to eliminate excess cells 39, 40, 45. Deletion of Sphk1 in the erythroid lineage of Sphk2−/− mice revealed that red blood cells generate ~95% of the S1P content in embryonic tissue, which is required for survival after E13.5 23. In addition to severe hemorrhaging and vascular malformations in the head and aorta, yolk sacs of these S1P-deficient embryos exhibited disorganized, hyper-branching capillary networks 23. Maternal administration of the S1PR1 agonist SEW2871 rescued lethality in embryos lacking RBC-derived S1P, further demonstrating the essential role of S1PR1 in developmental S1P signaling 23. However, these embryos lack Sphk2 in platelets, and more recent analysis suggested that RBC and platelets have redundant functions as suppliers of embryonic S1P needed for proper vascular development 73.
Detailed insights into EC S1PR signaling have been obtained using the retina as a model of vascular network formation and maturation. Over the first 9 days of postnatal murine life, vessels grow radially and form a network of arteries, capillaries, and veins extending to the retina periphery. In perfused vessels of the nascent vascular network, S1P/S1PR1 signaling promotes endothelial maturation and adherens junction assembly 39, 40, 45. ECs at the angiogenic front, including tip cells of blind-ended sprouts, are very different from those in the nascent network as they are poorly perfused and lack S1PR1 expression 39, 40, 45. Angiogenic front ECs engage in VEGFR signaling which drives expression of JunB, c-Jun, and other “tip cell genes” that contribute to EC proliferation, migration, full integration into the endothelial monolayer and proper patterning 6, 45, 74-76. Evidence suggests a mechanism by which S1PR-dependent VE-cadherin assembly promotes endothelial maturation in the nascent vascular network through adherens junction assembly as well as suppression of AP-1 45 and FOXO1 77 transcription factors.
In addition, EC S1PR signaling supports vascular maturation through positive regulation of proteins critical for neurovascular specialization (discussed below) 6, 45, 78-80. Concomitantly, EC S1PR signaling suppresses expression of migration-promoting genes often observed in tip cells, including Esm1, Igfbp3, and Angpt2 6, 74-76, 81. Ectopic expression of tip cell genes in the nascent network goes hand-in-hand with hypersprouting 39, 40, 45, 75. Inversely, inducible S1PR1 over-expression suppressed vascular sprouting and tip cell frequency, resulting in hypovascularization 39. Genetic mosaic studies showed that S1PR1-expressing ECs tend to incorporate into the mature regions of the vascular network rather than adopt a tip cell position 40, further supporting the notion that S1PR1 facilitates vascular maturation in a context dependent cell-autonomous manner.
Expression of Notch pathway components and target genes, which also inhibit hypersprouting 75, was unaffected in S1PR-KO retinal ECs 39, 40, 45. In addition, aortic hyperbranching was observed in S1pr1−/− and EC-specific S1PR1-KO embryos but not in Dll4+/− or EC-specific Rbpj-KO embryos. These distinct outcomes downstream of S1P or Notch inhibition suggest that these ligands inhibit hypersprouting through discrete mechanisms 39, 40, 45. However, S1PR and Notch signaling pathways may intersect and/or cross-regulate each other in additional biological contexts, such as biomechanical signaling 82, 83. This framework may also be understood in the context of interactions between different cell competition pathways, but these hypotheses are only now beginning to be tested46, 84.
LPA signaling in vascular development
Endothelial responses to LPA and S1P are very different, if not in direct opposition. In HUVECs, LPA signals through LPAR6 and rapidly induces actin stress fibers that decrease cell-cell adhesion and cause intercellular gaps 50, 85. After binding LPA, LPAR6 couples with Gα12/13 and activates the GTPase RhoA and its target Rho kinase (ROCKI/II) 50, 85. This is the mechanism by which LPA reduces endothelial barrier integrity and promotes vascular leak 50, 56, 85. Inhibition of any individual component in LPA signaling, including LPAR6, Gα13, RhoA, or ROCK abrogates LPA induction of stress fibers 50, 85. Like LPAR6, LPAR4 activates the Gα12/13/Rho/ROCK pathway 35, but can also couple with Gq/11, Gi/o, and Gs 35. Overlapping signaling components downstream of LPAR4 and LPAR6 in embryonic endothelium may contribute to their functional redundancy during embryogenesis 43. Indeed, all Lpar6−/− mice and most Lpar4−/− mice survive to term, but all Lpar4−/−Lpar6−/− double-KO mice die by E10.5 due to vascular defects 42, 43. LPA-deficient Atx−/− mice, EC-specific Gα13-knockout, and Rock1−/−Rock2−/− mice each die embryonically with impaired vascular development 86-90, suggesting that these genes encode essential components for EC LPAR signaling. Endothelial RhoA, however, is dispensable as EC-specific RhoA-knockout animals develop normally 91, suggesting that EC LPARs signal through one or more of the ~18 Rho GTPases expressed in endothelium 92.
Phenotypes associated with Lpar4−/− embryos include pericardial effusion, severe edema, general fragility, subcutaneous hemorrhage, and lethality (~35% penetrant) 42. Lpar4−/−Lpar6−/− phenotypes include pericardial effusion, severe developmental delay, poor vascular network formation in the head and intersomitic regions, absence of blood vessels in the yolk sac, and death by E10.5 (100% penetrant) 43. Both global and EC-specific Gα13-KO mice die between E9.5 and E11.5 and fail to form normal yolk sac vasculature 86, 87. This phenotype was also seen in Rock1−/−Rock2−/− embryos, which die between E8.5 and E9.5 88. Thus, the LPAR/Gα13/ROCK signaling axis is essential for early vascular development.
In contrast to the hypersprouting phenotypes in S1PR1-deficient retinas, EC-specific LPAR4/LPAR6 double-KO (Lpar4:Lpar6iΔEC) mice exhibit hyposprouting with reduced vascular density and branching 43. Tip cells in Lpar4:Lpar6iΔEC retinas were few in number and exhibited defects including reduced filopodia length and frequency 43. This hypovascular phenotype was reproduced in Lpar6−/− retinas, suggesting that LPAR4 and LPAR6 are not entirely redundant during sprouting angiogenesis and that LPAR6 may be the primary mediator of LPA-induced EC proliferation during retinal development 47.
ATX is widely expressed during embryogenesis and is essential for embryonic development 89, 93. Atx−/− mice are LPA-deficient and have vascular defects in the embryo proper, lack yolk sac vasculature and die from circulatory failure by E10.5 89, 90. These phenotypes were reproduced in embryos harboring a biallelic single amino acid substitution rendering ATX catalytically inactive, suggesting that enzymatic formation of LPA (or a related molecule) is the primary defect 94. This is in stark contrast to LPP3, for which there is substantial in vitro and in vivo evidence for an EC-autonomous LPA/LPP3/LPAR axis that controls the sub-cellular location of LPAR signaling.
Encoded by the gene Ppap2b, LPP3 is a glycoprotein with a channel-like structure composed of six putative transmembrane domains 95. LPP3 is best known for catalyzing dephosphorylation of phosphatidic acid, ceramide 1-phosphate (C1P), S1P, and LPA to generate diacylglycerol, ceramide, sphingosine, and monoacylglycerol, respectively 95. Biochemical analysis showed that human LPP3 has highest affinity (1/Km) and catalytic efficiency (Vmax/Km) for LPA and PA, whereas these values were 3-4 times lower for S1P and C1P 96. Reduction of LPP3 activity can result in local and/or systemic accumulation of LPA, phosphatidic acid, C1P, and S1P 97. For example, cardiac-specific LPP3-KO mice harbor ~3-fold higher [LPA] in circulation relative to wild-type counterparts 98.
Lpp3 is expressed in many cell types and structures during embryogenesis including, but not limited to, endothelial, cardiac, vascular, nervous, and mesenchymal tissues 99 (Figure 1). Both global and EC-specific LPP3-KO mice die by E10.5 and exhibit hemorrhage of the embryo proper, defective yolk sac vasculature, and failure to form a chorioallantoic placenta 51, 99.
In HUVECs, LPP3 appears to partition LPAR signaling between regions of strong and weak cell-cell contact 43, 47, 50-53. LPP3 knockdown enhanced sensitivity to LPA-induced stress fibers 50. This LPA response was more robust in sub-confluent HUVECs, indicating that cell-cell contacts are involved in inhibition of LPAR signaling. After treatment with forskolin to enhance cell-cell adhesion, LPP3 localized to sites of cell-cell contact and the LPA-induced stress fiber response was abolished, suggesting cross talk with cAMP-regulated signaling pathways 50. However, forskolin-treated HUVECs deficient in LPP3 were sensitive to LPA, suggesting that LPP3 inhibits LPAR signaling in ECs with strong cell-cell adhesion 50. In scratch assays, LPP3 localized to sites of cell-cell contact but was absent from non-contact sites in “leader cells” at the monolayer edge 50. The leading edges (non-contact sites) of these cells showed robust stress fiber responses after LPA treatment 50, suggesting that LPAR signaling is permitted in membrane compartments that lack cell-cell contact and therefore also lack LPP3 activity. Studies using LPP3-KO mouse embryonic ECs 51 or LPP3-KO human aortic ECs (HAECs) 52 reported that LPP3 promotes adherens junction assembly 51, 52 and inhibits intercellular gap formation 52. LPP3-deficient HAECs showed reduced barrier function 53, suggesting that LPP3 promotes endothelial barrier function, perhaps by attenuating LPA-dependent Rho GTPase activation.
These results demonstrate that LPP3 can localize to sites of endothelial cell-cell contact and inhibit LPA/LPAR signaling, restricting high levels of LPAR signaling to non-cell-cell contact sites. Non-contact sites of EC membranes have important migration-associated functions in tip cells and remodeling capillaries undergoing regression 55, a process promoted by LPA/LPAR signaling 50 (Figure 2).
EC-specific LPP3-KO (LPP3-ECKO) embryos die at or before E10.5 with hemorrhagic areas in the embryo proper, abnormalities of the aortic sac, outflow tract, irregular intersomitic vasculature, defective yolk sac vasculature, failure to form a chorioallantoic placenta, as well as decreased cardiac trabeculation and growth of the compact myocardial wall 51, 56, 99. These findings suggest that LPP3 function leads to spatial restriction of lysolipid receptor signaling to regulate vascular development (Figure 2).
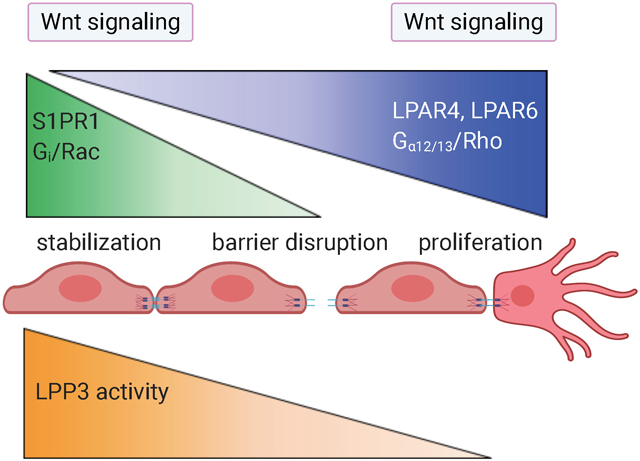
In summary, mechanistic studies that reveal the in vitro differences between S1P and LPA signaling explain, at least in part, the in vivo functions of these lysolipids. S1P signaling-deficient mice exhibit vascular hypersprouting, which is characterized by hyper-branching, disorganized vascular networks that are poorly perfused and lack barrier integrity. In contrast, LPA signaling-deficient embryos exhibit vascular hyposprouting with notable absence or apparent lack of blood vessels in some tissues, particularly in the yolk sac. Conversely, S1P1/Gi/Rac signaling promotes cell-cell contact, adherens junction assembly, and vascular stability (Figure 2).
Whether endothelial LPARs exhibit spatial expression gradients during vascular development remains to be determined. Our understanding of in vivo LPAR and S1PR distributions, including sub-cellular localization, has been limited by the lack of widely available high quality antibodies and the technical challenges associated with precise measurement of lysophospholipid gradients in complex tissues100.
C. Developmental studies suggest lysolipid and Wnt connections
As outlined above, many of the core processes influenced by lysolipid signaling have also been observed to be regulated, to some extent, by the central pathways of early development; Notch, BMP and in particular Wnt signaling. This is interesting given the dependence of Wnt signals on lipids and lipoproteins101, and the intricate relationships between Wnt signals, cell polarity, intercellular junctions, cytoskeletal structure and metabolism84, 102, 103. Wnt signaling’s role in central decisions is illustrated by the diverse effects of mutations in Wnt pathway genes during development and in disease104. Importantly, there are often parallels between the effects of Wnt gain of function and loss of function which suggest a tuning of a wide range of signaling pathways, though in many instances this may simply reflect our limited understanding of the relevant biology102, 105. There are several key downstream effectors of Wnt including the β-catenin destruction complex, the planar cell polarity pathway and a complex non-canonical pathway which appears to modulate the multifaceted effects of calcium signals throughout the cell102, 104.
Specific lipids and lipoproteins are required for the transmission of Wnt signals between cells, and inability to package Wnt ligands in appropriate lipoprotein essentially abolishes the downstream effects in receiving cells101. Classical Wnt effects are restricted to only a few cell diameters in range, but recent evidence of signaling roles for circulating lipoproteins in higher vertebrates raises the possibility that Wnt signals may also operate at a distance106, 107. The lipid requirements for Wnt signal transduction are poorly understood, but specific lipids or lipoproteins may be necessary for endocytosis in the receiving cell of the ligand-receptor/co-receptor complexes and/or subsequent gating of the signals by intraorganelle pH or other factors103, 108-110. The parallels between lysolipid pathways and Wnt in development warrant further studies to elucidate underlying mechanisms. Some recently-described mechanisms are discussed in detail below.
S1P and Wnt signaling in blood-retina-barrier development
Like lysolipid signaling, Wnt signaling is transduced by a family of receptors with seven transmembrane domains, some of which have been shown to couple to heterotrimeric G proteins 111. The protein Norrin (Ndp), a TGF-β family member produced by glia, is a high-affinity Wnt-like ligand for its EC receptor Frizzled4 (FZD4) 112. Norrin/FZD4 signaling increases the activity of β-catenin and TCF/LEF transcription factors (TFs) 113, which leads to induction of proteins that promote a functional blood-retina-barrier (BRB) and suppression of proteins that cause vascular leakage or fenestration 113, 114. The developing BRB becomes dysfunctional upon loss of Norrin, or EC-specific deletion of Fzd4, Cttnb1 and S1pr1 39, 40, 45, 113, 114. There are shared albeit distinct outcomes of endothelial S1P/S1PR and Norrin/FZD4/β-catenin signaling in BRB development, which suggests both convergent and divergent signaling, possibly as a function of underlying metabolic states, microenvironmental or humoral factors103.
Wnt-deficient retinas are hypovascular 113-116 whereas S1PR1 signaling-deficient retinas are hypervascular. Thus, Wnt signaling promotes EC proliferation and vascular branching 116 while S1P signaling stabilizes blood vessels 39, 40, 45. An in vivo reporter of canonical Wnt signaling was active throughout the developing retinal vascular ECs 80, suggesting that β-catenin and TCF/LEF factors are active in blind-ended vascular sprouts as well as in ECs undergoing maturation. Whether Wnt signaling is involved in expression of tip cell genes at the vascular front is not known. However, there is evidence suggesting that both Wnt and S1P signaling suppress a tip cell gene expression program in the nascent vascular network.
Deficiency of either Wnt or S1P signaling results in vascular leakage and hemorrhage 39, 45, 113, which can increase the concentration of VEGF and activation of VEGFRs. In fact, EC VEGFR signaling is required for expression of the prototypical tip cell genes Esm1, Apln, and Igfbp3 74, which are induced in retinal ECs that lack Wnt or S1P signaling (Figure 3). While tissue hypoxia and high VEGF might contribute to ectopic expression of tip cell genes in Ndp-KO and S1pr-KO retinal ECs, it is also possible that VEGFR-independent mechanisms contribute to S1P- and Wnt signaling-mediated suppression of these genes in the nascent vascular network. Indeed, tissue hypoxia might be anticipated to interrupt Wnt signaling, possibly explaining some of the selective phenomena observed103, 109.
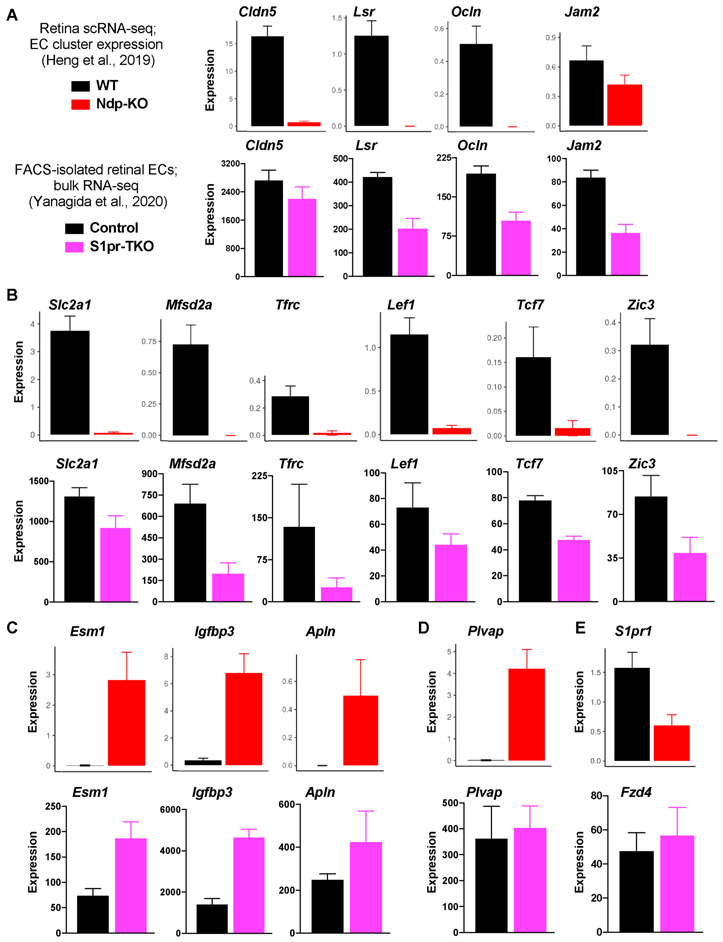
Figure 3. Gene expression in Norrin-deficient and S1PR-deficient retinal endothelial cells.
(A-E) Expression of selected genes in Norrin knockout (Ndp-KO) and WT retinal EC were acquired from https://jacobheng.shinyapps.io/cnshypoxia/ 117 . For control and S1PR-deficient retinal EC, data were acquired from GEO accession GSE14144045. (A-B) Expression of neurovascular-enriched transcripts that encode tight junction components (A), transporters or transcription factors (B). (C) Expression of genes that are enriched in tip cells. (D) Expression of Plvap. (E) S1pr1 or Fzd4 expression in Ndp-KO or S1PR-deficient retinal EC, respectively.
Yanagida et al. (2020) reported that 97 neurovasculature-enriched transcripts were down-regulated in S1PR-deficient retinal ECs 45. Ndp-KO retinal ECs also down-regulated many of these transcripts (Figure 3A,B) 117. For example, both Wnt and S1P signaling are required for normal expression of tight junction components (Lsr, Ocln), transporters (Mfsd2a, Tfrc), and transcription factors (Lef1, Tcf7, Zic3) that are enriched in the vasculature of the central nervous system (CNS) 45 (Figure 3). Expression of Cldn5 and Plvap, well-characterized targets of Wnt signaling in the BRB, were not significantly affected in S1PR signaling-deficient retinal ECs (Figure 3A,D) 45. Therefore, S1P and Wnt signaling regulate common and distinct gene sets that determine neurovascular structure and function. For example, suppression of Plvap (fenestrae) and induction of Cldn5 (tight junctions) are likely two mechanisms by which Wnt signaling, but not S1P signaling, promote BRB integrity (45 and Figure 3). However, S1PR1 signaling is upstream of CLDN5 expression in lymphatic ECs during developmental lymphangiogenesis 118. Thus, S1PR1 and other EC surface receptors do not exhibit a “one-size-fits-all” model of signaling and transcriptional outputs, but rather have unique functions according to developmental stage, vascular bed, microenvironmental context, and EC subtype.
Any epistatic relationship between endothelial Wnt and S1P signaling is anticipated to be multifaceted and insights will likely require analysis of novel mouse strains102, such as Ctnnb1flex3/+;S1p1f/f;Cdh5-CreERT2, which would address whether increasing β-catenin activity is sufficient to rescue maturation defects in S1PR1-KO retinal ECs. The Ctnnb1flex3 allele encodes a β-catenin protein that resists degradation and is sufficient for induction of a BRB-like endothelial phenotype (MFSD2A+, LEF1+, CLDN5+, PLVAP-) in the vasculature of circumventricular (e.g. neuroendocrine) organs 80. Additionally, R26-8xTCF/LEF-LSL-H2B-GFP;S1p1f/f;Cdh5-CreERT2 mice would likely address the effect of EC-specific S1pr1-knockout on β-catenin and TCF/LEF transcriptional activity in retinal ECs 80.
Mosaic deletion of Fzd4 in BRB and BBB vasculature showed that induction of CLDN5 and suppression of PLVAP occurs in a FZD4-dependent, cell-autonomous fashion and is unlikely secondary to hypoxia or high VEGF levels 80. Similar mosaic experiments in S1PR signaling-deficient mice might reveal cell-autonomous S1PR versus hypoxia-dependent effects on expression of BRB-enriched genes such as Mfsd2a, Tfrc, and Lef1. Alternatively, fms-like tyrosine kinase-1 (sFlt1) that blocks VEGF signaling maybe a useful reagent to investigate the role of this pathway in Wnt- and S1P signaling reporter mice. Because VEGF itself can induce vascular leak 119, adherens junctions (VE-cadherin) should also be examined to determine the role of aberrant VEGF signaling in junctional breakdown observed in S1PR-KO retinal vasculature 39, 40, 45. These experiments may provide novel mechanistic insights into S1PR- versus VEGFR-mediated gene expression and vascular function during BRB development in the context of Wnt signaling.
LPP3, Lysolipids and Wnt signaling in gastrulation and axial patterning
Some Lpp3−/− embryos exhibited defective gastrulation with axis duplication at E7.5 (30% penetrance), a phenotype reminiscent of ectopic Wnt signaling 99. Over-expression of Xwnt3a or Xwnt8 induces axis duplication in Xenopus embryos 99, as does over-expression of Cwnt8C 120 or ablation of the Wnt signaling inhibitor Axin 121 in mouse embryos. At E7.0, expression of brachyury, a WNT3 target gene, is restricted to the primitive streak 99; however, Lpp3−/− embryos with axis duplication have two brachyury-expressing primitive streak structures 99. In addition, expression of the Wnt signaling antagonist Dkk1 is markedly reduced in Lpp3−/− embryos 99. Importantly, axis duplication induced by injection of Xwnt3a or Xwnt8 mRNA was inhibited by co-injection with mouse Lpp3 mRNA, demonstrating that LPP3 can inhibit signaling by these Wnt ligands 99.
There are several open questions related to LPP3-mediated axial patterning, including whether specific lysolipid receptors are involved. To date, axis duplication has not been reported in S1PR- or LPAR-deficient mice, suggesting that LPP3-mediated axial patterning does not require signaling by individual lysolipid receptors. This notion is supported by insights from Drosophila, which lack orthologs of vertebrate G protein-coupled lysolipid receptors but express two LPP3 homologs, wun and wun2 (Wunens) that are essential for gastrulation 95, 99. In somatic cells, wun and wun2 produce phospholipid metabolites that serve as guidance cues by repelling germ cells (GCs), a process that is required for bilateral sorting of GCs away from the ventral midline 95, 122. Wunen-deficient embryos have scattered GCs and high frequency of GC death 123. Interestingly, a Drosophila GPCR called Tre1 is required for GC migration towards high concentrations of Wunen-generated phospholipids in a Gαo-dependent manner 124. In support of the notion that Tre1 binds Wunen metabolites, the human Tre1 homolog, GPR84, binds medium chain fatty acids 125. Thus, Tre1 might be the primary receptor for Wunen-generated phospholipids, though this would require confirmation by receptor signaling assays.
Tre1, in addition to guiding GC migration, is required for Rho-mediated protein polarization in GCs 124. Drosophila Tre1 may have conserved functions that are split among multiple proteins in vertebrates 124. The presence of compensatory mechanisms in vertebrates may explain why defective gastrulation is ~30% penetrant in Lpp3−/− mice but 100% penetrant in Wunen-null Drosophila embryos. In addition, the phosphatase activity of wun2 is essential for GC survival 126. Human LPP3 can rescue GC death in wun2-deficient embryos, suggesting an evolutionarily conserved phosphatase function for LPP3 in gastrulation 126.
Evidence to date strongly suggests that the phospholipids metabolized by LPP3 must be spatially compartmentalized to ensure normal axial patterning. Given that lipoprotein-derived precursors feed into lysolipid metabolic pathways, LDL, VLDL and other lipoproteins may be involved in spatial control of lysolipid signaling. However, identification of the specific lipids, the means of compartmentalization and clarification of downstream signaling mechanisms all remain a major challenge. Isolation of these molecules will provide mechanistic insight into Wunen and LPP3-mediated axial patterning, cell survival, and may also reveal how phospholipids regulate Wnt signaling in vertebrates and/or invertebrates127. A detailed mechanistic understanding of embryonic Wunen and LPP3 metabolites and cognate receptors may inform exploration of LPP3-mediated endothelial functions during development, postnatal homeostasis and disease.
D. Lysolipids in cardiovascular disease
Insights from human studies
In the 1970s, landmark studies reported negative correlations between coronary artery disease (CAD) severity and circulating HDL levels 128-130. Subsequent biochemical analyses of HDL particles have uncovered significant heterogeneity 131. For example, on a stoichiometric basis, 1 in 10 HDL particles contains S1P 132. Even though only a minority of studies in the HDL field have focused on lysolipids, recent work has linked HDL-S1P to CAD. For example, HDL-S1P, 1) correlates inversely with the severity of coronary atherosclerosis 133, 2) is an independent predictor of coronary in-stent restenosis 134, 3) is lower in patients with stable CAD than in healthy individuals 135,136, and 4) correlates negatively with the occurrence of CAD independently of HDL-cholesterol 136 (reviewed in 137). In HUVECs, HDL isolated from CAD patients was ineffective at stimulating S1PR1-dependent vasoprotective signaling events, including vasodilation, which was rescued by providing exogenous S1P 138.
Lipoprotein(a), which is highly predictive of cardiovascular dieases in humans, was shown to supply autotaxin and therefore involved in local signaling of LPA via its receptors. In calcific aortic valve disease, lipoprotein(a)-derived autotaxin directly induces valve fibrosis and calcification, presumably via a Rho GTPase signaling pathway139, 140. Inhibitors of this signaling axis may be useful in the medical management of aortic valve diseases.
S1PR1 genomic heterogeneity may contribute to cardiovascular disease risk. For example, SNPs in the N-terminal cap region of S1PR1 were associated with multivessel cardiovascular disease in a patient cohort, suggesting a potential regulatory function of this receptor141. Asthma is characterized by a chronic inflammatory process with increased vascular permeability. Several SNPs (rs2038366, rs3753194, rs59317557) in the putative enhancer and promoter regions of S1pr1 associate with increased risk of asthma development 142. The SNP rs2038366 was notable for conferring S1pr1 downregulation by luciferase assay in human pulmonary artery ECs 142.
S1PR1: Pharmacologic considerations
The FDA-approved S1PR functional antagonist FTY720 (fingolimod) is prescribed as an immunomodulatory agent to treat relapse-remitting multiple sclerosis 143. This drug’s mechanism of action is down-regulation of lymphocyte S1PR1 in lymphoid tissue, which sequesters lymphocytes by preventing chemotaxis towards high [S1P] in circulation 144. While this lymphocyte-targeted drug has clear immunological benefits, endothelial S1PR1 is also being explored as a therapeutic target in autoimmune and fibrotic conditions. Studies using experimental disease models (discussed below) suggest that endothelial S1PR1 agonism mitigates inflammation through at least two mechanisms: 1) enhancing the vascular barrier and 2) attenuating endothelial inflammatory responses, each limiting leukocyte recruitment to tissue parenchyma 145.
The notion that pharmacologic or endogenous agents elicit varying degress of “biased” engagement of S1PR1 with either Gi/Rac or β-arrestin pathways has both clarified and added complexity to our understanding of S1P signaling 38, 106, 146. For example, arterial ECs of the thoracic aorta express S1PR1 mRNA 147 and protein 106 in a homogenous manner and are exposed to circulatory S1P. However, S1PR1/β-arrestin coupling is heterogenous in aortic arterial endothelium of adult mice 106,147, and the frequency of S1PR1/β-arrestin coupling increases with temporal transition from early postnatal to young adult, which coincides with upregulation of thrombospondin-1 106,147. Despite high expression and the functional importance of S1PR1 in early postnatal mouse retinal ECs, we observe relatively low levels of S1PR1/β-arrestin coupling in these cells (unpublished observation). In contrast, developing embryonic lymphatic vessels show high levels of S1PR1/β-arrestin coupling 118. While these data suggest a spectrum of S1PR1/β-arrestin signaling among ECs, we lack in vivo evidence for such a spectrum of S1PR1/Gi signaling. We can hypothesize that cells highly engaged in β-arrestin signaling are relatively low in S1PR1/Gi activity. Endogenous mechanisms that skew S1PR1 towards β-arrestin versus Gi are unclear, but may involve concomitant signaling pathways, such as LPARs 148, VEGFRs, junctional signals, shear force responses, or S1PR1 association with presently unknown cofactors. A prototypical example of cofactor-dependent signaling in vascular endothelium occurs in the CNS, when ECs respond to WNT7 ligands with the multi-protein complex of FZD4/GPR124/RECK/LRP6 149,150,151,152.
Patients taking FTY720 risk complications from lymphopenia, which is a direct result of β-arrestin-mediated S1PR1 internalization in lymphocytes 144, 153. A similar mechanism in ocular endothelium may underpin macular edema that occurs in a small subset (0.8-1.5%) of patients 154. Vascular development is apparently unaffected in mice expressing two mutant S1PR1 alleles (S1pr1S5A/S5A) that encode a β-arrestin coupling- and internalization-defective receptor 153. Furthermore, S1pr1S5A/S5A mice are more resistant to FTY720-induced lung vascular leakage and S1PR1 degradation 61. Therefore, endothelium-targeted S1PR1 agonists would likely provide maximal therapeutic benefit if biased towards activation of Gαi/Rac to limit β-arrestin recruitment (i.e. mimic S1pr1S5A/S5A) and avoid receptor degradation and lymphopenia. A compound matching these criteria was recently described and showed therapeutic efficacy in preclinical models of coronary endothelial damage and renal ischemia/reperfusion injury 146. In a recent phase 1 clinical study of diabetics, this compound, SAR247799 stimulated myocardial perfusion without inducing lymphopenia in diabetics155.
Taken together, these studies of S1PR1 signaling highlight an important consideration for drug development and remind us that S1P measurement in patient fluids or tissue does not inform on S1PR1 expression or the extent of β-arrestin versus Gi/Rac signaling, which may have more functional significance than S1P levels alone.
S1P in experimental disease models
Rheumatoid arthritis and systemic lupus erythematosus, though etiologically complex, share the pathophysiologic mechanisms of neutrophil activation and immune complex (IC) deposition in tissues with resultant end-organ damage. S1PR1 agonism limits vascular barrier leakage associated with IC deposition64. Inversely, genetic inactivation of EC S1PR1 or pharmacologic S1PR1 antagonism resulted in more vascular leak and pulmonary neutrophil accumulation relative to control animals 64.
After organ damage, endothelial S1PR1 promotes recovery, regeneration and limits fibrosis. In a hydrochloric acid-induced model of lung injury, EC S1PR1 protected against vascular leak and limited fibrosis 156. Following partial hepatectomy, EC S1PR1 protected against fibrosis and improved liver vascular function, perfusibility, tissue regeneration, and animal survival 157. Perhaps it is not surprising that S1PR1 is important for tissue regeneration as the vasculature is a critical component of most major organ systems and S1PR1 is a central regulator of vascular network formation, but other roles for lysolipid signals in endothelial or epithelial biology may be involved.
In the murine Apoe−/− high-fat diet (HFD) model, EC-specific S1PR1 deficiency exacerbated disease severity and macrophage infiltration into atherosclerotic plaques106. While S1P signaling in ECs seems protective in the context of atherosclerosis, S1P regulation of macrophage phenotypes is more complex. S1PR2 158 or S1PR3 159 deficiency attenuated foam cell accumulation into lesions – an effect that is myeloid cell-intrinsic, as evidenced by bone marrow chimera experiments. In contrast, S1PR1-specific agonists confer an anti-inflammatory macrophage phenotype in vitro 160. Thus, we propose a model describing pro- and anti-inflammatory effects of S1P as first compartmentalized among cell types (vascular vs myeloid) 161, secondarily compartmentalized between different S1P receptors (S1PR1 vs S1PR2/3), and at a tertiary level when considering S1PR1 association with Gi/Rac versus β-arrestin pathways.
S1PR1 signaling is engaged in arterial ECs of the aorta intima as well as in adventitial lymphatic endothelium 147. In homeostasis, S1PR1 attenuates expression of pro-inflammatory transcripts in arterial (e.g. Cx3cl1/fractalkine, Vcam1, Ptgs2) and lymphatic ECs (Ccl21, Irf8, Il7). Furthermore, S1PR1 is a critical regulator of developmental lymphagiogenesis 118. Therefore, future studies might parse out arterial versus lymphatic S1PR1 signaling in mitigation of inflammation.
Consistent with an anti-inflammatory S1PR1 function, Teijara et al. demonstrated that the S1PR1 agonist CYM-5442 mitigates influenza virus-induced pulmonary cytokine storm and leukocyte infiltration 162. These effects of CYM-5442 were observed in Rag2−/− mice, which lack mature B and T cells, suggesting a minor role for lymphocyte S1PR1 162. Lung ECs from influenza virus-infected mice showed reduced CCL2 and CXCL10 expression in response to CYM-5442 162, suggesting that EC S1PR1 attenuates cytokine amplification during influenza virus infection. The expression of cytokines has been linked to Wnt-Ca2+ signaling in ECs, and there is initial evidence of bidirectional cross-talk between TLR2/4, inflammasome activation and canonical Wnt signals163-165, though the detailed molecular mechanisms have not been studied.
The primary Mendelian forms of atherosclerosis result from mutations in a small number of genes which cause familial dyslipidemias and premature vasculopathy. These genes (Ldlr, ApoB, Lrp6, Ldlrap, Pcsk9) not only all share the defining vascular phenotypes but also participate in different aspects of Wnt signaling, implying some commonality to the underlying mechanisms of atherosclerosis, but to date defining any shared mechanism has proven elusive108, 166, 167. Activation of canonical Wnt signaling has been observed in the endothelium of murine models prior to the emergence of focal atherosclerotic lesions and has been attributed to flow effects168. Disruption of physiological endothelial-smooth muscle interactions with proliferation of subjacent smooth muscle is associated with local canonical Wnt activation in reporter mice169. In later stages of the atherosclerotic process, several aspects of Wnt signaling have been directly implicated in vascular calcification, including evidence that LRP6 mitigates calcification in Ldlr−/− diabetic mice170. Ongoing work exploring the role of Wnt and lysolipids in the pathophysiology of atherosclerosis spans the full repertoire of Wnt signaling, but unifying generalizable insights have yet to emerge171.
LPA and vascular disease
In patients with acute coronary syndromes, culprit coronary arteries showed elevated LPA levels relative to the peripheral circulation 172. LPA accumulates in the lipid core region of human and mouse atherosclerotic lesions 173-175, and unstable plaques show high frequency of ATX immunostaining in the necrotic core 176.
In addition to disruption of endothelial junctions (discussed above), LPA also induces NFκB signaling and expression of downstream pro-inflammatory molecules in ECs177. Furthermore, LPA has been shown to positively regulate monocyte/macrophage uptake of oxidized-LDL 178, 179, expression of the pro-inflammatory molecule IL-1β 179, and inhibit apoptosis 180, which may inhibit macrophage clearance from subendothelial space. These effects are likely mediated by LPAR1 and/or LPAR2 181,182. Importantly, the LPA/LPAR5 signaling axis induces platelet activation, which may contribute to atherothrombosis 183, 184. Finally, a pharmacologic inhibitor of LPAR1/3 reduced plaque burden and myeloid infiltrate 185, 186 in two different models of murine atherosclerosis, whereas injection with LPA20:4 increased plaque burden and myeloid infiltrate 186. Collectively, these data imply that LPA enhances atherothrombosis and subendothelial foam cell accumulation, though careful studies of signaling events intrinsic to specific cell types are lacking in this field.
Consistent with human genetic studies suggesting a protective role for LPP3 in endothelium 53, 187-189, EC LPP3 expression protects against lung vascular leakage in homeostatic and endotoxemic conditions in mice 56. Global reduction of LPP3 in the postnatal period accelerates atherosclerosis development in a mouse model, which is largely attributable to the role of LPP3 in smooth muscles cells but unlikely related to LPP3 function in myeloid cells 190. Inhibition of LPA signaling by an ATX inhibitor or pan-LPAR inhibitor rescued the exacerbated lung vascular leakage in LPP3-ECKO animals. Conversely, mice with low levels of circulating ATX (and likely reduced LPA content) were resistant to LPS-induced lung vascular leakage 56. Intradermal LPA injection induced permeability of skin vasculature in a dose-dependent fashion 56, consistent with HUVEC responses to LPA 50, 85. In endotoxemia models, LPP3-ECKO mice showed ~3-fold increases plasma [IL-6] and peritoneal leukocyte recruitment after thioglycolate injection, suggesting that EC LPP3 attenuates inflammatory responses 56. Collectively, these data suggest that endothelial LPP3 attenuates LPA/LPAR-mediated vascular permeability and inflammation.
Several multi-ethnic genome-wide association studies (GWAS) found evidence of a strong association between CAD and a SNP (rs17114036) in intron 5 of Ppap2b (which encodes the LPP3 protein) 187-189. In HAECs, rs17114036 is within a ~1.2 kb peak of high chromatin accessibility and histone modification (H3K27ac, H3K4me2) associated with active enhancers 53. Other cell types (K562, GM12878, NHEK) lacked indications of active chromatin, suggesting a unique role for this cis-element in ECs 53. Luciferase assays demonstrated significant enhancer activity for the ~1.2 kb region at rs17114036 53. CRISPR-Cas9-mediated deletion of a ~66 bp region enclosing rs17114036 attenuated LPP3 expression 53. Consistently, mutagenesis of the risk allele (T/T) to the protective genotype (T/C) resulted in an ~6-fold increase in enhancer activity, establishing a causal relationship between the T/T variant and attenuated LPP3 expression 53. Taken together with information from GWAS studies, these experiments suggest that strategies which promote endothelial LPP3 activity or expression may have therapeutic benefit in cardiovascular disease (CVD).
E. Outstanding questions and perspectives for Future Research
S1P and LPA receptors, and molecules that regulate lysolipid bioavailability, are emerging as tractable targets for a range of pathologies that stem from autoimmune diseases, tissue injury, and pathogen infection. Animal models have yielded insights regarding downstream outcomes of single and collective receptor signaling, namely, S1PR1 as the primary endothelial S1PR promoting stabilization and maturation, while LPAR4 and LPAR6 promote EC proliferation and vascular front expansion. Further studies of sub-cellular location-specific LPAR activation, such as LPP3-regulated restriction of signaling to regions devoid of cell-cell contacts, warrant further study. Similarly, we lack mechanisms to describe S1PR1 biased signaling towards Gi/Rac or β-arrestin pathways. Understanding of these pathways may facilitate rational drug design as well as development of assays that, in addition to lipid measurements, will more precisely inform on the lysolipid signaling status of patients.
We lack a mechanism to explain why the rs17114036 SNP, which appears to regulate EC LPP3 expression, is strongly correlated with CVD risk. Is the mechanism as straightforward as aberrant LPAR signaling in coronary arteries downstream of increased local LPA levels? Or do individuals with this genetic variant have developmental defects, perhaps involving Wnt signaling, that compound with other factors and manifest as CVD? As we learn more about the basic science of lysolipid signaling using biochemical, cell culture, and animal models, we will be better prepared for rational design of therapeutic agents and evaluation of their efficacy.
Post-transcriptional regulation of lysolipid receptors in cell type-specific contexts is an active area of investigation. Several studies have identified microRNAs (miRNAs) that directly or indirectly downregulate S1pr1 expression in human cancer cell lines, including miR-148a in ovarian cancer 191, miR-149 in hepatocellular carcinoma 192, and miR-133b in nasopharyngeal carcinoma 193. At least two studies have shown that miR-155 targets S1pr1 in lymphocytes 194,195. miR-24 downregulates S1pr1 expression in HUVECs and human kidney epithelial cells 196, miR-24 antagonism improved survival and tissue vascularization in a model of renal ischemia/reperfusion injury 196. Similarly, the miR-17~92 cluster negatively regulates normal and ischemia-responsive arteriogenesis around mouse limbs, likely via inhibition of Wnt signaling components including FZD4 and LRP6 197. As compared to vascular S1P receptors, less is known about miRNA regulation of Lpar4 and Lpar6, though one study showed miR-139-5p targets Lpar4 in human umbilical cord mesenchymal stem cells 198. miRNA signaling in vascular biology has been reviewed elsewhere 199,200,201, and additional insights are likely to arise from unbiased profiling in a human 3D-culture angiogenesis model 202. Currently, data indicate that antagonism of endothelial miRNAs that downregulate S1pr1 may improve vascular function after tissue injury.
The enzyme S1P lyase (SPL) irreversibly catabolizes S1P to phosphoethanolamine and hexadecenal 203. Along with LPP3 204 and SPNS2 20, SPL is critical for T cell egress from lymphoid organs to circulation 205. Mechanistically, SPL expressed by thymic dendritic cells causes parenchymal S1P “sinks” that promote T cell chemotaxis towards relatively high [S1P] in the bloodstream 206. Ongoing research aims to determine whether SPL-targeted therapies may provide immunomodulatory benefits similar to those of FTY720 (fingolimod) without inhibiting vascular-protective S1P signaling 206,207,208,209.
Finally, understanding the complex and often contradictory effects of Wnt and lysolipid signaling in vascular biology is likely to require a much deeper exploration of the developmental effects of these convergent pathways on endothelial biology. Accumulating evidence suggests that the potential to connect mechanotransduction, metabolism, cell polarity and intercellular communication or competition with the fundamental processes of aging remains high, but mechanistic molecular models will require unraveling many of the most complex temporal and regional signaling hierarchies in development84, 102, 104.
Highlights.
Sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA) are secreted lipid mediators produced by metabolism of membrane phospholipids
S1P and LPA interact with specific G protein-coupled receptors to regulate vascular development, physiology and cardiovascular diseases
S1P/ LPA signaling axis intersects with fundamental developmental systems such as Wnt / ß-catenenin
Metabolic and signaling genes in the S1P/ LPA system show cardiovascular disease-specific heterogeneity
Acknowledgements
Sources of Funding: This work is supported by NIH grant R35HL135821 and Fondation Leducq transatlantic network grant – SphingoNet to T.H.
Nonstandard Abbreviations and Acronyms
- EC
Endothelial cells
- VEGF
Vascular endothelial growth factor
- GPCR’s
G protein-coupled receptors
- S1P
Sphingosine 1-phosphate
- LPA
Lysophosphatidic acid
- HDL
High density lipoprotein
- MFSD2B
Major facilitator family transporter 2b
- SPNS2
Spinster 2
- ATX
Autotaxin
- HUVECs
Human umbilical vein endothelial cells
- GCs
Germ cells
- SPL
S1P lyase
- CAD
Coronary artery disease
- BRB
Blood-retina-barrier
Footnotes
Disclosure: None
References:
- 1.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. Vegf guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rattner A, Williams J, Nathans J. Roles of hifs and vegf in angiogenesis in the retina and brain. J Clin Invest. 2019;130:3807–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur H, Carvalho J, Looso M, Singh P, Chennupati R, Preussner J, Gunther S, Albarran-Juarez J, Tischner D, Classen S, Offermanns S, Wettschureck N. Single-cell profiling reveals heterogeneity and functional patterning of gpcr expression in the vascular system. Nat Commun. 2017;8:15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalucka J, de Rooij L, Goveia J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779 e720 [DOI] [PubMed] [Google Scholar]
- 5.Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480 [DOI] [PubMed] [Google Scholar]
- 6.Sabbagh MF, Heng JS, Luo C, Castanon RG, Nery JR, Rattner A, Goff LA, Ecker JR, Nathans J. Transcriptional and epigenomic landscapes of cns and non-cns vascular endothelial cells. Elife. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yung YC, Stoddard NC, Chun J. Lpa receptor signaling: Pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagida K, Hla T. Vascular and immunobiology of the circulatory sphingosine 1-phosphate gradient. Annu Rev Physiol. 2017;79:67–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki S, Yatomi Y, Ohta M, Osada M, Kazama F, Satoh K, Nakahara K, Ozaki Y. Sphingosine 1-phosphate-related metabolism in the blood vessel. J Biochem. 2005;138:47–55 [DOI] [PubMed] [Google Scholar]
- 10.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by hdl-associated apolipoprotein m. Proc Natl Acad Sci U S A. 2011;108:9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraemer MP, Mao G, Hammill C, Yan B, Li Y, Onono F, Smyth SS, Morris AJ. Effects of diet and hyperlipidemia on levels and distribution of circulating lysophosphatidic acid. J Lipid Res. 2019;60:1818–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris AJ, Selim S, Salous A, Smyth SS. Blood relatives: Dynamic regulation of bioactive lysophosphatidic acid and sphingosine-1-phosphate metabolism in the circulation. Trends Cardiovasc Med. 2009;19:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obinata H, Kuo A, Wada Y, Swendeman S, Liu CH, Blaho VA, Nagumo R, Satoh K, Izumi T, Hla T. Identification of apoa4 as a sphingosine 1-phosphate chaperone in apom- and albumin-deficient mice. J Lipid Res. 2019;60:1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salous AK, Panchatcharam M, Sunkara M, Mueller P, Dong A, Wang Y, Graf GA, Smyth SS, Morris AJ. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J Lipid Res. 2013;54:2775–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675 [DOI] [PubMed] [Google Scholar]
- 18.Vu TM, Ishizu AN, Foo JC, et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature. 2017;550:524–528 [DOI] [PubMed] [Google Scholar]
- 19.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse spns2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 2012;7:e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuhara S, Simmons S, Kawamura S, et al. The sphingosine-1-phosphate transporter spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza A, Breart B, Ramos-Perez WD, Pitt LA, Gobert M, Sunkara M, Lafaille JJ, Morris AJ, Schwab SR. The transporter spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, Hait NC, Maceyka M, Milstien S, Takabe K, Spiegel S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong Y, Yang P, Proia RL, Hla T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J Clin Invest. 2014;124:4823–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons S, Sasaki N, Umemoto E, et al. High-endothelial cell-derived s1p regulates dendritic cell localization and vascular integrity in the lymph node. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrakis A, Moolenaar WH. Autotaxin: Structure-function and signaling. J Lipid Res. 2014;55:1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129:1239–1250 [DOI] [PubMed] [Google Scholar]
- 28.Yunoki K, Renaguli M, Kinoshita M, Matsuyama H, Mawatari S, Fujino T, Kodama Y, Sugiyama M, Ohnishi M. Dietary sphingolipids ameliorate disorders of lipid metabolism in zucker fatty rats. J Agric Food Chem. 2010;58:7030–7035 [DOI] [PubMed] [Google Scholar]
- 29.Norris GH, Porter CM, Jiang C, Millar CL, Blesso CN. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;40:36–43 [DOI] [PubMed] [Google Scholar]
- 30.Chung RW, Kamili A, Tandy S, Weir JM, Gaire R, Wong G, Meikle PJ, Cohn JS, Rye KA. Dietary sphingomyelin lowers hepatic lipid levels and inhibits intestinal cholesterol absorption in high-fat-fed mice. PLoS One. 2013;8:e55949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millar CL, Jiang C, Norris GH, Garcia C, Seibel S, Anto L, Lee JY, Blesso CN. Cow’s milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in ldl-receptor knockout mice fed a western-type diet. J Nutr Biochem. 2020;79:108351. [DOI] [PubMed] [Google Scholar]
- 32.Wexler HM. Bacteroides: The good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20:593–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson EL, Heaver SL, Waters JL, Kim BI, Bretin A, Goodman AL, Gewirtz AT, Worgall TS, Ley RE. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat Commun. 2020;11:2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the g protein-coupled receptor edg-1. Science. 1998;279:1552–1555 [DOI] [PubMed] [Google Scholar]
- 35.Yanagida K, Ishii S. Non-edg family lpa receptors: The cutting edge of lpa research. J Biochem. 2011;150:223–232 [DOI] [PubMed] [Google Scholar]
- 36.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: Receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55 [DOI] [PubMed] [Google Scholar]
- 37.Yanagida K, Kurikawa Y, Shimizu T, Ishii S. Current progress in non-edg family lpa receptor research. Biochim Biophys Acta. 2013;1831:33–41 [DOI] [PubMed] [Google Scholar]
- 38.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55:1596–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, Hla T. Flow-regulated endothelial s1p receptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaengel K, Niaudet C, Hagikura K, et al. The sphingosine-1-phosphate receptor s1pr1 restricts sprouting angiogenesis by regulating the interplay between ve-cadherin and vegfr2. Dev Cell. 2012;23:587–599 [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the g protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sumida H, Noguchi K, Kihara Y, et al. Lpa4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060–5070 [DOI] [PubMed] [Google Scholar]
- 43.Yasuda D, Kobayashi D, Akahoshi N, Ohto-Nakanishi T, Yoshioka K, Takuwa Y, Mizuno S, Takahashi S, Ishii S. Lysophosphatidic acid-induced yap/taz activation promotes developmental angiogenesis by repressing notch ligand dll4. J Clin Invest. 2019;130:4332–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor s1p1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667 [DOI] [PubMed] [Google Scholar]
- 45.Yanagida K, Engelbrecht E, Niaudet C, Jung B, Gaengel K, Holton K, Swendeman S, Liu CH, Levesque MV, Kuo A, Fu Z, Smith LEH, Betsholtz C, Hla T. Sphingosine 1-phosphate receptor signaling establishes ap-1 gradients to allow for retinal endothelial cell specialization. Dev Cell. 2020;52:779–793 e777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohsawa S, Vaughen J, Igaki T. Cell extrusion: A stress-responsive force for good or evil in epithelial homeostasis. Dev Cell. 2018;44:284–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kano K, Matsumoto H, Inoue A, Yukiura H, Kanai M, Chun J, Ishii S, Shimizu T, Aoki J. Molecular mechanism of lysophosphatidic acid-induced hypertensive response. Sci Rep. 2019;9:2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Im E, Motiejunaite R, Aranda J, Park EY, Federico L, Kim TI, Clair T, Stracke ML, Smyth S, Kazlauskas A. Phospholipase cgamma activation drives increased production of autotaxin in endothelial cells and lysophosphatidic acid-dependent regression. Mol Cell Biol. 2010;30:2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motiejunaite R, Aranda J, Kazlauskas A. Pericytes prevent regression of endothelial cell tubes by accelerating metabolism of lysophosphatidic acid. Microvasc Res. 2014;93:62–71 [DOI] [PubMed] [Google Scholar]
- 50.Yukiura H, Kano K, Kise R, Inoue A, Aoki J. Lpp3 localizes lpa6 signalling to non-contact sites in endothelial cells. J Cell Sci. 2015;128:3871–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee I, Baruah J, Lurie EE, Wary KK. Endothelial lipid phosphate phosphatase-3 deficiency that disrupts the endothelial barrier function is a modifier of cardiovascular development. Cardiovasc Res. 2016;111:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C, Huang RT, Kuo CH, et al. Mechanosensitive ppap2b regulates endothelial responses to atherorelevant hemodynamic forces. Circ Res. 2015;117:e41–e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krause MD, Huang RT, Wu D, Shentu TP, Harrison DL, Whalen MB, Stolze LK, Di Rienzo A, Moskowitz IP, Civelek M, Romanoski CE, Fang Y. Genetic variant at coronary artery disease and ischemic stroke locus 1p32.2 regulates endothelial responses to hemodynamics. Proc Natl Acad Sci U S A. 2018;115:E11349–E11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siemerink MJ, Klaassen I, Van Noorden CJ, Schlingemann RO. Endothelial tip cells in ocular angiogenesis: Potential target for anti-angiogenesis therapy. J Histochem Cytochem. 2013;61:101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korn C, Augustin HG. Mechanisms of vessel pruning and regression. Dev Cell. 2015;34:5–17 [DOI] [PubMed] [Google Scholar]
- 56.Panchatcharam M, Salous AK, Brandon J, Miriyala S, Wheeler J, Patil P, Sunkara M, Morris AJ, Escalante-Alcalde D, Smyth SS. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler Thromb Vasc Biol. 2014;34:837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humtsoe JO, Feng S, Thakker GD, Yang J, Hong J, Wary KK. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/vcip. EMBO J. 2003;22:1539–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312 [DOI] [PubMed] [Google Scholar]
- 59.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vouret-Craviari V, Bourcier C, Boulter E, van Obberghen-Schilling E. Distinct signals via rho gtpases and src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. J Cell Sci. 2002;115:2475–2484 [DOI] [PubMed] [Google Scholar]
- 61.Oo ML, Chang SH, Thangada S, Wu MT, Rezaul K, Blaho V, Hwang SI, Han DK, Hla T. Engagement of s1p(1)-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121:2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, Proia RL, Steinman L, Han MH, Hla T. Hdl-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523:342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burg N, Swendeman S, Worgall S, Hla T, Salmon JE. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 2018;70:1879–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swendeman SL, Xiong Y, Cantalupo A, et al. An engineered s1p chaperone attenuates hypertension and ischemic injury. Sci Signal. 2017;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanagida K, Liu CH, Faraco G, Galvani S, Smith HK, Burg N, Anrather J, Sanchez T, Iadecola C, Hla T. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc Natl Acad Sci U S A. 2017;114:4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma S, Santhosh D, Kumar TP, Huang Z. A brain-region-specific neural pathway regulating germinal matrix angiogenesis. Dev Cell. 2017;41:366–381 e364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T, Di Lorenzo A. S1pr1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension. 2017;70:426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates akt, nitric oxide production, and chemotaxis through a gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677 [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216 [DOI] [PubMed] [Google Scholar]
- 71.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–1838 [DOI] [PubMed] [Google Scholar]
- 72.Nohata N, Uchida Y, Stratman AN, Adams RH, Zheng Y, Weinstein BM, Mukouyama YS, Gutkind JS. Temporal-specific roles of rac1 during vascular development and retinal angiogenesis. Dev Biol. 2016;411:183–194 [DOI] [PubMed] [Google Scholar]
- 73.Gazit SL, Mariko B, Therond P, et al. Platelet and erythrocyte sources of s1p are redundant for vascular development and homeostasis, but both rendered essential after plasma s1p depletion in anaphylactic shock. Circ Res. 2016;119:e110–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, Penninger J, Eichmann A. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitulescu ME, Schmidt I, Giaimo BD, et al. Dll4 and notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol. 2017;19:915–927 [DOI] [PubMed] [Google Scholar]
- 76.Rocha SF, Schiller M, Jing D, Li H, Butz S, Vestweber D, Biljes D, Drexler HC, Nieminen-Kelha M, Vajkoczy P, Adams S, Benedito R, Adams RH. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing vegf bioavailability. Circ Res. 2014;115:581–590 [DOI] [PubMed] [Google Scholar]
- 77.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by ve-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934 [DOI] [PubMed] [Google Scholar]
- 78.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sabbagh MF, Nathans J. A genome-wide view of the de-differentiation of central nervous system endothelial cells in culture. Elife. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim YH, Choi J, Yang MJ, Hong SP, Lee CK, Kubota Y, Lim DS, Koh GY. A mst1-foxo1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat Commun. 2019;10:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, Hirschi KK, Chen CS. A non-canonical notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su SC, Mendoza EA, Kwak HI, Bayless KJ. Molecular profile of endothelial invasion of three-dimensional collagen matrices: Insights into angiogenic sprout induction in wound healing. Am J Physiol Cell Physiol. 2008;295:C1215–1229 [DOI] [PubMed] [Google Scholar]
- 84.Claveria C, Torres M. Cell competition: Mechanisms and physiological roles. Annu Rev Cell Dev Biol. 2016;32:411–439 [DOI] [PubMed] [Google Scholar]
- 85.van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of rhoa and rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:E127–133 [DOI] [PubMed] [Google Scholar]
- 86.Offermanns S, Mancino V, Revel JP, Simon MI. Vascular system defects and impaired cell chemokinesis as a result of galpha13 deficiency. Science. 1997;275:533–536 [DOI] [PubMed] [Google Scholar]
- 87.Ruppel KM, Willison D, Kataoka H, Wang A, Zheng YW, Cornelissen I, Yin L, Xu SM, Coughlin SR. Essential role for galpha13 in endothelial cells during embryonic development. Proc Natl Acad Sci U S A. 2005;102:8281–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamijo H, Matsumura Y, Thumkeo D, Koike S, Masu M, Shimizu Y, Ishizaki T, Narumiya S. Impaired vascular remodeling in the yolk sac of embryos deficient in rock-i and rock-ii. Genes Cells. 2011;16:1012–1021 [DOI] [PubMed] [Google Scholar]
- 89.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830 [DOI] [PubMed] [Google Scholar]
- 90.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase d, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zahra FT, Sajib MS, Ichiyama Y, Akwii RG, Tullar PE, Cobos C, Minchew SA, Doci CL, Zheng Y, Kubota Y, Gutkind JS, Mikelis CM. Endothelial rhoa gtpase is essential for in vitro endothelial functions but dispensable for physiological in vivo angiogenesis. Sci Rep. 2019;9:11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barlow HR, Cleaver O. Building blood vessels-one rho gtpase at a time. Cells. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bachner D, Ahrens M, Betat N, Schroder D, Gross G. Developmental expression analysis of murine autotaxin (atx). Mech Dev. 1999;84:121–125 [DOI] [PubMed] [Google Scholar]
- 94.Ferry G, Giganti A, Coge F, Bertaux F, Thiam K, Boutin JA. Functional invalidation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 2007;581:3572–3578 [DOI] [PubMed] [Google Scholar]
- 95.Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/phosphotransferases: Common structure and diverse functions. Biochem J. 2005;387:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts R, Sciorra VA, Morris AJ. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J Biol Chem. 1998;273:22059–22067 [DOI] [PubMed] [Google Scholar]
- 97.Brindley DN, Pilquil C. Lipid phosphate phosphatases and signaling. J Lipid Res. 2009;50 Suppl:S225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chandra M, Escalante-Alcalde D, Bhuiyan MS, Orr AW, Kevil C, Morris AJ, Nam H, Dominic P, McCarthy KJ, Miriyala S, Panchatcharam M. Cardiac-specific inactivation of lpp3 in mice leads to myocardial dysfunction and heart failure. Redox Biol. 2018;14:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Jr Gang C, Sciorra VA, Daar I, Spiegel S, Morris AJ, Stewart CL. The lipid phosphatase lpp3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637 [DOI] [PubMed] [Google Scholar]
- 100.Fang V, Chaluvadi VS, Ramos-Perez WD, Mendoza A, Baeyens A, Rivera R, Chun J, Cammer M, Schwab SR. Gradients of the signaling lipid s1p in lymph nodes position natural killer cells and regulate their interferon-gamma response. Nat Immunol. 2017;18:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for hedgehog and wingless signalling. Nature. 2005;435:58–65 [DOI] [PubMed] [Google Scholar]
- 102.Wiese KE, Nusse R, van Amerongen R. Wnt signalling: Conquering complexity. Development. 2018;145 [DOI] [PubMed] [Google Scholar]
- 103.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar h+-atpase-mediated acidification for wnt signaling. Science. 2010;327:459–463 [DOI] [PubMed] [Google Scholar]
- 104.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999 [DOI] [PubMed] [Google Scholar]
- 105.Kestler HA, Kuhl M. Generating a wnt switch: It’s all about the right dosage. J Cell Biol. 2011;193:431–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, Dahlback B, Kono M, Proia RL, Smith JD, Hla T. Hdl-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor s1p1 to limit vascular inflammation. Sci Signal. 2015;8:ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGough IJ, Vecchia L, Bishop B, Malinauskas T, Beckett K, Joshi D, O’Reilly N, Siebold C, Jones EY, Vincent JP. Glypicans shield the wnt lipid moiety to enable signalling at a distance. Nature. 2020;585:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ozhan G, Sezgin E, Wehner D, Pfister AS, Kuhl SJ, Kagermeier-Schenk B, Kuhl M, Schwille P, Weidinger G. Lypd6 enhances wnt/beta-catenin signaling by promoting lrp6 phosphorylation in raft plasma membrane domains. Dev Cell. 2013;26:331–345 [DOI] [PubMed] [Google Scholar]
- 109.Zhang CS, Jiang B, Li M, et al. The lysosomal v-atpase-ragulator complex is a common activator for ampk and mtorc1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20:526–540 [DOI] [PubMed] [Google Scholar]
- 110.Wang L, Chai Y, Li C, Liu H, Su W, Liu X, Yu B, Lei W, Yu B, Crane JL, Cao X, Wan M. Oxidized phospholipids are ligands for lrp6. Bone Res. 2018;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, Chang H, Rattner A, Nathans J. Frizzled receptors in development and disease. Curr Top Dev Biol. 2016;117:113–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: Control by norrin and frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895 [DOI] [PubMed] [Google Scholar]
- 113.Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical wnt signaling components in vascular development and barrier formation. J Clin Invest. 2014;124:3825–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H. Nrarp coordinates endothelial notch and wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martowicz A, Trusohamn M, Jensen N, Wisniewska-Kruk J, Corada M, Ning FC, Kele J, Dejana E, Nyqvist D. Endothelial beta-catenin signaling supports postnatal brain and retinal angiogenesis by promoting sprouting, tip cell formation, and vegfr (vascular endothelial growth factor receptor) 2 expression. Arterioscler Thromb Vasc Biol. 2019;39:2273–2288 [DOI] [PubMed] [Google Scholar]
- 117.Heng JS, Rattner A, Stein-O’Brien GL, Winer BL, Jones BW, Vernon HJ, Goff LA, Nathans J. Hypoxia tolerance in the norrin-deficient retina and the chronically hypoxic brain studied at single-cell resolution. Proc Natl Acad Sci U S A. 2019;116:9103–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geng X, Yanagida K, Akwii RG, et al. S1pr1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent vegf-c signaling. JCI Insight. 2020;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shibuya M, Claesson-Welsh L. Signal transduction by vegf receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560 [DOI] [PubMed] [Google Scholar]
- 120.Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, Krumlauf R, Beddington RS. Misexpression of cwnt8c in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005 [DOI] [PubMed] [Google Scholar]
- 121.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse fused locus encodes axin, an inhibitor of the wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192 [DOI] [PubMed] [Google Scholar]
- 122.Sano H, Renault AD, Lehmann R. Control of lateral migration and germ cell elimination by the drosophila melanogaster lipid phosphate phosphatases wunen and wunen 2. J Cell Biol. 2005;171:675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang N, Zhang J, Purcell KJ, Cheng Y, Howard K. The drosophila protein wunen repels migrating germ cells. Nature. 1997;385:64–67 [DOI] [PubMed] [Google Scholar]
- 124.LeBlanc MG, Lehmann R. Domain-specific control of germ cell polarity and migration by multifunction tre1 gpcr. J Cell Biol. 2017;216:2945–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan g protein-coupled receptor gpr84. J Biol Chem. 2006;281:34457–34464 [DOI] [PubMed] [Google Scholar]
- 126.Renault AD, Sigal YJ, Morris AJ, Lehmann R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 2004;305:1963–1966 [DOI] [PubMed] [Google Scholar]
- 127.Gong B, Shen W, Xiao W, Meng Y, Meng A, Jia S. The sec14-like phosphatidylinositol transfer proteins sec14l3/sec14l2 act as gtpase proteins to mediate wnt/ca(2+) signaling. Elife. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller NE, Thelle DS, Forde OH, Mjos OD. The tromso heart-study. High-density lipoprotein and coronary heart-disease: A prospective case-control study. Lancet. 1977;1:965–968 [DOI] [PubMed] [Google Scholar]
- 129.Rhoads GG, Gulbrandsen CL, Kagan A. Serum lipoproteins and coronary heart disease in a population study of hawaii japanese men. N Engl J Med. 1976;294:293–298 [DOI] [PubMed] [Google Scholar]
- 130.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am J Med. 1977;62:707–714 [DOI] [PubMed] [Google Scholar]
- 131.Rosenson RS, Brewer HB Jr., Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. Hdl measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410 [DOI] [PubMed] [Google Scholar]
- 132.Poti F, Simoni M, Nofer JR. Atheroprotective role of high-density lipoprotein (hdl)-associated sphingosine-1-phosphate (s1p). Cardiovasc Res. 2014;103:395–404 [DOI] [PubMed] [Google Scholar]
- 133.Sattler K, Lehmann I, Graler M, Brocker-Preuss M, Erbel R, Heusch G, Levkau B. Hdl-bound sphingosine 1-phosphate (s1p) predicts the severity of coronary artery atherosclerosis. Cell Physiol Biochem. 2014;34:172–184 [DOI] [PubMed] [Google Scholar]
- 134.Jing XD, Wei XM, Deng SB, Du JL, Liu YJ, She Q. The relationship between the high-density lipoprotein (hdl)-associated sphingosine-1-phosphate (s1p) and coronary in-stent restenosis. Clin Chim Acta. 2015;446:248–252 [DOI] [PubMed] [Google Scholar]
- 135.Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B. Sphingosine 1-phosphate levels in plasma and hdl are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–832 [DOI] [PubMed] [Google Scholar]
- 136.Argraves KM, Sethi AA, Gazzolo PJ, Wilkerson BA, Remaley AT, Tybjaerg-Hansen A, Nordestgaard BG, Yeatts SD, Nicholas KS, Barth JL, Argraves WS. S1p, dihydro-s1p and c24:1-ceramide levels in the hdl-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 2011;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Levkau B. Hdl-s1p: Cardiovascular functions, disease-associated alterations, and therapeutic applications. Front Pharmacol. 2015;6:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sattler K, Graler M, Keul P, Weske S, Reimann CM, Jindrova H, Kleinbongard P, Sabbadini R, Brocker-Preuss M, Erbel R, Heusch G, Levkau B. Defects of high-density lipoproteins in coronary artery disease caused by low sphingosine-1-phosphate content: Correction by sphingosine-1-phosphate-loading. J Am Coll Cardiol. 2015;66:1470–1485 [DOI] [PubMed] [Google Scholar]
- 139.Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:677–690 [DOI] [PubMed] [Google Scholar]
- 140.Bourgeois R, Devillers R, Perrot N, Despres AA, Boulanger MC, Mitchell PL, Guertin J, Couture P, Boffa MB, Scipione CA, Pibarot P, Koschinsky ML, Mathieu P, Arsenault BJ. Interaction of autotaxin with lipoprotein(a) in patients with calcific aortic valve stenosis. JACC Basic Transl Sci. 2020;5:888–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Obinata H, Gutkind S, Stitham J, Okuno T, Yokomizo T, Hwa J, Hla T. Individual variation of human s1p(1) coding sequence leads to heterogeneity in receptor function and drug interactions. J Lipid Res. 2014;55:2665–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun X, Ma SF, Wade MS, Flores C, Pino-Yanes M, Moitra J, Ober C, Kittles R, Husain AN, Ford JG, Garcia JG. Functional variants of the sphingosine-1-phosphate receptor 1 gene associate with asthma susceptibility. J Allergy Clin Immunol. 2010;126:241–249, 249 e241–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415 [DOI] [PubMed] [Google Scholar]
- 144.Chun J, Hartung HP. Mechanism of action of oral fingolimod (fty720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cartier A, Hla T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science. 2019;366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Poirier B, Briand V, Kadereit D, Schafer M, Wohlfart P, Philippo MC, Caillaud D, Gouraud L, Grailhe P, Bidouard JP, Trellu M, Muslin AJ, Janiak P, Parkar AA. A g protein-biased s1p1 agonist, sar247799, protects endothelial cells without affecting lymphocyte numbers. Sci Signal. 2020;13 [DOI] [PubMed] [Google Scholar]
- 147.Engelbrecht E, Levesque MV, He L, Vanlandewijck M, Nitzsche A, Niazi H, Kuo A, Singh SA, Aikawa M, Holton K, Proia RL, Kono M, Pu WT, Camerer E, Betsholtz C, Hla T. Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta. Elife. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hisano Y, Kono M, Cartier A, et al. Lysolipid receptor cross-talk regulates lymphatic endothelial junctions in lymph nodes. J Exp Med. 2019;216:1582–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vallon M, Yuki K, Nguyen TD, Chang J, Yuan J, Siepe D, Miao Y, Essler M, Noda M, Garcia KC, Kuo CJ. A reck-wnt7 receptor-ligand interaction enables isoform-specific regulation of wnt bioavailability. Cell Rep. 2018;25:339–349 e339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cho C, Smallwood PM, Nathans J. Reck and gpr124 are essential receptor cofactors for wnt7a/wnt7b-specific signaling in mammalian cns angiogenesis and blood-brain barrier regulation. Neuron. 2017;95:1221–1225 [DOI] [PubMed] [Google Scholar]
- 151.Cho C, Wang Y, Smallwood PM, Williams J, Nathans J. Molecular determinants in frizzled, reck, and wnt7a for ligand-specific signaling in neurovascular development. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical gpr124- and reck-dependent wnt/beta-catenin pathway during brain angiogenesis. Elife. 2015;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mandal P, Gupta A, Fusi-Rubiano W, Keane PA, Yang Y. Fingolimod: Therapeutic mechanisms and ocular adverse effects. Eye (Lond). 2017;31:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bergougnan L, Armani S, Golor G, et al. First-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple oral doses of sar247799, a selective g-protein-biased sphingosine-1 phosphate receptor-1 agonist for endothelial protection. Br J Clin Pharmacol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ding BS, Yang D, Swendeman SL, Christoffersen C, Nielsen LB, Friedman SL, Powell CA, Hla T, Cao Z. Aging suppresses sphingosine-1-phosphate chaperone apom in circulation resulting in maladaptive organ repair. Dev Cell. 2020;53:677–690 e674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding BS, Liu CH, Sun Y, Chen Y, Swendeman SL, Jung B, Chavez D, Cao Z, Christoffersen C, Nielsen LB, Schwab SR, Rafii S, Hla T. Hdl activation of endothelial sphingosine-1-phosphate receptor-1 (s1p1) promotes regeneration and suppresses fibrosis in the liver. JCI Insight. 2016;1:e87058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323 [DOI] [PubMed] [Google Scholar]
- 160.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weigert A, Olesch C, Brune B. Sphingosine-1-phosphate and macrophage biology-how the sphinx tames the big eater. Front Immunol. 2019;10:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Singh V, Holla S, Ramachandra SG, Balaji KN. Wnt-inflammasome signaling mediates nod2-induced development of acute arthritis in mice. J Immunol. 2015;194:3351–3360 [DOI] [PubMed] [Google Scholar]
- 164.Undi RB, Sarvothaman S, Narasaiah K, Gutti U, Gutti RK. Toll-like receptor 2 signalling: Significance in megakaryocyte development through wnt signalling cross-talk and cytokine induction. Cytokine. 2016;83:245–249 [DOI] [PubMed] [Google Scholar]
- 165.Huang L, Luo R, Li J, Wang D, Zhang Y, Liu L, Zhang N, Xu X, Lu B, Zhao K. Beta-catenin promotes nlrp3 inflammasome activation via increasing the association between nlrp3 and asc. Mol Immunol. 2020;121:186–194 [DOI] [PubMed] [Google Scholar]
- 166.Matthijs Blankesteijn W, Hermans KC. Wnt signaling in atherosclerosis. Eur J Pharmacol. 2015;763:122–130 [DOI] [PubMed] [Google Scholar]
- 167.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. Lrp6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, Blackman BR. Hemodynamic activation of beta-catenin and t-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol. 2011;31:1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/beta-catenin signaling induces vsmc proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436 [DOI] [PubMed] [Google Scholar]
- 170.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, Krchma K, Bello Arredondo Y, Kovacs A, Kapoor K, Brill LM, Perera R, Williams BO, Towler DA. Vascular smooth muscle lrp6 limits arteriosclerotic calcification in diabetic ldlr−/− mice by restraining noncanonical wnt signals. Circ Res. 2015;117:142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boucher P, Matz RL, Terrand J. Atherosclerosis: Gone with the wnt? Atherosclerosis. 2020;301:15–22 [DOI] [PubMed] [Google Scholar]
- 172.Dohi T, Miyauchi K, Ohkawa R, et al. Increased lysophosphatidic acid levels in culprit coronary arteries of patients with acute coronary syndrome. Atherosclerosis. 2013;229:192–197 [DOI] [PubMed] [Google Scholar]
- 173.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bot M, Bot I, Lopez-Vales R, van de Lest CH, Saulnier-Blache JS, Helms JB, David S, van Berkel TJ, Biessen EA. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am J Pathol. 2010;176:3073–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747 [DOI] [PubMed] [Google Scholar]
- 176.Gu C, Wang F, Zhao Z, Wang H, Cong X, Chen X. Lysophosphatidic acid is associated with atherosclerotic plaque instability by regulating nf-kappab dependent matrix metalloproteinase-9 expression via lpa2 in macrophages. Front Physiol. 2017;8:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Palmetshofer A, Robson SC, Nehls V. Lysophosphatidic acid activates nuclear factor kappa b and induces proinflammatory gene expression in endothelial cells. Thromb Haemost. 1999;82:1532–1537 [PubMed] [Google Scholar]
- 178.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chang CL, Lin ME, Hsu HY, Yao CL, Hwang SM, Pan CY, Hsu CY, Lee H. Lysophosphatidic acid-induced interleukin-1 beta expression is mediated through gi/rho and the generation of reactive oxygen species in macrophages. J Biomed Sci. 2008;15:357–363 [DOI] [PubMed] [Google Scholar]
- 180.Koh JS, Lieberthal W, Heydrick S, Levine JS. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Invest. 1998;102:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fueller M, Wang DA, Tigyi G, Siess W. Activation of human monocytic cells by lysophosphatidic acid and sphingosine-1-phosphate. Cell Signal. 2003;15:367–375 [DOI] [PubMed] [Google Scholar]
- 182.D’Aquilio F, Procaccini M, Izzi V, Chiurchiu V, Giambra V, Carotenuto F, Di Nardo P, Baldini PM. Activatory properties of lysophosphatidic acid on human thp-1 cells. Inflammation. 2007;30:167–177 [DOI] [PubMed] [Google Scholar]
- 183.Khandoga AL, Pandey D, Welsch U, Brandl R, Siess W. Gpr92/lpa(5) lysophosphatidate receptor mediates megakaryocytic cell shape change induced by human atherosclerotic plaques. Cardiovasc Res. 2011;90:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. Unique ligand selectivity of the gpr92/lpa5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304–17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kritikou E, van Puijvelde GH, van der Heijden T, van Santbrink PJ, Swart M, Schaftenaar FH, Kroner MJ, Kuiper J, Bot I. Inhibition of lysophosphatidic acid receptors 1 and 3 attenuates atherosclerosis development in ldl-receptor deficient mice. Sci Rep. 2016;6:37585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou Z, Subramanian P, Sevilmis G, Globke B, Soehnlein O, Karshovska E, Megens R, Heyll K, Chun J, Saulnier-Blache JS, Reinholz M, van Zandvoort M, Weber C, Schober A. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing cxcl1 from the endothelium. Cell Metab. 2011;13:592–600 [DOI] [PubMed] [Google Scholar]
- 187.Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Coronary Artery Disease Genetics C. A genome-wide association study in europeans and south asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344 [DOI] [PubMed] [Google Scholar]
- 189.Ke W, Rand KA, Conti DV, Setiawan VW, Stram DO, Wilkens L, Le Marchand L, Assimes TL, Haiman CA. Evaluation of 71 coronary artery disease risk variants in a multiethnic cohort. Front Cardiovasc Med. 2018;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mueller PA, Yang L, Ubele M, Mao G, Brandon J, Vandra J, Nichols TC, Escalante-Alcalde D, Morris AJ, Smyth SS. Coronary artery disease risk-associated plpp3 gene and its product lipid phosphate phosphatase 3 regulate experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wen Z, Zhao S, Liu S, Liu Y, Li X, Li S. Microrna-148a inhibits migration and invasion of ovarian cancer cells via targeting sphingosine-1-phosphate receptor 1. Mol Med Rep. 2015;12:3775–3780 [DOI] [PubMed] [Google Scholar]
- 192.Cheng JL, Li DJ, Lv MY, Pei YJ, Zhang XJ, Li L, Liu XY, Fan AH. Lncrna kcnq1ot1 regulates the invasion and migration of hepatocellular carcinoma by acting on s1pr1 through mir-149. Cancer Gene Ther. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cheng N, Wang GH. Mir-133b, a microrna targeting s1pr1, suppresses nasopharyngeal carcinoma cell proliferation. Exp Ther Med. 2016;11:1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xin Q, Li J, Dang J, et al. Mir-155 deficiency ameliorates autoimmune inflammation of systemic lupus erythematosus by targeting s1pr1 in faslpr/lpr mice. J Immunol. 2015;194:5437–5445 [DOI] [PubMed] [Google Scholar]
- 195.Okoye IS, Czieso S, Ktistaki E, Roderick K, Coomes SM, Pelly VS, Kannan Y, Perez-Lloret J, Zhao JL, Baltimore D, Langhorne J, Wilson MS. Transcriptomics identified a critical role for th2 cell-intrinsic mir-155 in mediating allergy and antihelminth immunity. Proc Natl Acad Sci U S A. 2014;111:E3081–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lorenzen JM, Kaucsar T, Schauerte C, et al. Microrna-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25:2717–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Landskroner-Eiger S, Qiu C, Perrotta P, Siragusa M, Lee MY, Ulrich V, Luciano AK, Zhuang ZW, Corti F, Simons M, Montgomery RL, Wu D, Yu J, Sessa WC. Endothelial mir-17 approximately 92 cluster negatively regulates arteriogenesis via mirna-19 repression of wnt signaling. Proc Natl Acad Sci U S A. 2015;112:12812–12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jiang C, Tong Z, Fang WL, Fu QB, Gu YJ, Lv TT, Liu DM, Xue W, Lv JW. Microrna-139–5p inhibits epithelial-mesenchymal transition and fibrosis in post-menopausal women with interstitial cystitis by targeting lpar4 via the pi3k/akt signaling pathway. J Cell Biochem. 2018;119:6429–6441 [DOI] [PubMed] [Google Scholar]
- 199.Landskroner-Eiger S, Moneke I, Sessa WC. Mirnas as modulators of angiogenesis. Cold Spring Harb Perspect Med. 2013;3:a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fernandez-Hernando C, Suarez Y. Micrornas in endothelial cell homeostasis and vascular disease. Curr Opin Hematol. 2018;25:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Aryal B, Suarez Y. Non-coding rna regulation of endothelial and macrophage functions during atherosclerosis. Vascul Pharmacol. 2019;114:64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rosano S, Cora D, Parab S, Zaffuto S, Isella C, Porporato R, Hoza RM, Calogero RA, Riganti C, Bussolino F, Noghero A. A regulatory microrna network controls endothelial cell phenotypic switch during sprouting angiogenesis. Elife. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50:349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Breart B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y, Adams RH, Lafaille JJ, Escalante-Alcalde D, Morris AJ, Schwab SR. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208:1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Saba JD. The low down on sphingosine-1-phosphate lyase as a regulator of thymic egress. J Immunol Sci. 2017;1:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zamora-Pineda J, Kumar A, Suh JH, Zhang M, Saba JD. Dendritic cell sphingosine-1-phosphate lyase regulates thymic egress. J Exp Med. 2016;213:2773–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chew WS, Wang W, Herr DR. To fingolimod and beyond: The rich pipeline of drug candidates that target s1p signaling. Pharmacol Res. 2016;113:521–532 [DOI] [PubMed] [Google Scholar]
- 208.Stepanovska B, Lange AI, Schwalm S, Pfeilschifter J, Coldewey SM, Huwiler A. Downregulation of s1p lyase improves barrier function in human cerebral microvascular endothelial cells following an inflammatory challenge. Int J Mol Sci. 2020;21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Weigel C, Huttner SS, Ludwig K, Krieg N, Hofmann S, Schroder NH, Robbe L, Kluge S, Nierhaus A, Winkler MS, Rubio I, von Maltzahn J, Spiegel S, Graler MH. S1p lyase inhibition protects against sepsis by promoting disease tolerance via the s1p/s1pr3 axis. EBioMedicine. 2020;58:102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, Zhang F, Mundlos S, Christiansen L, Steemers FJ, Trapnell C, Shendure J. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]