Abstract
The treatment of active cardiac sarcoidosis (CS) usually involves immunosuppressive therapy, with the goal of preventing inflammation-induced scar formation. In most cases, steroids remain the first-line treatment for CS. However, given the side effect profile of their long-term use, steroid-sparing therapies are increasingly used. There are no published randomized trials of steroid-sparing agents in CS. We sought to do a systematic review to evaluate the current published data on the use of non-steroidal treatments in the management of CS. We searched the Cochrane Library, Ovid Medline, Ovid Embase, PubMed, and Web of Science Core Collection databases from inception of database to August 2020 to identify the effectiveness of biological or synthetic disease-modifying antirheumatic agents (s- and bDMARDs). Secondary objectives include safety profile as well as the change in the average corticosteroid dose after treatment initiation. Twenty-three studies were ultimately selected for inclusion which included a total of 480 cases of CS treated with a range of both s- and bDMARDs. In all included studies, sDMARDs and bDMARDs were studied in combination with steroids or as second or higher-line treatments after therapeutic failure or intolerance to corticosteroid use. Methotrexate (MTX) and infliximab (IFX) were the most common synthetic and biologic DMARDs studied respectively, reported in about 35% of the studies reviewed. The use of steroid-sparing agents was associated with a reduction in the maintenance steroid dose used. In conclusion, steroids will remain as the cornerstone of anti-inflammatory management in patients with CS until trials on the use and safety profile of other immunosuppressive agents are completed and published.
Keywords: Cardiac sarcoidosis, Corticosteroids, Nonsteroidal treatment
1. Introduction
The treatment of active cardiac sarcoidosis (CS) usually involves immunosuppressive therapy [1], despite a paucity of high quality of evidence demonstrating benefit. Cardiac sarcoidosis is often seen in conjunction with systemic sarcoidosis, but cardiac involvement may also occur in the absence of other systemic manifestations.[2] Clinical CS is thought to occur in about 5% of patients with sarcoidosis. It is estimated that in patients with systemic sarcoidosis, asymptomatic CS ranges from 25% in U.S. studies to 58% in studies from Japan [3], [4], [5]. However, autopsy data suggest that the prevalence of cardiac involvement on pathology may be as high as 70% [4], [6], with isolated CS seen in almost 25% of the total cases [7], [8]. Identification of CS is important as the proportion of deaths from sarcoidosis attributed to cardiac causes may be as high as 58% [4].
The goal of immunosuppressive therapies in CS is to prevent clinical sequelae by preventing fibrosis and myocardial remodelling induced by inflammation as focal areas of inflammation can progress to scar formation. Adverse clinical sequalae arise from these pathological processes in CS, leading to consequences such as atrial/ventricular tachycardias, high-degree atrioventricular block, ventricular dysfunction, heart failure and/or sudden cardiac death. These sequelae can potentially be improved by treatment focused on reducing inflammation and subsequent scar formation [2]. In most cases, glucocorticoid therapy remains the first-line immunosuppressive treatment for CS [9], with serial cardiac imaging (such as 18F-FDG (fluorodeoxyglucose) PET (positron emission tomography) or cardiac MRI) often used for the longitudinal assessment of treatment response [10], [11]. However, given the side effect profile of long-term glucocorticoid use such as an increased risk of infections, diabetes, weight gain, osteoporosis, etc, steroid-sparing treatment options such as methotrexate (MTX), azathioprine, cyclophosphamide, mycophenolate mofetil (MMF) and anti-tumor necrosis factor (TNF) alpha therapies (infliximab (IFX), etanercept, adalimumab, certolizumab pegol) are increasingly used in CS. Local practice rather than high-quality evidence usually guides selection of steroid sparing agents, and there is limited data showing benefit of steroid-sparing agents in CS. There are no published randomized trials of steroid-sparing agents in systemic or cardiac sarcoidosis. The aim of this systematic review is to evaluate the current published data describing the use of non-steroidal treatments in the management of CS, focusing on their effectiveness in cardiac clinical and imaging parameters and safety profile when used as adjunct or alternative therapies to glucocorticoid management.
2. Methods
A systematic review assessing the effectiveness of DMARDs alone or in combination with corticosteroids in the treatment of CS was conducted according to the PRISMA (Preferred Reporting Items For Systematic Reviews And Meta-Analyses) statement [12]. We systematically searched the Cochrane Library, Ovid Medline, Ovid Embase, PubMed, and Web of Science Core Collection databases from inception of database to August 2020.
Databases were searched using a combination of controlled and free text terms (search strategy in the Supplement). The search was not limited by language, publication type, or publication date. The search was peer-reviewed by a second librarian using PRESS (Peer Review for Electronic Search Strategies) [13]. The final searches were performed in all the databases on August 29, 2020. Citations from all databases were imported into an Endnote X9 library and then ingested into Covidence (v1517 e4b75b54, 2020), a screening and data extraction tool. Two independent reviewers (CGK, BDY) performed a title abstract and full text review with disagreements resolved by a third reviewer (EJM).
The primary endpoint was to identify the effectiveness of nonsteroidal anti-inflammatory agents including, but not limited to, the incidence of adverse clinical events, changes in imaging parameters, such as cardiac FDG PET (myocardial FDG tracer uptake and/or perfusion defects), echocardiography (i.e. ventricular function), or cardiac MRI (i.e. late gadolinium enhancement; ventricular function) as well as changes in arrhythmic burden and circulating biomarkers. Secondary objectives included the safety profile of such interventions as well as the change in the average corticosteroid dose after treatment initiation. Studies were deemed eligible for inclusion if they included adult patients with CS diagnosed based on endomyocardial biopsy and/or systemic sarcoidosis with imaging or clinical findings indicative of cardiac involvement. Studies must have also included treatment with steroid-sparing medications with or without corticosteroids, and results regarding the efficacy of the steroid-sparing regimen. Meeting and conference abstracts as well as studies in any language were also included if a translation of the abstract was available in English. Studies without report of cardiac involvement, studies reporting treatment with steroids alone, or with insufficient information to extract data on the effectiveness of the steroid-sparing interventions were excluded. Case reports were also excluded to reduce the effect of selection/reporting bias. Finally, studies by the same research groups describing duplicate populations were also reviewed and the most recent one or the one with the largest population were included. The excluded studies and exclusion criteria for each study are summarized in the Supplement (Table S1).
Extracted data included patient and study characteristics as well as effectiveness on clinical, imaging, and safety outcomes. Baseline characteristics included demographics, baseline cardiac profile, steroidal, and non-steroidal treatments given (including dosing and frequency if provided), cardiac outcome measures, and safety profile (adverse events). Given the observational nature and heterogeneity of the studied interventions and populations, no quantitative summary (i.e. meta-analysis) was feasible.
Risk of bias was assessed using the ROBINS-I (“Risk Of Bias In Non-randomised Studies of Interventions”) tool [14], focusing on the following key domains: confounding, selection of participants (pre-intervention), measurement of interventions (at intervention), deviations from intended interventions, missing data, measurements of data, selection of the reported result (post-intervention).
3. Results
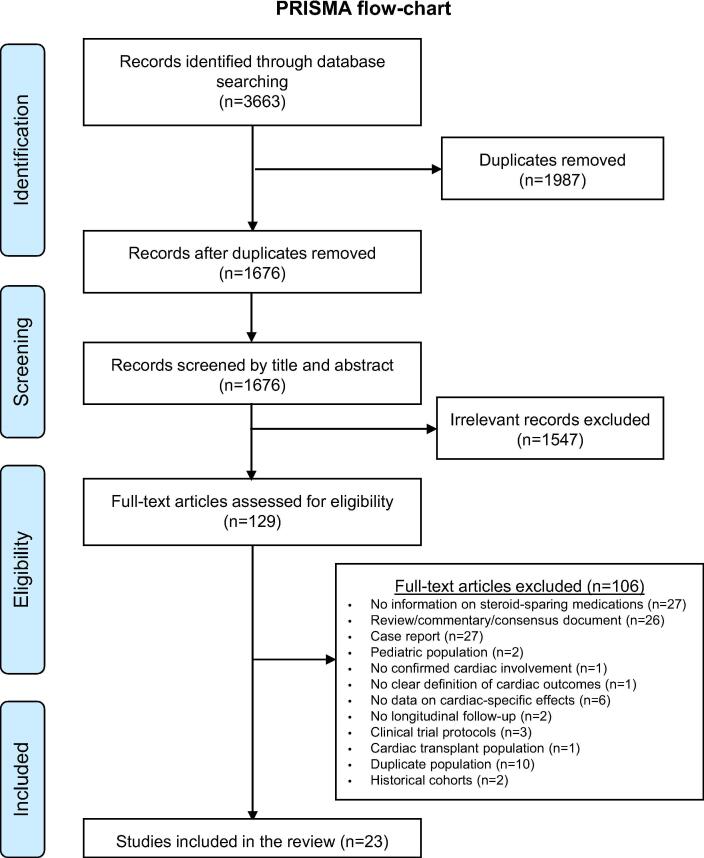
Our systematic search yielded 1676 unique titles following title and abstract screening, of which 129 were retrieved for full text review. Of these, 23 studies were ultimately selected for inclusion in our study (Fig. 1). These studies included a total of 480 cases of CS treated with a range of both synthetic (methotrexate, n = 83; MMF, n = 68; cyclophosphamide, n = 22; azathioprine, n = 19; cyclosporine A used post-heart transplant, n = 1) and biological disease-modifying antirheumatic agents (infliximab, n = 171; adalimumab, n = 64, rituximab, n = 6, etanercept, n = 1, golimumab = 1– excluding the study by Jamilloux et al. [15] which did not specify the biologics given in the CS patient subset. The median age ranged from 36 to 66 years and proportion of female patients ranged from 25.7% to 80% (Table 1). The cardiac outcome measures are summarized in Table 2, whereas the doses used and reported adverse events are presented in the Supplement (Table S2). Studies differed in the criteria used for diagnosis of CS, with some studies relying on the Heart Rhythm Society consensus criteria [1], while others used the Japanese Circulation Society [16], WASOG (World Association of Sarcoidosis and other Granulomatous Disorders) criteria [17] or Japanese Society of Cardiology consensus diagnostic criteria [18] (Table 3). Regardless of the diagnostic criteria used, studies showed cases with a high prevalence of ventricular dysfunction at baseline, high arrhythmic burden (predominantly ventricular tachycardias), high-grade atrioventricular block, and need for pacemaker or implantable cardioverter-defibrillator (Table S1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart.
Table 1.
Design and baseline demographics of the included studies.
| Author, year | Country | Study type | Publication type | Years | Study population | Non-steroidal treatment studied (n) | Patients receiving non-steroidal treatment (n) | Age (years) | Female sex (n, %) | Race (n, %) |
|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic disease-modifying antirheumatic drugs (sDMARD) | ||||||||||
| Yazaki et al, 2014 [19] | Japan | Retrospective, single-center cohort | abstract | N/A | CS patients with addition of MTX due to relapse, deterioration or steroid-related adverse effects | MTX | 7 | N/A | N/A | N/A |
| Yokomatsu et al, 2018 [20] | Japan | Retrospective, single-center cohort | abstract | N/A | CS patients treated with MTX with sequential PET scans at least 12 months apart | MTX | 6 | mean: 66 | 4 (66.7%) | N/A |
| Nagai et al, 2014 [21] | Japan | Retrospective, single-center cohort | full | N/A | CS patients followed every three months for five years in the CS clinic | MTX | 10 | 65.9 ± 7.7 | 8 (80%) | N/A |
| Ballul et al, 2019 [23] | France | Retrospective, single-center cohort | full | 2012–2016 | Consecutive patients with histologically proven sarcoidosis | MTX (5, 41.7%), AZA (5, 41.7%), CP (n = 2, 16.7%) | 12 | 50.6 (mean) | 6 (50%) | Black (12, 100%) |
| Chapelon-Abric et al, 2017 [24] | France | Retrospective, single-center cohort | full | 1995–2014 | Patients with CS treated in a single department | CP (20, 57.1%), MTX (12, 34.3%), MMF (2, 5.7%), cyclosporine A (1, 2.9% - transplant) | 35 | median: 42 (95% CI: 33–49) | 9 (25.7%) | Caucasian: 22 (63%), Black: 12 (34%), Asian: 1(3%) |
| Fussner et al, 2016 [25] | USA | Retrospective, two-center cohort | abstract | 1994–2014 | Patients with CS who received MMF in two large academic centers | MMF | 33 | median: 51 [IQR: 47–58] | 9 (27%) | Caucasian (26, 79%) |
| Griffin et al, 2018 [26] | USA | Retrospective, single-center cohort | abstract | N/A | CS patients who received combination therapy of MMF with prednisone | MMF | 25 | 51.5 ± 11.4 | 10 (40%) | Black: 10 (40%) |
| Biological disease-modifying antirheumatic drugs (bDMARD) | ||||||||||
| Rosenthal et al, 2019 [22] | USA | Retro-/prospective, single-center cohort | full | 2009–2018 | Treatment-naïve CS patients with two consecutive cardiac PET scans (6 months apart) | MTX (25) ± ADA (19, if persistent symptoms or intolerance to MTX) | 28 | 52 | 12 (42.8%) | N/A |
| Sethi et al, 2018 [38] | USA | Retrospective, single-center cohort | abstract | N/A | CS patients with at least two sequential cardiac PET scans | MTX (15, 100%), ADA (added in 8 [53%]) | 15 | N/A | N/A | N/A |
| Estephan et al, 2017 [27] | USA | Retrospective, single-center study | abstract | 2013–2014 | Sarcoidosis patients with cardiac PET suggesting cardiac involvement | IFX (8, 53.3%), ADA (1, 6.7%), MMF (7, 46.7%), AZA (2, 13.3%) | 15 | N/A | N/A | N/A |
| Kandolin et al, 2017 [28] | Finland | Retrospective, single-center cohort | abstract | 2012–2017 | Biopsy-proven CS patients receiving IFX as fourth-line treatment due to persistent disease activity, adverse events or intolerance to other medications | IFX | 9 | 53 ± 11.1 | 6 (67%) | N/A |
| Kowlgi et al, 2019 [29] | International | Retrospective, multi-center cohort | abstract | N/A | Refractory CS that have failed treatment with at least one immunosuppressant | IFX | 27 | 54 [45–59] | 8 (29.6%) | N/A |
| Chapelon-Abric et al, 2015 [30] | France | Retrospective, single-center cohort | full | 2005–2013 | Consecutive patients with biopsy-proven, severe and treatment-resistant sarcoidosis with cardiac and/or neuro involvement | IFX | 16 | median: 36 [range: 26–43] | 7 (43.8%) | Caucasian: 10 (62.5%), Black: 5 (31.3%), Asian: 1 (6.3%) |
| Harper et al, 2019 [31] | USA | Retrospective, single-center cohort | full | N/A | CS patients on IFX due to refractory arrythmias or persistently elevated 18F-FDG uptake with cardiac symptoms | IFX | 36 | 50 ± 11 | 10 (27.8%) | White (28, 77.8%); Black (8, 22.2%) |
| Cundiff et al, 2019 [32] | USA | Retrospective, single-center cohort | abstract | N/A | Patients with metabolically active CS (defined by cardiac PET) treated with TNFi due to disease progression or intolerance, contraindications to steroids. | IFX (8, 88.9%), ADA (1, 11.1%) | 9 | N/A | N/A | N/A |
| Sinokrot et al, 2019 [33] | USA | Retrospective, single-center cohort | abstract | 2016–2018 | Refractory CS disease (dysrhythmias, cardiomyopathy and persistent 18F-FDG uptake) despite 1st/2nd-line therapies | IFX | 5 | N/A | N/A | N/A |
| Devraj et al, 2020 [34] | USA | Retro-/prospective, single-center cohort | abstract | 2013–2018 | All CS patients treated with biologics due to disease progression or intolerance, contraindications to standard therapy.All had received steroids prior to initiation | ADA (7, 58.3%), IFX (4, 33.3%) and RXM (1, 8.3%) | 12 | 52 ± 8 | 50% | Black (9, 74%); White (3, 25%) |
| Puyraimond-Zemmour et al, 2017 [35] | France | Retrospective, multi-center cohort | abstract | N/A | Patients with definite histologically proven extra-thoracic sarcoidosis involving the heart who received a TNFi | IFX (24, 96%), ETN (1, 4%) | 25 | 38 | N/A | N/A |
| Jamilloux et al, 2017 [15] | France | Retrospective, multi-center cohort | full | 2014–2015 | Sarcoidosis patients treated with anti-TNF agents (28/132 [21.2%] had cardiac involvement) | IFX (120, 91%), ADA (8, 6%), ETN (3, 2%), CZP (1, 1%) | 132 (28 with CS) | mean: 45.5 [range: 14–78] | 76 (57.6%) | Caucasian: 88 (66.7%), Black: 37 (28%), Asian: 4 (3%), N/A: 3 (2.2%) |
| Krause et al, 2016 [41] | USA | Retrospective, single-center cohort | abstract | N/A | All CS cases treated with RXM due to failure of 1st/2nd-line treatment (corticosteroids ± MMF (80%), MTX (40%), AZA (20%), IFX (20%), leflunomide (20%)) with ≥ 1 follow-up | RXM | 5 | 50.9 ± 8.8 | 2 (40%) | N/A |
| Baker et al, 2019 [37] | USA | Retrospective, single-center cohort | full | 2009–2018 | All CS cases treated with TNFi for worsening imaging findings | IFX (10, 50%), ADA (10,50%-one patient had received IFX) Golimumab (1, 5%) | 77 (TNFi only 20) | Mean 55 (median 58 years) | 39% | 66% Whitie, 16% Black, 9% Asians, 9% Hispanics |
| Gilotra et al, 2020 [39] | USA | Retrospective, multi-center | full | 2014–2019 | All CS were treated with TNFi for 1) persistent cardiac inflammation on FDG-PET despite immunosuppression 2) clinically active CS and/or 3) into side effects from immunosuppression agents. | IFX (30, 79%), ADA (8, 21%) | 38 | Mean 49.9 | 42% | 53% Black |
| Injean et al, 2019 [36] | USA | Retrospective, single center | abstract | 2014–2019 | Not specified. | Multiple. IFX (3, 21%), ADA (2, 14%). Also, steroids, AZA, MTX, MMF, HCQ, CP, tacrolimus | 14 | 58 | 40% | N/A |
ADA: adalimumab; AZA: azathioprine; CP: cyclophosphamide; CS: cardiac sarcoidosis; CZP: certolizumab pegol; ETN: etanercept; FDG: fluorodeoxyglucose; HCQ: hydroxychloroquine; IFX: infliximab; MMF: mycophenolate mofetil; MTX: methotrexate; N/A: not available; PET: positron emission tomography; RXM: rituximab; TNF(i): tumor necrosis factor (inhibitor). Data presented as mean ± standard deviation and n (%) unless specified otherwise.
Table 2.
Cardiac effects of non-steroidal treatments in cardiac sarcoidosis.
| Author, year | Non-steroidal treatment studied | Other prior/concurrent treatments | Study size (n) | Follow-up (months) | Cardiac outcomes |
|---|---|---|---|---|---|
| Synthetic disease-modifying antirheumatic drugs (sDMARD) | |||||
| Yazaki et al, 2014 [19] | MTX | [Concurrent]: maintenance corticosteroids | 7 | N/A |
|
| Yokomatsu et al, 2018 [20] | MTX | [Concurrent]: prednisolone taper | 6 | mean: 17.3 |
|
| Nagai et al, 2014 [21] | MTX | [Concurrent]: prednisolone | 10 (7 steroid-only) | 12, 36, 60 |
|
| Ballul et al, 2019 [23] | MTX (5, 41.7%), AZA (5, 41.7%), CP (2, 16.7) | [Concurrent]: corticosteroids | 12 (24 steroid-only) | median: 3.6 [range: 1–15.2] months |
|
| Chapelon-Abric et al, 2017 [24] | CP (20, 57.1%), MTX (12, 34.3%), MMF (2, 5.7%), cyclosporine A (1, 2.9% - transplant) | [Concurrent]: corticosteroid taper | 59 (35 treated with steroid-sparing) | median: 60 (95% CI: 42–86) |
|
| Fussner et al, 2016 [25] | MMF | [Concurrent]: prednisone (32, 97%), TNFi (2, 6%), cyclosporine (1, 3%) | 33 | median: 22 [IQR: 13–104] |
|
| Griffin et al, 2018 [26] | MMF | [Concurrent]: prednisone | 25 (MMF), 12 prednisone only | 12 months (n = 21, 84%) |
|
| Biological disease modifying antirheumatic drugs (bDMARD) | |||||
| Rosenthal et al, 2019 [22] | MTX (25) ± ADA (19, if persistent symptoms or intolerance to MTX) | [Concurrent]: Prednisone taper | 28 | mean: 49.2 (±18) |
|
| Sethi et al, 2018 [38] | MTX (15, 100%), ADA (added in 8 [53%]) | [Concurrent]: Corticosteroids (94%) | 15 | median: 24 |
|
| Estephan et al, 2017 [27] | IFX (8, 53.3%), ADA (1, 6.7%), MMF (7, 46.7%), AZA (2, 13.3%) | [Concurrent]: Prednisone (14, 93.3%) | 15 (9 [60%] with PET follow-up) | 6–12 months |
|
| Kandolin et al, 2017 [28] | IFX | [Prior]: Steroids (9, 100%), MTX or AZA (n = 8, 88.9%) | 9 | mean: 14.8 [range: 4–37] |
|
| Kowlgi et al, 2019 [29] | IFX | [Prior]: steroids ± steroid-sparing agents: MTX (70%), AZA (25%) and HCQ (10%) | 27 | 21 [12.5–35.5] |
|
| Chapelon-Abric et al, 2015 [30] | IFX | [Prior]: CP (13, 81.3%), MTX (11, 68.8%), MMF (5, 31.3%), AZA (1, 6.3%), ETN (1, 6.3%) | 16 (4 with CS) | median: 57 [range: 2–91] |
|
| Harper et al, 2019 [31] | IFX | [At IFX initiation] steroids (32, 88.9%), MTX (25, 69.4%), leflunomide (9, 25%), AZA (1, 2.8%), HCQ (2, 5.6%) | 36 | 6 (in 35/36, 97.2%), 12 (in 29/38, 76.3%) |
|
| Cundiff et al, 2019 [32] | IFX (8, 88.9%), ADA (1, 11.1%) | [Prior]: steroids (8, 88.9%) | 9 | mean: 7 |
|
| Sinokrot et al, 2019 [33] | IFX | [Prior]: prednisone (5, 100%), MTX (4, 80%), HCQ (1, 20%) | 5 | mean: 12 (±6) |
|
| Devraj et al, 2020 [34] | ADA (7, 58.3%) IFX (4, 33.3%) and RXM (1, 8.3%%) | [Prior]: prednisone (12) | 12 | N/A |
|
| Puyraimond-Zemmour et al, 2017 [35] | IFX (24, 96%), ETN (1, 4%) | [Prior]: MTX (24, 96%), CP (12, 48%), AZA (8, 32%), and MMF (6, 24%) | 25 | 50.7 |
|
| Jamilloux et al, 2017 [15] | IFX (120, 91%), ADA (8, 6%), ETN (3, 2%), CZP (1, 1%) | [Prior]: Steroids (n = 113, 85.6%), MTX (n = 81, 61.4%), AZA (n = 10, 7.6%), MMF (n = 6, 4.5%) | 132 (28 with CS) | 20.5 [IQR 8–48] months |
|
| Krause et al, 2016 [41] | RXM | [Concurrent]: prednisone (all), MTX (20%) | 5 | Median: 9.6 (range 2.4–22.8) |
|
| Baker et al, 2019 [37] | IFX (20, 26%)ADA 10 (13%)Golimumab 1(1%) | [Concurrent/prior prednisone (69), MTX alone (2) , [Concurrent} MTX 55(71%), AZA 8(10%), HCQ 5 (7%), MMF 4 (5%), Leflunomide 2 (3%) | 77 (20 of these used TNF alfa inhibitors | Mean 4.8 years |
|
| Gilotra et al, 2020 [39] | IFX (30, 79%)ADA (8, 21%) | [Prior]: steroids (38, 100%), SSA (37, 99%) | 38 | 486 (IQR 405 days) |
|
| Injean et al, 2019 [36] | IFX (3,21%)MMF (1,7%)Steroids (10, 78%)AZA (5, 36%)MTX (3, 14%)HCQ (1, 7%)Tacrolimus (1, 7%) | N/A | 14 | N/A |
|
ADA: adalimumab; AZA: azathioprine; (NT-pro)-BNP: N-terminal pro-brain natriuretic peptide; CP: cyclophosphamide; CS: cardiac sarcoidosis; CZP: certolizumab pegol; EKG: electrocardiogram; ePOST: extrapulmonary physician organ severity tool; ETN: etanercept; FDG: fluorodeoxyglucose; HCQ: hydroxychloroquine; IFX: infliximab; IQR: interquartile range; MMF: mycophenolate mofetil; MRI: magnetic resonance imaging; MTX: methotrexate; N/A: not available; PET: positron emission tomography; RXM: rituximab; TNF(i): tumor necrosis factor (inhibitor); VAD: ventricular assist device.
Table 3.
Cardiac involvement at baseline.
| Author, year | Cardiac sarcoidosis definition | LV dysfunction (n, %) | Arrhythmias (n, %) | High-grade atrioventricular block (n, %) | Pacemaker/ICD (n, %) |
|---|---|---|---|---|---|
| Ballul et al, 2019 [23] | Heart Rhythm Society consensus criteria [1] | LVEF < 50%: 6 (50%) | 4 (33.3) with sustained AT/VT | 3 (25%) | Pacer/ICD in 22.2%/13.9% of the total population |
| Baker et al, 2019 [37] | Heart Rhythm Society consensus criteria | LVEF < 50% (80%) | 11 (55%) | 7 (35%) | N/A |
| Chapelon-Abric et al, 2015 [30] | International criteria [16] | N/A | N/A | N/A | N/A |
| Chapelon-Abric et al, 2017 [24] | International criteria [16] | 38 (64.4%) with LV dysfunction | 17 (28.8%) with ventricular arrhythmias | 15 (25.4%) for AV block | N/A |
| Cundiff et al, 2019 [32] | N/A | LVEF (mean ± SD): 45 ± 15% | N/A | N/A | N/A |
| Devraj et al, 2020 [34] | N/A | N/A | 3 (25%) with ventricular arrhythmias | 1 (8.3%) | N/A |
| Estephan et al, 2017 [27] | Biopsy-proven sarcoidosis with cardiac PET imaging for cardiac involvement | N/A | N/A | N/A | N/A |
| Fussner et al, 2016 [25] | Heart Rhythm Society consensus criteria [1] | LVEF < 40%: 17 (52%) | 11 (33%) with ventricular arrhythmias | 12 (36%) | N/A |
| Gilotra et al, 2020 [39] | Heart Rhythm Society consensus criteria or Japanese imaging criteria | 48.5% | 13 (34%) | 5 (13%) | 27 (71%) |
| Griffin et al, 2018 [26] | N/A | LVEF < 50%: 22 (88%) vs 8 (66.7%) | 8 (32%) vs 1 (8%) with VT | 7 (28%) vs 2 (16.7%) | N/A |
| Harper et al, 2019 [31] | WASOG criteria [17] | LVEF < 30%: 6 (16.7%) | 8 (22.2%) with VT | 7 (19.4%) | 4 (11.1%) with pacing |
| Injean et al, 2019 [36] | Heart Rhythm Society consensus criteria | N/A | 15% | 29% | N/A |
| Jamilloux et al, 2017 [15] | N/A | N/A | N/A | N/A | N/A |
| Kandolin et al, 2017 [28] | Biopsy-proven | LVEF (mean ± SD): 44 ± 12.7% | 2 (22.2%) with PVC's | 4 (44.4%) | N/A |
| Kowlgi et al, 2019 [29] | N/A | N/A | 10 (37%) with VT | 11 (41%) with CHB | N/A |
| Krause et al, 2016 [41] | Endomyocardial biopsy or biopsy of different organ plus consistent cardiac imaging | N/A | N/A | N/A | [ICD in all during follow-up] |
| Nagai et al, 2014 [21] | Japanese Society of Sarcoidosis and Other Granulomatous Disorders criteria [50] |
LVEF < 50% (4, 40%) | N/A | 6 (60%) with unspecified AV block | 8 (80%) on pacemaker |
| Puyraimond-Zemmour et al, 2017 [35] | Heart Rhythm Society consensus [1] | 9 (36%) had heart failure or chest pain | ~18 (72%) had abnormal findings on EKG | ~18 (72%) had abnormal findings on EKG | N/A |
| Rosenthal et al, 2019 [22] | Japanese Society of Cardiology expert consensus diagnostic criteria [18] | 11 (39.3%) with heart failure | 8 (26.6%) with atrial arrhythmias, 18 (64.3%) with sustained VT/VF, 10 (35.7%) with NSVT, 4 (14.3%) with cardiac arrest | 11 (39.2%) | 26 (92.9%) with ICD/pacer |
| Sethi et al, 2018 [38] | N/A | N/A | N/A | N/A | N/A |
| Sinokrot et al, 2019 [33] | N/A | N/A | N/A | N/A | N/A |
| Yazaki et al, 2014 [19] | N/A | N/A | N/A | N/A | N/A |
| Yokomatsu et al, 2018 [20] | N/A | mean LVEF: 41.8% | N/A | N/A | 1 (16.7%) with pacer, 2 (33.3%) with CRT-D |
CRT-D: cardiac resynchronization device-defibrillator; EKG: electrocardiogram; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; PVC: premature ventricular contraction; SD: standard deviation; (NS)VT: (non-sustained) ventricular tachycardia; VF: ventricular fibrillation; WASOG: World Association of Sarcoidosis and Other Granulomatous Diseases.
3.1. Methotrexate, cyclophosphamide and azathioprine
Three small retrospective, single-center cohort studies [19], [20], [21] have examined the cardiac effects of methotrexate when used as an adjunct therapy with low-dose maintenance steroids [19], or a prednisolone taper [20], [21] for CS relapse due to poor response to corticosteroid use or due to steroid-related adverse effects. In a study by Yazaki et al. [19], use of methotrexate (7.5 mg orally weekly) resulted in improved myocardial perfusion defects and reduced myocardial uptake of 67Ga on PET in two out of seven patients (28.6%). It was also associated with a significant decrease in the average daily prednisone dose (Table S2), as well as a significant decrease in HbA1c, and triglyceride levels (Table 2). In a study by Yokumatsu et al. [20], methotrexate use (initial dose of 6 mg per week together with a prednisolone taper) in six patients followed over a mean period of 17.3 months resulted in a significant reduction of 18F-FDG uptake in all cases with almost complete resolution of FDG uptake in three patients (50%). There was also improvement of left ventricular ejection fraction (LVEF) with an increase from 41.8% to 45.4% (level of significance not provided). In the latter study, pneumonia was reported as an adverse event in one patient (16.7%). A separate study which compared patients on methotrexate and prednisolone (n = 10) versus prednisolone alone (n = 7) showed that the combined regimen was associated with stabilization of LVEF and NT-proBNP (N-terminal pro-brain natriuretic peptide) over five years of follow-up, as opposed to the prednisolone group which experienced a gradual drop in LVEF and eventual increase in mean NT-proBNP which was statistically significant [21]. Also, in a separate retrospective study of 25 patients receiving methotrexate in addition to prednisone [22], methotrexate resulted in a reduction and resolution of 18F-FDG uptake in 88% and 60% of the total cases, respectively.
Two retrospective single-center cohort studies from France have looked at methotrexate and/or the addition of the synthetic disease-modifying antirheumatic drugs (sDMARDs) azathioprine and cyclophosphamide as adjunct therapies to corticosteroids. In a study by Ballul et al. [23], 12 patients receiving steroid-sparing treatment with methotrexate (15–20 mg/week; n = 5, 41.7%), azathioprine (2 mg/kg/day; n = 5, 41.7%) or cyclophosphamide (0.7 mg/m2 every 4 weeks for 24 weeks; n = 2, 16.7%) were compared to 24 patients receiving steroid-only therapy. Over a median follow-up of 3.6 months, clinical relapse (defined as LVEF reduction, 3rd degree atrioventricular block, atrial/ventricular tachycardia or sudden cardiac death) was seen in 6.7% of the sDMARD group versus 45.8% of the steroid-only group (p = 0.048). Severe infections were seen in 16.7% of patients in each study group (p = NS). Similarly, in a study by Chapelon-Abric et al. [24], use of cyclophosphamide (500–700 mg/m2/month intravenously, n = 20, 57.1%) followed by standard therapy with methotrexate (0.3 mg/kg/week) with a steroid taper also resulted in a higher cardiac recovery (absence of cardiac clinical symptoms and normalization of all abnormal baseline examinations: ECG, Holter monitor, echocardiogram, other cardiac imaging) rate (n = 29/35; 82.9%) compared to the steroid-only group (n = 18/24; 75%). No severe infections or other adverse events such as liver function test abnormalities were reported.
3.2. Mycophenolate mofetil (MMF)
Evidence on the use of MMF in the treatment of CS comes from two retrospective studies. One retrospective study of 33 patients who received MMF in addition to prednisone (n = 32, 97%), TNF inhibitors (n = 2, 6%) or cyclosporine (n = 1, 3%) [25] use of MMF allowed twenty (of 32, 63%) to be tapered off prednisone. After initiation of MMF, the median survival without the composite endpoint of ventricular-assist device, transplant or both, was 79 months suggesting benefit in immunosuppression [25]. A second study [26], compared 25 patients receiving MMF with prednisone to 12 patients on prednisone alone. Twelve month follow-up was available in 21 patients and demonstrated beneficial effects on cardiac function (mean delta[LVEF] of 6.95% in the MMF group versus −2.2% in the steroid-only group, p < 0.05), and follow-up 18F-FDG-PET in 19 patients in the MMF group showed complete resolution of inflammation in six (31.6%) patients and partial improvement in eight (42.1%) patients. Adverse events for sDMARDs include infection, diabetes, cytopenias (with MMF), rash, and tremor (Table S2).
3.3. TNF inhibitors (infliximab, adalimumab, etanercept)
Anti-TNF agents are increasingly used biological agents in the management of CS according to our review of the literature and knowledge of expert centers’ clinical practice. Most studies have focused on infliximab [15], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37] a chimeric monoclonal antibody biologic which acts by binding TNF-α, and adalimumab, a fully human monoclonal antibody [15], [22], [27], [32], [34], [36], [37], [38].
All studies reporting on TNF inhibitors were retrospective cohort studies, with TNF inhibitors used as second, third, or even fourth line treatments following the failure of corticosteroid- or sDMARD-based regimens [15], [22], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [38], [39], as summarized in Table 1, Table 2. Infliximab was often started at a dose of 3–5 mg/kg at weeks 0, 2 and 6, followed by a dose of 5 (less frequently 7.5 or 10) mg/kg every 4–8 weeks [15], [28], [30], [31] (Table S2). Where reported, adalimumab was given at a dose of 40 mg subcutaneously every other week [15], [22]. TNF inhibition was associated with a subsequent reduction (or resolution) in myocardial inflammation assessed by 18F-FDG PET [22], [27], [28], [29], [32], [34], [39], normalization of perfusion defects [38], significant decrease in NT-proBNP [28], remission of cardiac involvement by gadolinium-enhanced cardiac MRI [30], [33] as well as by a decrease in the cardiac extrapulmonary physician organ severity tool (ePOST) score [15], [40] and mean dose of corticosteroids used [30], [31], [33], [35]. Effects on left ventricular function were less consistent, with most studies demonstrating no significant change in LVEF, including the largest series by Gilotra et al. [28], [29], [31], [32], [33], [39], while others showed a modest longitudinal increase [38] (Table 2). Finally, some studies also suggest beneficial effects on arrhythmic burden [39], with a study by Kowlgi et al reporting a ten-fold reduction in ventricular tachycardia after initiation of infliximab [29], while other small studies reported similar decreases which did not reach statistical significance [22], [28], [31]. Reported adverse events while on TNF inhibitors (Table S2) included infections (pulmonary infections, prostatitis, aspergillosis, atypical mycobacterial disease, bacterial sepsis, multiviral reactivation, uterine infection, C. difficile infection, shingles, disseminated cryptococcus infection), elevated liver enzymes, pancytopenia, leukoencephalopathy, decompensated heart failure, and cardiac arrest [15], [28], [30], [31], [32], [35].
3.4. Rituximab
Rituximab, a chimeric monoclonal antibody against the protein CD20 which is primarily found on B cells, has also been used in the management of CS cases that have failed treatment with steroids ± sDMARDs or anti-TNF inhibitors, such as infliximab [41]. In a retrospective single-center cohort of five patients who received rituximab (at a dose of 1000 mg intravenously two weeks apart for a total of two doses) and were followed over a median period of 0.8 years (range: 0.2 to 1.9 years), rituximab use was associated with decreased 18F-FDG uptake in all five patients (100%), improved LVEF in three patients (60%), no change in LVEF in one patient (20%) and a drop from an LVEF of 45% to 28% in one patient (20%), with no reported adverse events [41]
3.5. Risk of bias assessment
All studies were found to be at moderate to high risk of bias given their retrospective nature, missing data, incomplete follow-up, lack of an appropriate comparator group and absence of a pre-defined research protocol (Table S3).
4. Discussion
Our systematic review demonstrates that steroid-sparing immunosuppressive agents, such as sDMARDs and bDMARDs are widely used as second- or third-line treatment options in the management of CS and in some cases, as first-line therapy in combination with corticosteroids. This may be due to treating clinicians’ assessment of corticosteroid treatment failure or as alternative agents when there are adverse effects or contraindications to steroid use. More importantly, our systematic review highlights a series of points related to the use of these agents, including significant variations in treatment and monitoring protocols and variable outcomes used to judge their effectiveness.
Corticosteroids remain the cornerstone of anti-inflammatory management in patients with CS and imaging or biopsy evidence concerning for myocardial inflammation.[9] In all included studies, sDMARDs and bDMARDs were studied in combination with steroids or as second or higher-line treatments after therapeutic failure or due to intolerance to corticosteroid use. Not surprisingly, the use of steroid-sparing agents was associated with a reduction in the maintenance steroid dose used.
sDMARDs, such as methotrexate [19], [20], [21], [22], [23], [24] seem to be the preferred second-line treatment option based on the number of publications describing case series with these agents. However, in our experience, we recommend considering concomitant use of methotrexate (or other sDMARDs) and steroids in complicated cases (i.e., moderate left ventricular dysfunction, large myocardial scar burden, high arrhythmic burden or in those anticipated to require long-term corticosteroid use). Alternative agents that have been used include hydroxychloroquine, azathioprine, cyclosporine, MMF and leflunomide [42], though these are much less studied for the treatment of CS. In cases of therapeutic failure, adverse effects or contraindications to their use, targeted bDMARDs, usually TNF inhibitors, such as infliximab or adalimumab have been used [15], [22], [27], [28], [29], [30], [31], [32], [33], [34], [35], [38]. Although TNF inhibitors are traditionally contraindicated in patients with heart failure, TNF inhibition may be a reasonable therapeutic target in sarcoidosis-induced cardiac dysfunction where myocardial inflammation is the basis of the mechanical cardiac dysfunction [43]. Although a less studied option, rituximab has been used in patients that have failed multiple lines of therapy. Newer agents, such as tofacitinib, a Janus kinase (JAK) inhibitor and tocilizumab, a human IL-6 receptor monoclonal antibody, merit further study in CS given beneficial effects on extracardiac disease [44].
Imaging studies suggest that most of these treatments seem to be effective in reducing myocardial inflammation as assessed by cardiac PET. The use of 18F-FDG PET [45], [46] is now recognized as a useful tool in guiding both the initiation and de-escalation of immunosuppressive therapies in these patients [10], [11]. With regards to myocardial function, retrospective studies without control groups have mostly shown stable LVEF values over the duration of follow-up. One study comparing methotrexate use with prednisolone versus prednisolone alone suggests that methotrexate use may prevent ventricular decompensation, such as a significant drop in LVEF or rise in NT-proBNP as seen in the steroid-only group over a period of three to five years [21].
While there is an absence of completed randomized controlled trials (RCT) for CS, some are under way (Table 4). Until these trials are completed and published in several years, data on the use and safety profile immunosuppressive treatment of CS will be limited to retrospective observational studies in CS or from experience with the same agents in other more prevalent conditions such as rheumatoid arthritis. The retrospective nature of the literature on CS therapy raises concern for ascertainment and selection bias. Moreover, within the observational studies, the decision to initiate a specific therapeutic regimen and deploy imaging modalities and/or Holter/EKG for longitudinal monitoring was guided by the clinical course of each patient and local institutional practices, which resulted in incomplete follow-up and missing data that is poorly amenable to formal quantitative meta-analysis.
Table 4.
Ongoing clinical trials of non-steroidal treatments in cardiac sarcoidosis.
| Clinical trial | ID | Sponsor location | Status | Expected completion date | Study size | Study type | Intervention studied | Comparator | Primary outcome measure | Secondary outcome measures | Eligibility criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-1 Blockade for Treatment of Cardiac Sarcoidosis (MAGiC-ART) [48] | NCT04017936 | Va, USA | Not recruiting yet | 12/2023 | 28 | RCT (double-blind) | Anakinra | Placebo | Change in CRP at 28 days | Change in cardiac PET uptake, LGE on MRI, hospitalizations/cardiac deaths at 28 days | Adults 21 years or older with a clinical diagnosis of cardiac sarcoidosis according to the Heart Rhythm Society criteria - Cardiac 18F-FDG uptake on recent PET (performed within the prior month). - CRP high-sensitivity assay > 2 mg/l. |
| Cardiac Sarcoidosis Randomized Trial (CHASM-CS-RCT) [47] | NCT03593759 | Ottawa, Canada | Recruiting | 12/2023 | 194 | RCT (open-label, non-inferiority, with blinded end-point analysis) | Methotrexate + folic acid + prednisone taper | Prednisone 0.5 mg kg/day for 6-months (max dose 30 mg per day) | SPRS on 18F-FDG-PET at 6 months | Mortality, cardiovascular hospitalizations, medication-related adverse events, QoL metrics and others at 6 months | Adults 18 years or older with CS and >=1 of the following: high-degree AV block, NSVT, sinus dysfunction, ventricular dysfunction & no alternative explanation for clinical features; & 18F-FDG-PET uptake suggestive of active CS within two months of enrollment (confirmed by PET core lab read) |
| Japanese Antibacterial Drug Management for Cardiac Sarcoidosis (J-ACNES) [49] | UMIN000025936 | Osaka, Japan | Active | – | 80 minimum | A multicenter, open-label RCT | Standard corticosteroid therapy plus antibacterial drug therapy | Standard corticosteroid therapy | Change in 18F-FDG‐PET/CT SUV at 6 months | Efficacy, prognosis and safety endpoints | Adults 20 years or older with CS according to the Japanese Society of Sarcoidosis and Other Granulomatous disease (JSSOG) 2015 criteria with cardiac histopathological findings or with histopathological findings of other organs (skin or lung) and clinical signs of cardiac involvement & abnormal cardiac uptake on 18F-FDG‐PET or gallium‐67 scintigraphy |
AV: atrioventricular; CRP: C-reactive protein; CS: cardiac sarcoidosis; CT: computed tomography; FDG: fluorodeoxyglucose; NSVT: non-sustained ventricular tachycardia; PET: positron emission tomography; RCT: randomized controlled trials; SPRS: summed perfusion rest score; SUV: standardized uptake values.
5. Study limitations
Several limitations of our study require mention. First, a formal meta-analysis was not pursued because most of the studies included were retrospective and single-center, and there were no randomized-control trials on this topic. Because of the small number of sites publishing on their treatment strategies, we also found that there may be an overlap in patients included in studies of sDMARDs versus bDMARDs, further limiting the analysis. Moreover, though we did search for unpublished articles and conference proceedings, 13 out of 20 (Table 1) were published abstracts without accompanying full publications, thus limiting the extent of available information and description of the methodology followed. Another important limitation is the small number of patients reported receiving non-steroidal treatment, which make these results and dosages, not generalizable. Lastly, we found that there is also limited data on hard endpoints, such as mortality, among patients treated with various immunosuppressant agents.
6. Conclusion
This systematic review shows two key points. First, steroid-sparing medications, including synthetic and biological agents, are widely used as second- or third-line agents in the management of refractory CS without standardization. Second, there is high variability in the use of steroid sparing immunosuppressive drugs as adjunctive or combination treatment for CS. In about 35% of the studies reviewed, MTX was the most commonly studied sDMARD with a dose ranging between 6 and 20 mg per week. IFX was the most common bDMARD studied. Overall, the evidence for their use among patients with CS is limited to retrospective observational case series and cohort studies, most of which were found to be at high risk of bias. However, RCTs are currently underway to provide more concrete evidence on the use of immunosuppressive therapies in CS patients (summarized in Table 4), such as methotrexate [47], anakinra [48], as well as antibacterial therapies targeting Propionibacterium acnes [49], a potential etiologic agent of CS. Such studies must overcome several obstacles, including the low number of CS cases and associated events requiring multi-center collaborations to achieve adequate power, or reliance on surrogate measures, such as a change in circulating inflammatory markers or imaging findings.
In the absence of high-quality evidence from randomized controlled trials, treatment decisions rely on the comprehensive assessment of each patient’s history and cardiac profile as further guided by longitudinal imaging (e.g. with 18F-FDG PET) for the appropriate and timely deployment of immunosuppressive medications.
References
- 1.Birnie D.H., Sauer W.H., Bogun F., Cooper J.M., Culver D.A., Duvernoy C.S., Judson M.A., Kron J., Mehta D., Cosedis Nielsen J., Patel A.R., Ohe T., Raatikainen P., Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 2.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016;68(4):411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 3.Silverman K.J., Hutchins G.M., Bulkley B.H. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y., Iwai K., Tachibana T., Fruie T., Shigematsu N., Izumi T., Homma A.H., Mikami R., Hongo O., Hiraga Y., Yamamoto M. Clinicopathological study of fatal myocardial sarcoidosis. Ann. N. Y. Acad. Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma O.P., Maheshwari A., Thaker K. Myocardial sarcoidosis. Chest. 1993;103(1):253–258. doi: 10.1378/chest.103.1.253. [DOI] [PubMed] [Google Scholar]
- 6.Yigla M., Badarna-Abu-Ria N., Tov N., Ravell-Weiller D., Rubin A.H. Sarcoidosis in northern Israel; clinical characteristics of 120 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19(3):220–226. [PubMed] [Google Scholar]
- 7.Kandolin R., Lehtonen J., Graner M., Schildt J., Salmenkivi K., Kivisto S.M., Kupari M. Diagnosing isolated cardiac sarcoidosis. J. Intern. Med. 2011;270(5):461–468. doi: 10.1111/j.1365-2796.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 8.Okada D.R., Bravo P.E., Vita T., Agarwal V., Osborne M.T., Taqueti V.R., Skali H., Chareonthaitawee P., Dorbala S., Stewart G., Di Carli M., Blankstein R. Isolated cardiac sarcoidosis: A focused review of an under-recognized entity. J Nucl Cardiol. 2018;25(4):1136–1146. doi: 10.1007/s12350-016-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadek M.M., Yung D., Birnie D.H., Beanlands R.S., Nery P.B. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can. J. Cardiol. 2013;29(9):1034–1041. doi: 10.1016/j.cjca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadian A., Pawar S., Govender P., Berman J., Ruberg F.L., Miller E.J. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017;24(2):413–424. doi: 10.1007/s12350-016-0490-7. [DOI] [PubMed] [Google Scholar]
- 11.Osborne M.T., Hulten E.A., Singh A., Waller A.H., Bittencourt M.S., Stewart G.C., Hainer J., Murthy V.L., Skali H., Dorbala S., Di Carli M.F., Blankstein R. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21(1):166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.J. McGowan, M. Sampson, D.M. Salzwedel, E. Cogo, V. Foerster, C. Lefebvre, PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement, J Clin Epidemiol 75 (2016) 40-6. [DOI] [PubMed]
- 14.Sterne J.A., Hernan M.A., Reeves B.C., Savovic J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., Carpenter J.R., Chan A.W., Churchill R., Deeks J.J., Hrobjartsson A., Kirkham J., Juni P., Loke Y.K., Pigott T.D., Ramsay C.R., Regidor D., Rothstein H.R., Sandhu L., Santaguida P.L., Schunemann H.J., Shea B., Shrier I., Tugwell P., Turner L., Valentine J.C., Waddington H., Waters E., Wells G.A., Whiting P.F., Higgins J.P. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamilloux Y., Cohen-Aubart F., Chapelon-Abric C., Maucort-Boulch D., Marquet A., Perard L., Bouillet L., Deroux A., Abad S., Bielefeld P., Bouvry D., Andre M., Noel N., Bienvenu B., Proux A., Vukusic S., Bodaghi B., Sarrot-Reynauld F., Iwaz J., Amoura Z., Broussolle C., Cacoub P., Saadoun D., Valeyre D., Seve P., Groupe Sarcoidose F. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin. Arthritis Rheum. 2017;47(2):288–294. doi: 10.1016/j.semarthrit.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Hiraga H., Yuwai K., Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 17.M.A. Judson, U. Costabel, M. Drent, A. Wells, L. Maier, L. Koth, H. Shigemitsu, D.A. Culver, J. Gelfand, D. Valeyre, N. Sweiss, E. Crouser, A.S. Morgenthau, E.E. Lower, A. Azuma, M. Ishihara, S. Morimoto, T. Tetsuo Yamaguchi, N. Shijubo, J.C. Grutters, M. Rosenbach, H.P. Li, P. Rottoli, Y. Inoue, A. Prasse, R.P. Baughman, T.W. Organ Assessment Instrument Investigators, The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool, Sarcoidosis Vasc Diffuse Lung Dis 31(1) (2014) 19-27. [PubMed]
- 18.Terasaki F., Yoshinaga K. New Guidelines for Diagnosis of Cardiac Sarcoidosis in Japan. Ann. Nucl. Cardiol. 2017;3(1) [Google Scholar]
- 19.Y. Yazaki, H. Kasai, M. Koshikawa, U. Ikeda, Effect of Additional Methotrexate Use on Clinical Findings and Metabolic Profile in Patients with Cardiac Sarcoidosis, Journal of Cardiac Failure 20(10, Supplement) (2014) S156.
- 20.Yokomatsu T., Hojo S., Kawaji T., Kushiyama A., Nakatsuma K., Kaneda K., Kato M., Miki S. P1603Efficacy of combination therapy of methotrexate and low-dose corticosteroid for cardiac sarcoidosis evaluated by fuluorine-18 fluorodeoxyglucose positron emission tomography. Eur. Heart J. 2018;39(suppl_1) [Google Scholar]
- 21.Nagai S., Yokomatsu T., Tanizawa K., Ikezoe K., Handa T., Ito Y., Ogino S., Izumi T. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern. Med. 2014;53(23):2761. doi: 10.2169/internalmedicine.53.3120. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal D.G., Parwani P., Murray T.O., Petek B.J., Benn B.S., De Marco T., Gerstenfeld E.P., Janmohamed M., Klein L., Lee B.K., Moss J.D., Scheinman M.M., Hsia H.H., Selby V., Koth L.L., Pampaloni M.H., Zikherman J., Vedantham V. Long-Term Corticosteroid-Sparing Immunosuppression for Cardiac Sarcoidosis. J. Am. Heart Assoc. 2019;8(18) doi: 10.1161/JAHA.118.010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballul T., Borie R., Crestani B., Daugas E., Descamps V., Dieude P., Dossier A., Extramiana F., van Gysel D., Papo T., Sacre K. Treatment of cardiac sarcoidosis: A comparative study of steroids and steroids plus immunosuppressive drugs. Int. J. Cardiol. 2019;276:208–211. doi: 10.1016/j.ijcard.2018.11.131. [DOI] [PubMed] [Google Scholar]
- 24.Chapelon-Abric C., Sene D., Saadoun D., Cluzel P., Vignaux O., Costedoat-Chalumeau N., Piette J.C., Cacoub P. Cardiac sarcoidosis: Diagnosis, therapeutic management and prognostic factors. Arch Cardiovasc. Dis. 2017;110(8–9):456–465. doi: 10.1016/j.acvd.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Fussner L., Karlstedt E., Fine N., Kalra S., Carmona E., Utz J., Isaac D., Cooper L. Multicenter Experience With Mycophenolate Mofetil in Cardiac Sarcoidosis. Chest. 2016;150(4):512A. [Google Scholar]
- 26.J.M.C. Griffin, E.; Tandri, H.; Kasper, E. K.; Gilotra, N. A., Combination Therapy with Prednisone and Mycophenolate Mofetil in the Management of Cardiac Sarcoidosis, Journal of Cardiac Failure 24(8) (2018) S101-S102.
- 27.M.M. Estephan, M.; Hamblin, M.; Magadan, J., Successful Treatment of Cardiac Sarcoidosis with Biologic and Immunosuppressive Combination Therapy, Arthritis & Rheumatology 67(S10) (2015) 1397.
- 28.R. Kandolin, J. Lehtonen, MIDFIN, 1961Infliximab in cardiac sarcoidosis, European Heart Journal 38(suppl_1) (2017).
- 29.Kowlgi G.N., Syed H., James E.W., Houston B.A., Rieter W.J., Crawford T.C., Wunderly K., Bogun F.M., Lehtonen J., Kandolin R., Chicos A.B., Birnie D.H., Meredith R., Abbate A., Ellebogen K.A., Kron J. Infliximab Stabilizes Ejection Fraction And Reduces Ventricular Tachycardia In Refractory Cardiac Sarcoidosis. Heart Rhythm. 2019;16(5):S32. [Google Scholar]
- 30.Chapelon-Abric C., Saadoun D., Biard L., Sene D., Resche-Rigon M., Hervier B., Costedoat-Chalumeau N., Drier A., Leger J.M., Cacoub P. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin. Exp. Rheumatol. 2015;33(4):509–515. [PubMed] [Google Scholar]
- 31.L.J. Harper, M. McCarthy, M.L. Ribeiro Neto, R. Hachamovitch, K. Pearson, B. Bonanno, J. Shaia, R. Brunken, E. Joyce, D.A. Culver, Infliximab for Refractory Cardiac Sarcoidosis, Am J Cardiol 124(10) (2019) 1630-1635. [DOI] [PubMed]
- 32.M.M. Cundiff, D.; Pincus, M.; Campagna, A.; Nghiem, L.; Dou, Y.; Patten, R.; Venesy, D.; Rybicki, M.; Shah, S. P., Treatment of Active Cardiac Sarcoidosis with TNF Alpha Inhibitors, Journal of Cardiac Failure 25 (2019) S27-S28.
- 33.O.P. Sinokrot, S.; Reyentovich, A.; Addrizzo-Harris, D.J., Efficacy of Infliximab Therapy in Cardiac Sarcoidosis: A Single Center Case Series, Am J Respir Crit Med 199 (2019) A3064.
- 34.Devraj M., Pillarisetty A., Constantinescu F., Sheikh F. Experience with Biologic Agents for the Treatment of Cardiac Sarcoidosis in a U.S. Academic Medical Center. J. Am. Coll. Cardiol. 2020;75:786. [Google Scholar]
- 35.Puyraimond-Zemmour D., Chapelon-Abric C., Saadoun D., Bouvry D., Ruivard M., Andre M., Perard L., Seve P., Cacoub P. Efficacy and tolerance of TNF alpha inhibitor (TNFI) treatment in cardiac sarcoidosis (CS) Arthritis Rheumatol. 2017;69 [Google Scholar]
- 36.Injean P., Lee Y., Hojjati M. Role of Alternative Immunosuppressant Therapy in Management of Cardiac Sarcoidosis [abstract] Arthritis Rheumatol. 2019;71 [Google Scholar]
- 37.Baker M., Sheth K., Simard J., Shoor S., Genovese M. Novel Approach to the Treatment of Cardiac Sarcoidosis with TNF-alpha Inhibition [abstract] Arthritis Rheumatol. 2019;71 [Google Scholar]
- 38.P. Sethi, D. Rosenthal, J. Zikherman, V. Vedantham, M. Pampaloni, Changes In Global Left Ventricular Ejection Fraction And Myocardial Perfusion By Rb-82 In Patients Treated For Cardiac Sarcoidosis J Nucl Cardiol 25(4) (2018) 1463.
- 39.Gilotra N.A., Wand A.L., Pillarisetty A., Devraj M., Pavlovic N., Ahmed S., Saad E., Solnes L., Garcia C., Okada D.R., Constantinescu F., Mohammed S.F., Griffin J.M., Kasper E.K., Chen E.S., Sheikh F.H. Clinical and Imaging Response to Tumor Necrosis Factor Alpha Inhibitors in Treatment of Cardiac Sarcoidosis: A Multicenter Experience. J. Card Fail. 2020 doi: 10.1016/j.cardfail.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Judson M.A., Baughman R.P., Costabel U., Flavin S., Lo K.H., Kavuru M.S., Drent M., Centocor T.S.I. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur. Respir. J. 2008;31(6):1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 41.Krause M., Cooper L., Chareonthaitawee P., Amin S. Rituximab in Refractory Cardiac Sarcoidosis - Single Center Experience. Arthritis Rheumatol. 2016;68:2. [Google Scholar]
- 42.Fussner L.A., Karlstedt E., Hodge D.O., Fine N.M., Kalra S., Carmona E.M., Utz J.P., Isaac D.L., Cooper L.T. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur. J. Heart Fail. 2018;20(12):1713–1720. doi: 10.1002/ejhf.1319. [DOI] [PubMed] [Google Scholar]
- 43.Listing J., Strangfeld A., Kekow J., Schneider M., Kapelle A., Wassenberg S., Zink A. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58(3):667–677. doi: 10.1002/art.23281. [DOI] [PubMed] [Google Scholar]
- 44.Damsky W., Young B.D., Sloan B., Miller E.J., Obando J.A., King B. Treatment of Multiorgan Sarcoidosis With Tofacitinib. ACR Open Rheumatol. 2020;2(2):106–109. doi: 10.1002/acr2.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller E.J., Culver D.A. Establishing an Evidence-Based Method to Diagnose Cardiac Sarcoidosis: The Complementary Use of Cardiac Magnetic Resonance Imaging and FDG-PET. Circ. Cardiovasc. Imaging. 2018;11(1) doi: 10.1161/CIRCIMAGING.117.007408. [DOI] [PubMed] [Google Scholar]
- 46.Sharp M.B., Soufer A., Abdelmessih M., Rosenfeld L.E., Baldassarre L.A., Miller E.J. Using FDG-PET to guide targeted cardiac magnetic resonance imaging in patients with suspected cardiac sarcoidosis. J. Nucl. Cardiol. 2019 doi: 10.1007/s12350-019-01640-z. [DOI] [PubMed] [Google Scholar]
- 47.Cardiac Sarcoidosis Randomized Trial, https://ClinicalTrials.gov/show/NCT03593759.
- 48.Interleukin-1 Blockade for Treatment of Cardiac Sarcoidosis, https://ClinicalTrials.gov/show/NCT04017936.
- 49.Ishibashi K., Eishi Y., Tahara N., Asakura M., Sakamoto N., Nakamura K., Takaya Y., Nakamura T., Yazaki Y., Yamaguchi T., Asakura K., Anzai T., Noguchi T., Yasuda S., Terasaki F., Hamasaki T., Kusano K. Japanese Antibacterial Drug Management for Cardiac Sarcoidosis (J-ACNES): A multicenter, open-label, randomized, controlled study. J. Arrhythm. 2018;34(5):520–526. doi: 10.1002/joa3.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Japan Society of Sarcoidosis and Other Granulomatous Disorders, the Diffuse Pulmonary Study Group of the Health and Labor Sciences Research Grant-supported Rare/Intractable Disease Project, et al. Committee for revision of the diagnostic standard for sarcoidosis. Diagnostic standard and guideline for sarcoidosis - 2006. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2007; 27: 89 – 102. [In Japanese]