Abstract
Ensuring that global warming remains <2 °C requires rapid CO2 emissions reduction. Additionally, 100–900 gigatons CO2 must be removed from the atmosphere by 2100 using a portfolio of CO2 removal (CDR) methods. Ocean afforestation, CDR through basin-scale seaweed farming in the open ocean, is seen as a key component of the marine portfolio. Here, we analyse the CDR potential of recent re-occurring trans-basin belts of the floating seaweed Sargassum in the (sub)tropical North Atlantic as a natural analogue for ocean afforestation. We show that two biogeochemical feedbacks, nutrient reallocation and calcification by encrusting marine life, reduce the CDR efficacy of Sargassum by 20–100%. Atmospheric CO2 influx into the surface seawater, after CO2-fixation by Sargassum, takes 2.5–18 times longer than the CO2-deficient seawater remains in contact with the atmosphere, potentially hindering CDR verification. Furthermore, we estimate that increased ocean albedo, due to floating Sargassum, could influence climate radiative forcing more than Sargassum-CDR. Our analysis shows that multifaceted Earth-system feedbacks determine the efficacy of ocean afforestation.
Subject terms: Carbon cycle, Ocean sciences, Marine biology
Ocean afforestation is considered as an important method to remove gigatons of CO2 from the atmosphere. Here the authors use the Great Atlantic Sargassum Belt as a natural analogue to show that the efficacy of ocean afforestation is determined by complicated feedbacks with the Earth system.
Introduction
Capturing atmospheric CO2 using ocean afforestation is receiving widespread attention in scientific and public discourse, due to the prospects of converting seemingly unproductive open ocean deserts into thriving seaweed ecosystems1–5. Indeed, high-level political and economic stakeholders are beginning to advocate the rapid implementation of offshore seaweed farming6,7, even though little is known about the CDR potential and side-effects of ocean afforestation when upscaled. Model simulations are the first step to explore these unknowns8, but they inevitably miss many complexities associated with real-world systems. Therefore, performing in situ experiments must follow to inform model developments. However, most of what is experimentally and logistically feasible is orders of magnitude lower than the scale envisioned for climate-relevant ocean afforestation9.
Studying natural analogues presents a low-risk, cost-effective way to evaluate the potential of ocean afforestation on the scale needed to contribute significantly to CDR. Indeed, there are prominent examples where studying natural analogues of climate intervention, such as the Mount Pinatubo volcanic eruption10 or glacial–interglacial ocean iron fertilisation11, has led to breakthroughs in our understanding of the response of the Earth System to perturbation. The recent blooms of floating seaweed Sargassum in the (sub)tropical North Atlantic12,13 provide the first natural analogue for seaweed farming distributed across multiple open ocean regions. While the extent of these patchy Sargassum blooms (up to 6100 km2) are still below the maximum extent envisoned by some for ocean afforestation (e.g., 9% of the surface ocean4), they are orders of magnitude larger than anything that is experimentally feasible. Thus, the recent emergence of this Great Atlantic Sargassum Belt (GASB)13 offers the unique, perhaps the only, opportunity to study large-scale ocean afforestation under real-world conditions before its potential application.
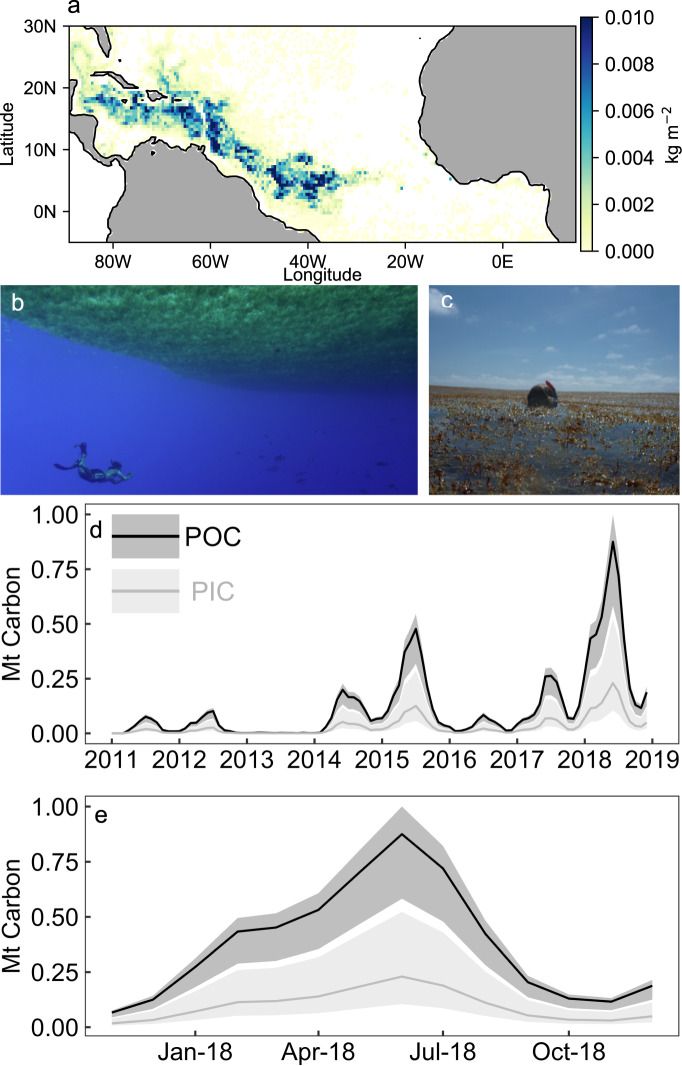
Floating Sargassum seaweed was historically found in the Sargasso Sea, but extended its bloom formations from 2011 onward across the Intra-Americas Sea and the (sub)tropical North Atlantic from West Africa to South America12,13 (Fig. 1a–c). This extension may have been initiated by an extreme negative phase of the North Atlantic Oscillation during 2009–2010 which shifted regional wind patterns14. The subsequent development of the GASB is hypothesized to be sustained by increased nutrient runoff from the Amazon River12,13. The Sargassum bloom, detected using satellites, follows a seasonal cycle with highest biomass in summer13,15 (Fig. 1d). The largest GASB bloom was observed in 2018 covering up to 6100 km2 distributed in numerous rafts over a ~9000 km belt just north of the Equator13,16. The 2018 bloom commenced in November 2017 and peaked in June 2018 with a net build-up of 0.81 million tons (Mt) of particulate organic carbon (POC; Fig. 1d and Supplementary Table 1). This constitutes a lower bound as satellites neither detect small Sargassum rafts13 nor biomass submerged transiently by wind-driven ocean circulation, which could increase Sargassum stocks17. POC formation in the GASB could be viewed as a first-order estimate for its capacity to absorb and sequester CO2. However, this CDR potential will be modified through biogeochemical feedbacks. Here, we utilize insights from the GASB to elucidate the complex influence of calcification and nutrient reallocation on ocean afforestation.
Fig. 1. Floating Sargassum biomass during the 2018 Great Atlantic Sargassum Belt (GASB) event.
a A satellite image of Sargassum wet weight biomass in the North Atlantic during June 201813. b, c Illustrative pictures of Sargassum rafts in the Sargasso Sea (2013) with a diver underneath or inside a raft for perspective. d, e Cumulative Sargassum particulate organic carbon (POC) and particulate inorganic carbon (PIC) within 5°S–25°N, 89°W–15°E from 2011 to 2018 or the 2018 growth cycle. The shaded areas indicate upper and lower bounds depending on Sargassum PIC:POC ratios.
Results and discussion
Biogeochemical feedbacks reduce CDR by ocean afforestation
POC formation via photosynthesis consumes CO2. In contrast, the formation of particulate inorganic carbon (PIC) through calcification (e.g., Ca2+ + CO32− → CaCO3) releases CO2 by reducing seawater alkalinity18,19. Thus, to accurately assess the CDR potential of the GASB, we must subtract the CO2 formed via calcification from the CO2 consumed via photosynthesis. Sargassum and other fleshy seaweeds do not calcify themselves, but provide a habitat for colonising epibiont calcifiers such as bryozoans20. Carbonate biominerals attached to Sargassum collected from the Sargasso Sea contribute 9.4% on an annual average to its wet weight biomass20, equivalent to a PIC:POC ratio of ~0.25 (mol:mol). This reduces the CO2 removal generated through photosynthetic POC formation by ~17% (Fig. 2a, c). It is currently unclear if 9.4% is applicable for the new Sargassum blooms occurring since 2011 in the GASB, or for other seaweeds potentially used for ocean afforestation. Slower/faster growing seaweeds may provide more/less time for epibiontic calcifiers to settle, which would affect the PIC:POC ratio accordingly. Over the range of wet weight CaCO3 percentages reported for individual Sargassum samples from the Sargasso Sea (i.e., 4.3–21.4%)20, the PIC:POC is 0.11–0.9 and the offset to CO2 removal 7–57% (Fig. 2c). This indicates that the calcification offset could range from being negligible to being a major factor reducing the CDR efficiency of ocean afforestation.
Fig. 2. Reduction of the theoretical CO2 removal potential (CDRtheoretical) through biogeochemical feedbacks.
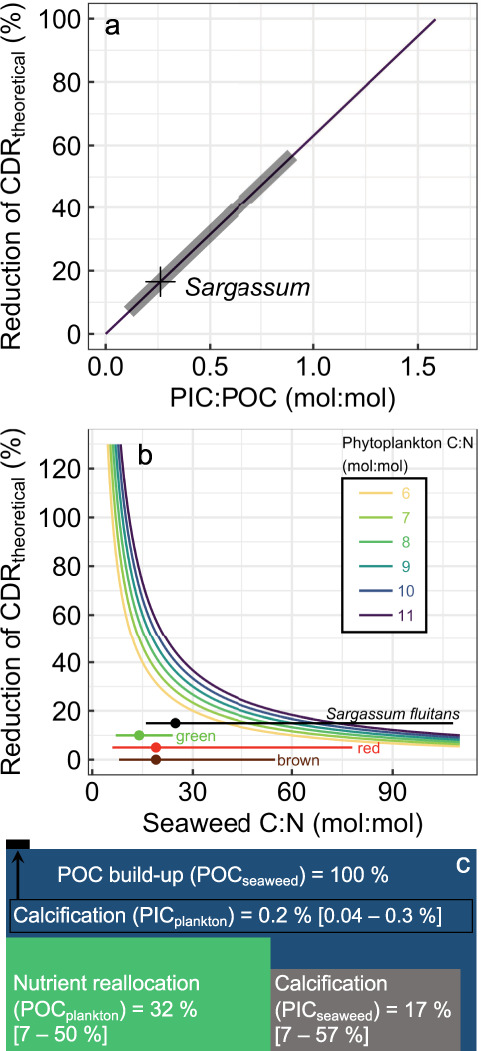
a Reduction of CDRtheoretical through particulate inorganic carbon (PIC) production by epibiont calcifiers associated with particulate organic carbon (POC) formed by Sargassum. The purple line shows the general relationship, the grey shaded part the range of Sargassum PIC:POC observations, and the cross the Sargassum mean. b Reduction of CDRtheoretical due to nutrient reallocation, which becomes more pronounced the more the seaweed carbon-to-nitrogen (C:N) ratio approaches the phytoplankton C:N ratio. We used a range of phytoplankton C:N ratios22 as indicated by the colour code. The horizontal lines display the range of C:N in Sargassum72 and green/red/brown seaweeds, respectively28 (the height of the lines on the y-axis has no meaning). The solid symbols on the horizontal lines are averages (Sargassum average = 24.8). c Summary of discounts and additions to CDRtheoretical due to calcification and nutrient reallocation following Eq. 1 (upper and lower bounds in square brackets). Percent reductions due to POC formed by plankton (POCplankton, blue); Percent reduction due to PIC associated with seaweeds (PICseaweed, grey); Percent increase due to PIC associated with plankton (PICplankton, black).
Another reduction in the CDR potential of the GASB is due to reallocation of nutrient resources from phytoplankton to Sargassum. Photosynthesis by Sargassum consumes nutrients which would otherwise fuel phytoplankton photosynthesis. Therefore, we must subtract the amount of natural phytoplankton CDR that would have been possible, with the amount of nutrients reallocated to Sargassum CDR. In the open (sub)tropical North Atlantic, Nitrogen (N) limits primary production while Phosphorus (P) and Iron (Fe) are occasionally co-limiting21. The 2018 GASB consumed 2.7, 0.12, and 0.003 Gmol of N, P, and Fe, respectively. With these resources, phytoplankton could photosynthesise 0.26 Mt POC (assuming N-limitation21 and a C:N ratio of 8 mol:mol22), thereby reducing Sargassum CDR by 32% (31% reduction assuming P-limitation and a C:P ratio of 170 mol:mol23; Supplementary Fig. 1). A key question is whether phytoplankton POC formation and the associated natural CDR would be equally effective as CDR by Sargassum. Phytoplankton in the open (sub)tropical North Atlantic maintain surface nutrient concentrations close to zero, which is indicative of a ~100% efficiency of the phytoplankton carbon sequestration in this region24,25. Thus, arguably, all phytoplankton POC that would have been fixed with the nutrients utilised by Sargassum must be subtracted from the Sargassum CDR, although this discount may be mitigated by the use of external nutrient sources (see also Supplementary discussion 1).
Nutrient reallocation from phytoplankton to Sargassum not only reduces phytoplankton POC, but also its PIC formation (i.e., calcification) and therefore the associated CO2 formation. Hence, less CO2 is formed by phytoplankton calcification because of the nutrient reallocation to Sargassum, and this saved amount of CO2 must be added to the CDR potential of Sargassum. However, this addition is minor because the planktonic PIC:POC is small in the (sub)tropical North Atlantic (~0.01)26,27 relative to Sargassum assemblages (PIC:POC ~0.25; see above).
Considering the offsets through calcification and nutrient reallocation we define the theoretical CDR potential of ocean afforestation as:
| 1 |
with the associated amounts of CO2 bound/released given in Mt C. With respect to the 2018 GASB, POCseaweed, PICseaweed, POCplankton, and PICplankton are 0.81, 0.13, 0.26, and 0.002 Mt C, respectively. Accordingly, the 2018 GASB generated a CDRtheoretical of ~0.42 Mt C due to the higher organic C:N ratio of Sargassum (C:N = ~25) than phytoplankton biomass (C:N = 8)22, and because this C:N “advantage” is not fully offset by epibiont calcification. The average C:N ratios (mol:mol, range in parentheses) of other brown, red, and green seaweeds are 19 (8–55), 19 (6–78), and 14 (7–24), respectively28, indicating that Sargassum is within the higher C:N range of seaweeds and closer to the upper bound of CDRtheoretical achievable with ocean afforestation (see also Supplementary discussion 1).
Another addition to CDRtheoretical by Sargassum is the photosynthetic formation of dissolved organic carbon (DOC) and its subsequent release into seawater29. Indeed, Sargassum may have produced ~1 Mt DOC during the 2018 GASB which exceeds the build-up of POC of 0.81 Mt C (Supplementary Table 1). However, seaweed-DOC mixes with the background DOC pool, is displaced by ocean currents, and generally only ~2% of DOC escapes remineralization for ≥20 years30. Therefore, accounting for CDR via DOC requires dedicated monitoring of vast ocean volumes with in situ sensors, that can differentiate DOC sources. So long as such monitoring is unfeasible, DOC may not be considered in CDRtheoretical because accountability and independent verification of DOC storage are likely essential to finance ocean afforestation via carbon trading31–34.
Multiple challenges to verify CDR by ocean afforestation
To this point, we have quantified how much CO2 dissolved in seawater is fixed by Sargassum during the 2018 GASB (i.e., CDRtheoretical). Ultimately, however, we need to quantify the permanent influx of atmospheric CO2 into the oceans after afforestation has generated a seawater CO2 deficit. As a first step, this requires traceable air-sea CO2 transfer following CO2 fixation by seaweeds8. We calculated air-sea equilibration timescales (τCO2) for the locations of pronounced Sargassum occurrence in the GASB region following Jones et al. (see ref. 35) and found that these range between 2.5–15 months (mean = 5; Fig. 3). This is 2.5–18 (mean = 5.5) times longer than the modelled residence time of seawater in the surface mixed-layer (τres) over much of the GASB region, which is between 0.3–1.5 months (mean = 0.9; Fig. 3b, c; see ref. 35; but note that large datagaps occur in the Intra-Americas Sea). For the entire GASB region between 5°S–25°N and 89°W–15°E13, τCO2 is 2–46 (mean = 8) times longer than τres, which is between 0.2–2 months (mean = 0.9; Fig. 3b, c; see ref. 35). Hence, seawater containing a CO2-deficit generated through afforestation has a high chance to lose contact with the atmosphere before the deficit is fully replenished with atmospheric CO2 (i.e., before equilibration is complete). Complete equilibration will likely occur some time in the future when seawater is eventually reexposed long enough to the surface but this may take >100 years8. The potential time-lag raises the question at what time-point can CDRtheoretical be considered to be realized, because CO2-fixation and seawater CO2-absorption can be spatially and temporarily uncoupled. In practice, CDR will likely only be rewarded in a carbon trading system when atmospheric CO2 uptake is accountable and independently verifiable31. Hence, a key challenge for the implementation of ocean afforestation (and other marine CDR methods) on a carbon trading market is to quantify the CO2 influx into the ocean, as this requires sophisticated measurements of seawater CO2 uptake over large spatial and temporal scales.
Fig. 3. Timescales of CO2 equilibration in the Great Atlantic Sargassum Belt.
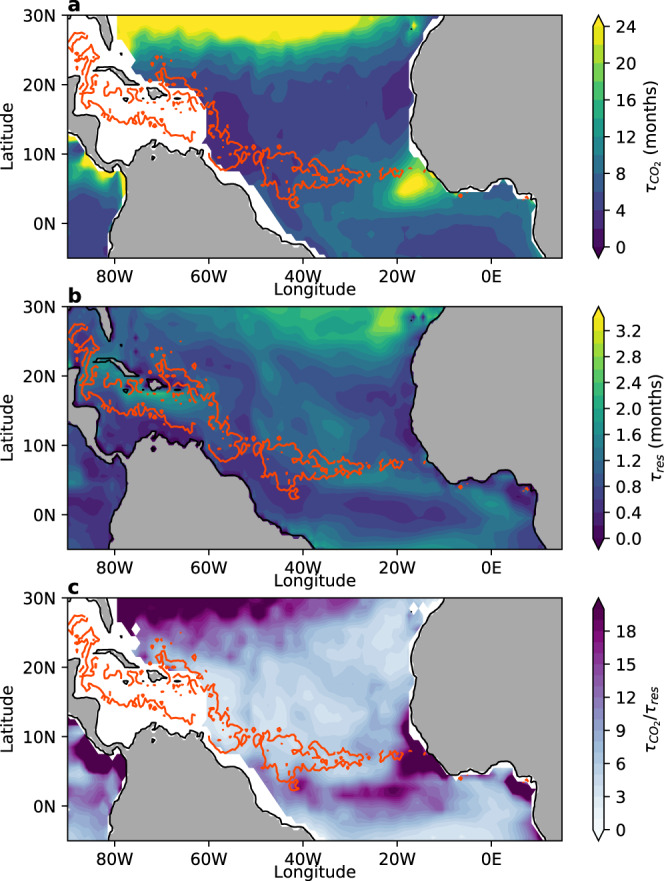
The orange mask indicates the occurrence of Sargassum with >0.002 kg/m2 during June 2018 as in Fig. 1a. a Annual mean timescales of CO2 equilibration (τCO2) between the ocean and atmosphere. b Residence time of seawater in the surface mixed layer (τres) before losing contact with the atmosphere. c Ratio of τCO2 and τres.
After quantifying air-sea CO2 transfer, seaweed carbon must be stored in a reservoir isolated from the atmosphere. The two storage methods widely anticipated are (i) biomass combustion followed by underground CO2 storage (BECCS) or (ii) biomass deposition on the deep seafloor1. Underground storage in appropriate geological formations comes with additional offsets to CDRtheoretical, for example through shipping Sargassum biomass (0.0014–0.017% t wetweight−1 km−1) or through CO2 separation after combustion (e.g., ~10–20% in the case of BECCS). Seafloor deposition may be associated with less process-related offsets to CDRtheoretical, when the deep sea is adjacent to the afforestation site. The value of geological and sea floor deposition depends upon a yet to be established policy framework that needs to clarify how sequestration permanence is factored into a carbon price, and how stringently CO2 leakage back into the atmosphere must be monitored36. Geological sequestration in appropriate formations can be considered long-term with a 66–90% chance of less than 1% CO2 leakage over 1000 years and possibly beyond37. Seafloor deposition will be less permanent than that. Sargassum sinks at 2500 m/d when its flotation bladders are removed38, so that there is little time for degradation until reaching the seafloor (see also Supplementary discussion 2). However, on the deep seafloor and within its sediments, >90% of deposited carbon is typically remineralized and released back into the water column25,29. Thus, a significant fraction will eventually be transported back to the surface as respired CO2 by ocean circulation and mixing. Data-constrained modelling suggests that the zonally-averaged mean time from deep seafloor remineralization to re-exposure to the atmosphere ranges between 700–900 years in the North Atlantic39, where GASB biomass is deposited38, and increases further along the global conveyor belt to >1400 years in the North Pacific39. These timescales are generally longer than those considered for some land-based CDR methods like terrestrial afforestation or soil carbon sequestration, which are on the order of decades to a century36,40. Nevertheless, the systematic differences reveal that some locations are better suited for seafloor deposition of seaweed biomass than others. The necessary monitoring of inevitable CO2 leakage appears at least equally challenging as the monitoring of air-sea CO2 uptake (see previous paragraph), because relatively small amounts of respired CO2 must be traced in a vast volume and be distinguished from the background CO2 pool. This monitoring challenge will likely influence the feasibility to implement seafloor seaweed deposition in carbon trading.
Albedo climate feedbacks of ocean afforestation
In addition to CO2-related feedbacks on green-house radiative forcing, afforestation also influences radiative forcing by changing the Earth’s albedo, the ratio between reflected and incident solar flux at the Earth surface. Afforestation in terrestrial environments reduces the albedo because forests are usually darker and reflect less short-wave radiation than other landscapes41. This negative feedback can substantially reduce or even offset the cooling potential of terrestrial afforestation generated by CDR41–43. In the case of ocean afforestation, albedo increases because seaweeds reflect more short-wave radiation than seawater, especially when they occur near the sea surface44. We estimate that (sub)tropical ocean afforestation of the area of the 2018 GASB (~6100 km2) would reduce radiative forcing in the range of 180 (seaweed at ~5 m depth) to 1800 (seaweed floating at the surface) peta J/y. This exceeds an estimated reduction of radiative forcing of 42 peta J/y caused by 0.42 million tonnes of CDRtheoretical. Accordingly, the magnitude of these numbers suggest that ocean afforestation would also need to be assessed in the context of solar radiation management, i.e., the deliberate reflection of solar radiation into space. However, ocean afforestation is typically envisioned with benthic seaweeds cultured several meters below the surface to avoid storm damage1,4. Such culturing practices would alleviate the albedo effect44. Furthermore, our estimates do not consider indirect albedo feedbacks, such as the release of bioaerosols, which may counteract or enhance the albedo effect45. Indeed, identifying and accounting for all albedo feedback mechanisms induced by ocean afforestation will likely be as challenging as the comprehensive assessment of CDR (see Supplementary discussion 3).
Synthesis and ramifications
The GASB analysis sets realistic bounds on ocean afforestation CDR and provides the first estimates of the relevance of the direct albedo effect. Two biogeochemical feedbacks, calcification and nutrient reallocation, reduce the theoretical CDR potential of ocean afforestation. Propagating both the upper and lower bounds for these feedbacks indicates that the CDRtheoretical of the 2018 GASB ranges from −0.03–0.8 Mt C. Accordingly, ocean afforestation at the scale of the GASB could constitute a net CO2 source or, at best, contribute 0.0001–0.001% to the amount of annual CDR required in 2100 under a low emission scenario. The large range of estimates—from positive to negative—underlines that CDR with ocean afforestation is heavily dependent on feedbacks with the Earth System, which need to be further constrained. In addition, our analysis confirms that verification of both air-sea CO2 flux and the permanence of carbon storage remain major challenges. Thus, significant physical and chemical oceanographic questions must be answered, along with establishing governance rules on verification and permanence to fully evaluate ocean afforestation. Moreover, the influence of ocean afforestation on ocean albedo could be more relevant than the climatic effect of CDR. However, albedo enhancements are currently not considered within carbon trading and thus would need additional legislation to be incentivised and rewarded46. Last, all the abovementioned feedbacks on the net climatic effect must also be evaluated for an environment that is constantly being modified due to ongoing climate change.
The assessment of terrestrial afforestation is further advanced than that of ocean afforestation, because ubiquitous natural analogues (forests) provide constraints on its efficacy. Thus, there is substantial knowledge of how Earth-system feedbacks modify the apparent CDR potential of terrestrial afforestation (see the wider discussion around ref. 47). The GASB provides a new opportunity to constrain ocean afforestation, and our analysis points towards a similar conclusion as for terrestrial afforestation; i.e., climate intervention through ocean afforestation must consider complicated Earth-system feedbacks (Fig. 4), which could influence the sign and magnitude of its cumulative climatic effect.
Fig. 4. Summary of feedbacks discussed in this study.
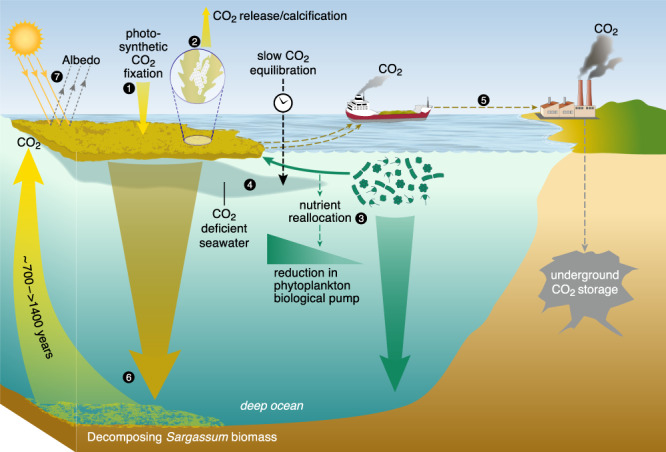
The numbers in the black circles denote individual processes in the order they were discussed. 1. Photosynthetic carbon fixation by Sargassum consumes CO2 dissolved in seawater. 2. Sargassum provides habitat for epibiontic encrusting organisms to calcify, thereby generating CO2 through complicated feedbacks in the seawater carbonate system. 3. Nutrients taken up by Sargassum become unavailable for phytoplankton thereby reducing the natural carbon sequestration by phytoplankton. 4. CO2 deficient seawater (driven by photosynthesis) can be subducted below the surface layer before it fully equilibrates with atmospheric CO2. 5. Sargassum biomass can be harvested and transported to shore and used for Bioenergy with Carbon Capture and Storage (BECCS) causing CO2 discounts. 6. Alternatively, Sargassum could be deposited on the seafloor but a large fraction of the respired Sargassum carbon would come back to surface on centennial-millenial timescales. 7. Sargassum increases albedo at the sea surface but could also induce complicated indirect albedo effects of unknown sign and magnitude.
Acceleration in CDR research and development is urgently needed as global negative emissions must be upscaled to gigatonnes within this decade (Supplementary Fig. 2). This requires rapid identification of tractable CDR solutions, which enable accurate and ongoing accounting of their overall climatic influence. The GASB analysis reveals that the net climatic impact of ocean afforestation is associated with major uncertainties, largely due to the inherent complexity of biological systems (Fig. 4). Other marine biological CDR approaches such as ocean iron fertilization exhibit similar complexity, and two decades of investigation has revealed comparable uncertainties about their net climatic impact. These ongoing unknowns raise the question of whether CDR using marine biota has the potential to be sufficiently well understood within this decade to prioritize development. Instead, the complexity associated with such CDR approaches may provide a compelling argument to focus on bottom-up engineered and better understood abiotic methods.
Methods
Build-up of seaweed biomass in the Great Atlantic Sargassum Belt (GASB)
Floating Sargassum biomass in the GASB has been quantified with satellites15,48. We downloaded monthly mean cumulative Sargassum wet weight data for the GASB region (Caribbean Sea and Central Atlantic Ocean) from ref. 16 under the following link (https://www.ncei.noaa.gov/access/metadata/landing-page/bin/iso?id=gov.noaa.nodc:0190272#). The cumulative wet weight biomass was converted to total particulate carbon (TPC) by multiplication with the wet weight to TPC conversion factor of 0.0543 (g:g) given in the ref. 49. Supplementary Table 1 provides wet weights16 and corresponding TPC values for the 2018 growth cycle, which commenced in November 2017 and constitutes our natural analogue case study for ocean afforestation.
Calcification by epibionts living on Sargassum
The precipitation of calcium carbonate (i.e., Ca2+ + CO32− → CaCO3) reduces seawater alkalinity, thereby shifting the carbonate chemistry equilibrium and increasing the concentration of CO2 (see refs. 18,50). Thus, calcification by epibiont calcifiers counteracts photosynthetic CO2 uptake by Sargassum and the desired CDR due to seaweed growth19. In the following, we go step-by-step through our calculation (and associated assumptions) to estimate the importance of the calcification-related offset by using the GASB as a natural analogue.
Wang et al. (see ref. 49) have measured wet weight and total particulate carbon (TPC) content of Sargassum for their GASB biomass analysis (Supplementary Table 1). They have not separated TPC into fractions of particulate organic carbon (POC) and particulate inorganic carbon (PIC, i.e., carbonates). Sargassum does not form PIC itself but it provides substrate for calcifying epibionts (e.g., bryozoans or polychaete worms like Spirorbis spp.), which form firmly-attached calcareous structures on seaweed tissues51,52. CaCO3 formed by these epibionts can constitute between 4.3–21.4% of the Sargassum wet weight biomass, with the average being 9.4% over the course of the year20. We used this average wet weight percentage to calculate the CaCO3 mass of the GASB based on total wet weights as:
| 2 |
where the wet weight values were GASB July averages from 2011 to 2017 reported by Wang et al. (see ref. 49). This range yields CaCO3 masses from 0.0032 to 0.415 Mt CaCO3, which were converted into mols by division with its molecular weight (i.e., 100 g/mol). (There is uncertainty associated with this as calcifying epibionts may also precipitate MgCO3 with a lower molecular weight (i.e., 84 g/mol). We neglect this uncertainty because we have no information about the fraction of MgCO3, and assuming all carbonates to be CaCO3 sets a lower bound to the CDR offset through calcification. Assuming all carbonates were MgCO3 would increase the offset further because the formation of 1 g of MgCO3 reduces alkalinity slightly more than 1 g of CaCO3). The calculation provided the mols of PIC associated with Sargassum which were subsequently subtracted from the mols of TPC reported by Wang et al. (see ref. 49) to calculate POC. This enabled us to calculate a PIC:POC ratio (mol:mol) of 0.265 for Sargassum and the associated epibiont biomass under the assumption that 9.4% of the wet weight is CaCO3 (see above). For the range of CaCO3 wet weight percentages reported by Pestana (4.3–21.4%)20, PIC:POC ranges from 0.11 to 0.9 (mol:mol).
The next step was to calculate how many moles CO2 would be formed per mol PIC precipitated by the epibionts. This ratio, known as psi, was calculated following Frankignoulle et al.18 using the psi-function of the carbonate chemistry calculation software seacarb53 for R with recommended default settings for carbonate chemistry constants53 (e.g., K1 and K2 from ref. 54). For this calculation, we assumed subtropical conditions with a total alkalinity of 2350 µmol kg−1, a dissolved inorganic carbon concentration of 2047.5 µmol kg−1, a salinity of 35, and a temperature of 25 °C (the following code was used: psi < - psi(flag = 15, var1 = 2350e−6, var2 = 2047.5e−6, S = 35, T = 25, P = 0, Pt = 0, Sit = 0, pHscale = “T”, kf = “pf”, k1k2 = “l”, ks = “d”). In this case psi is 0.63.
Finally, we multiplied psi with the mols of PIC precipitated by Sargassum epibionts and compared the amounts of generated CO2 with the amounts of fixed CO2 by photosynthetic POC production:
| 3 |
According to this, the calcification offset is 0.165 or 16.5% to the amount of POC fixed photosynthetically by Sargassum. The key assumption in this calculation is that 9.4% (ranging from 4.3 to 21.4%)20 of Sargassum wet weight is CaCO3, which is based on measurements with Sargassum collected in the Sargasso Sea20. These values provide a useful first estimate but they may differ for Sargassum occurring in the GASB since 2011, or for other species usef for ocean afforestation as pointed out in the discussion.
Nutrient reallocation from resident phytoplankton to Sargassum
Ocean afforestation requires macro-nutrients (Nitrogen (N) and Phosphorous (P)) and micro-nutrients such as iron (Fe), which are the primary limiting resources for primary production in the subtropical Atlantic21. Nutrients fixed by afforested seaweeds in offshore environments can no longer support carbon fixation by phytoplankton. Thus, nutrient reallocation from phytoplankton to seaweeds reduces phytoplankton carbon sequestration, a natural marine CO2 sink. The phytoplankton carbon sequestration that would have been possible with the reallocated nutrients must be subtracted from the CDR potential of ocean afforestation. In the following paragraphs, we go through the calculations and considerations made to assess the degree of reduction in seaweed CDR through nutrient reallocation.
The 2018 Sargassum bloom in the GASB commenced in November 2017 and peaked in June 2018 with a net biomass build-up of 18.8 Mt wet weight (Supplementary Table 1). Wet weight was converted into Mt N by multiplication with the Sargassum N:wet weight ratio (g:g) reported in Wang et al. (~0.002)49. Next, the mass of N was converted into gigamole (Gmol) by division with the molecular weight of N (i.e., 14.007 g/mol). The same calculation was done for P using the P:wet weight ratio of 0.0002 (g:g)49 and the molecular P weight of 30.974 g/mol. Fe removal was calculated the same way (molecular weight of Fe 55.845 g/mol), but we had to derive a Fe:wet weight ratio from other references. We used Fe:wet weight ratios of 1.009 × 10−5 (g:g) determined for the benthic Sargassum ilicifolium from the Red Sea55 (please note that Anton et al. (see ref. 55) provide Fe:dry weight ratios but also give a wet weight:dry weight ratio of 8.18, which we use for the conversion). While this may not be fully representative for holopelagic Sargassum from the GASB, it may provide a useful number within a plausible range.
Based on these calculations, Sargassum growth during the 2018 GASB consumed 2.7, 0.12, and 0.003 Gmol N, P, and Fe, respectively. In the sub(tropical), Atlantic phytoplankton is mostly N-limited21. Assuming a typical organic matter C:N ratio of 8 in the (sub)tropical Atlantic22, phytoplankton could fix 0.26 Mt C with the amount of N bound in Sargassum.
The C:N ratio for Sargassum as determined by Wang et al. (see ref. 49) is 31.3 (mol:mol). However, this value includes PIC from epibionts which needs to be subtracted assuming a wet weight percentage of CaCO3 of 9.4% (see previous section). With this correction the Sargassum organic C:N is 24.8 (i.e., (TPC-PIC)/N, where TPC and N is from Wang et al. (see ref. 49) and PIC calculated as described in the previous section) or 28.2–16.5 for wet weight CaCO3 percentages from 4.3 to 21.4% (see ref. 20). Thus, phytoplankton are able to fix around one third (i.e., 8/24.8 × 100 = 32%) of the carbon fixed by Sargassum seaweeds (or 28–48% for the range of CaCO3 wet weight percentages). The 32% must be subtracted from the CDR potential of afforestation, because phytoplankton carbon fixation with these nutrients would likely have occurred under the absence of the GASB. To test whether this percentage offset is sensitive to the assumption of N-limitation in the sub(tropical) North Atlantic21, we repeated the calculation assuming P-limitation. The molar C:P ratio of Sargassum is 550 (i.e., (TPC-PIC)/P where TPC and P is from Wang et al. (see ref. 49), and PIC calculated as described in the previous section) compared to 170 typically found in oligotrophic plankton communities23. Thus, as for N-limitation, phytoplankton are able to fix around one third (i.e., 170/550 × 100 = 31%) of the carbon fixed by Sargassum seaweeds when assuming P-limitation (Supplementary Fig. 1) (or 26–45% for the range of CaCO3 wet weight percentages). As the differences between N-limitation or P-limitation are marginal, we focussed on C:N ratios in our study.
Dissolved organic carbon (DOC) production
Powers et al. (see ref. 56) conducted incubation experiments with Sargassum collected in the Sargasso Sea under in situ light and temperature conditions, and reported DOC release rates of 288 ± 24 µg C d−1 gwetweight−1. Multiplying their daily rate with satellite derived biomasses yields a cumulative Sargassum DOC production for Nov. 2017—Dec. 2018 of 1.07 Mt C (Supplementary Table 1). Please note that this estimate is associated with several uncertainties that are difficult to quantify56. While the uncertainty of the reported rate is relatively minor (SD ± 24 µg C d−1 gwetweight−1), it has been determined only during a certain time of the year (September) when Sargassum was in a distinct growth stage. As mentioned by Powers et al., (see ref. 56) DOC production rates may be different for early or late season Sargassum, and also change with the environmental conditions such as light and temperature.
Air-sea CO2 exchange and equilibration timescales
To generate atmospheric CO2 removal, the CO2 deficit generated through afforestation needs to be balanced by atmospheric CO2 influx into the ocean. Air-sea CO2 fluxes in the open ocean primarily depend on wind speeds (U) and the air-sea pCO2 difference. Temperature (T) and salinity (S) also play a role as they influence the Schmidt number (Sc) and the solubility (K) of CO2 in seawater57. We followed the approach by Jones et al. (see ref. 35) to calculate the timescale of air-sea equilibration (τCO2) as:
| 4 |
where h is the mixed layer depth (in m), R is the ratio of dissolved CO2 + H2CO3 to total dissolved inorganic carbon (DIC), G is the gas transfer velocity (in m/s), and B is the Revelle Factor. In a first step, we calculated τCO2 for a range of plausible scenarios for (sub)tropical environments, where ocean afforestation could be applied. In these scenarios, we assumed typical mixed layer depths ranging from 10 to 110 m58. R and B were calculated with the seacarb package using the carb and buffergen functions with recommended default settings for equilibrium constants53 (e.g., K1 and K2 from ref. 54). As input variables we used a total alkalinity (TA) of 2350 µmol/kg59 and assumed equilibrated pCO2 of 410 µatm. Nutrients (P and Si) were set to zero and salinity to 35. Temperature varied between 20–30 °C depending on the scenario (see Supplementary Fig. 3). G was calculated following the empirical equation by Wanninkhof60 as:
| 5 |
where U10 is the wind speed 10 m above ground and Sc is the Schmidt Number. By convention, G (typically abbreviated as k) is reported in cm/h and U10 in m/s. Thus, the coefficient 0.251 has the unit (cm h−1) (m/s)−2. Sc is temperature-dependent and was calculated as in ref. 60. G was converted from cm/h to m/s by dividing it with 360,000.
Supplementary Fig. 3 shows τCO2 for a range of scenarios under (sub)tropical conditions. h and G have the largest influence on τCO2 as has been pointed out previously35. The driving force behind G is the wind speed while increasing h increases τCO2, because a larger volume of water needs to be equilibrated (Supplementary Fig. 3A). Temperature influences τCO2 through its influence on R, B, and Sc but its effect is small relative to h and wind speed (Supplementary Fig. 3B).
To further explore the constraints of air-sea CO2 exchange on CDR, we mapped τCO2 for the GASB region using observational datasets and ERA5 wind data products. R and B were calculated with the Python script PyCO2SYS version 1.0.1 by Matthew Humphreys et al. (see ref. 61) using the Takahashi et al. monthly climatology59 for TA and pCO2. Input values for phosphate, silicate, temperature, and salinity were also taken from the Takahashi et al. climatology59, with all variables on a 4° latitude by 5° longitude grid. We used the default constants recommended for seacarb53 (see above) also for the calculations with PyCO2SYS (e.g., K1 and K2 from ref. 54).
Mixed layer depth (h) is calculated from a 1° latitude by 1° longitude gridded Argo climatology62 of temperature and salinity provided as monthly means, averaged for the time period 2004 through 2018. Mixed layer depth is defined using a density threshold of 0.03 kg m−3 following de Boyer Montégut et al. (see ref. 63). Finally, G is calculated as in Eq. 5, using gridded Argo temperature to determine Sc following Wanninkhof (see ref. 60), and ERA5 monthly mean wind speeds for the year 2018 for U10. ERA5 winds, R, and G, are linearly interpolated onto the Argo 1° latitude by 1° longitude grid to compute G and τCO2. We produce an annual mean estimate of h, R, B, G, and τCO2 by averaging the monthly mean Argo and ERA5 data, noting that the time periods are different for these data and the Takahashi climatology59, with the interpolated annual mean components shown in Supplementary Fig. 4. Additionally, we make seasonal estimates of τCO2 by averaging the monthly data for all variables to 3-month means (Supplementary Fig. 5).
The τCO2 timescales calculated for the GASB region (Fig. 3 main text; Supplementary Fig. 5) are generally in good agreement with those calculated by Jones et al. and both analyses show the same regional features (compare Fig. 3 main text with Fig. 1a in the ref. 35). Nevertheless, our τCO2 timescales tend to be slightly longer, especially north of 25°N. We used the same underlying datasets for h, R, and B so these factors cannot explain the differences. However, we used a more recent parameterization of air-sea gas exchange, which increases G by about 20% relative to the one used by Jones et al. Furthermore, Jones et al. used a wind speed climatology based on a monthly mean QuikSCAT and National Centers of Environmental Prediction (NCEP) reanalysis averaged from January 2000 to July 2007 whereas we used ERA5 data for 2018. Sensitivity calculations show that τCO2 timescales are highly dependent on wind speed, especially in the critical range below 10 m/s (Supplementary Fig. 3A).
The residence time of seawater in the surface mixed layer was provided by Jones et al. (see ref. 35) from their simulation of an idealized surface age tracer using circulation fields from the Estimating the Circulation and Climate of the Ocean-Global Ocean Data Assimilation Experiment product, version 3 iteration 73, a 1° × 1° global data assimilating product based on the Massachusetts Institute of Technology General Circulation Model35. All relevant details on their calculations and assumptions can be found in their paper (see Section 2.2 in ref. 35).
CO2 offset through biomass transportation and processing associated with geological carbon storage
Seaweed carbon storage in geological reservoirs requires energy for transportation from offshore seaweed farms to the processing and storage sites. Biomass could be transported as wet weight, dry weight, or as some further refined product64. Storage could be in various depots and in various physical states such as biochar or liquid CO2 (see refs. 65,66). It is beyond the scope of this study to go through all possible scenarios and estimate the associated CO2 discounts for each of them in a full lifecycle analysis. We therefore consider possible CO2 discounts with two central steps (shipping, CO2 separation from flue gas stream) within one plausible scenario, where harvested seaweed wet weight biomass is transported from the oceans to the coast and combusted for bioenergy with carbon capture and storage (BECCS). Please note that we neglect possible CO2 emissions during harvesting, drying, (un)loading as well as other CO2 offsets associated with BECCS such as liquified CO2 transports.
CO2 emissions by bulk carriers (ships transporting unpackaged goods such as coal) depend on the vessel size but range from 2.7 to 33.9 g CO2 t cargo−1 km−1 with the higher value for smallest vessels67. One tonne of Sargassum wet weight contains 54,300 g of carbon49, equivalent to 200,000 g of CO2 fixed as carbon in biomass. Thus, the CO2 discount through shipping is 0.0014–0.017 % t wetweight−1 km−1, depending on the vessel size. Transport from an offshore macroalgae farm 100 km away from the processing facility would therefore reduce CDRtheoretical by 0.14–1.7% due to combustion of engine fuels. This sets a lower bound since energy consumption during (un)loading as well as fuel production and ship construction are not considered.
For BECCS, CO2 generated during macroalgae combustion needs to be separated from the flue gas, which has an efficiency of 80–90% depending on the applied method37,66. Accordingly, losses of CO2 during the separation process would reduce CDRtheoretical by 10–20%. The energy for processing the CO2 for storage could theoretically be derived from combusting the algae, but there are still other potential CO2 offsets along the process chain (see above and ref. 66). Thus, the 10–20% discount considered for BECCS is likely another lower bound of the potential offsets to CDRtheoretical.
Albedo modification by Sargassum
Albedo is defined as the ratio between reflected and incoming solar flux at the Earth surface68. It is given as a dimensionless number between 0 (absorption of all incident solar flux) and 1 (reflection of all solar flux). Seawater has a relatively low albedo compared to other surfaces69. Therefore, marine vegetation on top or slightly below the sea surface increases the albedo44. In the following, we estimate how much the albedo enhancement caused by afforestation could reduce radiative forcing. Afterwards, we estimate how much radiative forcing is reduced by CDR through afforestation. Last, we compare the reductions of radiative forcing. The calculations described below are based upon equations by Betts41 and Kirschbaum et al.43, who did a similar assessment in the context of terrestrial afforestation.
The 2018 GASB had initially a surface Sargassum coverage of ~870 km2 in December 2017 (Supplementary Table 2). It then extended to a maximum of 6093 km2 in June 2018 after which coverage declined to ~807 km2 (Supplementary Table 2; refs. 13,16). The seasonality in coverage as shown in Supplementary Table 2 prescribes the growth cycle for our ocean afforestation scenario, where we assume that (i) seaweed farms of 6093 km2 are maintained north of South America within the GASB region (lat = 10°N; 60°W), (ii) growth occurs during approximately the first half of the year, and (iii) harvest/processing during the second half matching biomass build-ups and declines as in Supplementary Table 2. The (sub)surface growth of marine vegetation increases the albedo of seawater44, thereby decreasing the daily average radiative forcing (ΔRFdaily) calculated as:
| 6 |
Here, Qs↓ is the total daily downward solar flux (in J m−2 d−1), Δa the change in albedo over the shortwave spectrum (i.e., ~0.28–2500 nm) due to seaweed coverage, and αatm is the proportion of shortwave-radiation absorbed by the atmosphere (~20%)43,70. Qs↓ data was obtained from the Giovanni online data system, developed and maintained by the NASA GES DISC. More specifically, we downloaded maps of monthly averages (December 2017–November 2018) of Surface incoming shortwave flux (0.5 × 0.625°) from the MERRA-2 Model M2TMNXRAD v5.12.4. (This data is provided in W m−2 and was multiplied with 86,400 to convert to J m−2 d−1 needed for Qs↓ in Eq. 6.) Afterwards we extracted representative monthly incoming solar fluxes for the anticipated study region (lat = 10°N; 60°W). Qs↓ changes over the course of a seasonal cycle but is relatively stable in the low latitude considered in our scenario, ranging from 180 to 280 W m−2 (Supplementary Table 2; equivalent to 15.5–24 MJ m−2 d−1). The increase of albedo, Δa, due to Sargassum blooms has, to the best of our knowledge, not been determined so far. However, there are assessments of Δa due to seagrass in shallow water published by Fogarty et al. (see ref. 44). These authors found that albedo of seagrass meadows is by about 0.01–0.07 higher than open water albedo with the highest increase observed, when the coverage of the canopy was dense and close to the surface (the closest they considered was 0.25 m below surface)44. While not fully representative for our Sargassum analogue, Fogarty et al.’s data (see ref. 44) provides a useful first indication how much seaweeds could increase albedo relative to open ocean seawater. Arguably, Sargassum rafts would be on the upper end or above of the 0.01–0.07 range, because they float right at the surface and have a very dense coverage49.
Applying Eq. (6), we first calculate cumulative monthly ΔRF (i.e., ΔRFdaily * days of the month) and then cumulative yearly ΔRF (i.e., sum of monthly ΔRF). We do this calculation for a range of plausible Δa (0.01–0.1)44, to account for different types of ocean afforestation where seaweeds could grow at different depths in the water column or even at the surface like Sargassum (Supplementary Tables 2 and 3). In all scenarios, ΔRFyearly decreases ranging from 181—1811 PJ y−1.
The reduction in radiative forcing per year−1 due to the removal of 1 tonne of carbon from the atmosphere (ΔRFC in J y−1) can be calculated as:
| 7 |
where Catm is the CO2 concentration in the atmosphere (we use 410 p.p.m.v. for our calculations), 86,400 is the number of seconds per day, 365 the number of days per year, 510 × 1012 the surface area of the Earth, and 2.124 × 109 is the tonnes of carbon that lead to a 1 p.p.m.v. increase of atmospheric CO2 (see ref. 43). The factor 5.35 and the natural logarithm account for the increase of radiative forcing with increasing atmospheric CO2 (see ref. 71). According to Eq. 7, ΔRFC equals −99 GJ tC−1 y−1. This number is slightly lower than −104 GJ tC−1 y−1 calculated by Kirschbaum et al.43 because they used a Catm of 390 p.p.m.v. and the climate sensitivity is a logarithmic function of Catm (see ref. 71).
The assessment shows that ocean afforestation increases albedo thereby constituting a positive feedback in addition to the reduction of radiative forcing caused by CDR. The CDR effect on radiative forcing, i.e., −99 GJ tC−1 y−1 × 420,000 t C = −41.6 PJ y−1, is considerably smaller than the albedo effect during the first years (420,000 t C = CDRtheoretical of the 2018 GASB; see main text). However, the reduction of radiative forcing through CDR is cumulative from season to season, so that it eventually becomes more important than the albedo effect. In our scenario seaweed farms totalling 6093 km2 in the tropics, CDR would outweigh the albedo effect after −181/−41.6 to −1811/−41.6 (i.e., 4.4–44) annual seaweed growth cycles assuming instantaneous (and therefore unrealistic) atmospheric CO2 invasion into seawater. Please note that this calculation of timescales neglects the change in Catm due to ongoing emissions and changes in atmospheric CO2 as this has only a small influence on the relatively short timescales considered here.
Propagating the upper and lower bounds for the reduction of CDRtheoretical through calcification and nutrient reallocation
In this section we propagate the wide range of assumptions for the calcification and nutrient reallocation offsets to estimate the upper and lower bounds of CDRtheoretical of afforestation with Sargassum. The range of assumptions are constrained by published data as described in methods on calcification and nutrient reallocation. The goal of such propagations is to ensure that the best-case and worst-case scenarios for ocean afforestation are represented to provide a balanced viewpoint. Furthermore, we aim to illustrate the degree of uncertainty of the theoretical CDR potential of ocean afforestation due to these biogeochemical feedbacks. Our estimation is based on Eq. 1.
To estimate the lower bound of CDRtheoretical, we first assume an arbitrary amount of 100 mol TPC associated with Sargassum. Assuming the maximum of CaCO3 contribution Sargassum wet weight by epibiontic calcifiers of 21.4%20 yields an upper value PIC:POC ratio of ~0.9 (mol:mol). Thus, 100 mol TPC would be split in ~47 mol PIC and ~53 mol POC. The 53 mols of POC would bind the same amount of CO2 whereas the formation of 47 mols PIC would release ~29.6 mols of CO2 (i.e., PICseaweed calculated as 47*psi). Accordingly, CDRtheoretical would decrease from 53 to 23.4 mols CO2 in this high calcification scenario. The 53 mols of POC would require ~3.3 mols of nitrogen assuming a lower bound C:N ratio of 16 (mol:mol) reported for holopelagic Sargassum fluitans72. These amounts of N would support 26.5 mols of POC fixation by phytoplankton assuming a phytoplankton C:N ratio of 8 (see ref. 22). Thus, CDRtheoretical would decrease by another 26.5 mols (POCplankton) and become slightly negative (i.e., 53−29.6−26.5 = −3.1). However, nutrient reallocation from phytoplankton to Sargassum would also reduce calcification by phytoplankton. The planktonic PIC:POC ratio in the (sub)tropical Atlantic is ~0.01 (see refs. 26,27), so that 26.5 mols of POC would be associated with 0.265 mols of PIC. This phytoplankton PIC is not formed due to the nutrient reallocation to Sargassum which saves ~0.17 mols of CO2 (PICplankton) from being released (0.265*psi). Overall, the CDRtheoretical would be close to zero under the abovementioned lower bound assumptions (i.e., 53 − 29.6 − 26.5 + 0.17 ≈ −3 mols for 100 mol TPC initially fixed by the Sargassum community). For the 2018 GASB with a production of ~1.02 Mt TPC (Supplementary Table 1), this would be −0.03 Mt C (formation of ~0.1 Mt CO2).
To estimate the upper bound of CDRtheoretical we do exactly the same calculations but with upper bound assumptions: CaCO3 contribution Sargassum wet weight by epibiontic calcifiers of 4.3%20 leading to a Sargassum PIC:POC of ~0.11 (mol:mol); C:N Sargassum = 108 mol:mol72; C:N phytoplankton = 8 (mol:mol, same as above); planktonic PIC:POC = 0.01 (mol:mol, same as above). Under these conditions, 10 of the initially 100 mol TPC formed in Sargassum habitats would be present as PIC and release 6.3 mol CO2 (i.e., PICseaweed). The 90 mols of POC would require 0.83 mols of N. This would support 6.6 mol of phytoplankton POC (i.e., POCplankton), which would form 0.066 mols of PIC thereby releasing 0.04 mols CO2. Thus, the upper bound CDRtheoretical is 90 − 6.3 − 6.6 + 0.04 ≈ 77 mols for 100 mol TPC initially fixed. For the 2018 GASB with a production of ~1.02 Mt TPC (Supplementary Table 1), this would be 0.79 Mt C (consumption of ~2.9 Mt CO2).
The amount of CO2 removal to limit global warming below 2 °C, and the hypothetical contribution of the GASB to these CO2 removal targets
Results from simulations of shared socioeconomic pathways (SSPs) 1–5 with Integrated Assessment Models (IAMs) were downloaded in November 2018 from the database of the International Institute of Applied Systems Analysis (IIASA) under the link https://secure.iiasa.ac.at/web-apps/ene/SspDb/dsd?Action=htmlpage&page=about. The database was filtered for SSP scenarios with an additional temperature forcing of 2.6 W/m2 (SSP-2.6) where global warming at the end of the simulation (i.e., 2100) was below 2 °C (transient temperature overshoots above 2 °C were not considered as an exclusion factor). This yielded 32 SSP-2.6 simulations. Afterwards, we examined each of the 32 simulations for the variable: Emissions | CO2|Carbon Capture and Storage | Biomass (please note that IAMs currently realize CO2 removal mostly with Bio Energy with Carbon Capture and Storage (BECCS) and terrestrial afforestation73). The time steps of IAMs shown here is 10 years (5 years from 2005 to 2010) so that the annual CO2 removal was calculated by linear interpolation between two datapoints.
All IAM runs simulating SSPs included CO2 removal, which generally commenced in the 2020’s, although earlier in a few simulations (Supplementary Fig. 2). The integrated amounts of CO2 removal from 2005 until 2100 were between 112–931 Gt CO2 (minimum–maximum) with a median of 423 Gt CO2 and a mean of 513 Gt CO2. In general, CO2 removal was lower in those scenarios that had a more ambitious emission reduction (e.g., SSP1; Supplementary Fig. 2).
Ocean afforestation at the scale of Sargassum growth in the GASB during 2018 could contribute −0.0001–0.0029 Gt CO2 of CO2 removal, if all of the seawater CO2 consumed through biomass formation is balanced by permanent influx of atmospheric CO2. The large range is due to propagating the upper and lower bounds of assumptions (see previous section) with respect to magnitude of calcification and nutrient reallocation.
Supplementary information
Acknowledgements
We thank Chuanmin Hu and Mengqiu Wang for providing their monthly composites of gridded Sargassum biomass data; Daniel Jones, Takamitsu Ito, Yohei Takano, and Wei-Ching Hsu for providing model output data on surface water residence time; Martin Jung and Jens Daniel Müller for their photographs of Sargassum rafts; Andrew Lenton for proof-reading the manuscript. This study was funded by the Australian Research Council by a Laureate awarded to P.W.B. (FL160100131). V.T. acknowledges support from CSHOR, a joint research Centre for Southern Hemisphere Ocean Research between QNLM and CSIRO. Argo data were collected and made freely available by the International Argo Program and the national programs that contribute to it (http://www.argo.ucsd.edu; http://argo.jcommops.org; 10.17882/42182). The Argo Program is part of the Global Ocean Observing System. The ERA5 dataset used for biomass and equilibration timescale estimations was developed by the European Centre for Medium-Range Weather Forecasts and was obtained from the Research Data Archive at the National Center for Atmospheric Research, Computational and Information Systems Laboratory. Analyses and visualizations used for albedo calculations were partially produced with the Giovanni online data system, developed and maintained by the NASA GES DISC. We acknowledge the mission scientists and Principal Investigators who provided the data used in the albedo calcuations.
Author contributions
L.T.B., V.T., and P.W.B. designed the study. L.T.B. and V.T. analysed the data. L.T.B. drafted and revised the manuscript. P.W.B. and V.T. worked on the different versions of the manuscript. J.G., C.L.H., and J.A.R. commented on and contributed to the improvement of the manuscript.
Data availability
Data used for calculations is provided in the supplementary material. In case calculations were based upon datasets from repositories, we provide the sources and/or necessary links in the methods or respective supplementary material.
Code availability
All calculations, equations, and applied software are detailed and referenced in the methods and supplementary material.
Competing interests
The authors declare no competing interests
Footnotes
Peer review information Nature Communications thanks Andreas Oschlies and other, anonymous, reviewers for their contributions ot the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22837-2.
References
- 1.Duarte, C. M., Wu, J., Xiao, X., Bruhn, A. & Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 4, 100 (2017).
- 2.Ritschard RL. Marine algae as a CO2 sink. Water Air Soil Pollut. 1992;64:289–303. doi: 10.1007/BF00477107. [DOI] [Google Scholar]
- 3.Flannery, T. Sunlight and Seaweed: An Argument for How to Feed, Power and Clean Up the World (Text Publishing Melbourne, 2017).
- 4.N’Yeurt ADR, Chynoweth DP, Capron ME, Stewart JR, Hasan MA. Negative carbon via ocean afforestation. Process Saf. Environ. Prot. 2012;90:467–474. doi: 10.1016/j.psep.2012.10.008. [DOI] [Google Scholar]
- 5.Smetacek V, Zingone A. Green and golden seaweed tides on the rise. Nature. 2013;504:84–88. doi: 10.1038/nature12860. [DOI] [PubMed] [Google Scholar]
- 6.Warren, E. We need a blue new deal for our oceans. https://elizabethwarren.com/plans/blue-new-deal. Accessed 14th August 2020 (2020).
- 7.Doumeizel, V. et al. Seaweed Revolution: A Manifesto for a Sustainable Future (Lloyd’s Register Foundation, 2020).
- 8.Orr JC, Sarmiento JL. Potential of marine macroalgae as a sink for CO2: constraints from a 3-D general circulation model of the global ocean. Water Air Soil Pollut. 1992;64:405–421. doi: 10.1007/BF00477113. [DOI] [Google Scholar]
- 9.Chung IK, et al. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013;70:1038–1044. doi: 10.1093/icesjms/fss206. [DOI] [Google Scholar]
- 10.Crutzen PJ. Albedo enhancement by stratospheric sulfur injections: a contribution to resolve a policy dilemma? Clim. Change. 2006;77:211–219. doi: 10.1007/s10584-006-9101-y. [DOI] [Google Scholar]
- 11.Martínez-García A, et al. Iron fertilization of the subantarctic ocean during the last ice age. Science. 2014;343:1347–1350. doi: 10.1126/science.1246848. [DOI] [PubMed] [Google Scholar]
- 12.Gower J, Young E, King S. Satellite images suggest a new Sargassum source region in 2011. Remote Sens. Lett. 2013;4:764–773. doi: 10.1080/2150704X.2013.796433. [DOI] [Google Scholar]
- 13.Wang M, et al. The great Atlantic Sargassum belt. Science. 2019;364:83–87. doi: 10.1126/science.aaw7912. [DOI] [PubMed] [Google Scholar]
- 14.Johns EM, et al. The establishment of a pelagic Sargassum population in the tropical Atlantic: biological consequences of a basin-scale long distance dispersal event. Prog. Oceanogr. 2020;182:102269. doi: 10.1016/j.pocean.2020.102269. [DOI] [Google Scholar]
- 15.Gower, J. & King, S. The distribution of pelagic Sargassum observed with OLCI. Int. J. Remote Sens. 10.1080/01431161.2019.1658240 (2019).
- 16.Wang, M., Hu, C. & Barnes, B. Sargassum density and coverage using Moderate Resolution Imaging Spectroradiometer (MODIS) satellite data from 2001-01-01 to 2018-12-31. NOAA National Centers for Environmental Informationhttps://accession.nodc.noaa.gov/0190272. Accessed 25th March 2020 (2019).
- 17.Woodcock AH. Winds subsurface pelagic Sargassum and Langmuir circulations. J. Exp. Mar. Bio. Ecol. 1993;170:117–125. doi: 10.1016/0022-0981(93)90132-8. [DOI] [Google Scholar]
- 18.Frankignoulle M, Canon C, Gattuso J-P. Marine calcification as a source of carbon dioxide: positive feedback of increasing atmospheric CO2. Limnol. Oceanogr. 1994;39:458–462. doi: 10.4319/lo.1994.39.2.0458. [DOI] [Google Scholar]
- 19.Macreadie PI, Serrano O, Maher DT, Duarte CM, Beardall J. Addressing calcium carbonate cycling in blue carbon accounting. Limnol. Oceanogr. Lett. 2017;2:195–201. doi: 10.1002/lol2.10052. [DOI] [Google Scholar]
- 20.Pestana H. Carbonate sediment production by Sargassum epibionts. J. Sediment. Petrol. 1985;55:184–186. [Google Scholar]
- 21.Moore CM, et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013;6:701–710. doi: 10.1038/ngeo1765. [DOI] [Google Scholar]
- 22.Martiny AC, Vrugt JA, Primeau FW, Lomas MW. Regional variation in the particulate organic carbon to nitrogen ratio in the surface ocean. Glob. Biogeochem. Cycles. 2013;27:723–731. doi: 10.1002/gbc.20061. [DOI] [Google Scholar]
- 23.Martiny AC, et al. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 2013;6:279–283. doi: 10.1038/ngeo1757. [DOI] [Google Scholar]
- 24.Devries T, Primeau F, Deutsch C. The sequestration efficiency of the biological pump. Geophys. Res. Lett. 2012;39:1–5. doi: 10.1029/2012GL051963. [DOI] [Google Scholar]
- 25.Sarmiento, J. L. & Gruber, N. Ocean biogeochemical dynamics. (Princeton University Press, 2006).
- 26.Poulton AJ, et al. Phytoplankton mineralization in the tropical and subtropical Atlantic Ocean. Glob. Biogeochem. Cycles. 2006;20:1–10. doi: 10.1029/2006GB002712. [DOI] [Google Scholar]
- 27.Sarmiento, J. L. et al. A new estimate of the CaCO3 to organic carbon export ratio. Global Biogeochem. Cycles16, 54–1 (2002).
- 28.Atkinson MJ, Smith SV. C:N:P ratios of benthic marine plants. Limnol. Oceanogr. 1983;28:568–574. doi: 10.4319/lo.1983.28.3.0568. [DOI] [Google Scholar]
- 29.Krause-Jensen D, Duarte CM. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016;9:737–742. doi: 10.1038/ngeo2790. [DOI] [Google Scholar]
- 30.Hansell DA. Recalcitrant dissolved organic carbon fractions. Ann. Rev. Mar. Sci. 2013;5:421–445. doi: 10.1146/annurev-marine-120710-100757. [DOI] [PubMed] [Google Scholar]
- 31.Peters GP, Geden O. Catalysing a political shift from low to negative carbon. Nat. Clim. Change. 2017;7:619–621. doi: 10.1038/nclimate3369. [DOI] [Google Scholar]
- 32.Herzog H, Caldeira K, Reilly J. An issue of permanence: Assessing the effectiveness of temporary carbon storage. Clim. Change. 2003;59:293–310. doi: 10.1023/A:1024801618900. [DOI] [Google Scholar]
- 33.Zakkour P, Kemper J, Dixon T. Incentivising and accounting for negative emission technologies. Energy Procedia. 2014;63:6824–6833. doi: 10.1016/j.egypro.2014.11.716. [DOI] [Google Scholar]
- 34.Bellamy R, Geden O. Govern CO2 removal from the ground up. Nat. Geosci. 2019;12:874–876. doi: 10.1038/s41561-019-0475-7. [DOI] [Google Scholar]
- 35.Jones DC, Ito T, Takano Y, Hsu WC. Spatial and seasonal variability of the air-sea equilibration timescale of carbon dioxide. Glob. Biogeochem. Cycles. 2014;28:1163–1178. doi: 10.1002/2014GB004813. [DOI] [Google Scholar]
- 36.Ruseva T, et al. Rethinking standards of permanence for terrestrial and coastal carbon: implications for governance and sustainability. Curr. Opin. Environ. Sustain. 2020;45:69–77. doi: 10.1016/j.cosust.2020.09.009. [DOI] [Google Scholar]
- 37.IPCC. The IPCC special report on carbon dioxide capture and storage. Working Group III of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2005).
- 38.Baker P, et al. Potential contribution of surface-dwelling Sargassum algae to deep-sea ecosystems in the southern North Atlantic. Deep. Res. Part II Top. Stud. Oceanogr. 2018;148:21–34. doi: 10.1016/j.dsr2.2017.10.002. [DOI] [Google Scholar]
- 39.DeVries T, Holzer M. Radiocarbon and helium isotope constraints on deep ocean ventilation and mantle-3He sources. J. Geophys. Res. Ocean. 2019;124:3036–3057. doi: 10.1029/2018JC014716. [DOI] [Google Scholar]
- 40.Smith P, Haszeldine RS, Smith SM. Preliminary assessment of the potential for, and limitations to, terrestrial negative emission technologies in the UK. Environ. Sci. Process. Impacts. 2016;18:1400–1405. doi: 10.1039/C6EM00386A. [DOI] [PubMed] [Google Scholar]
- 41.Betts RA. Offset of the potential carbon sink from boreal forestation by decreases in surface albedo. Nature. 2000;408:187–190. doi: 10.1038/35041545. [DOI] [PubMed] [Google Scholar]
- 42.Keller DP, Feng EY, Oschlies A. Potential climate engineering effectiveness and side effects during a high carbon dioxide-emission scenario. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirschbaum MUF, et al. Implications of albedo changes following afforestation on the benefits of forests as carbon sinks. Biogeosciences. 2011;8:3687–3696. doi: 10.5194/bg-8-3687-2011. [DOI] [Google Scholar]
- 44.Fogarty MC, Fewings MR, Paget AC, Dierssen HM. The influence of a sandy substrate, seagrass, or highly turbid water on Albedo and surface heat flux. J. Geophys. Res. Ocean. 2018;123:53–73. doi: 10.1002/2017JC013378. [DOI] [Google Scholar]
- 45.Brooks SD, Thornton DCO. Marine aerosols and clouds. Ann. Rev. Mar. Sci. 2018;10:289–313. doi: 10.1146/annurev-marine-121916-063148. [DOI] [PubMed] [Google Scholar]
- 46.Thompson M, Adams D, Johnson KN. The albedo effect and forest carbon offset design. J. For. 2009;107:425–431. [Google Scholar]
- 47.Bastin J-F, et al. The global tree restoration potential. Science. 2019;365:76–79. doi: 10.1126/science.aax0848. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Hu C. Mapping and quantifying Sargassum distribution and coverage in the Central West Atlantic using MODIS observations. Remote Sens. Environ. 2016;183:350–367. doi: 10.1016/j.rse.2016.04.019. [DOI] [Google Scholar]
- 49.Wang M, et al. Remote sensing of Sargassum biomass, nutrients, and pigments. Geophys. Res. Lett. 2018;45:359–12,367. doi: 10.1029/2018GL078858. [DOI] [Google Scholar]
- 50.Zeebe, R. E. & Wolf-Gladrow, D. A. CO2in seawater: Equilibrium, Kinetics, Isotopes. (Elsevier, 2001).
- 51.Schell JM, Goodwin DS, Siuda ANS. Recent Sargassum inundation events in the Caribbean: shipboard observations reveal dominance of a previously rare form. Oceanography. 2015;28:8–10. doi: 10.5670/oceanog.2015.70. [DOI] [Google Scholar]
- 52.Coston-Clements, L., Settle, L. R., Hoss, D. E. & Cross, F. A. Utilization of the Sargassum habitat by marine invertebrates and vertebrates—a review. NOAA Technical Memorandum NMFS-SEFSC-296 (1991).
- 53.Lavigne, H., Epitalon, J.-M. & Gattuso, J.-P. Seacarb: seawater carbonate chemistry with R. R package version 3.0. (2011).
- 54.Lueker TJ, Dickson AG, Keeling CD. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 2000;70:105–119. doi: 10.1016/S0304-4203(00)00022-0. [DOI] [Google Scholar]
- 55.Anton A, et al. Iron deficiency in seagrasses and macroalgae in the red sea is unrelated to latitude and physiological performance. Front. Mar. Sci. 2018;5:1–14. doi: 10.3389/fmars.2018.00074. [DOI] [Google Scholar]
- 56.Powers, L. C. et al. Sargassum sp. act as a large regional source of marine dissolved organic carbon and polyphenols. Global Biogeochem. Cycles10.1029/2019gb006225 (2019).
- 57.Wanninkhof R, Asher WE, Ho DT, Sweeney C, McGillis WR. Advances in quantifying air-sea gas exchange and environmental forcing. Ann. Rev. Mar. Sci. 2009;1:213–244. doi: 10.1146/annurev.marine.010908.163742. [DOI] [PubMed] [Google Scholar]
- 58.Holte J, Talley LD, Gilson J, Roemmich D. An Argo mixed layer climatology and database. Geophys. Res. Lett. 2017;44:5618–5626. doi: 10.1002/2017GL073426. [DOI] [Google Scholar]
- 59.Takahashi T, et al. Climatological distributions of pH, pCO2, total CO2, alkalinity, and CaCO3 saturation in the global surface ocean, and temporal changes at selected locations. Mar. Chem. 2014;164:95–125. doi: 10.1016/j.marchem.2014.06.004. [DOI] [Google Scholar]
- 60.Wanninkhof R. Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods. 2014;12:351–362. doi: 10.4319/lom.2014.12.351. [DOI] [Google Scholar]
- 61.Humphreys, M. P. et al. PyCO2SYS: marine carbonate system calculations in Python. 10.5281/zenodo.3744275 (2020).
- 62.Roemmich D, Gilson J. The 2004–2008 mean and annual cycle of temperature, salinity, and steric height in the global ocean from the Argo Program. Prog. Oceanogr. 2009;82:81–100. doi: 10.1016/j.pocean.2009.03.004. [DOI] [Google Scholar]
- 63.de Boyer Montégut C, Madec G, Fischer AS, Lazar A, Iudicone D. Mixed layer depth over the global ocean: an examination of profile data and a profile-based climatology. J. Geophys. Res. 2004;109:1–20. [Google Scholar]
- 64.Lehahn Y, Ingle KN, Golberg A. Global potential of offshore and shallow waters macroalgal biorefineries to provide for food, chemicals and energy: feasibility and sustainability. Algal Res. 2016;17:150–160. doi: 10.1016/j.algal.2016.03.031. [DOI] [Google Scholar]
- 65.Hughes AD, et al. Does seaweed offer a solution for bioenergy with biological carbon capture and storage? Greenh. Gases Sci. Technol. 2012;2:402–404. doi: 10.1002/ghg.1319. [DOI] [Google Scholar]
- 66.Leung DYC, Caramanna G, Maroto-Valer MM. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014;39:426–443. doi: 10.1016/j.rser.2014.07.093. [DOI] [Google Scholar]
- 67.Psaraftis HN, Kontovas CA. CO2 emission statistics for the world commercial fleet. WMU J. Marit. Aff. 2009;8:1–25. doi: 10.1007/BF03195150. [DOI] [Google Scholar]
- 68.Myhre, G. et al. Anthropogenic and natural radiative forcing. in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change (eds. Stocker, T. F. et al.) 658–740 (Cambridge University Press, 2013). 10.1017/CBO9781107415324.018.
- 69.Payne RE. Albedo of the sea surface. J. Atmos. Sci. 1972;29:959–970. doi: 10.1175/1520-0469(1972)029<0959:AOTSS>2.0.CO;2. [DOI] [Google Scholar]
- 70.Trenberth KE, Fasullo JT, Kiehl J. Earth’s global energy budget. Bull. Am. Meteorol. Soc. 2009;90:311–323. doi: 10.1175/2008BAMS2634.1. [DOI] [Google Scholar]
- 71.Myhre G, Highwood EJ, Shine KP. New estimates of radiative forcing due to well mixed greenhouse gases. Geophys. Res. Lett. 1998;25:2715–2718. doi: 10.1029/98GL01908. [DOI] [Google Scholar]
- 72.Lapointe BE, West LE, Sutton TT, Hu C. Ryther revisited: nutrient excretions by fishes enhance productivity of pelagic Sargassum in the western North Atlantic Ocean. J. Exp. Mar. Bio. Ecol. 2014;458:46–56. doi: 10.1016/j.jembe.2014.05.002. [DOI] [Google Scholar]
- 73.Fuhrman, J., McJeon, H., Doney, S. C., Shobe, W. & Clarens, A. F. From zero to hero?: Why integrated assessment modeling of negative emissions technologies is hard and how we can do better. Front. Clim. 1, 11 (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for calculations is provided in the supplementary material. In case calculations were based upon datasets from repositories, we provide the sources and/or necessary links in the methods or respective supplementary material.
All calculations, equations, and applied software are detailed and referenced in the methods and supplementary material.