Abstract
Saccharomyces cerevisiae has evolved diverse mechanisms to osmotic changes: the cell wall, ion and water transport systems, and signaling cascades. At the present time, little is known about the mechanisms involved in short-term responses of osmotic stress in yeast or their physiological state during this process. We conducted studies of flow cytometry, wet weight measurements, and electron microscopy to evaluate the modifications in cell volume and the cell wall induced by osmotic stress. In response to osmotic challenges, we show very fast and drastic changes in cell volume (up to 60%), which were completed in less than eight seconds. This dramatic change was completely reversible approximately 16 s after returning to an isosmotic solution. Cell volume changes were also accompanied by adaptations in yeast metabolism observed as a reduction by 50% in the respiratory rate, measured as oxygen consumption. This effect was also fully reversible upon returning to an isosmotic solution. It is noteworthy that we observed a significant recovery in oxygen consumption during the first 10 min of the osmotic shock. The rapid adjustment of the cellular volume may represent an evolutionary advantage, allowing greater flexibility for survival.
Keywords: Yeast, Cell volume, Osmotic stress
Introduction
Yeast has developed a cell wall as a barrier to isolate and protect itself from noxious changes in the extracellular milieu, primarily to survive in the absence of external solutes while maintaining a high internal concentration. Until recently, the cell wall was considered a rigid structure, capable of protecting cells from mechanical damage and osmotic stress, preventing volume changes induced by internal osmotic pressure. This last assumption is striking, considering that the internal osmolarity in resting yeast is nearly 0.59 M (equivalent to 13 atm), but the internal osmolarity of fermenting yeast is close to 0.9 M (equivalent to 21 atm). This increase in the internal osmolarity of fermenting yeast is due to the accumulation of several metabolites and ions, mainly K+ [1].
A relevant question arising from these facts concerns how yeast responds to changes in external osmolarity. In incubation periods of several minutes, Saccharomyces cerevisiae can modify its cell wall and change its volume quickly in response to mild osmotic shocks [2].
The mechanism regulating long-term volume adaptation in yeast is known as the high osmotic glycerol pathway (HOG) [3–6]. HOG activation takes place over several hours and includes the activation of more than 100 genes. Among these is GPD1, glycerol phosphate dehydrogenase, whose expression also results in glycerol synthesis and accumulation inside the cell [5–7].
Short-term (in seconds) volume alterations have been studied in yeast protoplasts, in relation to the role of aquaporins [8]. Rapid adaptive changes taking place a few seconds after the onset of temperature changes, including water efflux, have been previously documented [9]. Changes of the internal water content in intact cells, indicating shrinkage of the cells in the presence of high concentrations of sorbitol [10] and a decrease of the internal pH during shrinkage in hyperosmotic buffers [11] have been shown. It has been proposed that yeast can balance water efflux by extracting water from its vacuole [12, 13]. The important question remains whether the volume changes in hyperosmotic media are produced by the contraction of the cell within the cell wall (plasmolysis), by the existence of a rigid cell wall, or that this structure is more flexible than originally thought, capable of undergoing substantial reversible and rapid modifications. However, detailed studies documenting rapid cell volume changes in real time are lacking.
In an attempt to characterize the rapid changes that yeast undergoes after osmotic challenges, we conducted studies to characterize changes in cell volume and morphology during the first few seconds after osmotic shock.
Materials and methods
Yeast strain, growth, and stress conditions
S. cerevisiae strain W3031-B (Mat α, ade2, his3, ura3, leu2, and trp1) was used in this study. The strain was grown in a YPD medium (1% yeast extract, 1% bacto peptone, and 2% glucose). One liter of the YPD medium was inoculated with 20 mL of a previous culture that had grown overnight at 30 °C in an oscillatory shaker at 260 rpm. Yeast cells were harvested by centrifugation and washed twice with distilled water, suspended at w/v, and kept on ice until used. Cells were exposed to distilled water or the hyperosmotic solutions indicated below.
Wet weight of yeast cells
A 5-min osmotic shock was applied to 250 mg of yeast cells in 1.6-mL microtubes. Later, the microtubes were centrifuged, the supernatant was discarded, and the wet weight was determined using a scale. The solutions tested in these experiments were 0.05, 0.5, 1.0, and 1.33 M of KCl. Distilled water was used as a control in each trial or YPD.
Fluorescence-activated cell sorter (FACS)
After a 30-h growth period, yeast cells were sorted using a florescence-activated cell sorter apparatus (FACS; FACScalibur, Becton Dickinson). Acquisition and analysis of the FACS data were performed using CELLQUEST software (Becton Dickinson).
We monitored the changes in morphology and volume undergone by yeast when exposed to hyperosmotic shock, as well as the rate at which these changes occurred. Changes in cell volume were studied to explore the time it takes yeast to change its cell size when it is transferred from a hyperosmotic media to a hypo-osmotic media, and vice versa. To conduct these experiments, yeast was placed in either distilled water or 1 M KCl. A yeast aliquot from one of these conditions was placed in a plastic tube containing either distilled water or 1 M KCl. Data acquisition was initiated immediately after placement and maintained for the next 30 s. The acquired data were separated into files containing 5000 events each. The time resolution between each file was 4 s.
Light microscopy and scanning electron microscopy (SEM)
The yeast cells were fixed in 3% glutaraldehyde for 2 h in either H2O or 1 M KCl [14]. The cells were then covered with a thin coat of gold using an Ion Sputter FC 1100 (Jeol) operating at 1200 kV and 5 mA for 10 min. Samples were analyzed and micrographs were taken with scanning electron microscopy (SEM; JSM-54110LV, Jeol) at a magnification of 10,000x.
Respiration measurements
The rate of oxygen consumption was monitored using a Clark oxygen electrode (Yellow Springs Instr. Co., Ohio, U.S.A.) with a polarization device and a recorder.
We measured the rate of consumed oxygen in experimental conditions with an oxygen meter, as described by Schatz [15]. The east was suspended in a buffer solution composed of MES-TEA 3 mM pH 6.0 and glucose 50 mM to stimulate breathing at 30 °C. One hundred milligrams of yeast (200 μL) was added to 5 mL of buffer and placed in the experimental chamber.
Potassium determination
A flame photometer (Zeiss PF5) was used to monitor ion concentrations, as previously described [16]. After incubation under different concentrations, the cells (500 mg wet weight) were suspended in 5 mL of 10 mM MES-TEA (pH 6.0) and disrupted by boiling in a heating bath for 20 min and centrifuged for 5 min. Potassium was measured in an adequate dilution of the supernatant. The flame photometer was calibrated using a solution of 1 mM KCl or NaCl, as previously described [17].
Results
Morphological changes to different osmolarities
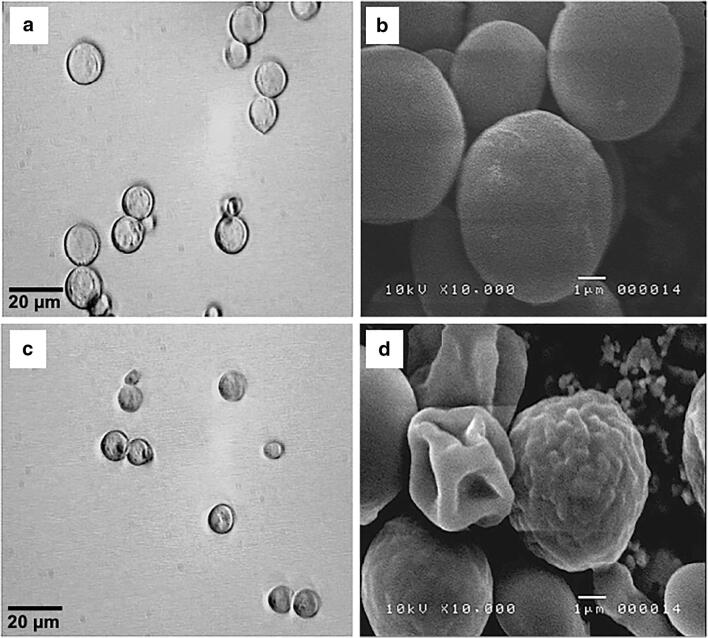
Observing the yeast under the phase contrast microscope allowed us to detect the cells’ morphologic changes when exposed to external solutions with different osmolarities. In distilled water, the cells showed a spherical appearance with a smooth surface (Fig. 1a). This morphology was similar to that displayed in the YPD culture medium (180 mOsm). In contrast, in the medium with 1 M KCl, the yeast showed an evident reduction in cell volume (Fig. 1c). Cell morphology was also altered under these experimental conditions; a rough cellular surface was observed, and the cells no longer maintained a spherical form (Fig. 1c).
Fig. 1.
Micrographs of yeast cells in response to osmotic stress. Comparative micrographs of light microscopy of yeast cells in water (a) and 1 M KCl (c) and scanning electron microscopy in water (b) and KCl (d)
SEM studies conducted with yeast exposed to water (Fig. 1b) or 1 M KCl external solutions highlighted the dramatic morphological changes in higher detail (Fig. 1d). In water, the cells showed an ellipsoidal morphology with a smooth surface. In contrast, yeast in 1 M KCl acquiring a wrinkled and shrunken surface, and the collapse of the cell wall was evident in many cells (Fig. 1d). These morphological changes were completely reversible upon return to water or YPD media (not shown).
Morphological and physiological changes were reversible
These dramatic changes in cell volume were also evident by weighing the pellets obtained after centrifuging the cells exposed to water or different KCl concentrations. As illustrated in Fig. 2a, an increasing reduction in wet weight was observed in higher KCl concentrations. The maximum reduction was observed with 1 M KCl; no further reduction was observed with 1.33 M KCl. Changes in wet weight were also fully reversible; it was possible to subject the same sample several times to changes in the extracellular solution repeatedly, from water to 1 M KCl and vice versa, with indistinguishable results. In the same way, the data had single exponential decay fits in wet weight
Fig. 2.

Volume changes in wet weight after different stress conditions. a Photographs illustrating the reduction in volume of the yeast pellet after exposing cells to different KCl concentrations or water (control). The lower panel shows the wet weight (mg) obtained with different KCl concentrations and the control (yeast exposed to distilled water). Lines above bars are the error rate. b A single exponential decay fits the reduction in wet weight
| 1 |
where y0 = 0.109 ± 0.001, A1 = 0.14 ± 0.002, t1 = 0.29 ± 0.014, Chi2 = 0.00003, and R2 = 0.99. Fitted data shows that yeast under the same challenge always responds with the same magnitude of shrinkage due to the osmotic shock (Fig. 2b). If the cells are placed in 1 M KCl and returned to water, the cell volume recovers. A similar series of experiments were performed using glycerol as the osmolyte instead of KCl; we observed a similar tendency, but the magnitude of the response was not comparable to that observed in cells grown with KCl.
Volume changes are reversible after osmotic challenges
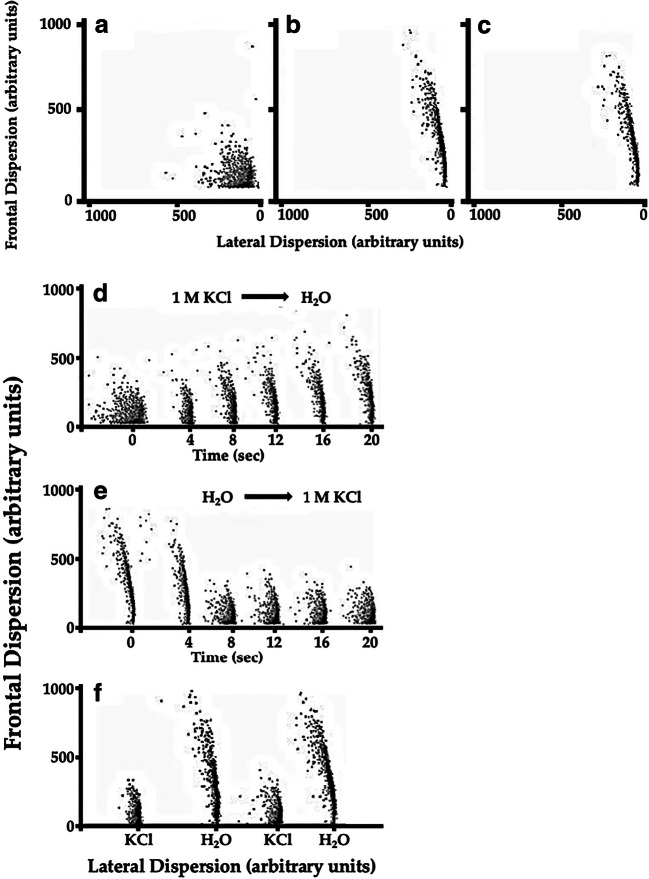
With the purpose of exploring changes in response to hypertonicity of the external media in greater detail and in real time, experiments were conducted using a flow cytometer. In this type of experiment, frontal dispersion reflects the single cell volume, and lateral dispersion reflects the roughness of the cellular surface.
As illustrated, cells exposed to 1 M KCl (Fig. 3a) showed a reduction in cell size, compared to those exposed to water (Fig. 3b) or YPD (Fig. 3c).
Fig. 3.
Volume changes in response to the hypertonicity of different external media. Typical bar plots were obtained using a fluorescence-activated cell sorter apparatus. Bar plots show frontal dispersion (reflecting cell size) and lateral dispersion (reflecting cell surface complexity, or roughness). Cells were maintained in 1 M KCl (a), distilled water (b), or YPD (c). Panel d illustrates the rapid modifications of cell size and shape when replacing the extracellular media (1 M KCl) with distilled water. Panel e illustrates the opposite experiment; cells maintained in distilled water were rapidly changed to 1 M KCl. (F) The same yeast sample was exposed to 1 M KCl then placed in distilled water and back to KCl and finally in distilled water. Histograms show the reproducibility of the response to these extreme osmolarities
To evaluate the rapid changes in cell volume, we conducted experiments in which cells in 1 M KCl were rapidly exposed to water, while measuring cell size over time. Immediately after exposing the cells to water, an increase in frontal dispersion was observed, reflecting an increase of cell volume. This was accompanied by a reduction in lateral dispersion, reflecting a transition to a smoother cell surface. This result agrees with our observations via SEM (Fig. 3c and d), in which cell roughness was dramatically increased upon exposure to 1 M KCl.
The increase of cell volume and surface roughness after exposing yeast previously incubated in KCl to water was completed within 16 s after changing the extracellular medium (Fig. 3d).
The opposite experiment yielded somewhat different results. Yeast maintained in water and rapidly exposed to 1 M KCl reduced its volume to a steady state within 8 s after KCl application. The exact time required for volume reduction was not possible to determine because of limitations in the sampling speed of the machine. However, no statistically significant differences in frontal dispersion were observed between 8 and 12 s (Fig. 3e). These results suggest that cell volume reduction was about twice as fast as the cell volume increase. Cell volume reduction was also accompanied by an increase in the roughness of the cellular surface, as expected from the electron microscopy results.
The reversibility of the observed phenomena was verified by repeating the changes from water to KCl and vice versa in the same yeast sample. To this purpose, yeast samples were centrifuged and washed to remove the extracellular solution. The bar plot obtained from repeated flow cytometry experiments was indistinguishable from that of fresh cells, indicating that the time series obtained were reproducible among osmotic stress challenges in the same sample (Fig. 3f).
Potassium ion was responsible for osmotic and volume changes
To evaluate modifications in the yeast’s ionic content as a result of osmotic stress, we measured intracellular K+, the most abundant intracellular ion. In order to do so, the yeast was exposed to increasing extracellular glycerol concentrations. In all cases, 5 mM KCl was present in addition to the glycerol (Fig. 4).
Fig. 4.
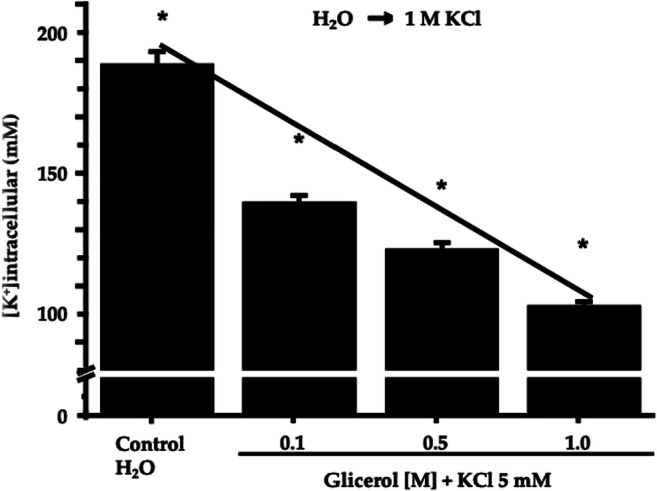
Determination of potassium content after osmotic stress with glycerol. The bar plot shows the reduction in the intracellular potassium concentration (in mM) obtained with increments in the extracellular osmolarity. Intracellular potassium was measured using a flame photometer, as described under Materials and Methods. In all cases, combined data represent the mean ± standard deviation from independent observations (N = 9). All data points were statistically different by a t-student test, as marked by * (p < 0.005)
The reduction in the intracellular potassium was insensitive to barium (up to 60 μM) and triethylammonium (up to 5 mM), two well-known blockers of potassium channels. One potassium channel has been identified thus far in S. cerevisiae (Tok-1). We performed similar experiments with a Tok-1 null yeast strain [14] and obtained similar results when compared to the wild strain. This indicates that extrusion of intracellular potassium upon hyperosmotic stress does not occur via Tok-1 potassium channels (data not shown).
Yeast respiration is regulated by osmotic changes
To evaluate a cell’s metabolic changes upon osmotic stress, we measured oxygen consumption at different extracellular potassium and sodium concentrations. As illustrated in Fig. 5a, yeast showed reduced oxygen consumption when directly exposed to osmotic stress; maximal reduction was observed at 2 M KCl. However, pre-incubating the cells for 10 min in 1 or 2 M KCl appeared to result in rapid adaptation to the stress, since the oxygen consumption recovered almost to control levels, even in the continuous presence of osmotic stress (Fig. 5a, subscript i).
Fig. 5.
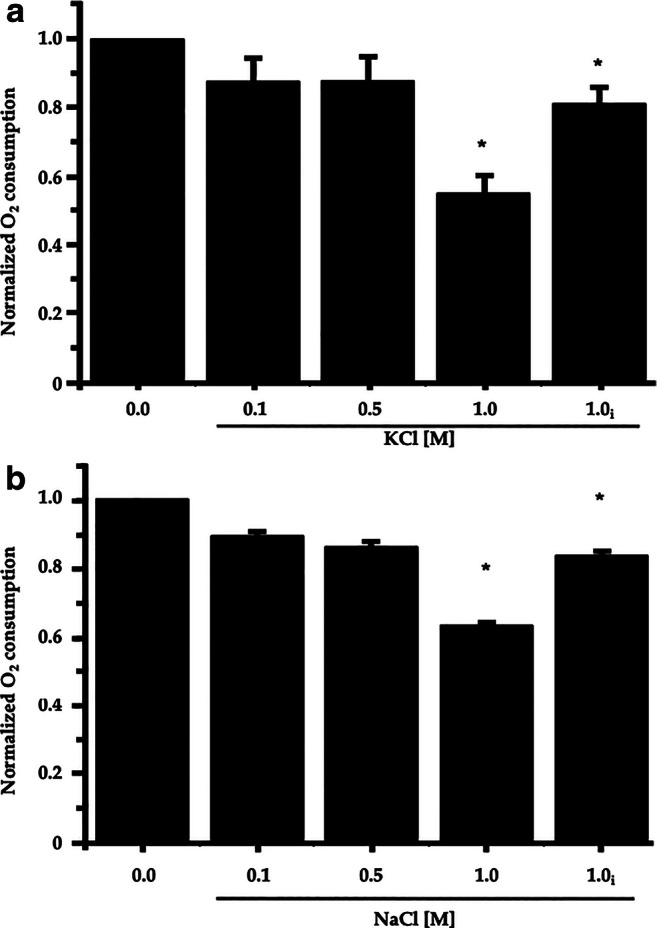
Cell respiration and partial metabolic adaptation to osmotic stress. Oxygen consumption was measured with increasing concentrations of KCl (A) and NaCl (B) in the medium. Cells were exposed to the indicated KCl (2 M) or NaCl (2 M) concentrations, and oxygen consumption was measured immediately after osmotic stress or 10 min after (subscript i for incubated). Cells (100 mg wet weight) were added at the beginning of the experiment to a medium containing 3 mM MES-TEA buffer, pH 6.0, final volume 5 mL. Twenty millimoles glucose was added at the beginning of each tracing. Dissolved oxygen saturation was 400 natg/mL in a final volume of 5 mL, amounting to a total of 2000 natg in the incubation chamber. Oxygen consumption was normalized to the consumption of oxygen in the absence of KCl or NaCl. Data show the means of four independent observations. *Asterisks show statistical differences among data points, compared to the values of the control without salt in a t-test
Similar results were obtained when Na+ was used as the osmolyte in the osmotic shock (Fig. 5b). However, with this cation, the reduction of oxygen consumption was smaller than with K+. The adaptation observed after incubating yeast for 10 min was also evident with this cation; the decreased oxygen consumption was near the control levels at 1 M if the cells were pre-incubated with the cation (Fig. 5b, subscript i).
Pre-incubating the cells for 30 min with 1 M KCl or 1 M NaCl resulted in a marked increment in oxygen consumption, showing values of around 1.5 times that of the control. When cells were incubated in the presence of 1 M KCl or NaCl and then centrifuged and washed with water, the original respiration rate was recovered.
Our results show that the cell wall of yeast is a remarkably flexible structure and can collapse under high osmotic pressure, resulting in a 60% reduction of the cell volume. This phenomenon is completed less than eight seconds after the onset of the osmotic stress. Twice as much time is required by the yeast to return to its original volume after replacing the extracellular media with an isosmotic solution. Our results show that changes in cell volume can take place immediately after the onset of an osmotic shock. The ion content measurement after a hyperosmotic shock suggests the participation of ion channels in volume regulation and yeast osmosensitivity. The changes in oxygen consumption rate before and after a hyperosmotic shock indicate that yeast respiration is also influenced by osmotic changes and that this adaptation is fully reversible.
Discussion
The plasticity of the yeast cell wall to quickly adapt to extracellular osmolarity stress is demonstrated in this work. The cells lost a great proportion of their volume (60%) after an osmotic shock. As a result of this, the yeast underwent important changes in size and shape during osmotic stress, a phenomenon that was completely reversible upon return to an isosmotic solution. These changes occurred within the first few seconds after replacing the extracellular solution. That the time points could be fitted by a single exponential relationship may suggest that the main mechanism involved is a one-component signal transduction system. These results are interesting, since they were observed in intact cells; most other authors have studied the effects of osmotic shock in yeast protoplasts [8, 9]. A steady-state study was also carried out in intact cells of S. cerevisiae through finding an important decrease of the cell water by incubating the cells in concentrations of sorbitol up to 0.5 M [10, 11].
It is important to point out that practically all cells showed size and general morphology changes when observed by phase contrast microscopy, but different types of responses were observed by SEM. Under SEM, some cells appeared to collapse, acquiring a wrinkled and shrunken surface, which indicates a higher sensitivity to osmotic shock. One possible explanation for this is that the more sensitive cells are the younger ones, which may possess softer cell walls.
The rapid adjustment (in seconds) of the cellular volume indicates that the cell wall is more flexible than originally suspected. This may represent an evolutionary advantage, allowing greater flexibility for survival.
The yeast cells lost more than 60% of their weight in the presence of 1 M KCl. This loss was much smaller in the medium containing 1 M glycerol, with only 36% of the weight lost. These differences are most likely because KCl is a soluble molecule. However, they may also be due to a higher permeability of the cells to glycerol at these high concentrations, and thus, its lower efficacy as an osmolyte.
When the volume changes were produced by glycerol, there was a significant loss of intracellular K+ ions accompanying the size and shape modifications, most likely reflecting changes in the membrane permeability to these ions induced by the osmotic shock [18]. The loss of water, and also these ions, is what may permit the reduction in cell volume.
In general, volume changes in eukaryotes result from the movement of ions [19] (mainly K+, Na+, and Cl−), amino acids, and water in and out of the cell [20]. In most eukaryotic cells, osmotically induced changes in volume are accompanied by rapid regulatory volume recovery (either regulatory volume increase, RVI, or decrease, RVD, depending on the osmolarity of the extracellular solution used). Conversely, yeast does not show a rapid volume recovery, but rather a slow response that involves the activation of several genes associated with the HOG cascade, mainly the activation of the GPD1 gene, which leads to the increased activity of glycerol phosphate dehydrogenase [7]. Thus, yeast lacks the immediate regulatory mechanisms observed in most eukaryotes, which may help compensate and recover the original cell volume in short periods of time (minutes). These rapid compensatory mechanisms involve the transport of ions, water, and amino acids in most eukaryotes, contrasting with the lower permeability of ions and molecules reported in yeast [20].
The pathways for Na+ and K+ extrusion after the osmotic stress induced in this study remain to be discovered. The involvement of Tok1p as an ionic channel was partly ruled out by the absence of effects of two K+-channel inhibitors, barium ions and triethylammonium. Preliminary experiments with the Tok-1 null strain also suggest that this channel is not involved in potassium movement after osmotic shock. Stretch-activated nonselective cationic channels may be activated by osmotic stress and could provide a pathway for both potassium and sodium fluxes. Future experiments may help elucidate this point.
A model has been published in an attempt to integrate the response of yeast to osmotic shock [21]. However, the model concentrates on the HOG signaling cascade without considering the very fast responses produced after the onset of osmotic shock because of the lack of studies exploring these rapid responses [22].
Currently, the HOG pathway is frequently studied to understand the responses of yeast to osmotic stress. We can see that in recent years, the literature has indicated the activation of translation as well as transcription.
The HOG signal pathway involves a large number of cellular events in S. cereviseae, such as the pheromone response in high osmolarity [23] and temperature stress [24]. In Candida albicans, Hog1 regulates the cell cycle progression in response to oxidative stress [25]; HOG has been classically related to the response to osmostress, and several of the proteins that participate in this response have been identified [see 26–30].
Yeast continually detects changes in the environment via osmosensors located on the cell surface. Environmental modification is finely modulated by receptors and transduced by GTPases into MAPK phosphorylation cascades [26, 31, 32]. Once these cascades are activated, the transcriptional process of the specific genes begins, and the production of osmoadaptive proteins follows [27]. Hog1 defines the response through molecular and nuclear targets [26]. In detail, activated Hog1 translocates to the nucleus and induces transcription of osmosensitive genes, adjusting the cell’s metabolism to produce glycerol essential for lasting osmotic adaptation [32]. Activation of the HOG pathway takes around 30 min and includes more than hundreds of genes [27, 30, 31]. However, there is evidence that adaptation to stress occurs differently at the transcriptional and translational levels [33, 34]. The translational response is fast because it allows the preferential use of pre-existing transcripts and avoids the need for a time-consuming gene transcription. The latter is the most durable, and it activates in approximately 30 min for the HOG pathway. In contrast, we show in this study that yeast is capable of triggering physiological responses in short times. This response probably involves translation mechanisms, as demonstrated by the activation of translation in short response times to 0.4 M NaCl; the cell contains polyribosomes that are preloaded and that synthesize proteins necessary to activate processes that respond quickly [33]. Subsequently, the HOG pathway is activated and culminates with the transcription of the proteins necessary to coordinate the HOG pathway in a coordinated manner. This precedes the initial changes required to respond to environmental fluctuations that do not involve gene transcription and that are necessary for the yeast to make decisions about its molecular response.
Conclusions
Our results on the effects of osmotic shock on yeast cellular respiration are also interesting, since the cells appear able to partially adapt in short periods of time (10 min). One possible explanation for this result might be related to the HOG signaling cascade, which directs the expression of many genes, resulting in profound alterations in yeast metabolism [7, 35]. However, it is improbable that this signaling cascade would act in such short periods of time. Rapid water movement at low temperatures may require aquaporins [8]; however, these experiments were performed with protoplasts at different concentrations of sorbitol. In our experiments, the permeability of yeast cells to high concentrations of K+ and Na+ is not known. With glycerol, it may be expected that the exit of potassium will be accompanied by water extrusion, most likely through aquaporins; the role of aquaporins in this phenomenon might be explored in future work. It is also possible that the effects on respiration may be due to internal pH changes in the cells [11]. Since different results were obtained with NaCl or KCl besides water movement, the involvement of monovalent cation transporters is possible. Yeast monovalent transport systems are much more specific for K+ than for Na+ [36]. Although it is not known if this specificity is still valid at high concentrations of the cations used, some discrimination may exist between both cations, and a preferential uptake of K+ may favor K+ over Na+.
Although this is the first of such studies, many questions remain unanswered that require further experimentation. However, our results underline the powerful mechanisms displayed by yeast to contend with rapid and extreme changes in external osmolarity.
Acknowledgments
The authors gratefully acknowledge the helpful discussion with Dr. Ataúlfo Martínez-Torres, Dr. Luis Vaca, and Dr. Antonio Peña. Thanks for technical support to Carlos Lozano and the Microscopy Unit (Instituto de Fisiología Celular, UNAM), M. in C. Adriana González-Gallardo (Instituto de Neurobiología, UNAM), Laboratorio Nacional de Visualización Científica Avanzada (LAVIS, UAQ), Estefany Vega Santo and Dr. Marco Sánchez Ramos (Faculty of Natural Science, UAQ). Special thanks for technical and administrative support to Luis Aguilar, Alejandro de León, Carlos Flores, and Jair García (Laboratorio Nacional de Visualización Científica Avanzada, UNAM).
Funding
This research was financed by SEP-CONACyT Ciencia Básica (grant number A1-S-26966 to C.S.), Laboratorios Nacionales CONACyT to C.S., and FONDEC-UAQ 2019 to C.S.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Conway EJ, Armstrong WM. The total intracellular concentration of solutes in yeast and other plant cells and the distensibility of the plant-cell wall. Biochem J. 1961;81(3):631–639. doi: 10.1042/bj0810631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez de Marañon I, Marechal PA, Gervais P. Passive response of Saccharomyces cerevisiae to osmotic shifts: cell volume variations depending on the physiological state. Biochem Biophys Res Commun. 1996;227(2):519–523. doi: 10.1006/bbrc.1996.1539. [DOI] [PubMed] [Google Scholar]
- 3.Tamás MJ, Rep M, Thevelein JM, Hohmann S. Stimulation of the yeast high osmolarity glycerol (HOG) pathway: evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett. 2000;472(1):159–165. doi: 10.1016/S0014-5793(00)01445-9. [DOI] [PubMed] [Google Scholar]
- 4.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66(2):300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warringer J, Hult M, Regot S, Posas F, Sunnerhagen P. The HOG pathway dictates the short-term translation response after hyperosmotic shock. Mol Biol Cell. 2010;21(17):3030–3092. doi: 10.1091/mbc.e10-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You T, Ingram P, Jacobsen MD, Cook E, McDonagh A, Thorne T, Lenardon MD, de Moura AP, Romano MC, Thiel M, Stumpf M, Gow NA, Haynes K, Grebogi C, Stark J, Brown AJ. A systems biology analysis of long and short-term memories of osmotic stress adaptation in fungi. BMC Res Notes. 2012;5:258. doi: 10.1186/1756-0500-5-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albertyn J, Hohman S, Thevelein JM, Prior BA. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high osmolarity glycerol response pathway. Mol Cell Biol. 1994;14(6):4135–4144. doi: 10.1128/MCB.14.6.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soveral G, Veiga A, Loureiro-Dias MC, Tanghe A, Van Dijck P, Moura TF. Water channels are important for osmotic adjustments of yeast cells at low temperature. Microbiology. 2006;152(Pt 5):1515–1152. doi: 10.1099/mic.0.28679-0. [DOI] [PubMed] [Google Scholar]
- 9.Gervais P, Martínez de Marañón I, Evrard C, Ferret E, Moundanga S. Cell volume changes during rapid temperature shifts. J Biotechnol. 2003;102(3):269–279. doi: 10.1016/S0168-1656(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 10.Ramos J, Haro R, Rodríguez-Navarro A. Regulation of potassium fluxes in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990;1029(2):211–217. doi: 10.1016/0005-2736(90)90156-I. [DOI] [PubMed] [Google Scholar]
- 11.Vindeløv J, Arneborg N. Saccharomyces cerevisiae and Zygosaccharomyces mellis exhibit different hyperosmotic shock responses. Yeast. 2002;19(5):429–439. doi: 10.1002/yea.844. [DOI] [PubMed] [Google Scholar]
- 12.Latterich M, Watson MD. Evidence for a dual osmoregulatory mechanism in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1993;191(3):1111–1117. doi: 10.1006/bbrc.1993.1331. [DOI] [PubMed] [Google Scholar]
- 13.Nass R, Rao R. The yeast endosomal Na+/H+ exchanger, Nhx1, confers osmotolerance following acute hypertonic shock. Microbiology. 1999;145(Pt 11):3221–3228. doi: 10.1099/00221287-145-11-3221. [DOI] [PubMed] [Google Scholar]
- 14.Saldaña C, Vázquez-Cuevas F, Garay E, Arellano RO. Epithelium and/or theca are required for ATP-elicited K+ current in follicle-enclosed Xenopus oocytes. J Cell Physiol. 2005;202(3):814–821. doi: 10.1002/jcp.20184. [DOI] [PubMed] [Google Scholar]
- 15.Schatz G, Racker E, Tyler DD, Gonze J, Estabrook RW. Studies of the DPNH-cytochrome b segment of the respiratory chain of baker's yeast. Biochem Biophys Res Commun. 1966;22(5):585–590. doi: 10.1016/0006-291X(66)90315-9. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Muñoz GA, Peña A. In situ study of K+ transport into the vacuole of Saccharomyces cerevisiae. Yeast. 2005;22(9):689–704. doi: 10.1002/yea.1238. [DOI] [PubMed] [Google Scholar]
- 17.González-Hernández JC, Cárdenas-Monroy CA, Peña A. Sodium and potassium transport in the halophilic yeast Debaryomyces hansenii. Yeast. 2004;21(5):403–412. doi: 10.1002/yea.1108. [DOI] [PubMed] [Google Scholar]
- 18.Saldaña C, Naranjo D, Coria R, Peña A, Vaca L. Splitting the two pore domains from TOK1 results in two cationic channels with novel functional properties. J Biol Chem. 2002;277(7):4797–4805. doi: 10.1074/jbc.M107957200. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong WM, Rothstein A. Discrimination between alkali metal cations by yeast. I. Effect of pH on uptake. J Gen Physiol. 1964;48(1):61–71. doi: 10.1085/jgp.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood JM. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev. 1999;63(1):230–262. doi: 10.1128/MMBR.63.1.230-261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakab M, Fürst J, Gschwentner M, Bottà G, Garavaglia ML, Bazzini C, Rodighiero S, Meyer G, Eichmueller S, Wöll E, Chwatal S, Ritter M, Paulmichl M. Mechanisms sensing and modulating signals arising from cell swelling. Cell Physiol Biochem. 2002;12(5–6):235–258. doi: 10.1159/000067895. [DOI] [PubMed] [Google Scholar]
- 22.Vázquez-Ibarra A, Subirana L, Ongay-Larios L, Kawasaki L, Rojas-Ortega E, Rodríguez-González M, de Nadal E, Posas F, Coria R. Activation of the Hog1 MAPK by the Ssk2/Ssk22 MAP3Ks, in the absence of the osmosensors, is not sufficient to trigger osmostress adaptation in Saccharomyces cerevisiae. FEBS J. 2018;285(6):1079–1096. doi: 10.1111/febs.14385. [DOI] [PubMed] [Google Scholar]
- 23.Baltanás R, Bush A, Couto A, Durrieu L, Hohmann S, Colman-Lerner A (2013) Pheromone-induced morphogenesis improves osmoadaptation capacity by activating the HOG MAPK pathway. Sci Signal 6(272):ra26. 10.1126/scisignal.2003312 [DOI] [PMC free article] [PubMed]
- 24.Shiraishi K, Hioki T, Habata A, Yurimoto H, Sakai Y (2018) Yeast Hog1 proteins are sequestered in stress granules during high-temperature stress. J Cell Sci 131(1):jcs209114. 10.1242/jcs.209114 [DOI] [PubMed]
- 25.Correia I, Alonso-Monge R, Pla J. The Hog1 MAP (2017) Kinase promotes the recovery from cell cycle arrest induced by hydrogen peroxide in Candida albicans. Front Microbiol 7:2133. 10.3389/fmicb.2016.02133 [DOI] [PMC free article] [PubMed]
- 26.Saito H, Posas F. Response to hyperosmotic stress. Genetics. 2012;192(2):289–318. doi: 10.1534/genetics.112.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stojanovski K, Ferrar T, Benisty H, Uschner F, Delfago J, Jimenez J, Solé C, de Nadal E, Klioo E, Posas F, Serrano L, Kiel C. Interaction dynamics determine signaling and output pathway responses. Cell Rep. 2017;19(1):136–149. doi: 10.1016/j.celrep.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Reiter W, Dohnal I, Gregori C, Beese-Sims S, Kuchler K, Ammerer G, Levin DE. MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev. 2013;27(23):2590–2601. doi: 10.1101/gad.229310.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Levin DE. Methylated metabolite of arsenite blocks glycerol production in yeast by inhibition of glycerol-3-phosphate dehydrogenase. Mol Biol Cell. 2019;30(17):2134–2140. doi: 10.1091/mbc.E19-04-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YL, Tseng SF, Huang YC, Shen ZJ, Hsu PH, Hsieh MH, Yang CW, Tognetti S, Canal B, Subirana L, Wang CW, Chen HT, Lin CY, Posas F, Teng SC. Yeast Cip1 is activated by environmental stress to inhibit Cdk1–G1 cyclins via Mcm1 and Msn2/4. Nat Commun. 2017;8(1):56. doi: 10.1038/s41467-017-00080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutt KL, Moseley JB. Transient activation of fission yeast AMPK is required for cell proliferation during osmotic stress. Mol Biol Cell. 2017;28(13):1804–1814. doi: 10.1091/mbc.e17-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Nadal E, Posas F. Osmostress-induced gene expression--a model to understand how stress-activated protein kinases (SAPKs) regulate transcription. FEBS J. 2015;282(17):3275–3285. doi: 10.1111/febs.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warringer J, Hult M, Regot S, Posas F, Sunnerhagen P. The HOG pathway dictates the short-term translational response after hyperosmotic shock. Mol Biol Cell. 2010;21(17):3080–3092. doi: 10.1091/mbc.E10-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng YL, Wang SA. Stress tolerance variations in Saccharomyces cerevisiae strains from diverse ecological sources and geographical locations. PLoS One. 2015;10(8):e0133889. doi: 10.1371/journal.pone.0133889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klipp E, Nordlander B, Krüger R, Gennemark P, Hohmann S. Integrative model of the response of yeast to osmotic shock. Nat Biotechnol. 2005;23(8):975–982. doi: 10.1038/nbt1114. [DOI] [PubMed] [Google Scholar]
- 36.Albertyn J, Hohmann S, Prior BA. Characterization of the osmotic-stress response in Saccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr Genet. 1994;25(1):12–18. doi: 10.1007/BF00712960. [DOI] [PubMed] [Google Scholar]