Abstract
We report here the synthesis of silver nanoparticles (AgNPs) from an aqueous extract of Juniperus excelsa and their use as an antimicrobial agent on their own or in combination with antibiotics in inhibiting multidrug-resistant bacteria (MDR). One strategy of bacterial infection control in wound healing is AgNP biosynthesis. We collected bacterial strains of patient skin infections from Al-Adwani Hospital. Phenotyping, biotyping, and molecular characterizations were applied using 16S rRNA gene analysis of bacterial isolates. Our results identified tested MDR bacteria Staphylococcus aureus strains (methicillin-resistant and methicillin-susceptible) and Proteus mirabilis. Gas chromatography/mass spectrometry (GC/MS) analysis was used to identify the Juniperus excelsa biomolecules in the leaf extract acting as both reducing and capping agents in the biosynthesis of AgNPs. The AgNPs appeared hexagonal and spherical in shape upon transmission electron microscope (TEM) analysis. The AgNP sizes ranged from 16.08 to 24.42 nm. X-ray diffraction (XRD) analysis confirmed the crystalline nature of the particles. The minimum inhibitory concentrations (MICs) of the AgNPs against the tested MDR bacteria ranged from 48 to 56 µg/ml, while the minimum bactericidal concentrations (MBCs) of the AgNPs against the tested strains ranged from 72 to 96 µg/ml. The AgNPs showed a good synergistic efficacy with Cefaclor, Cefoxitin, and Erythromycin. Their efficiency showed a threefold increase in the inhibition of tested strains when used in wound dressing, due to the AgNPs potentially activating the antibiotics. Consequently, we can use AgNPs with Cefaclor, Cefoxitin, and Erythromycin antibiotics as alternative antimicrobial agents, and they could be utilized in wound dressing to prevent microbial infections.
Keywords: Antibiotic resistance, Silver nanoparticles, Synergistic effect, Wound dressing
Introduction
Recently, some microorganisms were found to have developed strong resistance towards a broad-spectrum antibiotics (Pender et al. 2013; Huwaitat et al. 2016). Healthcare-associated infections have been found to be caused by the multidrug-resistant strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumanii, Enterobacter species, Enterococcus faecium, and Staphylococcus aureus, putting the medical community on the defensive against these strains (Hidron et al. 2008; Van Duin and Paterson 2016). Methicillin-resistant Staphylococcus aureus (MRSA) was first detected as a resistant strain in 1960, with patients treated with vancomycin as an effective agent (Bozdogan et al. 2003). Bacterial resistance challenges are serious, with Gram-negative strains of bacteria acting as causative agents. Additionally, these MDR bacteria present a threat level of urgent or serious concern (Centers for Disease Control and Prevention 2013).
Many researchers have abandoned this area of study for treating chronic diseases (Schuhmacher et al. 2016; Projan 2003); however, the discovery of antimicrobial nanomaterials, such as metal nanoparticles, is needed, as technology against which pathogens cannot develop a resistance mechanism is limited. These metal nanoparticles (MNPs) have unique properties, including a large surface-to-mass ratio, an ultra-small size, a high reactivity, and interactions with biological systems (Khan et al. 2019).
Ultra-small nano-sized particles have several applications in various areas, from electronic devices for medical and pharmaceutical sciences to plant-soil systems, due to their unique properties (Rahayu et al. 2020; Khan et al. 2019). The metal nanoparticle contains two parts: an inorganic central core and a biocompatible surface coating. The latter could be functionalized using multiple molecules at its surface for stabilization or under physiological conditions (Agnihotri et al. 2014). Several studies have previously demonstrated that MNPs have unique properties and facilitate antimicrobial delivery to microbial infection sites (Iravani et al. 2014).
There are various methods—both physical and chemical in nature—for the synthesis of AgNPs (with a particle size of < 100 nm), which can be used as highly concentrated reducing and stabilizing agents (Ashok et al. 2020; Yorseng et al. 2020). Previous methods appeared harmful effects on human health the environment (Ashok et al. 2020). Biological synthesis methods are safer due to the availability of more biological entities and eco-friendly procedures. Different aqueous plant extracts contain various secondary metabolites, and these biomolecules are responsible for reducing ionic into bulk MNP formation (Iravani et al. 2014). Moreover, AgNPs generally used in different applications, such as tooth cement, wound dressing, bone, and antimicrobial agents (Iravani et al. 2014). The experimental parameters were studied to identify desirable biosynthesis conditions.
This study investigated a biological approach to the synthesis of AgNPs using Juniperus excelsa leaf extract, which contains secondary metabolites as a reducing and stabilizing agent. The evaluation of their biomedical properties was obtained in terms of antimicrobial efficacy. The bio-synthesized AgNPs showed a synergism of Juniperus excelsa leaf extract and AgNPs in terms of a bactericidal effect. This hybrid nano-material provides an alternative material for use in antibacterials. Further, nano-materials synergize with antibiotics in inhibiting various MDR pathogenic bacteria, which makes them especially applicable as wound dressing materials.
Materials and methods
Juniperus excels leaf extract preparation
The fresh leaves of Juniperus excels used in this study were collected from AL-Shafa area, Taif, Saudi Arabia, in summer. A fresh aqueous extract plant was prepared. Then, 25 g of washed and finely cut Juniperus excels leaves was added to 100 ml of deionized water and heated at 80 °C for 30 min. Whatman filter paper no. 1 was used for the filtration of the Juniperus excels leaf extract (Halawani et al. 2020).
Gas chromatography/mass spectrometry analysis (GC/MS) of Juniperus excels leaf extract
For identifying the phytochemical active components, chemical analysis of the prepared Juniperus excels extract was performed using gas chromatography/mass spectrometry (GC/MS) (Ehsani et al. 2012; Eryigit et al. 2013). This medicinal Juniperus excels was used for the first time to prepare nanoparticles for medical applications.
Biosynthesis of nanoparticles
The effects of experimental parameters were studied according to Chung et al. (2016).
Biosynthesis of AgNPs using Juniperus excels extract
One ml of the prepared Juniperus excels extract was added to 10 ml of 1 mM AgNO3 with constant stirring at room temperature, mixed on a magnetic stirrer, and then allowed to settle at room temperature. Observation of the colour change from colourless to brown was made due to the reduction in the silver ions after exposure to the Juniperus excels extracts (Enan et al. 2021).
Characterization of AgNPs
UV–visible spectra
The formation of AgNPs using Juniperus excelsa leaf extract was monitored with UV–visible spectra as a function of time via double-beam spectrophotometry (Shimadzu UV-1650 pc spectrophotometry, Osaka, Japan) in a range of 300–800 nm and worked at a resolution of 1 nm (Gad El-Rab et al. 2021).
Factors affecting synthesis of AgNPs
The following factors were studied according to Patra and Baek (2014).
Effect of silver nitrate concentration
The effect of AgNO3 concentration was determined by UV–visible spectroscopy using various concentrations (1 mM–10 mM).
Effect of Juniperus excelsa leaf extract concentration
The effect of the Juniperus excelsa leaf extract concentration on the biosynthesis of the AgNPs was determined using various concentrations (2 ml, 4 ml, 6 ml, 8 ml, 10 ml, and 12 ml). This effect of Juniperus excelsa leaf extract concentration was analysed using UV–visible spectroscopy.
Effect of pH
The effect of the pH value was analysed by UV–visible spectroscopy using various pH levels (5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0).
Effect of temperature
The effect on the biosynthesis of the AgNPs was analysed via UV–visible spectroscopy with varying temperatures (25 °C, 35 °C, 45 °C, 60 °C, 80 °C, and 100 °C).
Effect of incubation time
This effect was analysed via UV–visible spectroscopy with various incubation times (0 min, 10 min, 15 min, 20 min, 25 min, 35 min, and 45 min).
Transmission electron microscopy (TEM)
For the determination of the AgNP shape and size, TEM was used. Furthermore, imaging of Proteus mirabilis (P. mirabilis) cells treated with the AgNPs was conducted. The TEM image was obtained using a JEOL 100CX II 100 kV transmission electron microscope at the Assiut Electron Microscope Unit. The AgNP sample was prepared via the piping of aqueous sample drops on carbon-coated copper grids and then dried (Gad El-Rab et al. 2018; Verma and Mehata 2016).
X-ray diffraction (XRD) analysis
The AgNP nature and size were interpreted using XRD (Shimadzu XD-3A, Japan), with the particle size calculation using the formula of Debye–Scherrer, as shown below:
where β is the full-width at half maximum of the diffraction peak, d is the mean AgNP diameter, λ is the X-ray wavelength, and θ is the diffraction angle (Dubey et al. 2010).
Fourier transform infrared spectroscopy (FTIR)
The stabilized AgNP solution was collected using centrifugation at 10,000 g for 20 min, washed to remove any unattached moieties, and then dried completely. The AgNP powder was used for FTIR measurement, which was performed using a Shimadzu IR-470 Spectrometer (Shimadzu, Japan). The FTIR peaks of the AgNPs were identified and expressed in wavenumbers (cm−1). To determine the probable reducing agent, the Juniperus excelsa leaf extract was also analysed (Panigrahi 2013).
Isolation and characterization
Collection of clinical isolates and growth conditions
The samples were collected by the staff of Microbiology Labs from patients with wound infection cases in Al-Edwani Hospital, Taif, Saudi Arabia. The wound infection swabs were collected by cleaning the patient’s infected site using sterile swabs of cotton and then transported to the bacteriological tests within one hour of collection. The wound infection swabs were spread on the surface of different media, such as blood agar, mannitol salt agar, and MacConkey agar, and incubated for 16–24 h at 37 °C. Gram-positive strains were identified using specific analysis techniques, such as catalysis reaction, Gram staining, haemolytic activity, and coagulase testing on sheep blood agar plates. Gram-negative bacteria were identified based on morphology of colony on blood agar, MacConkey agar and then followed by biochemical reactions, namely urease tests, citrate, oxidase, triple sugar iron (TSI), and Sulphur, Indole, Motility (SIM) (Holt et al. 1994).
Also, single bacterial colonies were identified via morphological, biochemical tests, and the API 20E Strep and API Staph test kit (BioMerieux, France) for P.mirabilis and S. aureus, respectively, according to the criteria of MaccFadin (2000); Loberto et al. (2004); and Chaudhary et al. (2007).
The predominant bacterial isolates were identified as P. mirabilis and methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSR). These bacterial strains were cultivated periodically in order to regulate the viability and were maintained on Muller Hinton agar slants in the microbiology laboratory, which were stored at 4 °C for antimicrobial efficacy study using AgNPs alone or with antibiotics. Bacterial strains were further cultivated with a Mueller Hinton Broth for 16–24 h at 37 °C. Cell suspensions were diluted with a sterile saline solution to obtain a final concentration of 107 CFU/ml for further antimicrobial efficacy study using AgNPs alone or AgNPs with antibiotics.
Antibiotic susceptibility testing
Disk diffusion method
Isolates were grown in peptone water at 37 °C. The bacterial culture was cultivated on Mueller Hinton agar plates, and antibiotic discs were placed. The plates were incubated for 16–24 h at 37 °C. The inhibition zones were explained according to the Clinical Laboratory Standard Institute (CLSI) guidelines (Clinical Laboratory Standard Institute 2014). The susceptibility of bacterial isolates was tested to 31 different antibiotics, obtained from (OXOID, UK). Amikacin (AK) 30 μg, amoxicillin-clavulanic acid (AMC) 30 μg, Cefaclor (CEC) 30 μg, Cefotaxime (CTX) 30 μg, Cefoxitin (FOX) 30 μg, Ceftazidime (CAZ) 30 μg, Cefuroxime (CXM) 30 μg, Doxycycline/HCL (DO) 30 μg, Ciprofloxacin (CIP) 30 μg, Levofloxacin (LVX) 30 μg, Clarthromycin (CLR) 30 μg, Ampicillin (AMP) 30 μg, Cefalexin (LEX), Cefadroxil (FEP) 30 μg, Cefaclor (CEC) 30 μg, Ceftriaxone (CRO) 30 g, Meropenem (MEM) 30 μg, Sulpha/Trimethoprim (SXT) 30 μg, Tigecycline (TGC) 30 μg, Gentamicin (GEM) 30 μg, Imipenem (IPM) 30 μg, Nitrofurantoin (NTF) 30 μg, Fluxocillin (FUX) 30 μg, Azithromycin (AZM) 30 μg, Clindamycin (CLI) 30 μg, Penicillin (P) 30 μg, Oxacillin (OXA) 30 μg, Erythromycin (ERY) 30 μg, Vancomycin (VAN) 30 μg, and Rifampicin 30 μg (RIF) were used.
Molecular typing
Molecular characterization using 16S rRNA gene
Wound bacterial cells were used for the isolation of genomic DNA using THE Wizard Genomic DNA Purification Kit (Promega). An overnight culture of 5 ml was pelleted VIA centrifugation at 15,000 xg for three minutes at 25 °C and re-suspended in 900 µl of 50 mM Ethylenediaminetetraacetic acid (EDTA). A volume of 120 µl (about 40 mg/ml of lysozyme solution (Sigma Chemical Co. USA) was mixed with the cell suspension to disrupt the cell wall and incubated in a water bath for 1 h at 37 °C. The bacterial suspension was centrifuged (15,000 xg for three minutes at 25 °C), and the pellet was gently resuspended in 900 µl nuclei lysis solution (Promega). The bacterial isolate cells were lysed after incubation at 80 °C for five minutes; then, four microliters of RNase A (50 mg/ml) (Sigma/USA) was added to the RNA lysis, and the tubes were inverted ten times before the mixture was kept again for 1 h at 37 °C with inversion. For the protein precipitation step, 300 µl precipitation solution (Promega) was put in the lysed mixture and vortexed for 30 s; then, the mixture was kept for 7 min on ice and centrifuged for 15 min at 15,000 xg. For the washing step, the supernatant was carried to an Eppendorf tube containing 600 µl isopropanol at 25 °C; the mixture was gently mixed by inverting the tube, which was followed by centrifugation at 15,000 xg for 10 min. Moreover, 70% ethanol was used to wash the pellet. In the final step, the pellet containing the genomic DNA was resuspended in 60 µl DNA rehydration solution (Promega, USA).
PCR of 16S rRNA genes:
In 20 µl of the Polymerase chain reaction (PCR) reaction solution, one microliter of template DNA was added. Forward primer 27F (AGA GTT TGA TCM TGG CTC AG) and reverse primer 1492R (TAC GGY TAC CTT GTT ACG ACT T) were used. Then, 35 amplification cycles of 16S rRNA genes were performed at 94 °C for 45 s in the denaturation step, at 55 °C for 60 s in the annealing step, and then at 72 °C for 60 s in the extension step. DNA fragments were amplified ~ 1,400 bp.
16S rRNA gene analysis
The purified PCR products of 16S rRNA of approximately 1400 bp were sequenced using forward primer 518F (5ʹ-CCA GCA GCC GCG GTA ATA CG-3ʹ) and reverse primer 800R (5ʹ-TAC CAG GGT ATC TAA TCC-3ʹ). The sequencing was achieved using a Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, USA). Sequencing products were analysed using an Applied Biosystems model 3730XL automated DNA sequencing system (Applied Biosystems, USA). After the sequences were analysed, similarity in the bacterial strains was aligned using the CLUSTAL W (1.81) phylogenetic tree and extracted from the nucleotide sequence databases. The 16S rRNA gene sequences of the isolates reported in this paper were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession numbers LC189112 (STA6 strain), LC189113 (STA7* strain), and LC189112 (PRO3 strain).
Antibacterial activity of AgNPs
Antimicrobial activity of AgNPs using agar diffusion method
For the determination of the AgNP antibacterial activity, the agar diffusion method was used for detection against MDR bacteria (Kanmani and Lim 2013). The MDR bacteria were initially propagated at 37 °C in a nutrient broth. The culture of MDR bacteria was then again cultivated in nutrient broth media for 2 h until 0.01 OD. Subsequently, 100 μL of inoculum was spread uniformly onto Mueller–Hinton agar plates. Then, 0.25 g/ml of Juniperus excelsa leaf extract and 0.083 µg/ml AgNPs were loaded into the wells after the cultivation of the strain inoculum on the plates and incubated at 37 °C. Finally, the inhibition zone was determined after 16 h. All samples were done in triplicate, and the test was five separate times.
Antimicrobial activity of AgNPs wound dressing
One hundred microliters (0.25 g/ml) of Juniperus excelsa leaf extract, antibiotics [cefoxitin, erythromycin or cefaclor (30 µg/ml)], and AgNPs with an antibiotic (0.083 µg/ml + 30 µg/ml) were added to the sterilized wound dressing of 1 cm2 under sterilized conditions (Hebeisha et al. 2014). The wound dressing was kept at 50 °C overnight to evaporate the excess water content and tested against the four MDR pathogenic bacteria [Proteus mirabilis, S. aureus (MRSA and MSSA)]. The bacterial inoculum was spread on Mueller Hinton Agar plates, and the wound dressing was placed over the inoculated medium. Petri dishes were then incubated for 24 h at 37 °C, and the inhibition zone was determined. All samples were done in triplicate, and the test was repeated five separate times.
Micro-dilution test for determination of MIC and MBC
The test was performed with dilutions of AgNPs arranged across the rows and inoculation of the wells of a micro-dilution plate with the bacterial culture. The AgNP concentrations were arranged in serial dilutions. The lower MIC value against the isolate was determined. Also, the MICs of AgNPs were determined using serial dilutions of AgNPs from 96 μg/mL to 8 μg/mL. Standards are available for measuring the MBC, but the test is more complicated and difficult to perform than the MIC determination. Therefore, MBC is rarely measured or reported in clinical laboratories (Balouiri et al. 2016). All samples of AgNPs were done in triplicate, and the test was repeated five separate times.
Results and discussion
Biosynthesis of AgNPs
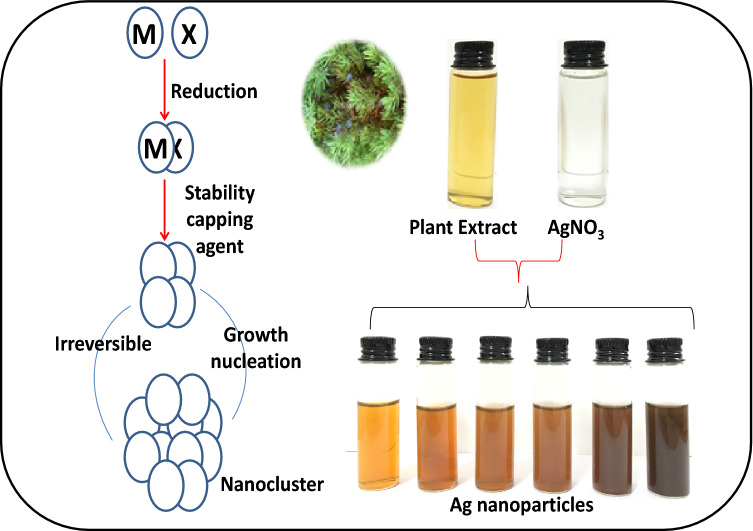
Adding aqueous Juniperus excelsa leaf extract to silver nitrate led to a colour change from colourless to dark brown due to the formation of AgNPs, as shown in Fig. 1. The reducing and stabilizing agents in the extract of Juniperus excelsa (medicinal plant) caused a reduction in silver ions and stabilized the AgNPs. Juniperus excelsa is used for bronchitis, the common cold, jaundice, and tuberculosis (Ehsani et al. 2012; Azzimonti et al. 2015; Nabi et al. 2012; Tumen et al. 2012; Khan et al. 2012). The Juniperus excelsa aqueous extract contains a large number of phenolic compounds, alcoholic compounds, esters, and amines (Table 1), which acted as reducing and stabilizing agents. The complete colour change took about 1 h, and silver nitrate present in the reaction mixture was reduced completely. As shown in Fig. 2, the maximum absorbance was obtained at 430 nm. Our results are in agreement with the results of Mahdieha et al. (2012).
Fig. 1.
Scheme of silver biosynthesis explained change of the solution colour to brown by adding Juniperus excelsa leaf extract to silver nitrate at optimum conditions
Table 1.
Chemical composition of aqueous leaf extract from wild-growing Juniperus excelsa in Taif, Saudi Arabia
| Component | Retention time | Rate (%) |
|---|---|---|
| Phenols | ||
| (4-ethynylphenyl)diphenylmethoxymethane | 36.850 | 4.668 |
| Carvacrol | 18.303 | 0.400 |
| 4-chloro-2,6-dimethylphenol | 20.582 | 0.279 |
| p-vinylphenol | 17.347 | 0.751 |
| Alcohols | ||
| 1-hexanol | 9.904 | 0.422 |
| 2-hexanol | 7.135 | 0.376 |
| 1-octen-3-ol | 12.393 | 0.653 |
| 3S,4R,5S,6R,7S)-Aristol-9-en-3-ol | 28.684 | 0.400 |
| 3-hexanol | 9.752 | 0.330 |
| 3-phenylpropanol | 17.668 | 1.025 |
| 4,4-dimethyltricyclo[6.3.2.0(2,5)]trideca-8-ene-1-ol | 26.766 | 0.583 |
| Aspidinol | 30.607 | 0.660 |
| Benzeneethanol | 15.692 | 0.987 |
| Benzyl alcohol | 14.532 | 0.614 |
| Borneol L | 16.001 | 0.510 |
| Dehydroxy-isocalamendiol | 27.704 | 0.298 |
| Isopropenylethyl alcohol | 5.381 | 1.130 |
| p-cymen-8-ol | 16.694 | 0.465 |
| Spathulenol | 27.984 | 0.907 |
| Esters | ||
| 2-ethylbutyric acid, heptadecyl ester | 38.843 | 0.507 |
| Fumaric acid dodecyl 2-methylcyclohex-1-enylmethyl ester | 25.688 | 0.525 |
| Hexadecanoic acid methyl ester | 27.460 | 0.244 |
| Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 37.910 | 16.008 |
| Octadecanoic acid methyl ester | 29.202 | 0.361 |
| Octadecanoic acid, 2,3-dihydroxypropyl ester | 40.055 | 12.624 |
| Oxalic acid, 6-ethyloct-3-yl propyl ester | 16.298 | 0.337 |
| Vanillic acid ethyl ester | 24.982 | 0.177 |
| Vanillic acid methyl ester | 24.032 | 0.86 |
| Amines | ||
| 2-(1-ethoxycarbonyl-2,2,2-trifluoro-1-pentanoy lamino-ethylamino)-4-ethyl-5-methy | 35.736 | 0.248 |
| Amino Alcohols | ||
| 1-(3′-aminobenzyl)-7-methoxyisoquinolin-8-ol | 37.403 | 2.682 |
| Pyridines | ||
| 2,5-diethoxy-4-piperidinopyridine | 35.993 | 0.996 |
| 3,4-dihydro-1-methyl-4-[1′-(3′-methylindolyl)]pyridin-2(1H)-one | 36.716 | 2.069 |
| 5-oxo-6-phenyl-1,2,3,5,6,7-hexahydroimidazo[1,2-a]pyridine-8-carbonitrile | 33.970 | 3.846 |
| Pyrroles or phenols | ||
| 2-amino-3-cyano-4-(p,p′-diphenyl)-4-phenyl-5-(4′-methoxyphenyl)-3H-pyrrole | 54.161 | 1.687 |
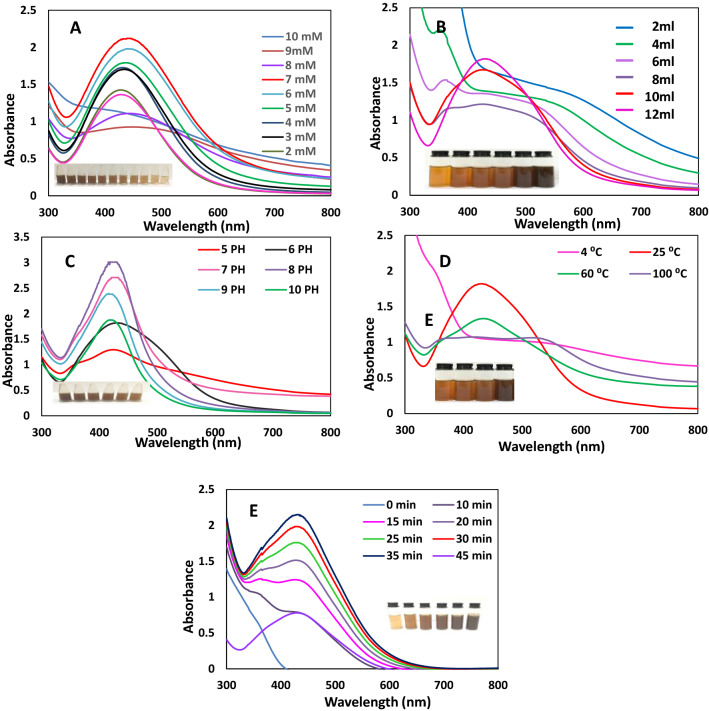
Fig. 2.
Effect of AgNO3 concentration (a), Juniperus excelsa leaf extract concentration (b), pH (c), temperature (d), and incubation time (e) on the formation of AgNPs as recorded by the UV–Vis spectroscopy
Characterization of AgNPs
UV–visible spectra
Effect of silver nitrate concentration
The main factor that significantly affected the AgNP biosynthesis was the Ag+ ion concentration. The AgNP formation was examined and confirmed by obtaining the respective absorption spectra. Figure 2a shows the AgNP absorption spectra at different silver concentrations from 1 to 10 mM. The spectrometry shows a sharp peak at 430 nm, which confirms the AgNP formation (Atta et al. 2014). Ag+ ion reduction was enhanced with the increase in the Ag+ ion concentration, as indicated by a change in the colour of the solution. The peak absorption wavelength shifted from 430 to 445 nm with an increase in the AgNO3 concentration. The absorption peak increased with an increase in the silver nitrate concentration from 1 m M to 7 mM, while the absorption peak decreased when increasing the concentration from 8 to 10 mM. The result shows a very narrow band appearing at an AgNO3 concentration of 1 mM, which means the optimum concentration of AgNO3 was 1 mM. Figure 2A shows that the narrow band was due to the small-size nanoparticles (Verma and Mehata 2016).
Effect of extract concentration on the formation of ANPs
Figure 2B displays the absorption spectra of the AgNPs at different concentrations of Juniperus excelsa extract. When 2 ml of Juniperus excelsa leaf extract was added to 10 ml silver nitrate, a relatively weak absorption band was observed. The absorption intensity increased monotonically with an increasing concentration of Juniperus excels extract until 12 ml, as shown in Fig. 2b, the peak intensity increased abruptly, indicating an enhancement in the production of AgNPs. Moreover, by increasing the Juniperus excels aqueous extract concentration, the absorption increased with a narrow peak, indicating an increase in the formation of AgNPs small in particle size. Variations in the biological extract and silver concentrations are known to influence nanoparticle synthesis (Christopher et al. 2015).
Effect of pH
The pH might have affected the capping as well as the stabilizing abilities of biomolecules, since pH has the ability to change the charge of biomolecules. A change in pH affected the size and shape of the AgNPs. Figure 2c shows a shift in the peak absorption wavelength and intensity with a varying pH. As the pH increased from 5 to 10, the absorption intensity increased up to pH 8. This revealed that pH 8 was the optimum pH for the synthesis of AgNPs when using Juniperus excelsa leaf extract. The pH of 8 enhanced the reduction rate, and a dark brown colour change was observed quite rapidly when AgNO3 was mixed with the aqueous Juniperus excels leaf extract. In the present study, the absorbance peak increased at pH 8, indicating that an alkaline pH8 is the optimum for AgNP synthesis. The absorption peak of the AgNPs increased with increasing pH up to 8, as also confirmed by Khalil et al. (2015).
Effect of temperature
Figure 2d displays the absorption spectra of the AgNPs at temperatures in the range of 25–100 °C. The AgNPs synthesis using Juniperus excels extract was enhanced, as indicated by a rapid change in the colour of AgNPs at room temperature (25 °C). The intensity of the absorption spectra decreased with increasing temperature, which indicated that room temperature is the optimum temperature for the biosynthesis of AgNPs. Our results agree with data obtained by Emam et al. (2015).
Effect of incubation time
Figure 2e displays the absorption spectra of the AgNPs obtained from the reaction of the Juniperus excelsa leaf extract with AgNO3, which was recorded in the range of 300–800 nm at different incubation times (0 min–45 min). A change in colour was observed initially after 5 min of adding the Juniperus excelsa leaf extract to the AgNO3 solution. Moreover, an increase in the absorbance over time until 35 min was observed, with the maximum absorption observed at 430 nm (Fig. 2e). After 35 min, the colour of the solution became nearly constant, indicating that no silver salt remained for further reaction. The results are in complete correlation with those reported by Saware et al. (2014) and Aravinthan et al. (2015), indicating that AgNPs have a uniform and small size distribution.
Transmission electron microscopy (TEM)
Figure 3A shows that the AgNPs were spherical and hexagonal in shape and were uniformly distributed (monodispersed) without significant agglomeration. The morphology and shape of the AgNPs were determined using TEM measurements. The particle distribution shows that most of the particles were in the 16.08–24.42 nm range. This datum is in agreement with Agnihotri et al. (2014).
Fig. 3.
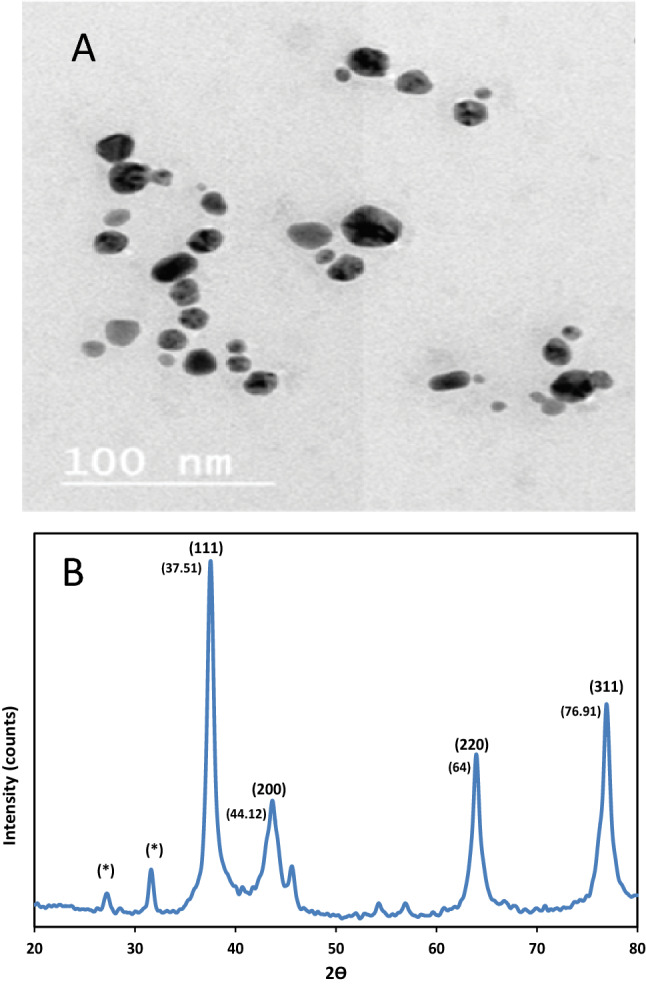
TEM images of AgNPs (a) and XRD analysis of phytosynthesized AgNPs using Juniperus excelsa leaf extract (b)
X-ray diffraction (XRD) of AgNPs
AgNP analysis using XRD patterns confirmed the purity and crystalline nature of the synthesized AgNPs. The XRD spectrum showed strong diffraction peaks at 37.51º, 44.12º, 64.00º, and 76.91º, which correspond to the AgNP diffraction planes of (111), (200), (220), and (311), respectively (Fig. 3b); these can be indexed according to the facets of the face-centred cubic crystal structure of AgNPs. The calculated the AgNPs size was ~ 16–24 nm, as determined via Debye Scherer’s equation (Dubey et al. 2010).
Fourier-transform infrared spectroscopy (FTIR)
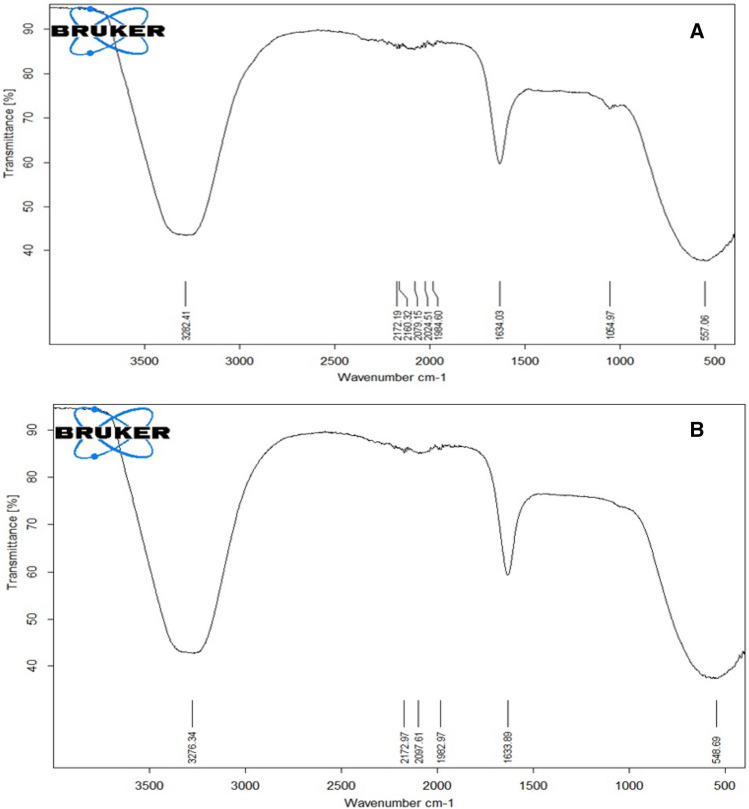
Figure 4 shows the FTIR analysis of the Juniperus excelsa leaf extract and AgNPs. FTIR spectra of the extract revealed major peaks at 3282.41, 1634.03, 1054.97, and 557.06 cm−1, which corresponded to the O–H a broad peak, N–H stretch, C-O stretch, and –C=C–H, respectively (Fig. 4a).
Fig. 4.
FTIR analysis of Juniperus excelsa leaf extract (a) and AgNPs (b)
The FTIR spectrum of the biosynthesized AgNPs was in the range of 548.69–3276.34 cm−1. The FTIR spectra of the AgNPs exhibited major peaks at 3276.34, 1633.89, and at 548.69 cm−1, which corresponded to the O–H stretching peak, N–H stretch, and –C=C–H, respectively (Fig. 4b). In the case of the AgNPs, a broad, strong, and characteristic peak was observed at 3276.34 cm−1. This peak observed the O–H peak at 3276.34 cm−1 (for extract), which is related to alcohol or phenols (Khatami et al. 2015), indicating that OH bonding is enhanced during NP formation. The amine peak observed at 1633.89 cm−1 is close to that reported for proteins (Khatami and Pourseyedi 2015). The C–O peak (at 1054.97 cm−1) appeared, and the –C=C–H was observed at 548.69 cm−1. The O–H, N–H, C–O, and –C=C–H functional groups indicated the groups involved in the reduction of AgNPs. The FTIR analysis showed that the formation of AgNPs might be stabilized by the existence of polyphenols, alcoholic compound, esters, and amine materials in the Juniperus excelsa leaf extract, which may play a role as a powerful bio-reducer and help in the formation of stabilized AgNPs.
Isolation and identification of drug-resistant bacteria
The bacterial strains PRO3, STA6, and STA7 were isolated from the skin (Table 2). The strains were morphologically and biochemically characterized as P. mirabilis and Staphylococcus aureus and designated as P. mirabilis PRO3, S. aureus STA6, and S. aureus STA7, respectively.
Table 2.
Morphological and biochemical characteristics of bacteria (STA6, *STA7 and PRO3) isolated from Skin
| Bacterial isolates | Characteristics | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram’s stain | Cell morphology | Motility | Catalase test | Coagulase test | Oxidase test | Indole test | Methyl Red test | Voges-Proskauer test | Citrate | H2S Production | Urease | Nitrate | Fermentation | Probable bacteria | |||||
| Rods | Cocci | Glucose | Lactose | Maltose | Mannitol | ||||||||||||||
| STA6 | + | − | + | − | + | + | − | − | + | + | + | − | + | + | + | + | + | − | S. aureus |
| STA7 | + | − | + | − | + | + | − | − | + | + | + | − | + | + | + | + | + | − | S. aureus |
| PRO3 | − | + | − | + | + | _ | − | − | + | _ | + | + | + | + | − | − | + | − | Proteus mirabilis |
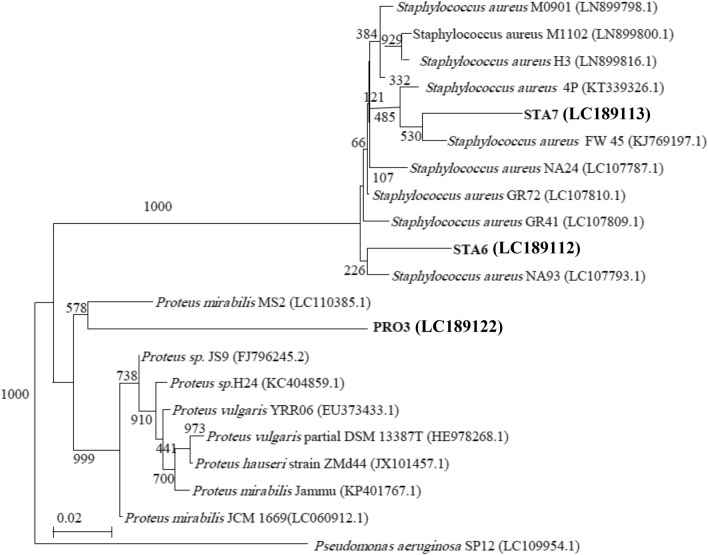
16S rRNA analysis
PCR amplification of the 16S rRNA encoding genes for multidrug-resistant bacterial isolates STA6, STA7, and PRO3 was carried and sequenced. The 16S rRNA gene sequences of the bacterial isolates from the wounds were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases with the following accession numbers (Table 3): LC189112 (STA6 strain), LC189113 (STA7 strain), and LC189122 (PRO3 strain). A dendrogram demonstrating the results of the 16S rRNA of S. aureus (STA6, and STA7) analysis is displayed in Fig. 5. The results show the highest similarity of isolates STA6 and STA7 to members of the Staphylococcus group, while they show the highest similarity of isolate PRO3 to members of the Proteus group. Moreover, the 16S rRNA sequences of the Staphylococcus isolates STA6 and STA7 are the most closely associated with S. aureus, while the 16S rRNA sequences of the isolate PRO3 are the most closely related to P. mirabilis (Table 3). The 16S rRNA gene of STA6 and STA7* displayed 99.28% and 99.43% similarities with S. aureus NA93 (LC107793.1) and S. aureus KW45 (KJ769197.1), respectively. Additionally, the 16S rRNA gene of PRO3 displayed a 89.77% similarity with P. mirabilis MS2 (LC110385.1).
Table 3.
The bacterial isolates (STA6, *STA7 and PRO3) of skin infections
| Bacterial isolates | Accession No | Closest neighbor |
|---|---|---|
| STA6 | LC189112 | S. aureus |
| STA7* | LC189113 | S. aureus |
| PRO3 | LC189122 | P. mirabilis |
*MRSA Methicillin-resistant Staphylococcus aureus
Fig. 5.
A phylogenetic tree of multidrug-resistant bacterial isolates from skin relied on the nucleotide sequences of 16S rRNA genes, constructed by neighbour-joining method. The scale bar displays the genetic distance. The number presented next to each node displays the percentage bootstrap value of 1000 replicates. The Pseudomonas aeruginosa SP12 was treated as the out-group. The GenBank accession numbers of the bacteria are presented in parentheses
These results show that S. aureus (STA6, STA7, and PRO3) is compatible with the conclusions of the morphological and biochemical characterization. Various studies have recorded that S. aureus and P. mirabilis can be isolated from the skin (Kobayashi et al. 2015; Mordi and Momoh 2009).
Antimicrobial susceptibility
The bacterial isolates recovered from the skin were screened for antimicrobial agent sensitivity testing. The MICs of these different antimicrobials against bacterial isolates were determined (Table 4). The obtained results were interpreted according to the CLSI guidelines. As presented in Table 4, isolate STA6 was methicillin-sensitive (MSSA) and resistant to four antimicrobials. Isolate STA7 was resistant to nine antimicrobials and methicillin-resistant (MRSA). The MRSA isolate STA7* was resistant to cefoxitin and oxacillin. Moreover, isolate PRO3 was resistant to 16 antibiotics. The antimicrobial resistance was 100% for Cefaclor, Erythromycin, and Cefoxitin. S. aureus isolates were multidrug-resistant. Moreover, the P. mirabilis isolate showed high resistance rates toantimicrobial agents (16 antimicrobials). The incidence of antibiotic resistance (Khalil et al. 2015) has increased due to the incorrect use of antibiotics. In 2014, the number of patients infected with MRSA was in the range of 50–64%, and they are more likely to die than patients infected with a non-resistant form (WHO 2015). MRSA bacteria contain a mecA gene carried on the chromosomal cassette mec (SCCmec) and coded to a penicillin-binding protein (PBP2a), which interferes with the β-lactam antibiotic effects on the bacterial cell walls of MRSA bacteria. This gives virtually complete resistance to all β-lactam antibiotics, including semi-synthetic penicillin, as shown in these results and those of other researchers (Al-Anazi 2009; Shahkarami et al. 2014; Gad El-Rab et al. 2020). The high resistance rates in isolates from skin infections indicate serious problems for immunosuppressed individuals, often cause death like many other members of the family Enterobacteriaceae. Multidrug-resistant strains of these bacteria generally produce extended-spectrum β-lactamases (ESBLs) or AmpC-type cephalosporin and, rarely, carbapenemases (Tumbarello et al. 2012).
Table 4.
Resistance patterns of multi-drug resistant isolates (STA6, *STA7 and PRO3) from skin infections
| Isolates | Resistance of antibiotic | No. of antibiotics |
|---|---|---|
| STA6 | CEC, PEN, ERY, FOX | 4 |
| *STA7 | AMP, AMC, CEC, FOX, CXM, MEM, OXA, PEN, ERY | 9 |
| PRO3 | AMP, AMC, CEC, FOX, CAZ, CRO, CTX, CXM, CIP, LVX, DO, SXT, GEM, AK, FEP, ERY | 16 |
AK Amikacin, AMC Amoxy/Clavulanic acid, CEC Cefaclor, FEP Cefepime, CTX Cefotaxime, FOX Cefoxitin, CAZ Ceftazidime, CRO Ceftriaxone, CXM Cefuroxime, DO Doxycycline/HCL, CIP Ciprofloxacin, LVX Levofloxacin, AMP Ampicillin, FEP Cefepim, MEM Meropenem, SXT Sulpha/Trimethoprim, GEM Gentamicin, PEN Penicillin, OXA Oxacillin, ERY Erythromycin
*MRSA Methicillin-resistant Staphylococcus aureus
Antibacterial activity
Determination of inhibition zone, MIC, and MBC for AgNPs against MDR P. mirabilis and S. aureus strains
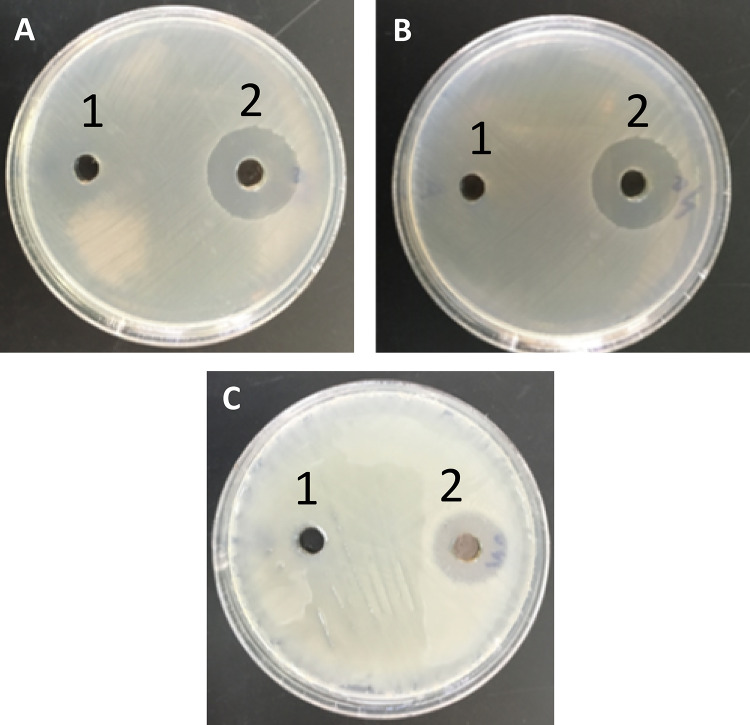
The AgNP antibacterial activities were studied against a range of multidrug-resistant pathogenic P. mirabilis and S. aureus (MRSA and MSSA) strains using a well diffusion assay and micro-dilution plate methods. The diameter of the inhibition zone in millimetres, MIC, and MBC are shown in Table 5 and Fig. 6. In the present study, the inhibition zone of the AgNPs was found to be highest (13 mm) against P. mirabilis PRO3 and lowest (11 mm) against S. aureus STA6. Finally, the inhibition zone of the AgNPs ranged from 11 to 13 mm, the MIC ranged from 48 to 56 μg/ml, and the MBC of the AgNPs ranged from 72 to 96 μg/ml against MDR strains. These results are in agreement with data obtained by Ghosh et al. (2012) and Jyoti et al. (2016).
Table 5.
Inhibition zone, MIC and MBC of AgNPs against MDR pathogenic strains
| Isolate | AgNPs (µg/ml) | ||
|---|---|---|---|
| aZIN (mm) | bMIC | cMBC | |
| STA6 | 13 ± 0.57 | 48 ± 2.02 | 72 ± 1.15 |
| STA7* | 13 ± 0.28 | 48 ± 1.15 | 72 ± 0.33 |
| PRO3 | 11 ± 0.57 | 56 ± 1.15 | 96 ± 0.57 |
| ANOVA, P value | 0.05 | 0.001 | 0.0001 |
*MRSA
aInhibition zone
bMIC Minimum inhibitory concentration
cMBC Minimum bactericidal concentration
Fig. 6.
Inhibition zone of Juniperus excelsa leaf extract (1) and AgNPs (2) against (a) S. aureus STA6, b S. aureus STA7 and Proteus mirabilis PRO3 (c).
Synergistic effect of AgNPs with antibiotics
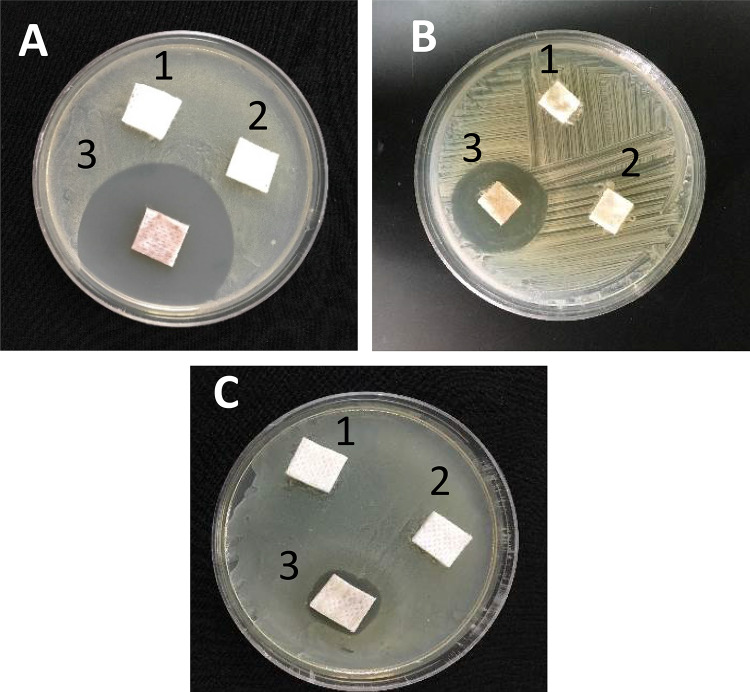
The synergistic effects of the AgNPs with three antibiotics were investigated against pathogenic bacteria P. mirabilis and s. aureus (MRSA and MSSA) using the agar disk diffusion method shown in Table 6 and Fig. 7. These strains were resistant to cefoxitin, erythromycin, and cefaclor. The synergistic interaction of the AgNPs with cefoxitin, erythromycin, and cefaclor showed a threefold increase in the inhibition zone against these pathogenic bacterial isolates. Our results are in correlation with previous studies on the synergistic effect of AgNPs alone and in combination with conventional antibiotics against MDR bacteria (Akram et al. 2016; Sangili and Gurunathan 2015). Multidrug-resistant pathogenic bacteria cannot develop a resistance mechanism against the combination of AgNPs and antibiotics. AgNPs enhance the bactericidal efficacy of antibiotics by increasing the generation of reactive oxygen species that cause the death of the bacterial cell (Wu et al. 2014).
Table 6.
Inhibition zone (mm) of different antibiotics (alone or with AgNPs) against MDR bacteria
| Bacterial isolates | 1ZIN of Antibiotic without or with AgNPs | |||
|---|---|---|---|---|
| Cefoxitin without or with AgNPs | Erythromycin without or with AgNPs | Cefaclor without or with AgNPs | ||
| STA6 | A | 06 ± 0.28 | 06 ± 0.16 | 06 ± 0.288 |
| B | 18 ± 0.28 | 17 ± 0.255 | 14 ± 0.88 | |
| C | 08 ± 044 | 07 ± 0.33 | 04 ± 0.44 | |
| STA7 | A | 06 ± 0.16 | 06 ± 0.57 | 06 ± 0.25 |
| B | 19 ± 044 | 17 ± 0.288 | 19 ± 0.72 | |
| C | 09 ± 0.28 | 07 ± 0.33 | 09 ± 0.57 | |
| PRO3 | A | 06 ± 0.33 | 06 ± 0.44 | 06 ± 0.44 |
| B | 18 ± 0.44 | 17 ± 0.255 | 19 ± 0.288 | |
| C | 08 ± 0.57 | 07.033 | 09 ± 0.72 | |
All agar disk diffusion method experiments were displayed in triplicate and each test was repeated five separate times—standard deviations were negligible. Increase in fold area of individual antibiotics was calculated as , where A and B are the inhibition zones (mm) obtained for antibiotic alone (Cefaclor, Cefoxitin, or Erythromycin) and antibiotics + AgNPs, respectively. In case of no inhibition zone, diameter of the disk (6 mm) was obtained for the calculation
*MRSA
Fig. 7.
Inhibition zone of Juniperus excelsa leaf extract dressing (1) antibiotic-dressing (2) and AgNPs + antibiotic-dressing (3) against (a) S. aureus STA6, b S. aureus STA7 and Proteus mirabilis PRO3 (c)
Mechanism of action
Figure 8 shows the TEM of P. mirabilis, which was incubated with the AgNPs. Specifically, it shows the binding sites of NPs with cell membranes and denotes the lysis of bacterial cells. The mechanism of the inhibitory action of the AgNP nanoparticles on multi-drug resistant bacteria is not still clearly known. The antibacterial activity could be due to the small size of the AgNPs synthesized by the Juniperus excelsa leaf extract. Small AgNPs (~ 16–24 nm) have an extremely large surface area and exhibit better contact and interaction with bacterial cells than large-size nanoparticles (Tamayo et al. 2014; Wan et al. 2016), and the results of the cell membrane rapture observed via TEM analysis confirmed this finding. The release of Ag ions from AgNPs could be reacted with sulfur and the phosphorus in proteins and DNA, respectively, which could inhibit DNA replication and cell viability. Such Ag ions could interact with the thiol group of enzymes. Furthermore, AgNPs form free radicals, which induced oxidative stress leading to cell death (Wan et al. 2016; Singh et al. 2014).
Fig. 8.
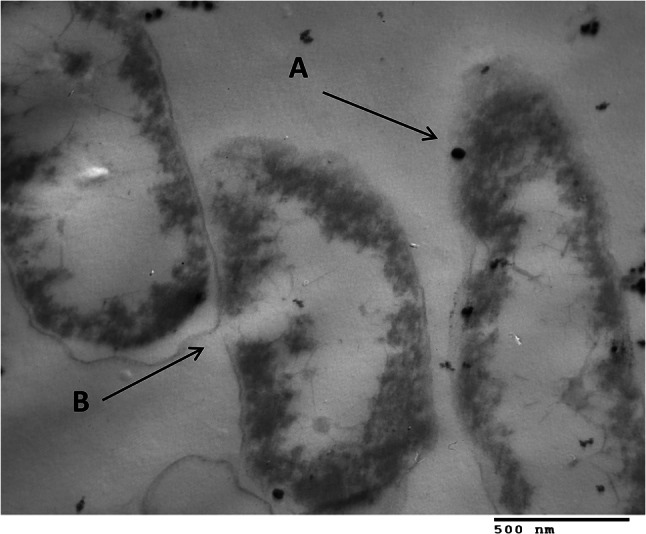
Transmission electron micrographs of Proteus mirabilis incubated with 56 μg mL−1 AgNPs + antibiotic. a Arrows point to binding sites of NPs with cells; b Arrows denote lysed cells
Conclusions
This work demonstrates a new strategy for controlling serious bacterial infections caused by MDR bacteria. The present research investigated a novel approach for the biosynthesis of AgNPs from Juniperus excelsa leaf extract, which contains reducing, stabilizing, and capping agents. The synthesized AgNPs were spherical and hexagonal in shape, with a size ranging around ~ 16–24 nm, as observed via the TEM and XRD analyses. The AgNPs were used as a dressing for the control of MDR bacteria in skin infections. Consequently, AgNPs and the combination of AgNPs with antibiotics are a promising therapy for infections caused by MDR and MRSA bacteria, which thus supports their application in biomedicine. The AgNPs synergized with antibiotics were more effective than the AgNPs alone against the MDR bacteria and could be used to preserve last-resort antibiotics, such as vancomycin. AgNPs synergized with antibiotics can be used in future studies to control the infections of diabetic wound ulcers, which are correlated with amputations in diabetic patients.
Acknowledgements
The authors would like to express their Acknowledge to Taif University Researchers Supporting Project number (TURSP-2020/273), Taif University, Taif, Saudi Arabia. Also, the authors are thankful to the Al-Edwani hospital for providing us the samples and King Abdulaziz City for Science and Technology for financial support.
Abbreviations
- AgNPs
Silver nanoparticles
- MDR
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-susceptible Staphylococcus aureus
- GC/MS
Gas chromatography/mass spectrometry
- FTIR
Fourier transform infrared spectroscopy
- TEM
Transmission electron microscope
- XRD
X-ray diffraction
- MICs
Minimum inhibitory concentrations
- MBCs
Minimum bactericidal concentrations
- MNPs
Metal nanoparticles
Authors contribution
EMH and SMFG contributed to the study’s concept and design. Study materials and their preparation, data collection, and analyses were performed by SA and SMFG. The first draft of the manuscript was written by EMH and SMFG. However, the comments of all authors on the previous versions of the manuscript have been included in this manuscript. The author(s) read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Agnihotri S, Mukherji S, Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014;8:3974–3983. doi: 10.1039/C3RA44507K. [DOI] [Google Scholar]
- Akram FE, El-Tayeb T, Abou Aisha K, El Azizi M. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) Ann Clin Microbiol Antimicrob. 2016;15:48. doi: 10.1186/s12941-016-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Anazi AR. Prevalence of methicillin-resistant Staphylococcus aureus in a teaching hospital in Riyadh, Saudi Arabia. Biomed Res. 2009;20:7–14. [Google Scholar]
- Aravinthan A, Govarthanan M, Selvam K, Praburaman L, Selvankumar T, Balamurugan R, Kamala-Kannan S, Kim J. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int J Nanomedicine. 2015;10:1977–1983. doi: 10.2147/IJN.S79106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok B, Hariram N, Siengchin S, Rajulu AV. Modification of tamarind fruit shell powder with in situ generated copper nanoparticles by single step hydrothermal method. J Biores Bioprod. 2020;5:180–185. doi: 10.1016/j.jobab.2020.07.003. [DOI] [Google Scholar]
- Atta AM, Al-Lohedan HA, Ezzat AO. Synthesis of silver nanoparticles by green method stabilized to synthetic human stomach fluid. Molecules. 2014;23:6737–6753. doi: 10.3390/molecules19056737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzimonti B, Cochis A, Beyrouthy ME, Iriti M, Uberti F, Sorrentino R, Landini MM, Rimondini L, Varoni EM. Essential oil from berries of lebanese Juniperus excels M. bieb displays similar antibacterial activity to chlorhexidine but higher cytocompatibility with human oral primary cells. Molecules. 2015;20:9344–9357. doi: 10.3390/molecules20059344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdogan B, Esel D, Whitener C, Browne FA, Appelbaum PC. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey medical center. J Antimicrobial Chemotherap. 2003;52:864–868. doi: 10.1093/jac/dkg457. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC): Antibiotic resistance threats in the United States . Atlanta. GA: Centers for Disease Control and Prevention; 2013. [Google Scholar]
- Chaudhary SD, Vives MJ, Reiter MF. Postoperative spinal wound infections and post procedural diskitis. J Spinal Cord Me. 2007;30:441–451. doi: 10.1080/10790268.2007.11753476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher J, Saswati B, Ezilrani PS. Optimization of parameters for biosynthesis of silver nanoparticles using leaf extract of Aegle marmelos. Braz Arch Biol Technol. 2015;58:702–710. doi: 10.1590/S1516-89132015050106. [DOI] [Google Scholar]
- Chung HL, Augustine GJ, Choi KW. Drosophila schip1 links expanded and tao-1 to regulate hippo signaling. Dev Cell. 2016;36:511–524. doi: 10.1016/j.devcel.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . Approved standard M02–A11. 11. Wayne, PA: National Committee for Clinical Laboratory Standards Institute, CLSI; 2014. Performance standards for antimicrobial disk susceptibility tests. [Google Scholar]
- Dubey SP, Lahtinen M, Sillanpaa M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010;45:1065–1071. doi: 10.1016/j.procbio.2010.03.024. [DOI] [Google Scholar]
- Ehsani E, Akbari K, Teimouri M, Khadem A. Chemical composition and antibacterial activity of two Juniperus species essential oils. Afr J Microbiol Res. 2012;6:6704–6710. doi: 10.5897/AJMR12.686. [DOI] [Google Scholar]
- Emam HE, Saleh NH, Nagy KS, Zahran MK. Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int J Biol Macromol. 2015;78:249–256. doi: 10.1016/j.ijbiomac.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Enan ET, Ashour AA, Basha S, Felemban NH, Gad El-Rab SMF. Antimicrobial activity of biosynthesized silver nanoparticles, Amoxicillin and glass-ionomer cement against Streptococcus mutans and Staphylococcus aureus. Nanotechnology. 2021;32:2151011. doi: 10.1088/1361-6528/abe577. [DOI] [PubMed] [Google Scholar]
- Eryigit T, Okut N, Ekici K, Yildirim B. Chemical composition and antibacterial activities of Juniperus horizontalis essential oil. Can J Plant Sci. 2013;2014:323–327. doi: 10.4141/cjps2013-242. [DOI] [Google Scholar]
- Gad El-Rab SMF, Halawani EM, Hassan AM. Formulation of ceftriaxone conjugated gold nanoparticles and their medical applications against extended-spectrum β-lactamase producing bacteria and breast cancer. World J Microbiol Biotechnol. 2018;28:1563–1572. doi: 10.4014/jmb.1711.11037. [DOI] [PubMed] [Google Scholar]
- Gad El-Rab SMF, Abo-Amer AE, Asiri AM. Biogenic synthesis of ZnO nanoparticles and its potential use as antimicrobial agent against multidrug-resistant pathogens. Curr Microbiol. 2020;77:1767–1779. doi: 10.1007/s00284-020-01991-8. [DOI] [PubMed] [Google Scholar]
- Gad El-Rab SMF, Enan ET, Basha S, Ashour AA, Alyamani AA, Felemban NH (2021) Biofabrication of silver-nanobiotic using Rosmarinus officinalis and its dental application with zinc phosphate luting cement against S. mutans. J Biomed Sci (inpress).
- Ghosh S, Patil S, Ahire M, Kitture R, Kale S, Pardesi K. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomedicine. 2012;7:483–496. doi: 10.2147/IJN.S24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halawani EM, Hassan AM, Gad El-Rab SMF. Nanoformulation of biogenic cefotaxime-conjugated-silver nanoparticles for enhanced antibacterial efficacy against multidrug-resistant bacteria and anticancer studies. Int J Nanomedicine. 2020;5:1889–1901. doi: 10.2147/IJN.S236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeisha A, El-Rafiea MH, EL-Sheikha MA, Seleemb AA, El-Naggar ME. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int J Biol Macromol. 2014;65:509–515. doi: 10.1016/j.ijbiomac.2014.01.071. [DOI] [PubMed] [Google Scholar]
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST, editors. Bergey’s manual of determinative bacteriology. 9. Baltimore, Maryland: Williams & Wilkins; 1994. pp. 527–555. [Google Scholar]
- Huwaitat R, McCloskey AP, Gilmore BF, Laverty G. Potential strategies for the eradication of multi-drug resistant Gram-negative bacterial infections. Future Microbiol. 2016;11:955–972. doi: 10.2217/fmb-2016-0035. [DOI] [PubMed] [Google Scholar]
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Int J Pharm Sci Res. 2014;9:385–406. [PMC free article] [PubMed] [Google Scholar]
- Jyoti K, Baunthiya M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. 2016;9:217–227. doi: 10.1016/j.jrras.2015.10.002. [DOI] [Google Scholar]
- Kanmani P, Lim ST. Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr Polym. 2013;12:421–428. doi: 10.1016/j.carbpol.2013.04.048. [DOI] [PubMed] [Google Scholar]
- Khalil FM, Sonbol A, Badr SA. Comparative study of virulence factors among ESβL producing and non-producing Pseudomonas aeruginosa clinical isolates. Turk J Med Sci. 2015;45:60–69. doi: 10.3906/sag-1311-102. [DOI] [PubMed] [Google Scholar]
- Khan M, Khan AU, Rehman NU, Gilani AH. Pharmacological explanation for the medicinal use of Juniperus excelsa in hyperactive gastrointestinal and respiratory disorders. J Natural Med. 2012;66:292–301. doi: 10.1007/s11418-011-0605-z. [DOI] [PubMed] [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Khatami M, Pourseyedi S. Phoenix dactylifera (date palm) pit aqueous extract mediated novel route for synthesis high stable AgNPs with high antifungal and antibacterial activity. IET Nanobiotechnol. 2015;9:1–7. doi: 10.1049/iet-nbt.2014.0052. [DOI] [PubMed] [Google Scholar]
- Khatami M, Pourseyedi S, Khatami M, Hamidi H, Zaeifi M, Soltani L. Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity. Bioresour Bioprocess. 2015;2:1–19. doi: 10.1186/s40643-015-0043-y. [DOI] [Google Scholar]
- Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberto JCS, Martins CAP, Santos SSF, Cortelli JR, Jorge AOC. Staphylococcus spp. in the oral cavity and periodontal pockets of chronic periodontitis patients. Braz J Microbiol. 2004;35:64–68. doi: 10.1590/S1517-83822004000100010. [DOI] [Google Scholar]
- MaccFadin JK. Biochemical Test for Identification of Medical Bacteria. 3. Philadelphia Baltimor. New York: Lippincott Williams and Winkins. Awolter Klumer Company; 2000. [Google Scholar]
- Mahdieha M, Zolanvari A, Azimeea AS, Mahdiehc M. Green biosynthesis of silver nanoparticles by Spirulina platensis. Scientia Iranica. 2012;19:926–929. doi: 10.1016/j.scient.2012.01.010. [DOI] [Google Scholar]
- Mordi RM, Momoh MI. Incidence of Proteus species in wound infections and their sensitivity pattern in the University of Benin teaching hospital. Afr J Biotechnol. 2009;8:725–730. [Google Scholar]
- Nabi SA, Ahmed N, Khan MJ, Bazai Z, Yasinzai M, KaharamanIn YAL. In vitro antileshmanial, antitumor activities and phytochemical studies of methanolic extract and its fractions of Juniperus excelsa berries. World Appl Sci J. 2012;19:1495–1500. [Google Scholar]
- Panigrahi AK. Relationship between inventory management and profitability: an empirical analysis of indian cement companies. Asia Pacific Manag Rev. 2013;2:7. [Google Scholar]
- Patra JK, Baek KH. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J. Nanomater. 2014 doi: 10.1155/2014/417305. [DOI] [Google Scholar]
- Pender DS, Vangala LM, Badwaik VD, Thompson H, Paripelly R, Dakshinamurthy R. A new class of gold nanoantibiotics—direct coating of ampicillin on gold nanoparticles. Pharmaceut Nanotech. 2013;1:126–135. doi: 10.2174/2211738511301020008. [DOI] [Google Scholar]
- Projan SJ. Why is big pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6:427–430. doi: 10.1016/j.mib.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Rahayu I, Darmawan W, Zaini LH, et al. Characteristics of fast-growing wood impregnated with nanoparticles. J for Res. 2020;31(2):677–685. [Google Scholar]
- Sangili Y, Gurunathan I. Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J Ind Eng Chem. 2015;29:217–226. doi: 10.1016/j.jiec.2015.04.005. [DOI] [Google Scholar]
- Saware K, Sawle B, Salimath B, Jayanthi K, Abbaraju V. Synthesis of stable silver nanoparticles (AgNPs) using Ficus religiosa leaf extract. Int J Res Eng Technol. 2014;3:2321–7308. doi: 10.1007/s10876-014-0697-1. [DOI] [Google Scholar]
- Schuhmacher A, Gassmann O, Hinder M. Changing R&D models in research-based pharmaceutical companies. J Transl Med. 2016;14:105. doi: 10.1186/s12967-016-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahkarami F, Rashki A, Ghalehnoo ZR. Microbial susceptibility and plasmid profiles of Methicillin-Resistant Staphylococcus aureus and Methicillin-Susceptible S. aureus. Jundishapur J Microbiol. 2014;7:169–184. doi: 10.5812/jjm.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav JP. Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multidrug-resistant clinical isolates of Pseudomonas aeruginosa. J Nanobiotechnology. 2014;12:1–9. doi: 10.1186/1477-3155-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo LA, Zapata PA, Vejar ND, Azócar MI, Gulppi MA, Zhou X, Thompson GE, Rabagliati FM, Páez MA. Release of silver and copper nanoparticles from polyethylene nano-composites and their penetration into Listeria monocytogenes. Mat Sci Eng. 2014;40:24–31. doi: 10.1016/j.msec.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. Predictors of mortality in bloodstream infections caused by Klebsiella pneumonaie carbapenemase-producing K. pneumonaie: importance of combination therapy. Clin Infect Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- Tumen I, Süntar I, Keleş H, Akkol EK. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid Based Complement Alternat Med. 2012;2012:1–7. doi: 10.1155/2012/728281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin D, Paterson D. Multidrug resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Mehata MS. Controllable synthesis of silver nanoparticles using neem leaves and their antimicrobial activity. J Radiat Res Appl Sci. 2016;9:109–115. doi: 10.1016/j.jrras.2015.11.001. [DOI] [Google Scholar]
- Wan G, Ruan L, Yin Y, Yang T, Ge M, Cheng X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int J Nanomedicine. 2016;11:3789–3800. doi: 10.2147/IJN.S104166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015) WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health
- Wu J, Kamaly N, Shi J, Zhao L, Xiao Z, Hollett G, John R, Ray S, Xu X, Zhang X, Kantoff PW, Farokhzad OC. Development of multinuclear polymeric nanoparticles as robust protein nanocarriers. Angew. Chem Int Ed. 2014;53:8975–8979. doi: 10.1002/anie.201404766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorseng K, Siengchin S, Ashok B, Rajulu AV. Nanocomposite egg shell powder with in situ generated silver nanoparticles using inherent collagen as reducing agent. J Biores Bioprod. 2020;5:101–107. doi: 10.1016/j.jobab.2020.04.003. [DOI] [Google Scholar]