Abstract
Azo dyes are widely used in the textile industry due to their resistance to light, moisture, and oxidants. They are also an important class of environmental contaminant because of the amount of dye that reaches natural water resources and because they can be toxic, mutagenic, and carcinogenic. Different technologies are used for the decolorization of wastewater containing dyes; among them, the biological processes are the most promising environmentally. The aim of this study was to evaluate the potential of Phanerochaete chrysosporium strain ME-446 to safely decolorize three azo dyes: Direct Yellow 27 (DY27), Reactive Black 5 (RB5), and Reactive Red 120 (RR120). Decolorization efficiency was determined by ultraviolet-visible spectrophotometry and the phytotoxicity of the solutions before and after the fungal treatment was analyzed using Lactuca sativa seeds. P. chrysosporium ME-446 was highly efficient in decolorizing DY27, RB5, and RR120 at 50 mg L−1, decreasing their colors by 82%, 89%, and 94% within 10 days. Removal of dyes was achieved through adsorption on the fungal mycelium as well as biodegradation, inferred by the changes in the dyes’ spectral peaks. The intensive decolorization of DY27 and RB5 corresponded to a decrease in phytotoxicity. However, phytotoxicity increased during the removal of color for the dye RR120. The ecotoxicity tests showed that the absence of color does not necessarily translate to an absence of toxicity.
Keywords: Phanerochaete chrysosporium, Azo dyes, Decolorization, Phytotoxicity
Introduction
The textile industry is one of the largest water-consuming industries worldwide and releases large amounts of effluent into river systems [1, 2]. To process about 1 kg of textile material, textile industries consume approximately 100 L of water, wasting 60–65 L of water as effluent [3]. The pollutants found in this wastewater are primarily persistent substances, namely, dyes, salts, heavy metals, phenolic compounds, softeners, and other chemicals used in the dyeing process [1, 2, 4]. During dyeing processes, almost 15% of the total dye used is discharged as effluent into the environment causing colored rivers [5–7]. Coloring hinders the penetration of sunlight into water and reduces photosynthesis of core primary producers in these eco-systems disrupting food webs and eco-system function [6–8]. Furthermore, some dyes can be toxic, either carcinogenic and/or mutagenic to various life forms [5–10].
Among the various classes of dyes, the azo dyes are extensively used in industrial applications but mainly in textile industries because of their wide spectrum of shades, covalent strong attachment to fibers, ease of application, brilliant colors, and minimal energy consumption [6]. The main characteristic of azo dyes is the presence of one or more azo bonds (―N═N―) in their structure which, added to sulfonated substitutions, contributes to their resistance to fading and to degradation [11–13] which is good for the textile industries but results in colored and recalcitrant effluent pollution. Usually, colored wastewater is treated by physical and chemical processes such as adsorption, coagulation, flocculation, oxidation, filtration, and electrochemical methods [3, 7]. However, these techniques are not 100% efficient at removing color for a broad range of dyes and often these methods generate other colored and sometimes toxic sludge and waste that need further treatment [14, 15].
To mitigate these challenges, microbial bioremediation processes have emerged as a solution to colored water pollution. Microorganisms such as bacteria, fungi, and algae are known to play a crucial role in dye removal [3–6, 12, 17–19]. Bioremediation of dyes can be achieved via biosorption and/or biodegradation. In the first case, dye molecules bind to the surface of the biomass; in the second case, enzymes are responsible for converting complex colorful molecules into simpler compounds [2, 13–16]. Most studies on azo dye biodegradation have focused on species of white-rot fungi, such as Phanerochaete chrysosporium, Trametes versicolor, Pleurotus ostreatus, and Bjerkandera adusta [5, 12, 20, 21]. These species produce extracellular non-specific ligninolytic enzymes (laccase, lignin peroxidase, and manganese peroxidase) which can cleave a wide variety of complex aromatic compounds including dyes [2, 13–16]. Phanerochaete chrysosporium has emerged as a model agent for textile effluent remediation due to its ability to degrade multiple complex compounds that can cause pollution [22]. This microorganism has been used to biodegrade: gaseous chlorobenzene [23], the pesticide endosulfan [24], the insecticide heptachlor [25], and a number of different dyes [12, 21, 26, 27]. As such, it is a good candidate for experimentation in case-specific bioremediation processes.
The harmful effects of azo dyes in water bodies extend far beyond visual pollution. Another issue stems from the metabolites produced when the azo dyes are degraded; in many cases, they are more toxic than the parent dye molecule. Azo dye degradation can produce amines, aromatic amines (benzidines), and phenols that can be highly toxic and slow to biodegrade [28–32]. Therefore, it is very important for any novel bioremediation technology to assess the toxicity of the pollutants and metabolites formed after dye degradation to determine the efficiency of the method.
Sensitive and simple ecotoxicological bioassays like seed germination and root elongation tests are commonly used to evaluate the phytotoxicity of waste and industrial discharge waters. Plants form an important group of organisms used in toxicity tests. Seeds from Lactuca sativa are excellent indicators of the effects of environments polluted by toxic substances including dyes according to USEPA’s Guidelines OPPTS 850.4200. Although they are not aquatic system species, the data afforded from seed toxicity test are informative on the possible effect of contaminants on adjacent plant communities. In these tests, the germination and the growth/ inhibition of the root are generally measured, acting as parameters to assess lethal and sublethal effects [33–35].
The limited number of reports in literature regarding fungal decolorization of the azo dyes DY27, RB5, and RR120 focus on decolorization and not the possible toxicological effects after biological treatment. The ecological risk of these dyes is therefore seldom reported. Thus, in this study, these three potentially carcinogenic and recalcitrant azo dyes were treated with Phanerochaete chrysosporium strain ME-446 and their decolorization and phytotoxicity is reported and discussed.
Material and methods
Azo dyes
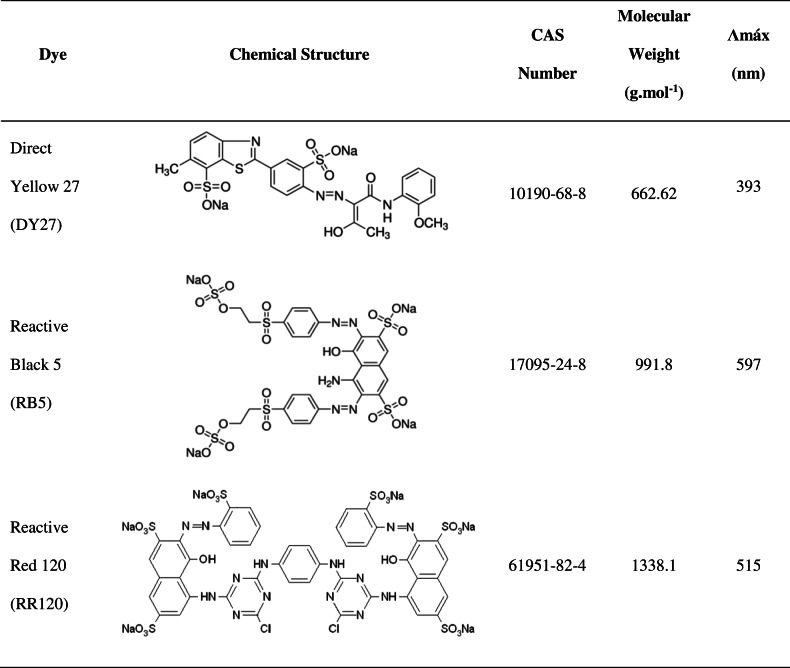
Direct Yellow 27 (DY27), Reactive Black 5 (RB5), and Reactive Red 120 (RR120) were obtained from Sigma-Aldrich Chemical Company, Inc. Properties and chemical structures of these azo dyes are shown in Table 1.
Table 1.
Properties and chemical structures of the azo dyes
Microorganism
P. chrysosporium ME-446 was purchased from the American Type Culture Collection (ATTC). The fungal strain was maintained on Potato Dextrose Agar (PDA) and sub-cultured periodically. The culture was grown at 28 °C for 10 days for obtaining fungal biomass.
Dye decolorization experiment
Two mycelial plugs of P. chrysosporium ME-446 were transferred to 500-mL Erlenmeyer flasks containing 120 mL Potato Dextrose Broth (PDB) supplemented with 50 and 100 mg L−1 of the textile dyes and enriched with 1.0 mL L−1 trace element solution (g L−1: CuSO4, 2.0; ZnSO4, 1.4; FeSO4, 5.0; MnSO4, 1.6), in triplicate. The flasks were incubated in the dark at 28 °C and 180 rpm for 10 days. Untreated (non-inoculated) dye solutions were designated as negative controls. 3.0 mL aliquots were sampled at determined time intervals (3, 5, 7, and 10 days), centrifuged (8000×g, 10 min) and the supernatant collected for spectrophotometric analysis. The cell-free extracts were analyzed at pre-determined maximum absorption wavelengths of the respective dyes (DY27 at 393 nm, RB5 at 597 nm, and RR120 at 515 nm) using an ultraviolet-visible spectrophotometer Shimadzu UV-1800. The dye removal was expressed as decolorization efficiency (DE, %) according to formula (1):
| 1 |
where Ai represents the initial absorbance and Ao represents the observed absorbance after treatment with P. chrysosporium ME-446, respectively.
Ultraviolet-visible spectral analysis
Ultraviolet-visible (UV-Vis) spectral analysis was used to infer the occurrence of biodegradation due to changes in peaks between control and treated dye samples. The culture supernatants at 3, 5, 7, and 10 days were scanned at wavelengths between 200 and 700 nm using an ultraviolet-visible spectrophotometer Shimadzu UV-1800. Spectral peaks for each dye before and after the P. chrysosporium treatment were plotted and compared.
Toxicity tests
Phytotoxicity tests were performed to evaluate the toxicity of the dyes and their metabolites before and after decolorization. The experiment was carried out using Lactuca sativa seeds according to the modified methodology described by Sobrero and Ronco [35]. For such, Petri dishes were lined with filter paper, to which 10 seeds of L. sativa (Feltrin®) and 4.0 mL of test solution were added, in triplicate. Samples after 10 days of the decolorization process were tested, as well as untreated dye solutions at a concentration of 50 and 100 mg L−1. Distilled water was the control. The plates were individually wrapped in plastic film to avoid the evaporation of moisture and incubated at 28 °C in the absence light for 7 days. At the end of the exposure period, seed germination (%), and root growth (cm) were measured and mean values reported.
Results
Dye decolorization experiment
Results of dye removal by P. chrysosporium ME-446 under submerged fermentation are shown in Fig. 1. The maximum decolorization efficiencies were 82% for DY27, 89% for RB5, and 94% for RR120 at a concentration of 50 mg L−1 after 10 days of treatment (Fig. 1a). The effect of initial dye concentration on the decolorization ability of P. chrysosporium ME-446 was also observed. It appeared to be more efficient at lower initial dye concentration (50 mg L−1) compared to higher concentration (100 mg L−1). The dye removal diminished to 53% for DY27 and to 69% for RB5 after 10 days of treatment. In the case of RR120, decolorization efficiency was significantly reduced from 94 to 5% when the dye concentration was increased to 100 mg L−1 (Fig. 1b).
Fig. 1.
Decolorization efficiency of DY27, RB5, and RR120 at a concentration of 50 mg L−1 (a) and 100 mg L−1 (b) after 3, 5, 7, and 10 days of treatment with P. chrysosporium. Bars indicate one standard deviation of mean (±SD)
Overall, decolorization efficiency increased with increased fermentation time. Part of this color removal was presumably reached via biosorption, since fungal cell walls adsorbed some of the dyes (Fig. 2).
Fig. 2.
Adsorption of DY27 (a), RB5 (b), and RR120 (c) on the fungal mycelium
Ultraviolet-visible spectral analysis
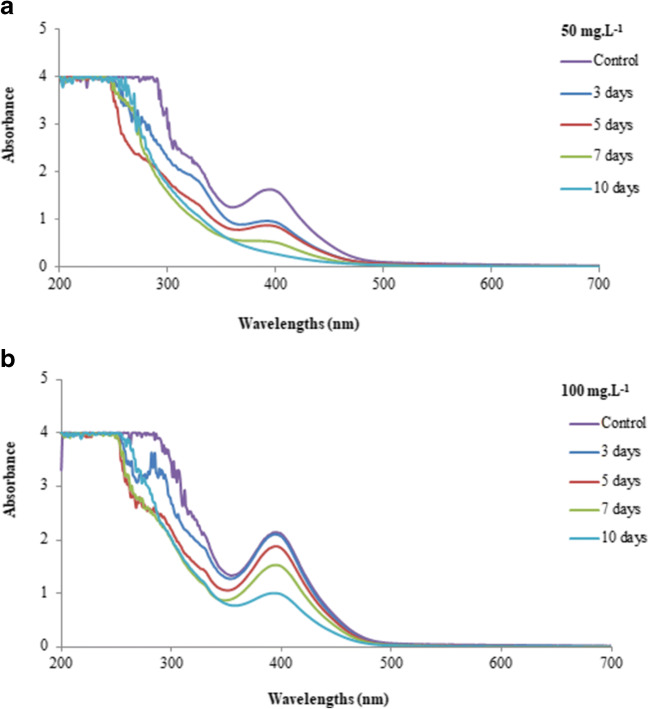
Absorption spectra of the dyes DY27, RB5, and RR120 at concentrations of 50 mg L−1 and 100 mg L−1 after the treatment with P. chrysosporium ME-446 for different times are shown in Figs. 3, 4, and 5, respectively.
Fig. 3.
Absorption spectra of DY27 at a concentration of 50 mg L−1 (a) and 100 mg L−1 (b) after interaction with P. chrysosporium for different times
Fig. 4.
Absorption spectra of RB5 at a concentration of 50 mg L−1 (a) and 100 mg L−1 (b) after interaction with P. chrysosporium for different times
Fig. 5.
Absorption spectra of RR120 at a concentration of 50 mg L−1 (a) and 100 mg L−1 (b) after interaction with P. chrysosporium for different times
Significant spectral changes were found during microbiological treatment. A prominent reduction of the peak at 393 nm (Fig. 3a, b), 597 nm (Fig. 4a, b), and 515 nm (Fig. 5a) corresponding to the maximum absorption wavelength of DY27, RB5, and RR120 was revealed by the analysis. There was virtually no change in the peak at 515 nm for the dye RR120 at a concentration of 100 mg L−1, which confirms the low discoloration obtained (Fig. 5b). The maximum removal of color achieved after 10 days of treatment for the three dyes at a concentration of 50 mg L−1 was followed by the total disappearance of the peak at their respective maximum absorption wavelengths (Figs. 3a, 4a, and 5a). Changes in the absorption spectra of the dyes were also observed at wavelengths of the ultraviolet region. By comparison of the spectra for the dyes DY27 and RB5, there was a reduction of the peaks over the range of 250–350 nm. The most significant reduction occurred in the peak around 320 nm (Figs. 3a, b, and 4a, b). The same was observed for the dye RR120 at 50 mg L−1. In addition, a new peak appeared in the range of 250–300 nm after 10 days of treatment (Fig. 5a). At the concentration of 100 mg L−1, treated samples showed higher absorbance values than the control in the range of 350–400 nm (Fig. 5b).
Toxicity tests
The results of the toxicity tests are presented in Table 2. The interpretation of the data followed a simple logic: the lower the seed germination and the root growth, the greater the toxicity of the tested sample.
Table 2.
Toxicity assessment with seeds from L. sativa
| Seed germination (%) | Root growth (cm) | |||||
|---|---|---|---|---|---|---|
| Solution | Dye test | |||||
| DY27 | RB5 | RR120 | DY27 | RB5 | RR120 | |
| Distilled water | 100 ± 0a | 100 ± 0a | 87± 5.7a | 1.76 ± 0.03a | 1.17 ± 0.10a | 1.48±0.15a |
| Untreated dye solution at 50 mg L−1 | 60 ± 0b | 50 ± 0b,d | 43± 5.7b | 0.95± 0.09b,d | 0.46± 0.22b | 0.45 ± 0.05b,c |
| Treated dye solution at 50 mg L−1 | 73 ± 5.7c | 70 ± 10c,e | 20 ± 10c,f | 1.23 ± 0.19b | 0.71 ± 0.07b | 0.20 ± 0.10b,e |
| Untreated dye solution at 100 mg L−1 | 40 ± 0d | 37 ± 5.7b | 23 ± 5.7d,f | 0.62 ± 0.03c,e | 0.32 ± 0.05b | 0.25 ± 0.10c,e |
| Treated dye solution at 100 mg L−1 | 53 ± 5.7b | 63 ± 5.7d,e | 20±0e,f | 0.8 ± 0.13d,e | 0.57 ± 0.25b | 0.17± 0.03d,e |
Values are the mean ± SD. Values followed by letters (a, b, c, …) in the same column are significantly different at p < 0.05 by one-way analysis of variance (ANOVA) with Tukey’s test
In general, the presence of dyes in the tested samples caused an increase in toxicity. The treatments with highest color removal (dye solutions at 50 mg L−1) showed lower germination rates and root elongations than the control seeds treated with water. The increase in dye concentration from 50 to 100 mg L−1 also seemed to cause greater toxicity.
From Table 2, it was possible to observe that seed germination after the treatment of DY27 and RB5 with P. chrysosporium ME-446 was significantly higher than the respective untreated solutions at both concentrations. Treated solutions of the dye DY27 showed seed germination rates of 73% and 53% compared with 60% and 40% of untreated dye solutions at 50 mg L−1 and 100 mg L−1, respectively. For the dye RB5, treated solutions showed seed germination rates of 70% and 63% against 50% and 37% of untreated dye solutions. These results indicated the microbial treatment resulted in a less toxic solution.
On the other hand, the opposite occurred for the dye RR120. Seed germination after the treatment with P. chrysosporium ME-446 was significantly lower than the respective untreated solution at a concentration of 50 mg L−1. The treated solution had a seed germination rate of 20% against 43% of the untreated solution. These results indicated that the fungal treatment resulted in a more toxic effluent even though much of the color had been removed. No significant difference was observed for the dye RR120 at a concentration of 100 mg L−1 before and after the treatment.
Analyzing root growth, treated solutions of the dye DY27 showed elongation of 1.23 cm and 0.8 cm against 0.95 cm and 0.62 cm for untreated dye solutions at 50 mg L−1 and 100 mg L−1, respectively. For the dye RB5, treated solutions showed root growth of 0.71 cm and 0.57 cm against 0.46 cm and 0.32 cm of untreated dye solutions. Treated solutions of the dye RR120 showed elongation of 0.20 cm and 0.17 cm against 0.45 cm and 0.25 cm of untreated dye solutions at 50 mg L−1 and 100 mg L−1, respectively. These results showed no significant differences, indicating that the fungal treatment had no effect on seedling development.
Discussion
Dye decolorization
The fungal strain of P. chrysosporium ME-446 proved to be versatile and effective in the decolorization of the azo dyes Direct Yellow 27 and Reactive Black 5, decreasing their colors by more than 50% even at a concentration of 100 mg L−1 (53% and 69%, respectively) and more than 80% at a concentration of 50 mg L−1 (82% and 89%, respectively) within 10 days of treatment. To the best of our knowledge, Pramanik and Chaudhuri’s [36] findings are the only other report in literature about discoloration of Direct Yellow 27. The authors discussed the decolorization potentiality of five high molecular weight azo dyes, by the macro-fungus Podoscypha elegans. Their results for DY27 showed decolorization efficiency below 50% after 28 days. In comparison, Phanerochaete chrysosporium ME-446 proved to be a more promising agent for DY27 decolorization, being able to discolor more in less time and under non-optimized conditions. Discoloration of Reactive Black 5 by fungal strains has been described by other authors. Permpornsakul et al. [37] evaluated the discoloration capability of resupinate fungi from Thailand on RB5. Decolorization ranged from 43 to 100% within 3 days. One isolate, identified as Phanerochaete sordida PBU 0057, completely decolored the solution. Martínez-Sánchez et al. [38] applied a factorial design to increase the specific decolorization rate of RB5 by Trametes versicolor, using free and immobilized cells in a batch reactor. The results showed that immobilized cells (84% color removed in 24 h) were decolorized faster than free cells (85% in 144 h). A new efficient RB5 dye decolorizing white-rot fungus Cerrena sp. WICC F39 was described by Hanapi et al. [39]. The isolate exhibited decolorization of 86% of the dye in 8 days. The performance of P. chrysosporium ME-446 in our experiments is comparable. Ibrahim et al. [40] investigated the decolorization of the same dye by Aspergillus and Pleurotus strains. At 10 mg L−1 of dye concentration, the maximum performance of decolorization ranged from 60 to 70% after 21 days. In comparison, Phanerochaete chrysosporium ME-446 is a better decolorizing agent, being able to discolor more and in less time at 50 mg L−1. On the other hand, there was practically no discoloration when the RR120 dye concentration was increased to 100 mg L−1.
Some studies demonstrate that under optimized growth conditions other fungal species decolor RR120 efficiently and perhaps better than the strain P. chrysosporium ME-446. Yang et al. [41] investigated the decolorization of different acid, disperse, and reactive dyes by Trametes versicolor CBR43. That strain decolorized more than 90% of RR120 solutions at a concentration of 200 mg L−1 within 6 days. Different Aspergillus species isolated from soil samples contaminated with industrial effluent were also analyzed for RR120 biodegradation by Ameen and Alshehrei [42]. The results for RR120 degradation were found to be 86% by A. flavus, 84% by A. fumigatus, 85% by A. niger, and 86% by A. terreus, with a fixed initial concentration of the dye equal to 100 mg L−1, and an incubation period of 7 days. Harazono et al. [43] investigated the decolorization of RR120 by a white-rot basidiomycete, Phanerochaete sordida strain YK-624. In a liquid culture containing 200 mg L−1 of the dye, 90.6% was decolorized after 7 days.
In this study, similar decolorization profiles were observed for all three dyes with this fungal strain. The decolorization efficiencies increased with increased fermentation time and were significantly reduced with the increase of the initial dye concentration. The concentration of dye in the medium was crucial for decolorization by the fungus. This result is in line with reported studies that demonstrated that initial dye concentrations can exert negative impacts on decolorization efficiency [3, 6, 17, 36, 37, 40, 41, 44]. Besides the initial dye concentration, several environmental and physiological factors can influence the microbial activity and consequently the efficacy and effectiveness of the complete biodegradation processes, such as the amount of fungal biomass, oxygen requirement, nutrients and co-metabolic induction, pH, temperature, and salinity [3, 6, 17, 36, 37, 40, 41, 44, 45]. Thus, small structural differences between the dyes have been shown to significantly affect their decolorization. This might be due to differences in electron distribution, charge density, or steric factors [5, 46]. It has been reported that the presence of substituent groups at different positions on the aromatic ring may accelerate or reduce the rate of discoloration or cause its complete inhibition [46–49]. The presence of more electron-withdrawing groups (-SO3 groups) promotes rapid degradation, while dye molecules with more electron-donating groups (-CH3 groups) are degraded less rapidly [50, 51]. With this, it is to be expected that different dyes are degraded differently by the same fungus and different fungi degrade the same dye differently. In terms of a meaningful application, we seek a fungal strain that degrades broadly and effectively a number of dyes but that its decolorization process does not increase or create a new environmental pollution.
Primarily, the fungal biomass appeared to be responsible for removing the azo dyes through biosorption. It was possible to observe a certain coloration of the fungal biomass from the beginning of the treatment, suggesting that the cell wall of P. chrysosporium ME-446 has an adsorption capacity for these dyes. Coriolopsis sp., Fusarium solani, Aspergillus niger, Aspergillus fumigatus, and Trichoderma sp. are examples of other fungi where dye biosorption has been demonstrated [44, 52–55]. Biosorption is reported to be the primary dye removal process in wood-rotting basidiomycetes [46, 56–58] and has been linked to electrostatic pull between the negatively charged dyes and the positively charged cell wall components [58].
Similar mechanisms were evident in this study; however, biosorption alone is not sufficient to explain the high color removals achieved since the fungal biomass remained very pale colored (Fig. 2). Furthermore, analysis of the dyes’ absorption spectra allows us to infer that the dyes were in fact biodegraded. These observations are in line with the findings of Balan and Monterio [56], where they reported the removal of Indigo dye by fungal adsorption and extracellular degradation. The bio-removal abilities for different dyes have been separately related to biosorption and biodegradation in the case of P. chrysosporium. Fungal biomass showing biosorption of a specific dye remained extremely colored; otherwise, it kept colorless [59]. Moreover, biodegradation of metal complex dyes (Grey lanaset G.) revealed dye degradation in three successive stages: (1st) primary dye adsorption to the fungus leading; (2nd) the splitting of metal complex bond from the dye; and (3rd) dye degradation with the release of products into the culture medium [60].
Ultraviolet-visible spectral analysis
Changes in UV-Vis spectral analyses were used as evidence to infer the occurrence of biodegradation. As was seen, decolorization can be achieved via biosorption, when the dye is adsorbed on the fungal cell and/or biodegradation, when the dye molecule is converted into a simpler one. When adsorption is the main mechanism of removal of color, then an examination of the light absorption spectrum reveals that all peaks decrease approximately in proportion to each other. However, when transformations in the dye structure occur there is either a complete removal of the major visible light absorbance peak or a significant spectral change (e.g., a change in the relative absorbance of two peaks or development of a new peak) [46].
The prominent reduction/total disappearance of the peaks corresponding to the maximum absorption wavelength of DY27, RB5, and RR120 (393 nm, 597 nm, and 515 nm, respectively) observed after 10 days of treatment indicated that P. chrysosporium ME-446 was also able to transform the chemical structure of the dyes. The breakdown of the chromophore group led to the disappearance of the absorbance peaks [44]. Besides that, disruption of the azo dye structure results in loss of its color [37, 61]. The peak reduction occurring around 320 nm may be also related to this breakdown, since this wavelength corresponds to the azo bond [21, 62]. So, these results strongly indicated the occurrence of biodegradation as one of the mechanisms that removed the dye color. Changes in the absorption spectra of the dye RR120 were also observed in the ultraviolet region, mainly in the range of 250–400 nm. The appearance of a peak in this region suggests that the transformation of dye molecule produced aromatic intermediates that absorb energy in UV range. An absorbance peak at 250 nm, for example, relates to aromatic hydrocarbon, or polycyclic aromatic hydrocarbon groups and an absorbance peak at 340 nm is the extended peak because of interactions between aromatic hydrocarbon or polycyclic aromatic hydrocarbon groups and other chromophores [63]. Zille et al. [64] also postulated that azo dye degradation by laccase from T. villosa produces the formation of peaks in the UV region. This could be attributed to the conversion of the degraded dye into different reaction products such as phenolic compounds and polymerized products.
In this study, decolorization of dye solution seems to be due to both adsorption and biodegradation mechanisms. Moreover, the results revealed dye removal in successive stages. Adsorption to the fungal biomass appeared to be more effective at the first moment, while with time biodegradation took place with the release of products into the culture medium. A consolidated measure of that biodegradation potential would require FTIR analysis and the quantification of enzymatic activities, not studied nor reported here.
As discussed above, fungal strains degrading dyes are not new. Here, we highlight the potential of P. chrysosporium ME-446 to be incorporated into biotechnologies for the decolorization and biodegradation of the azo dyes DY27, RB5, and RR120. To move beyond “potential” and on to an applicable clean-up biotechnology, there is a need for strain-specific toxicity testing.
Toxicity tests
Despite the apparent decolorization achieved after the treatment with P. chrysosporium ME-446, it was important to make sure that the treated solutions are less toxic than the original dye solution. For this, toxicity tests were performed on seeds of L. sativa to evaluate the phytotoxic effect of the three dyes solutions before the fungal treatment and their metabolites produced after decolorization.
Plants are sensitive indicators of remediation by-products by showing either inhibition or stimulation of plant growth and development. By measuring seed germination and root growth, it was possible to analyze the toxicity effect at two levels: lethal and sublethal. According to Sobrero and Ronco [35], germination and seed development are very sensitive to external factors, so a contaminant can decisively interfere in these essential processes for survival of the species. The root elongation test evaluates the effects of very low concentrations of contaminant that might not inhibit germination, but delay seedling development.
The intensive removal of the color from dyes DY27 and RB5 corresponded to a decrease in lethality, indicating that the treatment resulted in less toxic metabolites. For these dyes, seed germinations after the treatment with P. chrysosporium ME-446 were significantly higher than the respective untreated solutions. This is the first report that demonstrates fungal detoxification of the azo dye DY27. Fungal detoxification of the azo dye RB5 was also observed by Permpornsakul et al. [37]. The biodegradation of RR120, however, resulted in increased phytotoxicity. Seed germination after the treatment with P. chrysosporium ME-446 was significantly lower than the respective untreated solution at a concentration of 50 mg L−1 even though much of the color had been removed. Almeida and Corso [53] reported that incomplete biodegradation by Aspergillus terreus of Porcion Red MX-5, a reactive dye that has a similar chemical structure to RR120, also led to the formation of toxic metabolites. In summary, the degradation products of the dyes DY27 and RB5 seem to be not toxic and the metabolites produced after decolorization of RR120 seem to be toxic. Therefore, we can say that our fungal strain is a candidate to be used in solution to clean up the dyes DY27 and RB5, but not to RR120.
The dye Reactive Red 120 has a complex chemical structure with several aromatic rings and two azo groups. Ameen and Alshehrei [42] analyzed the metabolites formed during RR120 biodegradation by different Aspergillus species using GC-MS analysis. Their results demonstrated that sodium 2-aminobenzenesulfonate is produced as a metabolic intermediate. However, the authors did not correlate this data with its toxicity. Previously, Almeida and Corso [53] analyzed the metabolites formed during Procion Red MX-5B biodegradation by A. niger and A. terreus using FTIR analysis. They observed several structural changes in the dye molecule, indicating that the degradation led to the formation of different secondary metabolites including primary and secondary amines, which were more toxic than the original dye. Thus, the increase in toxicity observed in the degradation of the dye RR120 can be explained by the formation of more toxic intermediates, which reinforces the occurrence of biodegradation as one of the decolorization mechanisms. That observation made, the formation of a more toxic effluent is highly undesirable and can exert a more severe impact on the environment. The ecological risk of these dyes is seldom reported because there is very little literature that combines novel strain and dye-specific decolorization data with ecotoxicity testing. So, more studies should be encouraged to provide a better correlation between decolorization and ecotoxicity. The results of the experiments reported here underscore the importance of determining the toxicity of effluents following a decolorization process before discharging wastewater into the environment. Thus, the absence of color does not necessarily translate to an absence of toxicity.
Conclusions
This study demonstrated that P. chrysosporium ME-446 is an efficient decolorizer of the three azo dyes, Reactive Black 5, Reactive Red 120, and Direct Yellow 27, decreasing their colors by more than 80% within 10 days of treatment. The influence and importance of the initial dye concentration were observed. Removal of dyes was achieved through both adsorption on the fungal mycelium and biodegradation. The UV-Vis spectrophotometry analyses were quick and easy to perform and a useful way to confirm biodegradation. Seeds from L. sativa proved to be good indicators of acute toxicity and were sensitive to changes in the toxicity of the solutions after the fungal treatment. Toxicity tests indicated that the degradation products of Reactive Black 5 and Direct Yellow 27 were less toxic than the untreated dye. These results support the use of this strain to be used in novel biotechnologies to help clean and protect water supplies and consequently the food and biological webs that water supports. Conversely, the decolorization and degradation of Reactive Red 120 generated more toxic metabolites, requiring further studies.
Acknowledgments
The authors thank the support from the National Council for Scientific and Technological Development (CNPq) and from the Coordination of Superior Level Staff Improvement (CAPES), Brazil’s Federal funding agencies.
Code availability
Not applicable.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alana Pereira. The first draft of the manuscript was written by Alana Pereira and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Council for Scientific and Technological Development (CNPq) and by the Coordination of Superior Level Staff Improvement (CAPES).
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hai FI, Yamamoto K, Fukushi K. Hybrid treatment systems for dye wastewater. Crit Rev Environ Sci Technol. 2007;37(4):315–377. doi: 10.1080/10643380601174723. [DOI] [Google Scholar]
- 2.Siddique K, Rizwan M, Shahid MJ, Ali S, Ahmad R, Rizvi H. Textile wastewater treatment options: a critical review. In: Anjum N, Gill S, Tuteja N, editors. Enhancing Cleanup of Environmental Pollutants. Cham: Springer; 2017. pp. 183–207. [Google Scholar]
- 3.Kurade MB, Waghmode TR, Khandare RV, Jeon BH, Govindwar SP. Biodegradation and detoxification of textile dye Disperse Red 54 by Brevibacillus laterosporus and determination of its metabolic fate. J Biosci Bioeng. 2016;121(4):442–449. doi: 10.1016/j.jbiosc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Vijayalakshmidevi SR, Muthukumar K. Improved biodegradation of textile dye effluent by coculture. Ecotoxicol Environ Saf. 2015;114:23–30. doi: 10.1016/j.ecoenv.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Chao Y, Yang X, Bao H, Qian S. Biodecolorization of azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. J Ind Microbiol Biotechnol. 2004;31:127–132. doi: 10.1007/s10295-004-0123-z. [DOI] [PubMed] [Google Scholar]
- 6.Saroj S, Kumar K, Pareek N, Prasad R, Singh RP. Biodegradation of azo dyes Acid Red 183, Direct Blue 15 and Direct Red 75 by the isolate Penicillium oxalicum. Chemosph. 2014;107:240–248. doi: 10.1016/j.chemosphere.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Przystaś W, Zabłocka-Godlewska E, Grabińska-Sota E. Efficiency of decolorization of different dyes using fungal biomass immobilized on different solid supports. Braz J Microbiol. 2018;49(2):285–295. doi: 10.1016/j.bjm.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Boyjoo Y, Choueib A, Zhu ZH. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005;39:129–138. doi: 10.1016/j.watres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Suteu D, Zaharia C, Muresan A, Muresan R, Popescu A. Using of industrial waste materials for textile wastewater treatment. Environ Eng Manag J. 2009;8(5):1097–1102. doi: 10.30638/eemj.2009.160. [DOI] [Google Scholar]
- 10.Zaharia C, Suteu D, Muresan A, Muresan R, Popescu A. Textile wastewater treatment by homogeneous oxidation with hydrogen peroxide. Environ Eng Manag J. 2009;8(6):1359–1369. doi: 10.30638/eemj.2009.199. [DOI] [Google Scholar]
- 11.Hu TL, Wu SC. Assessment of the effect of azo dye RP2B on the growth of a nitrogen fixing cyanobacterium – Anabaena sp. Bioresour Technol. 2001;77(1):93–95. doi: 10.1016/S0960-8524(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 12.Martins MAM, Ferreira IC, Santos IM, Queiroz MJ, Lima N. Biodegradation of bioaccessible textile azo dyes by Phanerochaete chrysosporium. J Biotechnol. 2001;89:91–98. doi: 10.1016/S0168-1656(01)00318-2. [DOI] [PubMed] [Google Scholar]
- 13.Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. App Microbiol Biotechnol. 2001;56(1-2):69–80. doi: 10.1007/s002530100686. [DOI] [PubMed] [Google Scholar]
- 14.Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol. 2001;77(3):247–255. doi: 10.1016/S0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- 15.Singh R, Singh P, Singh RP. Enzymatic decolorization and degradation of azo dyes: a review. Int Biodeterior Biodegrad. 2015;104:21–31. doi: 10.1016/j.ibiod.2015.04.027. [DOI] [Google Scholar]
- 16.Banat IM, Nigam P, Singh D, Marchant R. Microbial decolorization of textile-dye containing effluents: a review. Bioresour Technol. 1996;58(3):217–227. doi: 10.1016/S0960-8524(96)00113-7. [DOI] [Google Scholar]
- 17.Jadhav JP, Govindwar SP. Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463. Yeast. 2006;23(4):315–323. doi: 10.1002/yea.1356. [DOI] [PubMed] [Google Scholar]
- 18.Sharma DK, Saini HS, Singh M, Chimni SS, Chandha BS. Isolation and characterization of microorganisms capable of decolorizing various triphenylmethane dyes. J Basic Microbiol. 2004;44(1):59–65. doi: 10.1002/jobm.200310334. [DOI] [PubMed] [Google Scholar]
- 19.Deng D, Guo J, Zeng G, Sun G. Decolorization of anthraquinone, triphenylmethane and azo dyes by a new isolated Bacillus cereus strain DC11q. Int Biodeter Biodegr. 2008;62:263–269. doi: 10.1016/j.ibiod.2008.01.017. [DOI] [Google Scholar]
- 20.Salame TM, Knop D, Levinson D, Mabjeesh SJ, Yarden O, Yitzhak H. Release of Pleurotus ostreatus versatile-peroxidase from Mn2+ repression enhances anthropogenic and natural substrate degradation. PLoS One. 2012;7(12):e52446. doi: 10.1371/journal.pone.0052446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos GC, Corso CR. Comparative analysis of azo dye biodegradation by Aspergillus oryzae and Phanerochaete chrysosporium. Water Air Soil Pollut. 2014;225(7):2026. doi: 10.1007/s11270-014-2026-6. [DOI] [Google Scholar]
- 22.Asamudo N, Daba AS, Ezeronye OU. Bioremediation of textile effluent using Phanerochaete chrysosporium. Afr J Biotechnol. 2006;4:1548–1553. doi: 10.4314/ajfand.v4i13.71767. [DOI] [Google Scholar]
- 23.Wang C, Xi JY, Hu HY, Wen XH. Biodegradation of gaseous chlorobenzene by white-rot fungus Phanerochaete chrysosporium. Biomed Environ Sci. 2008;21:474–478. doi: 10.1016/S0895-3988(09)60005-2. [DOI] [PubMed] [Google Scholar]
- 24.Kullman SW, Matsumura F. Metabolic pathways utilized by Phanerochaete chrysosporium for degradation of the cyclodiene pesticide endosulfan. App Environ Microbiol. 1996;62:593–600. doi: 10.1128/AEM.62.2.593-600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arisoy M, Kolankaya N. Biodegradation of heptachlor by Phanerochaete chrysosporium ME 446: the toxic effects of heptachlor and its metabolites on mice. Turkish J Biol. 1998;22:427–434. [Google Scholar]
- 26.Cripps C, Bumpus JA, Aust SD. Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990;56:1114–1118. doi: 10.1128/AEM.56.4.1114-1118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capalash N, Sharma P. Biodegradation of textile azodyes by Phanerochaete chrysosporium. World J Microbiol Biotechnol. 1992;8:309–312. doi: 10.1007/BF01201886. [DOI] [PubMed] [Google Scholar]
- 28.Rizzo L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011;45:4311–4340. doi: 10.1016/j.watres.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Sen SK, Raut S, Bandyopadhyay P, Raut S. Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev. 2016;30(3):112–133. doi: 10.1016/j.fbr.2016.06.003. [DOI] [Google Scholar]
- 30.Wang L, Yan J, Hardy W, Mosley C, Wang S, Yu H. Light-induced mutagenicity in Salmonella TA102 and genotoxicity/cytotoxicity in human T-cells by 3,3’-dichlorobenzidine: a chemical used in the manufacture of dyes and pigments and in tattoo inks. Toxicol. 2005;207:411–418. doi: 10.1016/j.tox.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Sabti K. Chlorotriazine reactive azo Red 120 textile dyes induces micronuclei in fish. Ecotox Environ Saf. 2000;147:149–155. doi: 10.1006/eesa.2000.1931. [DOI] [PubMed] [Google Scholar]
- 32.Gottilieb A, Shaw C, Smith A, Wheatley A, Forsythe S. The toxicity of textile reactive azo dyes after hydrolysis and decolourization. J Biotechnol. 2003;101:49–56. doi: 10.1016/S0168-1656(02)00302-4. [DOI] [PubMed] [Google Scholar]
- 33.Fiskesjö G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102:99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 34.Yi H, Meng Z. Genotoxicity of hydrated sulfur dioxide on root tips of Allium sativum and Vicia faba. Mutat Res. 2003;537:109–114. doi: 10.1016/s1383-5718(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 35.Sobrero MS, Ronco A. Ensayo de toxidad aguda consemillas de lechuga (Lactuca sativa L) In: Castillo G, editor. Ensayos toxicológicos y métodos de evaluación de calidad de aguas. Estandarización, intercalibración, resultados y aplicaciones. Canadá: IDRC, IMTA; 2004. pp. 71–79. [Google Scholar]
- 36.Pramanik S, Chaudhuri S. Laccase activity and azo dye decolorization potential of Podoscypha elegans. Mycobiol. 2018;46(1):79–83. doi: 10.1080/12298093.2018.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Permpornsakul P, Prasongsuk S, Lotrakul P, Eveleigh DE, Kobayashi DY, Imai T, Punnapayak H. Biological treatment of reactive black 5 by resupinate white rot fungus Phanerochaete sordida PBU 0057. Polish J Environ Stud. 2016;25(3):1167–1176. doi: 10.15244/pjoes/61625. [DOI] [Google Scholar]
- 38.Martínez-Sánchez J, Membrillo-Venegas I, Martínez-Trujillo A, García-Rivero AM. Decolorization of reactive black 5 by immobilized Trametes versicolor. Rev Mex Ing Química. 2018;17(1):107–121. doi: 10.24275/uam/izt/dcbi/revmexingquim/2018v17n1/Martinez. [DOI] [Google Scholar]
- 39.Hanapi SZ, Abdelgalil SA, Hatti-Kaul R, Aziz R, El Enshasy HA. Isolation of a new efficient dye decolorizing white rot fungus Cerrena sp. WICC F39. J Sci Ind Res. 2018;77:399–404. [Google Scholar]
- 40.Ibrahim NN, Talib SA, Ismail HN, Tay CC. Decolorization of reactive red-120 by using macrofungus and microfungus. J Fundam Appl Sci. 2017;9:6–954. doi: 10.4314/jfas.v9i6s.11. [DOI] [Google Scholar]
- 41.Yang S, Sodaneath H, Lee J, Jung H, Choi J, Ryu HW, Cho K. Decolorization of acid, disperse and reactive dyes by Trametes versicolor CBR43. J Environ Sci Health. 2017;52:862–872. doi: 10.1080/10934529.2017.1316164. [DOI] [PubMed] [Google Scholar]
- 42.Ameen F, Alshehrei F. Biodegradation optimization and metabolite elucidation of Reactive Red 120 by four different Aspergillus species isolated from soil contaminated with industrial effluent. Ann Microbiol. 2017;67(4):303–312. doi: 10.1007/s13213-017-1259-1. [DOI] [Google Scholar]
- 43.Harazono K, Watanabe Y, Nakamura K. Decolorization of azo dye by the white-rot basidiomycete Phanerochaete sordida and by its manganese peroxidase. J Biosci Bioeng. 2003;95(5):455–459. doi: 10.1016/S1389-1723(03)80044-0. [DOI] [PubMed] [Google Scholar]
- 44.Chen SH, Ting ASY. Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. isolated from compost. J Environ Manage. 2015;150:274–280. doi: 10.1016/j.jenvman.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Bardi L, Marzona M. Factors affecting the complete mineralization of azo dyes. In: Atacag Erkurt H, editor. Biodegradation of azo dyes. Berlin: Springer; 2010. pp. 195–210. [Google Scholar]
- 46.Knapp JS, Newby PS, Reece LP. Decolorization of dyes by wood-rotting basidiomycete fungi. Enzyme Microb Technol. 1995;17:664–668. doi: 10.1016/0141-0229(94)00112-5. [DOI] [Google Scholar]
- 47.Dawkar VV, Jadhav UU, Telke AA, Govindwar SP. Peroxidase from Bacillus sp. VUS and its role in the decolorization of textile dyes. Biotechnol Bioprocess Eng. 2009;14:361–368. doi: 10.1007/s12257-008-0242-x. [DOI] [Google Scholar]
- 48.Pasti-Grigsby MB, Paszczynski A, Goszczynski S, Crawford DL, Crawford RL. Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl Environ Microbial. 1992;58:3605–3613. doi: 10.1128/AEM.58.11.3605-3613.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaffiqu TS, Roy JJ, Nair R, Abraham TE. Degradation of textile dyes mediated by plant peroxidases. Appl Biochem. 2002;102:315–326. doi: 10.1385/ABAB:102-103:1-6:315. [DOI] [PubMed] [Google Scholar]
- 50.Chen CH, Chang CF, Liu SM. Partial degradation mechanisms of malachite green and methyl violet B by Shewanella decolorationis NTOU1 under anaerobic conditions. J Haz Mat. 2010;177:281–289. doi: 10.1016/j.jhazmat.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 51.Hsueh CC, Chen BY, Yen CY. Understanding effects of chemical structure on azo dye decolorization characteristics by Aeromonas hydrophila. J Haz Mater. 2009;167:995–1001. doi: 10.1016/j.jhazmat.2009.01.077. [DOI] [PubMed] [Google Scholar]
- 52.Abedin RM. Decolorization and biodegradation of crystal violet and malachite green by Fusarium solani (Martius) Saccardo. A comparative study on biosorption of dyes by the dead fungal biomass. Am-EurasJ Bot. 2008;12:17–31. [Google Scholar]
- 53.Almeida EJR, Corso CR. Comparative study of toxicity of azo dye Procion Red MX-5B following biosorption and biodegradation treatments with the fungi Aspergillus niger and Aspergillus terreus. Chemosphere. 2014;112:317–322. doi: 10.1016/j.chemosphere.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhry MT, Zohaib M, Rauf N, Tahir SS, Parvez S. Biosorption characteristics of Aspergillus fumigatus for the decolorization of triphenylmethane dye acid violet 49. Appl Microbiol Biotechnol. 2014;98:3133–3141. doi: 10.1007/s00253-013-5306-y. [DOI] [PubMed] [Google Scholar]
- 55.Sivasamy A, Sundarabal N. Biosorption of an azo dye by Aspergillus niger and Trichoderma sp. fungal biomasses. Current microbial. 2011;62(2):351–357. doi: 10.1007/s00284-010-9713-3. [DOI] [PubMed] [Google Scholar]
- 56.Balan DSL, Monteiro RTR. Decolorization of textile indigo dye by ligninolytic fungi. J Biotechnol. 2001;89:141–145. doi: 10.1016/S0168-1656(01)00304-2. [DOI] [PubMed] [Google Scholar]
- 57.Fu YZ, Viraraghavan T. Removal of a dye from aqueous solution by the fungus Aspergillus niger. Wat Qual Res J Can. 2000;35:95–111. doi: 10.2166/wqrj.2000.006. [DOI] [Google Scholar]
- 58.Ali N, Hameed A, Ahmed S. Role of brown-rot fungi in the bioremoval of azo dyes under different conditions. Braz J Microbiol. 2010;41(4):907–915. doi: 10.1590/S1517-83822010000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sani RK, Azmi W, Banerjee UC. Comparison of static and shake culture in the decolorization of textile dyes and dye effluents by Phanerochaete chrysosporium. Folia Microbiol. 1998;43(1):85–88. doi: 10.1007/BF02815550. [DOI] [PubMed] [Google Scholar]
- 60.Blanquez P, Casas N, Font X, Gabarrell X, Sarra M, Caminal G, Vicent T. Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res. 2004;38:2166–2172. doi: 10.1016/j.watres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez LVG, Alatorre GG, Silva EME. Proposed pathways for the reduction of a reactive azo dye in an anaerobic fixed bed reactor. World J Microbiol Biotechnol. 2009;25:415–426. doi: 10.1007/s11274-008-9906-0. [DOI] [Google Scholar]
- 62.Silverstein RM, Bassler GC, Morril TC. Identificação espectrométrica de compostos orgânicos. 5. Rio de Janeiro: Guanabara Koogan; 1994. [Google Scholar]
- 63.Wang N, Chu Y, Zhao Z, Xu X. Decolorization and degradation of Congo red by a newly isolated white rot fungus, Ceriporiala cerata, from decayed mulberry branches. Int Biodeterior Biodegrad. 2017;117:236–244. doi: 10.1016/j.ibiod.2016.12.015. [DOI] [Google Scholar]
- 64.Zille A, Gornacka B, Rehorek A, Cavaco-Paulo A. Degradation of azo dyes by Trametes villosa laccase over long periods of oxidative conditions. Appl Environ Microbiol. 2005;71(11):6711–6718. doi: 10.1128/AEM.71.11.6711-6718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.