Abstract
During our conveying the microbial structures of phycosphere microbiota (PM) derived from diverse marine harmful algal bloom (HAB) dinoflagellates, a new rod-sharped, white-colored cultivable bacterial strain, designated as LZ-15-2, was isolated from the PM of highly toxic Alexandrium catenella LZT09. Phylogenetic analysis of 16S rRNA gene sequence indicated that strain LZ-15-2 belonged to the genus Marivita within the family Rhodobacteraceae, and demonstrated the highest gene similarity of 99.2% to M. cryptomonadis CL-SK44T, and less than 98.65% with other type strains of Marivita. Phylogenomic calculations on average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values between the new isolate and M. cryptomonadis CL-SK44T were 99.86% and 99.88%, respectively. Genomic comparison of strain LZ-15-2 with available genomes of Marivita species further verified its taxonomic position within the genus of Marivita. Moreover, comparative genomics analysis showed a proximal similarity of strain LZ-15-2 with M. cryptomonadis CL-SK44T, and it also revealed an open pan-genome status based on constructed gene accumulation curves among Marivita members with 9,361 and 1,712 genes for the pan- and core-genome analysis, respectively. Based on combined polyphasic taxonomic characteristics, strain LZ-15-2 represents a new member of M. cryptomonadis, and proposed as a potential candidate for further exploration of the detailed mechanisms governing the dynamic cross-kingdom algae–bacteria interactions (ABI) between PM and their algal host LZT09.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00463-w.
Keywords: Phycosphere microbiota, Alexandrium catenella LZT09 , Marine HAB dinoflagellates, Paralytic shellfish poisoning, toxins, Algae–bacteria interactions
Introduction
The genus Marivita as a member of Roseobacter group was erected by Hwang et al. [1] in 2009 with Marivita cryptomonadis as the type species [1]. Marivita members are abundant bacterial group to mediate key biogeochemical processes in the oceans [1]. Previously, five type strains with validated names including M. hallyeonensis [2], M. geojedonensis [3], M. roseacus [4], and M. lacus [5] were described, and later Gaetbulicola byunsanensis [6] was reclassified as M. byunsanensis by Yoon et al. [7]. This genus also has a type strain M. roseacus invalidly published [4] (https://lpsn.dsmz.de/genus/marivita). Members of the genus Marivita were isolated from diverse marine environments in which M. cryptomonadis CL-SK44T were from culture of marine phytoplankton Cryptomonas sp. [1]. Mostly, the cells of members within this genus are rod-shaped, strictly aerobic, Gram-negative, non-motile, non-spore forming, and positive for both oxidase and catalase. The predominant fatty acid is C18:1ω7c, and major respiratory quinone is Q-10. Phosphatidylcholine, phosphatidylglycerol, and phosphatidylethanolamine are the main polar lipid profile of Marivita [1–6].
Phycosphere harbors inter-kingdom exchanges of diverse nutrients, infochemicals, and genetic transposable elements through dynamic algae–bacteria interactions (ABI) [7]. Those complex cross-kingdom associations occurs within this unique ecological niche are the keys to reveal biosynthesis mechanism of paralytic shellfish poisoning toxins (PSTs), also for the prevention and control of harmful algal blooms (HABs) [8]. Previously, during our Global Phycosphere Microbiome (GPM) Project to convey the microbial community structures of phycosphere microbiota (PM) derived from diverse marine HAB dinoflagellates which produce varied kinds of phycotoxins such as PSTs [9–13], a new white-colored bacterial strain, designated as LZ-15-2, was isolated from cultivable PM of Alexandrium catenella LZT09 which was sampled in the Zhoushan Archipelago area of East China Sea during an algal bloom in July of 2018, and produces highly toxic PSTs [12, 13]. The present study aims to clarify the taxonomic position of strain LZ-15-2 using combined polyphasic approaches along with comparative and phylogenomic analysis.
Materials and methods
Bacterial strains and culture conditions
Strain LZ-15-2 was isolated from highly toxic A. catenella LZT09 according to our previous procedures [9–13]. Colonies were isolated and purified using MA medium (Difco). The plates were incubated at 25 °C for 3 days. The six reference strains, M. cryptomonadis CL-SK44T (KCCM 90070T =DSM 21340T =JCM 15447T), M. litorea CL-JM1T (=KCCM 90071T =DSM 21329T =JCM 15446T), M. roseacus CB1052T (=ATCC BAA 1914T =DSM 21329T), M. byusanensis SMK-114T (=KCTC 22632T =CCUG 57612T =DSM 28225T =KCTC 22632T), M. geojedonensis DPG-138T (=KCTC 23882T =CCUG 62112T =DSM 29432T), and M. hallyeonensis DPG-28T (=KCTC 23421T =CCUG 60522T =DSM 29431T) purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) were selected for comparative purposes. All the strains were grown in the same condition for the experiments.
Morphology, physiology, and biochemical analysis
Cell morphology was examined by transmission electron microscopy (JEOL, JEM1010). The Gram reaction was determined using the Gram-staining Kit (bioMérieux, Shanghai, China) according to the manufacturer’s instructions. Oxidase and catalase activities were examined by the addition of 1% (w/v) tetramethyl-p-phenylene diamine and 3% (w/v) H2O2 solution, respectively [14]. Growth was tested at a range of different pHs using marine broth (MB) with varying pH values (4–10, 0.5 pH intervals) and different salt concentrations (0–10% [w/v, %], 1% intervals) at 25 °C. The pH was adjusted using 0.1 M HCl/NaOH. Growth at different temperatures (4, 10, 15, 20, 25, 28, 30, 32, 35, 37, 40, 45, and 50 °C) was assessed on marine agar (MA) plates for 3 days. The enzyme activities and carbon utilization were measured using API ZYM, API 20NE, and API 50CH Kits (bioMérieux), respectively, according to the manufacturer’s instructions.
Phylogenetic analysis
The genomic DNA was extracted with Ezup Bacterial Genomic DNA Extraction Kit (Sangon, Shanghai, China) according to the manufacturer’s instructions. PCR amplification of 16S rRNA gene was performed as previously described by Yang et al. [15] using the bacterial universal primer 27F/1492R. The identification of phylogenetic neighbors and calculation of pairwise 16S rRNA gene sequence similarity were performed using EzBioCloud online server (http://www.ezbiocloud.net) and NCBI database (www.ncbi.nlm.nih.gov). Multiple alignments were performed with the CLUSTAL_W program [16]. Phylogenetic trees were constructed using the neighbor joining (NJ), maximum-parsimony (MP), and maximum-likelihood (ML) algorithms by MEGA 7.0 software [17]. The constancy of the groupings was estimated by bootstrap analysis based on 1000 replicates.
Chemotaxonomic characterization
Respiratory quinones were extracted with Sep-Pak Vac cartridges (Waters, USA) and menaquinones were analyzed by high performance lipid chromatography (HPLC) based on previous methods [18]. To identify fatty acids, cells were incubated in MB for 3 days at 25 °C. Fatty acids were purified by saponification, methylation, and extraction according to the procedures described previously [19]. The fatty acid methyl esters (FAME) were identified using the Sherlock Microbial Identification System version 6.1 (MIS, MIDI; database: TSBA 6.0). Polar lipids were extracted and separated using two-dimensional thin layer chromatography (TLC) using silica gel plates (10×10 cm, Merck 5554), and the different spots were observed by spraying with appropriate detection reagents as described previously [20].
Genome sequencing, assembly, and annotation
Genomic DNA (gDNA) of strain LZ-15-2 was sequenced using the Illumina HiSeq 4000 platform (Illumina) following the manufacturer’s protocols [10–13]. Genome assembly of the raw sequence data generated was performed with SOAPdenovo 2 [21]. The estimate of genome completeness and contamination was performed using checkM software [22]. The genome was then processed using RNAmmer and tRNAscan-SE [23] for RNA gene prediction. Model genes were predicted in assembled genome using GLIMMER 3.0 [24]. The predicted coding sequences (CDSs) were then translated into protein sequence and submitted to COG (Clusters of Orthologous Groups) database to generate the function category and summary statistics [25]. For pathway analysis, the predicted ORFs were uploaded to KEGG Automatic Annotation Server (KAAS) for alignment with the KEGG database. The GENES data for alignment was set to “for prokaryotes” and the assignment method as “bi-directional best hit”. Genome annotation was also performed on the web tools RAST [26].
Comparative genomics
For genomic comparison, the available genomes of six member of Marivita, including three type strains, M. cryptomonadis CL-SK44T (JFKD00000000), M. geojedonensis DPG-138T (JFKC00000000), and M. hallyeonensis DPG-28T (FQXC00000000), and three non-type ones, M. geojedonensis strain DSM 29432 (PVTN00000000), Marivita sp. strain CAU 1492 (VCPC00000000), and strain XM-24bin2 (QCWH000000000) were selected. For each selected genome, complete genome sequence, proteome sequences, and Orfeome sequences were retrieved from NCBI. Pan-core genome analysis was performed using Bacterial Pan Genomes Analysis Pipeline (BPGA) [27]. Then, the function analysis of core, accessory, and unique sequences were annotated by EggNOG. The shared gene-clusters between strain LZ-15-2 and other Marivita members were identified by Orthovenn 2 [28]. The digital DNA–DNA hybridization (dDDH) was conducted using genome-to-genome distance calculator (GGDC) [27]. The fasta files were downloaded to the GGDC 2.1 web-server (https://ggdc.dsmz.de/ggdc.php), and dDDH was calculated using Formula 2. The Orthologous Average Nucleotide Identity (OrthoANI) was calculated using Orthologous ANI Tool (OAT) [29].
Results and discussion
Morphology, physiology, and biochemical analysis
The colonies of strain LZ-15-2 were observed convex, smooth, circular, and white colored after 3-day growth at 25 °C on MA. Cells are Gram-negative, non-motile, non-spore forming, rod-shaped, and approximately 0.98–1.22 μm wide and 1.71–3.04 μm long (Fig. 1). Growth occurs at 15–37 °C (optimum, 25 °C), pH 6.0–9.0 (optimum, 7.5) and with 1–7.0% (w/v) NaCl (optimum, 4.0%). No growth was observed in the presence of NaCl (10%) in MB. The strain was positive for oxidase and catalase. Phenotypic characteristics of strain LZ-15-2 are shown in Table 1 with species description and comparison with those of the type strains of the genus Marivita.
Fig. 1.
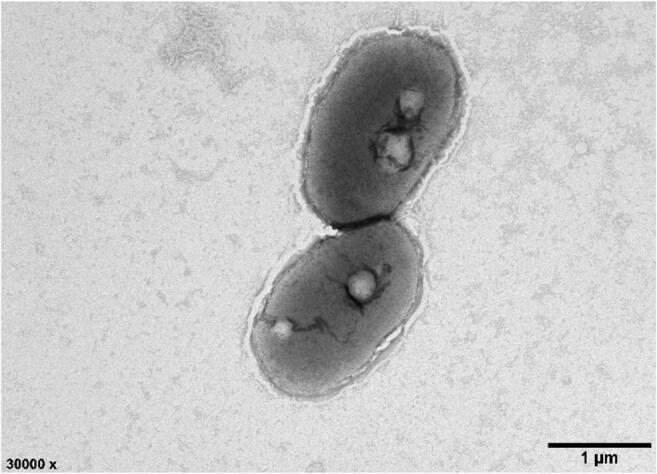
Transmission electron micrograph of the cells of strain LZ-15-2
Table 1.
Comparison of phenotypic characteristics of strain LZ-15-2 and other type strains within the genus Marivita
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Isolation source | Toxic marine dinoflagellate phycosphere | Marine phytoplankton | Seawater | Temperate estuary | Tidal flat sediment | Seawater | Seawater |
| Cell shape | Rod-shaped | Rod-shaped | Rod-shaped | Rod-shaped | Pleomorphic | Rod-shaped | Rod-shaped |
| Poly-β-hydroxybutyrate | + | + | + | − | − | + | + |
| Cell size (μm) | |||||||
| Length | 1.71–3.04 | 1.9–3.5 a | 1.0–3.5 a | 3–15 b | 1.5–15.0 c | 0.8–8.0 d | 1.0–8.0 e |
| Width | 0.98–1.22 | 0.4–1.2 a | 0.3–0.9 a | 0.5 b | 1.0–3.0 c | 0.4–1.3 d | 0.4–1.3 e |
| Motility | − | + | + | − | − | − | − |
| Growth | |||||||
| Temperature range (optimum, °C) | 15–37 (25) | 15–35 (30) | 15–33 (30) | 10–30 (28) | 10–40 (25–30) | 10–40 (30) | 10–40 (30) |
| pH range | 6–9 (7.5) | 6–10 (7–9) | 6–10 (7–8) | 6.5–9 (7–8) | 5.5–9.5 (7–8) | 6–9.5 (7–7.5) | 6–9.5 (7–7.5) |
| NaCl range (w/v, %) | 1–7 (4) | 2–10 (3–5) | 1–10 (3–5) | 0.5–6 (2–3) | 1–5 (2) | 0.5–9 (2–3) | 0.5–8 (2) |
| Oxygen requirement | Strictly aerobic | Strictly aerobic | Strictly aerobic | Strictly aerobic | Strictly aerobic | Aerobic | Aerobic |
| API 20NE tests | |||||||
| Nitrate reduction | + | − | − | − | + | + | + |
| Aesculin hydrolysis | + | + | + | - | − | + | + |
| Gelatinase | − | + | − | − | − | − | |
| Β-Galactosidase | + | + | + | − | − | − | − |
| Assimilation of glucose | − | + | − | − | − | − | |
| Arabinose | − | + | − | − | − | − | − |
| API ZYM tests | |||||||
| Alkaline phosphatase | + | + | w | w | − | + | − |
| Esterase (C4) | + | w | w | − | − | + | w |
| Esterase lipase (C8) | + | w | + | − | − | + | − |
| Leucine arylamidase | + | + | + | − | − | + | w |
| Valine arylamidase | − | w | w | w | − | − | w |
| Acid phosphatase | + | + | w | − | − | + | − |
| Naphthol-AS-BI-phosphohydrolase | + | w | w | − | w | + | − |
| API 50 CH tests | |||||||
| D-Trehalose | − | + | − | − | − | − | − |
| D-Cellobiose | − | + | − | + | + | − | − |
| α-D-Lactose | − | + | − | − | − | − | − |
| N-Acetyl neuraminic acid | + | − | − | − | − | − | − |
| α-D-Glucose | − | + | − | + | + | − | − |
| D-Mannose | − | − | − | − | + | − | − |
| D-Galactose | − | + | − | + | − | − | − |
| Glycerol | − | − | − | + | − | − | − |
| Glucuronamide | w | − | − | − | − | − | − |
| Methyl pyruvate | − | − | − | − | + | − | − |
| Tween 40 | − | + | + | − | + | + | |
| G + C content (mol %) | 59.0 | 58.6a | 61a | 59.6b | 60c | 59.9d | 65.1e |
Strains: 1, LZ-15-2; 2, M. cryptomonadis CL-SK44T; 3, M. litorea CL-JM1T; 4, M. roseacus CB1052T; 5, M. byusanensis SMK-114T; 6, M. geojedonensis DPG-138T; 7, M. hallyeonensis DPG-28T
All data were obtained from this study unless otherwise indicated. +, positive; −, negative; w, weakly positive
Data from the original study: a, Hwang et al. [1]; b, Buinoff et al. [3]; c, Yoon et al. [6]; d, Yoon et al. [4]; e, Yoon et al. [2], respectively
Strain LZ-15-2 was positive for nitrate reduction to nitrite, β-galactosidase (PNPG), and aesculin hydrolysis, whereas negative for indole production (tryptophane), fermentation of glucose, arginine dihydrolase, urease, gelatinase, assimilation of glucose, arabinose, mannose, mannitol, N-acetyl- glucosamine, maltose, potassium gluconate, capric acid, adipic acid, malate, trisodium citrate, and phenylacetic acid. Enzyme activities were positive for alkaline phosphatase, esterase (C4), esterase lipase (C8) leucine arylamidase, acid phosphatase, and naphthol-AS-BI-phosphohydrolase, but negative for lipase (C14), valine arylamidase, cystine arylamidase, trypsin, α-chymotrypsin, α-galactosidase, β-galactosidase, β-glucuronidase, α-glucosidase, β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase activities. N-Acetyl-neuraminic acid and glucuronamide were available for strain LZ-15-2 as carbon sources for growth.
Phylogenetic analysis
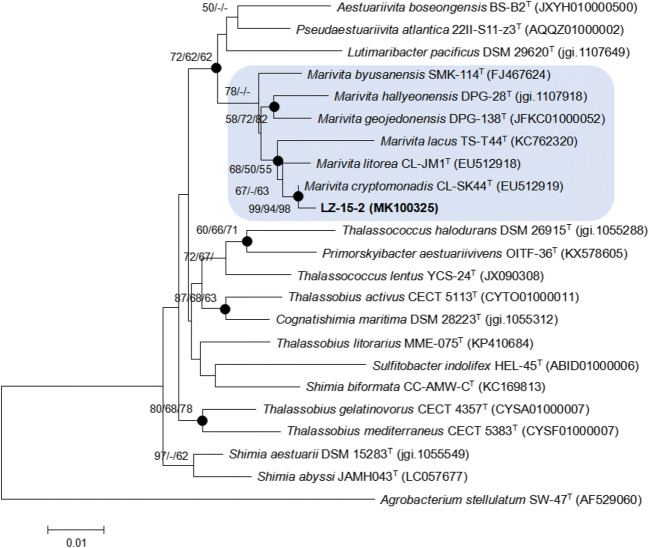
The length of the 16S rRNA gene sequence of strain LZ-15-2 is 1347 bp. The gene sequence of strain LZ-15-2 was compared with those of type strains within the genus Marivita and closely related taxa. Strain LZ-15-2 shared high 16S rRNA gene sequence similarities with M. cryptomonadis CL-SK44T (99.2% 16S rRNA gene similarity), M. litorea CL-JM1T (98.6%), M. roseacus CB1052T (97.4%), M. byusanensis SMK-114T (97.2%), M. geojedonensis DPG-138T (97.0%), and M. hallyeonensis DPG-28T (97.0%). Based on the constructed phylogenetic tree by neighbor-joining method (Fig. 2), strain LZ-15-2 clustered with M. cryptomonadis CL-SK44T within the genus Marivita. Other two phylogenetic trees based on maximum likelihood (Fig. S1) and maximum parsimony (Fig. S2) both supported this result. The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequence of strain LZ-15-2 is MK100325.
Fig. 2.
Neighbor-joining (NJ) tree showing the phylogenetic position of strain LZ-15-2 and related taxa based on 16S rRNA gene sequences. Accession numbers for the type strains are shown in parenthesis. Filled circles indicate nodes that were also recovered in maximum-parsimony (MP) tree and maximum-likelihood (ML) tree based on the same sequences. Bootstrap values (expressed as percentages of 1000 replications) ≥50% are shown at branching points for NJ/ML/MP trees. Agrobacterium stellulatum SW-47T is used as used as an outgroup
Chemotaxonomic characterization
The major cellular fatty acids of strain LZ-15-2 were determined as C18:1ω7c and C18:1ω7c-11-methyl (over 10%). The comparison of fatty acid profiles of strain LZ-15-2 and six reference type strains is given in Table 2. It can be seen that strain LZ-15-2 exhibited similar profile with other members of the genus Marivita, yet their proportions between strain LZ-15-2 and the close relates slightly differed (Table 2). The polar lipid profile of strain LZ-15-2 was diphosphatidylglycerol (DPG), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), unidentified aminolipid (AL), and two unidentified polar lipids (Ls) (Fig. S3). The predominant respiratory quinone of strain LZ-15-2 was Q-10. The results of these chemotaxonomic characteristics are consistent with other members of the genus Marivita [1–6].
Table 2.
Cellular fatty acid compositions of strains LZ-15-2 and type strains of the genus Marivita
| Fatty acids | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Saturated | |||||||
| C16:0 | 7.0 | 2.6 | 3.7 | 1.8 | 2.8 | 3.5 | 2.8 |
| C17:0 | 2.5 | 1.3 | 1.4 | − | 2.5 | 2.8 | 3 |
| C18:0 | 6.3 | 3.7 | 3.4 | 3.5 | 2.9 | 5.9 | 7.7 |
| Hydroxyl | |||||||
| C12:0 3-OH | 0.1 | 2.9 | 5.8 | − | − | − | − |
| C12:1 3-OH | 3.6 | − | 3.6 | 4.7 | 3.3 | − | 3.2 |
| Branched-chain | |||||||
| iso-C18:0 | − | − | − | 12.6 | − | − | − |
| C18:1 ω9c | 1.7 | − | − | − | − | − | − |
| C18:1 ω7c11-methyl | 12.3 | 11.4 | 9 | 5.6 | 9.8 | 18.6 | 10.5 |
| C18:1 ω7c | 62.9 | 73.5 | 76.1 | 68.8 | 75.3 | 65.6 | 69.9 |
| C18:1 ω9c | − | tr | 1.3 | − | 0.9 | 0.5 | 1.2 |
| C20:1 ω7c | 0.4 | − | − | − | − | − | − |
| cyclo-C19:0 ω8c | − | − | − | − | 1.5 | − | − |
| Summed feature* | |||||||
| 3 | 0.6 | tr | tr | − | − | − | − |
| 7 | 0.8 | tr | tr | − | 1.1 | 1.6 | 0.9 |
All data were obtained from this study unless otherwise specified. Strains: 1, LZ-15-2; 2, M. cryptomonadis CL-SK44T; 3, M. litorea CL-JM1T; 4, M. roseacus CB1052T; 5, M. byusanensis SMK-114T; 6, M. geojedonensis DPG-138T; 7, M. hallyeonensis DPG-28T. Values represent percentage of total fatty acids contents. −, not detected. tr, trace amounts (<1 %)
*Summed feature 3 contains C16:1 ω7c and/or C16:1 ω6c; summed feature 7 comprises C19:1ω7c/.846/19cy
Genome properties
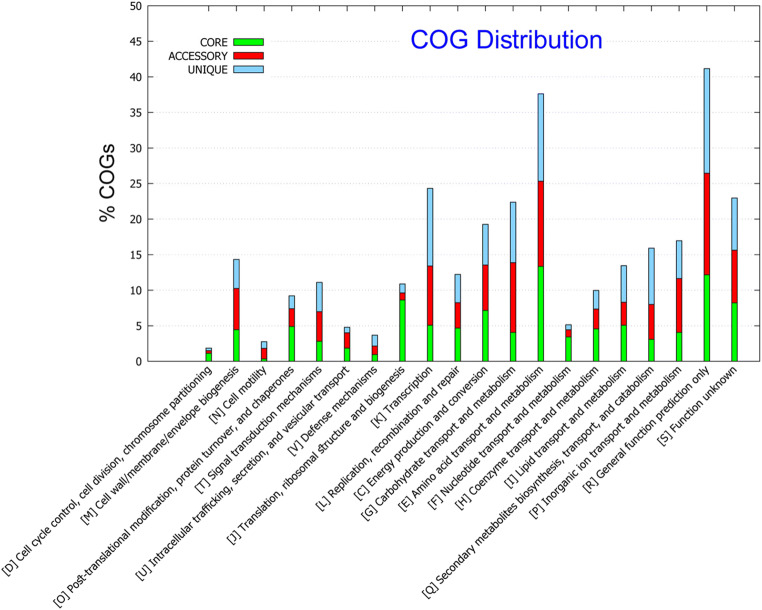
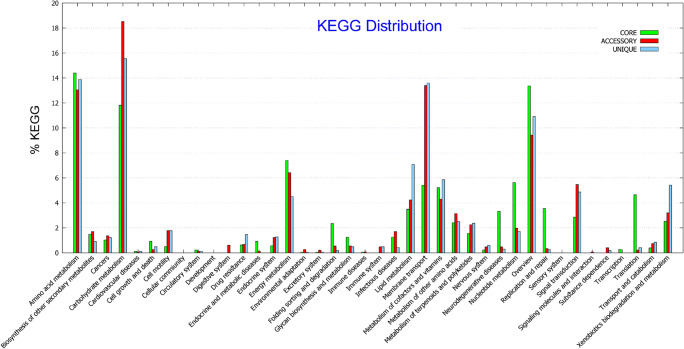
Genome sequence of strain LZ-15-2 was deposited in GenBank/EMBL/DDBJ under accession number SWKO00000000. The draft genome is 4,829,700 bp long with a 59% G+C content (Table 3). The estimates of genome completeness and contamination values were 98.72% and 1.23%, respectively. It is composed of 65 contigs. The genome has 4,782 predicted genes, including 4,643 protein-coding genes, 52 RNAs genes (6 rRNA, 43 tRNA, and 3 other RNA) and 87 pseudogenes. Among the protein-coding genes, 3,510 genes (75.6%) were functionally annotated within the COG database, indicating that a large part of the genome is dominant. However, only 2,231 genes (48.1%) were annotated by KEGG metabolic pathways, indicating that a number of functional genes were still indistinct. The properties and statistics of genes into the COG functional category and KEGG classification are shown in Fig. S4 and Fig. S5. The most abundant CDS in the genome of strain LZ-15-2 are general function (R), followed by genes dedicated to amino acid transport and metabolism (COG category E) and Function unknown (S) group. These two categories were also presented in higher proportions in the genomes of other Marivita members (Table S1). These findings also indicted that considerable portion of the genes still needs to be further discovered for revealing their functions. In KEGG pathway, metabolism occupies as the dominant proportion (Fig. S4). Furthermore, amino acid metabolism and carbohydrate metabolism were two prominent groups. These results obtained by KEGG classification were also verified and in accordance with the results by COG category analysis (Fig. S5).
Table 3.
General genomic features of seven Marivita strains with available genomes
| Attributes | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Genome size (Mb) | 4.83 | 4.61 | 4.31 | 4.33 | 4.19 | 4.63 | 4.43 |
| Number of scaffolds | 63 | 115 | 73 | 67 | 20 | 15 | 117 |
| Number of contigs | 65 | 115 | 73 | 67 | 20 | 17 | 220 |
| N50 length of contigs (bp) | 380,371 | 196,262 | 125,395 | 125,109 | 726,303 | 1,177,115 | 39,056 |
| G+C content (mol%) | 59 | 58.6 | 60.0 | 59.9 | 65.1 | 63.1 | 57.9 |
| Predicted genes | 4,833 | 4,518 | 4,224 | 4,275 | 4,137 | 4,426 | 4,375 |
| Protein-coding genes | 4,765 | 4,380 | 4,115 | 4,157 | 4,046 | 4,310 | 4,236 |
| RNA genes | 52 | 57 | 49 | 49 | 49 | 50 | 43 |
| tRNA genes | 43 | 45 | 43 | 43 | 42 | 42 | 39 |
| rRNA genes | 6 | 9 | 3 | 3 | 4 | 5 | 1 |
| Other RNA genes | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Pseudogenes | 87 | 81 | 60 | 69 | 42 | 66 | 96 |
| Genome accession no. | SWKO00000000 | JFKD00000000 | JFKC00000000 | PVTN00000000 | FQXC00000000 | VCPC00000000 | QCWH000000000 |
Strains: 1, LZ-15-2; 2, M. cryptomonadis CL-SK44T; 3, M. geojedonensis DPG-138T; 4, M. geojedonensis DSM 29431; 5, M. hallyeonensis DPG-28T; 6, Marivita sp. CAU 1492; 7, Marivita sp. XM-24bin2
Comparative genomics profile of Marivita members
The genome size of strain LZ-15-2 is the largest among the genomes that are currently available for Marivita members, while M. hallyeonensis DPG-28T has the smallest (Table 3). The difference in the number of genes between strain LZ-15-2 and M. hallyeonensis DPG-28T was more prominent in the metabolism-related genes, particularly inorganic ion and amino acid transport and metabolism (Table S1). By contrast, a smaller genome of M. hallyeonensis DPG-28T may correlate to tight packing of its genome as a means for reproductive efficiency against stress environments [29]. The genetic difference within the members of the genus Marivita can be further determined from the distribution of the core (conserved), accessory (dispensable), and unique (species) genes (Fig. 3). In addition, the COG functional category and KEGG pathway of core, accessory, and unique genes are displayed in Fig. 4 and Fig. 5. The most abundant group represented in the core genes was amino acid transport and metabolism (COG category E), followed by general function (R) and function unknown (S). Nevertheless, the accessory and unique genes devoted to amino acid metabolism, carbohydrate transport, and metabolism (COG category E and G) were in consistent with the results obtained by KEGG category as shown in Fig. 5. It clearly indicated the genetic difference presented among Marivita members.
Fig. 3.
Venn diagram of the distribution patterns of the core, accessory, and unique genes in the genomes of seven bacterial strains within the genus Marivita
Fig. 4.
Functional distributions of the core, accessory, and unique genes of Marivita members based on the COG assignments
Fig. 5.
Functional distributions of the core, accessory, and unique genes of Marivita members based on the KEGG pathway
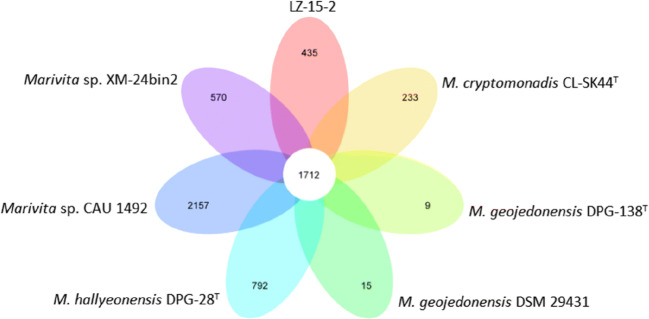
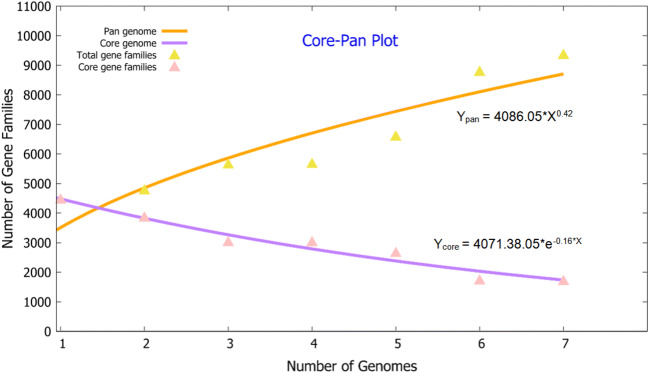
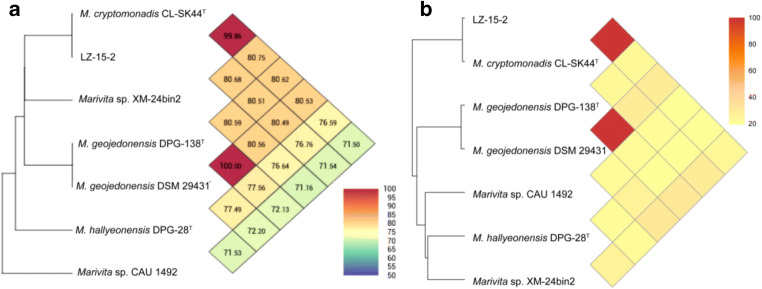
The pan-core genome of the Marivita species comprised 9,361 and 1,712 genes, respectively (Fig. 6). This considerable number of core genes may be an indication for their involvement in metabolic mechanisms for survival [30]. Based on calculation of nucleus–universal genome fitting curve (Table S2), it can be concluded that the universal genome of this species is open, meaning that in different environments, the species can exchange genetic material with other species in many ways to acquire new genes [25]. To analyze small genomic differences among Marivita members, the core-phylogenomic tree was constructed and shown in Fig. 7. It can be seen that an adjacent similarity between strain LZ-15-2 and M. cryptomonadis CL-SK44T was observed, while demonstrating a high variance with Marivita sp. strain CAU 1492. This finding was in accordance with the heatmap generated with OrthoANI value calculation among Marivita members (Fig. 8). The Venn diagram for the number of shared and unique orthologous gene clusters between strain LZ-15-2 and other Marivita members is displayed in Fig. 9. It indicated that the number of shared gene clusters between each pair of species was highly variable. Maximum clusters were shared between the genomes of strains LZ-15-2 and M. cryptomonadis CL-SK44T (3,925 shared clusters), further verifying the high similarity between the two strains. An insight into the pan-genome of Marivita members suggested that the third abundant genes were functionally unknown group (Fig. 9). Furthermore, the majority of these genes were identified to encode hypothetical proteins, indicating that functions of large sections of these ortholog clusters in Marivita genomes still remain to be further determined. In addition, specific genes occupy the largest numbers in metabolism, indicating that specific genes may play a crucial role in environmental adaptation [31]. Besides, unique genes in environmental information processing ranking only second to metabolism further confirm this point. The ANI and dDDH values between strain LZ-15-2 with other Marivita members ranged from 71.5 to 99.8% (panel a, Fig. 8), and 19.1 to 98.8% (panel b, Fig. 8), respectively. The maximum values are higher than the threshold values (ANI, 95–96%; dDDH, 70%) recommended to delineate prokaryotic species [32, 33].
Fig. 6.
The distribution of pan-core genes of the Marivita members
Strains: 1, LZ-15-2; 2, M. cryptomonadis CL-SK44T; 3, M. geojedonensis DPG-138T; 4, M. geojedonensis DSM 29432; 5, M. hallyeonensis DPG-28T; 6, Marivita sp. CAU 1492; 7, Marivita sp. XM-24bin2
Fig. 7.
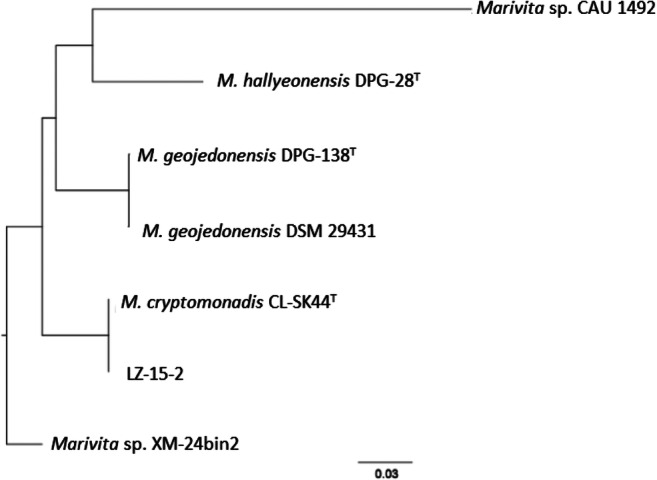
Phylogenomic tree constructed based on the core genes using seven genomes of Marivita members
Fig. 8.
Heatmaps of comparison of ANI (Pane A) and dDDH (Pane B) values between strain LZ-15-2 and others Marivita members Strains: 1, LZ-15-2; 2, M. cryptomonadis CL-SK44T; 3, M. geojedonensis DPG-138T; 4, M. geojedonensis DSM 29431; 5, M. hallyeonensis DPG-28T; 6, Marivita sp. CAU 1492; 7, Marivita sp. XM-24bin2
Fig. 9.
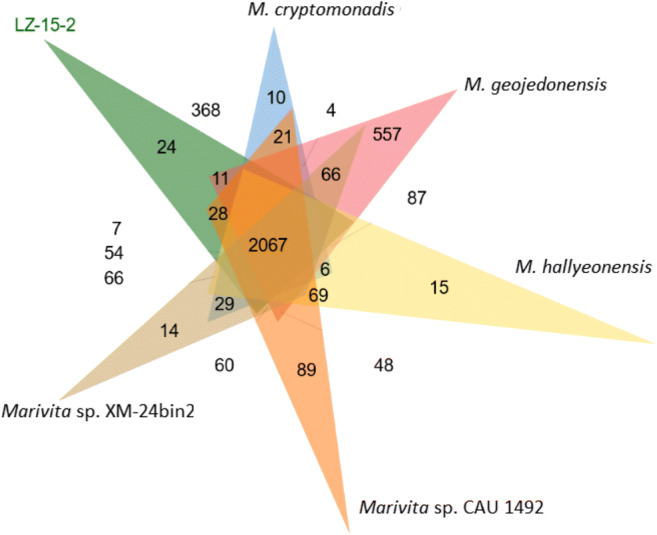
Venn diagram of the shared and unique orthologs between strain LZ-15-2 and others Marivita members with available genomes
In summary, based on combined characterizations by phenotypic, chemotaxonomic, phylogenetic analysis, and genome comparison, strain LZ-15-2 is a new member of M. cryptomonadis. The new isolate is a novel potential candidate for further exploration of the detailed mechanisms governing the dynamic cross-kingdom associations between the PM and their algal host.
Supplementary Information
(DOCX 651 kb)
Funding
This work was supported by the National Natural Science Foundation of China (41876114), NSF of Zhejiang Province (LY18D060007), Guangzhou Municipal Science and Technology Bureau (201804010338), and Presidential Foundation of Guangdong Academy of Agricultural Sciences (201723).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Accession numbers: The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence and the draft genome sequence of strain LZ-15-2 are MK100325 and SWKO00000000, respectively.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Zhou and Xiao-Ai Zhang contributed equally to this work.
References
- 1.Hwang CY, Bae GD, Yih W, Cho BC. Marivita cryptomonadis gen. nov., sp. nov. and Marivita litorea sp. nov., of the family Rhodobacteraceae, isolated from marine habitats. Int J Syst Evol Microbiol. 2009;59:1568–1575. doi: 10.1099/ijs.0.005462-0. [DOI] [PubMed] [Google Scholar]
- 2.Yoon JH, Kang SJ, Lee SY, Jung YT, Lee JS, Oh TK. Marivita hallyeonensis sp. nov., isolated from seawater, reclassification of Gaetbulicola byunsanensis as Marivita byunsanensis comb. nov. and emended description of the genus Marivita Hwang et al. 2009. Int J Syst Evol Microbiol. 2012;62:839–843. doi: 10.1099/ijs.0.032086-0. [DOI] [PubMed] [Google Scholar]
- 3.Budinoff CR, Dunlap JR, Hadden M, et al. Marivita roseacus sp. nov. of the family Rhodobacteraceae, isolated from a temperate estuary and an emended description of the genus Marivita. J Gen Appl Microbiol. 2011;57(5):259–267. doi: 10.2323/jgam.57.259. [DOI] [PubMed] [Google Scholar]
- 4.Yoon JH, Kang SJ, Lee JS. Marivita geojedonensis sp. nov. isolated from seawater. Int J Syst Evol Microbiol. 2013;63:423–427. doi: 10.1099/ijs.0.039065-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhong ZP, Liu Y, Hou TT, Liu HC, Zhou YG, Wang F, Liu ZP. Marivita lacus sp. nov., isolated from a saline lake. Int J Syst Evol Microbiol. 2015;65:1889–1894. doi: 10.1099/ijs.0.000195. [DOI] [PubMed] [Google Scholar]
- 6.Yoon JH, Kang SJ, Jung YT, Oh TK. Gaetbulicola byunsanensis gen. nov., sp. nov., isolated from tidal flat sediment. Int J Syst Evol Microbiol. 2010;60:196–199. doi: 10.1099/ijs.0.011015-0. [DOI] [PubMed] [Google Scholar]
- 7.Ramanan R, Kim BH, Cho DH, Oh HM, Kim HS. Algae-bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv. 2016;34(1):14–29. doi: 10.1016/j.biotechadv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Yang J, Ge Y, et al. Acute effects of CH3NH3PbI3 perovskite on Scenedesmus obliquus and Daphnia magana in aquatic environment. Ecotoxicol Environ Saf. 2021;28:111677. doi: 10.1016/j.ecoenv.2020.111677. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Ma L, Tian X, Huang H, Yang Q. Biodiversity study of intracellular bacteria closely associated with paralytic shellfish poisoning dinoflagellates Alexandrium tamarense and A. minutum. Int J Environ Resour. 2015;4:23–27. doi: 10.14355/ijer.2015.04.004. [DOI] [Google Scholar]
- 10.Duan Y, Jiang Z, Wu Z, Sheng Z, Yang X, Sun J, Zhang X, Yang Q, Yu X, Yan J. Limnobacter alexandrii sp. nov., a thiosulfate-oxidizing, heterotrophic and EPS-bearing Burkholderiaceae isolated from cultivable phycosphere microbiota of toxic Alexandrium catenella LZT09. Antonie Van Leeuwenhoek. 2020;113:1689–1698. doi: 10.1007/s10482-020-01473-8. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Jiang Z, Zhou X, Zhang R, Wu Y, Lou L, Ma Z, Wang D, Ge Y, Zhang X, Yu X. Nioella ostreopsis sp. nov., isolated from toxic dinoflagellate Ostreopsis lenticularis. Int J Syst Evol Microbiol. 2020;70:759–765. doi: 10.1099/ijsem.0.003816. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Jiang Z, Zhou X, Xie Z, Wang Y, Wang D, Feng L, Yang G, Ge Y, Zhang X. Saccharospirillum alexandrii sp. nov., isolated from the toxigenic marine dinoflagellate Alexandrium catenella LZT09. Int J Syst Evol Microbiol. 2020;70:820–826. doi: 10.1099/ijsem.0.003832. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Jiang Z, Zhou X, Zhang R, Xie Z, Zhang S, Wu Y, Ge Y, Zhang X. Haliea alexandrii sp. nov., isolated from phycosphere microbiota of the toxin-producing dinoflagellate Alexandrium catenella. Int J Syst Evol Microbiol. 2020;70:1133–1138. doi: 10.1099/ijsem.0.003890. [DOI] [PubMed] [Google Scholar]
- 14.Perin AP, Martins BTF, Barreiros MAB, Yamatogi RS, Nero LA, dos Santos Bersot L. Occurrence, quantification, pulse types, and antimicrobial susceptibility of Salmonella sp. isolated from chicken meat in the state of Paraná, Brazil. Braz J Microbiol. 2020;51:335–345. doi: 10.1007/s42770-019-00188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Jiang Z, Zhang J, Zhou X, Zhang X, Wang L, Yu T, Wang Z, Bei J, Dong B, Dai Z, Yang Q, Chen Z. Mesorhizobium alexandrii sp. nov. isolated from phycosphere microbiota of PSTs-producing marine dinoflagellate Alexandrium minutum amtk4. Antonie Van Leeuwenhoek. 2020;113:907–917. doi: 10.1007/s10482-020-01400-x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882 [DOI] [PMC free article] [PubMed]
- 17.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraishi A, Ueda Y, Ishihara J, et al. Comparative lipoquinone analysis of influent sewage and activated sludge by high performance liquid chromatography and photodiode array detection. J Gen Appl Microbiol. 2006;42:457–469. doi: 10.2323/jgam.42.457. [DOI] [Google Scholar]
- 19.Yang Q, Jiang Z, Huang C, et al. Hoeflea prorocentri sp. nov., isolated from a culture of the marine dinoflagellate Prorocentrum mexicanum PM01. Antonie Van Leeuwenhoek. 2018;111(10):1845–1853. doi: 10.1007/s10482-018-1074-0. [DOI] [PubMed] [Google Scholar]
- 20.Vizzotto CS, Peixoto J, Green SJ et al (2020) Muricauda brasiliensis sp. nov., isolated from a mat-forming cyanobacterial culture. Braz J Microbiol. 10.1007/s42770-020-00400-3 [DOI] [PMC free article] [PubMed]
- 21.Zhang X, Yang X, Wang S, et al. Draft genome sequences of nine cultivable heterotrophic proteobacteria isolated from phycosphere microbiota of toxic Alexandrium catenella LZT09. Microbiol Resour Announc. 2020;9:e00281–e00220. doi: 10.1128/MRA.00281-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks DH, Imelfort M, Skennerton CT, et al. Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2014;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:0955–0964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delcher AL, Harmon D, Kasuf S, et al. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27:4636–4641. doi: 10.1093/nar/27.23.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kremer FS, Teodoro DSJI, Guimarães, et al. Genome sequence of Xanthomonas fuscans subsp. fuscans strain Xff49: a new isolate obtained from common beans in Southern Brazil. Braz J Microbiol. 2019;50:357–367. doi: 10.1007/s42770-019-00050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhari NM, Gupta VK, Dutta C (2016) BPGA-an ultra-fast pan-genome analysis pipeline. Sci Rep 6:24373 [DOI] [PMC free article] [PubMed]
- 28.Yi W, Devin CD, Guoping C, et al. Orthovenn: a web server for genome wide comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2015;43(W1):W78–W84. doi: 10.1093/nar/gkv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I, Ouk KY, Park SC, et al. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 30.Jiao JY, Salam N, Liu L, Rao MPN, Zhang XT, Fang BZ, Han MX, Zhang ZT, Chen J, Zhao J, Zhou Y, Alkhalifah DHM, Liu Q, Xiao M, Klenk HP, Li WJ. Genome sequence and comparative analysis of Jiangella alba YIM 61503T isolated from a medicinal plant Maytenus austroyunnanensis. Antonie Van Leeuwenhoek. 2018;111:667–678. doi: 10.1007/s10482-017-1010-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun R, Tu Z, Fan L, Qiao Z, Liu X, Hu S, Zheng G, Wu Y, Wang R, Mi X. The correlation analyses of bacterial community composition and spatial factors between freshwater and sediment in Poyang Lake wetland by using artificial neural network (ANN) modeling. Braz J Microbiol. 2020;51:1191–1207. doi: 10.1007/s42770-020-00285-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu XW, de Meyer S, Trujillo ME. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 33.Alves KJ, da Silva MCP, Cotta SR, Ottoni JR, van Elsas JD, de Oliveira VM, Andreote FD. Mangrove soil as a source for novel xylanase and amylase as determined by cultivation-dependent and cultivation-independent methods. Braz J Microbiol. 2020;51:217–228. doi: 10.1007/s42770-019-00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 651 kb)