Abstract
Nowadays when conventional plastic is being looked as a menace, the possibility of it being replaced with polyhydroxyalkanoates (PHAs) which are biodegradable, environment friendly and biocompatible thermoplastics is not remote. PHAs are a fascinating group of biopolyesters stored within the cytoplasm of numerous bacterial cells as energy and carbon reserves. PHAs signify the best promising biological substitute to certain conventional petrochemical plastics which have wide range of applications in different industries such as biomedical sector, packaging, toners for printing, and adhesives for coating, etc. In the present study, PHAs producing bacterial strains were screened by Sudan black B staining and confirmed by Nile blue A staining. Out of forty bacterial strains showing positive results, six bacterial strains exhibited comparatively higher PHAs production. The highest PHAs producing bacterial strain was identified using 16s rRNA sequencing. Optimization of process parameters was performed by using one factor at a time (OFAT) approach. The isolated bacterium was able to synthesize PHAs when various agro-industrial wastes such as domestic kitchen waste, mixed fruit pulp, sugarcane molasses, and waste flour from bread factory were screened as a carbon substrate in the growth medium. The results showed accumulation of 44.5% PHAs of cell dry weight using domestic kitchen waste as carbon substrate. The characterization of biopolymers was performed using FTIR and XRD analysis. The commercial exploitation of results of this study may serve twin purposes of addressing the challenge of high production cost of PHAs being the major constraint in replacing petro-based plastics as well as address the problem of disposal of recurring domestic kitchen waste and other agro-industrial waste.
Keywords: Agro-industrial waste, Bacillus tropicus, Bio-plastics, Domestic kitchen waste, Polyhydroxyalkanoates
Introduction
Petroleum-based conventional plastic materials play inevitable role in every sphere of human life. However, these recalcitrant plastics are a matter of great concern as they are non-degradable rendering their disposal difficult which has led to mammoth accumulation of synthetic plastics [1, 2]. PHAs are regarded as green plastics and have positive social and environmental impact as compared to conventional plastics. Therefore, the concept of biodegradable plastic which could be produced and degraded naturally by microorganisms evolved as an effective alternative [3].
The PHAs are microbially produced polyoxoesters which possess properties similar to various synthetic plastics like polypropylene [4, 5]. There are evidences that PHAs have the required potential to replace some of the today’s petro-plastics, owing to their inherent biodegradability, thermo-plasticity, biocompatibility [6], and mechanical properties like flexibility, elasticity, and versatility [7–9]. The PHAs Market Research Report, 2019, indicated that by the year 2024, the market prospect for PHAs is expected to reach nearly USD 98 million [10].
In general, synthesis of PHAs takes place under unfavorable growth conditions and imbalanced nutrient supply. It implies that on one hand, there is abundant availability of carbon source in the surrounding medium for the microorganism, but on the other hand, there is limited supply of other growth essential elements like nitrogen, phosphorous, dissolved oxygen, or certain micro-components like sulfur or certain metals with essential functions in cell growth metabolism. These circumstances induce PHA synthesis as carbon and energy reserves in the cells [11].
Many Gram-positive as well as Gram-negative bacteria possess the ability to synthesize PHAs as energy and carbon reserve material under nutrient limiting conditions in the presence of excess carbon [12, 13]. Numerous bacteria including Alcaligenes, Bacillus, Pseudomonas, recombinant Escherichia coli, and Methylotrophs accumulate PHAs in their cytoplasm at a high productivity rate.
The most commonly produced PHAs by microbes are polyhydroxybutyrate (PHB). Apart from this, microbes also synthesize polyhydroxyvalerate and various copolymers like P(HB-co-HV) [14]. These biopolymers have miscellaneous applications such as domestic plastic commodities, packaging, skin and tissue implants, 3D printing in various photographic materials, drugs, drug coating, nutritional dietary supplements, and small chemical constituents [15]. Owing to their biocompatibility, PHAs also have extensive applications in drug delivery and tissue engineering [6, 16].
Although PHAs accumulating bacteria have high potential for commercial manufacturing of bio-plastics, high cost of raw materials used in the process is a hurdle [17]. To reduce the manufacturing cost of PHA bio-plastics, use of cheaper and renewable agro-industrial by-products as carbon substrates may be an asset. Many different organic wastes from food industry, agricultural sector, municipal waste, and waste water as carbon substrate feed have been studied [18].
Also, selection of high PHAs producing bacterial strain along with optimized culture conditions can remove the constraints in large-scale economic production biosynthesis. Although the industrial production of this bio-plastic commenced decades ago, its large-scale production is still debilitated by factors such as rate of production by the bacteria and the raw materials used for its production, which finally escalates the production cost of PHAs [3, 19, 20].
Henceforth, the objective of the present study was to isolate bacteria capable of accumulating PHAs. For enhanced PHAs production, nutritional and physical parameters of culture conditions were optimized. One factor at a time approach was used to study the effects of different carbon and nitrogen sources in media along with physical parameters like pH and temperature. The data obtained were statistically analyzed using ANOVA single factor and Tukey post hoc test. The highest PHAs producing isolate was checked for its ability to utilize different agro-industrial wastes as carbon substrate for the cost-effective fermentative production of PHAs. The low-cost renewable agro-industrial wastes used were domestic kitchen waste, sugarcane molasses, waste flour from bread factory, and mixed fruit pulp from fruit juice shops. The use of agro-industrial waste may serve two purposes, one being the disposal of abundant surplus agricultural and industrial waste materials and another being economic utilization of such waste by its conversion into sustainable high-valued bio-plastics. The extracted biopolymer was confirmed using crotonic acid assay, and its characterization was done using FTIR and XRD technique.
Materials and methods
Sampling and isolation of bacteria
All the media components, chemicals, and salts used were of higher quality analytical grade, purchased from Hi-media, India. The biopolymer PHAs (Goodfellow Cambridge limited, England) was purchased from Sigma-Aldrich, USA. Soil samples were collected aseptically in clean zip lock polybags from five different sites in Indore City. These sites were truck parking site having leaked oil in Transport Nagar area; plastic garbage dumping site at Bypass Road, Lasudia; plastic enriched site near Khajrana Lake; Auto garage oil-contaminated site at Palasia and garbage-dumping site near Palasia. Isolation of bacterial isolates was carried out by using serial dilution plating method. Five- to sevenfold dilution range was used for isolation purpose. A 0.1 ml volume of each dilution was spread on carbon-enriched nutrient agar plates. The composition of carbon-enriched nutrient agar media was 6.0g of Na2HPO4, 3.0g KH2PO4, 1.0g NH4Cl, 0.5g NaCl, 0.05g yeast extract, 17g agar-agar, and 10g glucose per liter. The pH was adjusted to 7.0. After streaking, plates were kept for incubation at 37oC for 24 h [21].
Screening of PHAs producing bacteria
After 24 h of incubation, PHAs producing bacterial colonies were primarily screened by pouring 0.02% alcoholic solution of Sudan black B onto the petri plates. The plates were kept undisturbed for 20 min. The excess dye from the plates was carefully decanted without disturbing the bacterial colonies. Then, the plates were rinsed gently by adding absolute ethanol. The colonies that had ability to produce PHAs incorporated Sudan black B and appeared bluish black. The remaining colonies which were not PHA producers appeared white [22]. After the primary screening, Sudan black B-positive isolates were further confirmed by viable colony method using Nile blue A stain [23]. The Sudan black B-positive bacterial isolates were streaked in different petri plates containing carbon rich (1% glucose) nutrient agar along with Nile blue A stain at concentrations of 0.5 μg/ml. Nile blue A stain ensures a powerful discrimination between PHA-negative and PHA-positive strains. The PHAs accumulating colonies fluoresce bright orange color after Nile blue A staining on irradiation with UV light and their fluorescence intensity increased with the increase in PHA contents of the bacterial cells. The isolates which showed bright orange fluorescence on irradiation with UV light after Nile blue A staining were selected as PHA accumulators [22]. Thereafter, PHAs accumulating bacteria were streaked to obtain pure culture.
Culture inoculation and bacterial growth
For inoculum preparation, 100 ml of sterile nutrient broth containing 1% glucose was used. The composition of nutrient broth was as follows (g/l): peptone 5.0, sodium chloride 5.0, beef extract 1.5, and yeast extract 1.5. To prepare 100 ml of nutrient broth, 1.3 g of nutrient broth powder (purchased from Hi-Media, India) was suspended in 100 ml distilled water. One percent of glucose powder was also added to it. Final pH was adjusted to 7.0±0.2 and, thereafter, autoclaved. The autoclaved froth was inoculated using a loop full of pure culture and allowed to incubate overnight at 37oC with shaking at 120 rpm speed in an incubator shaker. This liquid culture of the bacteria was used as an inoculum (seed culture) in further experiments.
The growth pattern of the isolate was observed in mineral salt medium (MSM) containing 1% glucose at an initial pH of 7.0 at 37°C with agitation speed of 120 rpm. The composition of MSM was as follows (g/l): urea (1.0), yeast extract (0.16), KH2PO4 (1.52), Na2HPO4 (4.0), MgSO4·7H2O (0.52), CaCl2 (0.02), glucose (40), and trace element solution, 0.1 ml. The trace element solution contained (g/l): ZnSO4·7H2O (0.13), FeSO4·7H2O (0.02), (NH4)6MO7O2.4H2O (0.06), and H3BO3 (0.06). Both glucose and trace element solutions were autoclaved separately and reconstituted prior to inoculation. Inoculum size of 1% was used to study the growth pattern. Absorbance was observed at regular intervals. Cell growth was monitored over time at regular intervals by measuring absorbance at 600 nm. All the experiments were performed in triplicates.
PHAs accumulation
The fermentation for PHAs production was carried out using basal mineral salt medium (MSM). Ten grams of carbon substrate per liter was added in basal MSM. The pH of the medium was adjusted to 7.0 using a pH meter. The medium was sterilized at 15 lb pressure for 20 min in an autoclave. Thereafter, fermentation medium was inoculated using 20 ml inoculum under sterile conditions using a laminar bench. The inoculated fermentation flasks were kept on a rotary shaker for 96 h at 37oC with shaking at 120 rpm [24].
Extraction and quantification of PHAs
The PHAs were directly extracted using dispersion method of sodium hypochlorite and chloroform with slight modifications [25–28]. For that, 10 ml of bacterial culture was centrifuged at 8500×g for 15 min. The supernatant was discarded, and pellet was washed with phosphate buffered saline of pH 7.4. The cell pellet was air dried for 2 h and, thereafter, weighed accurately. The weight was designated as dry cell weight (DCW). Thereafter, the cell pellet was suspended in solvent having 12.5 μl chloroform and 12.5 μl of 4% sodium hypochlorite solution per mg of pellet weight. The suspension was incubated at 37oC for 90 min under shaking conditions for complete digestion of cell components except PHAs. The dispersion was centrifuged at 6500×g for 10 min at the room temperature resulting in the formation of different phases. The bottom phase of chloroform contains PHAs. This phase was transferred to another fresh tube, and its volume was measured. To it, five volumes of methanol and water (7:3 v/v) was added, and thereafter, it was centrifuged at 8500 rpm for 15 min resulting in the formation of a precipitate of PHAs. The precipitate was air dried for 1 h. The amount of PHAs extracted was quantified by weighing the precipitate and designated as dry weight of extracted PHAs (g/l). For quantitative analysis of PHAs, cell pellet was dried to estimate the dry cell weight (DCW). Residual biomass was estimated as difference between the DCW and dry weight of extracted PHAs. Residual biomass was calculated to find out the cellular weight and accumulation of other than PHAs. The percentage of intracellular PHAs accumulation was estimated as the percent composition of PHAs present in the DCW [22].
Crotonic acid assay
The extracted powder was transferred in a clean test tube, and 10ml of 36N sulfuric acid was added to it, capped, and heated for 20 min at 100oC in a boiling water bath. During this process, PHAs granules were converted into crotonic acid by dehydration. The resultant brown-colored solution of crotonic acid was cooled and thereafter mixed thoroughly by shaking. The absorbance of the sample was measured at 235 nm using an UV-VIS spectrophotometer using sulfuric acid as a control. The biopolymer PHAs was used as a standard. The monomeric composition of standard biopolymeric PHAs used was PHB (polyhydroxybutyrate) [27].
Morphological, physiological, and biochemical characterization
Morphological and physiological characterization of the highest PHAs producing bacteria was performed according to Bergey’s Manual of Systematic Bacteriology [29]. Multiple biochemical tests were also performed. Vitek-2 compact system of biochemical characterization and identification was used [30].
Identification of the bacterial strain
Identification of the bacterial strain was done by using 16s rRNA gene sequencing. The 16s rRNA gene was sequenced at the National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory, Pune, India. Chromosomal DNA was extracted by using spin column kit (Hi-Media, India). The 16s rRNA sequences contained hyper-variable regions that can provide species-specific signature sequences useful for bacterial identification. The primer used was 704F_907RC (1258bp). Bacterial 16S rRNA gene (1500 bp) [31] was amplified using polymerase chain reaction in a thermal cycler and was purified using Exonuclease I-Shrimp Alkaline Phosphatase (Exo-SAP) [32]. Purified amplicons were sequenced by using Sanger’s method in an ABI 3500xL genetic analyzer (Life Technologies, USA).
Phylogenetic analysis
Sequencing files (.ab1) edited by using CHROMASLITE (version 1.5) were analyzed by Basic Local Alignment Search Tool (BLAST) with closest culture sequence retrieved from the National Centre for Biotechnology Information (NCBI) database that finds regions of local similarity between sequences [33].
Further multiple sequence alignment and phylogenetic analysis were carried out for accurate species prediction and evolutionary relationship. Neighbor-joining method was used to study evolutionary history. The analysis involved 8 nucleotide sequences. All the positions with gaps and missing data were eliminated. Evolutionary analysis was performed in MEGA 7.0.20 [34].
Optimization studies using OFAT method
The optimization of physical and nutritional culture conditions for PHAs production was performed by using one factor at a time approach (OFAT). It is a method of designing experiments involving the testing of factors, one at a time instead of all simultaneously.
Evaluation of PHAs production using different carbon, nitrogen sources, and renewable substrates
In desire of higher PHAs production, the isolate M12 was selected to study the PHAs production in a range of carbon substrates, nitrogen sources, and various renewable agro-industrial wastes at different temperatures. For studies on carbon substrates, cells were first grown in nutrient broth at 37°C, 120 rpm for 24 h. Thereafter, cells were transferred to the PHAs production medium (MSM medium) containing the test carbon substrate (2% w/v). The physical parameters such as temperature 37°C, pH 7, and agitation speed 120 rpm were kept constant during optimization of carbon and nitrogen sources. The carbon substrates tested were glucose, fructose, lactose, sucrose, maltose, and arabinose that covered the constituents of different cost-effective waste materials. Experiments were performed in 250 ml flasks containing 50 ml of MSM. The total biomass and PHAs production were measured for each of the six test carbon substrates. All the experiments were performed in triplicates. The best carbon source was determined on the basis of higher PHAs yield.
To determine the best nitrogen source, the isolate M12 was inoculated in MSM containing the best carbon source 2% and various nitrogen sources such as ammonium chloride, ammonium sulfate, peptone, yeast extract, urea, potassium nitrate, sodium nitrate, casein, and malt extract at a concentration of 0.2 % in different flasks. The flasks were incubated at 37°C and 120 rpm. All the experiments were performed in triplicates. The best nitrogen source was determined on the basis of higher PHAs yield.
To determine the capability of the isolate M12 to utilize different renewable agro-industrial wastes as carbon substrate, the MSM was used as production medium supplemented with 2% of dried and powdered agro-industrial waste like domestic kitchen waste, sugarcane molasses, waste flour from bread factory, and mixed fruit pulp from fruit juice shops in separate flasks. The flasks were incubated at 37°C and 120 rpm. The best renewable agro-industrial waste was determined based on higher PHAs accumulation.
Evaluation of PHAs production in varied range of initial pH and temperature
The effect of physical variables such as initial pH (5 to 9) and incubation temperature (25 to 40°C) on PHAs production was also studied.
Characterization of PHAs
Fourier transform infrared (FTIR) spectroscopic analysis
The chemical structure and the functional groups of the extracted PHAs were analyzed using attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy. The infrared spectra of the samples were recorded in the wavelength range from 600 to 4000/cm using a Bruker ATR-FTIR spectrophotometer with Zn-Se disc [24, 35].
X-ray diffraction studies
To get an insight into the structure of PHAs produced by the bacteria, XRD measurements were carried out using Bruker D8 Advance X-ray diffractometer. The X-rays of the wavelength 0.154 were used (Cu K-alpha). A fast counting detector based on silicon strip technology was used to detect X-rays (Bruker LynxEye detector).
Results
Isolation and screening of PHAs producing bacteria
PHAs producing bacterial colonies from various soil samples collected from various sites in Indore city as mentioned under Materials and Methods were identified by using primary screening.
Based on the amount of PHAs accumulation and secondary screening, a total of six bacterial colonies were selected as PHAs producers. Among these, one colony named as M12 was further studied and identified based on the highest quantity of PHAs accumulation of 0.4 g/ml.
Identification of the highest PHAs accumulator isolate
Identification of the isolate M12 was done on the basis of morphological, biochemical, as well as physiological characteristics. Besides, 16s rRNA sequencing was also done for molecular identification of the selected strain.
The bacterium was phenotypically characterized as a Gram positive with rod shape (Bacillus sp.). It appeared as off-white-colored spore former and grown under the aerobic conditions on the agar plate. The various morphological and biochemical characteristics of the bacteria are shown in Table 1.
Table 1.
Morphological and biochemical characteristics of isolate M12
| Characteristics | Observation |
|---|---|
| Colony shape | Circular |
| Color | Off-white, translucent |
| Gram reaction | Gram positive |
| Cell shape | Rod |
| Motility | Motile |
| Catalase test | Positive |
| Oxidase test | Positive |
| Voges Proskauer | Positive |
| Gelatin hydrolysis | Positive |
| Arginine dihydrolase | Positive |
| Esculin hydrolysis | Positive |
| Beta galactosidase | Negative |
| Lysine decarboxylase | Negative |
| H2S production | Negative |
| Urease activity | Negative |
| Tryptophan desaminase | Negative |
| Indole production | Negative |
| Polymixin_B and kanamycin resistance | Positive |
| Ellman reaction | Positive |
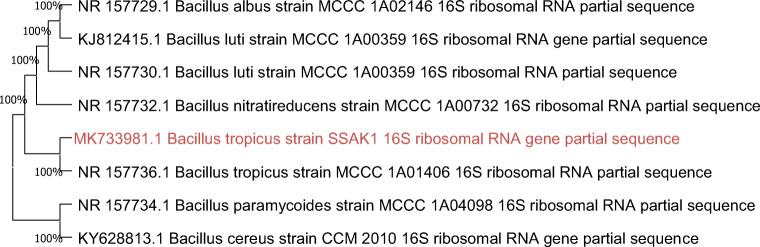
The detailed sequence results obtained from the 16s rRNA sequencing was submitted to the NCBI GenBank database, and the given accession number is MK733981.1. Further, the NCBI BLAST program was used for the comparison of 16s rRNA sequence of the isolated bacterium with the database sequences. The phylogenetic tree was built with neighbor joining method with MEGA version 7.0.20. The blast sequences revealed that the isolated bacterium displayed 100% similarity with the sequence of Bacillus tropicus MCCC 1A01406 and the microorganism was identified to be Bacillus tropicus SSAK1 (Fig. 1)
Fig. 1.
Phylogenetic relationship based on 16S rRNA of the isolate M 12
Growth curve of the isolate SSAK1 (M12) and PHAs quantification
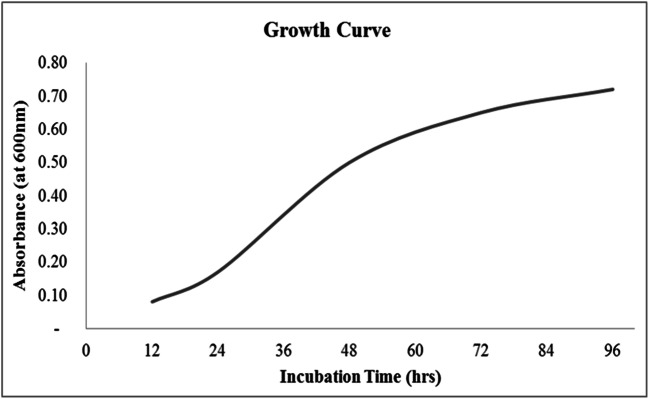
The results of the growth curve study of the isolate SSAK1 (M12) indicated that the microorganism remained in the lag phase until 12 h. After 18 h of incubation, a steep rise in the curve was observed. Also, a gradual increase in biomass was noted. Commencement of PHAs production began at the 25th hour (mid-log phase) and gradually increased until mid-stationary phase. Highest PHAs accumulation was observed after 48 h. Maximum bacterial growth occurred up to 42 h, and thereafter bacterial growth ceased (stationary phase) (Fig. 2).
Fig. 2.
Growth curve of B. Tropicus SSAK1 (M12)
Effect of different carbon sources on growth and PHAs production
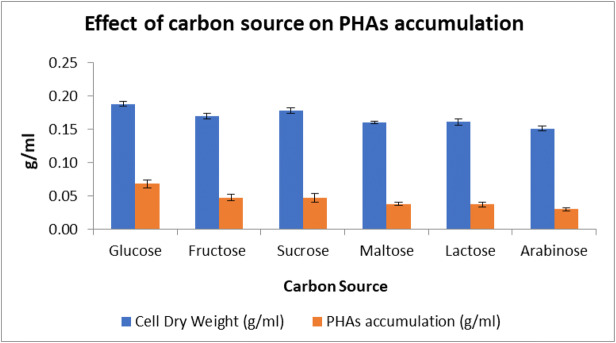
The study of effects of all the commercial carbon sources such as glucose, fructose, maltose, sucrose, lactose, and arabinose on PHAs production revealed that B. Tropicus SSAK1 produced the highest DCW and PHAs yield when glucose was used as a carbon source, followed by fructose, sucrose, lactose, and maltose, whereas arabinose had the least biomass yield and PHAs accumulation. A bar diagram indicating percent of PHAs accumulation in the presence of various sugars as sole carbon source is shown as Fig. 3.
Fig. 3.
Effect of different carbon sources on PHAs accumulation and dry cell weight. Data represent mean ± SD (n=3); P ˂ 0.05
Effect of different nitrogen sources
To study the effect of different nitrogen sources on PHAs accumulation, MSM containing glucose 20 g/l and various nitrogen sources such as ammonium chloride, ammonium sulfate, potassium nitrate, sodium nitrate, peptone, yeast extract, urea, malt extract, and casein at 2 g/l were screened. The results revealed that maximum PHAs yield was obtained when ammonium sulfate was used as nitrogen source (Fig. 4). To conclude, the isolate SSAK1 produced the highest amount of PHAs when glucose was used as the sole carbon source and ammonium sulfate as the nitrogen source.
Fig. 4.
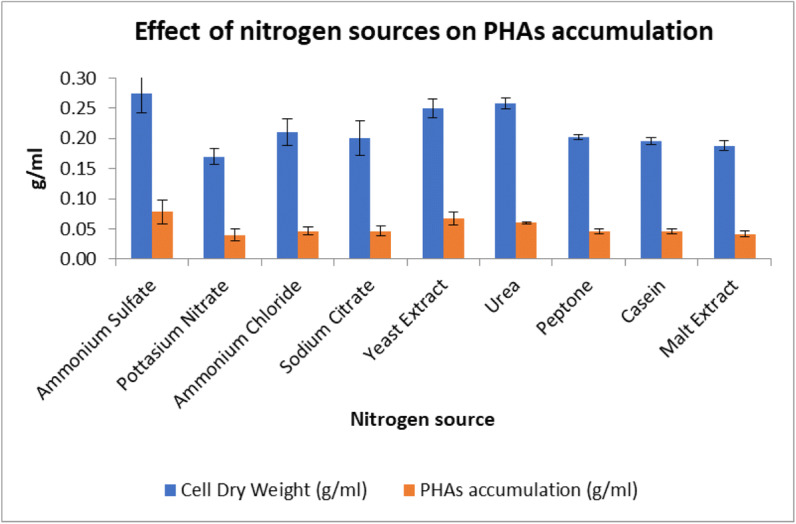
Effect of different nitrogen sources on PHA accumulation and dry cell weight by isolate M12. Data represent mean ± SD (n=3); P ˂ 0.05
Screening of renewable agro-industrial wastes for PHAs production
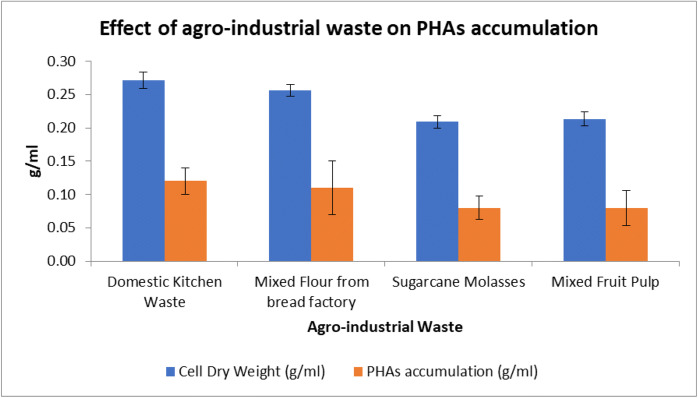
The usage of renewable agro-industrial wastes as sole source of carbon during fermentative production of PHAs can greatly reduce the production cost. In the present study, among the agro-industrial wastes tested, dried and powdered domestic kitchen waste gave the highest yield, followed by mixed flour from bread factory, sugarcane molasses, and mixed fruit pulp from fruit juice factory (Fig. 5). Therefore, glucose can be replaced with domestic kitchen waste at 2%, and ammonium sulfate 2% may be used as the optimum nitrogen source.
Fig. 5.
Effect of different agro-industrial waste on PHAs accumulation and dry cell weight. Data represent mean ± SD (n=3); P ˂ 0.05
Effect of initial pH, temperature, and inoculum size
In this study, effect of initial pH in range of 5 to 9 on PHAs production was investigated (Fig. 6). The effect of temperature in the range of 25 to 45°C was also studied (Fig. 7). Based on observations by one factor at a time method, it was inferred that optimal pH is 7.0 and optimal temperature is 37°C.
Fig. 6.
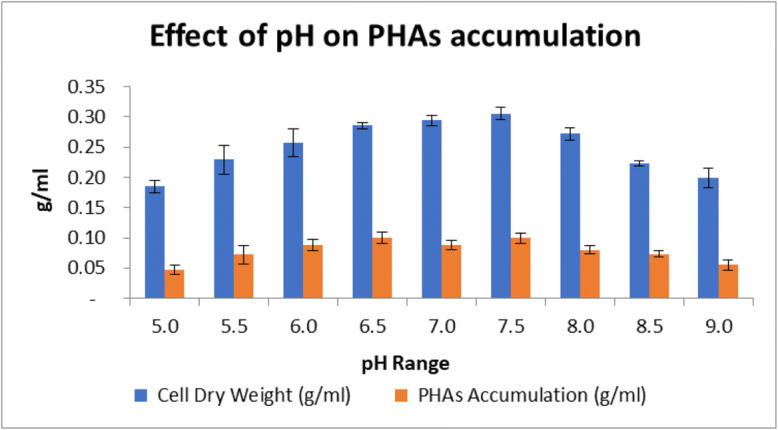
Effect of different pH on PHAs accumulation and dry cell weight. Data represent mean ± SD (n=3); P ˂ 0.05
Fig. 7.
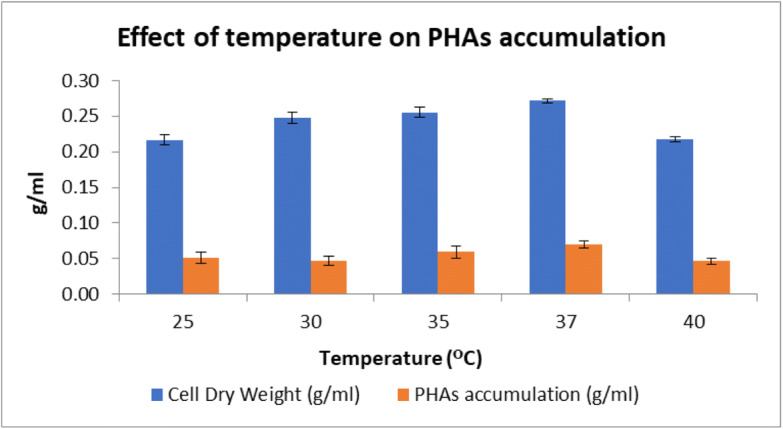
Effect of different temperature on PHAs accumulation and dry cell weight. Data represent mean ± SD (n=3); P ˂ 0.05
The inoculum size was tested in the range of 0.5 to 3%. It was observed that inoculum size 2% was optimum for higher production of PHAs. It showed 36.5% PHAs production. The inoculum size 0.5%, 1%, and 1.5% when used exhibited 33, 34, and 35.5% PHAs production. On increasing inoculum size from 2 to 2.5 or 3%, there was decreased PHAs production (Fig. 8).
Fig. 8.
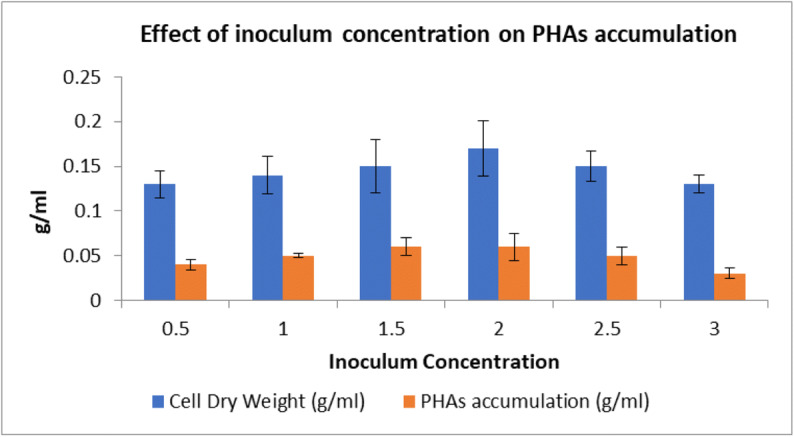
Effect of inoculum percentage on PHAs accumulation and dry cell weight. Data represent mean ± SD (n=3); P ˂ 0.05
All the data were statistically analyzed using ANOVA single factor analysis. It revealed that all results obtained were statistically significant. Data were also analyzed using Tukey post hoc test which revealed the two means to be significantly different as the calculated critical value in Tukey test was found to be larger than the critical value from critical table.
Characterization of PHAs
FTIR results
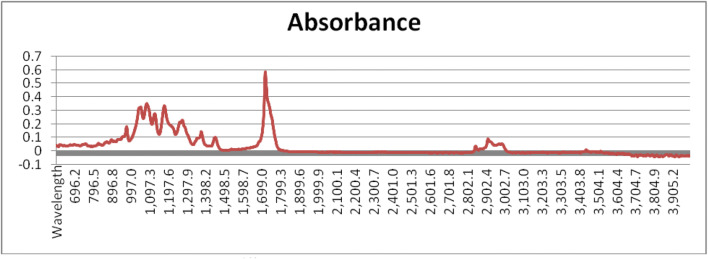
The FTIR spectrum of the PHAs synthesized by the isolate SSAK1 showed prominent peaks at different wavelength characteristics of PHAs. The peaks at 2844 and 2914 to 2966 cm−1 were due to the C-H stretch of alkanes. The presence of C=O and C-O stretch of ester could be confirmed from the absorption band at 1718.3 cm−1 and from the series of intense peaks located at 1080–1176 cm−1, respectively (Fig. 9). These are the characteristic stretch of short-chain length monomers of PHB. Hence, the PHAs produced by the isolate SSAK1 may be considered as polyhydroxybutyrate (PHB).
Fig. 9.
FTIR: absorbance at different wavelengths
XRD results
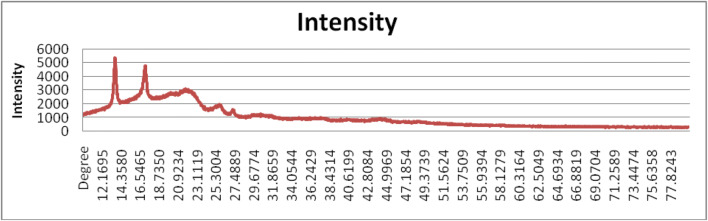
The XRD study was carried out to check crystalline structure of PHB. The XRD diffractogram (Fig. 10) showed four prominent peaks at 13.69°, 17.11°, 21.67°, and 25.62°. The presence of intense peak at 13.69° indicated the crystalline nature of the polymer.
Fig. 10.
XRD: intensity at different degrees
Discussion
It is known in the literature that PHAs are water-insoluble lipid granules synthesized by numerous bacteria under nutrient-deficient conditions. The PHAs are the key products to be used as bio-plastics in order to create a better environment with lesser plastic pollution and more environmentally sustainable solution for future generations. They have the veritable advantage of biodegradability and biocompatibility as compared to conventional petro-based synthetic plastics. Diverse PHAs producing bacteria from varied types of soil and niches have been reported [36]. The biopolymeric PHAs have a great impact as an alternative for petroleum-derived plastics. Therefore, present work was carried out to isolate and identify potent PHAs producing bacteria, and for that, soil samples from the sites having dump of plastic and/or oil were used for isolation of the potent bacteria.
In the present study, three different media, namely, carbon-enriched nutrient agar, nutrient broth, and MSM for isolation, inoculum preparation, and growth pattern studies, respectively, for Bacillus tropicus were used. Carbon-enriched nutrient agar medium was used for isolation being deficient in nutrients like nitrogen and phosphorus and excess in carbon source, a condition required for production of PHAs. Nutrient broth was considered to provide better results for the growth of Bacillus tropicus used as inoculum. The MSM medium enriched in glucose (40 g/l) was used as production medium during fermentation process for better growth and PHAs production. Earlier reports also showed production of more PHAs under excess carbon source like glucose and limiting nitrogen and phosphorus [37, 38].
The nitrogen source plays an important role during PHAs synthesis. The limitation of nitrogen source favors PHAs accumulation. Verlinden et al. [5] reported that during nutrient-limiting condition, acetyl-CoA instead of entering into Krebs cycle is directed towards PHAs biosynthesis.
The results of the present study corroborated with that of Evangeline and Sridharan [39], who also reported that Bacillus cereus VIT-SSR1 accumulated maximum PHAs amount when glucose was used as carbon source and ammonium sulfate as nitrogen source. Since PHAs are a growth-associated product, optimization of physical and cultural parameters is considered to be essential for efficient growth and PHA synthesis.
The growth curve study of the isolate B. tropicus SSAK1 is in accordance with previously reported growth curve study of Bacillus sp. [35, 39]. Process optimization was done to screen the important variables for enhanced PHAs production. Previous studies on effects of commercial carbon sources in PHAs production also revealed that Bacillus species accumulated high amount of PHAs when fed with glucose. There are reports on Bacillus species for PHAs production where yields have been reported ranging from 1.60 to 6.07 g/l [40–45]. Reddy et al. [46] reported that Bacillus megaterium OU303A produced 58.6 % PHAs when fed on glucose; also it produced the copolymer P(HB-co-HV). Glucose exhibits positive effects on PHAs biosynthesis which could be credited to the increased supply of the reduced cofactor, NADPH, which consecutively leads to the inhibition of enzymes of Krebs cycle [47]. Mohapatra et al. [38] isolated a potent PHAs producing microbe from mangrove forest and identified it as Bacillus megaterium using in-silico analysis and 16s rRNA sequencing. They optimized conditions for PHAs production in MSM medium using submerged and solid-state fermentation processes. They showed higher PHAs production by solid-state fermentation through sonication and mono-solvent extraction. They also characterized the produced PHAs using various techniques. Pati et al. [48] studied PHB production from Bacillus sp. using submerged as well as solid-state fermentation processes, and extraction was done using different downstream processing. They also found solid-state fermentation as more effective in PHB production compared to submerged fermentation. The PHB produced was also characterized using techniques like FTIR and NMR, and properties like melting temperature, degradation temperature, and crystallinity were determined. The nitrogen source also plays an important role during PHAs synthesis. The limitation of nitrogen source favors PHAs accumulation. Verlinden et al. [5] reported that during nutrient-limiting conditions, acetyl-CoA instead of entering into Krebs cycle is directed towards PHAs biosynthesis. The results of the present study corroborated with that of Evangeline and Sridharan [39], who also reported that Bacillus cereus VIT-SSR1 accumulated maximum PHAs amount when glucose was used as carbon source and ammonium sulfate as nitrogen source. Since PHAs are a growth-associated product, optimization of physical and cultural parameters is very beneficial for efficient growth and PHAs synthesis.
Getachew and Woldesenbet [37] as well as Stavroula et al. [20] suggested that the production cost of PHAs may be reduced by using agro-industrial waste as inexpensive carbon substrate. The results of our study are in conformity indicating that Bacillus tropicus SSAK1 bacterial strain has potential to synthesize PHAs using lesser expensive carbon source such as domestic kitchen wastes, sugarcane molasses, waste flour from bread factory, and mixed fruit pulp from fruit juice shops with reasonable yields.
The FTIR graph depicts the characteristic stretch of short-chain length monomers of PHB. The FTIR spectrum of the extracted biogenic PHAs in the present study is well comparable with the FTIR spectrum for the standard and sample PHAs [49]. The results are also consistent with previous studies which reported the presence of similar functional groups [24, 50, 51]. Hence, PHAs extracted from the isolate SSAK1 may be considered as polyhydroxybutyrate (PHB).
The XRD diffractogram of extracted biopolymer is almost identical with that obtained by Devi et al. [24] which depicted its crystalline nature. Similar results for the diffractogram of PHB were reported previously by other groups [50, 52].
The usage of cheaper carbon sources, effective fermentation techniques, and modified downstream processes can substantially cut down the production cost of the biopolymer. Moreover, concerning the environmental issues, the major problem of waste disposal all over the world may also be minimized.
Conclusions
A potent PHA accumulating bacterial isolate Bacillus tropicus SSAK1 capable of utilizing various agro-industrial wastes as carbon source for PHA production was illustrated in this study. The study revealed that higher PHA accumulation occurred when domestic kitchen waste was used as source of carbon in fermentation media. Optimization of process parameters was also performed which depicted higher PHA yield in the presence of glucose as sole source of carbon. Among different nitrogen sources screened, bacterial strain was able to produce considerable higher amount of PHA in the presence of ammonium sulfate. Other parameters that resulted in higher PHA yield were pH 7.0, temperature 37°C, and bacterial inoculum at concentration of 2 %. The FTIR and XRD analysis revealed the chemical nature of the bio-synthesized polymer. The commercial usage of green plastics (PHAs) can act as environmentally sustainable alternative to petro-based conventional plastics and can greatly lessen plastic pollution. Moreover, the problem of disposal of recurring domestic kitchen waste and other agro-industrial waste can be resolved.
Acknowledgements
SS is grateful to the Devi Ahilya University, Indore, for the award of the Golden Jubilee Fellowship. The authors acknowledge the facilities of the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi (DBT), under the Bioinformatics Sub Centre as well as M.Sc. Biotechnology program present in our School of Biotechnology and used in the present work. Authors acknowledge the facilities of FTIR and XRD at the Inter University Consortium, UGC-DAE available in our University campus used in the present work.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/20/2021
A Correction to this paper has been published: 10.1007/s42770-021-00467-6
References
- 1.Brandl H, Gross RA, Lenz RW, Fuller RC (1990) Plastics from bacteria and for bacteria: poly(beta-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. Adv Biochem Eng Biotechnol 41:77-93. 10.1007/BFb0010232 [DOI] [PubMed]
- 2.Mohapatra S, Samantaray DP, Samantaray SM. Study on polyhydroxyalkanoates production using rhizospheric soil bacterial isolates of sweet potato. Indian J Sci Technol. 2015;8:57–62. doi: 10.17485/ijst/2015/v8iS7/64027. [DOI] [Google Scholar]
- 3.Khanna S, Srivastava AK. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005;40:607–619. doi: 10.1016/j.procbio.2004.01.053. [DOI] [Google Scholar]
- 4.Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Mol Biol Rev 54:450–472 [DOI] [PMC free article] [PubMed]
- 5.Verlinden RA, Hill DJ, Kenward MA, Williams CD, Radecka I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol. 2007;102:1437–1449. doi: 10.1111/j.1365-2672.2007.03335.x. [DOI] [PubMed] [Google Scholar]
- 6.Dwivedi R, Pandey R, Kumar S, Mehrotra D. Polyhydroxyalkanoates (PHA): role in bone scaffolds. J Oral Biol Craniofac Res. 2020;10(1):389–392. doi: 10.1016/j.jobcr.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinbüchel A. Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol Biosci. 2001;1:1–24. doi: 10.1002/1616-5195(200101)1:1<1::AID-MABI1>3.0.CO;2-B. [DOI] [Google Scholar]
- 8.Lee WH, Loo CY, Nomura CT, Sudesh K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour Technol. 2008;99:6844–6851. doi: 10.1016/j.biortech.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen C, Rahman A, Rehman AU, Walsh MK, Miller CD. Food waste conversion to microbial polyhydroxyalkanoates. Microb Biotechnol. 2017;10:1338–1352. doi: 10.1111/1751-7915.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Market MA (2019) Polyhydroxyalkanoate (PHA) market by type, short chain length, medium chain length, production method, sugar fermentation, vegetable oil fermentation, methane fermentation, application, and region - Global Forecast to 2024 [Online]. Markets and Markets Research Private Ltd; online available at: https://www.marketsandmarkets.com/Market-Reports/pha-market-5.html?gclid=Cj0KCQjwoqDtBRD-ARIsAL4pviDVMGJlVibGAdbu0O60hVVk9gNpJ-Zs0NnJ-v9792cuGBpuyRLaO_AaAklKEALw_wcB Accessed October 10, 2019.
- 11.Kabasci S. Biobased plastics. In Plastic Waste and Recycling. Cambridge: Academic Press; 2020. pp. 67–96. [Google Scholar]
- 12.Steinbüchel A, Füchtenbusch B. Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998;16:419–427. doi: 10.1016/S0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- 13.Surendran A, Lakshmanan M, Chee JY, Sulaiman AM, Van Thuoc D, Sudesh K (2020) Can polyhydroxyalkanoates be produced efficiently from waste plant and animal oils? Front Bioeng Biotechnol 8. 10.3389/fbioe.2020.00169 [DOI] [PMC free article] [PubMed]
- 14.Reddy CSK, Ghai R, Kalia VC. Study on polyhydroxyalkanoate, PHA, production in pilot scale continuous mode wastewater treatment system. Bioresour Technol. 2003;87:37–146. doi: 10.1016/S0960-8524(02)00212-2. [DOI] [Google Scholar]
- 15.Maity S, Das S, Samantaray DP. Effect of vitamin on accumulation of PHB by Zobellella species under submerged fermentation process. Int J Curr Microbiol App Sci. 2017;6:1310–1316. [Google Scholar]
- 16.Byrom D. Biomaterials. London: Palgrave Macmillan; 1991. Miscellaneous biomaterials; pp. 333–359. [Google Scholar]
- 17.Chen GQ, Chen XY, Wu FQ, Chen JC (2019) Polyhydroxyalkanoates (PHA) towards cost competitiveness and functionality. Adv Indus Polym Res 3:1– 7. 10.1016/j.aiepr.2019.11.001
- 18.Koller M, Salerno A, Braunegg G (2013) Polyhydroxyalkanoates: basics, production and applications of microbial biopolyesters. In Bio-based plastics: materials and applications. Wiley, Germany, pp 137-170
- 19.Bugnicourt E, Cinelli P, Lazzeri A, Alvarez VA. Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett. 2014;8:791–808. doi: 10.3144/expresspolymlett.2014.82. [DOI] [Google Scholar]
- 20.Stavroula K, Simos M, Joanne HK (2020) Polyhydroxyalkanoates (PHAs) from household food waste: research over the last decade. Intl J Biotechnol Bioeng 6:26–36
- 21.Swathi K, Ranjani NS. Production, isolation, screening and extraction of polyhydroxybutyrate (PHB) from Bacillus sps using treated sewage sample. Int J Pharm Bio Sci. 2015;5:58–64. [Google Scholar]
- 22.Bhuwal AK, Singh G, Aggarwal NK, Goyal V, Yadav A (2013) Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Intl J Biomaterials Vol. 2013, Article ID 752821. 10.1155/2013/752821 [DOI] [PMC free article] [PubMed]
- 23.Ostle AG, Holt JG. Nile blue A as a fluorescent stain for poly-beta-hydroxybutyrate. Appl Environ Microbiol. 1982;44:238–241. doi: 10.1128/aem.44.1.238-241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devi AB, Nachiyar CV, Kaviyarasi T, Samrot AV. Characterization of polyhydroxybutyrate synthesized by Bacillus cereus. Int J Pharm Pharm Sci. 2015;7:140–144. [Google Scholar]
- 25.Hahn SK, Chang YK, Kim BS, Chang HN. Optimization of microbial poly 3-hydroxybutyrate recover using dispersions of sodium hypochlorite solution and chloroform. Biotechnol Bioeng. 1994;44:256–261. doi: 10.1002/bit.260440215. [DOI] [PubMed] [Google Scholar]
- 26.Slepecky RA, Law JH. A rapid spectrophotometric assay of alpha, beta-unsaturated acids and beta-hydroxy acids. Anal Chem. 1960;32:1697–1699. doi: 10.1021/ac60168a046. [DOI] [Google Scholar]
- 27.Singh P, Parmar N. Isolation and characterization of two novel polyhydroxybutyrate (PHB) producing bacteria. Afr J Biotechnol. 2011;10:4907–4919. [Google Scholar]
- 28.Sayyed RZ, Gangurde NS, Chincholkar SB. Hypochlorite digestion method for efficient recovery of PHB from Alcaligenes faecalis. Indian J Microbiol. 2009;49:230–232. doi: 10.1007/s12088-009-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergey DH. Bergey’s Manual of Systematic Bacteriology. Philadelphia: Williams and Wilkins; 1989. [Google Scholar]
- 30.Navas M, Pincus DH, Wilkey K, Sercia L, LaSalvia M, Wilson D, Procop GW, Richter SS. Identification of aerobic Gram-positive bacilli by use of Vitek MS. J Clin Microbiol. 2014;52:1274–1277. doi: 10.1128/JCM.03483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarridge JE. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17:840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darby AC, Chandler SM, Welburn SC, Douglas AE. Aphid-symbiotic bacteria cultured in insect cell lines. Appl Environ Microbiol. 2005;71:4833–4839. doi: 10.1128/AEM.71.8.4833-4839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomaa EZ. Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Braz Arch Biol Technol. 2014;57:145–154. doi: 10.1590/S1516-89132014000100020. [DOI] [Google Scholar]
- 36.Wu Q, Wang Y, Chen GQ (2009) Medical application of microbial bio-polyesters polyhydroxyalkanoates. Artif Cells Blood Substit Immobil Biotechnol 37:1–12. 10.1080/10731190802664429 [DOI] [PubMed]
- 37.Getachew A, Woldesenbet F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes. 2016;9:1–9. doi: 10.1186/s13104-016-2321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohapatra S, Pattnaik S, Maity S, Sharma S, Akhtar J, Pati S, Samantaray DP, Varma A (2020) Comparative analysis of PHAs production by Bacillus megaterium OUAT 016 under submerged and solid-state fermentation. Saudi J Biol Sciences 27:1242–1250. 10.1016/j.sjbs.2020.02.001 [DOI] [PMC free article] [PubMed]
- 39.Evangeline S, Sridharan TB. Biosynthesis and statistical optimization of polyhydroxyalkanoates (PHA) produced by Bacillus cereus VIT-SSR1 and fabrication of biopolymer films for sustained drug release. Int J Biol Macromol. 2019;135:945–958. doi: 10.1016/j.ijbiomac.2019.05.163. [DOI] [PubMed] [Google Scholar]
- 40.Mohapatra S, Samantaray DP, Samantaray SM. Phylogenetic heterogeneity of the rhizospheric soil bacterial isolates producing PHAs revealed by comparative analysis of 16s-rRNA. Int J Curr Microbiol App Sci. 2014;3:680–690. [Google Scholar]
- 41.Mohapatra S, Mohanta PR, Sarkar B, Daware A, Kumar C, Samantaray DP (2015) Production of polyhydroxyalkanoates (PHAs) by Bacillus strain isolated from waste water and its biochemical characterization. Proc Natl Acad Sci India Section B: Biol Sci 87:459–466. 10.1007/s40011-015-0626-6
- 42.Desouky SE, El-Shiekh HH, Elabd MA, Shehab AM (2014) Screening, optimization and extraction of polyhydroxyalkanoates (PHAs) from Bacillus thuringienesis. J Adv Biol Biotechnol 1:40–54. 10.9734/JABB/2014/12286
- 43.Israni N, Shivakumar S. Combinatorial screening of hydrolytic enzyme/s and PHA producing Bacillus spp. for cost effective production of PHAs. Int J Pharm Bio Sci. 2013;4:B934–B945. [Google Scholar]
- 44.Valappil SP, Misra SK, Boccaccini AR, Keshavarz T, Bucke C, Roy I. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterized Bacillus cereus SPV. J Biotechnol. 2007;132:251–258. doi: 10.1016/j.jbiotec.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Musa H, Bolanle BB, Kasim FH, Arbain D (2016) Screening and production of polyhydroxybutyrate (PHB) by bacterial strains isolated from rhizosphere soil of groundnut plants. Sains Malaysiana 45:1469–1476
- 46.Reddy SV, Thirumala M, Mahmood SK. Production of PHB and P(3HB-co-3HV) biopolymers by Bacillus megaterium strain OU303A isolated from municipal sewage sludge. World J Microbiol Biotechnol. 2009;25:391–397. doi: 10.1007/s11274-008-9903-3. [DOI] [Google Scholar]
- 47.Jangra MR, Nehra KS (2017) Isolation, screening and characterisation of new strains with optimisation studies to augment bacterial PHB production. Bull Env Pharmacol Life Sci 6:34–44
- 48.Pati S, Maity S, Dash A, Jema S, Mohapatra S, Das S, Samantaray DP. Biocompatible PHB production from bacillus species under submerged and solid-state fermentation and extraction through different downstream processing. Curr Microbiol. 2020;77:1203–1209. doi: 10.1007/s00284-020-01922-7. [DOI] [PubMed] [Google Scholar]
- 49.Shamala TR, Chandrashekar A, Vijayendra SVN, Kshama L. Identification of polyhydroxyalkanoates (PHA) producing Bacillus spp. using the polymerase chain reaction (PCR) J Appl Microbiol. 2003;94:369–374. doi: 10.1046/j.1365-2672.2003.01838.x. [DOI] [PubMed] [Google Scholar]
- 50.Nair AM, Annamalai K, Kannan SK, Kuppusamy S (2014) Characterization of polyhydroxyalkanoates produced by Bacillus subtilis isolated from soil samples. Malaya J Biosciences 1:8–12
- 51.Hong K, Sun S, Tian W, Chen GQ, Huang W. A rapid method for detecting bacterial polyhydroxyalkanoates in intact cells by Fourier transform infrared spectroscopy. Appl Microbiol Biotechnol. 1999;51:523–526. doi: 10.1007/s002530051427. [DOI] [Google Scholar]
- 52.Liau CP, Bin Ahmad M, Shameli K, Yunus WMZW, Ibrahim NA, Zainuddin N, Then YY. Preparation and characterization of polyhydroxybutyrate/polycaprolactone nanocomposites. Sci World J. 2014;2014:1–9. doi: 10.1155/2014/572726. [DOI] [Google Scholar]