Abstract
Objectives
The purpose of this study was to detect cardiovascular changes after mild severe acute respiratory syndrome-coronavirus-2 infection.
Background
Concern exists that mild coronavirus disease 2019 may cause myocardial and vascular disease.
Methods
Participants were recruited from COVIDsortium, a 3-hospital prospective study of 731 health care workers who underwent first-wave weekly symptom, polymerase chain reaction, and serology assessment over 4 months, with seroconversion in 21.5% (n = 157). At 6 months post-infection, 74 seropositive and 75 age-, sex-, and ethnicity-matched seronegative control subjects were recruited for cardiovascular phenotyping (comprehensive phantom-calibrated cardiovascular magnetic resonance and blood biomarkers). Analysis was blinded, using objective artificial intelligence analytics where available.
Results
A total of 149 subjects (mean age 37 years, range 18 to 63 years, 58% women) were recruited. Seropositive infections had been mild with case definition, noncase definition, and asymptomatic disease in 45 (61%), 18 (24%), and 11 (15%), respectively, with 1 person hospitalized (for 2 days). Between seropositive and seronegative groups, there were no differences in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide). With abnormal defined by the 75 seronegatives (2 SDs from mean, e.g., ejection fraction <54%, septal T1 >1,072 ms, septal T2 >52.4 ms), individuals had abnormalities including reduced ejection fraction (n = 2, minimum 50%), T1 elevation (n = 6), T2 elevation (n = 9), late gadolinium enhancement (n = 13, median 1%, max 5% of myocardium), biomarker elevation (borderline troponin elevation in 4; all N-terminal pro–B-type natriuretic peptide normal). These were distributed equally between seropositive and seronegative individuals.
Conclusions
Cardiovascular abnormalities are no more common in seropositive versus seronegative otherwise healthy, workforce representative individuals 6 months post–mild severe acute respiratory syndrome-coronavirus-2 infection.
Key Words: cardiovascular magnetic resonance, COVID-19, late gadolinium enhancement, myocardial edema, myocarditis, SARS-CoV-2, troponin
Abbreviations and Acronyms: CMR, cardiovascular magnetic resonance; COVID-19, coronavirus disease-2019; hsTnT, high-sensitivity troponin T; LGE, late gadolinium enhancement; LV, left ventricular; NT-proBNP, N-terminal pro–B-type natriuretic peptide; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
The coronavirus disease-2019 (COVID-19) pandemic caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has variable clinical impact. The majority of cases are mild and often asymptomatic, but a minority of individuals have severe acute respiratory syndrome, the most frequent cause of death (1). Multiorgan involvement occurs in severe disease, and the cardiovascular system is often involved in hospitalized COVID-19. Mechanisms include acute coronary syndrome, exacerbation of pre-existing cardiovascular disease, arrhythmia, myocarditis, and microangiopathic thrombosis (2). Pathophysiological mechanisms include disordered clotting, superimposed infection, cytokine storm, and hemophagocytic lymphohistiocytosis (3,4). Troponin elevation is common in severely ill hospitalized patients (5) and associated with adverse outcomes (6). However, cardiovascular disease is also a known risk factor for severe disease (7), so disentangling association and causation is challenging.
Cardiovascular magnetic resonance (CMR) has proven utility for diagnosis in patients with elevated troponin from unclear causes (8,9) by measuring cardiac structure, function, myocardial scar (late gadolinium enhancement), and edema (T1 and T2 mapping). Despite the logistical challenges of CMR during acute severe hospitalized COVID-19, studies reported multiple CMR abnormalities in these patients (10,11).
During convalescence, long-term cardiovascular effects and their (12) mechanisms are currently unclear, but chronic myocarditis has been proposed following severe hospitalized COVID-19 (13). Recent CMR studies have reported cardiac abnormalities after COVID-19 in up to 78% of patients, even after mild, nonhospitalized illness with evidence of ongoing myocardial inflammation in 60% (14). Such a prevalence of chronic myocarditis after mild disease has prompted societal concerns in diverse domains and suggests that screening should be considered post–COVID-19, even in asymptomatic individuals. However, study design issues (mixed severe and mild patients, historic control subjects, CMR sequence choice, and the definition of normal for mapping), concern that an isolated elevation of a parameter such as T1 or T2 is not disease, and errors requiring post-publication revision (15) have stimulated further investigation, with particular focus on mild disease. Furthermore, autopsy studies have suggested that myocarditis may not be as common as initially thought (16).
We therefore aimed to determine the prevalence and extent of late cardiac and cardiovascular sequelae after mild nonhospitalized SARS-CoV-2 infection.
Methods
This was a nested case-control study within COVIDsortium (ethical approval: South Central-Oxford A Committee, 20/SC/0149, NCT04318314; diffuse fibrosis CMR ethics: 07/H0715/101). Details have been previously described (17). In brief, the parent COVIDsortium study is a prospective study of 731 health care workers from 3 London hospitals starting early in the first COVID-19 wave. Participants underwent serial weekly polymerase chain reaction (PCR) and serology testing over 16 weeks (n = 6,495 Roche cobas, SARS-CoV-2 PCR; n = 12,990 Eurimmun anti spike S1 and Roche anti nucleocapsid tests [Roche, Basel, Switzerland]), with symptom ascertainment (median 10 visits). Comorbidities were relatively low (18% smokers, 13% body mass index >30 kg/m2, 11% asthma, 7% hypertension, 2% diabetes, 1% rheumatological disease, 1% cancer). A total of 38% were non-White, including 6% of Black ethnicity). Across the overall study, 21% (n = 157) seroconverted. Disease was mild in 99%, with 25% asymptomatic and only 2 hospitalized (2 days, no deaths). All infections occurred prior to May 1, 2020 (no new seroconversions, no new PCR-positive tests), with more than 90% of infections understood (from PCR, symptoms, and antibody data) to have been between March 1, 2020, and April 14, 2020 (18,19).
Participants
Participants were invited to the cardiovascular nested substudy. In total, 74 seropositive participants (47% of total seropositive patients; 51% of available seropositive patients [2 withdrew, 6 left the hospital, and 5 had CMR contraindications (2 pregnancy, 1 implant, 1 metallic fragment, and 1 prior contrast reaction)]) were recruited, along with 75 age-, sex-, and ethnicity-matched control subjects selected from volunteering seronegative subjects. Control subjects were recruited blinded to clinical data.
Study protocol
Blood was taken for cardiac biomarkers (high-sensitivity troponin T [hsTnT] and N-terminal pro–B-type natriuretic peptide [NT-proBNP]) and hematocrit. Peripheral and central blood pressures were recorded as the average of suprasystolic oscillometric blood pressure measurements acquired over 10 s at 200 Hz in duplicate after a period of rest in the semisupine position, as per international guidelines (Cardioscope II BP+, Uscom Ltd., Sydney, Australia).
Cardiac magnetic resonance
Scans were acquired at 2 sites: Royal Free (1 scanner) or Barts (2 scanners) between September 3, 2020, and November 7, 2020, in accordance with infection control guidelines (20) on 1.5-T CMR scanners (Magnetom Aera, Siemens Healthcare, Erlangen, Germany). Given pandemic pressures, an adapted 30-min protocol was used. This comprised anatomic images, long- and short-axis cines, T1, T2, and extracellular volume fraction (ECV) mapping (a mid-short axis and 4-chamber view each) with late gadolinium enhancement and aortic pulse wave velocity. T1 mapping used a modified Look-Locker inversion recovery sequence (5s[3s]3s pre-contrast, 4s[1s]3s[1s]2s post-contrast). The contrast was 0.1 mmol/kg Gadoterate meglumine (gadolinium-DOTA, Dotarem, Guerbet S.A., Paris, France). Mapping acquisition/analysis followed international consensus statements (21,22) with additional quality assurance steps as indicated in the Supplemental Appendix.
CMR analysis
All quantitative analyses were performed blinded to participant status. All scans were initially clinically reported (J.C.M.) and significant incidental extracardiac/cardiac findings and blood test abnormalities were committee adjudicated (J.C.M., C.M., T.A.T.) with appropriate action as needed (further testing, clinical review). Left ventricular (LV) structure and function was analyzed using a clinically validated artificial intelligence (AI) platform (23). Left atrial (LA) area and global longitudinal shortening was analyzed by further validated AI approaches (Supplemental Methods) (24). Aortic stiffness, distensibility, and pulse wave velocity were measured using validated software (ArtFun+, Imageens, Paris, France) and central pulse pressure. A standard operating procedure was developed for T1, T2, ECV, and late gadolinium enhancement (LGE), with all analyses performed by 2 observers (GJ and RA) using Circle CVI42 version 5.12.1 (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada). Quality assurance procedures included review of motion correction, artifact review, the use of field maps for potential bias errors, and a blinded contour review by an independent U.S.-based team for technical errors (P.K.), and clinical plausibility (E.B.S.). In brief, endocardial and epicardial contours (10% offset) were drawn and automatically divided into 6 segments. For LGE, a 3-SD approach was taken. LGE of <1% of the myocardium at the right ventricular (RV) insertion points only was ignored. ECV was derived using a same-day hematocrit. Magnet quality assurance used the T1MES phantom to ensure no differences between the 3 magnets and no temporal drift (25,26). Across the 3 magnets, temperature-corrected T1 and T2 was within 0.33% and 0.95%, respectively, with temporal drift of <1% (Supplemental Methods).
Power calculation
For information about power calculations, please see the Supplemental Methods.
Statistical analysis
Analyses were pre-specified. The 5 primary analyses were LV ejection fraction (EF), indexed end-diastolic volume, percentage LGE, septal T1, and septal T2. The 5 secondary analyses were indexed LV mass, indexed LA area, global longitudinal shortening (average of 3 views), septal ECV, and aortic distensibility (diaphragm level). Planned exploratory analyses were global and segmental mapping, heart rate, NT-proBNP and hsTnT levels, and blood pressure. Correlations of any SARS-CoV-2–associated cardiovascular abnormalities were planned with: 1) demographics; 2) immune response (peak antibody); and 3) symptoms (case defining, noncase defining, asymptomatic).
Analyses used SPSS Statistics version 26 (IBM, Armonk, New York). Data were examined for normality using the Kolmogorov-Smirnov test and visual inspection of histograms and Q-Q plots. Normally distributed variables were expressed as mean ± SD; non-normal as median (interquartile range [IQR]). Proportions were expressed as absolute frequencies and percentages. Independent and paired Student’s tests (2-tailed), the Mann-Whitney U test, Wilcoxon signed-rank test, and chi-square and Fisher exact test were used as appropriate. The 5 primary and 5 secondary endpoints were to be considered separately, with each having a critical 2-sided Benjamini-Hochberg’s p value to be considered significant, assuming a false discovery rate of 5%.
Data access
All individual participant deidentified data (serology, PCR results, Digital Imaging and Communications in Medicine images, meta-data, data dictionaries) are available upon reasonable request (Supplemental Methods).
Results
Patients
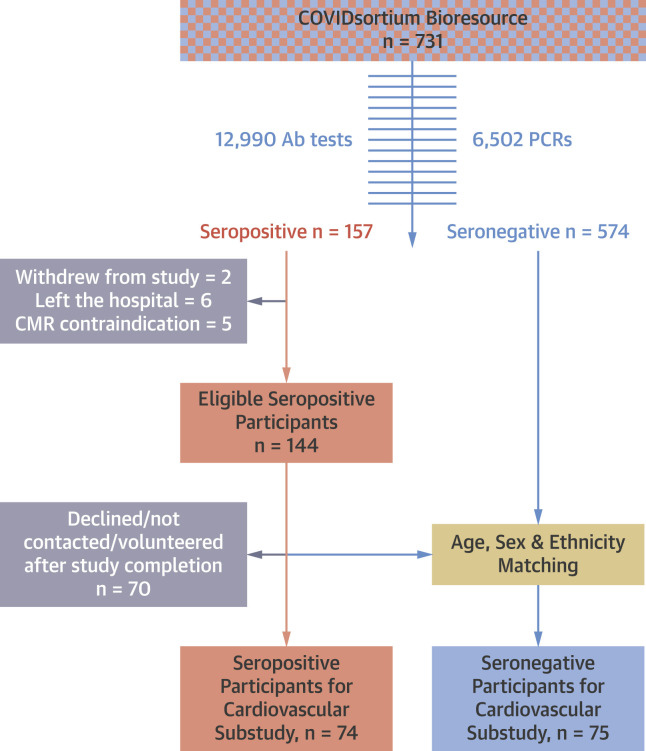
The study CONSORT diagram is shown in Figure 1 . A total of 149 subjects (74 seropositive vs. 75 seronegative; median age 37 years [range 18 to 63 years], 42% men, 32% non-White ethnicity) were recruited from COVIDsortium and underwent cardiovascular phenotyping 6 months 9 days (IQR: 5 months 26 days to 6 months 20 days) post–SARS-CoV-2 infection. Cases were well matched (Table 1 ).
Figure 1.
CONSORT Diagram
The study design: a nested substudy of the parent COVIDsortium Bioresource. Ab = antibody; CMR = cardiac magnetic resonance; PCR = polymerase chain reaction.
Table 1.
Cohort Characteristics
| Whole Cohort (N = 149) | Seropositive (n = 74) | Seronegative (n = 75) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs | 37 (31–48) | 39 (30–48) | 37 (31–47) | 0.89 |
| Female | 86 (58) | 46 (62) | 40 (53) | 0.28 |
| BSA, m2 | 1.89 ± 0.22 | 1.88 ± 0.21 | 1.90 ± 0.23 | 0.51 |
| Ethnicity | ||||
| White | 103 (69) | 50 (68) | 53 (71) | 0.68 |
| Black | 17 (11) | 10 (14) | 7 (9) | 0.42 |
| Asian | 20 (13) | 11 (15) | 9 (12) | 0.61 |
| Mixed race | 9 (6) | 3 (4) | 6 (8) | 0.49 |
| Role | ||||
| Doctor | 40 (27) | 20 (27) | 20 (27) | 0.96 |
| Nurse | 48 (32) | 25 (34) | 23 (31) | 0.68 |
| Ancillary staff | 53 (36) | 26 (35) | 27 (36) | 0.91 |
| Other | 8 (5) | 3 (4) | 5 (7) | 0.71 |
| Past medical history | ||||
| Hypertension | 14 (9) | 10 (14) | 4 (5) | 0.09 |
| Hyperlipidemia | 7 (5) | 4 (5) | 3 (4) | 0.69 |
| Diabetes mellitus | 3 (2) | 2 (3) | 1 (1) | 0.62 |
| Smoker (previous or current) | 24 (16) | 10 (14) | 14 (19) | 0.39 |
| Family history of CAD | 21 (14) | 9 (12) | 12 (16) | 0.5 |
| Asthma/COPD | 18 (12) | 13 (17) | 5 (7) | 0.04 |
| Exercise, h/week | 3.5 (2.0–5.0) | 3.5 (2.0–5.0) | 3 (1.5–6.0) | 0.84 |
| Symptoms | ||||
| Asymptomatic | 11 (15) | |||
| Noncase definitiona | 18 (24) | |||
| Case definitiona | 45 (61) |
Values are median (interquartile range), n (%), or mean ± SD.
BSA = body surface area; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease;
Case definition—at least one of the following (fever, cough, shortness of breath, anosmia, ageusia, or dysgeusia).
Seropositive infections had been mild with case definition symptoms (fever, new dry cough, anosmia, ageusia, or dysgeusia), noncase definition symptoms, and asymptomatic disease in 45 (61%), 18 (24%) and 11 (15%) respectively, with 1 person hospitalized (for 2 days). Symptoms had been recorded throughout the parent study weekly. At the time of scanning (6 months), 16 (11%) reported symptoms: 5 (3%) sore throat; 4 (3%) fatigue; 4 (3%) rhinorrhea; 3 (2%) shortness of breath; and 1 (1%) each of productive cough, chills, diarrhea, anosmia, and ageusia, with no difference between seropositive and seronegative subjects (6 [8%] vs. 10 [13%]; p = 0.47).
Study
CMR scanning took a median 30 min (IQR: 27 to 32 min). Example images and the CMR protocol are shown in Supplemental Figure 1. All data were >99% complete (see details in the Supplemental Methods). Segmentation of LV structure, function, LGE, T1, and T2 are shown in Supplemental Figure 2.
Primary and secondary endpoints
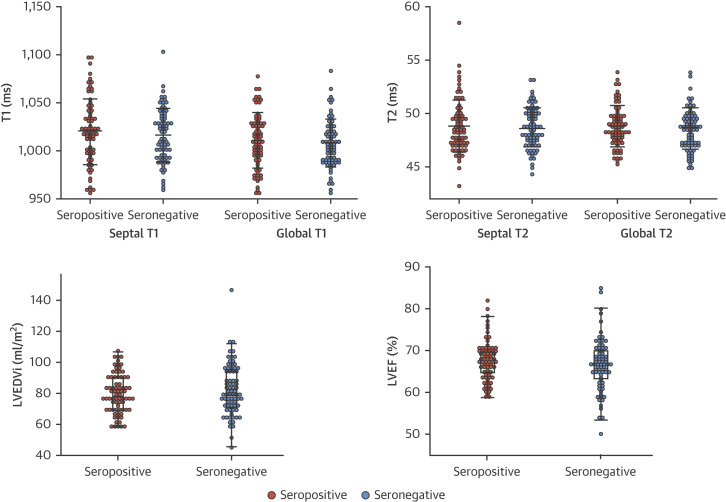
Between seropositive and seronegative groups, there were no statistically significant differences in any of the 5 pre-specified primary endpoints (LVEF, indexed end-diastolic volume, LGE%, septal T1, septal T2, marked as dagger in Table 2 ) or 5 pre-specified secondary endpoints (LV mass indexed, LA area indexed, global longitudinal shortening [average of 3 views], septal ECV, and aortic distensibility marked as double daggers in Table 2), even without correction for multiple testing (Benjamini-Hochberg’s method), with all p values >0.05 (Central Illustration ). All other comparisons were also nonsignificant (Table 2). Between-group differences between T1 and T2 are shown in Figure 2 .
Table 2.
Primary and Secondary Analyses Results
| Whole Cohort (N = 149) | Seropositive (n = 74) | Seronegative (n = 75) | p Value | |
|---|---|---|---|---|
| Blood pressure | ||||
| Peripheral sBP, mm Hg | 119 (109–130) | 115 (109–130) | 121 (110–131) | 0.3 |
| Peripheral dBP, mm Hg | 75 ± 9 | 74 ± 9 | 75 ± 10 | 0.58 |
| Laboratory investigations | ||||
| Hs troponin T (normal <14) | 4 (3–7) | 4 (3–7) | 4 (3–7) | 0.57 |
| NT-proBNP (normal <400) | 31 (18–54) | 36 (18–53) | 28 (17–56) | 0.24 |
| Function | ||||
| LVEF, % | 67.1 (63.7–70.1) | 67.5 (64.4–70.2) | 66.8 (62.8–70.1) | 0.28∗ |
| GLS mean, % | 17.4 ± 2.1 | 17.5 ± 1.8 | 17.3 ± 2.4 | 0.62† |
| Structure | ||||
| LVEDV indexed, ml/m2 | 78.8 (70.2–90.9) | 78.1 (69.7–90.3) | 80.0 (71.3–94.9) | 0.37∗ |
| LA area indexed, ml/m2 | 12.0 (10.9–13.1) | 12.0 (11.0–13.2) | 11.9 (10.6–13.1) | 0.87† |
| LV mass index, g/m2 | 46.6 (41.2–56.7) | 47.5 (41.5–57.6) | 47.5 (41.5–56.7) | 0.56† |
| Tissue characterization | ||||
| LGE, % | 0.29 ± 0.86 | 0.27 ± 0.78 | 0.32 ± 0.93 | 0.72∗ |
| RV insertion point | 14 (9) | 8 (11) | 6 (8) | 0.56 |
| Non-RV insertion point | 13 (9) | 6 (8) | 7 (9) | 0.79 |
| T1 septum, ms | 1,018 ± 31 | 1,020 ± 34 | 1,016 ± 28 | 0.42∗ |
| High | 6 (4) | 5 (7) | 1 (1) | 0.12 |
| T1 global, ms | 1,009 ± 27 | 1,010 ± 28 | 1,007 ± 25 | 0.47 |
| High | 6 (4) | 4 (5) | 2 (3) | 0.44 |
| T2 septum, ms | 48.7 ± 2.2 | 48.8 ± 2.5 | 48.6 ± 1.9 | 0.63∗ |
| High | 9 (6) | 7 (9) | 2 (3) | 0.098 |
| T2 global, ms | 48.6 ± 1.9 | 48.7 ± 1.9 | 48.4 ± 1.9 | 0.3 |
| High | 7 (5) | 4 (5) | 3 (4) | 0.72 |
| ECV septal, % | 22.2 ± 2.1 | 22.3 ± 2.0 | 22.1 ± 2.2 | 0.57† |
| High | 5 (3) | 3 (4) | 2 (3) | 0.68 |
| ECV global, % | 21.5 ± 2.0 | 21.6 ± 1.9 | 21.5 ± 2.1 | 0.73 |
| High | 4 (3) | 3 (4) | 1 (1) | 0.37 |
| Aortic stiffness | ||||
| Distensibility, mmHg-1·10-3 | 12.3 (8.7–17.5) | 12.6 (9.1–18.3) | 12.0 (8.7–17.1) | 0.74† |
Values are median (interquartile range), mean ± SD, or n (%). NT-proBNP in pg/ml and hs-troponin T in ng/ml. All indexing is to body surface area.
BSA = body surface area; COPD = chronic obstructive pulmonary disease; dBP = diastolic blood pressure; ECV = extracellular volume fraction; EDV = end-diastolic volume; EF = ejection fraction; GLS = global longitudinal shortening; IQR = interquartile range; LGE = late gadolinium enhancement; LV = left ventricle; LVM = left ventricular mass; NT-proBNP = N-terminal pro-brain natriuretic peptide; RV = right ventricular; sBP = systolic blood pressure.
Pre-specified primary endpoint.
Pre-specified secondary endpoint.
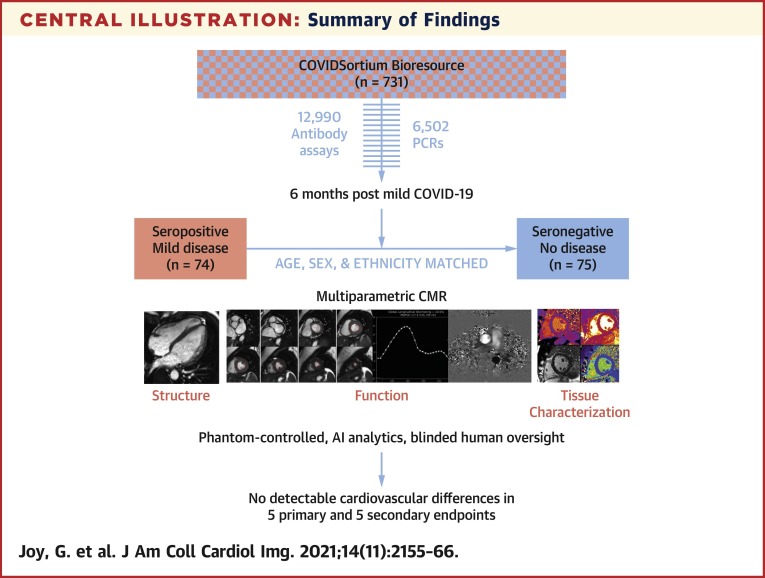
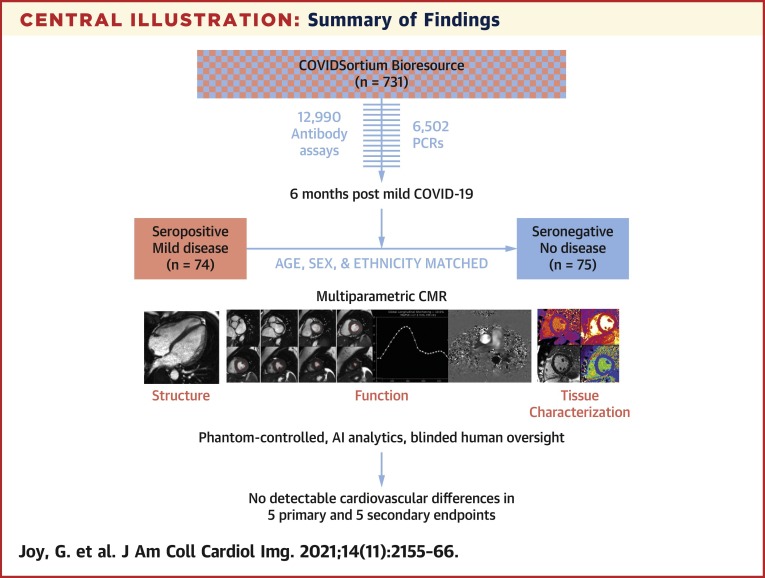
Central Illustration.
Summary of Findings
Participants were recruited from COVIDsortium, a 3-hospital prospective study of 731 health care workers who underwent first-wave weekly symptom, polymerase chain reaction, and serology assessment over 4 months, with seroconversion in 21.5% (n = 157). At 6 months post–mild severe acute respiratory syndrome coronavirus 2 infection, 74 seropositive and 75 age-, sex-, and ethnicity-matched seronegative health care workers underwent multiparametric cardiac magnetic resonance (CMR). This was phantom controlled, using artificial intelligence analytics with blinded human oversight. Our main finding was that there were no detectable cardiovascular differences in 5 primary and 5 secondary endpoints.
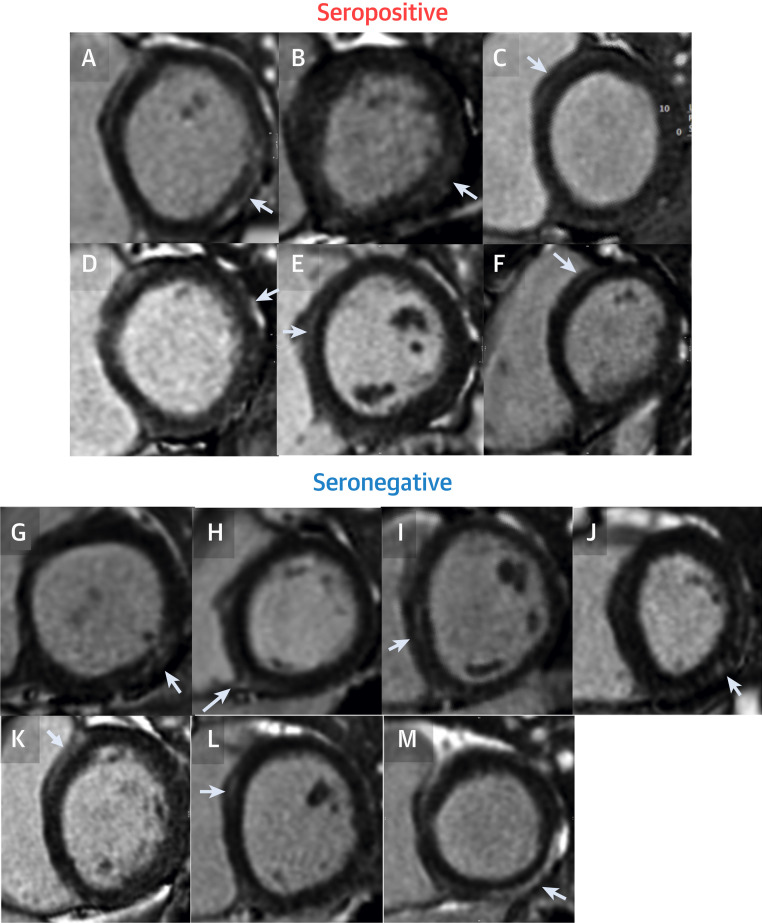
Figure 2.
All Subjects With Reported LGE Outside of the RV Insertion Point
There were 13 subjects with reported LGE outside of the RV insertion point. We show a single slice from all (i.e., no freedom to select cases). In total, 6 were found in seropositive cases (A to F), and 7 in seronegative cases (G to M). In addition to the low frequency of LGE abnormalities, the LGE was mainly small-volume, noninfarct pattern and nonspecific. Some may be normal (e.g., a likely septal perforator vessel visible in the anteroseptum) (A). In 2, the abnormality was 3 segments (M and J). For the rest of these subject results, see Figure 4. For LGE segmentation, see Supplemental Figure 1. LGE = late gadolinium enhancement; RV = right ventricular.
Additional endpoints
There were no between-group differences (seropositive vs. seronegative) across any exploratory analyses, including global (rather than septal) T1, T2 or ECV, RV volumes or RVEF, ascending and descending thoracic aortic pulse wave velocity, heart rate, NT-proBNP, hsTnT, or blood pressure. As no CMR or biomarker abnormalities were associated with prior SARS-CoV-2 infection, further correlations with demographics, immune response, or symptoms could not be performed.
Other CMR abnormalities
An abnormality was defined by either departmental standard clinical cutpoints (e.g., aortic root 36 mm), laboratory test normal ranges (hsTnT <14 ng/l, NT-proBNP <400 pg/l), or for primary/secondary endpoints, by using the 75 seronegative subjects to define abnormal (2 SDs from mean: e.g., EF <54%, T1 >1,072 ms, T2 >52.4 ms). Mild abnormalities were found including: aortic root dilatation n = 2, all mild), LA dilatation (n = 6, all mild) reduced LV function (n = 2, EF 50%–53%); T1 elevation (n = 6), T2 elevation (n = 9), non-RV insertion point LGE (n = 13, median 1%, maximum 5% of myocardium). All 13 cases with positive LGE are shown in Figure 2 (6 were seropositive, 7 were seronegative: all were noninfarct pattern, and some of the LGE patterns were nonspecific [2 were 3 segments, 1 was 4 segments]). Prevalence of myocarditis-like scar was 4% both in the seropositive and seronegative groups (i.e., no significant difference). There was no pericardial thickening seen in any subject. Individual participant septal T1 and T2 data in both groups along with the other primary endpoints and global T1 and T2 are shown in Figure 3 . Note: there is a single T2 septal outlier (4SD) that is considered on post hoc review to be erroneously high from poor motion correction, but has been left in the results. No subject had NT-proBNP elevation. A total of 4 subjects had borderline troponin elevation (maximum 26 ng/l, 3 seronegative, 1 seropositive). None of these were statistically more common in the seropositive group. Clinical results review recommended 2 people to have notes/full study data review, further imaging (1 echocardiography for aortic valve review, 1 chest radiography—both required no further follow-up) and 4 underwent clinical review—2 for reduced LV function, 2 for noninfarct pattern LGE (Figures 2J and 2M [2M was found to have been chronic and unchanged since a healthy volunteer study in 2011]).
Figure 3.
Dot Plots of the Pre-Specified Primary Endpoints
There are no statistical differences in any between group comparisons. LGE is not drawn (as 136 were negative with 6 in the seropositive and 7 in the seronegative; p = NS – making it hard to graph). Septal T1 and T2 were pre-specified endpoints, but global T1 and T2 were also measured and are displayed here (also with no statistical differences). LGE = late gadolinium enhancement; LVEDVi = left ventricular end-diastolic volume indexed; LVEF = left ventricular ejection fraction.
Abnormality clustering
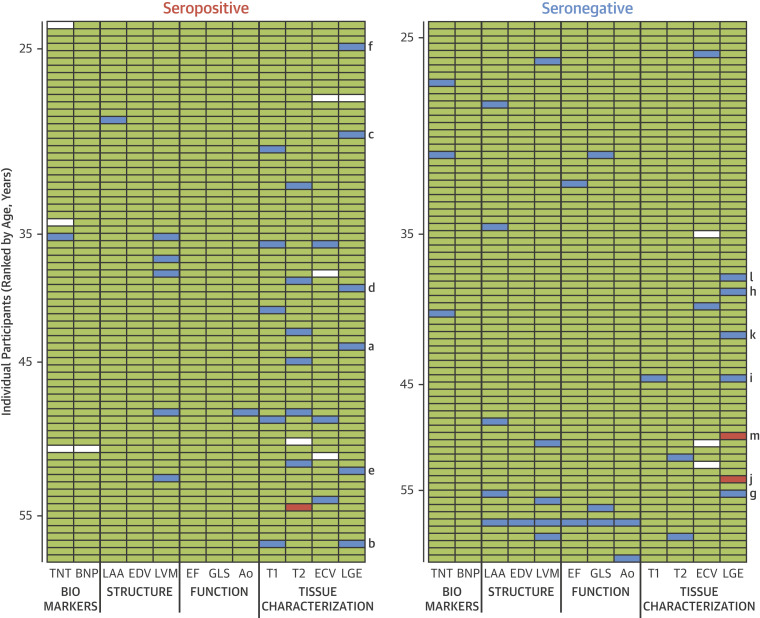
Figure 4 shows results for all 149 subjects (rows) and across the 12 key biomarkers (10 primary/secondary imaging endpoints plus blood biomarkers [columns]). Each horizontal row (12 across) is a health care worker split by serostatus (right/left) and in age order (top/bottom), for a total of 1,776 results. A green cell is a normal result; amber 2SDs abnormal, LGE present or biomarker above lab reference range cut-off; red 4 SDs abnormal or LGE ≥3 segments; and white is missing data. LGE subjects are marked with the letters a to m cross-referencing Figure 3, but only 2 participants (both seronegative) had LGE that looked clinically significant. A disease phenotype is likely to have multiple abnormalities cosegregating within individual patients in a horizontal line, as was found in 1 subject (seronegative subject, age >55 years) (Figure 4). There were more individual seropositive participants with isolated values for septal T1 and septal T2 outside of 2 SDs from the mean than for seronegative participants, but these differences were nonsignificant, they did not cosegregate with LGE, and elevated values were not reproduced with global rather than septal measurement (Table 2) or with T1 and T2 as a continuous variable (Figure 2).
Figure 4.
Graphical Representation of Abnormalities in Blood and Imaging Within Individuals by Serostatus
All 149 subjects ranked by age with the results of the 5 primary and 5 secondary endpoints, plus biomarkers. Each horizontal row (12 across) is a health care worker, with seropositives (left) and seronegatives (right). A green cell is a normal result, blue is 2 SDs abnormal or LGE present, red is 4 SDs abnormal or LGE ≥3 segments, and white is missing data. (A to M) Cross-reference the LGE images in Figure 2. Of the 1,776 results ([12 × 149] – 12 missing datapoints), abnormalities cluster in only a few (see the >55-year-old seronegative subject, a horizontal line of 6 abnormal results). Note that when T1 and T2 are abnormal (15 times), in no case were T1 and T2 abnormal at the same time. There is a single T2 septal outlier (4 SD) considered on post hoc review to be erroneously from poor motion correction, but has been left in the pre-specified analyses. BNP = B-type natriuretic peptide; ECV = extracellular volume fraction; EDV = left ventricular end diastolic volume indexed; EF = ejection fraction; GLS = global longitudinal shortening; LAA = left atrial area indexed; LGE = late gadolinium enhancement; LVM = left ventricular mass index; TNT = troponin T.
Discussion
This study demonstrates that in healthy people, measured cardiovascular abnormalities are common, but no more common in those who had had mild SARS-CoV-2 6 months previously compared with those who had not. In other words, in this population, mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers. The cardiovascular phenotyping employed was comprehensive and measured parameters that reflected cardiac structure (LV volumes, mass, LA area), function (LVEF, global longitudinal shortening, mitral annular plane systolic excursion), inflammation (T1 and T2), focal fibrosis (LGE), diffuse fibrosis (ECV), aortic compliance, heart rate, blood pressure, and high-sensitivity troponin and NT-proBNP.
The study was designed to focus on late effects after mild (rather than hospitalized) disease, following an earlier pathfinder study (14) that indicated high (78%) rates of post COVID-19 cardiac involvement with ongoing myocardial inflammation in 60%, even after nonhospitalized disease, based on mapping and late gadolinium enhancement findings. Subsequent studies have generally been small and included either convalescent symptomatic patients (27), post-hospitalized patients (28), or specific populations (recovering athletes) (29). These results have collectively attracted widespread attention, with mainstream media coverage and downstream societal impact. Clinicians and experts managing viral myocarditis patients have queried the atypical duration of disease and rates of involvement with mild disease. In line with this, where histology is available from autopsy, meta-analyses have only occasionally shown lymphocytic infiltrates suggestive of myocarditis (30,31), but have more commonly found interstitial macrophage infiltration, consistent with other forms of sepsis. In parallel, there has been discussion across several stakeholder groups recognizing limitations and differences between the design and technical methodology and analysis of some studies (32). Studies have generally included only severe disease or mixed populations without inclusion of asymptomatic seroconverters, and have scanned early following disease or at variable time points, meaning it is challenging to draw clear conclusions. Technical limitations include also include the T1 mapping sequence used (high read-out flip angle rendering it prone to confounding by T2, heart rate, off resonance, and magnetization transfer) (33) and the use of historic controls –time, sequence, scanner, institution, observer, and analysis method affect T1 measurement.
The current study has a number of advantages and was designed to address the knowledge gaps arising from prior studies. As a nested substudy of COVIDsortium, participants were prospectively recruited predominantly prior to infection, hence minimizing recruitment bias. This enabled identification of asymptomatic individuals with the mildest phenotype of disease who have not previously been studied for cardiovascular effects. Bias from symptom recall was low, and serological testing was comprehensive due to the parent study design with serial testing at a median of 10 time points using 2 assays. Control subjects were recruited from the same study contemporaneously, and were well matched with cases. The substudy recruited ∼50% of seropositive individuals and matched control subjects. Scanning of case and control subjects was performed in parallel, and all acquisitions and analysis was performed blinded to serostatus. Quality control of T1 and T2 measurement was performed via U.S. Food and Drug Administration– and European Medicines Agency–approved phantom calibration showing near identical measurement performance and no temporal drift during the study. Application of validated AI analytic tools improves the ability to detect normal (23,24). New quality assurance methods were used including field map measurement in a subset and blinded review for region of interest identification by 2 separate U.S. based teams and review of T1 SD maps to validate performance (34). All analyses were pre-specified, and all images and metadata are available on reasonable request.
Study limitations
The current study provides insight only into the short- to medium-term sequelae of community COVID-19 in workforce representative subjects age 18 to 69 years with low levels of comorbidities, and not the cardiovascular effects post–severe hospitalized infection or in those with multimorbidity. It does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis. The study design would not distinguish between individuals who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected; a cross-sectional athlete study at 1 month post–mild community COVID-19 reported a significant pericardial involvement (late enhancement and/or pericardial effusion), but this study is at 6 months (when the pericardium was normal). No baseline pre–COVID-19 imaging was performed (35). We did not analyze other cardiovascular measures (including exercise testing). It would not be feasible or appropriate to obtain histological data from overwise healthy subjects.
Conclusions
In a workforce representative population, using best-available study design (prospective recruitment, contemporaneous control subjects, phantom scanner calibration, blinded analysis, full data availability to other researchers), there are no detectable persistent cardiovascular abnormalities 6 months post–mild infection with SARS-CoV-2 compared with matched case control subjects. Thus, screening in asymptomatic patients following nonhospitalized COVID-19 is currently not indicated.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Mild SARS-CoV-2 infection has not been found to cause detectable cardiovascular abnormalities 6 months post-infection in our study.
COMPETENCY IN PATIENT CARE: Our study provides societal reassurance for the cardiovascular health of working-age individuals with convalescence from mild SARS-CoV-2. Screening asymptomatic individuals following mild diseases is not indicated.
COMPETENCY IN INTERPERSONAL AND COMMUNICATION SKILLS: It is important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects.
TRANSLATIONAL OUTLOOK: Efforts to understand cardiovascular injury from SARS-CoV-2 infection require meticulous attention to study design, including inclusion of contemporary control groups and measurement quality control. This study suggests such research should focus on patients with acute infections or following severe hospitalized disease, with little benefit from screening asymptomatic patients following mild community infection.
Funding Support and Author Disclosures
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. Wider support is acknowledged on the COVIDsortium web site. Institutional support from Barts Health NHS Trust and Royal Free NHS Foundation Trust facilitated study processes, in partnership with University College London and Queen Mary University London. Serology tests (anti-S1 and anti-NP) were funded by Public Health England. This study forms part of the portfolio of COVID-Heart, a UKRI UKRI-DHSC funded study (ISRCTN58667920). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Dr Seraphim is supported by a doctoral research fellowship from the British Heart Foundation (FS/18/83/34025). Dr Augusto is supported by an EACVI grant. Prof. McKnight is supported by Rosetrees trust, The John Black Charitable Foundation, and Medical College of St. Bartholomew’s Hospital Trust. Prof. Noursadeghi is supported by the Wellcome Trust (207511/Z/17/Z) and by NIHR Biomedical Research Funding to UCL and UCLH. Prof. Fontana is supported by a BHF Intermediate Research Fellowship (FS FS/18/21/33447). Dr Treibel is funded by a BHF Intermediate Research Fellowship (FS/19/35/34374). Drs Treibel and Manisty and Prof. Moon are directly and indirectly supported by the University College London Hospitals (UCLH) and Barts NIHR Biomedical Research Centres and through the British Heart Foundation (BHF) Accelerator Award (AA/18/6/34223). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Cheelo Simaanya, Lizette Cash, Matt Davies, Cavan McGurk, Nadine Miller, Salma Mohammed, Marisa Bairos, Rosie Goddard, Fiona Hamilton, Jed Gibbs, Joanne Vickers, Federica Perinu, Kevin Konickal, and Mohammed Mahmoodi.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris G., Bortolasci C.C., Puri B.K. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life Sci. 2020;258:118166. doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 4.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 5.Long B., Long D.A., Tannenbaum L., Koyfman A. An emergency medicine approach to troponin elevation due to causes other than occlusion myocardial infarction. Am J Emerg Med. 2020;38:998–1006. doi: 10.1016/j.ajem.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S., Anstine C., Jaffe A.S. Cardiac magnetic resonance in patients with elevated troponin and normal coronary angiography. Heart. 2019;105:1231–1236. doi: 10.1136/heartjnl-2018-314631. [DOI] [PubMed] [Google Scholar]
- 9.Dastidar A.G., Baritussio A., De Garate E. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. J Am Coll Cardiol Img. 2019;12:1973–1982. doi: 10.1016/j.jcmg.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Esposito A., Palmisano A., Natale L. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. J Am Coll Cardiol Img. 2020;13:2462–2465. doi: 10.1016/j.jcmg.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojha V., Verma M., Pandey N.N. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging. 2021;36:73–83. doi: 10.1097/RTI.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 12.Yelin D., Wirtheim E., Vetter P. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight D.S., Kotecha T., Razvi Y. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel E., Puntmann V.O. Errors in statistical numbers and data in study of cardiovascular magnetic resonance imaging in patients recently recovered from COVID-19. JAMA Cardiol. 2020;5:1307–1308. doi: 10.1001/jamacardio.2020.4661. [DOI] [PubMed] [Google Scholar]
- 16.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augusto J., Menacho K., Andiapen M. Healthcare Workers Bioresource: study outline and baseline characteristics of a prospective healthcare worker cohort to study immune protection and pathogenesis in COVID-19. Wellcome Open Res. 2020:179. doi: 10.12688/wellcomeopenres.16051.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manisty C., Treibel T., Jensen M. Characterising heterogeneity and sero-reversion in antibody responses to mild SARS CoV-2 infection: a cohort study using time series analysis and mechanistic modelling. medRxiv. 2020 doi: 10.1016/j.ebiom.2021.103259. 2020.11.04.20225920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treibel T.A., Manisty C., Burton M. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395:1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y., Chen T., Bryant J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020;22:26. doi: 10.1186/s12968-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messroghli D.R., Moon J.C., Ferreira V.M. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2∗ and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon J.C., Messroghli D.R., Kellman P. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augusto J.B., Davies R.H., Bhuva A.N. Diagnosis and risk stratification in hypertrophic cardiomyopathy using machine learning wall thickness measurement: a comparison with human test-retest performance. The Lancet Digital Health. 2021;3:e20–e28. doi: 10.1016/S2589-7500(20)30267-3. [DOI] [PubMed] [Google Scholar]
- 24.Xue H, Artico J, Fontana M, Moon JC, Davies RH, Kellman P. Landmark detection in Cardiac Magnetic Resonance Imaging Using A Convolutional Neural Network. August 14, 2020. Accessed May 3, 2021. https://arxiv.org/abs/2008.06142. [DOI] [PMC free article] [PubMed]
- 25.Captur G., Gatehouse P., Keenan K.E. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance-the T1 Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18:58. doi: 10.1186/s12968-016-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Captur G., Bhandari A., Bruhl R. T1 mapping performance and measurement repeatability: results from the multi-national T1 mapping standardization phantom program (T1MES) J Cardiovasc Magn Reson. 2020;22:31. doi: 10.1186/s12968-020-00613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L., Zhao P., Tang D. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng M.Y., Ferreira V.M., Leung S.T. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2476–2478. doi: 10.1016/j.jcmg.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajpal S., Tong M.S., Borchers J. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salamanca J., Diez-Villanueva P., Martinez P. COVID-19 “fulminant myocarditis” successfully treated with temporary mechanical circulatory support. J Am Coll Cardiol Img. 2020;13:2457–2459. doi: 10.1016/j.jcmg.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basso C., Leone O., Rizzo S. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengupta P.P., Chandrashekhar Y.S. Cardiac involvement in the COVID-19 pandemic: hazy lessons from cardiac imaging? J Am Coll Cardiol Img. 2020;13:2480–2483. doi: 10.1016/j.jcmg.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellman P., Hansen M.S. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellman P., Arai A.E., Xue H. T1 and extracellular volume mapping in the heart: estimation of error maps and the influence of noise on precision. J Cardiovasc Magn Reson. 2013;15:56. doi: 10.1186/1532-429X-15-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brito D., Meester S., Yanamala Y. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. J Am Coll Cardiol Img. 2021;14:541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.