Abstract
Objective
To evaluate the trends in incident premature myocardial infarction (MI) and prevalence of cardiac risk factors in a population-based cohort.
Methods
We studied a population-based cohort of incident premature MIs among residents (MI in men aged 18-55 years and women aged 18-65 years) in Olmsted County, Minnesota, during a 26-year period from January 1, 1987 through December 31, 2012. Recurrent MI and death after incident premature MI were enumerated through September 30, 2018.
Results
Of 3276 MI cases, 850 were premature events (37.9% [322/850] women). Age-adjusted premature MI incidence rates (2012 vs 1987) declined by 39% in men (rate ratio, 0.61; 95% CI, 0.46 to 0.81]) and 61% in women (rate ratio, 0.39; 95% CI, 0.27 to 0.57). Among men with premature MI, the prevalence of hypertension, diabetes, and hyperlipidemia increased over time, whereas in women, only the prevalence of hyperlipidemia increased. During a mean follow-up of 13.3 years, there was no temporal decline in recurrent MI in men and women. Women showed 66% decreased risk for mortality (hazard ratio, 0.34; 95% CI, 0.17 to 0.68) over time, whereas men showed no change.
Conclusion
The incidence of premature MI declined over a 26-year period for both men and women. The risk factor profile of persons presenting with MI worsened over time, especially in men. Death following incident MI declined only in women. These results underscore the importance of primary prevention in young adults and of sex-specific approaches.
Abbreviations and Acronyms: ARIC, Atherosclerosis Risk in Communities; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio; MI, myocardial infarction; MINOCA, myocardial infarction with nonobstructive coronary arteries; REP, Rochester Epidemiology Project; RR, rate ratio
During the last 4 decades, overall myocardial infarction (MI) mortality rates in the United States have declined without apparent benefit in adults aged 25 to 64 years, in whom mortality rates have leveled off.1, 2, 3, 4 Several factors may contribute to the suboptimal trends in these adults, including changes in incidence of MI and prevalence of its risk factors, acute management, and use of preventive therapies.2,4, 5, 6 Despite this, there is sparse information on trends in incident premature MI and associated cardiac risk factors.
In a US nationwide sample of adults aged 30 to 54 years with first/recurrent MI from 2001 to 2010, there was no change in MI-related hospitalization rates for men and women.7 The Atherosclerosis Risk in Communities (ARIC) surveillance study, which included first/recurrent MI from 4 US communities from 1995 to 2014, showed that women aged 35 to 54 years had an increase in MI-related hospitalization, but similarly aged men showed a decline.1 During the same period, the prevalence of hypertension and diabetes increased in men and women, but the prevalence of smoking decreased.1 In addition to traditional cardiovascular risk factors, other studies have examined the role of recreational drugs (cocaine and marijuana) and hemostatic dysfunction in young adults with MI.8, 9, 10
Although these studies are informative, they were based on convenience samples, included adults with first-ever but also recurrent MI, or had limited follow-up for cardiovascular events and/or death following the initial MI. Therefore, there is a paucity of information on temporal trends for incident (first-ever) MI, the prevalence of risk factors, and recurrent events following premature MI. To address these knowledge gaps, we conducted a community-based study of incident premature MI over a 26-year period. We examined temporal trends in incidence, patient characteristics, and outcomes (recurrent MI and death).
Methods
Study Design and Setting
This study was conducted in Olmsted County, Minnesota. Because Olmsted County is relatively isolated from other urban centers and medical care is practically self-contained within the community, it provides a highly suitable setting for epidemiologic research. This study used resources of the Rochester Epidemiology Project (REP).11,12 The REP is a medical records linkage system that links the medical records of nearly all persons living in the county. All medical diagnoses are maintained through an electronic index, and patients can be identified through their in- and outpatient contacts across the local medical providers.12 This study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center.
Cohort Identification and Validation
Using consistent data collection methods that were available from January 1, 1987, to December 31, 2012, residents admitted to Olmsted County hospitals with possible MI and who provided general authorization to review medical records for research as part of Minnesota law (Statute 144.335) were identified using methods previously described.13 Briefly, all events with International Classification of Diseases, Ninth Revision code 410 (acute MI) were reviewed. The MIs were validated using standard epidemiologic criteria that integrate cardiac pain, electrocardiogram changes, and elevated levels of biomarkers.14 From 1987 to 2000, creatine kinase (CK) and creatine kinase–myoblast isoenzyme were used, and 2000 onward, troponin was used, as previously described.13
Cases were manually reviewed to ensure that there were no alternative causes resulting in biomarker level elevation. Only incident (first-ever) cases were studied; thus, patients with MI diagnosed before 1987 were excluded. Patients who died before being admitted to the hospital were not included in the study; cases who died during the hospitalization were included. In this study, we focused on premature MI, defined using age cut-points based on the definition for family history of premature coronary artery disease (CAD; aged <55 years in men and <65 years in women).15,16
Clinical Characteristics
Medical records were reviewed by trained nurse abstractors to abstract information on clinical characteristics at the time of incident MI, as previously described.17 Consistent definitions for risk factors were used throughout the study period; therefore, risk factor ascertainment was not affected by changes in clinical practice. Body mass index (calculated as the weight in kilograms divided by the height in meters squared) was calculated using the current weight and earliest adult height. Current smoking was defined based on smoking (yes/no) within 6 months before the incident MI.18 Comorbid conditions (hypertension, hyperlipidemia, and diabetes mellitus) were identified by clinician-documented diagnoses, as previously described.18 Familial coronary heart disease was defined as the presence of a first-degree relative (men aged <55 years or women <65 years) with a history of MI or coronary bypass grafting. The Charlson Comorbidity Index was measured, as previously described.18,19 Risk factors were allocated to individuals based on presence at the time of presenting with MI.
Data for ST-segment elevation, anterior MI, Killip class, participation in cardiac rehabilitation, and medications at discharge were collected. The presence of Q waves was determined from serial comparisons of the electrocardiograms. The Global Registry of Acute Coronary Events (GRACE) score was calculated using 9 bedside clinical variables (older age, history of MI, history of heart failure, increased pulse, low systolic blood pressure, elevated creatinine level, elevated cardiac enzyme levels, ST-segment depression, and not having percutaneous coronary intervention in the hospital) that are predictive of 6-month mortality in patients with acute coronary syndrome.20 Medications prescribed at the time of hospital discharge were abstracted, as was participation in cardiac rehabilitation, defined as attending the first session of an outpatient cardiac rehabilitation program.
The extent of CAD was expressed by the number of major coronary arteries with significant obstruction (0-, 1-, 2-, or 3-vessel disease) obtained from coronary angiograms at a median of 2 (25th-75th percentile, 0-2) days after MI. A significant obstruction was defined as angiographic evidence of 50% or greater luminal stenosis of any of the epicardial coronary vessels, including side branches.21 Retrieval of all coronary angiography procedures, both diagnostic and therapeutic, was possible through a registry of all coronary angiographies performed at Mayo Clinic (the sole provider of coronary angiographies in Olmsted County).
Outcome Ascertainment
Participants were followed up using their complete inpatient and outpatient medical records in the community from the index MI through September 30, 2018 (date of last follow-up), for recurrent MI and death. Recurrent MI was based on a clinical diagnosis.22 Information on death was obtained using death certificate data for the state of Minnesota available through the REP.
Statistical Analyses
All analyses were stratified by sex. Patient characteristics are presented as frequency with percentage or mean ± SD according to period of the MI (1987-1995, 1996-2004, and 2005-2012). Differences across periods were tested using analysis of variance for continuous variables and Mantel-Haenszel χ2 tests for categorical variables. Age- and year-specific incidence rates of MI were calculated. The counts of validated cases were used as the numerators, and the denominators were the Olmsted County population 18 years or older as determined by census data for 1980, 1990, 2000, and 2010, with linear interpolation for the intercensal years and extrapolation after 2010.23 The rates were directly standardized to the age distribution of the 2010 US male and female population. Poisson regression was used to examine trends over time. Linear and quadratic components of calendar year and age were tested, along with the year × age interaction.
Mortality was analyzed using the Kaplan-Meier method according to time, with differences tested using the log-rank test. Cumulative incidence curves for recurrent MI treating death as a competing risk were constructed; differences across periods were tested using the method by Grey.24 Cox proportional hazards regression models examined temporal trends in outcomes, adjusting for age, comorbid conditions, MI characteristics, and GRACE score. A quadratic calendar year effect was tested and found to be nonsignificant. The proportional hazards assumption was tested using the scaled Schoenfeld residuals and found to be valid. Data analyses were performed using SAS software, version 9.4 (SAS Institute), and R, version 3.4 (R Foundation for Statistical Computing).
Results
In Olmsted County, Minnesota, from January 1, 1987, through December 31, 2012, a total of 3276 participants (41.0% [n=1343] women) experienced an incident first-ever MI. Among these, 850 were premature events, including 528 (62.1%) men with a mean ± SD age of 47±6.3 years and 322 (37.9%) women with a mean ± SD age of 55±7.8 years.
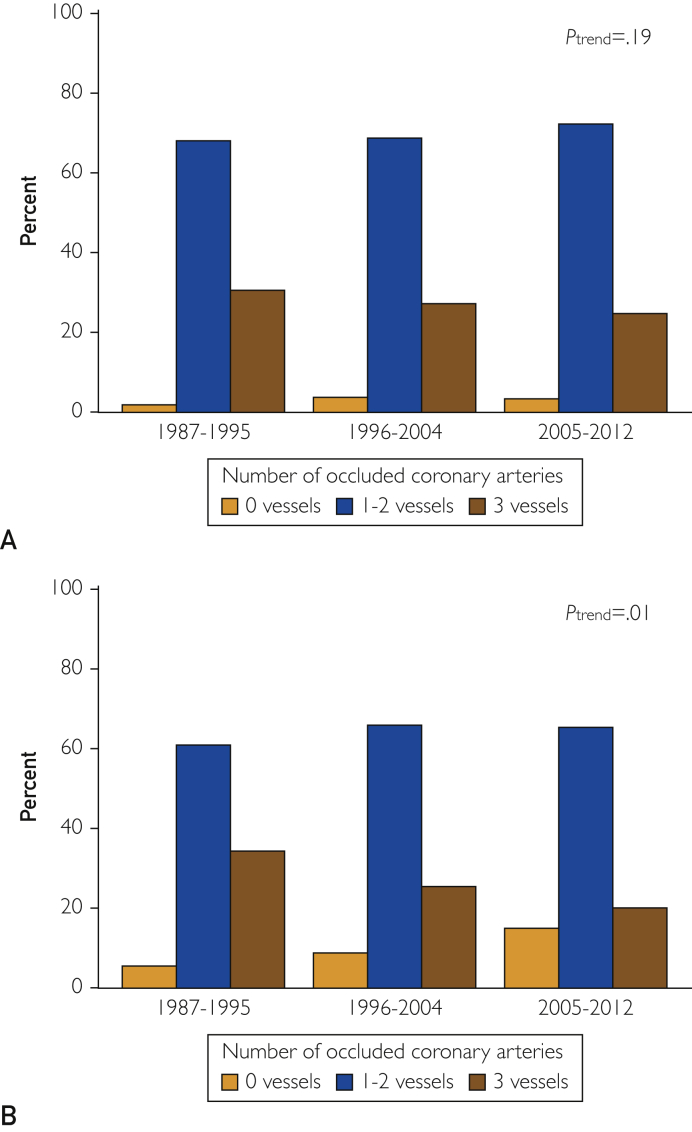
Among men, the prevalence of hypertension, diabetes, and hyperlipidemia increased over time (Table 1). Comorbidity as measured using the Charlson Comorbidity Index increased as well. At the time of MI presentation, the GRACE score and the proportion of men in higher Killip classes decreased over time. The severity of CAD did not change over time (Ptrend=.19; Figure 1A). The use of reperfusion or revascularization during the index hospitalization increased, as did the use of aspirin, β-blockers, and statins at hospital discharge. Participation in cardiac rehabilitation decreased over time (Table 1).
Table 1.
Characteristics of Men Aged 18 to 55 Years Presenting With Incident MI, Categorized by Time
| Characteristic | 1987-1995 (N=145) | 1996-2004 (N=195) | 2005-2012 (N=188) | Ptrend |
|---|---|---|---|---|
| Age at MI (y), mean ± SD | 47.3±6.2 | 47.3±6.1 | 47.5±6.7 | .771 |
| Risk factors | ||||
| Current smoker, no. (%) | 77 (53.5) | 100 (51.3) | 85 (45.2) | .126 |
| Body mass index (kg/m2), mean ± SD | 29.3±6.8 | 30.4±5.8 | 30.4±6.0 | .116 |
| Familial coronary heart disease, no. (%) | 50 (36.5) | 60 (30.8) | 57 (30.5) | .277 |
| Hypertension, no. (%) | 53 (36.6) | 70 (35.9) | 88 (46.8) | .045 |
| Hyperlipidemia, no. (%) | 37 (25.5) | 84 (43.1) | 109 (58.0) | <.001 |
| Diabetes mellitus, no. (%) | 12 (8.3) | 22 (11.3) | 31 (16.5) | .022 |
| Charlson Comorbidity Index score, no. (%) | .036 | |||
| 0 | 106 (73.1) | 145 (74.4) | 123 (65.4) | |
| 1-2 | 32 (22.1) | 39 (20.0) | 45 (23.9) | |
| ≥3 | 7 (4.8) | 11 (5.6) | 20 (10.6) | |
| MI characteristics, no. (%) | ||||
| Cardiac pain | 142 (97.9) | 190 (97.4) | 183 (97.3) | .738 |
| Killip class II-IV | 37 (26.1) | 34 (17.7) | 23 (12.2) | .001 |
| ST-segment elevation | 62 (43.1) | 84 (44.2) | 83 (44.1) | .850 |
| Presence of Q waves | 62 (44.0) | 116 (64.4) | 108 (61.0) | .004 |
| GRACE score, mean ± SD | 75.6±18.7 | 74.0±15.6 | 71.9±18.3 | .057 |
| MI management, no. (%) | ||||
| Reperfusion or revascularization during hospitalization | 113 (78.5) | 158 (81.0) | 166 (88.3) | .016 |
| Aspirin at discharge | 120 (84.5) | 184 (95.3) | 180 (96.8) | <.001 |
| β-blockers at discharge | 99 (69.7) | 173 (89.6) | 178 (95.7) | <.001 |
| Statins at discharge | 5 (3.5) | 122 (63.2) | 180 (96.8) | <.001 |
| Attended ≥1 cardiac rehabiliation session | 120 (85.1) | 157 (81.8) | 136 (73.1) | .006 |
GRACE, Global Registry of Acute Coronary Events; MI, myocardial infarction.
Figure 1.
Distribution of number of occluded arteries (0, 1-2, and 3 vessels) at the time of the incident myocardial infarction, categorized by time period for (A) men aged 18 to 55 years and (B) women aged 18 to 65 years.
Among women, with regard to cardiovascular risk factors, the prevalence of hyperlipidemia increased over time, whereas no change in hypertension and diabetes was detected. The GRACE score decreased, as did the proportion with Killip classes II to IV and ST-segment elevation MI (Table 2). Women experienced less severe CAD over time (Ptrend=.010; Figure 1B). The use of aspirin, β-blockers, and statins at hospital discharge increased, whereas there was no change in the use of reperfusion or revascularization or in participation in cardiac rehabilitation (Table 2).
Table 2.
Characteristics of Women Aged 18 to 65 Years Presenting With Incident MI, Categorized By Time Periods
| Characteristic | 1987-1995 (N=96) | 1996-2004 (N=135) | 2005-2012 (N=90) | Ptrend |
|---|---|---|---|---|
| Age at MI (y), mean ± SD | 56.4±7.8 | 54.5±7.4 | 54.6±8.2 | .103 |
| Risk factors | ||||
| Current smoker, no. (%) | 44 (45.4) | 60 (44.4) | 38 (42.2) | .668 |
| Body mass index (kg/m2), mean ± SD | 28.6±6.9 | 30.4±8.7 | 31.2±7.9 | .024 |
| Familial coronary heart disease, no. (%) | 25 (26.6) | 45 (34.4) | 33 (37.1) | .130 |
| Hypertension, no. (%) | 52 (53.6) | 74 (54.8) | 56 (62.2) | .241 |
| Hyperlipidemia, no. (%) | 29 (30.2) | 72 (53.3) | 59 (65.6) | <.001 |
| Diabetes mellitus, no. (%) | 23 (23.7) | 51 (37.8) | 25 (27.8) | .511 |
| Charlson Comorbidity Index score, no. (%) | .884 | |||
| 0 | 47 (49.0) | 49 (36.3) | 48 (53.3) | |
| 1-2 | 34 (35.4) | 54 (40.0) | 23 (25.6) | |
| ≥3 | 15 (15.6) | 32 (23.7) | 19 (21.1) | |
| MI characteristics, no. (%) | ||||
| Cardiac pain | 93 (95.9) | 117 (86.7) | 87 (96.7) | .901 |
| Killip class II-IV | 29 (30.9) | 39 (29.1) | 12 (13.3) | .007 |
| ST-segment elevation | 38 (39.6) | 50 (37.9) | 19 (21.3) | .010 |
| Presence of Q waves | 38 (40.0) | 79 (63.2) | 37 (43.5) | .546 |
| GRACE score, mean ± SD | 97.0±22.3 | 93.3±22.8 | 89.4±21.0 | .021 |
| MI management, no. (%) | ||||
| Reperfusion or revascularization during hospitalization | 69 (71.9) | 79 (58.5) | 63 (70.0) | .751 |
| Aspirin at discharge | 67 (73.6) | 106 (86.9) | 84 (93.3) | <.001 |
| β-blockers at discharge | 48 (52.7) | 96 (78.7) | 80 (88.9) | <.001 |
| Statins at discharge | 5 (5.5) | 67 (54.9) | 82 (91.1) | <.001 |
| Participation in cardiac rehabiliation | 59 (66.3) | 70 (57.4) | 55 (61.8) | .539 |
GRACE, Global Registry of Acute Coronary Events; MI, myocardial infarction.
MI Incidence
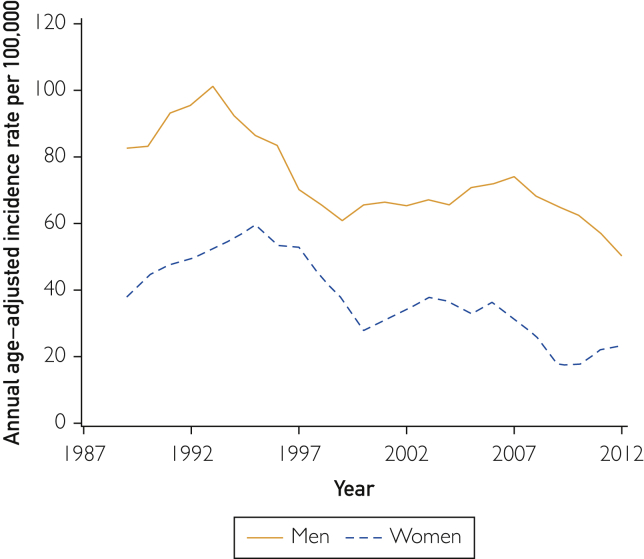
The age-adjusted incidence rates of premature MI declined over time for both men (P<.001) and women (P<.001; Figure 2). The incidence decreased by 39% during the study period for men (rate ratio [RR], 0.61; 95% CI, 0.46 to 0.81, for 2012 vs 1987) and 61% for women (RR, 0.39; 95% CI, 0.27 to 0.57, for 2012 vs 1987), but the trends differed by age (men: year × age interaction P=.036; women: year × age interaction P=.022) with a larger decline for older ages. For example, comparing 2012 to 1987, there was 31% decreased risk for MI in men aged 45 years (RR, 0.69; 95% CI, 0.51 to 0.93) and 57% decreased risk in men aged 55 years (RR, 0.43; 95% CI, 0.28 to 0.66). For women, there was 59% decreased risk for MI in women aged 55 years (RR, 0.41; 95% CI, 0.29 to 0.58) and 76% decreased risk for women aged 65 years (RR, 0.24; 95% CI, 0.14 to 0.42).
Figure 2.
Incidence of premature myocardial infarction in adults (men aged 18-55 years; women aged 18-65 years). Yearly rates (smoothed using 3-year moving average) per 100,000 persons were standardized by the direct method to the age distribution of the US population in 2010.
Outcomes after MI
The mean ± SD follow-up was 13.3±7.9 years. Altogether, 232 recurrent MIs and 311 deaths were enumerated. Men experienced 140 recurrent MIs and 159 deaths and women experienced 92 recurrent MIs and 152 deaths.
Recurrent MI
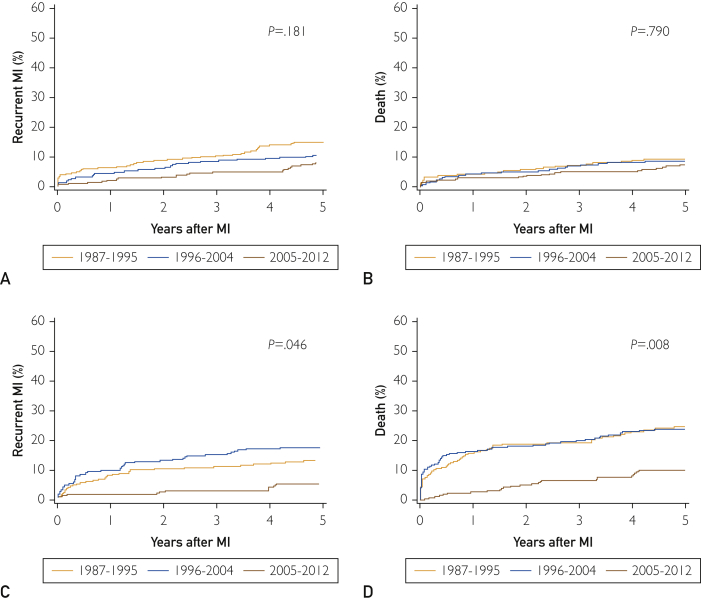
Among men, the cumulative incidence of recurrent MI at 5 years was 11.0% (58/528) overall. No change in the cumulative incidence was observed during the study period, with 5-year estimates of 15%, 11%, and 8% for the periods 1987 to 1995, 1996 to 2004, and 2005 to 2012, respectively (P=.18; Figure 3A). The unadjusted hazard ratio (HR) for 2012 vs 1987 was 0.59; 95% CI, 0.31 to 1.10 (Table 3). No change in the association between year of MI and recurrent MI was observed after adjustment for the residual effect of age or after further adjustment for diabetes mellitus and GRACE score.
Figure 3.
Cumulative incidence by time period of (A) recurrent myocardial infarction (MI), treating death as a competing risk for men; (B) cumulative incidence of death for men; (C) cumulative incidence of recurrent MI, treating death as a competing risk for women; and (D) cumulative incidence of death for women.
Table 3.
Associations Between Year of Incident MI and Outcomes (recurrent MI and death) for Men Aged 18 to 55 Years and Women Aged 18 to 65 Years at Time of Incident MIa
| Recurrent MI |
Death |
|||
|---|---|---|---|---|
| HR (95% CI) for 2012 vs 1987 | P | HR (95% CI) for 2012 vs 1987 | P | |
| Men | ||||
| Unadjusted | 0.59 (0.31-1.10) | .099 | 0.75 (0.39-1.46) | .398 |
| Model 1b | 0.59 (0.31-1.10) | .099 | 0.75 (0.39-1.46) | .397 |
| Model 2c | 0.57 (0.30-1.08) | .084 | 0.70 (0.36-1.36) | .297 |
| Model 3d | 0.60 (0.32-1.14) | .118 | 0.76 (0.39-1.49) | .424 |
| Women | ||||
| Unadjusted | 0.49 (0.22-1.11) | .088 | 0.29 (0.15-0.58) | <.001 |
| Model 1b | 0.47 (0.21-1.08) | .076 | 0.34 (0.17-0.68) | .002 |
| Model 2c | 0.43 (0.19-1.00) | 0.051 | 0.31 (0.15-0.62) | 0.001 |
| Model 3d | 0.50 (0.21-1.16) | 0.107 | 0.37 (0.18-0.74) | 0.005 |
HR, hazard ratio; MI, myocardial infarction.
Model 1 adjusted for age.
Model 2 adjusted for age and diabetes mellitus.
Model 3 adjusted for age, diabetes mellitus, and Global Registry of Acute Coronary Events score.
Among women, the cumulative incidence of recurrent MI at 5 years was 13.0% (42/322) overall and declined in the last period, with 5-year estimates of 13%, 18%, and 6% for 1987 to 1995, 1996 to 2004, and 2005 to 2012, respectively (P=.046; Figure 3C). The unadjusted HR for 2012 vs 1987 was 0.47; 95% CI, 0.21 to 1.08 (Table 3). As observed for men, the temporal trends did not materially change after adjustment for age, diabetes mellitus, and GRACE score.
Mortality
Among men, 5-year mortality was 8.9% (47/528) and did not change over time (10%, 9%, and 8% for 1987-1995, 1996-2004, and 2005-2012, respectively; P=.79; Figure 3B). After adjusting for age, there was no temporal decline in mortality among men (HR, 0.75; 95% CI, 0.39 to 1.46 for 2012 vs 1987; Table 3). The temporal trends did not materially change after adding diabetes mellitus and GRACE score to the model.
For women, 5-year mortality was 19.9% (64/322) and declined over time. The 5-year mortality estimates were 25%, 24%, and 10% for 1987 to 1995, 1996 to 2004, and 2005 to 2012, respectively (P=.008; Figure 3D). Comparing 2012 with 1987, women experienced 66% decreased risk for mortality after adjustment for age (HR, 0.34; 05% CI, 0.17 to 0.68) and 63% decreased risk after further adjustment for diabetes mellitus and GRACE score (HR, 0.37; 95% CI, 0.18 to 0.74; Table 3).
Discussion
In this large community study during a 26-year period, the incidence of first-ever premature MI declined among men and women. The prevalence of cardiovascular risk factors, specifically hypertension, hyperlipidemia, and diabetes, increased over time for men, whereas only hyperlipidemia increased for women. The prevalence of smoking did not change. Mortality after MI declined markedly over time in women, contrasting with the absence of changes in men.
The high prevalence of modifiable risk factors in men and women who present early in their life with a first MI identifies opportunities for better lifestyle choices, which can lower risk for heart disease by approximately 50%.25 This also highlights the importance of campaigns including Life’s Simple 7 to promote better cardiovascular health.26 Life’s Simple 7 recommends physical activity, weight management, smoking cessation, healthful diet, and better management of blood pressure, cholesterol level, and blood glucose level.26 The 2020 Heart Disease and Stroke Statistics reported that the age-standardized prevalence of obesity among US adults has increased from 2007 to 2008 to 2015 to 2016.27 Obesity is a major risk factor for cardiovascular disease and influences/is influenced by the other Life’s Simple 7 recommendations. Recently, a Canadian task force identified obesity as a complex chronic disease that requires a biopsychosocial approach for effective management.28
Here, the decline in age-adjusted incidence rates of first-ever premature MI for both men and women differs from previous reports of unchanged incidence over time7 or different trends for men and women.1 In a study of administrative claims data from the National Inpatient Sample from 2001 to 2010, MI hospitalization rates for adults aged 30 to 54 years were unchanged.7 In ARIC, from 1995 to 2014, MI hospitalization rates increased for women aged 35 to 54 years but declined for similarly aged men. Both studies included samples of hospitalized patients, included patients with first-ever and/or recurrent MI, and the nationwide study relied on discharage diagnosis codes to identify MI.1,7,29 In contrast, this study captures all first-ever validated MIs in a well-defined community.
Our observation that women experienced less severe CAD over time and had a lower proportion of obstructive CAD compared with men supports emerging literature on angiographic findings in younger women with MI.30, 31, 32 Younger women with MI, compared with older women and similarly aged men with MI, have a higher prevalence of MI with nonobstructive coronary arteries (MINOCA), a clinical entity of MI with less than 50% coronary occlusion.31 MINOCA may result from coronary plaque disruption and other causes, although the predisposing factors are incompletely understood. Patients with MINOCA are less likely to have traditional risk factors such as hypertension and diabetes compared with patients with coronary occlusion of 50% or greater.31
Randomized clinical trials on pharmacologic interventions for MINOCA are lacking; however, observational data suggest that the use of statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers was associated with better outcomes, whereas dual antiplatelet therapy had no effect.33,34
Recently, we showed that atherosclerotic burden quanitifed by the number of coronary arteries with significant obstruction (0-, 1-, 2-, or 3-vessel disease) is associated with a higher incidence of post-MI heart failure.35 The trends observed here underscore the need to better understand risk factors, clinical presentation, and outcomes of MINOCA.
Our observation that women were less likely than men to receive reperfusion or revascularization during hospitalization for MI supports observations from ARIC and Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients.1,29,36 Despite higher GRACE scores, women were less likely than men to receive evidence-based therapies including aspirin, β-blockers, and lipid-lowering statins. Improving prescription of therapies represents an opportunity for improved secondary prevention to reduce the risk for subsequent cardiovascular events.
In this study, women experienced less severe CAD over time and were less likely than men to receive secondary prevention therapies. Our observation that women, but not men, had a lower mortality risk over time contrasts with findings from ARIC, which showed no differences in 1-year mortality between women and men after MI.1,29,36 This study assessed mortality among men (aged 18-55 years) and women (aged 18-65 years) during a mean 13.3 years of follow-up, whereas ARIC assessed 1-year mortality among adults aged 35 to 54 years. Other studies have shown mixed results on the association of sex and post-MI mortality. Among 14,434 adults admitted to an intensive care unit with MI, women had similar 30-day mortality compared with men but lower 20-year mortality (adjusted HR, 0.77; 95% CI, 0.66 to 0.90]).37 Similarly, a study of 658,110 individuals with a first coronary heart disease event showed that women aged 75 to 84 years who survived to 28 days post-MI had lower 1-year mortality compared with similarly aged men.38
Several time-varying factors may account for these sex-based differences, including sex differences in lifetysle changes, adherence to secondary prevention therapies, and development of comorbid conditions (eg, cancer and cardiomyopathy), which are beyond the scope of this study. In addition, factors such as drugs (eg, cocaine, marijuana, opioids, and oncologic medications) may influence the risk for recurrent MI or post-MI mortality and need further study in the context of traditional cardiovascular risk factors. Further studies are required to determine the influence of age and sex on mortality after MI.
Some limitations must be acknowledged to aid in the interpretation of the data. We did not have data for lifestyle factors (eg, diet and physical activity) or biomarkers (eg, coronary artery calcium score). Most residents in Olmsted County are White, and these results will need replication in populations with different racial/ethnic composition. The numbers of recurrent MIs and deaths were limited, which limited statistical power.
Our study has several strengths. We leveraged a comprehensive data linkage system to capture incident MI, patient characteristics, and outcomes within an entire community population. The comprehensive longitudinal study over more than 2 decades allowed us to assess temporal trends in characteristics in a contemporary community cohort. Finally, through careful abstraction, we focused on validated cases of incident MI (compared with most studies that included incident/recurrent MI) and described risk factors and outcomes.
Conclusion
In this large community-based study, the incidence rates of first-ever premature MI declined over time for both men and women. Changes in recurrent MI rates among men and women over time were statistically nonsignificant, and only women showed decreased risk over time in mortality following a first-ever MI. Despite this, both men and women had a high burden of modifiable cardiovascular risk factors amenable to interventions at the person and population levels. Such interventions could help reduce cardiovascular events and mortality, which remains a significant public health problem in the United States.
Acknowledgments
We thank Ellen E. Koepsell, RN, and Debbi Strain, BS, for study support.
Footnotes
Grant Support: This work was supported by a grant from the National Heart, Lung, and Blood Institute (R01-HL120957) and was made possible by using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (grant number R01-AG034676; Principal Investigators: Walter A. Rocca, MD, MPH, and Jennifer L. St Sauver, PhD). This study received funding from the Division of Hospital Internal Medicine, Mayo Clinic Small Grants Program. The funding sources played no role in the design, conduct, or reporting of this study.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Arora S., Stouffer G.A., Kucharska-Newton A.M., et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchey M.D., Wall H.K., George M.G., Wright J.S. US trends in premature heart disease mortality over the past 50 years: where do we go from here? Trends Cardiovasc Med. 2019;30(6):364–374. doi: 10.1016/j.tcm.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilmot K.A., O'Flaherty M., Capewell S., Ford E.S., Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132(11):997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford E.S., Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50(22):2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Awad H.H., McManus D.D., Anderson F.A., Jr., Gore J.M., Goldberg R.J. Young patients hospitalized with an acute coronary syndrome. Coron Artery Dis. 2013;24(1):54–60. doi: 10.1097/MCA.0b013e32835b0bf7. [DOI] [PubMed] [Google Scholar]
- 6.Dugani S.B., Murad W., Damilig K., et al. Premature myocardial infarction in the Middle East and North Africa: rationale for the Gulf PREVENT study. Angiology. 2020;71(1):17–26. doi: 10.1177/0003319719849737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A., Wang Y., Spertus J.A., et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64(4):337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Biery D.W., Singh A., et al. Risk factors and outcomes of very young adults who experience myocardial infarction: the Partners YOUNG-MI Registry. Am J Med. 2019;133(5):605–612.e1. doi: 10.1016/j.amjmed.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFilippis E.M., Singh A., Divakaran S., et al. Cocaine and marijuana use among young adults with myocardial infarction. J Am Coll Cardiol. 2018;71(22):2540–2551. doi: 10.1016/j.jacc.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson L., Wilcox J., Kim D., Benton S., Fredi J., Vaughan D. Clinical features of precocious acute coronary syndrome. Am J Med. 2014;127(2):140–144. doi: 10.1016/j.amjmed.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Rocca W.A., Yawn B.P., St Sauver J.L., Grossardt B.R., Melton L.J., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver J.L., Grossardt B.R., Leibson C.L., Yawn B.P., Melton L.J., 3rd, Rocca W.A. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roger V.L., Weston S.A., Gerber Y., et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White A.D., Folsom A.R., Chambless L.E., et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 15.De Sutter J., De Bacquer D., Kotseva K., et al. EUROpean Action on Secondary Prevention through Intervention to Reduce Events II study group. Screening of family members of patients with premature coronary heart disease; results from the EUROASPIRE II family survey. Eur Heart J. 2003;24(3):249–257. doi: 10.1016/s0195-668x(02)00386-x. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones D.M., Nam B.H., D'Agostino R.B., Sr., et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 17.Gerber Y., Weston S.A., Killian J.M., Jacobsen S.J., Roger V.L. Sex and classic risk factors after myocardial infarction: a community study. Am Heart J. 2006;152(3):461–468. doi: 10.1016/j.ahj.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Jimenez F., Jacobsen S.J., Reeder G.S., Weston S.A., Meverden R.A., Roger V.L. Prevalence and secular trends of excess body weight and impact on outcomes after myocardial infarction in the community. Chest. 2004;125(4):1205–1212. doi: 10.1378/chest.125.4.1205. [DOI] [PubMed] [Google Scholar]
- 19.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Eagle K.A., Lim M.J., Dabbous O.H., et al. GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 21.Scanlon P.J., Faxon D.P., Audet A.M., et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33(6):1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 22.Jokhadar M., Jacobsen S., Reeder G., Weston S., Roger V. Sudden death and recurrent ischemic events after myocardial infarction in the community. Am J Epidemiol. 2004;159(11):1040–1046. doi: 10.1093/aje/kwh147. [DOI] [PubMed] [Google Scholar]
- 23.Bergstrahl E.J., Offord K.P., Chu C.P., Beard C.M., O'Fallon W.M., Melton L.J., 3rd Calculating incidence, prevalence and mortality rates for Olmsted County, Minnesota residents: an update. 1992. https://www.mayo.edu/research/documents/biostat-49pdf/doc-10027851 Rochester, MN: Mayo Clinic. Accessed March 13, 2020.
- 24.Gray R.J. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 25.Khera A.V., Emdin C.A., Drake I., et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Life's Simple 7 American Heart Association. https://www.heart.org/en/professional/workplace-health/lifes-simple-7 Accessed May 7, 2020.
- 27.Virani S.S., Alonso A., Benjamin E.J., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 28.Wharton S., Lau D.C.W., Vallis M., et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaccarino V. Myocardial infarction in young women. Circulation. 2019;139(8):1057–1059. doi: 10.1161/CIRCULATIONAHA.118.039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safdar B., Spatz E.S., Dreyer R.P., et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc. 2018;7(13):e009174. doi: 10.1161/JAHA.118.009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 32.Shah N., Kelly A.M., Cox N., Wong C., Soon K. Myocardial infarction in the "young": risk factors, presentation, management and prognosis. Heart Lung Circ. 2016;25(10):955–960. doi: 10.1016/j.hlc.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Lindahl B., Baron T., Erlinge D., et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336. [DOI] [PubMed] [Google Scholar]
- 34.Pasupathy S., Tavella R., Beltrame J.F. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation. 2017;135(16):1490–1493. doi: 10.1161/CIRCULATIONAHA.117.027666. [DOI] [PubMed] [Google Scholar]
- 35.Gerber Y., Weston S.A., Enriquez-Sarano M., et al. Atherosclerotic burden and heart failure after myocardial infarction. JAMA Cardiol. 2016;1(2):156–162. doi: 10.1001/jamacardio.2016.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bucholz E.M., Strait K.M., Dreyer R.P., et al. Editor's choice-sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610–622. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nauta S.T., Deckers J.W., van Domburg R.T., Akkerhuis K.M. Sex-related trends in mortality in hospitalized men and women after myocardial infarction between 1985 and 2008: equal benefit for women and men. Circulation. 2012;126(18):2184–2189. doi: 10.1161/CIRCULATIONAHA.112.113811. [DOI] [PubMed] [Google Scholar]
- 38.Berg J., Bjorck L., Nielsen S., Lappas G., Rosengren A. Sex differences in survival after myocardial infarction in Sweden, 1987-2010. Heart. 2017;103(20):1625–1630. doi: 10.1136/heartjnl-2016-310281. [DOI] [PMC free article] [PubMed] [Google Scholar]