Abstract
Objective
To systematically evaluate the prevalence of disclosed and undisclosed financial conflicts of interest (FCOI) among clinical practice guidelines (CPGs).
Methods
In this systematic review, we ascertained the prevalence and types of FCOI for CPGs from January 1, 1980, to March 3, 2019. The primary outcome was the prevalence of FCOI among authors of CPGs. FCOI disclosures were compared between medical subspecialties and societies producing CPGs.
Results
Among the 37 studies including 14,764 total guideline authors, 45% had at least one FCOI. The prevalence of FCOI per study ranged from 6% to 100%. More authors had FCOI involving general payments (39%) compared with research payments (29%). Oncology, neurology, and gastroenterology had the highest prevalence of FCOI compared with other medical specialties. Among the 8 studies that included the monetary values in US dollars of FCOI, average payments per author ranged from $578 to $242,300. Among the 10 studies that included data on undisclosed FCOI, 32% of authors had undisclosed industry payments.
Conclusion
There are numerous FCOI among authors of CPGs, many of which are undisclosed. Our study found a significant difference in FCOI prevalence based on types of FCOI and CPG sponsor society. Additional research is required to quantify the implications of FCOI on clinical judgment and patient care.
Abbreviations and Acronyms: CMS-OP, Centers for Medicare & Medicaid Services Open Payments; CPG, clinical practice guideline; FCOI, financial conflicts of interest
Article Highlights
-
•
Financial conflicts of interest (FCOI) may have an impact on the objectivity of clinical practice guidelines.
-
•
Among the 37 studies included in this systematic review, 45% of the 14,764 guideline authors had an FCOI.
-
•
Authors of oncology, neurology, and gastroenterology guidelines had higher prevalence of FCOI compared with other guidelines.
-
•
Eight studies included monetary value of FCOI, which ranged from $578 to $242,300 per author.
-
•
Little is known about the direct impact of FCOI on how authors of clinical practice guidelines vote on recommendations during guideline development.
Article Highlights.
-
•
Financial conflicts of interest (FCOI) may have an impact on the objectivity of clinical practice guidelines.
-
•
Among the 37 studies included in this systematic review, 45% of the 14,764 guideline authors had an FCOI.
-
•
Authors of oncology, neurology, and gastroenterology guidelines had higher prevalence of FCOI compared with other guidelines. Eight studies included monetary value of FCOI, which ranged from $578 to $242,300 per author. Little is known about the direct impact of FCOI on how authors of clinical practice guidelines vote on recommendations during guideline development.
Financial conflicts of interest (FCOI) exist across the spectrum of academia and medicine.1, 2, 3 One area that has been evaluated is FCOI in clinical practice guidelines (CPGs). These documents are intended to guide clinicians in decision-making and can be highly influential. There are concerns, however, that FCOI among CPG panel members can introduce bias and jeopardize objectivity.1
Various approaches have been suggested to mitigate industry influence among CPGs. At a policy level, the National Academy of Medicine in the United States has recommended that guideline chairs be free of FCOI and that authors with FCOI compose less than half of the CPG panel.2 In addition, the US government has aimed to make all industry payments to physicians more transparent through the Centers for Medicare & Medicaid Services Open Payments (CMS-OP) database. This database publishes data on payments provided to physicians by pharmaceutical and medical device companies in the United States as part of the Physician Payments Sunshine Act.3 Some societies, such as the American College of Chest Physicians, use CMS-OP to verify the accuracy of guideline authors’ disclosures.4
Despite these efforts, a large body of literature suggests that there are substantial FCOI in CPGs.5 To summarize and to contextualize the evidence, we conducted a systematic review of studies examining FCOI among CPGs.
Methods
We registered this systematic review using PROSPERO (CRD42019129060). We aimed to characterize the prevalence of disclosed and undisclosed FCOI among authors of CPGs and to evaluate the impact of FCOI among guideline authors on guideline recommendations.
Search Strategy
We conducted a systematic search in MEDLINE for studies that reported the prevalence of FCOI among CPGs published between January 1980 and March 2019. The complete search strategy is summarized in the Supplemental Table (available online at http://mcpiqojournal.org). In addition, we hand searched the reference lists of any included studies.
Study Selection
Two authors (S.T., R.K.) independently screened all titles and abstracts for inclusion and a third author (S.C.G.) adjudicated any disagreements. We included studies if they were observational cohort or cross-sectional studies examining the prevalence of FCOI among a subset of CPGs or examining the association of FCOI with guideline recommendations. To determine whether studies examined CPGs, we used the definition of CPG published by the National Academy of Medicine.2 We excluded any studies published in a non-English language for which full study details could not be retrieved.
Outcomes
Our primary outcome was the prevalence of FCOI among authors of CPGs in the included studies. We defined conflicts of interest, in keeping with the National Academy of Medicine definition, as “circumstances that create a risk that professional judgments or actions regarding a primary interest will be unduly influenced by a secondary interest.”2 We further categorized payments according to the CMS-OP classification as general payments, such as fees paid for speaking engagements, consulting, travel and accommodation, food and beverage, honoraria, and gifts; research payments for the funding and implementation of research studies, through compensation provided directly to a guideline author or to an author’s institution; and equity, such as investments, ownership stakes, and royalties. When applicable, we separately reported disclosed and undisclosed FCOI. As the majority of studies did not follow these CMS-OP definitions, two authors (S.T., R.K.) independently categorized all payments as general payments, research payments, or equity, and a third author (S.C.G.) resolved any disagreements.
We also reported the monetary value of FCOI in US dollars, separated when possible into disclosed and undisclosed payments and general, research, and equity payments.
The secondary outcome was the association between FCOI and guideline recommendations. For this outcome, we included data from studies that assessed a relationship between FCOI and voting on recommendation by guideline members. We did not assess this outcome in studies that provided only FCOI prevalence data as the majority of guidelines do not report individual voting patterns.
Data Extraction and Assessment of Quality
Two authors (S.T., R.K.) independently extracted data using a standardized data collection. For each study, we determined the year of publication, field of medicine, study design, CPG sponsor, clinical focus, prevalence of disclosed and undisclosed FCOI, types of FCOI (general payments, research payments, equity, other), and effect of FCOI on guideline recommendations. A third author (S.C.G.) resolved any disagreements.
We initially aimed to perform a quality assessment of included studies using the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.6 We decided, however, not to perform this quality assessment. As all included studies used a descriptive design with no comparison group, we thought that such an assessment would not contribute meaningfully to our ability to discriminate between studies. Specifically, all studies would have been considered uncontrolled observational studies or case series and thus would be considered level 4 evidence on the basis of the Oxford Centre for Evidence-Based Medicine Levels of Evidence guidelines.7
Deviations From Protocol
We included several observational studies that were not strictly cross-sectional and cohort studies. We did this as after initial review of abstracts, we appreciated that several important studies in this field were document reviews that could contribute to the understanding of FCOI in CPGs. In addition, we reported dollar value of FCOI when available. This information provides insight into the magnitude of FCOI.
Data Synthesis
We conducted a qualitative and narrative synthesis across included studies structured around study design, medical specialty, and presence of disclosed and undisclosed FCOI. We also calculated raw prevalence data, reported as the percentage of authors with FCOI among the total number of authors.
For the association between conflict of interest and guideline recommendations, we aimed to summarize the effect of conflicts on guideline recommendations through a random effects model and by calculating risk ratios (for dichotomous outcomes) and standardized mean differences (for continuous outcomes). We also aimed to assess CPG author characteristics associated with having disclosed and undisclosed FCOI. We did not, however, perform these meta-analyses because of a lack of data in the included studies.
Results
We identified 1595 potentially relevant records through our search strategy, of which 1102 remained after removal of duplicates, and 75 articles through hand searching. We retrieved 78 full-text articles or conference abstracts for review. We excluded 41 articles, which left 37 eligible studies.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 Our search flow is outlined in Figure 1.
Figure 1.
Study flow diagram.
The 37 included studies were published between 2001 and 2019. The median year of publication was 2015. Twenty-seven studies evaluated guidelines in specific subject areas and 10 evaluated guidelines in general medicine. Twenty-one studies evaluated guidelines published by US-based organizations or housed in US-based databases. Three studies evaluated German guidelines, 2 evaluated Canadian guidelines, and 1 each evaluated Japanese, Australian, and Danish guidelines. The remaining 8 studies included guidelines from more than one country. The studies evaluated a total of 14,764 guideline authors. The number of authors included in each study ranged from 6 to 2495. General characteristics of studies are summarized in Table 1, with study level characteristics available in Table 2.
Table 1.
General Characteristics of Included Studies
| Characteristic | No. of studies |
|---|---|
| Year of publication | 2001-2005: 2 2006-2010: 3 2011-2015: 13 2016-2019: 20 |
| Countrya | United States: 21 Germany: 3 Canada: 2 Australia: 1 Denmark: 1 Japan: 1 Multiple countries: 8 |
| Study design | Cross-sectional: 21 Single-group cohort: 9 Systematic search: 6 Document review: 1 |
| Medical specialty | General: 10 Gastroenterology: 5 Cardiology: 4 Psychiatry: 4 Neurology: 2 Oncology: 3 Orthopedic surgery: 2 Otherb: 7 |
| Guideline authors | Median, 172 (interquartile range, 83-402) |
Country of society or organization producing or housing target guidelines.
One guideline each in dermatology, endocrinology, family medicine, interventional specialties, pain medicine, respirology, and urology.
Table 2.
Study Characteristics
| Study | Year published | Clinical area | Country | Data collection method | No. of included authors |
|---|---|---|---|---|---|
| Akl et al11 | 2014 | Respirology | United States | Author declaration | 104 |
| Alhamoud et al23 | 2016 | Cardiology | United States | External sources | 537 |
| Allan et al39 | 2015 | Family medicine | Canada | Author declaration | 2495 |
| Andreatos et al9 | 2017 | General | United States | National database | 1329 |
| Bindslev et al24 | 2013 | General | Denmark | Author declaration | 254 |
| Carlisle et al25 | 2018 | Urology | United States | National database | 54 |
| Checketts et al27 | 2017 | Dermatology | United States | National database | 49 |
| Checketts et al26 | 2018 | Orthopedic surgery | United States | National database | 106 |
| Choudhry et al41 | 2002 | General | >1 country | Author declaration | 100 |
| Combs et al33 | 2018 | Gastroenterology | United States | National database | 83 |
| Cosgrove et al28 | 2006 | Psychiatry | United States | External sources | 170 |
| Cosgrove et al21 | 2009 | Psychiatry | United States | External sources | 20 |
| Cosgrove et al20 | 2013 | Psychiatry | United States | External sources | 6 |
| Cosgrove et al22 | 2017 | Psychiatry | United States | External sources | 172 |
| Feuerstein et al12 | 2013 | Gastroenterology | >1 country | Author declaration | 113 |
| Feuerstein et al13 | 2013 | Gastroenterology | United States | Author declaration | 83 |
| Feuerstein et al14 | 2016 | Gastroenterology | >1 country | Author declaration | 47 |
| Feuerstein et al15 | 2016 | Orthopedic surgery | >1 country | Author declaration | 80 |
| Feuerstein et al16 | 2014 | Subspecialty interventional fields | United States | Author declaration | 697 |
| Grindal et al10 | 2018 | Gastroenterology | >1 country | Author declaration | 173 |
| Hauser et al37 | 2017 | Pain medicine | Germany | Author declaration | 42 |
| Holloway et al36 | 2008 | Neurology | United States | Author declaration | 351 |
| Jefferson and Pearson42 | 2017 | Cardiology, gastroenterology | United States | External sources | 45 |
| Khan et al8 | 2018 | General | >1 country | National database | 160 |
| Langer et al34 | 2012 | General | Germany | Author declaration | 1379 |
| Liu et al17 | 2019 | Oncology | United States | National database | 542 |
| Mendelson et al29 | 2011 | Cardiology | United States | Author declaration | 651 |
| Mitchell et al30 | 2016 | Oncology | United States | National database | 125 |
| Moynihan et al19 | 2019 | General | Australia | National database | 402 |
| Neuman et al35 | 2011 | Cardiology, endocrinology | >1 country | External sources | 288 |
| Nifaratos and Pescatore18 | 2019 | Neurology | United States | National database | 76 |
| Norris et al32 | 2013 | Endocrinology | United States | Author declaration | 192 |
| Norris et al31 | 2012 | General | United States | Author declaration | 731 |
| Papanikolaou et al40 | 2001 | General | >1 country | Author declaration | 242 |
| Saito et al44 | 2019 | Oncology | Japan | Author declaration | 326 |
| Schott et al38 | 2015 | General | Germany | Author declaration | 2190 |
| Shnier et al43 | 2016 | General | Canada | External sources | 350 |
Identification of Conflicts of Interest
Nineteen studies relied on author declarations of FCOI, either through surveys sent to the authors36,41 or through the declaration section of included guidelines or the sponsoring society of the guideline.10, 11, 12, 13, 14, 15, 16,24,29,31,32,34,37, 38, 39, 40,43 Eleven studies evaluated conflict of interest using the CMS-OP or other national databases.8,9,17,18,25, 26, 27,30,33,44,45
Seven studies evaluated conflicts of interest through external searches.20, 21, 22, 23,28,35,42 Four of these studies searched through guideline authors’ academic publications in which financial ties with industry were disclosed, US Patent and Trademark Office records that signaled patents pending or awarded for intellectual property in a drug or device whose sales could be affected by guideline recommendations, and disclosures made at peer-reviewed conferences.20, 21, 22,28 One study used disclosures from pharmaceutical companies regarding payments made to physicians to identify FCOI.23 Another report identified guideline authors’ FCOI by using Google’s search engine, combining each author’s name with the name of pharmaceutical companies that produced drugs affected by the guideline and looking through the first 50 search results for reported financial relationships.35 The other study using an external source reviewed FCOI disclosures in guideline authors’ academic publications related to the topic of the guideline and in the same time of guideline development.42
Of the 18 studies that used either national databases or external sources, 10 also collected FCOI data from the declaration section of guidelines with the aim of identifying FCOI that were not disclosed by authors.8,9,19, 20, 21, 22,25,27,35,42
Prevalence of Conflicts of Interest
Of the 14,764 total authors included in the 37 studies, 45% (n = 6589) had at least one financial conflict of interest. The prevalence of FCOI ranged from 6%40 to 100%.20 In total, 22 studies provided data on general payments with a prevalence of 39% (3312/8469). Twenty-one studies provided data on research payments with a prevalence of 29% (1516/5295).
In comparing FCOI prevalence based on method of data collection (ie, author declarations, national databases, external searches), studies that used national databases had the highest prevalence of total payments (53% of authors [1720/3276]). Detailed data prevalence stratified by FCOI identification method is provided in Table 3.
Table 3.
| Prevalence of FCOI | Method used to identify FCOI |
||
|---|---|---|---|
| Group 1 Guideline author declarations |
Group 2 Open payment databases |
Group 3 External sources |
|
| Total | 43 (4439/10,250) | 53 (1720/3276) | 35 (430/1238) |
| General | 32 (2222/6481) | 75 (836/1112) | 29 (254/876) |
| Research | 29 (860/2938) | 42 (465/1119) | 15 (191/1238) |
| Undisclosed | – | 37 (729/1936) | 9 (46/495) |
FCOI, financial conflicts of interest.
Data are presented as percentage (number of authors with FCOI/total number of authors).
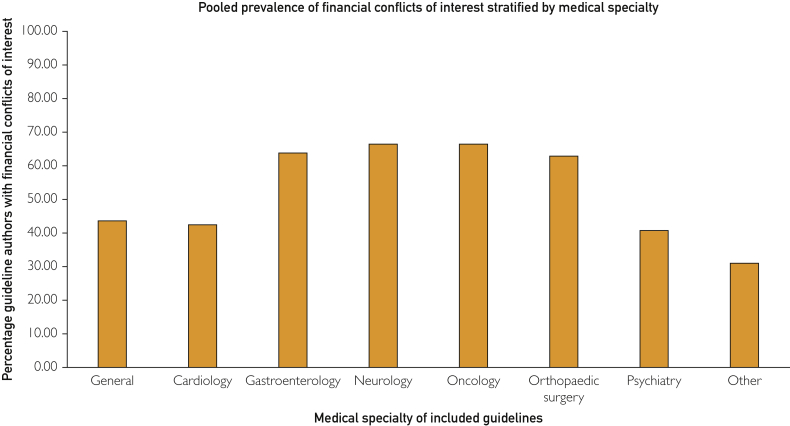
Authors of oncology, neurology, and gastroenterology guidelines had higher FCOI prevalence compared with other medicine subspecialties (Figure 2).
Figure 2.
Prevalence data stratified by medicine subspecialty.
Nine studies included data on monetary value in US dollars. Four studies reported median payments per guideline author of $578 (interquartile range, $0-$19,228),25 $1000 ($0-$39,938),33 $522 ($0-$40,444),8 and $3233 ($506-$10,873).44 Five studies reported mean payments of $67,547 (SD = $125,751),23 $93,537 ($415,203),26 $157,777 ($332,829),27 $219,557 (no SD data provided),17 and $242,300 (no SD data provided).30
Undisclosed Conflicts of Interest
Ten studies included data on undisclosed FCOI for 2431 authors. In addition, 5 studies provided undisclosed payment data on general payments for 539 authors, and 4 reported on undisclosed research payments for 533 authors. The prevalence of FCOI was 32% (775/2431) for total undisclosed FCOI, 36% (195/539) for undisclosed general payments, and 24% (130/533) for undisclosed research payments.
Association Between Conflicts of Interest and Guideline Recommendations
As only 2 studies with heterogeneous methodology reported on an association between FCOI and guideline recommendations, we did not perform a meta-analysis. One study found that in a German clinical guideline committee, when authors with financial or academic conflicts of interest were excluded, there was a change in 1 of 6 evidence-based findings, 0 of 3 guideline consensus statements, and 2 of 23 evidence-based recommendations.37 Another study reported that among National Comprehensive Cancer Network guidelines, there was no association between the prevalence of general or research payments among authors and the percentage of recommendations derived from low-level evidence per guideline.17
Discussion
Among the 37 studies including 14,764 total guideline authors, 45% had at least one FCOI. In addition, more authors had FCOI involving general payments (39%) compared with research payments (29%). Authors involved in guidelines for oncology, neurology, and gastroenterology had the highest prevalence of FCOI. Among the 8 studies that included the monetary values in US dollars of FCOI, average payments per author ranged from $578 to $242,300. Among the 10 studies that included data on undisclosed FCOI, 32% of authors had undisclosed industry payments.
There has been one previous systematic review examining FCOI among authors of CPGs.5 Our study has several strengths compared with this previous review. First, our updated review contains 23 new studies. Second, we performed independent dual data abstraction. This level of rigor is important for this review in particular as the data are heterogeneously collected, analyzed, and reported and thus prone to misinterpretation. Third, we considered differences between studies with respect to methodology and medical subspecialty. Finally, we systematically examined the reporting and prevalence of undisclosed conflicts of interest.
There may be several underlying reasons for the substantial variation in FCOI prevalence between the studies included in this review. The included studies used different data collection methods to identify FCOI. In general, they used 1 of 3 strategies: reporting through CPG author declarations, performing manual searches of external sources, or searching national payment databases for individual authors. Studies that used a national database had the highest prevalence of FCOI as this database lists payments as reported by pharmaceutical and medical device companies rather than by individual authors. A reliance on author declarations may underestimate the prevalence of FCOI as individuals may not recall all their financial interactions with drug and device companies. In addition, small payments, such as meals, may not even be considered to be a payment by physicians despite evidence that they can influence behaviour.46
Studies in certain specialties may be prone to identifying more payments as physicians in these specialties, such as gastroenterology, are more likely to receive industry payments.47 Some studies also considered more types of payments compared with others. The studies that included general and research payments, for example, identified more total FCOI compared with studies that included general payments alone. These studies may overestimate the burden of FCOI by combining general and research payments and treating them as equally influential. Previous reports have suggested that research payments are not as likely as general payments to affect behavior.48
Our results should be interpreted with caution because of several important limitations of the included studies. First, there was heterogeneity in the definitions of FCOI used and methodology to identify industry payments, which precludes quantitative analysis of the data. Many studies are also inherently biased because of their descriptive study designs with no comparators. Furthermore, bias may be introduced in the included studies by guideline author declarations because of recall bias and the potential for omission of FCOI. Several studies used external search methods that have no evidence of validity to identify FCOI, such as a search of guideline authors’ external publications. In addition, this review itself may be limited by a search of only one database, MEDLINE.
Nevertheless, these data suggest that there are numerous FCOI among authors of CPGs, many of which are undisclosed. Previous studies have shown that industry payments are associated with various outcomes favoring industry sponsors, including more positive findings in research, higher prescription volumes of certain companies’ drugs, and poorer methodologic quality.49, 50, 51 Institutions such as the National Academy of Medicine, World Health Organization, and National Health and Medical Research Council in Australia are increasingly recognizing the influence of for-profit entities on CPGs. For example, the World Health Organization and National Health and Medical Research Council have published and implemented policies to ensure disclosure and management of FCOI among their panelists and expert committees.52,53 In addition, several countries have created databases to systematically track physicians’ financial interactions with industry.54,55 Using these databases to identify FCOI as opposed to relying on author declarations may yield more accurate data on physician-industry relationships. Journals are also taking a more active role by using external sources to verify guideline authors’ disclosed FCOI.4 Whereas the impact of these measures is not yet known, increasing efforts to mitigate FCOI may help curb the potential of undue industry influence on clinical guidelines.
Conclusion
There are numerous FCOI among authors of CPGs, many of which are undisclosed. We found that the method of assessing FCOI can have an impact on the prevalence. Using national databases populated by pharmaceutical company records may lead to a higher prevalence of FCOI, whereas relying on author declarations may underestimate conflicts. Future research should determine the accuracy and impact of different types of payments (eg, general, research) on authors’ voting patterns during CPG development and explore the potential of undisclosed FCOI among guidelines. In addition, researchers should examine the impact of such measures as the implementation of National Academy of Medicine standards on the prevalence of FCOI among CPGs. Finally, additional research is required to quantify the implications of FCOI on clinical judgment and patient care.
Footnotes
Potential Competing Interests: Rishad Khan has received research grants from AbbVie and Ferring Pharmaceuticals and research funding from Pendopharm. Samir C. Grover has received research grants and personal fees from AbbVie and Ferring Pharmaceuticals, personal fees from Takeda, and education grants from Janssen and has equity in Volo Healthcare. All other authors have no relevant disclosures.
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Laupacis A. On bias and transparency in the development of influential recommendations. CMAJ. 2006;174(3):335–336. doi: 10.1503/cmaj.051622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E., editors. Clinical Practice Guidelines We Can Trust. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 3.Kirschner N.M., Sulmasy L.S., Kesselheim A.S. Health policy basics: the physician payment sunshine act and the open payments program. Ann Intern Med. 2014;161(7):519–521. doi: 10.7326/M14-1303. [DOI] [PubMed] [Google Scholar]
- 4.O’Neil K., Moores L., Detterback F. From the American College of Chest Physicians: guidelines on conflict-of-interest management. JAMA Intern Med. 2019;179(4):594–595. doi: 10.1001/jamainternmed.2019.0167. [DOI] [PubMed] [Google Scholar]
- 5.Norris S.L., Holmer H.K., Ogden L.A., Burda B.U. Conflict of interest in clinical practice guideline development: a systematic review. PLoS One. 2011;6(10):e25153. doi: 10.1371/journal.pone.0025153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Heart Lung and Blood Institute Quality assessment tool for observational cohort and cross-sectional studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Accessed June 29, 2020.
- 7.Howick J., Chalmers I., Glasziou P., Greenhalgh T., Heneghan C., Liberati A., et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence/ Accessed June 29, 2020.
- 8.Khan R., Scaffidi M.A., Rumman A., Grindal A.W., Plener I.S., Grover S.C. Prevalence of financial conflicts of interest among authors of clinical guidelines related to high-revenue medications. JAMA Intern Med. 2018;178(12):1712–1715. doi: 10.1001/jamainternmed.2018.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreatos N., Zacharioudakis I.M., Zervou F.N., Muhammed M., Mylonakis E. Discrepancy between financial disclosures of authors of clinical practice guidelines and reports by industry. Medicine (Baltimore) 2017;96(2):e5711. doi: 10.1097/MD.0000000000005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grindal A.W., Khan R., Scaffidi M.A., Rumman A., Grover S.C. Financial conflicts of interest in inflammatory bowel disease guidelines. Inflamm Bowel Dis. 2018;25(4):642–645. doi: 10.1093/ibd/izy315. [DOI] [PubMed] [Google Scholar]
- 11.Akl E.A., El-Hachem P., Abou-Haidar H., Neumann I., Schunemann H.J., Guyatt G.H. Considering intellectual, in addition to financial, conflicts of interest proved important in a clinical practice guideline: a descriptive study. J Clin Epidemiol. 2014;67(11):1222–1228. doi: 10.1016/j.jclinepi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Feuerstein J.D., Akbari M., Gifford A.E., et al. Systematic review: the quality of the scientific evidence and conflicts of interest in international inflammatory bowel disease practice guidelines. Aliment Pharmacol Ther. 2013;37(10):937–946. doi: 10.1111/apt.12290. [DOI] [PubMed] [Google Scholar]
- 13.Feuerstein J.D., Gifford A.E., Akbari M., et al. Systematic analysis underlying the quality of the scientific evidence and conflicts of interest in gastroenterology practice guidelines. Am J Gastroenterol. 2013;108(11):1686–1693. doi: 10.1038/ajg.2013.150. [DOI] [PubMed] [Google Scholar]
- 14.Feuerstein J.D., Castillo N.E., Akbari M., et al. Systematic analysis and critical appraisal of the quality of the scientific evidence and conflicts of interest in practice guidelines (2005-2013) for Barrett’s esophagus. Dig Dis Sci. 2016;61(10):2812–2822. doi: 10.1007/s10620-016-4222-2. [DOI] [PubMed] [Google Scholar]
- 15.Feuerstein J.D., Pelsis J.R., Lloyd S., Cheifetz A.S., Stone K.R. Systematic analysis of the quality of the scientific evidence and conflicts of interest in osteoarthritis of the hip and knee practice guidelines. Semin Arthritis Rheum. 2016;45(4):379–385. doi: 10.1016/j.semarthrit.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Feuerstein J.D., Akbari M., Gifford A.E., et al. Systematic analysis underlying the quality of the scientific evidence and conflicts of interest in interventional medicine subspecialty guidelines. Mayo Clin Proc. 2014;89(1):16–24. doi: 10.1016/j.mayocp.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Tang L.L., Mao Y.P., et al. Evidence underlying recommendations and payments from industry to authors of the National Comprehensive Cancer Network guidelines. Oncologist. 2019;24(4):498–504. doi: 10.1634/theoncologist.2017-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niforatos J.D., Pescatore R.M. Financial relationships with industry among guideline authors for the management of acute ischemic stroke. Am J Emerg Med. 2019;37(5):921–923. doi: 10.1016/j.ajem.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Moynihan R., Lai A., Jarvis H., et al. Undisclosed financial ties between guideline writers and pharmaceutical companies: a cross-sectional study across 10 disease categories. BMJ Open. 2019;9(2):e025864. doi: 10.1136/bmjopen-2018-025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosgrove L., Bursztajn H.J., Erlich D.R., Wheeler E.E., Shaughnessy A.F. Conflicts of interest and the quality of recommendations in clinical guidelines. J Eval Clin Pract. 2013;19(4):674–681. doi: 10.1111/jep.12016. [DOI] [PubMed] [Google Scholar]
- 21.Cosgrove L., Bursztajn H.J., Krimsky S., Anaya M., Walker J. Conflicts of interest and disclosure in the American Psychiatric Association’s clinical practice guidelines. Psychother Psychosom. 2009;78(4):228–232. doi: 10.1159/000214444. [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove L., Krimsky S., Wheeler E.E., Peters S.M., Brodt M., Shaughnessy A.F. Conflict of interest policies and industry relationships of guideline development group members: a cross-sectional study of clinical practice guidelines for depression. Account Res. 2017;24(2):99–115. doi: 10.1080/08989621.2016.1251319. [DOI] [PubMed] [Google Scholar]
- 23.Alhamoud H.A., Dudum R., Young H.A., Choi B.G. Author self-disclosure compared with pharmaceutical company reporting of physician payments. Am J Med. 2016;129(1):59–63. doi: 10.1016/j.amjmed.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Bindslev J.B.B., Schroll J., Gøtzsche P.C., Lundh A. Underreporting of conflicts of interest in clinical practice guidelines: cross sectional study. BMC Med Ethics. 2013;14(1):19. doi: 10.1186/1472-6939-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlisle A., Bowers A., Wayant C., Meyer C., Vassar M. Financial conflicts of interest among authors of urology clinical practice guidelines. Eur Urol. 2018;74(3):348–354. doi: 10.1016/j.eururo.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Checketts J.X., Cook C., Vassar M. An evaluation of industry relationships among contributors to AAOS clinical practice guidelines and appropriate use criteria. J Bone Joint Surg. 2018;100(2):e10. doi: 10.2106/JBJS.17.00184. [DOI] [PubMed] [Google Scholar]
- 27.Checketts J.X., Sims M.T., Vassar M. Evaluating industry payments among dermatology clinical practice guidelines authors. JAMA Dermatol. 2017;153(12):1229. doi: 10.1001/jamadermatol.2017.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosgrove L., Krimsky S., Vijayaraghavan M., Schneider L. Financial ties between DSM-IV panel members and the pharmaceutical industry. Psychother Psychosom. 2006;75(3):154–160. doi: 10.1159/000091772. [DOI] [PubMed] [Google Scholar]
- 29.Mendelson T.B., Meltzer M., Campbell E.G., Caplan A.L., Kirkpatrick J.N. Conflicts of interest in cardiovascular clinical practice guidelines. Arch Intern Med. 2011;171(6):577–584. doi: 10.1001/archinternmed.2011.96. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell A.P., Basch E.M., Dusetzina S.B. Financial relationships with industry among National Comprehensive Cancer Network guideline authors. JAMA Oncol. 2016;2(12):1628–1631. doi: 10.1001/jamaoncol.2016.2710. [DOI] [PubMed] [Google Scholar]
- 31.Norris S.L., Holmer H.K., Ogden L.A., Selph S.S., Fu R. Conflict of interest disclosures for clinical practice guidelines in the national guideline clearinghouse. PLoS One. 2012;7(11):e47343. doi: 10.1371/journal.pone.0047343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris S.L., Holmer H.K., Ogden L.A., Burda B.U., Fu R. Conflicts of interest among authors of clinical practice guidelines for glycemic control in type 2 diabetes mellitus. PLoS One. 2013;8(10):e75284. doi: 10.1371/journal.pone.0075284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combs T.R., Scott J., Jorski A., Heavener T., Vassar M. Evaluation of industry relationships among authors of clinical practice guidelines in gastroenterology. JAMA Intern Med. 2018;178(12):1711–1712. doi: 10.1001/jamainternmed.2018.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langer T., Conrad S., Fishman L., et al. Conflicts of interest among authors of medical guidelines: an analysis of guidelines produced by German specialist societies. Dtsch Arztebl Int. 2012;109(48):836–842. doi: 10.3238/arztebl.2012.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuman J., Korenstein D., Ross J.S., Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ. 2011;343:d5621. doi: 10.1136/bmj.d5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holloway R.G., Mooney C.J., Getchius T.S.D., Edlund W.S., JO Miyasaki. Invited Article: Conflicts of interest for authors of American Academy of Neurology clinical practice guidelines. Neurology. 2008;71(1):57–63. doi: 10.1212/01.wnl.0000316319.19159.c3. [DOI] [PubMed] [Google Scholar]
- 37.Hauser W., Petzke F., Kopp I., Nothacker M. [Impact of conflicts of interest on guideline recommendations: empirical study within the second update of the German interdisciplinary S3 guidelines on fibromyalgia syndrome] Schmerz. 2017;31(3):308–318. doi: 10.1007/s00482-017-0218-x. [DOI] [PubMed] [Google Scholar]
- 38.Schott G., Lieb K., Konig J., et al. Declaration and handling of conflicts of interest in guidelines: a study of S1 guidelines from German specialist societies from 2010-2013. Dtsch Arztebl Int. 2015;112(26):445–451. doi: 10.3238/arztebl.2015.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allan G.M., Kraut R., Crawshay A., Korownyk C., Vandermeer B., Kolber M.R. Contributors to primary care guidelines: what are their professions and how many of them have conflicts of interest? Can Fam Physician. 2015;61(1):52–58. [PMC free article] [PubMed] [Google Scholar]
- 40.Papanikolaou G.N., Baltogianni M.S., Contopoulos-Ioannidis D.G., Haidich A.B., Giannakakis I.A., Ioannidis J.P. Reporting of conflicts of interest in guidelines of preventive and therapeutic interventions. BMC Med Res Methodol. 2001;1:3. doi: 10.1186/1471-2288-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choudhry N.K., Stelfox H.T., Detsky A.S. Relationships between authors of clinical practice guidelines and the pharmaceutical industry. JAMA. 2002;287(5):612–617. doi: 10.1001/jama.287.5.612. [DOI] [PubMed] [Google Scholar]
- 42.Jefferson A.A., Pearson S.D. Conflict of interest in seminal hepatitis C virus and cholesterol management guidelines. JAMA Intern Med. 2017;177(3):352–357. doi: 10.1001/jamainternmed.2016.8439. [DOI] [PubMed] [Google Scholar]
- 43.Shnier A., Lexchin J., Romero M., Brown K. Reporting of financial conflicts of interest in clinical practice guidelines: a case study analysis of guidelines from the Canadian Medical Association Infobase. BMC Health Serv Res. 2016;16(a):383. doi: 10.1186/s12913-016-1646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito H., Ozaki A., Sawano T., Shimada Y., Tanimoto T. Evaluation of pharmaceutical company payments and conflict of interest disclosures among oncology clinical practice guideline authors in Japan. JAMA Netw Open. 2019;2(4):e192834. doi: 10.1001/jamanetworkopen.2019.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moynihan R. Who pays for the pizza? Redefining the relationships between doctors and drug companies. 2: disentanglement. BMJ. 2003;326(7400):1193–1196. doi: 10.1136/bmj.326.7400.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeJong C., Aguilar T., Tseng C.W., Lin G.A., Boscardin W.J., Dudley R.A. Pharmaceutical industry–sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med. 2016;176(8):1114–1122. doi: 10.1001/jamainternmed.2016.2765. [DOI] [PubMed] [Google Scholar]
- 47.Marshall E. Financial conflict. Universities puncture modest regulatory trial balloon. Science. 2001;291(5511):2060. doi: 10.1126/science.291.5511.2060a. [DOI] [PubMed] [Google Scholar]
- 48.Yeh J.S., Franklin J.M., Avorn J., Landon J., Kesselheim A.S. Association of industry payments to physicians with the prescribing of brand-name statins in Massachusetts. JAMA Intern Med. 2016;176(6):763–768. doi: 10.1001/jamainternmed.2016.1709. [DOI] [PubMed] [Google Scholar]
- 49.Lundh A., Lexchin J., Mintzes B., Schroll J.B., Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2:MR000033. doi: 10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan R., Nugent C.M., Scaffidi M.A., Gimpaya N., Grover S.C. Association of biologic prescribing for inflammatory bowel disease with industry payments to physicians. JAMA Intern Med. 2019;179(10):1424–1425. doi: 10.1001/jamainternmed.2019.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James J.E. Reviving Cochrane’s contribution to evidence-based medicine: bridging the gap between evidence of efficacy and evidence of effectiveness and cost-effectiveness. Eur J Clin Invest. 2017;47(9):617–621. doi: 10.1111/eci.12782. [DOI] [PubMed] [Google Scholar]
- 52.Guidelines for declaration of interests (WHO Experts). World Health Organization. 2017. https://www.who.int/about/ethics/doi-guide-EN.pdf?ua=1 Accessed June 29, 2020.
- 53.Disclosure of interests and management of conflicts of interest: a guide supporting the Australian Code for the Responsible Conduct of Research. Commonwealth of Australia. National Health and Medical Research Council, Australian Research Council and Universities Australia; Canberra: 2019. [Google Scholar]
- 54.Mulinari S., Ozieranski P. Disclosure of payments by pharmaceutical companies to healthcare professionals in the UK: analysis of the Association of the British Pharmaceutical Industry’s Disclosure UK database, 2015 and 2016 cohorts. BMJ Open. 2018;8(10):e023094. doi: 10.1136/bmjopen-2018-023094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fabbri A., la Santos A., Mezinska S., Mulinari S., Mintzes B. Sunshine policies and murky shadows in Europe: disclosure of pharmaceutical industry payments to health professionals in nine European countries. Int J Health Policy Manag. 2018;7(6):504–509. doi: 10.15171/ijhpm.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.