Abstract
Objective
To distinguish between sepsis only vs progressive lymphoma in patients with a history of lymphoma who present to the hospital with lactic acidosis.
Patients and Methods
We identified patients with non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma from January 2014 to December 2015. Patients were categorized into 2 groups: sepsis only or progressive lymphoma. Two-sided Wilcoxon rank sum test and χ1/Fisher exact test were used to compare the continuous and categorical variables, respectively. Kaplan-Meier analysis was used to estimate overall survival (OS).
Results
A total of 51 patients were identified; 33 (65%) patients were categorized into the sepsis only group, and 18 (35%), into the progressive lymphoma group. Values for serum lactate dehydrogenase (LDH) drawn during hospitalization were statistically different between the sepsis only and progressive lymphoma groups (median, 262 vs 665 U/L; P=.005), respectively. The sensitivity and specificity of serum LDH level 2 or more times the upper limit of normal for progressive lymphoma were 56% (95% CI, 33% to 79%) and 85% (95% CI, 73% to 97%), respectively. Serum LDH level was independently predictive of inferior OS (hazard ratio, 27.8; 95% CI, 4.0 to 160.1; P<.001), while serum albumin level (hazard ratio, 0.05; 95% CI, 0.01 to 0.27; P<.001) was independently predictive of improved OS.
Conclusion
Serum LDH levels used in conjunction with serial serum lactate values may be reliable markers to differentiate patients with progressive lymphomatous disease from patients with lymphoma with sepsis only. The LDH levels should be obtained in all patients with lymphoma who present to the hospital with lactic acidosis.
Abbreviations and Acronyms: ATP, adenosine triphosphate; CoA, coenzyme A; CT, computed tomography; DLBCL, diffuse large B-cell lymphoma; GLUT, facilitative glucose transporter; HR, hazard ratio; IQR, interquartile range; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; NAD, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide and hydrogen; O2, oxygen; NHL, non-Hodgkin lymphoma; OS, overall survival; TCA, tricarboxylic acid; ULN, upper limit of normal
Lactic acidosis is a significant concern in hospitalized patients and occurs when the production of lactic acid (lactate) exceeds the ability of the body to metabolize lactate. Lactate can be metabolized in the liver, kidney, and other tissues by the enzyme lactic acid dehydrogenase (LDH) to form pyruvate, which can then feed into the Cori cycle or tricarboxylic acid cycle and undergo oxidative phosphorylation.2 When homeostasis is disrupted, lactate accumulates and lactic acidosis ensues. Two types of lactic acidosis exist. Type A lactic acidosis is caused by tissue hypoperfusion or acute severe hypoxemia; type B is due to toxin-induced impairment of cellular metabolism and/or regional areas of ischemia without evidence of systemic hypoperfusion.2 Differentiating between types A and B lactic acidosis can be challenging,1 and the literature is replete with case reports demonstrating the danger of missing a type B lactic acidosis from a progressive malignancy.3, 4, 5.
In patients with a diagnosis of lymphoma, differentiating between type A lactic acidosis from sepsis and type B lactic acidosis from progressive lymphoma adds an additional challenge because patients are often immunocompromised and prone to infections.6,7 Although no studies have investigated methods to differentiate between sepsis alone vs progressive disease in patients with lymphoma, it is well established that malignant cells can exhibit the Warburg effect, wherein malignant cells change their metabolome by increasing glycolysis and lactate production to allow for uncontrolled proliferation.8,9 In lymphoma, a characteristic feature of aggressive disease is increased uptake on 18F-labeled fluoro-2-deoxyglucose positron emission tomography computed tomography (CT). In vitro studies have shown that lymphomas can upregulate enzymes involved in glucose metabolism and exhibit altered levels of metabolites,10, 11, 12 whereas case reports have demonstrated that profound systemic lactic acidosis is associated with high mortality in lymphoma.13 Retrospective studies have also observed that increased serum LDH levels are associated with aggressive lymphomatous disease and poor survival.14, 15, 16
We hypothesized that among patients with a history of lymphoma and lactic acidosis, serum LDH level may help differentiate between patients with sepsis only vs progressive malignant disease. Lactate dehydrogenase is found in blood cells and lymph tissue, has been used as a prognostic marker in patients with hematologic malignancy for many years, and is a part of the International Prognostic Index.17 Lactate dehydrogenase is believed to be a marker of increased cell turnover, significant tissue injury, and inflammation.18 However, because LDH exists in other organs such as skeletal muscle, liver, and heart, LDH values above the upper limit of normal (ULN) can also be seen in patients with significant ischemia, such as ischemic hepatitis19 and severe coronavirus disease 201920 and was previously used as a biomarker in myocardial infarction before the discovery of cardiac troponin.21 Our retrospective study aimed to investigate differences among patients with sepsis alone vs progressive lymphoma, assess whether LDH level could be a possible marker to differentiate between the 2 groups, and define the prognosis of patients with elevations of both LDH and lactate levels.
Patients and Methods
We used the Mayo Clinic Lymphoma Database to identify all patients with non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma from January 1, 2014 to December 31, 2015. Patients were included in the study if they were hospitalized with an initial diagnosis of sepsis, had at least 2 peripheral-blood cultures drawn during hospitalization, and received empiric treatment with intravenous antibiotics. Patients were also required to have lactic acidosis as defined as serum lactate level greater than 2.2 mmol/L (normal level is ≤2.2 mmol/L) and serum LDH level (normal level is <222 U/L). All other patients were excluded.
Patients who met our criteria were categorized into 2 groups that we established a priori: (1) sepsis only: those with documented infection (organism identified in a peripheral-blood culture) or patients who did not have documented infection (blood cultures were no growth to date after 5 days) but had improvement with antibiotics; and (2) progressive lymphoma: those with progressive malignant disease and no documented infection (all blood cultures were negative during hospitalization; no improvement with antibiotics). Progressive disease was determined using CT, bone marrow biopsy, and/or lymph node biopsy. The NHLs were considered aggressive if they were diffuse large B-cell lymphoma (DLBCL), Richter’s transformation from a low-grade lymphoma, Burkitt lymphoma, posttransplant lymphoproliferative disease, mantle cell lymphoma, and B-cell lymphoma not otherwise specified, whereas mucosa-associated lymphoid tissue lymphoma, Waldenström macroglobulinemia, and marginal zone lymphoma were considered low-grade lymphomas.
Two-sided Wilcoxon rank sum test and χ1/Fisher exact test were used to compare the continuous and categorical variables, respectively. Kaplan-Meier analysis was used to estimate overall survival (OS). The OS was calculated from the date of hospitalization to the date of last follow-up if alive or the date of death due to any cause. Hazard ratios (HRs) and CIs were also calculated. Statistical analyses were performed using JMP, version 14.0 (SAS).
Results
We identified 51 patients who met our inclusion criteria; 47 patients (92%) with B-cell NHL, 3 (6%) with T-cell NHL, and 1 (2%) with Hodgkin lymphoma. Among those with B-cell NHL, 24 patients (47%) had DLBCL, 14 (27%) had Richter’s transformation from a low-grade lymphoma, 2 (4%) had Burkitt lymphoma, 2 had posttransplant lymphoproliferative disease, and the remaining (1 patient each) had either mucosa-associated lymphoid tissue lymphoma, mantle cell lymphoma, marginal zone lymphoma, Waldenström macroglobulinemia, or B-cell lymphoma not otherwise specified.
Before hospitalization, all patients in our cohort had been previously treated with chemotherapy. The most common treatment regimen was rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone. Upon hospitalization, all patients received intravenous crystalloid fluids with 1 or a combination of the following intravenous antibiotics: piperacillin/tazobactam, cefepime, levofloxacin, tobramycin, daptomycin, and/or vancomycin. By use of our criteria, 33 (65%) patients were categorized as sepsis only and 18 (35%) were categorized as progressive lymphoma. From the date of lymphoma diagnosis, the median follow-up for the entire cohort was 6.3 (95% CI, 4.5 to 7.0) years, while the median follow-up from the date of hospitalization was 3.3 (95% CI, 2.5 to 4.7) years.
Progressive Lymphoma Group
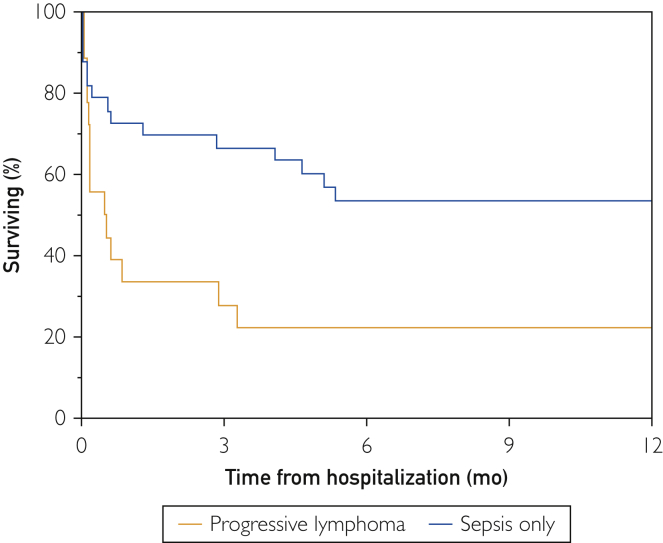
In the progressive lymphoma group (n=18), 13 patients (72%) were men. At the time of hospitalization, median age was 63 (interquartile range [IQR], 48-78) years. Median OS was 0.5 (95% CI, 0.16 to 2.9) months (Figure 1). The median number of chemotherapeutic treatments before hospitalization was 2 (IQR, 1-4), and 8 (44%) patients had liver involvement from their lymphoma.
Figure 1.
Kaplan-Meier curve illustrates overall survival of progressive lymphoma vs sepsis only groups.
The median initial lactate level drawn during hospitalization was 3.25 (IQR, 2.9-5.0) mmol/L. Lactate values 3, 6, 12, and 48 hours after the first lactate value were greater than the ULN in all patients who had serial lactate samples drawn. The median peak lactate level was 7.35 (IQR, 5.1-15.2) mmol/L, and the median time to peak lactate level was 13 (IQR, 0-78.5) hours. Fourteen patients (78%) had an LDH value greater than the ULN and the median LDH value during hospitalization was 665 (IQR, 275-2980) U/L. The median time to collecting a serum LDH value from the initial lactate value was 4 (IQR, 1-25) days. Laboratory evaluation at the time of admission was as follows: median hemoglobin, 10.3 (IQR, 9.5-11.2) g/dL (to convert to g/L, multiply by 10); median platelet level, 83 (IQR, 38-192) ×109/L; median white blood cell count, 7.2 (IQR, 5.6-18.8) ×109/L; median creatinine, 0.96 (IQR, 0.73-1.1) mg/dL (to convert to μmol/L, multiply by 88.4); and median serum albumin, 3.2 (IQR 2.9-3.9) g/dL (to convert to g/L, multiply by 10).
Nine patients (50%) had biopsies confirming progressive lymphoma, while the rest were confirmed with radiologic imaging. Of the 9 who underwent biopsy, 6 (33%) had Ki-67 staining, and all were in the high proliferative rate range (median, 75%; IQR, 60%-90%).
Of these 18 patients with progressive lymphoma, 4 (22%) survived the hospitalization. Among these patients, LDH values were 191, 192, 318, and 364 U/L, with initial lactate levels of 3.1, 3.4, 2.5, and 2.9 mmol/L, respectively. These 4 patients were able to receive subsequent chemotherapy, with the median time to chemotherapy from hospital discharge being 13 (range, 7-19) days and the median time to last known follow-up being 42 (range, 20-54) months. The remaining 14 patients (78%) died during the hospitalization, with a median time to death from hospitalization of 6 (IQR, 2-16) days. Among those who died, none received chemotherapy, and the causes of death were multiorgan failure from progressive lymphoma.
Sepsis Group
In the sepsis group (n=33), 20 (61%) were men. At the time of hospitalization, the median age was 68 (IQR, 60-80) years. Median OS was 24.8 (95% CI, 2.0 to not reached) months (Figure 1). The median number of chemotherapeutic treatments before hospitalization was 2 (IQR, 1-3), and 3 (9%) patients had liver involvement from their lymphoma.
The median lactate level for the first lactate specimen to be drawn during hospitalization was 3.5 (IQR, 2.9-4.4) mmol/L. Twenty-three (70%) patients had serial lactate samples drawn, and serum lactate levels 3, 6, 12, and 48 hours after the first lactate level continued to be elevated in 83% (19/23), 61% (14/23), 48% (11/23), and 43% (10/23), respectively. The median peak lactate level was 6.9 (IQR, 5.4-8.1) mmol/L, and the median time to peak lactate level was 28 (IQR, 6.5-66) hours. The median LDH value was 262 (IQR, 217-373) U/L, and the median time to collecting a serum LDH value from the initial lactate value was 9 (IQR, 1-22) days. Twenty-two patients (67%) had LDH values greater than the ULN. Laboratory evaluation at the time of admission was as follows: hemoglobin, 9.9 (IQR, 8.1-11.6) g/dL; platelets, 126 (IQR, 98-223) ×109/L; white blood cell count, 7.9 (IQR, 3.5-17.9) ×109/L; creatinine, 1.2 (IQR, 0.8-1.6) mg/dL; and serum albumin, 3.4 (2.8-3.9) g/dL.
Bacterial organisms identified on peripheral-blood culture included Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Citrobacter species, methicillin-sensitive Staphylococcus aureus, Parvimonas micra, Nocardia farcinica, Streptococcus mitis, and Streptococcus viridans. A bacterial organism was not identified in 12 patients (36%).
Nine of 33 patients (27%) died during hospitalization. Among those who died, the median time to death from hospitalization was 4 (IQR, 1-12) days, the median LDH level was 300 (IQR, 231-443) U/L, and the median initial lactate level was 3.7 (IQR, 3-4.6) mmol/L. Causes of death were attributed to multiorgan failure from septic shock.
Comparative Analysis
When comparing the 2 groups, the LDH level obtained during hospitalization was the only statistically significant variable observed (P=.005; Table 1). There was no statistical difference when comparing age at the time of hospitalization, sex, history of NHL, frequency of aggressive NHL, total previous lines of treatment, and values for hemoglobin, white blood cell count, platelets, creatinine, serum albumin, initial serum lactate, and peak serum lactate between the 2 groups.
Table 1.
| Parameters | Progressive Lymphoma (n=18) | Sepsis Only (n=33) | P (univariate) | P (multivariate) |
|---|---|---|---|---|
| Hospitalization | ||||
| Age at time of hospitalization (y), median (IQR) | 63 (48-78) | 68 (60-80) | .23 | .8 |
| Male, no. (%) | 13 (72) | 20 (61) | .54 | .58 |
| Non-Hodgkin lymphoma, no. (%) | 18 (100) | 32 (97) | .28 | |
| Aggressive non-Hodgkin lymphoma, no. (%) | 18 (100) | 29 (88) | 0.54 | |
| Previous lines of treatment, median (IQR) | 2 (1-4) | 2 (1-3) | .23 | |
| Hemoglobin (g/dL), median (IQR) | 10.3 (9.5-11.2) | 9.9 (8.1-11.6) | .64 | .52 |
| White blood cell count (109/L), median (IQR) | 7.2 (5.6-18.8) | 7.9 (3.5-17.9) | .69 | .66 |
| Platelets (109/L), median (IQR) | 83 (38-192) | 126 (98-223) | .4 | .72 |
| Creatinine (mg/dL), median (IQR) | 0.96 (0.73-1.1) | 1.2 (0.8-1.6) | .09 | .22 |
| Serum albumin (g/dL), median (IQR) | 3.2 (2.9-3.9) | 3.4 (2.8-3.9) | .44 | .38 |
| Lactate, initial (mmol/L; normal <2.2 mmol/L), median (IQR) | 3.25 (2.9-5.0) | 3.5 (2.9-4.4) | .89 | .49 |
| Peak lactate (mmol/L), median (IQR) | 7.35 (5.1-15.2) | 6.9 (5.4-8.1) | .83 | |
| LDH (U/L; normal <222 U/L), median (IQR) | 665 (275-2980) | 262 (217-373) | .005c | .003c |
| Before hospitalization | ||||
| Age (y) at time of lymphoma diagnosis, median (IQR) | 62.5 (48-77) | 68 (57-74) | .42 | |
| LDH (U/L) at time of lymphoma diagnosis, median (IQR) | 318 (200-569) | 309 (207-483.5) | .85 | |
IQR, interquartile range; LDH, lactate dehydrogenase.
SI conversion factors: To convert creatinine values to μmol/L, multiply by 88.4; to convert albumin values to g/L, multiply by 10.
Statistically significant.
The LDH levels continued to be independently significant in multivariate analysis (P=.003) that included age at the time of hospitalization, sex, and values for hemoglobin, white blood cell count, platelets, creatinine, serum albumin, LDH, and serum lactate. The sensitivity and specificity of serum LDH levels 2 or more times the ULN for progressive disease were 56% (95% CI, 33%-79%) and 85% (95% CI, 73%-97%) respectively.
Cox Regression Analysis
On univariate analysis, the factor predictive of improved OS for the entire cohort was higher serum albumin level (HR, 0.08; 95% CI, 0.02 to 0.44; P=.003), whereas higher serum LDH level (HR, 25.3; 95% CI, 4.8 to 116.5; P<.001) was predictive of inferior OS. These factors were included in a multivariate analysis that revealed serum LDH level (HR, 27.8; 95% CI, 4.0 to 160.1; P<.001) to be independently predictive of inferior OS, whereas higher serum albumin level (HR, 0.05; 95% CI 0.01-0.27; P<.001) was independently predictive of improved OS (Table 2).
Table 2.
Univariate and Multivariate Analysis for Overall Survival From Date of Hospitalization (Cox proportional hazards regression model)
| Variable | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Age at hospitalization | 1.4 | 0.34-6.6 | .66 | |||
| Sex (male/female) | 1.62 | 0.75-3.51 | .22 | |||
| Hemoglobin | 0.88 | 0.73-1.05 | .11 | |||
| White blood cell count | 1.1 | 0.26-4.2 | .87 | |||
| Platelets | 0.37 | 0.07-1.53 | .20 | |||
| Creatinine | 0.66 | 0.09-2.94 | .63 | |||
| Serum albumin | 0.08 | 0.02-0.44 | .0034 | .05 | 0.01-0.27 | <.001 |
| Lactate (mmol/L) | 2.64 | 0.6-8.67 | .15 | |||
| Lactate dehydrogenase (U/L) | 25.3 | 4.8-116.5 | <.001a | 27.8 | 4.0-160.1 | <.001a |
Statistically significant.
Discussion
Our study demonstrates that in patients with lymphoma with lactic acidosis, a serum LDH level may be a useful biomarker to alert physicians that the patient may not have sepsis alone but could have progressive lymphomatous disease. In this cohort, patients with progressive lymphoma had serum LDH levels that were 2.5 times higher than those for patients with sepsis alone. Elevated LDH level is usually seen only when there is marked tissue death, such as in hemolysis, myocardial infarction, ischemic hepatitis, and cancers in which cell turnover is high.6,19,21 Among our patients who had biopsies confirming tumor progression, all had a high cell proliferative rate by Ki-67 staining. Thus, it is not surprising that patients who have aggressive lymphomatous disease are likely to have significantly elevated LDH levels due to increased cell turnover and tumor hypermetabolism, whereas patients with sepsis should not have elevated LDH levels unless they have overwhelming multiorgan failure.
Additional information that may help differentiate between sepsis alone vs progressive lymphoma is the time to lactate level normalization. In patients with sepsis who are improving with standard care, including intravenous fluid resuscitation, antibiotic therapy, source control, and other supportive measures, improvement in the distributive shock state generally results in resolution of the lactic acidosis.22,23 However, in patients with type B lactic acidosis from progressive lymphoma, the lactic acidosis does not resolve with these measures because the lactic acidosis is caused by ongoing increased tumor cell metabolism. As observed in our study, 57% (13/23) of patients in the sepsis only group had normal lactate levels by 48 hours, whereas 0% (0/14) of patients had normal lactate levels in the progressive lymphoma group by 48 hours.
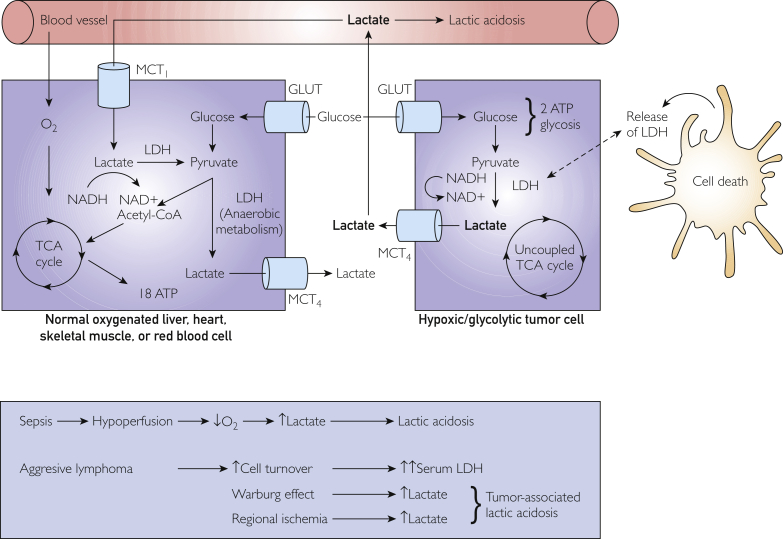
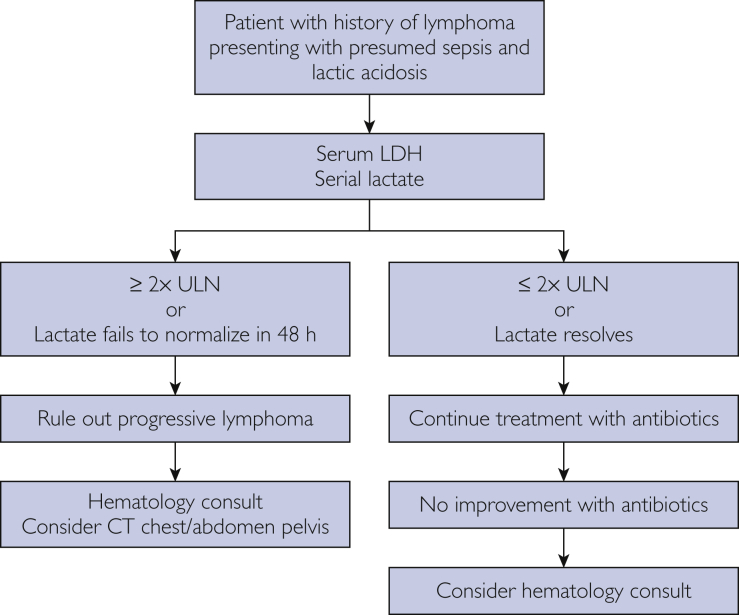
In Figure 2,24 we provide an illustration of the interrelationship between lactate and LDH levels in normal cells and tumor cells. In Figure 3, we propose an algorithm to assist physicians in distinguishing between progressive lymphoma vs sepsis in patient with known lymphoma and lactic acidosis. In particular, we highlight the importance of obtaining a serum LDH level as soon as possible in patients with lymphoma who present to the hospital with sepsis. In our study, serum LDH levels 2 or more times the ULN had a high specificity for progressive lymphoma. These patients need urgent evaluation and hematologic consultation because early recognition of progressive lymphoma may allow for prompt initiation of treatment that would likely lead to improved patient outcomes.
Figure 2.
Illustration shows the role of lactate and lactate dehydrogenase (LDH) in tumor and normal cells. ATP, adenosine triphosphate; CoA, coenzyme A; GLUT, facilitative glucose transporter; MCT, monocarboxylate transporter; NAD, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide and hydrogen; O2, oxygen; TCA, tricarboxylic acid. Adapted from Martel et al.24
Figure 3.
Algorithm outlines an approach to differentiating sepsis only vs progressive lymphoma in patients with a history of lymphoma. LDH, lactate dehydrogenase; ULN, upper limit of normal.
However, it should be noted that we had 8 patients with progressive lymphoma who did not meet the recommended serum LDH level cutoff, and among these patients, 4 died during the hospitalization. All these patients had an aggressive NHL (DLBCL, Richter’s transformation, mantle cell lymphoma, and T-cell lymphoma). Thus, even if a patient with an aggressive lymphoma has an LDH level less than 2 times the ULN, the differential diagnosis of progressive disease should still be considered, especially if the patient does not improve with intravenous antibiotics. Further studies in the metabolomics of lymphoma are needed to investigate why a subset of patients do not have elevated LDH levels despite progressive lymphoma and an elevated serum lactate level.
In our cohort, patients with lactic acidosis with progressive lymphoma had significantly inferior OS compared with those with lactic acidosis with sepsis alone. This finding is not surprising because most patients with progressive lymphoma were not medically fit to undergo subsequent chemotherapy due to being too ill from their lymphoma. Without being able to address the progressive disease, it is expected that patients would die of complications related to their lymphoma. We also observed that a higher serum LDH level was predictive of inferior OS, whereas higher serum albumin level was predictive of better OS for the overall cohort. A higher serum LDH level suggests increased cell death and higher LDH levels have been shown to be a prognostic marker for poor outcomes.15,16,20,25 However, higher serum albumin level was predictive of improved OS. A possible explanation for this finding is that additional comorbid conditions and worse nutritional intake may contribute to hypoalbuminemia and negatively affect OS.26
Finally, it should be noted that our study had 4 patients in the progressive lymphoma category who survived the hospitalization, were eventually discharged, received subsequent chemotherapy, and had long-term survival. This emphasizes the importance of early differentiation of progressive lymphoma. Of note, these patients had serum LDH levels less than 2 times the ULN.
Our study has limitations due to its retrospective nature. Not all patients with sepsis had an identifiable organism, although blood cultures are not perfectly sensitive for bacteremia27 and sepsis may result from pulmonary, urinary tract, and abdominal sources, among others, without concurrent bacteremia. We may have introduced selection bias because patients were excluded if they did not have an LDH sample drawn during hospitalization. However, the lack of an available serum LDH level is likely an indicator that physicians are not aware of type B lactic acidosis and are not considering progressive lymphomatous disease in their differential diagnosis. Most patients in our progressive lymphoma group were unable to undergo 18F-labeled fluoro-2-deoxyglucose positron emission tomography CT due to critical illness. Finally, our study is subject to tertiary bias. We also note that we were unable to further validate our findings with biobank samples because both lactate and LDH samples must be analyzed fresh. Thus, validation of our results will need to be performed prospectively. Finally, given that serum LDH level has been used as a prognostic tool in other malignancies,16,28 future studies should investigate whether serum LDH level can be used as a marker to distinguish between sepsis only vs progressive disease in patients with a pre-existing history of other types of cancer.
Conclusion
It is challenging to differentiate between type A and type B lactic acidosis in patients with lymphoma. Our study suggests that serum LDH and serial lactate values may be reliable markers to differentiate patients who have progressive lymphoma from those who have sepsis only. Serum LDH levels should be obtained in all hospitalized patients with lymphoma who have lactic acidosis and those with LDH levels 2 or more times the ULN should be promptly referred for hematologic consultation and consideration for tumor imaging, biopsy, and/or empiric treatment to slow or stop tumor hypermetabolism.
Footnotes
Grant Support: Supported by the University of Iowa/Mayo Clinic Specialized Programs of Research Excellence in Lymphoma (P50 CA97274).
Potential Competing Interests: The authors report no competing interests.
References
- 1.Redant S., Hussein H., Mugisha A., et al. Differentiating hyperlactatemia type A from type B: how does the lactate/pyruvate ratio help? J Transl Int Med. 2019;7(2):43–45. doi: 10.2478/jtim-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraut J.A., Madias N.E. Lactic acidosis. N Engl J Med. 2014;371(24):2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 3.Singh M., Ajmeri A.N., Suliman M.S., Zaheer K., Al-Astal A.K. A challenging case of coexisting type A and type B lactic acidosis: a case report. Cureus. 2019;11(1):e3944. doi: 10.7759/cureus.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz J.P., Singh A.K., Hart P. Type B lactic acidosis secondary to malignancy: case report, review of published cases, insights into pathogenesis, and prospects for therapy. ScientificWorldJournal. 2011;11:1316–1324. doi: 10.1100/tsw.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M., Kim T.Y., Pessegueiro A.M. Elevated lactate levels in a non-critically ill patient. JAMA. 2015;313(8):849–850. doi: 10.1001/jama.2014.14074. [DOI] [PubMed] [Google Scholar]
- 6.Sillos E.M., Shenep J.L., Burghen G.A., Pui C.H., Behm F.G., Sandlund J.T. Lactic acidosis: a metabolic complication of hematologic malignancies: case report and review of the literature. Cancer. 2001;92(9):2237–2246. doi: 10.1002/1097-0142(20011101)92:9<2237::aid-cncr1569>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Friedenberg A.S., Brandoff D.E., Schiffman F.J. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore) 2007;86(4):225–232. doi: 10.1097/MD.0b013e318125759a. [DOI] [PubMed] [Google Scholar]
- 8.Cairns R.A. Drivers of the Warburg phenotype. Cancer J. 2015;21(2):56–61. doi: 10.1097/PPO.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 9.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104(3):275–281. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X., Stenson M., Abeykoon J., et al. Targeting glycogen synthase kinase 3 for therapeutic benefit in lymphoma. Blood. 2019;134(4):363–373. doi: 10.1182/blood.2018874560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu J.J., Singh A., Xue K., et al. Up-regulation of hexokinase II contributes to rituximab-chemotherapy resistance and is a clinically relevant target for therapeutic development. Oncotarget. 2017;9(3):4020–4033. doi: 10.18632/oncotarget.23425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalali S., Shi J., Buko A., et al. Increased glutathione utilization augments tumor cell proliferation in Waldenstrom macroglobulinemia. Redox Biol. 2020;36:101657. doi: 10.1016/j.redox.2020.101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soleja M., Mims M., Rivero G. Uncovering molecular abnormalities leading to the Warburg effect in primary refractory diffuse large B-cell lymphoma. Blood Cancer J. 2016;6(12):e502. doi: 10.1038/bcj.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suki S., Swan F., Jr., Tucker S., et al. Risk classification for large cell lymphoma using lactate dehydrogenase, beta-2 microglobulin, and thymidine kinase. Leuk Lymphoma. 1995;18(1-2):87–92. doi: 10.3109/10428199509064927. [DOI] [PubMed] [Google Scholar]
- 15.Jung S.H., Yang D.H., Ahn J.S., Kim Y.K., Kim H.J., Lee J.J. Serum lactate dehydrogenase with a systemic inflammation score is useful for predicting response and survival in patients with newly diagnosed diffuse large B-cell lymphoma. Acta Haematol. 2015;133(1):10–17. doi: 10.1159/000360068. [DOI] [PubMed] [Google Scholar]
- 16.Huang B., Lu J., Wang X., et al. Prognostic value of lactate dehydrogenase in Chinese patients with newly diagnosed transplant eligible multiple myeloma. Leuk Lymphoma. 2017;58(7):1740–1742. doi: 10.1080/10428194.2016.1252975. [DOI] [PubMed] [Google Scholar]
- 17.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 18.Plagemann P.G., Gregory K.F., Wroblewski F. The electrophoretically distinct forms of mammalian lactic dehydrogenase. 1. Distribution of lactic dehydrogenase. 1. Distribution of lactic dehydrogenases in rabbit and human tissue. J Biol Chem. 1960;235:2282–2287. [PubMed] [Google Scholar]
- 19.Cassidy W.M., Reynolds T.B. Serum lactic dehydrogenase in the differential diagnosis of acute hepatocellular injury. J Clin Gastroenterol. 1994;19(2):118–121. doi: 10.1097/00004836-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Henry B.M., Aggarwal G., Wong J., et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danese E., Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med. 2016;4(10):194. doi: 10.21037/atm.2016.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryoo S.M., Ahn R., Shin T.G., et al. Korean Shock Society (KoSS) Investigators. Lactate normalization within 6 hours of bundle therapy and 24 hours of delayed achievement were associated with 28-day mortality in septic shock patients. PLoS One. 2019;14(6):e0217857. doi: 10.1371/journal.pone.0217857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 24.Martel F., Guedes M., Keating E. Effect of polyphenols on glucose and lactate transport by breast cancer cells. Breast Cancer Res Treat. 2016;157(1):1–11. doi: 10.1007/s10549-016-3794-z. [DOI] [PubMed] [Google Scholar]
- 25.Duman A., Akoz A., Kapci M., et al. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: predictive role of lactate dehydrogenase. Am J Emerg Med. 2016;34(11):2167–2171. doi: 10.1016/j.ajem.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Don B.R., Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee A., Mirrett S., Reller L.B., Weinstein M.P. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol. 2007;45(11):3546–3548. doi: 10.1128/JCM.01555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwala S.S., Keilholz U., Gilles E., et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951) Eur J Cancer. 2009;45(10):1807–1814. doi: 10.1016/j.ejca.2009.04.016. [DOI] [PubMed] [Google Scholar]