Abstract
Objective
To assess 4 adverse renal outcomes in a heterogeneous cohort of patients with systolic heart failure (HF) who were prescribed sacubitril-valsartan vs angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB).
Patients and Methods
The OptumLabs Database Warehouse, which contains linked administrative claims and laboratory results, was used to identify patients with systolic HF who were prescribed sacubitril-valsartan or ACEi/ARB between July 1, 2015, and September 30, 2019. One-to-one propensity score matching and inverse probability of treatment weighting was used to balance baseline variables. Cox proportional hazards modeling was performed to compare renal outcomes in both medication groups, including 30% or more decline in estimated glomerular filtration rate (eGFR), doubling of serum creatinine, acute kidney injury (AKI), and kidney failure (eGFR < 15 mL/min per 1.73 m2, kidney transplant, or dialysis initiation).
Results
A total of 4667 matched pairs receiving sacubitril-valsartan or ACEi/ARB were included; the mean follow-up period was 7.8±7.8 months. The mean age was 69.4±11 years; 35% were female, 19% black, and 15% Hispanic. The cumulative risk at 1 year was 6% for 30% or more decline in eGFR, 2% for doubling of serum creatinine, 3% for AKI, and 2% to 3% for kidney failure. Furthermore, no significant differences in risk were observed with sacubitril-valsartan compared with ACEi/ARB for a 30% or more decline in eGFR (hazard ratio [HR], 0.96; 95% CI, 0.79 to 1.10), doubling of serum creatinine (HR, 0.94; 95% CI, 0.69 to 1.27); AKI (HR, 0.80; 95% CI, 0.63 to 1.03), and kidney failure (HR 0.80; 95% CI, 0.59 to 1.08).
Conclusion
Among patients with systolic HF, the risk of adverse renal outcomes was similar between patients prescribed sacubitril-valsartan and those prescribed ACEi/ARB.
Abbreviations and Acronyms: ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision; IPTW, inverse probability of treatment weighting; NP, natriuretic peptide; RAAS, renin-angiotensin-aldosterone system; RCT, randomized controlled trial
Hyperactivation of the renin-angiotensin-aldosterone system (RAAS) is a hallmark feature of heart failure with reduced ejection fraction (HFrEF).1 Therapies that provide RAAS inhibition, namely, angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), have therefore formed one of the foundations for HF treatment.2, 3, 4 Despite the demonstrable benefits of ACEi/ARB therapy, many patients with HFrEF often have prognostically significant comorbidities including renal dysfunction.5, 6, 7, 8 Furthermore, the use of ACEi/ARB may precipitate acute kidney injury (AKI) and a decline in renal function,9,10 preventing effective doses from being achieved. Given the high risk of adverse outcomes associated with the coexistence of cardiorenal dysfunction in HF,5 therapeutic strategies that mediate protection of both heart and kidneys may reduce the risk of unfavorable outcomes.
Sacubitril-valsartan is a new class of HF therapeutics with a class I recommendation in cardiology guidelines for patients with chronic symptomatic HFrEF.2,6 This recommendation was based on the PARADIGM-HF (Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor with Angiotensin Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in HF) trial, which found that long-term therapy with sacubitril-valsartan improved cardiovascular morbidity and mortality compared with enalapril in patients with HFrEF.7 From a renal perspective, post hoc analysis of the PARADIGM-HF study reported that sacubitril-valsartan had a more favorable effect than did enalapril on renal function, particularly estimated glomerular filtration rate (eGFR).8
Despite the clinical benefits of sacubitril-valsartan, uncertainty and evidence gaps in the data exist in assessing the effects of sacubitril-valsartan on renal outcomes in real-world patient populations, which often comprise patient groups that are typically underrepresented in randomized controlled trials (RCTs), such as elderly individuals, women, and racial minorities. To this end, we sought to determine whether renal outcomes differed between patients with HFrEF treated with sacubitril-valsartan and those treated with ACEi/ARB by using a large and representative national US cohort.
Patients and Methods
Data Source
This study was a retrospective cohort study using de-identified administrative claims data from the OptumLabs Data Warehouse, which includes medical and pharmacy claims, laboratory results, and enrollment records for commercial and Medicare Advantage enrollees. The database contains longitudinal health information on enrollees and patients, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States.9,11 This study was institutional review board exempt; informed consent was not required as the study used preexisting de-identified data.
Adult patients (≥18 years of age) who had a prescription fill for sacubitril-valsartan or ACEi/ARB between July 1, 2015, and September 30, 2019, were identified. Patients were restricted to those with a previous diagnosis of systolic HF using International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) billing codes (ICD-9 code, 428.2X; ICD-10 code, I50.2X); this definition was found to have a 97.7% specificity for patients with HF and ejection fraction less than 45%.10 Patients were required to have at least 6 months of continuous enrollment in both medical and pharmacy insurance plans before the index date to ensure adequate capture of baseline characteristics. The index date of the sacubitril-valsartan cohort was defined as the patient’s first prescription fill of sacubitril-valsartan, whereas the index date of the ACEi/ARB cohort was the first prescription fill of ACEi/ARB in the study period after they met the 6-month continuous enrollment requirement. Patients in both the ACEi/ARB and sacubitril-valsartan cohorts could have ACEi/ARB prescriptions before their index date.
Baseline Characteristics
Demographic and clinical variables were defined by the presence of a claim with corresponding diagnosis codes, procedure codes, or prescription fills. Comorbidities were captured using ICD-9 and ICD-10 codes within 6 months before the index date, and the Charlson comorbidity index was calculated using the methods by Quan et al.12 Previous medication use was defined as having a prescription fill within 120 days before the index date. The total daily dose of ACEi/ARB was categorized as low, medium, and high (Supplemental Table 1, available online at http://www.mcpiqojournal.org). Previous hospitalizations and office visits with a cardiologist or primary care provider (physician, nurse practioner, or physician assistant) were captured within 6 months before the index date. The serum creatinine result closest to the index date, occurring up to 6 months before, was used as the baseline value. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.13 Patients with kidney failure (defined as eGFR < 15 mL/min per 1.73 m2, kidney transplant, or dialysis initiation) or AKI during the 6 months before the index date were excluded.
Follow-up
Follow-up began at the index date and continued until the end of treatment, which was defined as the earliest date of discontinuation of index medication, end of enrollment in health insurance plan, death, or end of the study period (October 31, 2019). All available serum creatinine levels and eGFR from the index date of inclusion until the end of follow-up period were recorded. Discontinuation of index medication was defined as not refilling a prescription within 45 days of the end of the last filled prescription. Adherence was calculated using the medical possession ratio.14 Specifically, we used the prescription fill dates and days’ supply for each medication group to calculate total days’ supply and divided by the number of days in the follow-up period.
Renal Outcomes
We assessed 4 renal outcomes: 30% or more decline in eGFR, doubling of serum creatinine, AKI, and kidney failure.15 The first 2 were approved by the Food and Drug Administration as acceptable surrogate end points for assessing kidney disease progression in clinical trials,16 whereas AKI17 and kidney failure15 are clinically important end points that have been validated in previous studies.15
The 30% or more decline in eGFR and doubling of serum creatinine were defined as changes from baseline at any time point during the follow-up period. Because these 2 end points relied entirely on laboratory data, when examining these 2 outcomes, we censored patients at their last laboratory measurement. Acute kidney injury was defined as a hospitalization or emergency department visit with a diagnosis code of AKI at any position.17 Kidney failure was defined as eGFR less than 15 mL/min per 1.73 m2, kidney transplant, or dialysis initiation.18
Statistical Methods
One-to-one propensity score matching was used to identify patients treated with ACEi/ARB who were similar to those treated with sacubitril-valsartan. Specifically, we estimated propensity to receive sacubitril-valsartan vs ACEi/ARB using logistic regression. Covariates included in the logistic modeling were age, sex, race, baseline eGFR, patient census region, comorbidities (depression, renal disease [defined by ICD codes],12 cardiac arrhythmia, peripheral vascular disease, valvular heart disease, anemia, hypertension, diabetes, history of myocardial infarction, dementia, cerebrovascular disease, chronic obstructive pulmonary disease, and implantable cardioverter-defibrillator), previous use of HF medications (β-blockers, loop diuretics, aldosterone antagonists, and digoxin), previous use of ACEi/ARB, strength of ACEi/ARB in previous/current users, Charlson comorbidity index, office visit with a cardiologist, office visit with a primary care provider, and hospitalizations (all-cause and HF). One-to-one nearest-neighbor caliper matching was used to match patients on the basis of the logit of the propensity score using a caliper equal to 0.2 of the SD of the logit of the propensity score.19 To account for potential effects of newly initiating renin-angiotensin therapy, we exactly matched new users of sacubitril-valsartan who had not used an ACEi/ARB in the past 120 days to new users of ACEi/ARB. Patients who switched to sacubitril-valsartan from ACEi/ARB (had filled a prescription for ACEi/ARB in previous 6 months) were matched to prevalent ACEi/ARB users. Standardized mean difference was used to assess the balance of covariates after matching, with a difference of no more than 10% considered acceptable.
We calculated event rates, plotted Kaplan-Meier curves, and estimated the cumulative risks of renal outcomes at 6, 12, 18, and 24 months of follow-up. Cox proportional hazards regression was used to compare the risk of outcomes between treatment groups in the matched cohort. Robust sandwich estimates were included to account for clustering within matched sets.20 The proportional hazards assumption was tested on the basis of Schoenfeld residuals and found to be valid.21 Differences in hazard ratios (HRs) by subgroups of interest (age, sex, race, previous ACEi/ARB use, diabetes, and baseline eGFR) were tested using interaction terms.
As new users of sacubitril-valsartan may differ significantly in terms of HF severity from those who were previously prescribed ACEi/ARB, we conducted a sensitivity analysis by separately analyzing patients who were and were not previously treated with ACEi/ARB. Furthermore, we also used inverse probability of treatment weighting (IPTW) as an additional approach to 1-to-1 propensity score for minimizing confounding.
All analyses were conducted using SAS 9.4 (SAS Institute Inc.) and Stata version 14.1 (StataCorp LLC).
Results
Patient Characteristics
A total of 24,034 and 6424 patients filling a prescription for ACEi/ARB and sacubitril-valsartan, respectively, were initially identified (Table 1). Before matching, patients treated with sacubitril-valsartan were more likely to be male, be treated with HF guideline–directed medications, and to have visited a cardiologist in the past 6 months. Our postmatch cohort included 4667 patients in each treatment group, and baseline characteristics between them were overall well balanced (standardized mean difference, ≤0.04) (Table 1) during which an average of 2.7 ± 3.5 creatinine values was obtained over the follow-up period. The mean age was 69.4±11.2 years, with approximately one-third women. There was diverse racial representation, with 19% black and 15% Hispanic. There was a high prevalence of medical comorbidities including 95% with hypertension, 59% with diabetes, and 38% with renal disease. The eGFR in both groups was 64.4 mL/min per 1.73 m2. Almost 40% of patients who received sacubitril-valsartan had not filled a prescription for ACEi/ARB in the preceding 120 days. Adherence in both treatment groups were high, with a mean MPR of 0.95±0.09 in the sacubitril-valsartan group and 0.97±SD 0.07 in the ACEi/ARB group, respectively.
Table 1.
| Characteristic | Prematch |
Standard mean difference | Postmatch |
Standard mean difference | ||
|---|---|---|---|---|---|---|
| Sacubitril-valsartan (n=6424) | ACEi/ARB (n=24,034) | Sacubitril-valsartan (n=4667) | ACEi/ARB (n=4667) | |||
| Age (y) | ||||||
| Mean ± SD | 69.5±11.3 | 70.6±10.9 | 0.10 | 69.4±11.2 | 69.4±11.3 | 0.00 |
| Median (Q1, Q3) | 71 (63, 78) | 72 (64, 79) | 71 (63, 78) | 70 (63, 78) | ||
| Age group | ||||||
| 18-44 y | 176 (3) | 468 (2) | 0.05 | 118 (3) | 111 (2) | 0.01 |
| 45-54 y | 486 (8) | 1489 (6) | 0.05 | 366 (8) | 378 (8) | 0.01 |
| 55-64 y | 1224 (19) | 4418 (18) | 0.02 | 891 (19) | 887 (19) | 0.00 |
| 65-74 y | 2180 (34) | 8028 (33) | 0.01 | 1624 (35) | 1628 (35) | 0.00 |
| ≥75 y | 2358 (37) | 9631 (40) | 0.07 | 1668 (36) | 1663 (36) | 0.00 |
| Sex | ||||||
| Female | 2099 (33) | 10,075 (42) | 0.19 | 1643 (35) | 1630 (35) | 0.01 |
| Male | 4325 (67) | 13,959 (58) | 0.19 | 3024 (69) | 3037 (65) | 0.01 |
| Race | ||||||
| White | 3535 (55) | 13,986 (58) | 0.06 | 2558 (55) | 2567 (55) | 0.00 |
| Black | 1268 (20) | 4677 (20) | 0.01 | 868 (19) | 850 (18) | 0.01 |
| Hispanic | 1005 (16) | 2747 (11) | 0.12 | 700 (15) | 674 (14) | 0.02 |
| Asian | 145 (2) | 548 (2) | 0.00 | 95 (2) | 92 (2) | 0.01 |
| Unknown | 471 (7) | 2076 (9) | 0.05 | 446 (10) | 484 (10) | 0.03 |
| Serum Cr level (mg/dL) | ||||||
| Mean ± SD | 1.2±0.4 | 1.2±0.4 | 0.03 | 1.2±0.4 | 1.2±0.4 | 0.00 |
| Median (Q1, Q3) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.4) | ||
| eGFR (mL/min per 1.73 m2) | ||||||
| Mean ± SD | 64.6±21.1 | 63.9±22.1 | 0.03 | 64.4±21.6 | 64.4±21.7 | 0.00 |
| Median (Q1, Q3) | 63.8 (48.6, 80.4) | 63.1 (47.0, 80.4) | 63.5 (48.0, 80.8) | 63.6 (47.6, 80.9) | ||
| Patient census region | ||||||
| Midwest | 579 (9) | 3379 (14) | 0.16 | 448 (10) | 459 (10) | 0.01 |
| Northeast | 961 (15) | 4283 (18) | 0.08 | 686 (15) | 683 (15) | 0.00 |
| South | 4448 (69) | 14,393 (60) | 0.20 | 3185 (68) | 3185 (68) | 0.00 |
| West | 436 (7) | 1979 (8) | 0.06 | 348 (8) | 340 (7) | 0.01 |
| Medical comorbidities | ||||||
| Hypertension | 6166 (96) | 22,846 (95) | 0.05 | 4434 (95) | 4442 (95) | 0.01 |
| History of myocardial infarction | 2472 (39) | 7700 (32) | 0.14 | 1479 (32) | 1516 (33) | 0.02 |
| Valvular disease | 4024 (63) | 12,245 (51) | 0.24 | 2536 (54) | 2548 (55) | 0.01 |
| Peripheral vascular disease | 3822 (60) | 11,068 (46) | 0.27 | 2464 (53) | 2469 (53) | 0.00 |
| Cardiac arrhythmia | 5011 (78) | 17,156 (71) | 0.15 | 3443 (74) | 3459 (74) | 0.01 |
| Cerebrovascular disease | 2046 (32) | 7852 (33) | 0.02 | 1273 (27) | 1304 (28) | 0.02 |
| Diabetes | 3867 (60) | 14,463 (60) | 0.00 | 2727 (58) | 2739 (59) | 0.01 |
| Renal disease | 2569 (40) | 9159 (38) | 0.04 | 1730 (37) | 1761 (38) | 0.01 |
| COPD | 3197 (50) | 11,904 (50) | 0.01 | 2098 (45) | 2158 (46) | 0.03 |
| Anemia | 1405 (22) | 5325 (22) | 0.01 | 871 (19) | 913 (20) | 0.02 |
| Depression | 1399 (22) | 5151 (21) | 0.01 | 886 (19) | 872 (19) | 0.01 |
| Dementia | 298 (5) | 2321 (10) | 0.20 | 210 (5) | 214 (5) | 0.00 |
| Pacemaker/ICD | 2870 (45) | 7279 (30) | 0.30 | 1872 (40) | 1898 (41) | 0.01 |
| Medications | ||||||
| β-Blockers | 5648 (88) | 17,720 (74) | 0.37 | 3969 (85) | 3989 (86) | 0.01 |
| Loop diuretics | 4200 (65) | 13,064 (54) | 0.23 | 2899 (62) | 2944 (63) | 0.02 |
| Aldosterone antagonist | 2135 (33) | 4013 (17) | 0.39 | 1244 (27) | 1292 (28) | 0.02 |
| Digoxin | 746 (112) | 2217 (9) | 0.08 | 485 (10) | 495 (11) | 0.01 |
| Previous ACEi/ARB (120 d earlier) | 4248 (66) | 14,123 (59) | 0.15 | 2702 (60) | 2702 (58) | 0.00 |
| ACEi | 2335 (36) | 8832 (37) | 0.01 | 1538 (33) | 1542 (33) | 0.00 |
| ARB | 1913 (30) | 5291 (22+) | 0.18 | 1164 (25) | 1160 (25) | 0.00 |
| Previous dose (ACEi/ARB) | ||||||
| Low dose | 1380 (333) | 4296 (30) | 0.05 | 853 (32) | 852 (32) | 0.00 |
| Medium dose | 1661 (39) | 5737 (41) | 0.03 | 1073 (30) | 1110 (41) | 0.03 |
| High dose | 1207 (28) | 4090 (29) | 0.01 | 776 (29) | 740 (27) | 0.03 |
| Charlson comorbidity index | ||||||
| Mean ± SD | 5.9±3.2 | 5.7±3.2 | 0.07 | 5.4±3.1 | 5.5±3.0 | 0.02 |
| Office visit | ||||||
| Cardiologist | 5397 (84) | 15,127 (63) | 0.49 | 3778 (81) | 3848 (83) | 0.04 |
| Primary care | 3143 (49) | 11,354 (47) | 0.03 | 2277 (49) | 2264 (49) | 0.01 |
| Previous hospitalization | ||||||
| 0 | 3865 (60) | 14,425 (60) | 0.01 | 2811 (60) | 2795 (60) | 0.01 |
| 1 | 1787 (28) | 6854 (28) | 0.02 | 1313 (28) | 1346 (29) | 0.02 |
| ≥2 | 772 (12) | 2755 (12) | 0.02 | 543 (12) | 526 (11) | 0.01 |
| Previous HF hospitalization | ||||||
| 0 | 5133 (80) | 20,223 (84) | 0.15 | 3815 (82) | 3773 (81) | 0.03 |
| 1 | 1066 (16) | 3270 (14) | 0.12 | 719 (115) | 752 (16) | 0.03 |
| ≥2 | 225 (4) | 541 (2) | 0.10 | 133 (3) | 142 (3) | 0.02 |
| Year of index | ||||||
| 2015 | 47 (1) | 9275 (39) | 1.08 | 47 (1) | 48 (1) | 0.00 |
| 2016 | 735 (11) | 4910 (20) | 0.25 | 715 (15) | 734 (16) | 0.01 |
| 2017 | 1760 (27) | 4443 (19) | 0.21 | 1487 (32) | 1400 (30) | 0.04 |
| 2018 | 2327 (36) | 3644 (15) | 0.50 | 1584 (34) | 1602 (34) | 0.01 |
| 2019 | 1555 (24) | 1762 (7) | 0.48 | 834 (18) | 883 (19) | 0.03 |
ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; Cr = creatinine; eGFR = estimated glomerular filtration rate; HF = heart failure; ICD = implantable cardioverter-defibrillator; Q1 = quartile 1; Q3 = quartile 3.
SI conversion factor: To convert to mg/dL values to mmol/L, multiply by 0.0259.
Data are presented as No. (percentage) unless otherwise stated.
Outcomes
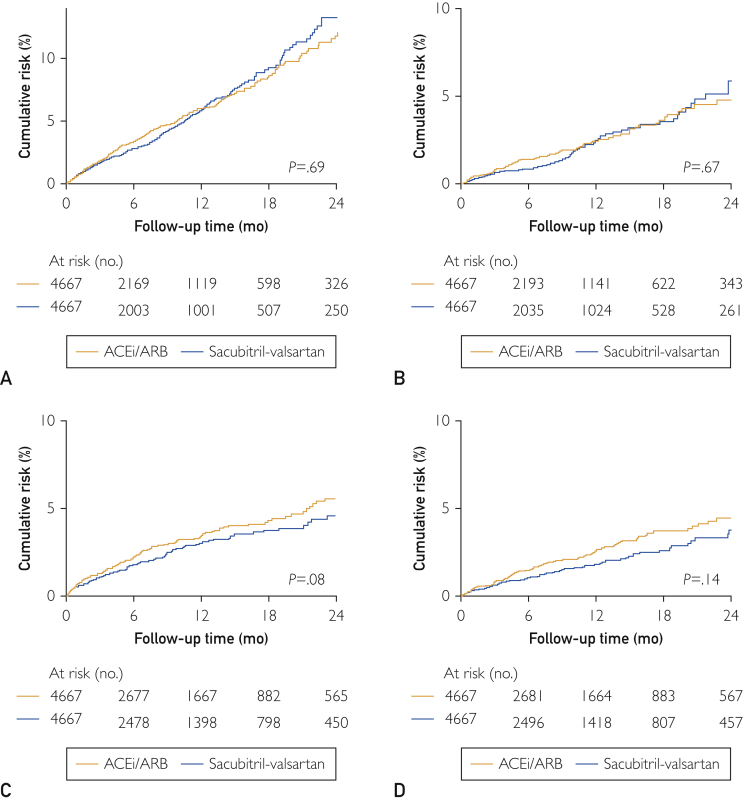
The mean on-treatment follow-up period was 7.8 ± 7.8 months. The event rates per 100 person-years and corresponding HRs (95% CI; P value) for the sacubitril-valsartan and ACEi/ARB groups were, respectively, 6.60 and 6.86 (HR, 0.96; 95% CI, 0.79 to 1.10; P=.69) for 30% or more decline in eGFR, 2.62 and 2.79 (HR, 0.94; 95% CI, 0.69 to 1.27; P=.67) for doubling of serum creatinine, 2.91 and 3.55 (HR, 0.80; 95% CI, 0.63 to 1.03; P=.08) for AKI, and 2.00 and 2.48 (HR 0.80; 95% CI, 0.59 to 1.08; P=.14) for kidney failure. The cumulative risk at 1 year of each renal outcome in the sacubitril-valsartan and ACEi/ARB groups was, respectively, 6% and 6% for 30% or more decline in eGFR, 2% and 2% for doubling of serum creatinine, 3% and 3% for AKI, and 2% and 3% for kidney failure (Figure 1).
Figure 1.
Comparative cumulative risk curves for (A) 30% or more decline in estimated glomerular filtration rate, (B) doubling of serum creatinine, (C) acute kidney injury, and (D) kidney failure in patients on sacubitril-valsartan or angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB).
Subgroup Analyses
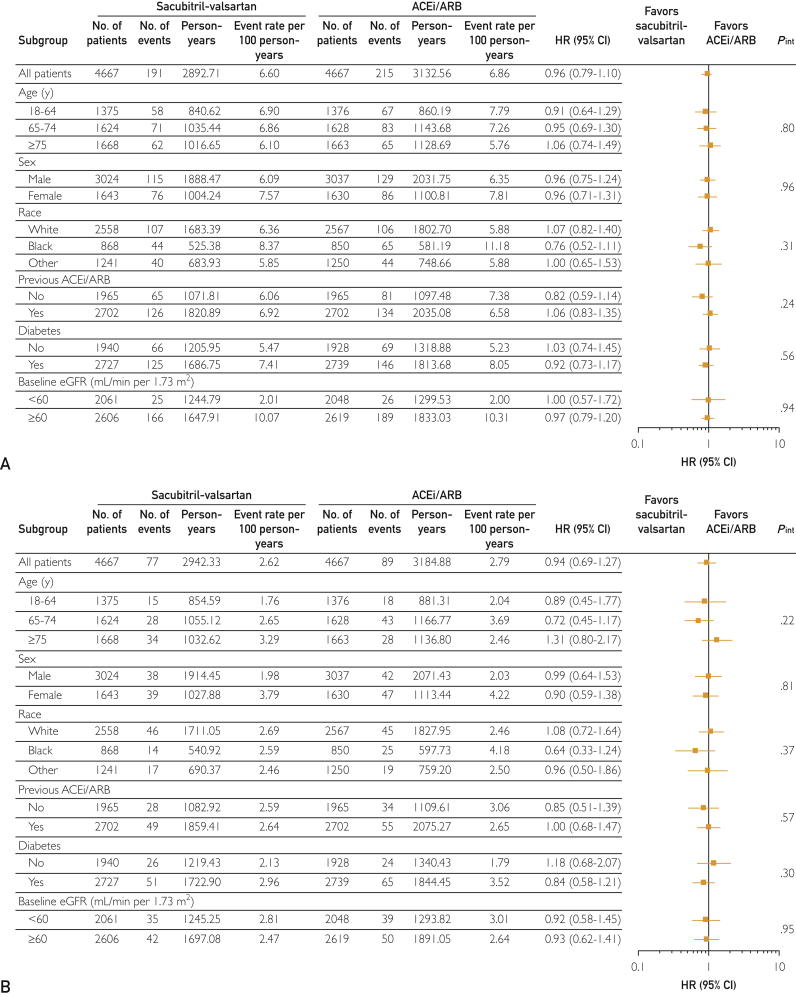
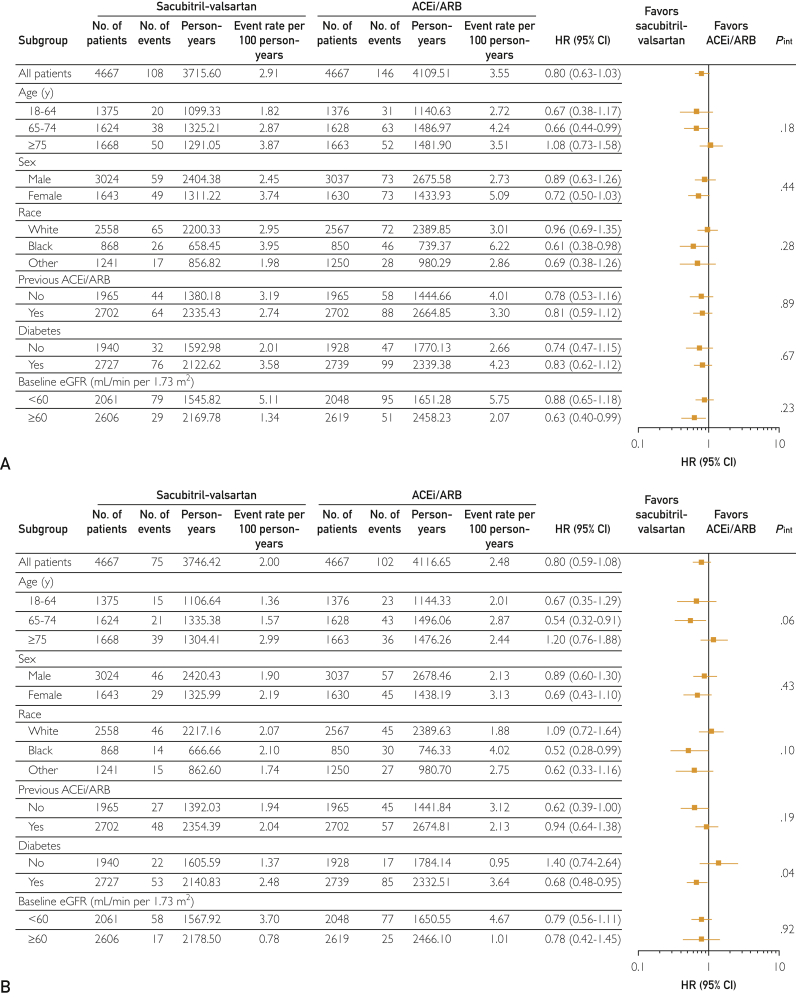
Figures 2 and 3 highlight the subgroup analyses performed for 30% or more decline in eGFR (Figure 2A), doubling of serum creatinine (Figure 2B), AKI (Figure 3A), and kidney failure (Figure 3B). No significant interactions with the examined clinical/demographic variables for each renal outcome were observed, except between diabetes and kidney failure. Specifically, there was a decreased risk of kidney failure associated with sacubitril-valsartan therapy in patients with diabetes as compared with ACEi/ARB therapy (interaction, P=.04) (Figure 3B).
Figure 2.
Differences in the risk of (A) 30% or more decline in estimated glomerular filtration rate (eGFR) and (B) doubling of serum creatinine according to patient baseline characteristics. ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HR = hazard ratio.
Figure 3.
Differences in the risk of (A) acute kidney injury and (B) kidney failure according to patient baseline characteristics. ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; eGFR = estimated glomerular filtration rate; HR = hazard ratio.
Sensitivity Analyses
Renal outcomes were also similar between treatment groups when IPTW was used and supports our overall findings (Table 2). Additionally, there were no significant differences in renal outcomes between patients prescribed sacubitril-valsartan vs their matched ACEi/ARB counterparts, regardless of whether they were previously prescribed ACEi/ARB (Supplemental Tables 2 and 3, available online at http://www.mcpiqojournal.org).
Table 2.
Comparison of Renal Outcomes Between Patients Prescribed ACEi/ARB or Sacubitril-Valsartan Using Inverse Probability of Treatment Weighting
| Outcome | Sacubitril-valsartan (n=6424) |
ACEi/ARB (n=24,034) |
Hazard ratio (95% CI) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| No. of events | Person-years | Rate per 100 person-years | No. of events | Person-years | Rate per 100 person-years | |||
| ≥30% decline in eGFR | 256 | 3691.63 | 6.93 | 315 | 3944.64 | 7.98 | 0.87 (0.70-1.07) | .18 |
| Doubling of serum creatinine | 103 | 3755.38 | 2.74 | 112 | 4014.89 | 2.78 | 0.99 (0.74-1.32) | .95 |
| Acute kidney injury | 139 | 4777.48 | 2.91 | 186 | 5161.62 | 3.60 | 0.80 (0.62-1.02) | .08 |
| Kidney failure | 98 | 4817.39 | 2.03 | 129 | 5187.32 | 2.48 | 0.82 (0.61-1.10) | .18 |
ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; eGFR = estimated glomerular filtration rate.
Discussion
In this study of more than 9000 US patients with HFrEF treated in routine clinical practice, we observe that patients prescribed either sacubitril-valsartan or ACEi/ARB had similar risks of adverse renal outcomes over an average of 7.8 months of follow-up. Moreover, in comparison to ACEi/ARB, treatment with sacubitril-valsartan was associated with a decreased risk of kidney failure in patients with HFrEF and diabetes, whereas no significant differences in treatment effects were observed for other patient subgroups examined.
The PARADIGM-HF trial found significant cardiovascular morbidity and mortality reduction in patients with HFrEF receiving sacubitril-valsartan in comparison with enalapril, heralding its swift approval by the Food and Drug Administration and incorporation into major cardiology guidelines.2,6 Indeed, we recently reported lower risks of mortality and hospitalization in US patients prescribed sacubitril-valsartan vs ACEi/ARB in clinical practice, which complemented the findings of the PARADIGM-HF trial.22 However, the effects of sacubitril-valsartan vs RAAS inhibition on renal outcomes in the real-world setting of HFrEF remain incompletely defined.
Sacubitril-valsartan holds promise with regard to renal benefits as this novel drug combines the favorable actions derived from the inhibition of both the RAAS and degradation of natriuretic peptides (NPs), which are known to have renal enhancing effects. To this end, several RCTs have investigated whether sacubitril-valsartan differed from ACEi/ARB with respect to renal outcomes. In the PARADIGM-HF trial, patients treated with sacubitril-valsartan did not have a significantly different decline in renal function (defined by end-stage renal disease, ≥50% decline in eGFR, or eGFR ≥ 30 mL/min per 1.73 m2) compared with those treated with enalapril.7 Subsequent focused post hoc analyses of the PARADIGM-HF trial found that there were similar rates of decline in eGFR between sacubitril-valsartan and enalapril treatment over 8 months of follow-up. However, beginning at 12 months after treatment initiation, sacubitril-valsartan was associated with a slower rate of decline in eGFR over the next 36 months. In contrast, there was a modest increase in urinary albumin-to-creatinine ratio,23 which serves as a marker of progressive renal dysfunction in chronic kidney disease,24 after 1 and 8 months in patients assigned to sacubitril-valsartan vs enalapril. Furthermore, in the United Kingdom Heart and Renal Protection-III (UK HARP III) trial, which recruited patients with established moderate to severe chronic kidney disease, with less than 5% having HF, no significant differences in measured GFR or albuminuria were noted between patients treated with sacubitril-valsartan and irbesartan at 12 months.25 Finally, in the Comparison of Sacubitril-Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode trial, there were no significant differences with respect to worsening renal function when patients with HFrEF presenting with acute decompensated HF were treated with either sacubitril-valsartan or enalapril.26
Notably, our results in the real-world setting are consistent with the general trend of the data reported from these RCTs. In contrast to RCTs, our patient cohort was diverse and well-represented in terms of age, sex, comorbidities, and ethnicity. Compared with participants from the PARADIGM-HF trial, our study cohort was older (mean age, 69 years vs 64 years) and had higher proportions of women (35% vs 22%), black patients (19% vs 5%), hypertensive patients (95% vs 71%), and diabetic patients (59% vs 35%).7 Herein, 4 clinically meaningful renal outcomes were investigated, which allowed for a comprehensive assessment of renal dysfunction in a setting outside of RCTs. It is therefore reassuring that despite the heterogeneity of our larger representative cohort of patients with HFrEF in the United States, we observed similar rates of renal outcomes in both treatment groups by the measures examined over an average of 7.8 months of follow-up. Additionally, there were no significant differences in treatment effect, for the most part, observed across most of the prespecified subgroups including baseline eGFR. However, our observation that patients with HFrEF and diabetes had a lower risk of kidney failure with sacubitril-valsartan than with ACEi/ARB is an interesting finding that warrants closer investigation. Indeed, increasing evidence has suggested that NPs have not only renal protective effects but are also closely associated with insulin sensitivity and glucose metabolism.27,28 As neprilysin inhibition via sacubitril increases bioavailability of NPs, particularly atrial NP,29 it is tempting to speculate that this may, in part, be a mechanism by which the risk of kidney failure in patients with diabetes reduces. However, further dedicated studies on the treatment effect and biological mechanism(s) mediating this difference are clearly needed.
Our study embodies several notable strengths. To our knowledge, this is the first study to compare renal outcomes in patients prescribed sacubitril-valsartan and ACEi/ARB in diverse clinical practice settings. Our cohort was derived from patients across the United States, with demographic and clinical characteristics that are more heterogeneous and representative than usually seen in clinical trials. The patient sample herein was also similar in size to that of the PARADIGM-HF trial but much greater than that of theUK HARP-III or Comparison of Sacubitril-Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode trials. Rigorous efforts were made to minimize potential confounding by balancing treatment group characteristics with propensity score matching. Multiple adverse renal outcomes, previously used and validated methodologically, were assessed in this study.
Limitations
Despite these strengths, several limitations should be noted. First, despite careful adjustments with propensity score matching and IPTW, our study may still be subject to residual confounding. However, the treatment groups were balanced on numerous important variables and the observation of similar results between propensity score matching and IPTW provides confidence that we have an optimally balanced cohort and minimized confounding. Second, serum creatinine levels were not obtained at prespecified time points. Third, the small number of events in each treatment group limited our power to detect significant differences. Finally, the average on-treatment follow-up period was relatively short (7.8 ± 7.8 months); thus, the long-term risk of renal outcomes requires further evaluation.
Conclusion
In a large and heterogeneous cohort of US patients with HFrEF, the overall rates of renal dysfunction were similar in patients prescribed sacubitril-valsartan or ACEi/ARB over a mean follow-up period of 7.8 months. Further research is needed to investigate the risk of renal dysfunction associated with sacubitril-valsartan or ACEi/ARB over the long term as well as to optimize renal protective therapies that would be of benefit in diverse patient populations with HFrEF.
Footnotes
Grant Support: The work was supported, in part, by grant R01 HL132854 awarded to Dr Sangaralingham from the National Institutes of Health (NIH). Over the past 36 months, Dr Shah has received research support through Mayo Clinic from the Centers of Medicare and Medicaid Innovation, from the Food and Drug Administration (U01FD004585), from the Agency for Healthcare Research and Quality (R01HS025164, R01HS025402, R03HS025517, and U19HS024075), from the NIH (R56HL130496 and R01HL131535), from the Medical Devices Innovation Consortium/National Evaluation System for health Technology, from the National Science Foundation, and from the Patient-Centered Outcomes Research Institute. Dr Burnett Jr was supported by NIH grants R01 HL36634 and R01 HL134668. Dr Dunlay was supported by NIH grants R03 HL135225 and R01 HL144529.
Potential Competing Interests: Dr Burnett Jr has served as a consultant to Novartis, Ironwood, and Theravance outside the submitted work. The other authors report no competing interests.
Supplemental material can be found online at: http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Verbrugge F.H., Tang W.H.W., Mullens W. Renin-angiotensin-aldosterone system activation during decongestion in acute heart failure: friend or foe? JACC Heart Fail. 2015;3(2):108–111. doi: 10.1016/j.jchf.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W., Jessup M., Bozkurt B., et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America [published correction appears in J Am Coll Cardiol. 2016;68(13):1495] J Am Coll Cardiol. 2016;68(13):1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 3.SOLVD Investigators. Yusuf S., Pitt B., Davis C.E., Hood W.B., Cohn J.N. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 4.Cohn J.N., Tognoni G., Valsartan Heart Failure Trial Invetsigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 5.Schefold J.C., Filippatos G., Hasenfuss G., Anker S.D., von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–623. doi: 10.1038/nrneph.2016.113. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P., Voors A.A., Anker S.D., et al. ESC Scientific Document Group 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2018;39(10):860] Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 7.McMurray J.J.V., Packer M., Desai A.S., et al. PARADIGM-HF Investigators and Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 8.Packer M., Claggett B., Lefkowitz M.P., et al. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018;6(7):547–554. doi: 10.1016/S2213-8587(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 9.Wallace P.J., Shah N.D., Dennen T., Bleicher P.A., Crown W.H. Optum Labs: building a novel node in the learning health care system [published correction appears in Health Aff (Millwood). 2014;33(9):1703] Health Aff (Millwood) 2014;33(7):1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 10.Sangaralingham L.R., Sangaralingham S.J., Shah N.D., Yao X., Dunlay S.M. Adoption of sacubitril/valsartan for the management of patients with heart failure. Circ Heart Fail. 2018;11(2):e004302. doi: 10.1161/CIRCHEARTFAILURE.117.004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Optum Labs. Real World Healthcare Experiences. 2015. Available at: https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed June 1 2020.
- 12.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 13.Levey A.S., Stevens L.A., Schmid C.H. et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155(6):408] Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson A.M., Nau D.P., Cramer J.A., Benner J., Gwadry-Sridhar F., Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Yao X., Tangri N., Gersh B.J., et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–2632. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 16.Levey A.S., Inker L.A., Matsushita K., et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Hwang Y.J., Shariff S.Z., Gandhi S., et al. Validity of the International Classification of Diseases, Tenth Revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open. 2012;2(6):e001821. doi: 10.1136/bmjopen-2012-001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens P.E., Levin A., Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Austin P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gayat E., Resche-Rigon M., Mary J.Y., Porcher R. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat. 2012;11(3):222–229. doi: 10.1002/pst.537. [DOI] [PubMed] [Google Scholar]
- 21.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan N.Y., Sangaralingham L.R., Sangaralingham S.J., Yao X., Shah N.D., Dunlay S.M. Comparative effectiveness of sacubitril-valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8(1):43–54. doi: 10.1016/j.jchf.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damman K., Gori M., Claggett B., et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Stehouwer C.D., Gall M.A., Twisk J.W., Knudsen E., Emeis J.J., Parving H.H. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 25.Haynes R., Judge P.K., Staplin N., et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138(15):1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez E.J., Morrow D.A., DeVore A.D., et al. PIONEER-HF Investigators Angiotensin-neprilysin inhibition in acute decompensated heart failure [published correction appears in N Engl J Med. 2019;380(11):1090] N Engl J Med. 2019;380(6):539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 27.Arora P., Wu C., Hamid T., et al. Acute metabolic influences on the natriuretic peptide system in humans. J Am Coll Cardiol. 2016;67(7):804–812. doi: 10.1016/j.jacc.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan A.M., Cheng S., Magnusson M., et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96(10):3242–3249. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim N.E., McCarthy C.P., Shrestha S., et al. Effect of neprilysin inhibition on various natriuretic peptide assays. J Am Coll Cardiol. 2019;73(11):1273–1284. doi: 10.1016/j.jacc.2018.12.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.