Abstract
Solitary plasmacytoma is a rare clonal plasma cell tumor, representing 2-5% of plasma cell disorders. The standard treatment is local radiotherapy. However, in some cases, its use is limited by the size and/or location of the mass. Systemic chemotherapy may be a useful therapeutic alternative. We describe a case of a 27-year-old male with a bulky solitary plasmacytoma arising in the posterior mediastinum, causing spinal cord compression. Radiotherapy was considered risky as the mass was located in the heart and left lung fields. Systemic treatment was given. After the first cycle of cyclophosphamide, bortezomib, and dexamethasone (VCD), the patient attained full neurological recovery. After four VCD cycles, complete remission was achieved. Autologous stem cell transplantation was given as consolidation therapy. At 3 months post-transplantation, the patient is in full clinical recovery and complete metabolic remission on 18FDG PET-CT. Although infrequent, plasma cell disorders must be considered in adult patients with a bulky tumoral mass in the posterior mediastinum. PET-CT is the whole-body imaging technique of choice to detect SP, to evaluate response to treatment and during follow-up.
Keywords: Multiple myeloma, Solitary plasmacytoma, Extramedullary solitary plasmacytoma, Posterior mediastinal mass
1. Introduction
Solitary plasmacytoma (SP) is a rare clonal plasma cell tumor, representing 2-5% plasma cell disorders. It usually presents as a single lesion frequently involving the axial skeleton [1,2].
SP is classified in solitary bone plasmacytoma (SBP) or solitary extramedullary plasmacytoma (SEP), depending on whether the tumor mass arises in bone or soft tissues [3].
The most usual symptom is pain. It can also present as spinal cord and/or nerve root compression, including cranial nerve palsies when the base of the skull is affected. A monoclonal paraprotein (M protein) has been reported in 24%–72% of patients, which helps to monitor the response to treatment if > 1 g/dL at diagnosis [3].
SBP evolves to multiple myeloma in most patients, with a median time to progression of 2–4 years [4]. Adverse factors associated with progression include bone involvement, large tumor size (>5 cm), elevated β2 microglobulin (>3.5 mg/L), abnormal serum free light chain (FLC) ratio at diagnosis, M-protein presence, persistence post-radiotherapy, and bone marrow involvement [5,6].
The standard treatment consists of local radiotherapy. However, in some scenarios, chemotherapy may be required.
This case report describes the clinical presentation and evolution of a young patient affected by a SBP located in the posterior mediastinum, presenting with spinal cord compression. The location and size of the tumor limited the possibility of using frontline radiotherapy, as the risk of pulmonary and cardiac toxicity was high. Therefore, he was treated with a regimen based on Bortezomib, Ciclofosfamide, Dexamethasone (VCD), and consolidation with autologous stem cell transplantation, achieving complete response (CR]. The patient signed informed consent authorizing the publication of this manuscript.
2. Case report
A 27-year-old man, previously healthy, was admitted with a two-month burning pain located in the left costal arches, intensity 7 of 10. No previous local trauma or effort. Two weeks before admittance, he noticed an increase in pain radiating to the left upper limb and toes, progressive bilateral muscle weakness in the legs with gait disturbance, instability in standing, and urinary incontinence. He had no fever, no anorexia, and no weight loss.
On physical examination, he presented lucid, no anemia, and no adenopathies. The cardiac and pulmonary examination was normal. The abdomen was painless, without organomegaly. The neurologic exam showed allodynia in T6-T8 dermatomes and bilateral absence of abdominal cutaneous reflexes. In the lower extremities, bilateral spasticity, grade 2 paraparesis, bilateral patellar hyperreflexia, and Babinsky's sign on the left were detected.
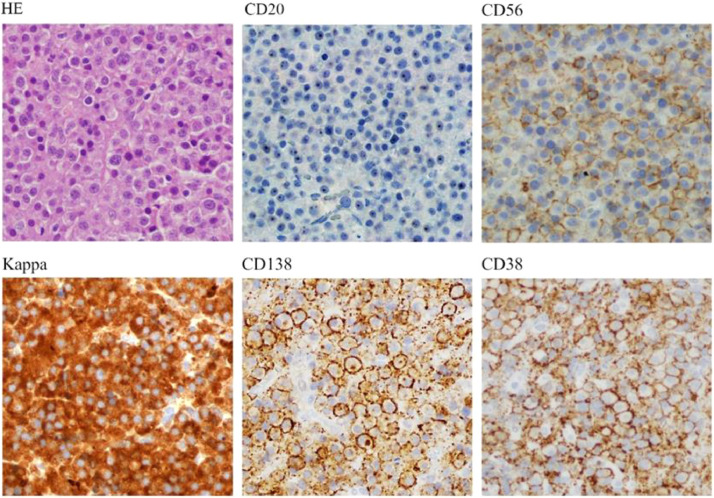
MRI showed an expansile lesion in the posterior mediastinum, from T5 to T8 vertebral bodies, involving the adjacent ribs and the transverse process of T7. The mass extended into the spinal canal through T5-T6, T6-T7, and T7-T8 neuroforamen, with a length of 48 mm, displacing the spinal cord forward and to the right. (Fig. 1). Initial diagnosis included tumors arising in the posterior mediastinal compartment: neurogenic tumors, vascular abnormalities, bone tumors, and lymphoma. The patient underwent immediate neurosurgery, a biopsy of the tumor was taken, and a posterior laminectomy was done. Dexamethasone was initiated. The histopathological study demonstrated a diffuse pattern of cell proliferation composed of medium-sized cells, rounded excentric nuclei, without nucleolus, and eosinophilic cytoplasm with plasmacytic appearance (Fig. 2) Immunohistochemistry showed diffuse positivity for CD38, CD138, and lambda light chain, with weak and focal co-expression of CD56 (Fig. 3). Cytogenetics (conventional and FISH for t;(4;14), t(14;16) and del17p) was normal. The evaluation was completed with protein electrophoresis showing an M component of 0.3 g/dl in the gammaglobulin area, IgA lambda on immunofixation. Bone marrow aspirate immunophenotype and bone marrow biopsy showed no plasma cell infiltration. Blood counts, calcemia, and renal function were normal. HIV, hepatitis B, and C were negative. No proteinuria. B2 microglobulin was normal.
Fig. 1.
A and B, on MRI, a poly-lobulated expansive process (white arrow) is observed in posterior mediastinum at the level of the dorsal vertebral bodies from T5 to T8, involves the adjacent coastal arches and the transverse process of T 7 and is presented inside the T5-T6, T6-T7, and T7 -T8 neuroforamen channel. It occupies the spinal canal (48 mm); C and D; on CT, a voluminous (85mm x 85mm x 35mm) solid tumor is observed at the middle third of the posterior mediastinum (white arrow), which contacts and displaces the descending thoracic aorta and displaces the left inferior pulmonary vein, and the middle third of the esophagus, without signs of infiltration; E and F, 18FDG PET-CT imaging which demonstrates increased uptake in the posterior mediastinum mass. (white arrow).
Fig. 2.
A, hematoxylin and eosin (HE) staining of the tumoral mass biopsy; B, immunohistochemistry directed to the detection of the antigens CD20, CD56, Kappa, CD138, and CD38 (40x Olympus BX51).
Fig. 3.
A and B, MRI control after 6 cycles of VCD; C and D, 18FDG PET-TC after 6 cycles of VCD, E and F, 18FDG PET-CT at 3 months post-transplantation.
An 18FDG PET-CT was performed showing a hypermetabolic soft tissue tumor with a heterogeneous distribution of the radiotracer, lobulated, located in the middle third of the posterior mediastinum, lateralized to the left, of 78 mm x 65mm x 80mm AP x Tx CC diameters, with SUVmax 4.9. The mass extended to contiguous ribs causing a structural alteration of the 7th left costal arch in its posterior sector with thinning of the cortical bone and a small area of disruption, with a SUVmax 2.6. (Fig. 1)
The diagnosis of bulky solitary plasmacytoma was established. Whether its initial origin was soft tissue or bone was difficult to determine. Adverse prognostic factors were neurologic clinical presentation, bone involvement, size >5 cm, and M component in serum.
After a thorough evaluation of the size and location of the tumor, radiotherapists considered that the risk of cardiac and pulmonary toxicity was too high. Semen preservation was done. Then he started cyclophosphamide, bortezomib, and dexamethasone (VCD). After the first cycle, the patient achieved full neurological recovery and complete remission of pain. After 4 cycles, the complete disappearance of the monoclonal spike was shown. MRI and 18FDG PET-CT showed >50% reduction in mass, with no metabolic uptake. (Fig. 3) The patient underwent autologous stem cell transplantation without complications. At 3 months from transplantation he is clinically asymptomatic, monoclonal spike is absent and serum FLC are normal. On 18FDG PET-CT he is in complete remission.
Discussion
SP is an uncommon clonal plasma cell neoplasm defined by the International Myeloma Working Group (IMWG) as a biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells, along with clonal bone marrow plasma cells under 10%, normal skeletal survey, and MRI (or CT) of spine and pelvis (except for the primary solitary lesion), and absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a plasma cell proliferative disorder [7]. Tumors of the posterior mediastinum are most frequently neurogenic tumors, lymphomas, or non-neoplastic entities, while plasmacytoma is rare.
SP show non-specific CT and MRI findings, which are complementary techniques. CT is preferred to assess the bony lesion and occasional lymphadenopathies that may appear in the context of SP. MRI is the preferred modality for evaluation due to better soft-tissue contrast and is the gold standard to evaluate spinal cord compression. Features that may suggest the diagnosis of plasmacytoma are the bulky soft tissue mass with low T1 and high T2 signals due to high cellularity. Whole-body low dose CT is the recommended imaging modality for MM diagnosis. However, its role in SP has not yet been defined. The use of 18-FDG PET-CT in SBP has not been extensively studied, although some studies reported that, at diagnosis, sensitivity and specificity of PET-CT is higher than that of MRI of the spine and pelvis. Higher uptake was associated with larger masses and increased risk of progression, adding a prognostic role for PET-CT compared to MRI. Based on these small prospective studies, the IMWG recommends MRI and/or PET-CT as mandatory imaging techniques for SP assessment. In the context of EMP, PET-CT is the preferred option. Imaging provides local assessment and staging, but they cannot distinguish SP from other malignancies. Histopathology is required for diagnosis [8], [9], [10].
In this case, histology is typical, and so is immunohistochemistry. No cytogenetic abnormalities were found in the plasmacytoma and bone marrow. Cytogenetic abnormalities have shown no impact on SP´s outcome.
Radiation therapy using a total fractionated dose of 40–50 Gy over approximately 4-5 weeks is the standard treatment for SP. The radiation field should include all involved tissues identified on imaging with an additional 2 cm of healthy tissue, to prevent a recurrence. The evidence for radiation therapy in SP is based on retrospective studies. The local response is ≥80% and it is higher in tumors ≤ 5cm. Tsang et al. reported a 100% local control rate in tumors < 5 cm vs only 38% in tumors > 5cm [11,12].
No prospective trials have compared radiotherapy to chemotherapy.
Surgery is reserved for the treatment of pathological fractures, neurologic complications, or lesions with a high chance of fracture or instability in patients with SBP. Complete or partial tumor resection must be followed by radiotherapy to reduce the chance of recurrence.
The role of adjuvant chemotherapy and autologous stem cell transplantation is controversial. Some authors recommend its use for bulky SP (>5 cm), and in patients with persistent disease, based on PET/CT, after initial radiotherapy [13]. Biphosphonates have not been studied in SP. They are recommended for osteoporosis and SBP with a high risk of fracture.
Response criteria have not been validated in SP. For secretory SBP, International Myeloma Working Group criteria have been recommended, whereas RECIST criteria are used for extramedullary plasmacytoma. In this patient, the tumor disappeared, the monoclonal spike and immunofixation are negative, and PET-CT uptake was negative in the pre- and post-transplant evaluation [14].
The best treatment for bulky SP in a young patient is long from being standardized. The particular location and size of this SP made radiotherapy too toxic. Surgery followed by chemotherapy and consolidation with high-dose melphalan and auto-SCT was done. The patient achieved complete imaging and metabolic remission.
Commentaries
This case is a useful reference for the semiological analysis of neoplasms in the posterior mediastinum with spinal cord compression, which require immediate diagnosis and treatment initiation, to prevent irreversible neurologic damage.
Although rare, plasmacytoma should be considered. Appropriate imaging techniques must be done to define its origin, neurologic and soft tissues involvement and assess response to therapy.
Chemotherapy is an effective strategy in managing SBP when radiotherapy is not suitable.
Footnotes
Competing interests statement: Authors refers no relevant financial or non-financial competing interests to report.
Funding statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest: No conflicts of interest to declare.
References
- 1.Ohana N, Rouvio O, Nalbandyan K, Sheinis D, Benharrich D. Classification of solitary plasmacytoma, is it more intricate than presently suggested? A commentary. J Cance. 2018;9(21):3894–3897. doi: 10.7150/jca.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos MA1, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol. 2002;3:255–259. doi: 10.1007/s11864-002-0015-2. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96:2037–2044. [PubMed] [Google Scholar]
- 4.Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol. 2004;124(6):717–726. doi: 10.1111/j.1365-2141.2004.04834.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharpley FA, Neffa P, Panitsas F, Kothari J, Subesinghe M, Cutter D. Long-term clinical outcomes in a cohort of patients with solitary plasmacytoma treated in the modern era [published correction appears in PLoS One. 2019 Nov 7;14(11):e0225184] PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0219857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustapha Khalife. Solitary plasmacytoma with minimal marrow involvement. Biomed J Sci & Tech Res. 2019;17(3) -2019. BJSTR. MS.ID.003017. [Google Scholar]
- 7.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 8.Fouquet G, Guidez S, Herbaux C. Impact of initial FDG PET/CT and serum free light chai non transformation of conventionally defined solitary plasmacytoma to Multiple Myeloma. Clin Cancer Res. 2014;20:3254–3260. doi: 10.1158/1078-0432.CCR-13-2910. [DOI] [PubMed] [Google Scholar]
- 9.Schirrmeister H, Buck AK, Bergman L. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm. 2003;18:841–845. doi: 10.1089/108497803770418382. [DOI] [PubMed] [Google Scholar]
- 10.Moulopoulos LA, Dimopoulos MA, Weber D. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol. 1993;11:1311–1315. doi: 10.1200/JCO.1993.11.7.1311. [DOI] [PubMed] [Google Scholar]
- 11.Aviles A, Huerta-Guzman J, Delgado Improved outcome in solitary bone plasmacytoma with combined therapy. Hematol Oncol. 1996;14:111–117. doi: 10.1002/(SICI)1099-1069(199609)14:3<111::AID-HON575>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Jantunen E, Koivunen E, Putkonen M. Autologous stem cell transplantation in patients with high-risk plasmacytoma. Eur J Haematol. 2005;74:402–406. doi: 10.1111/j.1600-0609.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 13.Caers J, Paiva B, Zamagni E. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018;11(1):10. doi: 10.1186/s13045-017-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]