Abstract
Findings from recent studies revealed a significant anti-inflammatory effect of polysaccharide-based excipients when formulated with classical drugs in experimental inflammatory bowel disease models. In this study, acacia and guar gum were investigated beyond their typical functionality for a possible additive anti-inflammatory effect when administered with 5-amino salicylic acid (5ASA) in murine experimental colitis. Anti-inflammatory effects of acacia and guar gum-based aqueous suspensions of 5ASA were evaluated in a murine experimental colitis. Acacia or guar gum (30 or 300 mg/kg) were administered via rectal administration alone or in combination with 5ASA (30 mg/kg). Disease activity, myeloperoxidase activity (MPO) and intratissue concentrations of various cytokines were assessed. Both acacia and guar gum separately showed significant effects in reducing the inflammatory markers in murine colitis model in vivo. When combined with the anti-inflammatory drug 5ASA, acacia showed a stronger therapeutic effect than guar gum, especially at the higher dose of acacia (300 mg/kg) which significantly reduced the inflammation in vivo compared to 5ASA alone (MPO, 5ASA: 5743 ± 1334, 5ASA + 30 mg/kg acacia: 3762 ± 2342; 5ASA + 30 mg/kg guar gum: 7373 ± 2115, 5ASA + 300 mg/kg acacia: 3131 ± 1012, 5ASA + 300 mg/kg guar gum: 6358 ± 2379; all U/g tissue). Acacia and guar gum separately showed significant anti-inflammatory effects in murine colitis, and furthermore, high dose acacia led to an additional therapeutic benefit when co-administered with 5ASA. These results indicate that further investigations are surely warranted in the search of better colitis therapy.
Keywords: Acacia, Guar gum, Colitis, Inflammatory bowel disease, 5-Amino salicylic acid
Graphical abstract
Highlights
-
•
Guar gum and acacia gum exhibit anti-inflammatory effect in murine colitis.
-
•
5 amino salicylic acid (5ASA) combined with Acacia gum shows therapeutic benefit over 5ASA alone.
-
•
Adhesion of guar gum and acacia gum stronger to inflamed colonic mucosa than to healthy controls.
-
•
Overall evaluation indicates a slightly better therapeutic effect with acacia gum.
1. Introduction
Inflammatory bowel diseases (IBD) are chronic and immunologically-mediated diseases of the gastrointestinal tract that can be categorized into two distinct idiopathic inflammatory diseases, namely Ulcerative Colitis and Crohn's Disease (Podolsky, 2002). Treatment of IBD involves the use of anti-inflammatory drugs, such as salicylates with 5-aminosalicylic acid (5ASA) as the active moiety.
Recent studies revealed that excipients that are usually incorporated during formulation design for their pharmaceutical functionalities, such as tablet binders, granulation aids, gel forming materials etc., can possess an intrinsic anti-inflammatory activity and additionally, contribute to the mitigating effect of the active drug. Previous findings indicated that chitosan and its oligosaccharides can contribute to the anti-inflammatory effects in experimental therapy of IBD in such a way (Azuma et al., 2015; Bautzová et al., 2012; Jhundoo et al., 2020; Rabišková et al., 2012; Yousef et al., 2012). In addition, chitosan has been found to show strong bioadhesion as well as influence on the opening of tight junctions due to changes in the intracellular pH hence affecting paracellular drug transport (Jhundoo et al., 2020; Rosenthal et al., 2012). Thus, mucoadhesive rectally administered hydrogels have been shown to be more targeted, effective and safer than typically used oral formulations (Xu et al., 2017). It would be logical to screen additional excipients from similar classes of polysaccharides to identify other polymer candidates for a broader applicability of this approach. Although “inert” excipients such as acacia or guar gum have long been used in various pharmaceutical dosage forms, their anti-inflammatory potential in IBD has not yet been fully investigated. Gums such as acacia and guar gum are heterogenous in composition and yield simple sugars when hydrolyzed. Acacia is a branched-chain anionic polysaccharide derived from the dried gummy exudate of the Acacia tree (Acacia Senegal (L) Willdenow) (Doi et al., 2006). It consists of a backbone made up of 1,3-linked β-D-galactopyranosyl units and side chains made up of two to five similar units joined to the backbone by 1,6-linkages. Acacia is used as a stabilizer, emulsifier and thickening agent in liquid and semi-solid formulations, but also as a tablet binder in solids (Dürig and Karan, 2018). However, Acacia extracts exhibited protective qualities in experimental IBD via inhibition of inflammatory mediators and prevention of oxidative damage whilst minimizing side effects, making the polymer a promising candidate for co-formulations in IBD therapy (Sakthivel and Chandrasekaran, 2014; Stohs and Bagchi, 2015). In addition, an improvement of the intestinal barrier function could be shown in vitro (Daguet et al., 2015). Guar gum consists of high molecular weight non-ionic polysaccharides with a molecular weight range of 50 to 8000 kDa and is composed of a linear chain of β-1,4-linked mannose units with α-1,6-linked galactose units as side chains. The ratio of galactose to mannose units in guar gum is 1:2 (Mudgil et al., 2012). The typical use of guar gum in oral dosage forms ranges from applications as a binder to disintegrant (Duru et al., 1992; Feinstein and Bartilucci, 1966; Sakr and El Sabbagh, 1977), although guar gum and its metabolites were also found to reduce mucosal inflammation and improve intestinal barrier properties (Hung and Suzuki, 2016; Naito et al., 2006; Takagi et al., 2016), suggesting this polymer to be an interesting option for the treatment of IBD. Therefore here, acacia and guar gum were analyzed for their anti-inflammatory efficacy in a murine experimental colitis and the usefulness in a combination therapy with the well-established therapeutic agent, 5-aminosalicylic acid (5ASA). The therapeutic outcome of the two excipients was evaluated at different doses, and combinations of the polymers with 5ASA were also assessed for potential enhanced therapeutic effects of the combination.
2. Materials and methods
2.1. Materials
Acacia gum, guar gum and 5ASA were purchased from Sigma-Aldrich Chemie GmbH, Steinheim, Germany. RAW-Blue™ cells were purchased from InvivoGen (San Diego, CA, USA). All other reagents for cell culture were purchased from Biochrom GmbH (Berlin, Germany). The Mouse Ready-Set-Go® TNF-α, IL-6 and IL-1β ELISA kits were obtained from eBioscience (Vienna, Austria). The InstantOne™ total mouse NFKB p65 ELISA 96 well kit was purchased from Affymetrix (Vienna, Austria). All other chemicals used for the in vivo studies were obtained from Sigma Aldrich (Deisenhofen, Germany).
2.2. Cell culture studies
RAW-Blue™ cells are derived from murine RAW 264.7 macrophages. They contain a chromosomal integration of a Secreted Embryonic Alkaline Phosphatase (SEAP) reporter construct, which is driven by nuclear factor kappa-B (NF-κB) and alkaline phosphatase-1 (AP-1). Cells were cultured in DMEM with l-glutamine, 100 μg/ml Normocin and 100 μg/ml Zeocin according to the manufacturer's recommendations. RAW-Blue™ cells (n = 6) were seeded in 96-well plates at a density of 1*105 cells/well and grown for 24 h at 37 °C in 5% CO2 (day 1). Cytotoxity of the polymers (10 μg/ml to 2000 μg/ml) was assessed by measuring cell viability using the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide] assay. After 24 h incubation with the polymer solutions, the RAW 264.7 macrophages cell culture supernatants were replaced by serum-free culture medium containing 0.5 mg/ml MTT and incubated for 2 h at 37 °C. Thereafter, the medium was aspirated, and the formazan crystals were dissolved in DMSO prior to the measurement of the absorbance at 544 nm using a microplate spectrophotometer (Victor 3 V, Perkin Elmer, MA, USA). The data was calculated as percent cell viability using the untreated control as the reference and the IC50 values were calculated for each test compound. To investigate the anti-inflammatory potential of the polymers and combinations the culture medium was either replaced after 24 h by serum-free medium containing 1 μg/ml lipopolysaccharides (LPS) derived from Escherichia coli 0111:B4 or by polymer solutions of each polymer alone or in combination with 5ASA at concentrations of 30 μg/ml made up in serum-free medium. The cells were incubated for another 24 h at 37 °C (day 2). On day 3, the culture medium from the RAW-Blue™ cells that had been previously stimulated with 1 μg/ml LPS was aspirated before washing of the cells twice with PBS warmed to 37 °C. Subsequently, the RAW-Blue™ cells were incubated with polymer solutions alone or in combination with 5ASA at concentrations of 30 and 300 μg/ml made up in serum-free medium. The supernatants from the non-stimulated (untreated) and stimulated cells were collected after 24 h of incubation (day 4) and used for quantification of NF-κB activity that was assessed from the levels of SEAP released in the cell supernatant using a colorimetric enzymatic assay (n = 3). The supernatants were incubated with Quanti-Blue™ (InvivoGen, San Diego, CA, USA) 50% (v/v) for 24 h at 37 °C and the absorbance was measured at 650 nm (Victor 3 V, Perkin Elmer, MA, USA).
2.3. Animal treatment
Experiments were conducted using male Swiss/CD-1 mice (4–6 weeks, average weight = 25 g) purchased from JANVIER (Saint-Berthevin, France), which have proven to be a realistic model for testing anti-inflammatory formulations (Barone et al., 2018). All animal experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, US). Experiments were conducted at the University of Franche-Comté in Besançon, France in compliance with the French legislation on animal experimentation under the experimentation authorization no. A-25-48. The single injection 2,4,6-trinitrobenzenesulfonic acid (TNBS) colitis model was used as it is a reproducible and reliable model that enables acute colitis induction at an exact location (Moulari et al., 2014). The mice were acclimatized to laboratory conditions for one week preceding the start of the experiments with food and water ad libitum. Food was withheld from the animals 24 h before the start of the experiment although water was provided. The mice were lightly narcotized using isoflurane prior to intrarectal catheterization (4 cm) to insert 100 μl of TNBS in 50% ethanol at a dose of 120 mg/kg body weight. The mice were kept for 24 h without any treatment in order to allow the full development of colitis. Subsequently, they were treated with 30 or 300 mg/kg body weight of polymer solution separately or in combination with 30 mg/kg body weight of 5ASA also administered using intrarectal catheterization (4 cm) in a volume of 100 μl for three consecutive days. The control group received saline solution for three consecutive days after induction of colitis with TNBS. The mice were sacrificed 24 h after the last treatment (total of 5 days following the intrarectal instillation of TNBS) and the colon was resected and washed with 1 ml cold PBS.
2.4. Disease activity index and colon-weight to length ratio
A disease activity index (DAI) was used to determine the extent of inflammation in the animals from the assessment of the body weight, stool consistency and presence of rectal bleeding. No weight loss was assigned a score of 0, 1 to 5% weight loss was assigned a score of 1, 5 to 10% weight loss was assigned a score of 2, 10 to 20% weight loss was assigned a score of 3 and > 20% weight loss was assigned a score of 4. The stool consistency was monitored as a score of 0 for well-formed pellets, 2 for pasty and semi-formed stools that were not stuck to the anus and 4 for liquid stools that were stuck to the anus. In addition, the presence of rectal bleeding was recorded as 0 for no bleeding, 2 for presence of blood and 4 points for intense bleeding. A dead animal was attributed the score of 6. The mean of these 3 parameters formed the disease activity index which ranged from 0 (healthy) to 6 (maximum colitis) (Lamprecht et al., 2001). Besides, the colon-weight to length ratio (CWL) was calculated as the ratio of the wet weight of the inflamed colon to the total length of the colon.
2.5. Assessment of the inflammatory biomarkers
The inflammatory response was assessed from minced distal colonic tissues that were stored in 1 ml buffer solutions. The samples were frozen, thawed and sonicated for 1 min and subjected to homogenization using the Ultra-Turrax® (IKA, Staufen, Germany) at 10000 rpm for one min and this procedure was conducted for three freeze-thaw cycles. The homogenates were centrifuged at 10000 rpm at 4 °C for 10 min and the supernatant was collected and used for the analysis of the inflammatory biomarkers. The extent of neutrophil infiltration, which quantifies the severity of the inflammation, was determined from the homogenates using the myeloperoxidase (MPO) assay, which was conducted according to a standard method (Krawisz et al., 1984). The colon specimens were minced in 1 ml of hexadecyltrimethylammonium bromide buffer at a concentration of 0.5% in 50 mM phosphate buffer as described above prior to the spectrophotometric measurement of the myeloperoxidase activity. 0.1 ml of the supernatant was added to 0.167 g/ml of o-dianisidine hydrochloride and 0.005% hydrogen peroxide. The change in absorbance at 460 nm was measured over one minute. One unit of myeloperoxidase activity was defined as the amount of enzyme that degraded 1 μmol of peroxide per minute at 25 °C.
The concentrations of pro-inflammatory cytokines, IL-1β, TNF-α and IL-6 were determined from the homogenates using a commercial ELISA kit (eBioscience, Vienna, Austria). Furthermore, the total mouse NF-κB p65 levels in the homogenates were measured using a commercial ELISA kit engineered for a fast analysis of samples (Affymetrix, Vienna, Austria).
2.6. Bioadhesion studies
Acacia and guar gum were labeled using fluoresceinamine and administered intrarectally after the induction of colitis as described in Section 2.3. Briefly, 1 ml of acetonitrile was added to 1.33 mg of 1-ethyl-3-(3-Dimethylaminopropyl)-carbodiimide hydrochloride (DMAP). 500 μl of the DMAP solution was added to 2 mg of fluoresceinamine and the remaining 500 μl was added to 100 mg of the polymer. Polymer and fluoresceinamine were well dissolved prior to mixing the two solutions and incubating the mixture at room temperature for 24 h away from light under slight agitation. The labeled polymer mixture was precipitated using deionized water at a ratio of 1:2 and the polymer was recuperated by centrifugation at 20000 g for 30 min at 4 °C. The supernatant was eliminated and the pellet was washed multiple times by first dissolving it into acetone and precipitating it with ethanol. This step was repeated until a clear supernatant was obtained and the pellet was re-dissolved into acetone and water. The mixture was subjected to evaporation using the RotoVapor in order to eliminate the acetone prior to the lyophilization of the sample. The fluoresceinamine labeling efficiency was determined by measuring the fluorescence emission of labeled-gum solutions against standard solutions of acacia or guar gum. The labelling efficiency was found to be 1.7 ± 0.7 w/w % for guar gum and 1.1 ± 0.4 w/w % for acacia. 100 μl of the polymer solution was administered intrarectally at a dose of 30 mg/kg and the colon was resected 24 h later and stored at −20 °C. Cryosections of a thickness of 13 μm were prepared using a cryomicrotome (Slee, Mainz, Germany) prior to the visualization of the sample using Confocal Laser Scanning Microscopy (CLSM) (Nikon Instruments Europe B.V., Amsterdam, Netherlands).
2.7. Statistical analysis
All cell culture experiments were performed in replicates of six for each concentration tested. Statistical analysis was conducted using Sigmastat 4.0 software (Systat Software, Inc., San Jose, California, USA) and Graphpad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA). Statistical difference was determined using ordinary one-way ANOVA and followed by multiple comparisons using Dunnett's test. The data were expressed as mean ± SD and treatments were considered significantly different if p < 0.05.
2.8. Normalized inflammation index
In order to better visualize the overall therapeutic benefit of each drug/excipient combination relative changes of CWL, MPO, and cytokine level compared to untreated colitis control (considered as 100%) were taken together to build a mean value for each group and normalized to 100% (colitis control) according to the following formula:
3. Results
3.1. Cell culture
Acacia did not show significant reduction of cell viability at concentrations ≤300 μg/ml in both untreated and LPS-stimulated RAW 264.7 cells. The IC50 value for acacia in untreated and LPS-stimulated cells mg/ml was found to be 1026 ± 64.3 and 1095 ± 76.9 μg/ml in RAW 264.7 macrophage cells. No significant difference was noted in the IC50 value of acacia after LPS treatment of the cells (Fig. 1). On the other hand, guar gum did not show a significant reduction of cell viability at concentrations ≤800 μg/ml in both untreated and LPS-stimulated RAW 264.7 cells (Fig. 2). The IC50 values for GG were 3246 ± 326.5 and 3498 ± 486.1 μg/ml in untreated and LPS-stimulated RAW 264.7 cells respectively, which confirms its low cytotoxicity. NF-κB activity was measured in LPS-activated RAW-Blue™ cells after treatment with either acacia or guar gum alone or in combination with 5ASA. All treatments were significantly different from the untreated LPS-activated RAW-Blue™ control. The treatment with acacia alone at a low dose of 30 μg/ml or in combination with 5ASA did not result in any significant changes in NF-κB activity when it was compared with 5ASA (Fig. 1). However, a significant lowering in NF-κB activity was observed when a high dose of acacia (300 μg/ml) was combined with 5ASA compared to the 5ASA (p < 0.05) control.
Fig. 1.
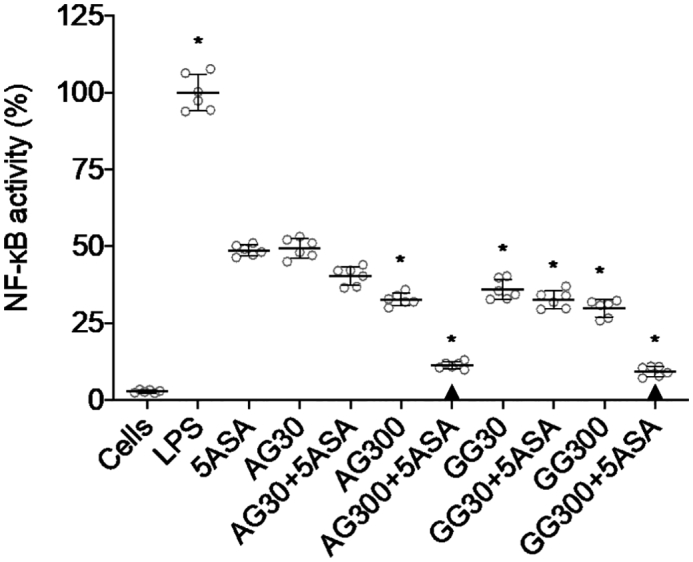
NF-κB activity from LPS-activated RAW-Blue™ cells after incubation with 30 and 300 μg/ml of acacia (AG), 30 and 300 μg/ml of guar gum (GG) and 30 μg/ml of 5ASA for 24 h in serum-free medium (mean ± SD; n = 6). * indicates p < 0.05 compared to 5ASA 30 μg/ml. All treatments were significantly different from LPS (+) control (p < 0.05), while ▲ below the respective columns indicates treatment is not significantly different from LPS-free control (p > 0.05).
Fig. 2.
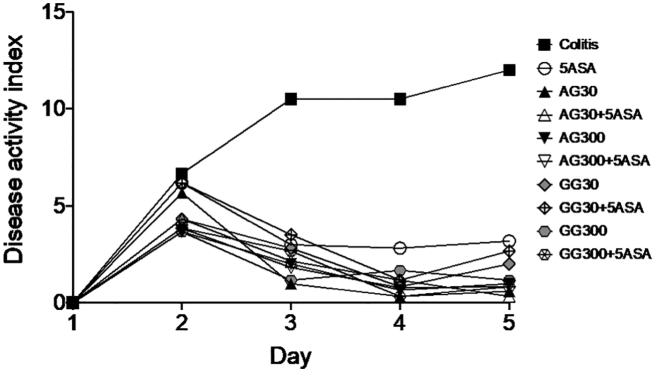
Disease activity index during experimental period (5 days) determined for n = 8 animals after treatment of severe colitis (120 mg/kg TNBS) with acacia (AG) or guar gum (GG) at a dose of 30 or 300 mg/kg and in combination with 5ASA at a dose of 30 mg/kg. Error bars were omitted for clarity reasons.
Similarly, the combination of a high dose of guar gum (300 μg/ml) with 5ASA was found to significantly suppress the NF-κB activity in RAW-Blue™ cells to 9% (Fig. 1) compared to 5ASA control (p < 0.05). In contrast to acacia, the low dose (30 μg/ml) guar gum in combination with 5ASA also showed significant differences when compared to 5ASA alone. It is noteworthy that with both gums at high doses, the combinations with 5ASA were not statistically different from LPS-free controls.
3.2. Experimental colitis
3.2.1. DAI and CWL and macroscopic tissue evaluation
Rectal administration of acacia and guar gum alone or as combinations with 5ASA showed the highest reduction in disease activity compared to the untreated colitis control group (Fig. 2). Due to the high variability, statistically significant differences were only observed between groups receiving any of the treatments and the untreated control group. Interestingly, most of the gum-5ASA combinations reach DAI levels close to 0 which reflects the situation of a remission or healthy control.
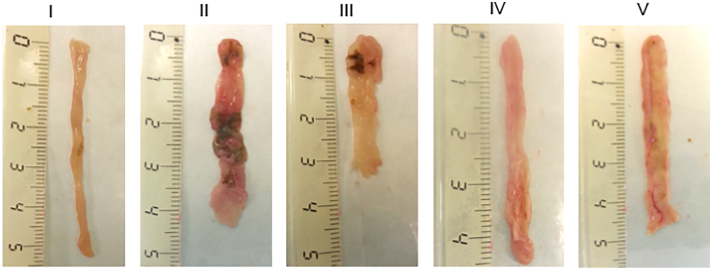
In colon tissue sections, treatments with acacia (IV) or guar gum (V) in combination with 5ASA resulted in minimal swelling of the colon tissue with its integrity and length preserved after 5 days (Fig. 3). Results from macroscopic tissue evaluation are in line with findings in the CWL measurements. All treatments, including both gums at both doses without 5ASA led to significantly lower CWL levels compared to colitis controls (Fig. 4). Surprisingly, no significant dose-dependent effects were observed in these measurements, however similar to the qualitative results from the macroscopic histology, in combination groups no significant differences were seen compared to healthy controls.
Fig. 3.
Photographs representative of the mouse colon show tissue sections from the healthy control (I) colitis group (II), group treated with 30 mg/kg 5ASA (III), 30 mg/kg acacia and 30 mg/kg 5ASA (IV) and 30 mg/kg guar gum and 30 mg/kg 5ASA (V). The scale shown indicates the length in cm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
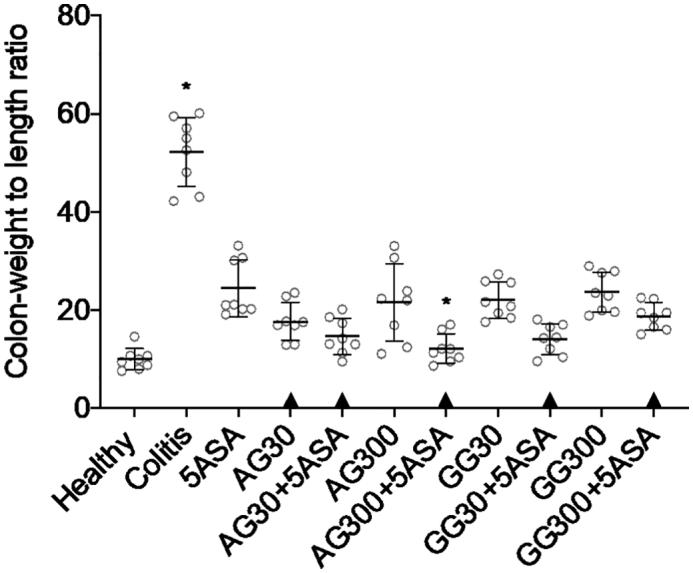
Determination of colon weight to length ratio after treatment of severe colitis (120 mg/kg TNBS) with acacia (AG) or guar gum (GG) at a dose of 30 or 300 mg/kg and in combination with 5ASA at a dose of 30 mg/kg (mean ± SD; n = 6). * indicates p < 0.05 compared to 5ASA 30 mg/kg. All treatments were significantly different from untreated colitis controls (p < 0.05), while ▲ below the respective columns indicates treatment is not significantly different from healthy controls (p > 0.05).
3.2.2. MPO activity
A significant reduction in MPO activity was found for all treated groups compared to the untreated colitis group (Fig. 5). However, no significant difference was observed between the groups treated with 5ASA combinations compared to 5ASA alone. In contrary to findings with the before mentioned disease parameters, guar gum was gradually less efficacious in lowering MPO. Accordingly, only 5ASA control and acacia treated groups were found not significantly different from healthy control levels.
Fig. 5.
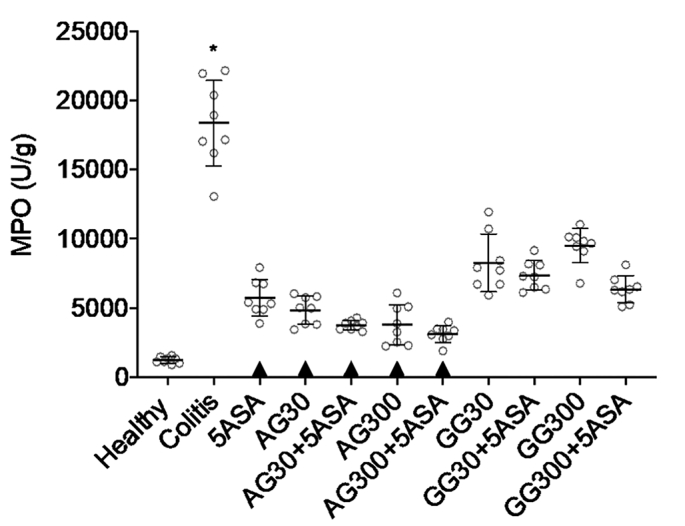
MPO activity after treatment of severe colitis (120 mg/kg TNBS) with acacia (AG) or guar gum (GG) at a dose of 30 or 300 mg/kg and in combination with 5ASA at a dose of 30 mg/kg (mean ± SD; n = 6). * indicates p < 0.05 compared to 5ASA 30 mg/kg. All treatments were significantly different from untreated colitis controls (p < 0.05), while ▲ below the respective columns indicates treatment is not significantly different from healthy controls (p > 0.05).
3.2.3. Pro-inflammatory cytokines
Similar trends in anti-inflammatory efficacy were observed when determining intratissue levels of different cytokines. Generally, both gums at both doses, irrespective whether they were applied in combination with 5ASA or not, led to significantly lower levels of IL-1β and TNF-α, and IL-6, respectively. Besides, combinations of acacia or guar gum and 5ASA were found to be more effective in suppressing the secretion of IL-1β and TNF-α levels when compared to 5ASA alone (Fig. 6A+B), while no significant difference was observed between the combination groups and 5ASA alone in the levels of IL-6 (Fig. 6C). Again, similar to CWL values, there were no significant dose-dependent differences in cytokine levels with neither acacia nor guar gum.
Fig. 6.
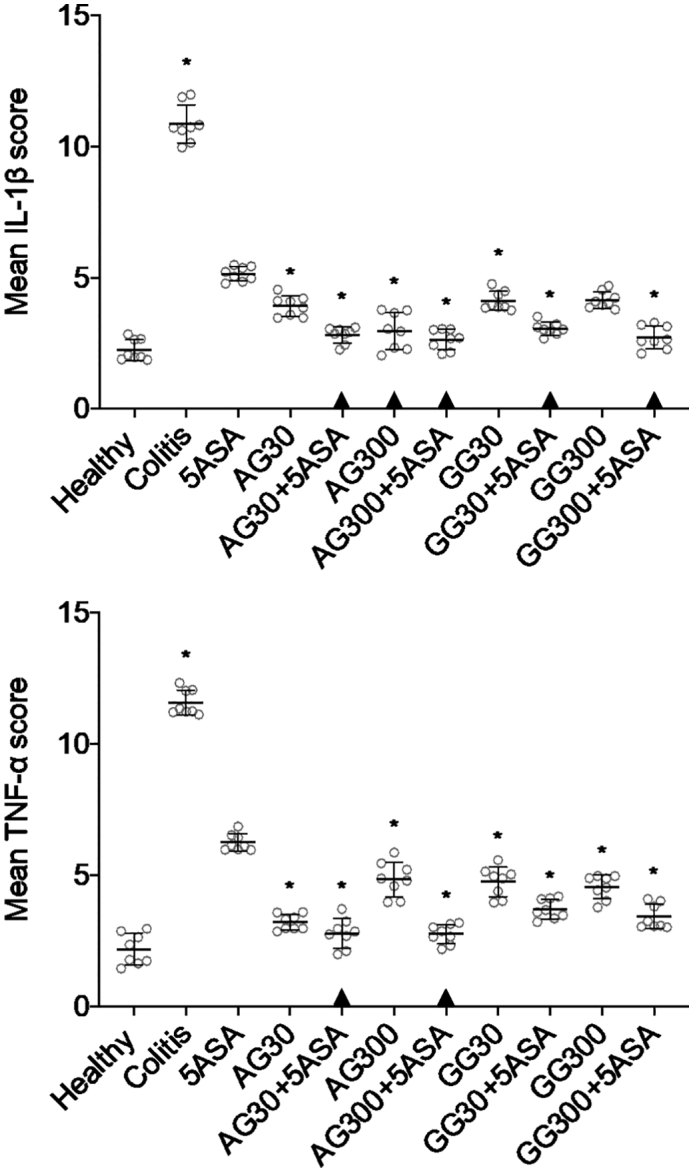
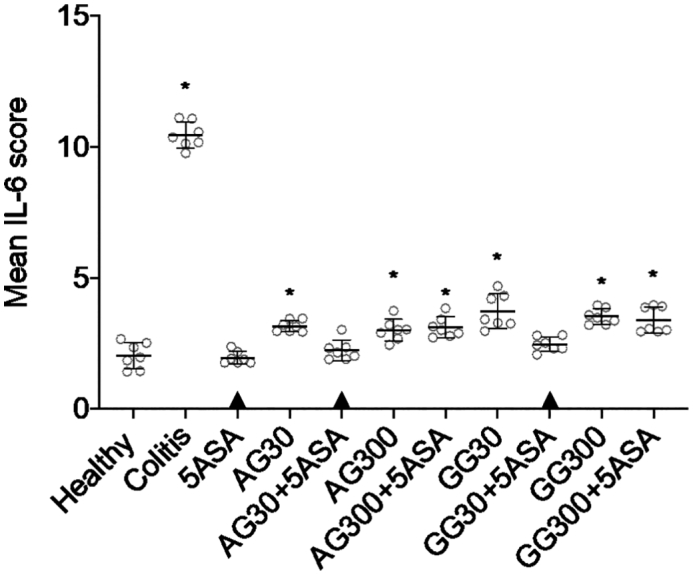
IL-1β (A), TNF-α (B) and IL-6 (C) secretion in colonic tissue homogenates after treatment of severe colitis (120 mg/kg TNBS) with acacia (AG) or guar gum (GG) at a dose of 30 or 300 mg/kg and in combination with 5ASA at a dose of 30 mg/kg (mean ± SD; n = 6). * indicates p < 0.05 compared to 5ASA 30 mg/kg. All treatments were significantly different from untreated colitis controls (p < 0.05), while ▲ below the respective columns indicates treatment is not significantly different from healthy controls (p > 0.05).
In line with the preceding cytokines, both gums lowered the NF-κB levels significantly compared to untreated colitis in tissue homogenates, but the effect of acacia was slightly stronger than that of guar gum on NF-κB levels (Fig. 7). Combining acacia with 5ASA led to lower NF-κB activity compared to the untreated control but no significant difference was noted between these combination groups and the 5ASA group (p > 0.05). Both 5ASA alone and combinations of acacia gum with 5ASA were not significantly different from healthy controls.
Fig. 7.
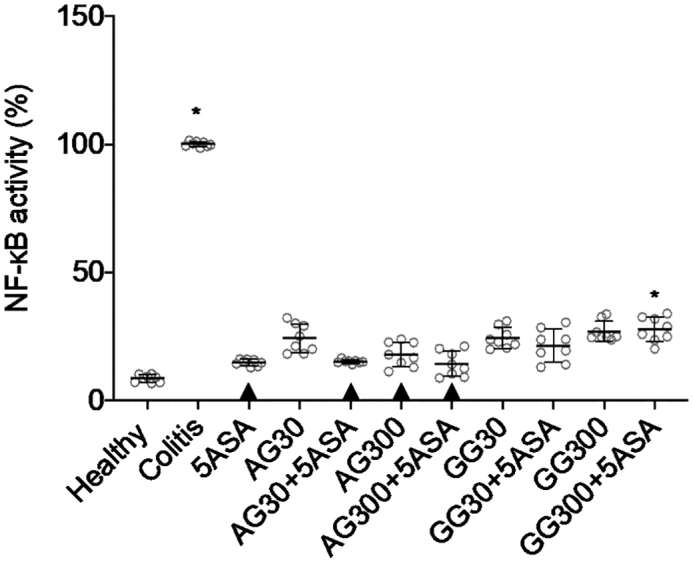
NF-κB activity in colonic tissue homogenates after treatment of severe colitis (120 mg/kg TNBS) with acacia (AG) or guar gum (GG) at a dose of 30 or 300 mg/kg in combination with 5ASA at a dose of 30 mg/kg (mean ± SD; n = 6). * indicates p < 0.05 compared to 5ASA 30 mg/kg. All treatments were significantly different from untreated colitis controls (p < 0.05), while ▲ below the respective columns indicates treatment is not significantly different from healthy controls (p > 0.05).
3.3. Bioadhesion studies
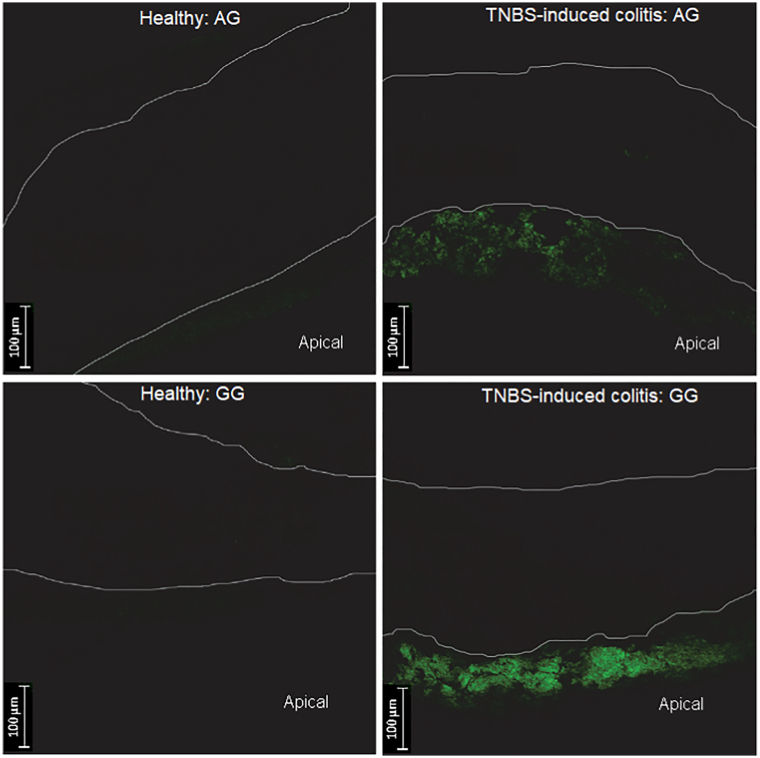
For both gums, acacia as well as guar gum, CLSM data revealed adhesive properties to inflamed colonic tissues while in healthy tissues much lower mucoadhesion was found (Fig. 8). Besides, a much higher fluorescence intensity was obtained with guar gum compared to acacia. Semi-quantitative image analyses showed a significantly enhanced bioadhesion for guar gum to the inflamed colonic tissue compared to the healthy tissue, while for acacia the differences were not statistically significant due to the large variability of the values. In both cases, approximatively 5- to 6-fold higher adhesion to the inflamed gut was obtained (Fig. 9).
Fig. 8.
CLSM images obtained after administration of labeled acacia (AG) and guar gum (GG) to healthy and inflamed colonic tissue in mice.
Fig. 9.
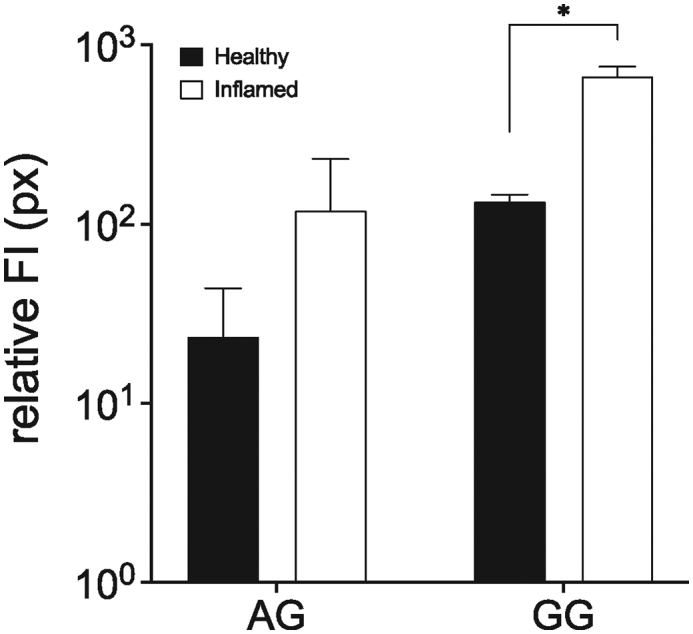
Relative fluorescence intensity (relative FI) in inflamed tissue for acacia and guar gum measured using Image J. Pictures were taken using the same settings and thresholding tool settings were duplicated in every image compared. (mean ± SD; n = 3; * indicates p < 0.05 compared to healthy tissue compared to inflamed tissue for each gum).
4. Discussion
Polysaccharides such as acacia and guar gum exhibit high stability and biodegradability, which make them excellent excipients for colonic drug delivery (Chourasia and Jain, 2004). Although recently an increasing number of studies revealed that certain polysaccharides can have a pharmacological effect besides their pharmaceutical functions in formulation design, the potential anti-inflammatory benefit of these compounds in IBD had not been investigated and may offer some interesting findings.
This study revealed significant anti-inflammatory efficacy of acacia and guar gum in experimental colitis in mice. In most pathophysiological parameters the trend suggested a better outcome when gums were combined with 5ASA. Although some of the data indicate a slight superiority of acacia, to our opinion there is no clear conclusive difference in the effect when compared to guar gum. Key indicators such as MPO and NF-κB activity in the in vivo model showed a superior effect with acacia compared to guar gum and particularly at a higher dose of 300 mg/kg. However, others did not lead to significant differences between the two gums. This was also in line with the results from cell culture experiments that indicated comparable anti-inflammatory potential for the two gums. Although the complexity of the in vivo environment makes it difficult to single out a specific type of anti-inflammatory mechanism and cell culture studies are often inadequate for an accurate simulation of the in vivo situation, the general outcome of this study highlights a significant therapeutic effect for both gums.
Although both acacia and guar gum are polysaccharides with similar galactose residues, they differ in their backbone structure as acacia consists of a highly branched chain of arabinose and rhamnose moieties whereas guar gum has a linear structure consisting of mannose residues. These structural differences and the differences in viscosities can also affect the stacking of the polymer chains and polymer conformation within the mucus layer, which in turns influences the ability of the polymer to form hydrogen bonds or electrostatic interactions with the mucin proteins. For instance, guar gum can lead to extensive hydrogen bonding due to its linear 1-4-β-linkages which enable stacking of the polymer chain within the mucus layer. Aside from hydrogen bonding, charge interactions also play an important role in influencing the binding of polymers to inflamed tissues. It has been reported that negatively charged molecules may show enhanced adhesion to inflamed tissues due to the fact that ulcerated tissues consist of a high number of positively charged proteins (Lamprecht et al., 2001) and this may lead in a very general way to the strongly increased tendency of such polymers to adhere to inflamed mucosa, similar to the observations in this study. The reason for the dramatic differences in adhesion between the two gums is not fully clear, however the much higher viscosity of guar gum could be one of the reasons. It has been postulated before that highly viscous polysaccharides adhere to a great extent to the inflamed gut and can form an additional gel layer on top of the mucus (Jhundoo et al., 2020; Popov et al., 2006). On the other hand, better mucoadhesion does not necessarily translate into better therapeutic effects, since highly viscous formulations can be subject to rapid elimination with the increased defecation tendencies observed in IBD.
However, the general mechanism of the anti-inflammatory effect still needs to be identified. It is known that acacia and guar gum affect the digestive process in the large intestine and lead to the production of several different volatile fatty acids. For instance, guar gum enhances fermentation into butyric and acetic acids whilst acacia enhances the production of propionic acid, which can be absorbed in the colon (McConnell et al., 2009). Such short-chain fatty acids can restore the levels of non-pathogenic microbiota and therefore exert a prebiotic effect, which would under acute inflammatory conditions mitigate the inflammatory cascade (Tulung et al., 1987).
As a consequence, both gums would also require further in-depth characterization for elucidating the impact of excipient grades, especially different molecular weights, but also whether different origins will lead to comparable therapeutic outcome. As the efficacy of the two gums did not extremely differ, these findings offer a “tool box” whereby tailor-made composition for either oral or rectal administration could be designed. In order to better elucidate the slight differences between the two gums, we calculated a normalized inflammatory index, which took into account all quantitative pathological indicators indexed relative to untreated colitis control (Fig. 10). This revealed a gradual superiority of acacia over guar gum combinations as only AG + 5ASA combinations were significantly different from 5ASA alone as well as not significantly different from healthy controls. Typically, acacia can be used as a stabilizer, emulsifier, but also as a tablet binder in solid dosage forms, and especially its low viscosity is seemingly advantageous as a therapeutic effect required relatively elevated doses. Accordingly, the presence of acacia in the dosage form would not lead to a major modification of the drug release profiles. On the other hand, guar gum possessing a relatively high viscosity, the use as an “anti-inflammatory” excipient in a gel form, such as a rectal lavage formulation where generally a higher viscosity is desirable, would be potentially more suitable due to the fact that guar gum would provoke a modified release when used in an oral solid dosage form. Finally, even a mix of both excipients formulated in the same dosage form could modulate the physicochemical properties to the desired level.
Fig. 10.
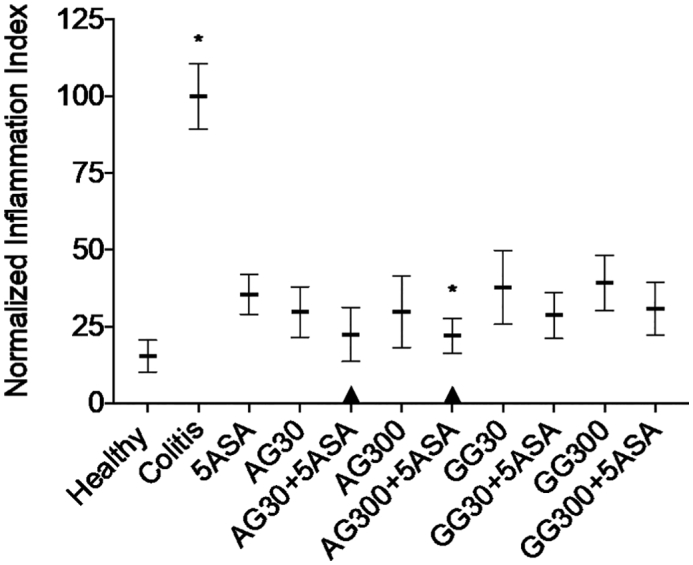
Normalized index over all in-vivo inflammatory indicators expressed as a relative change compared to untreated colitis controls. * indicates p < 0.05 compared to 5ASA 30 mg/kg. All treatments were significantly different from untreated colitis controls (p < 0.05), while ▲ below the respective columns indicates treatment is not significantly different from healthy controls (p > 0.05).
5. Conclusions
Acacia and guar gum are promising candidates for the colonic delivery of 5ASA in IBD therapy as these natural polymers exhibit mild anti-inflammatory effects in experimental colitis, which could be exploited to design a combination dosage form that allows for a more effective product.
Declaration of Competing Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgements
Henusha D. Jhundoo gratefully acknowledges the German Academic Exchange Service (Deutscher Akademischer Austauschdienst, DAAD) (91525759) for financial support. Alf Lamprecht is grateful to the support from a French Government grant managed by the French National Research Agency under the program “Investissements d'Avenir” with reference ANR-11-LABX-0021. Ferring Pharmaceuticals Inc. partially sponsored the research project that is the subject of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpx.2021.100080.
Appendix A. Supplementary data
Supplementary material
References
- Azuma K., Osaki T., Kurozumi S., Kiyose M., Tsuka T., Murahata Y., Imagawa T., Itoh N., Minami S., Sato K., Okamoto Y. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr. Polym. 2015;115:448–456. doi: 10.1016/j.carbpol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Barone M., Chain F., Sokol H., Brigidi P., Bermúdez-Humarán L.G., Langella P., Martín R. A versatile new model of chemically induced chronic colitis using an outbred murine strain. Front. Microbiol. 2018;9:565. doi: 10.3389/fmicb.2018.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautzová T., Rabišková M., Béduneau A., Pellequer Y., Lamprecht A. Bioadhesive pellets increase local 5-aminosalicylic acid concentration in experimental colitis. Eur. J. Pharm. Biopharm. 2012;81:379–385. doi: 10.1016/j.ejpb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Chourasia M.K., Jain S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004;11:129–148. doi: 10.1080/10717540490280778. [DOI] [PubMed] [Google Scholar]
- Daguet D., Pinheiro I., Verhelst A., Possemiers S., Marzorati M. Acacia gum improves the gut barrier functionality in vitro. Agro Food Ind. Hi-Tech. 2015;26:29–33. [Google Scholar]
- Doi Y., Ichihara T., Hagiwara A., Imai N., Tamano S., Orikoshi H., Ogasawara K., Sasaki Y., Nakamura M., Shirai T. A ninety-day oral toxicity study of a new type of processed gum arabic, from Acacia tree (Acacia senegal) exudates, in F344 rats. Food Chem. Toxicol. 2006;44:560–566. doi: 10.1016/j.fct.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Dürig T., Karan K. Ashland LLC; Wilmington, DE, United States: 2018. Chapter 9 - Binders in Wet Granulation, in: Handbook of Pharmaceutical Wet Granulation, Theory and Practice in a Quality by Design Paradigm; pp. 317–349. [Google Scholar]
- Duru C., Colombo P., Gaudy D., Massimo G., Barthelemy P. Comparative study of the disintegrating efficiency of polysaccharides in a directly table formulation. Pharm. Technol. Int. 1992;4:15–16. (20, 22–23) [Google Scholar]
- Feinstein W., Bartilucci A.J. Comparative study of selected disintegrating agents. J. Pharm. Sci. 1966;55:332–333. doi: 10.1002/jps.2600550313. [DOI] [PubMed] [Google Scholar]
- Hung T.V., Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2016;146:1970–1979. doi: 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- Jhundoo H.D., Siefen T., Liang A., Schmidt C., Lokhnauth J., Béduneau A., Pellequer Y., Larsen C.C., Lamprecht A. Anti-inflammatory activity of chitosan and 5-amino salicylic acid combinations in experimental colitis. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12111038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz J.E., Sharon P., Stenson W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology. 1984;87:1344–1350. doi: 10.1016/0016-5085(84)90202-6. [DOI] [PubMed] [Google Scholar]
- Lamprecht A., Ubrich N., Yamamoto H., Schäfer U., Takeuchi H., Maincent P., Kawashima Y., Lehr C.M. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001;299:775–781. [PubMed] [Google Scholar]
- McConnell E.L., Liu F., Basit A.W. Colonic treatments and targets: issues and opportunities. J. Drug Target. 2009;17:335–363. doi: 10.1080/10611860902839502. [DOI] [PubMed] [Google Scholar]
- Moulari B., Béduneau A., Pellequer Y., Lamprecht A. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J. Control. Release. 2014;188:9–17. doi: 10.1016/j.jconrel.2014.05.046. [DOI] [PubMed] [Google Scholar]
- Mudgil D., Barak S., Khatkar B.S. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012;50:1035–1039. doi: 10.1016/j.ijbiomac.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Naito Y., Takagi T., Katada K., Uchiyama K., Kuroda M., Kokura S., Ichikawa H., Watabe J., Yoshida N., Okanoue T., Yoshikawa T. Partially hydrolyzed guar gum down-regulates colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. J. Nutr. Biochem. 2006;17:402–409. doi: 10.1016/j.jnutbio.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Podolsky D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Popov S.V., Ovodova R.G., Markov P.A., Nikitina I.R., Ovodov Y.S. Protective effect of comaruman, a pectin of cinquefoil Comarum palustre L., on acetic acid-induced colitis in mice. Dig. Dis. Sci. 2006;51:1532. doi: 10.1007/s10620-005-9034-8. [DOI] [PubMed] [Google Scholar]
- Rabišková M., Bautzová T., Gajdziok J., Dvořáčková K., Lamprecht A., Pellequer Y., Spilková J. Coated chitosan pellets containing rutin intended for the treatment of inflammatory bowel disease: in vitro characteristics and in vivo evaluation. Int. J. Pharm. 2012;422:151–159. doi: 10.1016/j.ijpharm.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Rosenthal R., Günzel D., Finger C., Krug S.M., Richter J.F., Schulzke J.-D., Fromm M., Amasheh S. The effect of chitosan on transcellular and paracellular mechanisms in the intestinal epithelial barrier. Biomaterials. 2012;33:2791–2800. doi: 10.1016/j.biomaterials.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Sakr A.M., El Sabbagh H.M. Evaluation of guar gum as a tablet additive: a preliminary report. Pharm. Ind. 1977;39:399–403. [Google Scholar]
- Sakthivel K.M., Chandrasekaran G. Protective effect of acacia ferruginea against ulcerative colitis via modulating inflammatory mediators, cytokine profile and NF-κB signal transduction pathways. J. Environ. Pathol. Toxicol. Oncol. 2014;33:83–98. doi: 10.1615/JEnvironPatholToxicolOncol.2014008425. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D. Antioxidant, anti-inflammatory, and chemoprotective properties of Acacia catechu heartwood extracts. Phytother. Res. 2015;29:818–824. doi: 10.1002/ptr.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T., Naito Y., Higashimura Y., Ushiroda C., Mizushima K., Ohashi Y., Yasukawa Z., Ozeki M., Tokunaga M., Okubo T., Katada K., Kamada K., Uchiyama K., Handa O., Itoh Y., Yoshikawa T. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br. J. Nutr. 2016;116:1199–1205. doi: 10.1017/S0007114516003068. [DOI] [PubMed] [Google Scholar]
- Tulung B., Rémésy C., Demigné C. Specific effect of guar gum or gum arabic on adaptation of cecal digestion to high fiber diets in the rat. J. Nutr. 1987;117:1556–1561. doi: 10.1093/jn/117.9.1556. [DOI] [PubMed] [Google Scholar]
- Xu J., Tam M., Samaei S., Lerouge S., Barralet J., Stevenson M.M., Cerruti M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017;48:247–257. doi: 10.1016/j.actbio.2016.10.026. [DOI] [PubMed] [Google Scholar]
- Yousef M., Pichyangkura R., Soodvilai S., Chatsudthipong V., Muanprasat C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012;66:66–79. doi: 10.1016/j.phrs.2012.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material