Abstract
Importance
Changes in dietary habits and lifestyle can reduce the risk of cardiovascular disease which is the leading cause of death worldwide. Objectives of the MoKaRi study The MoKaRi (modulation of cardiovascular risk factors) intervention study is designed to evaluate the effectiveness and potential of the developed MoKaRi concept. The MoKaRi concept comprises three components, each designed to improve dietary behavior. The first component entails using daily menu plans to implement a defined “cardioprotective diet”. This diet consists of seasonal menu plans which are characterized by:
(i) a personalized energy supply depending on his or her age, gender, level of physical activity.
(ii) an adequate intake of carbohydrates, protein, fat, vitamins, minerals, and trace elements according to the guidelines of the German Society of Nutrition (DGE).
(iii) a recommended intake of saturated fatty acids (SFA; < 7% of caloric intake (En%)), monounsaturated fatty acids (MUFA; > 10 En%), polyunsaturated fatty acids.
(PUFA; approx. 10 En%), and long-chain n-3 PUFA (≥500 mg per day).
(iv) measures to encourage consumption of vegetables and fruits, and.
(v) eating more than 40 g dietary fiber every day.
Half of the participants will be scheduled to consume an additional 3 g of long-chain n- 3 PUFA every day in the form of fish oil.
The second component consists of regular one-on-one nutritional counseling, while a variety of further incentives make up the third component of the MoKaRi concept.
The MoKaRi study will provide essential insights into the relationship between defined nutrient intake, markers of food intake and health status. Our specific aim is to investigate the influence of dietary and lifestyle choices have on cardiovascular health.
The information and practical tools suitable for daily use, such as the personalized menu plans, could help to transfer knowledge on nutritional facts to the general population. In this way, the validated MoKaRi concept may contribute to the prevention and therapy of cardiovascular diseases.
Methods
In line with our power calculation, we will enroll 60 participants and randomly assign them to one of two parallel arms. Each participant will receive personalized menu plans for each day of the study and will be provided with one-on-one nutritional counseling sessions every two weeks for a study period of 20 weeks (140 days). During this period, blood samples will be taken every 14 days (11 time points) and twice during a 20-weeks follow-up period. Incentives such as a supply of foods approved according t the standards of the study, a sports program, individual feedback on study parameters reflecting health status, and group activities round off the MoKaRi concept.
Low-density cholesterol is the primary outcome measure of the MoKaRi study, and the secondary endpoints comprise markers of nutrient status (e.g. fatty acid distribution in plasma and erythrocyte lipids), a metabolomic profiling, diabetes risk markers, clotting markers, and further cardiovascular risk factors, such as blood lipids, homocysteine and high-sensitive c-reactive protein.
The MoKaRi study was registered before launch at ClinicalTrials.gov (identifier NCT02637778; https://clinicaltrials.gov/ct2/show/NCT02637778).
Keywords: Defined nutrient intake, Dietary fat, Dietary fibers, Nutritional counseling, Cardiovascular risk factors
Highlights
-
•
Study protocol to evaluate the cardioprotective potential of a new concept addressing nutritional and lifestyle behavior.
-
•
The MoKaRi concept is based on personalized menu plans, nutritional counselling, and incentives to increase motivation.
-
•
Regularly assessment of the outcome measures on 11 time points over the intervention period (every 14 days).
-
•
The validated MoKaRi concept may contribute to the prevention and the support of therapy of cardiovascular diseases.
1. Introduction
1.1. Background
The percentage of deaths worldwide attributed to cardiovascular disease (CVD) has risen from 21% in 2007 to 31% in 2016 [1]. Several risk factors contribute to the earlier onset and accelerated progression of CVD [2]. While gender, age, and genetic predisposition are beyond our control, most risk factors depend at least partly on lifestyle choices. Current CVD prevention guidelines focus on reductions in blood pressure, low-density lipoprotein (LDL) cholesterol, triglycerides, diabetes, weight, smoking, psychosocial stress, and on improving physical activity. Particular attention has been directed towards correcting unhealthy eating habits since diet is a significant determinant of this disease [3,4]. This finding is in line with the results of the Global Burden of Disease Study. In 2017, this study attributed 11 million deaths and 255 million disease-adjusted life years (DALYs) to poor diet [5]. On a global and local scale, the leading dietary risk factors for death and loss of DALYs were high intake of sodium and low intake of whole grains and fruits [5].
The traditional Western diet is characterized by high intake of calories, salt, saturated fat, simple sugar, and refined starch. At the same time, consumption of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA), whole-grain fibers, fish, vegetables, fruits, vitamin D or potassium is inadequate [[6], [7], [8]]. Reducing the energy density of the diet and the intake of saturated fatty acids (SFA) plays a crucial role in preventing CVD. Thus, both European and American experts recommend that less than 10% of caloric intake (En%) should be in the form of SFA; for persons with hypercholesterolemia this figure falls to 7% [3,4,9]. Based on the health claims for eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which have been approved by the European Food Safety Authority (1924/2006 and 432/2012), a regularly intake of 2–3 g long-chain n-3 PUFA is associated with a reduction of blood pressure and triglycerides [10,11]. Further, 3 g EPA and DHA are suitable to reduce inflammatory markers which will also contribute to the prevention of CVD [12].
In addition to the unfavorable nutrient profiles of many popular foods, the western eating culture is characterized by a lack of natural appetite, hunger or satiety, an oversupply of food, and a lack of awareness regarding the link between diet and health risks.
Optimizing diet might be an effective means to reduce the risk of CVD [13]. Up to now, most efforts, based on guidelines or recommendations from nutritional societies, to convince healthy individuals to consume a healthy diet to reduce cardiovascular risk in later years failed, because CVD stay one of the leading causes of death in Europe and the United States [1,2,14,15]. The efforts have failed in part because they have not focused enough on motivation and on generating recommendations that are suitable for daily use. We, therefore, developed the concept for modulation of cardiovascular risk factors by diet (MoKaRi) to address the challenges presented by adherence to the traditional Western diet.
MoKaRi aims to improve the diet by explicitly addressing the practical day-to-day needs of the participants and by including several strategies to increase motivation.
1.2. Rationale and objectives of the MoKaRi trial
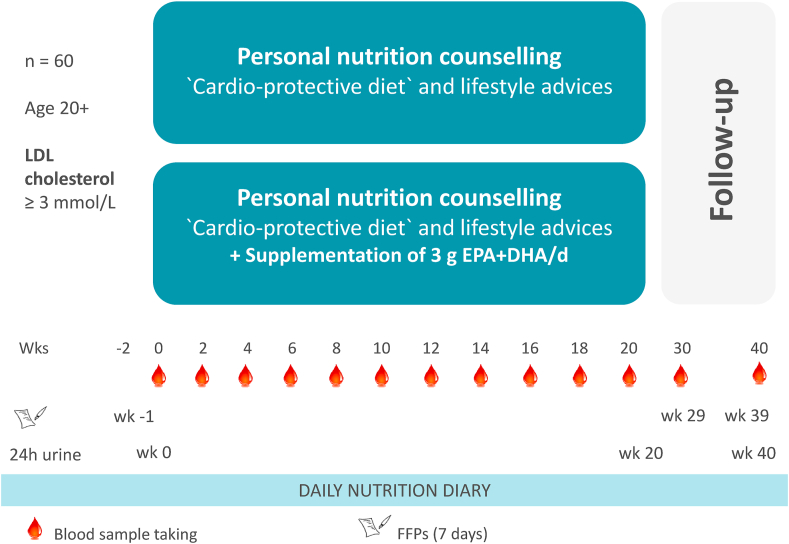
The proposed randomized clinical trial is designed to assess and evaluate the effectiveness and potential of the MoKaRi concept (Fig. 1) in adults with increased risk for CVD. Moreover, the study will provide data about the association between the food intake defined by the menu plans and measurable markers reflecting food intake (nutritional biomarkers) as well as cardiovascular biomarkers or risk factors.
Fig. 1.
Study design of the MoKaRi trial.
Besides the analysis of well-known nutritional biomarkers, such as B vitamins, vitamin D, vitamin E, minerals, and fatty acids, and established cardiovascular risk factors, such as blood lipids, fasting plasma glucose, and inflammation markers, we plan to conduct a metabolomic profiling. The controlled food intake by the menu plans in combination with the metabolomics approach offers the possibility to identify new potential biomarkers reflecting food intake.
The MoKaRi study will allow the identification of variables influencing people's nutritional behavior and offers the possibility to address these variables within the recommendations that will be developed for adults with increased cardiovascular risk.
1.3. Detailed issues addressed by the MoKaRi trial
-
●
Evaluating the influence of dietary/lifestyle interventions on cardiovascular risk factors and achievement of additive effects by additional intervention with long-chain n-3 PUFA
The menu plans are characterized by an optimized intake of SFA, MUFA, and PUFA to reduce cardiovascular risk factors such as total cholesterol and LDL cholesterol [3,4,9]. Based on the health claims which are authorized for EPA and DHA, a daily intake of 2–3 g long-chain n-3 fatty acids will result in an improvement of further cardiovascular risk factors, such as triglycerides and blood pressure [10,11].
-
●
Identification of time-dependent changes in cardiovascular risk factors
-
●
Evaluation of time-dependent incorporation of long-chain n-3 PUFA into plasma lipids, lipid fractions (phospholipids, triglycerides, free fatty acids, and cholesterol esters) and erythrocyte lipids
-
●
Identification of variables (e.g. problems, conflicts, incentives, possibilities for motivation) influencing nutritional behavior of study participants and reasons for failing to comply
-
●
Identification of targets for treatment goals of dietary interventions with a focus on cardiovascular risk
-
●
Derivation of tailor-made, practicable recommendations for the target group
The MoKaRi trial will provide valuable information for planning and implementing a healthy diet. Furthermore, valuable data will be obtained to develop or improve standards for conducting diet-related human intervention studies focusing on biomarkers for nutrition intake, establishing compliance with study interventions, and encouraging observation of the background diet.
2. Methods
2.1. Study design
The MoKaRi trial will be conducted as a randomized, single-center intervention study in parallel design (two arms: prepared menu plans with 3 g long-chain n-3 fatty acids by fish oil per day vs prepared menu plans without additional fish oil; Fig. 1). The MoKaRi study will be take place from February 2016 to July 2016.
The MoKaRi trial will start with a run-in period of one week to document the dietary habits of the study participants using questionnaires on food preferences and a food frequency protocol (FFP). The FFP consist of a list of foods that are normally consumed in the Western diet and the corresponding portion information. The participants mark the consumption of the listed foods with a line. Food and beverages that are not listed can be added. The daily nutrient intake will be calculated for seven days with the software package PRODI® version 6.4 (Nutri-Science, Stuttgart, Germany).
The intervention periods will start with a 24-h urine collection, fasting blood sampling, and an assessment of the health status (i.e., anthropometric data (height, weight, body mass index, and waist circumference), blood pressure, ankle-brachial index, pulse variability, Harvard-step-test, and bioelectric impedance measurement). At this time point, participants will receive their personalized menu plans for the first 14 days of the study as well as information material.
During the 20 weeks of the MoKaRi trial, we will take fasting blood samples and assess health status every 14 days. Also, 14 new menu plans and appropriate amounts of study foods will be handed out at these time points (i.e., 11 times). At the end of the intervention period, participants have to collect 24-h urine once again.
For fasting blood sampling, the follow-up period of 20 weeks will be split into two periods i.e. every 10 weeks. (Fig. 1). In the follow-up period, the participants can use the menu plans from the intervention period, but further measures of the MoKaRi concepts, such as nutritional counseling, providing study foods, visualization of the cardiovascular risk, feedback, sports program, and events encouraging group feeling are not planned in this period.
Over the entire period of the MoKaRi trial, the study participants have to keep a nutrition diary to document (i) their experiences with the menu plans, (ii) changes and deviations from the menu plans, and (iii) for evaluating all meals.
2.2. Setting and population, eligibility criteria and exclusion criteria
The study will be carried out in Jena (Thuringia, Germany) and the surrounding area.
Participants of both sexes aged 20–80 years will be eligible for inclusion of the MoKaRi study. As further inclusion criteria, study participants with plasma LDL cholesterol concentrations ≥3 mmol/L will be enrolled after written informed consent.
The study protocol listed the following exclusion criteria:
-
●
Intake of lipid-lowering medications, glucocorticoids, drugs that interfere with glucose metabolism
-
●
Gastrointestinal diseases, known allergies or food intolerances
-
●
Known familial hypercholesterolemia
-
●
Intake of additional dietary supplements (e.g. fish oil capsules or vitamin E)
-
●
Pregnancy, lactation
-
●
Patient's request or if patient compliance with the study protocol is doubtful
-
●
Regular abuse of alcohol or drugs
-
●
Body mass index (BMI) ≥ 25 kg/m2
Medication during the trial: Any sporadic and systemic use of medications because of other diseases will be allowed if it did not interfere with the study results. In this context, the regularly intake of lipid-lowering drugs, anti-inflammatory drugs, and drugs that interfere with glucose metabolism belongs to the exclusion criteria, but the regularly intake of thyroid hormones or the sporadic intake of antibiotics will be allowed. The regularly intake of antihypertensive drugs will be allowed if the dosage stay stable over the course of the study. All medication taken are recorded in the medication diary. The systemic intake of medications owing to other diseases is to remain unchanged over the study period.
The study leader will decide individually if the sporadic and systemic use of medications because of other diseases will be allowed or not as it interfere with the study results.
2.3. Intervention - the MoKaRi concept
The MoKaRi concept is based on a ‘cardioprotective diet‘ which is characterized by.
-
1.
Adequate intake of energy, carbohydrates, protein, and fat according to the guidelines of the German Nutrition Society (DGE) [9],
-
2.
Optimized intake of SFA (≤7 En%), MUFA (≥10 En%), PUFA (≥10 En%), and long-chain n-3 PUFA (≥500 mg/d) [16],
-
3.
Encouraged consumption of vegetables, fruits, and cereals,
-
4.
Intake of >40 g dietary fiber per day,
-
5.
Reduced intake of salt (limitation on a maximum of 6 g per day) and sugar (limitation on a maximum of 50 g per day) [9],
-
6.
Reduced intake of (highly) processed, calorie-rich, nutrient-poor foods (e.g. fast food, convenience products),
-
7.
Appropriate intake of vitamins, minerals, and trace elements through commercially available foods, and
-
8.
Moderate physical activity (moderate circle training with eight exercises in two intensities (1 or 1.5 h per week)).
Half of the participants will consume an additional 3 g long-chain n-3 PUFA (i.e., 3 g EPA and DHA) per day which corresponds to about 10 ml/d fish oil. The dietary regimes of both groups (with and without fish oil) will be isocaloric.
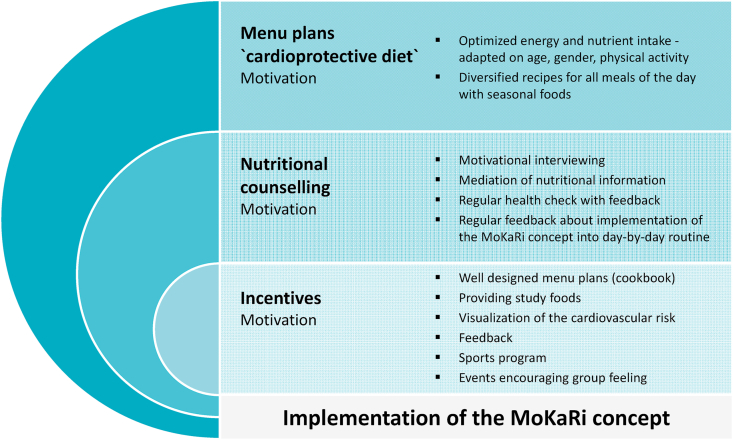
The MoKaRi concept is designed to improve nutritional behavior using the following three approaches:
-
I)personalized menu plans for implementing a cardioprotective diet,
-
II)regular, individual nutritional counseling, and
-
III)incentives.
- These incentives are (Fig. 2):
-
i)dissemination of knowledge
-
II)
-
ii)
the possibility to participate in a sports program adapted to target group-specific needs and personal capacities (cardioprotective circle training)
-
iii)
providing of chosen foods which are approved by the study guidelines, e.g. extra virgin olive oil, mixed nuts, yoghurt enriched with fish oil, chia seeds, barley flakes among others
-
iv)
regular feedback on the course of study parameters (with a focus on cardiovascular risk factors) and
-
v)
activities to encourage group feeling, e.g. engaging in cooking events or the sports program once a week.
Fig. 2.
The MoKaRi concept.
2.3.1. Preparation of MoKaRi menu plans
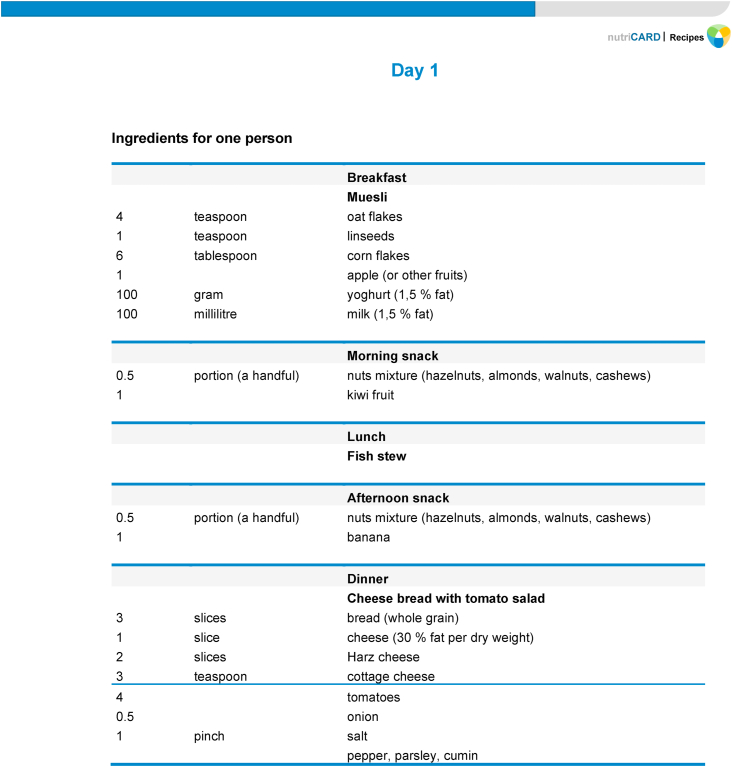
The daily recipes and menu plans will be adapted to the individual energy requirements of each study participant depending on age, gender, and physical activity level (PAL), and whether they belonged to the fish oil group or not. Therefore, the menu plans will be prepared in eight energy categories (1800 kcal, 1900 kcal, 2000 kcal, 2100 kcal, 2400 kcal, 2500 kcal, 2600 kcal, 2700 kcal) and will include detailed information about type and amount of foods for breakfast, morning snack, lunch, afternoon snack, and dinner (Fig. 3). For each meal, a well-designed detailed description for preparation will be provided.
Fig. 3.
Example of a menu plan according to the MoKaRi concept. The above daily menu plan is adapted to fit the requirements of a woman aged between 51 ≤ 65 yrs with a PAL of 1.6 which corresponds to a daily energy intake of 2000 kcal.
The software PRODI version 6.4 for professional dietary counseling and therapy will be used to calculate the nutrient content of recipes, meals, and menu plans. The recipes for each meal originate from cookbooks and personal experience. A unique hallmark of the menu plans will be the balanced nutrient profile. To achieve this, we will add carefully chosen nutrient-rich foods to meet the MoKaRi criteria for daily nutrient intake. For example, we will include fatty cold-water fish to ensure intake of long-chain n-3 PUFA, vitamin B12, vitamin D, iodine, and protein. The consumption of dairy products will be encouraged to achieve the recommended intake of B vitamins, high-value protein, calcium, and other minerals. Linseed, rapeseed, and olive oils, mixed nuts and seeds will be recommended to meet the criteria for fat quality. Vegetables, selected fruits, nuts, and seeds, and, in particular, pulses such as peas, beans, or lentils, will be inserted to ensure optimal intake of vitamins, minerals, trace elements, dietary fiber, and secondary plant molecules, e.g., carotenoids and polyphenols.
The participants of the MoKaRi trial will receive daily menu plans with optimized nutrient profiles over the entire study period of 20 weeks (14 new plans every two weeks, Fig. 1). An example of the MoKaRi menu plans is shown in Fig. 3.
2.3.2. Nutritional counseling and nutritional-lifestyle coaching
In addition to the daily menu plans for the implementation of the ‘cardioprotective diet' over the 140 days of the MoKaRi trial, participants will receive regular individual nutritional counseling every two weeks throughout the full study period (Fig. 1). The study leader will conduct all nutritional counseling sessions and addressed the following issues:
-
1.
Providing information about relevant nutritional topics, such as (i) energy requirements, (ii) dietary fats and oils, (iii) nuts and dietary fibers, (iv) sausage and meat, (v) sugar and salt, (vi) natural sources of omega-3 fatty acids (plant oils, seafood), (vii) (seasonal) vegetables and fruits, (viii) dairy products, (ix) drinking behavior, (x) individual diet-related issues, (xi) and various practical advice and preparation conditions
-
2.
Personal insights and feedback regarding the course of the study parameters, reflecting on health status and cardiovascular risk (e.g. anthropometric parameters, blood pressure, ankle-brachial index, pulse variability, Harvard-Step test (heart fitness test), blood lipids)
-
3.
Defining personal goals depending on the individual course of study parameters
-
4.
Evaluation of the applicability of the MoKaRi concept into everyday life
The consultations will be conducted using the motivational interviewing technique [17]. Personal information about the participant's life circumstances as well as needs and possible tips for better implementation of the MoKaRi concepts will be discussed. Based on this information, individual solutions for improving compliance will be explored. The consultations will be conducted in a casual environment to improve the well-being of the study participants.
2.3.3. Further incentives
Over the entire study period of 20 weeks, the study participants will receive study-approved products as an incentive to implement the daily menu plans. The following foods will be provided: extra virgin olive oil (recommendation: 25 ml/d, i.e. 233 kcal/d; O'zeite Tradicional, bottles á 750-ml for four weeks, Fairfruit Portugal – Unipessoal, Beja, Portugal), mixed nuts (almonds, cashews, walnuts, and hazelnuts; 200 g per 14 days, i.e. 128 kcal/d, Alesto, Übach-Palenberg, Germany), fruit yoghurt enriched with fish oil (Omeghurt®, 125 g per serving, i.e. 110 kcal, 500 mg EPA + DHA; four portions of yoghurt per week; Herzgut Landmolkerei, Rudolstadt, Germany), chia seeds, and Gerstoni® barley flakes as required according to the menu plans.
The participants of the fish oil group will consume 10 ml of fish oil per day (i.e. 93 kcal/d, MarisOmega-3 1570 Liquid Lemon, Imperial-Oel-Import Handelsgesellschaft, Hamburg, Germany).
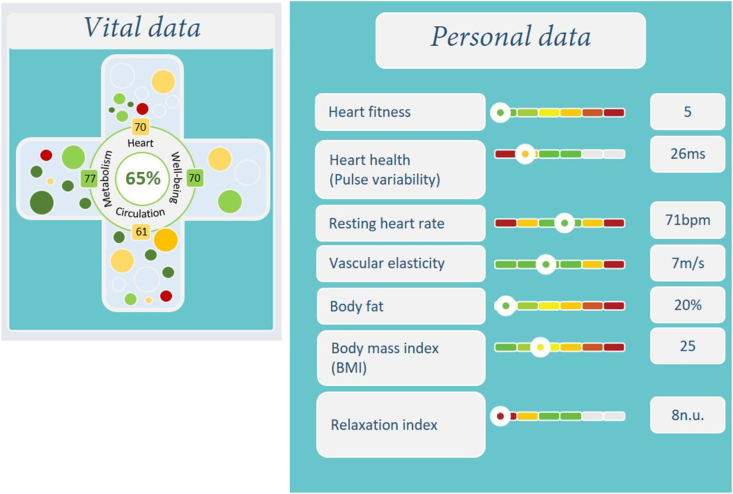
The cardiovascular risk profile of each participant will be visualized using a smartphone app developed by Preventicus® (Jena, Germany). With this tool, the participants of the MoKaRi study can track their risk factors (including blood pressure and blood lipids) which are displayed as icons in different colors. These icons change color depending on the individual values in comparison with reference values. They change from green (normal) over yellow (moderate deviation) to red (substantial deviation; Fig. 4).
Fig. 4.
Screenshots of the smartphone app to track risk factors.
Over the study period, the participants can participate in a weekly sports program. The offer will include a moderate circuit training with eight exercises in two intensities (1 h or 1.5 h). There will be four groups, with 15 participants per group.
Activities, such as organized cooking events and the sports program will serve the purpose of fostering group feeling and regular exchanges between the study participants and as incentives to improve compliance with the MoKaRi concept.
2.4. Study parameters
2.4.1. Primary outcome measures
The primary outcome measure of the MoKaRi study is LDL cholesterol [mmol/L].
2.4.2. Secondary outcome measures
-
1.
Fatty acid distributions in plasma lipids, plasma lipid fractions, erythrocyte lipids [% fatty acid methyl ester, FAME]
-
2.
Diabetes risk markers in fasting blood (insulin [units/ml], HbA1c [%], glucose [mmol/l])
-
3.
Clotting parameters (α-prothrombin time [s], fibrinogen [g/l]), and blood count
-
4.
Vitamins in serum/plasma: vitamins A [mmol/l], D [nmol/l], E [μmol/l], folic acid [μg/l], vitamins B1 and B6 [nmol/l], vitamin B2 [μg/l], vitamin B12 [pmol/l], and vitamin B12 status marker (holotranscobalamin [pmol/l], homocysteine [μmol/l]), biotin [ng/l], vitamin C [mg/l]
-
5.
Minerals and trace elements in serum/plasma: iron [μmol/l] and iron status marker (ferritin [μg/l], transferrin [g/l]), potassium [mmol/l], iodine [μg/l]
-
6.
Cardiovascular risk factors/markers in serum/plasma: blood lipids, including total cholesterol, HDL cholesterol, and triglycerides [mmol/L], high sensitive C-reactive protein [mg/dl], lipoprotein(a) [mmol/L], apolipoprotein A1 [g/l], apolipoprotein B [g/l], malondialdehyd-modified LDL cholesterol [mmol/l])
-
7.
Metabolomic profiling (186 metabolites; Biocrates Life Sciences, Innsbruck, Austria) and lipidome profiling (598 metabolites; Zora Biosciences, Espoo, Finland)
-
8.
24-hour urine sample: creatinine [mmol/24 h], albumin [mg/dl], methyl malonic acid [mg/24 h], sodium [mmol/24 h], magnesium [mmol/24 h], manganese [μg/l], selenium [μmol/24 h], zinc [μmol/24 h]
All study parameters will be analyzed according to standardized methods, mainly in cooperation with reference laboratories, e.g. the Institute of Clinical Chemistry and Laboratory Diagnostics, University Hospital Jena, Germany.
Anthropometric measurements will be undertaken by the observers using standardized methods and include height (m), weight (kg), and waist circumference (cm). The parameters will be measured by calibrated instruments (seca, Hamburg, Germany). Height will be measured with a stadiometer to the nearest 0.5 cm without shoes, head upright, and eyes looking straight ahead. Waist circumference (cm) will be measured using a finger width above the belly button. Body mass index (kg/m2) will be calculated. Arterial blood pressure will be measured with the subject in sitting position after least 5 min at rest. Blood pressure measurements will be taken twice at the relaxed right arm and the mean of the two values will be recorded. The ankle-brachial index will be calculated by dividing systolic blood pressure at the ankle by the systolic blood pressure value in the upper arm (brachium). A lower blood pressure in the leg than in the arm suggests the presence of arterial blockage, most likely due to atherosclerotic peripheral artery disease [18].
The primary and secondary outcome measures will be obtained regularly every two weeks over the intervention period (11 time points) and every ten weeks in the follow-up (two time points, Fig. 1). Blood samples will be collected via venipuncture after an overnight fast (a minimum of 12 h).
2.5. Statistical analysis
2.5.1. Primary outcome measure
The primary measure is the difference in plasma LDL cholesterol concentration during the implementation of the MoKaRi concept as estimated using a generalized linear model.
2.5.2. Primary and secondary outcome measures
Variables will be examined for normality using the Shapiro-Wilkes test. The Levene test will be used for variance homogeneity. Differences in the measured parameters within groups will be analyzed by Student's t-test or Wilcoxon signed-rank test. Furthermore, one-way ANOVA with repeated measurement will be conducted if data are normally distributed. Linear regression models will be used to assess the correlation between continuous variables. p-Values <0.05 (two-tailed) will be considered significant. The analyses will be performed with the statistical program R in the current version.
2.5.3. Power calculation, randomization, and enrollment
The single-center, randomized, phase II proof-of-concept study will examine the potential of a dietary concept to influence cardiovascular risk factors such as blood lipids, with LDL cholesterol, as the primary outcome. The two-arm parallel design will allow comparison between both groups (Fig. 1).
Jenkins et al. [19] conducted an intervention study with increased intake of plant sterols, vegetable proteins, and fiber. Due to the dietary intervention, the LDL cholesterol concentration was reduced from 3.80 mmol/L (run-in) to 3.01 mmol/L at the end of the treatment period. Based on these data, a group size of 27 subjects has 80% power to achieve a difference of 0.7 mmol/L (difference between μ1 = 3.8 mmol/L and μ2 = 3.1 mmol/L), assuming that the standard deviation is 0.9 (using a two-side t-test with 0.05 as significance level). Considering a dropout rate of 10%, we aim to enroll 30 participants per group which will be sufficient to evaluate two-sided standardized differences. The power calculation was performed using nQuery version 7.0 (Statistical Solutions, Boston, U.S.A.). Study participants who meet the eligibility criteria and gave their written informed consent will be randomly assigned to one of the two study groups and will receive either menu plans with 10 ml fish oil per day or menu plans without fish oil. Randomization will be conducted with the programming language R (package blockrand, block size 8). Blinding was not possible due to the study design.
3. Ethical considerations and dissemination
All participants will be informed about the aims and scope of the MoKaRi trial by the study leader and, if they agreed, they will provide written informed consent. All the study procedures will be carried out following the Declaration of Helsinki from 1989 [20]. The ethics committee of the Friedrich Schiller University Jena has approved the study protocol (number 4656-01/16).
The MoKaRi study was registered before launch at ClinicalTrials.gov (identifier NCT02637778).
A manuscript with the results of the primary outcomes will be published in a peer-reviewed journal. Separate manuscripts will be written on the secondary outcomes measures and published in peer-reviewed journals.
4. Discussion
4.1. Strengths of the MoKaRi trial
Because CVD is the leading cause of death in Europe, the development and sustainable implementation of validated strategies for prevention and support of therapy of CVD is of great importance. Dietary strategies are promising in this context [1,2,15].
Despite the widely known impact of diet on cardiovascular risk, its effects are often underestimated due to the lack of effective clinical trials that evaluate the potential of cardioprotective diets. The proposed MoKaRi study aims to counteract this insufficiency.
The MoKaRi concept is based on three approaches (Fig. 2) that address a wide variety of needs to modulate sustainable nutritional behavior. The three approaches have been developed to motivate participants to improve their nutritional behavior. Because motivating humans to change their behavior is not easy, the MoKaRi concept includes numerous approaches to achieving this ambitious aim.
The first approach includes the provision of suitable recipes, information, and tips on how to apply a cardioprotective diet in a daily routine. There are 140 menu plans with different recipes for each meal of the day considering the seasonal availability of foods and individual energy and nutrient requirements depending on age, gender, and physical activity. These adjustments make each menu plan unique. The variety of available menu plans ensures that each study participant can choose plans according to his/her food preferences. We hypothesize that these features of the MoKaRi concept will have a significant impact on the sustainable implementation of the cardioprotective diet.
The second approach of the MoKaRi strategy is regular and individual nutritional counseling. This method will also strengthen the implementation of the MoKaRi strategy during the trial. The design of the MoKaRi trial facilitates the evaluation of the impact of the counseling units by comparing the course of the study parameters between the intervention period (with menu plans and nutritional counseling) and the follow-up (with menu plans from the intervention period but no nutritional counseling).
Nutritional counseling consultations will be conducted every 14 days at each of the eleven time points by the study leader, who will use motivational interviewing as a technique to influence the study participants' behavior. In particular, the following principles of this technique will be used as part of the MoKaRi concept: (i) express empathy, (ii) client-centered accepting attitude, (iii) sincere interest in the participants and their life circumstances through active or reflective listening, and (iv) develop discrepancy using targeted (open) questions to help the participants to develop arguments for self-directed change. If the participants realize that the current behavior conflicts with critical goals (e.g. reduction of cardiovascular risk factors) this will strengthen the willingness to change. Confrontational behaviors will be avoided. Instead, different de-escalating strategies will be used, such as reflection a shift in focus, or a modification of the original strategy. The counseling sessions will aim to increase self-confidence and self-reliance in the participants to help them to achieve the goals of the study. This step is a vital issue of motivation which has generally proven to be necessary for the success of treatment [17]. Furthermore, the study leader will elicit and selectively reinforce self-motivating attitudes of the participants regarding problem insight, concerns, and willingness to change. At all times, the study leader will convey acceptance for and validation of the participant's thoughts and attitudes and will always communicate that the participants have agency and freedom of choice.Whereas the nutritional counseling process aims to stimulate intrinsic motivation, the incentive theory proposes that people are pulled towards behaviors that offer rewards to support desirable behaviors and avoid those that might lead to negative consequences [21]. Therefore, as a third approach, incentives, such as provision of chosen study foods, well-designed menu plans in the form of a cookbook, the possibility to participate in a sports program adapted to target group-specific needs and resources (cardioprotective circuit training) and, in particular, the regular feedback loops with a focus on the achieved changes on cardiovascular risk factors will be used to further improve compliance with the MoKaRi concept.
Specifically, the regular feedback loops (i) on changes of the study parameters with a focus on cardiovascular risk factors and (ii) on the applicability of the MoKaRi concept in day-by-day routine signalize our interest in individual life-circumstances and achievements. Moreover, the visualization of changes, e.g., on weight or cardiovascular risk factors will encourage study participants to continue.
Finally, the activities to encourage group feeling, e.g., cooking events, sports program, and the MoKaRi closing party will contribute to the adherence of the study participants to the recommendations. We hypothesize that the study participants motivate each other which will have a noticeable impact on sustainable behavioral changes (Fig. 2).
The design of the MoKaRi trial is unique due to the regular blood sampling which will be conducted every 14 days. This sampling will allow for a comprehensive monitoring of health status and cardiovascular risk factors during the implementation of the MoKaRi concept. Thus, valuable information about time-dependent changes of the study parameters will result. Moreover, this type of close-knit feedback on the achieved changes will encourage and motivate participants to continue. In this same manner, deviations from the concept (e.g. due to holidays, private parties or public holidays) will be discussed during the regularly talks.
The analysis of the fatty acid distribution in plasma lipids, plasma lipid fractions, and erythrocyte lipids as markers for the intake of dietary fat as well as the regular analysis of vitamins and minerals over the trial course are further markers for compliance with the MoKaRi concept. The possibility to observe compliance is a further essential strength of the MoKaRi study.
4.2. Limitations of the MoKaRi trial
Despite the numerous strengths of the study design, there are also several limitations, such as:
-
(i)
there will be no control group without menu plans available,
-
(ii)
variations (e.g., by sorts, soils, preparation, and feeding conditions) between calculated nutrient profiles available from the nutrition tables and the nutrient composition of the foods that are really consumed cannot be ruled out,
-
(iii)
the MoKaRi study is designed to evaluate the effectiveness and potential of the developed MoKaRi concept which comprises of different modules (menu plans, nutritional counseling, incentives, moderate circle training with eight exercise in two levels/1–1.5 h per week). The evaluation of the effectiveness of the individual modules is not possible,
-
(iv)
the individual compliance with dietary strategies is the most significant limitation and this uncertainty factor cannot be captured in total,
-
(v)
potential effects of the microbiome which has a significant impact on physiological markers in response to dietary modifications will not be considered,
-
(vi)
it is not clear whether the implementation of the MoKaRi concept will be sustainable, because we are not able to estimate sustainability without care (i.e., after the follow-up) and the questions remain what will happen when motivational strategies and incentives are stopped.
The MoKaRi trial will be conducted with participants at elevated cardiovascular risk. Therefore, sensibility for the topic and intrinsic motivation for improving cardiovascular risk factors, in particular, is relatively high. On the other hand, motivating healthy young individuals to consume a healthy diet for reducing their prospective cardiovascular risk at a later, undefined point in time remains challenging.
4.3. Options and perspective
For optimizing the study design of dietary interventions, the preparation and supply of study foods may have a high impact on compliance. Therefore, the allocation of study foods in an associated study restaurant or by a catering service could be a promising approach for improving compliance, particularly for healthy people. Therefore, the study design of diet-related studies could be improved and thus, valuable data will result that gives a reliable insight into the impact and potential of dietary interventions on health status and disease risk.
In addition, motivational strategies for stimulating intrinsic and extrinsic motivations as well as using client-centric methods of negotiation, such as motivational interviewing are promising concerning the improvement of nutritional behavior, e.g. by the implementation of the MoKaRi concept.
5. Conclusions
The MoKaRi trial may provide essential insights into the relationship between diet and health as well as disease status, particularly cardiovascular risk. The results will interest the general population as well as patients with increased cardiovascular risk because the implementation of the MoKaRi concept will be possible for everyone. The dissemination of information and practical tools, ready-to-use in day-by-day routine (e.g., menu plans) which are evaluated by the MoKaRi trial, may enable the scaling up of the transfer of knowledge to the general population. Therefore, the validated MoKaRi concept may contribute to the prevention and the support of therapy of CVD.
Funding
This work was supported by the German Federal Ministry of Research and Education (Competence Cluster for Nutrition and Cardiovascular Health (nutriCARD) Halle-Jena-Leipzig, grant number 01EA1411C).
Contributions
CD is responsible for the conception and trial design and writing the manuscript. SL was involved in the conception of the trial design and drafting the manuscript. PS provided statistical expertise. PC was responsible for linguistic finalization. CD, SL, and PC are responsible for critical revision of the article and contributing intellectual content.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Lorkowski reports personal fees from Akcea Therapeutics, amedes, AMGEN, Berlin-Chemie, Boehringer Ingelheim Pharma, Danone, Daiichi Sankyo, Lilly, MSD Sharp & Dohme, Novo Nordisk Pharma, Roche Pharma, Sanofi-Aventis, Swedish Orphan Biovitrum, Synlab, Unilever, and Upfield, and non-financial support from Preventicus outside the submitted work.
Acknowledgements
The authors thank Herzgut Landmolkerei (Rudolstadt, Germany) for their intention to provide fruit yoghurts enriched with long-chain n-3 PUFA (Omeghurt®) for free as part of the MoKaRi trial. We also thank Dieckmann Cereals (Rinteln, Germany) for their intention to provide barley flakes (Gerstoni®) at reduced costs to support the MoKaRi trial. The further needed study products will be bought at commercial prices. We will also thank Preventicus® (Jena, Germany) for providing the app for visualization of the cardiovascular risk profile for free.
Contributor Information
Christine Dawczynski, Email: Christine.Dawczynski@uni-jena.de.
Stefan Lorkowski, Email: Stefan.Lorkowski@uni-jena.de.
References
- 1.GBD 2017 Causes of Death Collaborators, Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendis S., Puska P., Norrving B., World Health Organization . Shanthi Mendis et al. World Health Organization; 2011. Global Atlas on Cardiovascular Disease Prevention and Control/https://apps.who.int/iris/handle/10665/44701 World Heart Federation et al. accessed. [Google Scholar]
- 3.Alberico L.C., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., Hoes A.W., Jennings C.S., Landmesser U., Pedersen T.R., Reiner Ž., Riccardi G., Taskinen M.R., Tokgozoglu L., Verschuren W.M.M., Vlachopoulos C., Wood D.A., Zamorano J.L., Cooney M.T. ESC scientific document group 2016, ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 2016;37:2999–3058. doi: 10.1016/j.rec.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 4.DeSalvo K.B., Olson R., Casavale K.O. Dietary Guidelines Am. JAMA. 2016;315:457–458. doi: 10.1001/jama.2015.18396. https://doi:10.1001/jama.2015.18396 [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 diet collaborators, health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith S.C., Jackson R., Pearson T.A., Fuster V., Yusuf S., Faergeman O., Wood D.A., Alderman M., Horgan J., Home P., Hunn M., Grundy S.M. Principles for national and regional guidelines on cardiovascular disease prevention. A scientific statement from the World Heart and Stroke Forum. Circulation. 2004;109:3112–3121. doi: 10.1161/01.CIR.0000133427.35111.67. [DOI] [PubMed] [Google Scholar]
- 7.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O'Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 8.MRI Nationale Verzehrsstudie II. Max-Rubner-Institut Bundesforschungsinstitut für Ernährung und Lebensmittel. 2008. https://www.bmel.de/SharedDocs/Downloads/Ernaehrung/NVS_ErgebnisberichtTeil2.pdf?__blob=publicationFile/.2008 accessed.
- 9.D-A-CH . aktualisierte Ausgabe; Bonn: 2018. Referenzwerte für die Nährstoffzufuhr der DGE, ÖGE, SGE/SVE, 2. Auflage, 4. [Google Scholar]
- 10.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Scientific Opinion on the substantiation of health claims related to EPA, DHA, DPA and maintenance of normal blood pressure (ID 502), maintenance of normal HDL-cholesterol concentrations (ID 515), maintenance of normal (fasting) blood concentrations of triglycerides (ID 517), maintenance of normal LDL-cholesterol concentrations (ID 528, 698) and maintenance of joints (ID 503, 505, 507, 511, 518, 524, 526, 535, 537) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009;7:1263. doi: 10.2903/j.efsa.2009.1263. [DOI] [Google Scholar]
- 11.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), docosapentaenoic acid (DPA) and maintenance of normal cardiac function (ID 504, 506, 516, 527, 538, 703, 1128, 1317, 1324, 1325), maintenance of normal blood glucose concentrations (ID 566), maintenance of normal blood pressure (ID 506, 516, 703, 1317, 1324), maintenance of normal blood HDL-cholesterol concentrations (ID 506), maintenance of normal (fasting) blood concentrations of triglycerides (ID 506, 527, 538, 1317, 1324, 1325), maintenance of normal blood LDL-cholesterol concentrations (ID 527, 538, 1317, 1325, 4689), protection of the skin from photo-oxidative (UV-induced) damage (ID 530), improved absorption of EPA and DHA (ID 522, 523), contribution to the normal function of the immune system by decreasing the levels of eicosanoids, arachidonic acid-derived mediators and pro-inflammatory cytokines (ID 520, 2914), and “immunomodulating agent” (4690) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1796. doi: 10.2903/j.efsa.2010.1796. [DOI] [Google Scholar]
- 12.Dawczynski C., Dittrich M., Neumann T., Goetze K., Oelzner P., Völker S., Schaible A.M., Troisi F., Thomas L., Pace S., Koeberle A., Werz O., Schlattmann P., Lorkoswki S., Jahreis G. Docosahexaenoic acid in the treatment of rheumatoid arthritis: a double-Blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018;37:494–504. doi: 10.1016/j.clnu.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Lim S.S., Vos T., Flaxman A.D. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heron M. Deaths: leading causes for 2017. Natl. Vital Stat. Rep. 2019;68:6. [PubMed] [Google Scholar]
- 15.Rees K., Dyakova M., Ward K., Thorogood M., Brunner E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2013;3 doi: 10.1002/14651858.CD002128.pub3. CD002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Society for the Study of Fatty Acids and Lipids (ISSFAL). Report of the Sub-committee on: Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. ISSFAL; Brighton: 2004. https://www.issfal.org/assets/issfal%2003%20pufaintakereccomdfinalreport.pdf [Google Scholar]
- 17.Bandura A. W H Freeman/Times Books/Henry Holt & Co; 1997. Self-efficacy: the Exercise of Control. [Google Scholar]
- 18.Al-Qaisi M., Nott D.M., King D.H., Kaddoura S. Ankle brachial pressure index (ABPI): an update for practitioners. Vasc. Health Risk Manag. 2009;5:833–841. doi: 10.2147/VHRM.S6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins D.J., Kendall C.W., Faulkner D., Vidgen E., Trautwein E.A., Parker T.L., Marchie A., Koumbridis G., Lapsley K.G., Josse R.G., Leiter L.A., Connelly P.W. A dietary portfolio approach to cholesterol reduction: combined effects of plant sterols, vegetable proteins, and viscous fibers in hypercholesterolemia. Metabolism. 2002;51:1596–1604. doi: 10.1053/meta.2002.35578. [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki: ethical principles for medical Research involving human subjects. J. Am. Med. Assoc. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 21.Cherry K. The incentive theory of motivation - are actions B a desire for rewards? 2018. https://www.verywellmind.com/the-incentive-theory-of-motivation-2795382 Updated November 20. 2 March 2019.