Summary
Iron deposition is one of the key factors in the etiology of Parkinson's disease (PD). Iron-free-apoferritin has the ability to store iron by combining with a ferric hydroxide-phosphate compound to form ferritin. In this study, we investigated the role of apoferritin in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice models and elucidated the possible underlying mechanisms. Results showed that apoferritin remarkably improved MPTP-induced motor deficits by rescuing dopaminergic neurodegeneration in the substantia nigra. Apoferritin inhibited MPTP-induced iron aggregation by down-regulating iron importer divalent metal transporter 1 (DMT1). Meanwhile, we also showed that apoferritin prevented MPTP-induced ferroptosis effectively by inhibiting the up-regulation of long-chain acyl-CoA synthetase 4 (ACSL4) and the down-regulation of ferroptosis suppressor protein 1 (FSP1). These results indicate that apoferritin exerts a neuroprotective effect against MPTP by inhibiting iron aggregation and modulating ferroptosis. This provides a promising therapeutic target for the treatment of PD.
Subject Areas: Animal Physiology, Neuroscience, Cellular Neuroscience
Graphical abstract
Highlights
-
•
Apoferritin improved MPTP-induced motor deficits.
-
•
Apoferritin rescued dopaminergic neurodegeneration in the SN of MPTP-treated mice.
-
•
Apoferritin inhibited MPTP-induced iron aggregation.
-
•
Apoferritin prevented MPTP-induced ferroptosis by regulation of ACSL4 and FSP1.
Animal Physiology ; Neuroscience ; Cellular Neuroscience ;
Introduction
Parkinson's disease (PD) is a neurodegenerative disease that is common in middle-aged and elderly people. The pathological feature of PD is the damage of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc), resulting in the progressive loss of motor functions. However, the etiology and pathogenesis are currently not fully understood. Increasing evidence has confirmed that iron content in the substantia nigra (SN) of patients with PD increased significantly compared with the control (Gerlach et al., 2006; Chen et al., 2019; Bergsland et al., 2019; Hirsch, 2009; Dexter et al., 1989; Hopes et al., 2016). Excessive labile iron in the SN resulted in oxidative stress and increased the production of reactive oxygen species (ROS) by the Fenton reaction (Wypijewska et al., 2010; Dixon and Stockwell, 2014; Weinreb et al., 2013). Iron is also an important participant in ferroptosis, which is a newly discovered iron-dependent cell death and has been found in patients with PD (Mahoney-Sánchez et al., 2021; Bellinger et al., 2011; Vallerga et al., 2020). It has also been reported that ferroptosis is related to the pathogenic changes observed in PD models, including elevated iron deposit in the SN, consumption of glutathione (GSH), lipid peroxidation, increased production of ROS, and oxidation of DA (Wypijewska et al., 2010; Dixon et al., 2012). Therefore, it is important to find therapeutic target to prevent iron deposition and iron-dependent ferroptosis in PD.
Studies have confirmed that the cellular mechanism leading to iron accumulation in the SN of PD might be related to abnormal brain iron metabolism (Hentze et al., 2010; Wang and Pantopoulos, 2011; Zecca et al., 2001, 2004). Iron homeostasis is maintained by interactions between iron transporters and iron storage protein. Impaired iron transport or altered iron storage could disrupt the balance of iron homeostasis. Transferrin (Tf)-transferrin receptor (TfR) and the divalent metal transporter 1 (DMT1)-mediated non-transferrin binding iron (NTBI) are two major pathways responsible for iron uptake (Moos and Morgan, 2000; Kielmanowicz et al., 2005). A large number of experiments have confirmed that the up-regulation of iron importer DMT1 might be involved in nigral iron accumulation and the degeneration of DA neurons in PD (Salazar et al., 2008; Saadat et al., 2015; Hirsch, 2009). Iron storage protein ferritin was known to play an important role in maintaining iron homeostasis. Ferritin is composed of 24 subunits of two types: H-ferritin (FTH) and L-ferritin (FTL) (Arosio et al., 1978; Harrison and Arosio, 1996). FTH has ferrous oxidase activity and can oxidize ferrous iron into ferric iron. FTL has a nucleation site to promote the formation of iron core and complete iron storage. Evidence has shown that the load of ferritin is significantly increased in PD models (Kaur et al., 2007; Goto et al., 1996). It has been reported that the extracellular ferritin interacts with the cell through the specific binding of FTH to transferrin receptor 1 (TfR1) (Fan et al., 2012; Daniels et al., 2006). The discovery of extracellular ferritin indicated that ferritin might be an important factor in the regulation of brain iron homeostasis.
Apoferritin is an iron-free form of ferritin, which has been used as a non-toxic nanomaterial in clinical treatments such as drug delivery, in vivo imaging, and photothermal therapy (Truffi et al., 2016; Srinivasan et al., 2014; Domínguez-Vera et al., 2010). Owing to the iron-binding function, apoferritin might play a role in chelating excess iron to protect DA neurons against PD. In this study, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was used to induce PD mice models. We explored the effects of apoferritin on MPTP-induced motor deficits and elucidated the possible underlying mechanisms. Our results showed that apoferritin could remarkably improve the MPTP-induced motor deficits and inhibit nigral iron aggregation and ferroptosis. This provides new discoveries and possibilities for the prevention and treatment of PD.
Results
Apoferritin pretreatment rescued the weight loss and improved motor deficits induced by MPTP
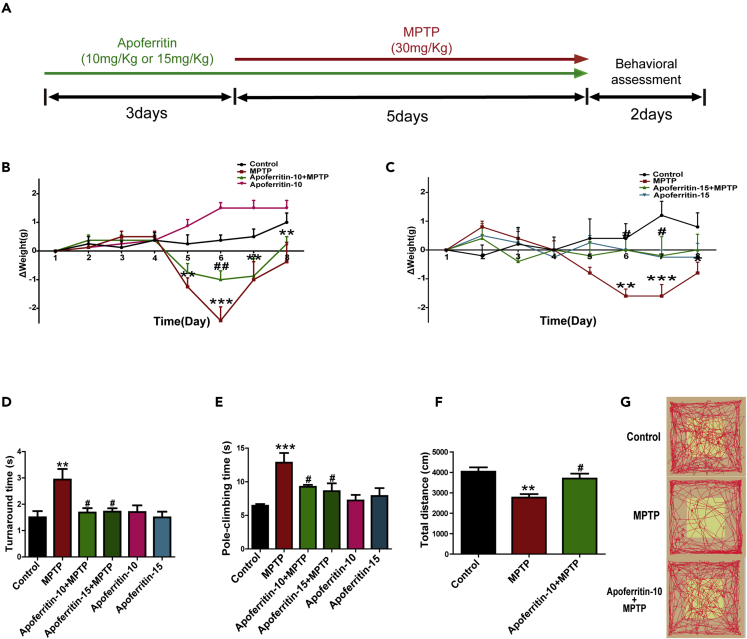
In this study, we investigated the potential therapeutic effects of apoferritin in MPTP-induced PD mice model. The experimental paradigm is shown in Figure 1A. Results showed that the body weight of MPTP-treated mice decreased significantly compared with the control. And pretreatment with 10 or 15 mg/kg apoferritin had a significant recovery on the weight loss induced by MPTP. There was no difference between the apoferritin group and the control group (Figures 1B and 1C).
Figure 1.
Apoferritin increased the body weight and improved motor deficits of MPTP-induced mice
(A) Experimental scheme of MPTP model.
(B and C) Apoferritin inhibited MPTP-induced weight loss in mice. ΔWeight means the weight change of mice.
(D and E) Injection of MPTP (intraperitoneal) induced a significant increase of pole-climbing time and turnaround time in pole-climbing test, which was inhibited by apoferritin.
(F and G) In the open-field test, the decrease in the total movement distance in 10 min induced by MPTP was also improved by apoferritin.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared with the control; #p < 0.05, ##p < 0.01, compared with the MPTP. Data were expressed as mean ± SEM (n = 5–9 in each group).
Then, we tested the effect of apoferritin on motor ability of MPTP-treated mice using the open-field test and pole-climbing test. Our results showed that MPTP mice exhibited a significantly reduced capacity for pole-climbing, including a significantly longer climb time and longer time to turn their heads. Pretreatment with 10 or 15 mg/kg apoferritin improved the pole-climbing ability (Figures 1D and 1E). There is no significant difference between 10 mg/kg apoferritin and 15 mg/kg apoferritin pretreatment. Therefore, 10 mg/kg apoferritin pretreatment was used in the following experiments. Results of open-field test showed that compared with the healthy control group, the total distance in MPTP-treated mice decreased significantly, whereas the total distance in the mice with apoferritin pretreatment was significantly increased compared with the MPTP group (Figures 1F and 1G).
Apoferritin effectively inhibited MPTP-induced degeneration of DA neurons
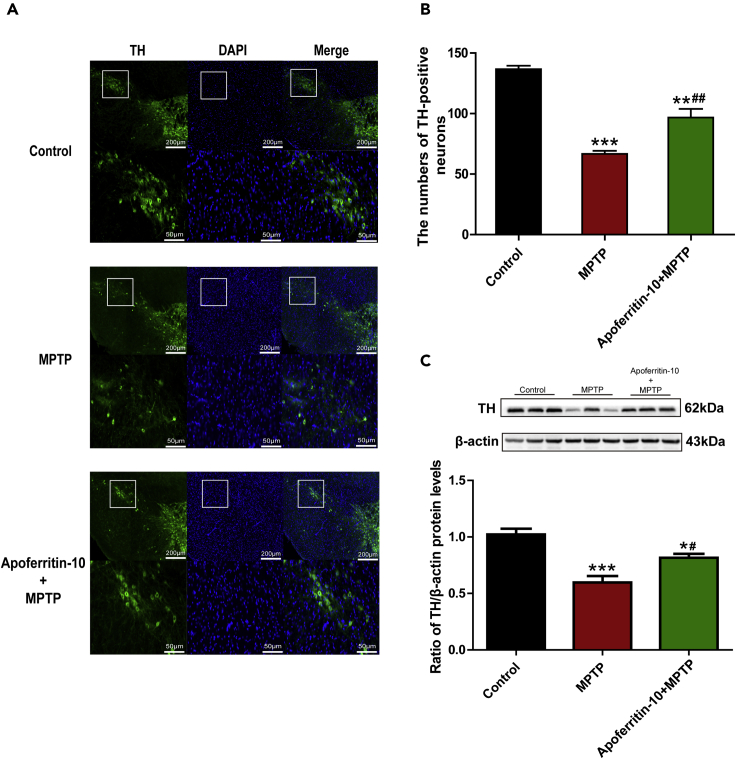
To further verify whether apoferritin pretreatment has a neuroprotective effect on DA neurons in the SN of MPTP-induced PD mice, tyrosine hydroxylase (TH) staining and western blots were used to evaluate the survival of DA neurons and the level of TH protein in the SN. As shown in Figures 2A and 2B, TH-positive neurons in the SN of MPTP-treated mice were significantly decreased compared with the healthy control. Conversely, pretreatment with apoferritin could effectively inhibit MPTP-induced loss of TH-positive neurons. Furthermore, we also detected the protein level of TH in the SN of mice in different groups. Results showed that the protein level of TH in the SN of MPTP-treated mice was significantly decreased compared with the control. Consistent with the effect on rescuing the loss of TH-positive neurons caused by MPTP, pretreatment with apoferritin could significantly attenuate the decrease in TH protein level in the SN of MPTP-treated mice (Figure 2C). Thus, these results suggested that apoferritin could protect DA neurons against MPTP neurotoxicity.
Figure 2.
Apoferritin ameliorated MPTP-induced loss of dopaminergic neurons in the SN
(A) The number of TH-positive neurons in the SNpc were significantly reduced after MPTP treatment. Apoferritin ameliorated the loss of TH-positive neurons caused by MPTP. The scale bar represents 200 μm (top) and 50 μm (below).
(B) Statistical analysis of TH-positive neurons as shown in (A).
(C) The expression of TH was reduced in the SN in MPTP-treated mice. Apoferritin suppressed MPTP-induced decrease in the expression of TH. Data were presented as percentage of control.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared with the control. #p < 0.05, ##p < 0.01, compared with the MPTP. Data were expressed as mean ± SEM (n = 8, 9 in each group).
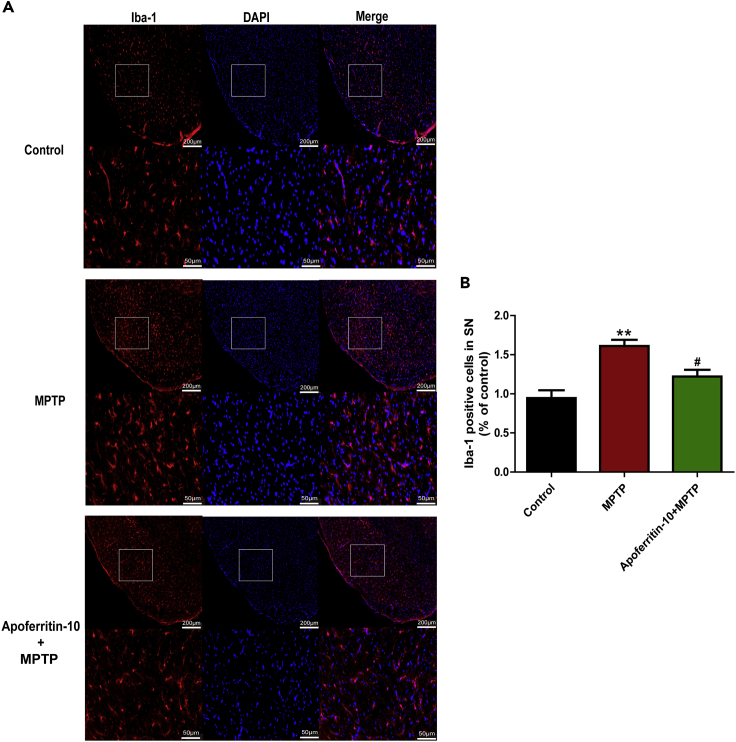
Apoferritin decreased the number of Iba-1-positive microglia in the SN of MPTP-induced mice
The expression of Iba-1 is a hallmark of microglia in the CNS. In this study, immunofluorescence was used to stain Iba-1-positive microglia. Results showed that MPTP treatment induced a robust increase in the number of Iba-1-positive microglia in the SN as detected by Iba-1 staining, compared with the control. The number of Iba-1-positive microglia markedly decreased after 10 mg/kg apoferritin pretreatment (Figures 3A and 3B). This suggested that the effect of apoferritin on the number of Iba-1-positive microglia in the SN might also contribute to its neuroprotection against MPTP.
Figure 3.
Apoferritin suppressed MPTP-induced increase of Iba1-positive microglia in the SN
(A) MPTP treatment caused a significant increase in the number of Iba1-positive microglia in the SN, which was suppressed by apoferritin. Scale bars, 200 μm (top) and 50 μm (below).
(B) Statistical analysis.
∗∗p < 0.01, compared with the control. #p < 0.05, compared with the MPTP. Data were expressed as mean ± SEM (n = 6 in each group).
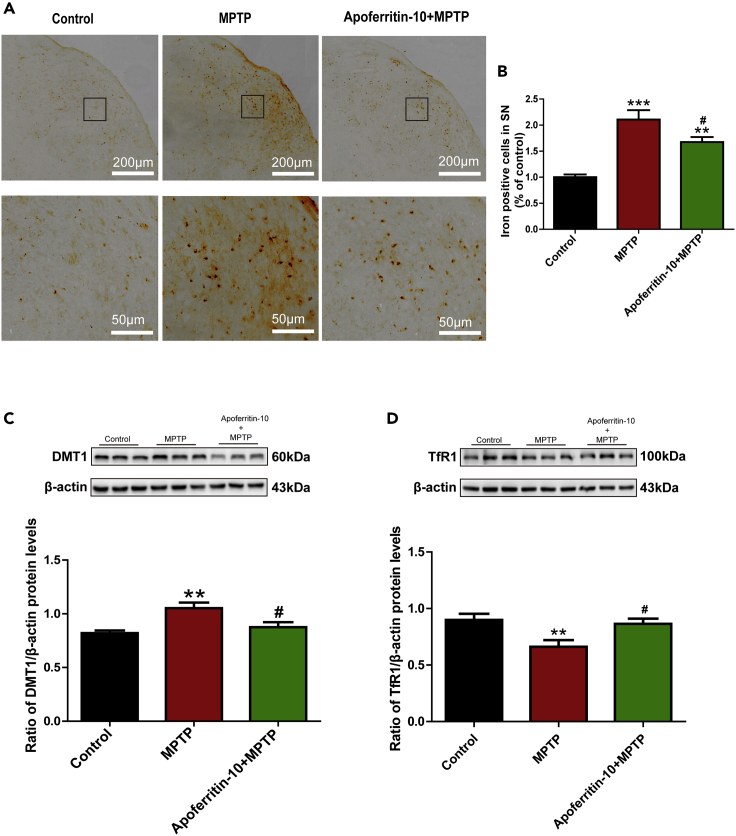
Apoferritin decreased iron content in the SN and regulated iron transporters
Increased iron content in the SN of PD has been indicated in patients with PD and PD animal models. Apoferritin is an iron-free ferritin and might exert its neuroprotective effect in PD by chelating excess iron. Therefore, in this study, we investigated the effect of apoferritin on the number of iron-positive cells and the expression of iron transporters in MPTP-induced PD models. Results showed that the number of iron-positive cells in the SN as measured by iron staining was higher in MPTP-treated mice compared with the control. On the contrary, pretreatment with apoferritin suppressed MPTP-induced increase in the number of iron-positive cells in the SN of MPTP-treated mice (Figures 4A and 4B). This indicated that the protection of apoferritin might be related to reducing iron accumulation in the SN of MPTP-induced PD mice.
Figure 4.
Apoferritin suppressed MPTP-induced iron accumulation in the SN via regulating iron-transport proteins
(A) Perls' iron staining revealed that MPTP treatment resulted in the increase in iron-positive cells in the SN, which could be suppressed by apoferritin. Scale bars, 200 μm (top) and 50 μm (below).
(B) Statistical analysis of iron positive cells as shown in (A).
(C) DMT1 was up-regulated in the SN of MPTP-induced mice. Apoferritin suppressed MPTP-induced up-regulation of DMT1.
(D) The expression of TfR1 decreased in the SN of MPTP-induced mice. Apoferritin suppressed MPTP-induced decrease in the expression of TfR1.
∗∗p < 0.01, ∗∗∗p < 0.001, compared with the control. #p < 0.05, compared with the MPTP. Data were expressed as mean ± SEM (n = 5–7 in each group).
Consistently, altered expressions of iron-related proteins have been reported to be involved in the nigral iron accumulation in PD. We further investigated whether the effect of apoferritin on iron levels was related to the regulation on the expression of iron-related proteins. Therefore, the expressions of iron importer DMT1 and TfR1 were detected by western blot analysis. Results showed that MPTP-induced PD mice expressed higher levels of DMT1 in the SN than the control mice, and pretreatment with apoferritin drastically inhibited the up-regulation of DMT1 induced by MPTP (Figure 4C). However, the expression of TfR1 decreased after MPTP treatment, and apoferritin restored the expression of TfR1 in MPTP-treated mice (Figure 4D). Thus, the effect of apoferritin on suppression of nigral iron accumulation in MPTP-induced PD mice might be associated with regulating the abnormal expression of iron import protein DMT1.
Apoferritin protected DA neurons against MPTP by inhibiting ferroptosis
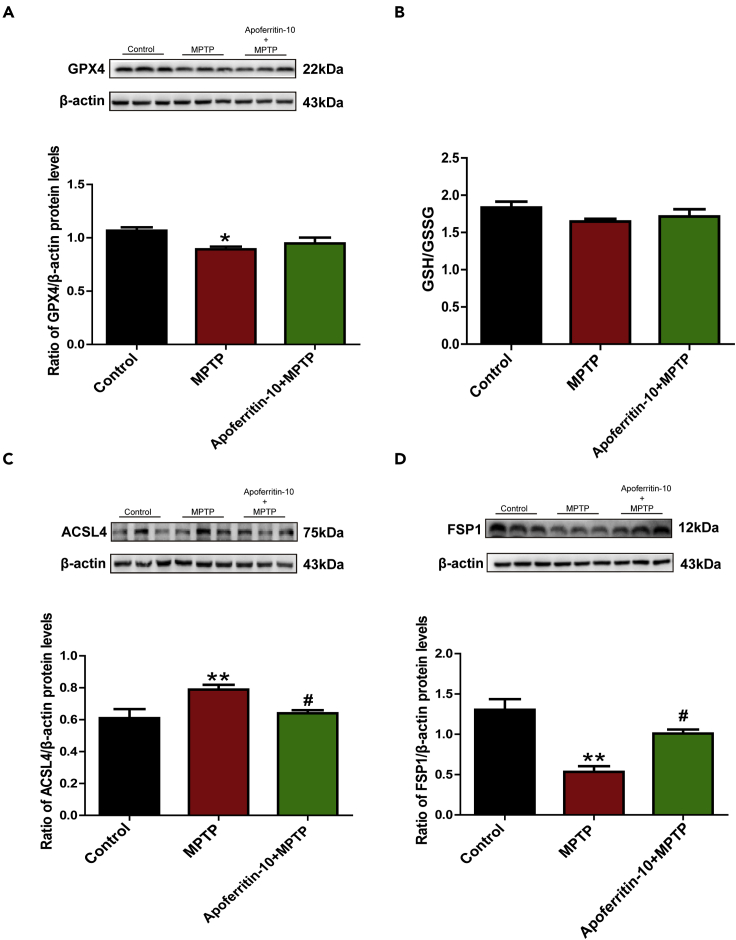
Ferroptosis is an iron-dependent cell death. Studies have confirmed that ferroptosis is involved in the pathology of PD. To further investigate whether apoferritin could inhibit ferroptosis, we conducted the experiments to detect the expressions of proteins related to ferroptosis including glutathione peroxidase 4 (GPX4), long-chain acyl-CoA synthetase 4 (ACSL4), and ferroptosis suppressor protein 1 (FSP1). Results showed that the expression of GPX4 decreased significantly in the SN of MPTP-treated PD mice. However, pretreatment with apoferritin did not restore MPTP-induced decrease in the expression of GPX4 as shown in the Figure 5A. Further study showed that MPTP and pretreatment with apoferritin did not affect the levels of GSH/oxidized glutathione (GSH/GSSG) (Figure 5B). In addition, results showed that the expression of ACSL4 increased, whereas the expression of FSP1 decreased significantly in the SN of MPTP-treated PD mice. Apoferritin pretreatment suppressed MPTP-induced increase in the expression of ACSL4 and decrease in the expression of FSP1 (Figures 5C and 5D). These results indicated that the neuroprotective effect of apoferritin might be associated with the regulation of ferroptosis by affecting the expression of ACSL4 and FSP1.
Figure 5.
Apoferritin suppressed MPTP-induced ferroptosis in the SN by regulating ferroptosis associated protein
(A) Apoferritin did not restore MPTP-induced decrease in the expression of GPX4.
(B) MPTP and pretreatment with apoferritin did not affect the levels of GSH/GSSG Figures 5C and 5D.
(C) Apoferritin suppressed MPTP-induced up-regulation of ACSL4.
(D) The expression of FSP1 decreased in the SN of MPTP-induced mice, which could be suppressed by apoferritin.
∗p < 0.05, ∗∗p < 0.01, compared with the control. #p < 0.05, compared with the MPTP. Data were expressed as mean ± SEM (n = 5–8 in each group).
Discussion
Evidence suggests that iron deposition in the SN is involved in the pathogenesis of PD (Hirsch et al., 1991; Dexter et al., 1987; Riederer et al., 1989). Treatment strategies that inhibit iron accumulation and iron-dependent cell damage might halt the progression of PD. Apoferritin is an iron-free ferritin that has the function of storing iron. Here, we investigated the neuroprotective effect and the possible underlying mechanisms of apoferritin against MPTP toxicity. We presented evidence supporting that apoferritin can effectively improve motor deficits and protect against the degeneration of DA neurons of MPTP-induced PD mice. More importantly, we found that suppression of nigral iron accumulation and regulation of iron transporter DMT1 might contribute to the neuroprotection of apoferritin against MPTP toxicity. Furthermore, we showed here that apoferritin could inhibit MPTP-induced ferroptosis by up-regulating FSP1 and down-regulating ACSL4.
MPTP is a by-product in the synthesis of 1-methyl-4-phenyl-4-propionoxypiperidine (MPPP) (Ziering and Lee, 1947). MPTP itself is not toxic, but after entering the brain, it is converted into toxic 1-methyl-4-phenylpyridiniumion (MPP+) under the action of monoamine oxidase B. MPP+ is specifically transported to dopaminergic neurons of the SN by DA transporter and then induces the occurrence of PD by inhibiting mitochondrial complex I (Langston et al., 1984; Chiba et al., 1984; Castagnoli et al., 1985; Lee et al., 2011). Our results demonstrated that apoferritin can prevent MPTP-induced weight loss and improve motor deficits. And there was no significant difference in the protection against MPTP-induced motor deficits between intragastric gavage and intravenous injection of apoferritin (Figure S1). In addition, apoferritin protected against MPTP-induced degeneration of DA neurons, including restoring TH-positive neurons and increasing the expression of TH protein of the SN in MPTP-induced PD mice. Further results showed that apoferritin decreased the numbers of Iba-1-positive microglia in the SN of MPTP-induced mice. These results indicate that apoferritin has a protective effect against MPTP-induced neurotoxicity. In vivo and in vitro results also showed that the expression of L-ferritin in the SN of mice and primary cultured ventral mesencephalon (VM) neurons increased significantly after apoferritin treatment compared with the control. This indicated that apoferritin might exert its effect by entering neurons and capturing intracellular iron (Figure S2).
Studies have confirmed that MPTP induced iron deposition in the SN of mice (Shi et al., 2019; Li et al., 2020; Goto et al., 1996; Temlett et al., 1994). In this study, we also observed the increased iron-positive cells in the SN of MPTP-induced PD mice. And we further proved that apoferritin pretreatment could inhibit MPTP-induced increase of iron-positive cells. DMT1 and TfR1 are two major iron import proteins, responsible for non-transferrin-bound iron import and transferrin-bound iron import, respectively. Our previous study has shown that MPTP induced iron accumulation and up-regulation of DMT1, whereas iron chelator deferoxamine (DFO) abolished these effects (Zhang et al., 2009). In this study, our results showed that up-regulation of DMT1 in the MPTP-induced PD model was inhibited by apoferritin. This indicated that the iron-suppressing effect of apoferritin might be achieved through the suppression of DMT1, but not TfR1.
Up-regulation of DMT1 might increase iron uptake by neurons, which in turn leads to lipid peroxidation and ROS production by the Fenton reaction (Wypijewska et al., 2010; Aguirre et al., 2012; Dixon and Stockwell, 2014; De Farias et al., 2016). Ferroptosis is an iron-dependent and regulated process of cell death, which is characterized by the accumulation of ROS and lipid peroxidation products. It has been reported that ferroptosis was involved in the neuropathology of MPTP neurotoxicity (Do Van et al., 2016). Previous studies have shown that ferroptosis inhibitors ferrostatin-1 (Fer-1) and liproxstatin-1 can effectively prevent the loss of DA neurons in the SN of PD (Do Van et al., 2016; Dixon et al., 2012), whereas the iron chelator DFO can effectively inhibit the occurrence of ferroptosis (Cheng et al., 2019). Therefore, apoferritin might protect DA neurons against MPTP by modulating iron homeostasis and iron-dependent ferroptosis.
Glutathione peroxidase 4 (GPX4) is one of the regulator of ferroptosis (Yang et al., 2014) and is essential for maintaining the redox balance of cells (Matsushita et al., 2015). In this study, we investigated the effect of apoferritin on the expression of GPX4 in MPTP-induced PD mice. Results showed that the expression of GPX4 decreased compared with the control. However, apoferritin did not restore MPTP-induced decrease in the expression of GPX4. To further confirm the possible mechanisms underlying the effect of apoferritin on ferroptosis in MPTP-induced PD mice model, we further detected other proteins related to ferroptosis. Recent studies showed that the balance of redox states was regulated by FSP1-NAD (P) H-CoQ10 axis (Bersuker et al., 2019; Doll et al., 2019), which is in parallel with the typical GPX4 pathway to suppress lipid peroxidation and ferroptosis (Doll et al., 2019). As a novel CoQ10 plasma membrane oxidoreductase, FSP1 protects cells from ferroptosis by reduced form of CoQ10, which is a potent antioxidant to prevent lipid peroxidation. Our results showed that MPTP induced decreased FSP1 in the SN, which can be inhibited by apoferritin pretreatment. This indicated that decreased FSP1 might cause CoQ10 to be in an oxidized state and further aggravated lipid peroxidation, leading to the occurrence of ferroptosis and ultimately the degeneration of DA neurons in PD. However, apoferritin can effectively inhibit ferroptosis by up-regulating FSP1, thereby inhibiting the production of lipid peroxidation to exert its neuroprotective effects against MPTP.
In addition, we also detected the expression of ACSL4, a member of the long-chain family of acyl-CoA synthetase proteins, which have recently been shown to play an important role in ferroptosis (Doll et al., 2017). ACSL4 catalyzes arachidonic acid and adrenaline to produce coenzyme A derivatives, which is a process associated with ferroptosis. Free iron or iron-containing lipoxygenase enzymes are responsible for oxidizing membrane polyunsaturated fatty acids (PUFAs), potentially leading to the formation of lipid ROS. It has been reported that ACSL4 was required for the activation or incorporation of PUFAs into membrane phospholipids, which is important in ferroptosis. Inhibiting the expression of ACSL4 prevents lipid peroxidation in ferroptosis and related cell death (Doll et al., 2017). Our results showed that MPTP induced up-regulation of ACSL4, which might aggravate lipid peroxidation, whereas apoferritin can effectively inhibit ferroptosis by inhibiting the expression of ACSL4. The results together with previous studies suggest that ferroptosis is probably an important cell death pathway of DA neurons in MPTP-induced PD mice models, and apoferritin might be a potential drug candidate to pharmacologically modulate the ferroptosis via regulation of ACSL4 and FSP1.
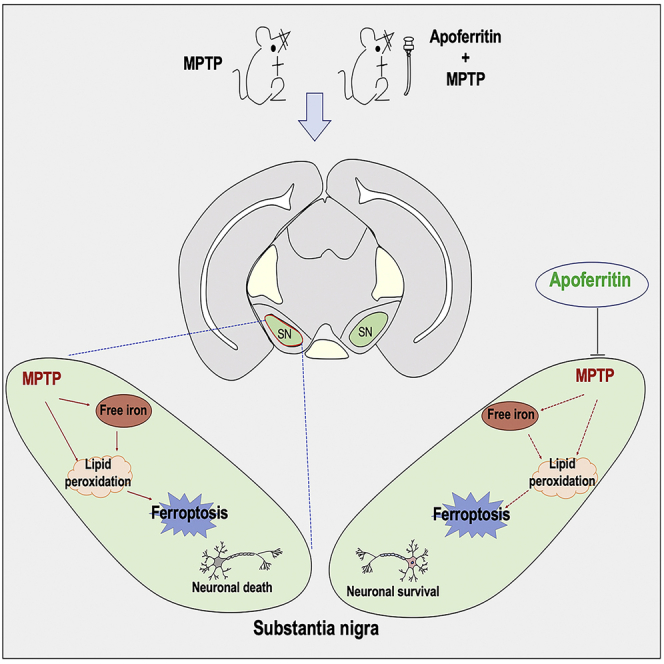
In conclusion, our study demonstrates the effect of apoferritin in MPTP-induced PD animal models and elucidates the possible mechanisms underlying its neuroprotection on DA neurons, which is summarized in Figure 6. Our study supports the pathophysiological significance of ferroptosis in the pathogenic process of MPTP-induced PD animal models. Suppression of iron accumulation and iron-dependent ferroptosis in the brain contributes to the neuroprotection of apoferritin against MPTP. This provides a promising therapeutic direction for the clinical prevention and treatment of PD.
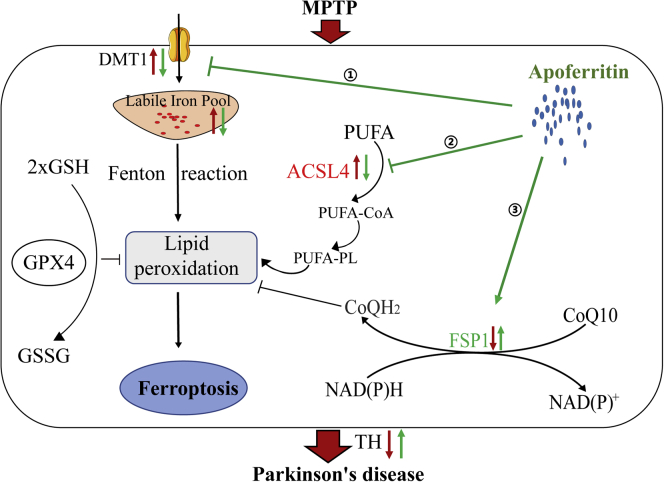
Figure 6.
A model of the possible mechanisms underlying the neuroprotective effect of apoferritin against MPTP
MPTP might result in the degeneration of DA neurons through the following pathways: (1) The increase of DMT1 in the SN leads to an increase in iron uptake, which increased the production of ROS by the Fenton reaction, resulting in lipid peroxidation. (2) The increased expression of ACSL4 protein in the SN results in the activation of PUFA, which further aggravates lipid peroxidation. (3) The decreased expression of FSP1 in the SN results in a decrease in the regeneration of reduced CoQ10, which traps lipid peroxidation free radicals, thus leading to ferroptosis. The effect of apoferritin is to abolish the increase in DMT1 and ACSL4 induced by MPTP, and to up-regulate the expression of FSP1 to inhibit lipid peroxidation and ferroptosis. The red arrow represents the changes induced by MPTP, and the blue arrow represents the effect of apoferritin pretreatment.
Limitations of the study
The mechanisms underlying the effect of apoferritin on MPTP-induced ferroptosis still need to be elucidated. In addition, the transport of apoferritin in the brain is not experimentally demonstrated.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Hua-Min Xu (huamin102@163.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Raw data will be shared upon receipt of a reasonable request.
Methods
All methods can be found in the accompanying transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31871202, 31771124), the Department of Science and Technology of Shandong Province (ZR2019MC057), Excellent Innovative Team of Shandong Province (2020KJK007), and Taishan Scholars Construction Project, Shandong.
Author contribution
H.-M.X. conceived the project, designed the experiments, and supervised the project. L.-M.S., Z.-X.X., N.Z., X.-Q.Y., and W.C. performed the experiments. L.-M.S. analyzed the data, prepared the figures, and wrote the manuscript. J.-X.X. revised the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102431.
Contributor Information
Jun-Xia Xie, Email: jxiaxie@public.qd.sd.cn.
Hua-Min Xu, Email: huamin102@163.com.
Supplemental information
References
- Aguirre P., Urrutia P., Tapia V., Villa M., Paris I., Segura-Aguilar J., Núñez M.T. The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals. 2012;25:795–803. doi: 10.1007/s10534-012-9525-y. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T.G., Drysdale J.W. On ferritin heterogeneity. Further evidence for heteropolymers. J. Biol. Chem. 1978;253:4451–4458. [PubMed] [Google Scholar]
- Bellinger F.P., Bellinger M.T., Seale L.A., Takemoto A.S., Raman A.V., Miki T., Manning-Boğ A.B., Berry M.J., White L.R., Ross G.W. Glutathione peroxidase 4 is associated with neuromelanin in substantia nigra and dystrophic axons in putamen of Parkinson's brain. Mol. Neurodegener. 2011;6:8. doi: 10.1186/1750-1326-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland N., Zivadinov R., Schweser F., Hagemeier J., Lichter D., Guttuso T., Jr. Ventral posterior substantia nigra iron increases over 3 years in Parkinson's disease. Mov Disord. 2019;34:1006–1013. doi: 10.1002/mds.27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli N., Jr., Chiba K., Trevor A.J. Potential bioactivation pathways for the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life Sci. 1985;36:225–230. doi: 10.1016/0024-3205(85)90063-3. [DOI] [PubMed] [Google Scholar]
- Chen Q., Chen Y., Zhang Y., Wang F., Yu H., Zhang C., Jiang Z., Luo W. Iron deposition in Parkinson's disease by quantitative susceptibility mapping. BMC Neurosci. 2019;20:23. doi: 10.1186/s12868-019-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L.T., Li Z., Ren J.J., Niu Q., Yu H.M., Liang R.F. [The role of DFO in Al (mal) (3)-induced ferroptosis in PC12 cells] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2019;37:722–727. doi: 10.3760/cma.j.issn.1001-9391.2019.10.002. [DOI] [PubMed] [Google Scholar]
- Chiba K., Trevor A., Castagnoli N., Jr. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 1984;120:574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- Daniels T.R., Delgado T., Rodriguez J.A., Helguera G., Penichet M.L. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- De Farias C.C., Maes M., Bonifácio K.L., Bortolasci C.C., De Souza Nogueira A., Brinholi F.F., Matsumoto A.K., Do Nascimento M.A., De Melo L.B., Nixdorf S.L. Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson's disease and its progression: disease and staging biomarkers and new drug targets. Neurosci. Lett. 2016;617:66–71. doi: 10.1016/j.neulet.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Dexter D.T., Wells F.R., Agid F., Agid Y., Lees A.J., Jenner P., Marsden C.D. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2:1219–1220. doi: 10.1016/s0140-6736(87)91361-4. [DOI] [PubMed] [Google Scholar]
- Dexter D.T., Wells F.R., Lees A.J., Agid F., Agid Y., Jenner P., Marsden C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J. Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., Bastide M., Laloux C., Moreau C., Bordet R. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol. Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Doll S., Freitas F.P., Shah R., Aldrovandi M., Da Silva M.C., Ingold I., Goya Grocin A., Xavier Da Silva T.N., Panzilius E., Scheel C.H. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Vera J.M., Fernández B., Gálvez N. Native and synthetic ferritins for nanobiomedical applications: recent advances and new perspectives. Future Med. Chem. 2010;2:609–618. doi: 10.4155/fmc.09.171. [DOI] [PubMed] [Google Scholar]
- Fan K., Cao C., Pan Y., Lu D., Yang D., Feng J., Song L., Liang M., Yan X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012;7:459–464. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- Gerlach M., Double K.L., Youdim M.B., Riederer P. Potential sources of increased iron in the substantia nigra of parkinsonian patients. J. Neural Transm. Suppl. 2006:133–142. doi: 10.1007/978-3-211-45295-0_21. [DOI] [PubMed] [Google Scholar]
- Goto K., Mochizuki H., Imai H., Akiyama H., Mizuno Y. An immuno-histochemical study of ferritin in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced hemiparkinsonian monkeys. Brain Res. 1996;724:125–128. doi: 10.1016/0006-8993(96)00284-3. [DOI] [PubMed] [Google Scholar]
- Harrison P.M., Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hentze M.W., Muckenthaler M.U., Galy B., Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Hirsch E.C. Iron transport in Parkinson's disease. Parkinsonism Relat. Disord. 2009;15(Suppl 3):S209–S211. doi: 10.1016/S1353-8020(09)70816-8. [DOI] [PubMed] [Google Scholar]
- Hirsch E.C., Brandel J.P., Galle P., Javoy-Agid F., Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson's disease: an X-ray microanalysis. J. Neurochem. 1991;56:446–451. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- Hopes L., Grolez G., Moreau C., Lopes R., Ryckewaert G., Carrière N., Auger F., Laloux C., Petrault M., Devedjian J.C. Magnetic resonance imaging features of the nigrostriatal system: biomarkers of Parkinson's disease stages? PLoS One. 2016;11:e0147947. doi: 10.1371/journal.pone.0147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D., Rajagopalan S., Chinta S., Kumar J., Di Monte D., Cherny R.A., Andersen J.K. Chronic ferritin expression within murine dopaminergic midbrain neurons results in a progressive age-related neurodegeneration. Brain Res. 2007;1140:188–194. doi: 10.1016/j.brainres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kielmanowicz M.G., Laham N., Coligan J.E., Lemonnier F., Ehrlich R. Mouse HFE inhibits Tf-uptake and iron accumulation but induces non-transferrin bound iron (NTBI)-uptake in transformed mouse fibroblasts. J. Cell. Physiol. 2005;202:105–114. doi: 10.1002/jcp.20095. [DOI] [PubMed] [Google Scholar]
- Langston J.W., Irwin I., Langston E.B., Forno L.S. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci. Lett. 1984;48:87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- Lee D.H., Kim C.S., Lee Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011;49:271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.J., Ren Y.D., Li J., Cao B., Ma C., Qin S.S., Li X.R. The role of iron in Parkinson's disease monkeys assessed by susceptibility weighted imaging and inductively coupled plasma mass spectrometry. Life Sci. 2020;240:117091. doi: 10.1016/j.lfs.2019.117091. [DOI] [PubMed] [Google Scholar]
- Mahoney-Sánchez L., Bouchaoui H., Ayton S., Devos D., Duce J.A., Devedjian J.C. Ferroptosis and its potential role in the physiopathology of Parkinson's Disease. Prog. Neurobiol. 2021;196:101890. doi: 10.1016/j.pneurobio.2020.101890. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Freigang S., Schneider C., Conrad M., Bornkamm G.W., Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T., Morgan E.H. Transferrin and transferrin receptor function in brain barrier systems. Cell. Mol. Neurobiol. 2000;20:77–95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P., Sofic E., Rausch W.D., Schmidt B., Reynolds G.P., Jellinger K., Youdim M.B. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Saadat S.M., Değirmenci İ., Özkan S., Saydam F., Özdemir Köroğlu Z., Çolak E., Güneş H.V. Is the 1254T>C polymorphism in the DMT1 gene associated with Parkinson's disease? Neurosci. Lett. 2015;594:51–54. doi: 10.1016/j.neulet.2015.03.054. [DOI] [PubMed] [Google Scholar]
- Salazar J., Mena N., Hunot S., Prigent A., Alvarez-Fischer D., Arredondo M., Duyckaerts C., Sazdovitch V., Zhao L., Garrick L.M. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc. Natl. Acad. Sci. U S A. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Huang C., Luo Q., Rogers E., Xia Y., Liu W., Ma W., Zeng W., Gong L., Fang J. The association of iron and the pathologies of Parkinson's diseases in MPTP/MPP(+)-Induced neuronal degeneration in non-human primates and in cell culture. Front. Aging Neurosci. 2019;11:215. doi: 10.3389/fnagi.2019.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Rastogi A., Ayyavoo V., Srivastava S. Nanotechnology-based approaches for the development of diagnostics, therapeutics, and vaccines. Monoclon. Antib. Immunodiagn. Immunother. 2014;33:186–191. doi: 10.1089/mab.2014.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temlett J.A., Landsberg J.P., Watt F., Grime G.W. Increased iron in the substantia nigra compacta of the MPTP-lesioned hemiparkinsonian African green monkey: evidence from proton microprobe elemental microanalysis. J. Neurochem. 1994;62:134–146. doi: 10.1046/j.1471-4159.1994.62010134.x. [DOI] [PubMed] [Google Scholar]
- Truffi M., Fiandra L., Sorrentino L., Monieri M., Corsi F., Mazzucchelli S. Ferritin nanocages: a biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016;107:57–65. doi: 10.1016/j.phrs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Vallerga C.L., Zhang F., Fowdar J., Mcrae A.F., Qi T., Nabais M.F., Zhang Q., Kassam I., Henders A.K., Wallace L. Analysis of DNA methylation associates the cystine-glutamate antiporter SLC7A11 with risk of Parkinson's disease. Nat. Commun. 2020;11:1238. doi: 10.1038/s41467-020-15065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Pantopoulos K. Regulation of cellular iron metabolism. Biochem. J. 2011;434:365–381. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O., Mandel S., Youdim M.B.H., Amit T. Targeting dysregulation of brain iron homeostasis in Parkinson's disease by iron chelators. Free Radic. Biol. Med. 2013;62:52–64. doi: 10.1016/j.freeradbiomed.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Wypijewska A., Galazka-Friedman J., Bauminger E.R., Wszolek Z.K., Schweitzer K.J., Dickson D.W., Jaklewicz A., Elbaum D., Friedman A. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat. Disord. 2010;16:329–333. doi: 10.1016/j.parkreldis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Yang W.S., Sriramaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L., Gallorini M., Schünemann V., Trautwein A.X., Gerlach M., Riederer P., Vezzoni P., Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J. Neurochem. 2001;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- Zecca L., Stroppolo A., Gatti A., Tampellini D., Toscani M., Gallorini M., Giaveri G., Arosio P., Santambrogio P., Fariello R.G. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. U S A. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang J., Song N., Xie J., Jiang H. Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol. Aging. 2009;30:1466–1476. doi: 10.1016/j.neurobiolaging.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Ziering A., Lee J. Piperidine derivatives; 1,3-dialkyl-4-aryl-4-acyloxypiperidines. J. Org. Chem. 1947;12:911–914. doi: 10.1021/jo01170a024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data will be shared upon receipt of a reasonable request.