Abstract
Recent research has tried to maximize broiler chick health and performance by utilizing commercial in-feed probiotics to inoculate fertile hatching eggs, and thus expose birds earlier to beneficial bacteria. However, the in ovo inoculation of a specific serotype of Bacillus subtilis was detrimental for broiler hatchability. Therefore, the objective of this study was to determine if other B. subtilis serotypes negatively affect hatchability or if it is associated with a specific serotype. It was also of interest to determine if the B. subtilis serotype influence chick performance and intestinal microflora. On d18 of incubation, 1886 fertile broiler eggs were in ovo inoculated with the following treatments (T): T1 = Marek's vaccine (MV), T2 = MV + B. subtilis (ATCC 6051), T3 = MV + B. subtilis (ATCC 8473), and T4 = MV + B. subtilis (ATCC 9466). It should be noted that in a previous study, T2 was detrimental to hatchability. Inoculated eggs were transferred to 3 hatchers/T. At hatch, chicks were weighed, feather sexed, and hatch residue analysis was conducted. Male chicks were randomly assigned to 40 raised wire cage so that there were 10 birds/cage. On d 0, 7, 14, and 21 of the grow-out, chicks and feed were weighed to calculate performance data. On these days, the ileum and ceca were aseptically collected to enumerate total aerobes and coliforms. No differences were observed for percentage of mid dead embryos, cracked eggs, and cull chicks (P > 0.05). However, hatch of transfer was significantly reduced by T2 compared to T1, T3, and T4 (P < 0.001). T2 had significantly higher percentages of late dead embryos and pips when compared to the other treatments (P = 0.002 and P < 0.001, respectively). Chicks hatched from T2 were not vigorous and, thus, not used for the grow-out trial. No differences were observed for growth performance characteristics for any of the treatments (P > 0.05). For bacterial enumeration, the ileum had equal or fewer bacterial counts for T3 and T4 when compared to T1 on most sampling days, except on d21 where T4 had higher aerobic and coliform counts (P ≤ 0.0001). For the ceca, T3 and T4 had equal or fewer bacterial counts than T1 on every sampling day (P ≤ 0.0001). These data demonstrate that not all B. subtilis evaluated are detrimental to hatchability, but rather, serotype dependent. In addition, different B. subtilis serotypes can modify the intestinal microflora with potential to reduce pathogenic bacteria present in young broiler, without impacting overall performance.
Key words: probiotic, in ovo, B. subtilis serotype, hatchability, microflora
INTRODUCTION
For many years, the poultry industry has been interested in the use of probiotics as an alternative to antibiotics. The World Health Organization previously described probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (FAO/WHO, 2001). In wild poultry production, probiotics or beneficial bacterial cultures were acquired naturally as part of the hen's microflora. This microflora was transferred to the eggs through the laying process (Ding et al., 2017; Lee et al., 2019) and during the brooding period (Kabir, 2009). However, due to the commercial settings for fertile egg production, there is no long-term direct contact with the hen after the eggs is laid, and the delivery of maternally provided beneficial cultures to the hatchlings is limited (Kabir, 2009). Therefore, the administration of beneficial bacteria to the chicks before hatch could result in health benefits for the bird, reducing the susceptibility to incoming challenges, and improving the bird's livability during the first weeks of life.
Competitive exclusion cultures obtained from the intestinal tract of broiler or broiler breeder hens have been thought to be beneficial if administered to the egg or after hatch by outcompeting pathogens (Cox et al., 1992; Meijerhof and Hulet, 1997; Al-Zenki et al., 2009; Schneitz and Hakkinen, 2016). Probiotic cultures have also been evaluated to determine their effectiveness at controlling specific pathogens such as Salmonella, Campylobacter, E. coli, and Clostridium in the broiler's gut, thus preventing or reducing the incidence of infections (La Ragione and Woodward, 2003; Wine et al., 2009; Youssef et al., 2011; Zhang et al., 2014; Ding et al., 2017). Probiotics, therefore, modulate the microbial environment in the bird's gut as well as their immune system, allowing for better nutrient and energy utilization, resulting in improved performance (Kabir, 2009; Eckert et al., 2010; Mountzouris et al., 2010; Torshizi et al., 2010; Youssef et al., 2011; Liu et al., 2012; Lee et al., 2013).
The most commonly used probiotic species in broiler production are Lactobacillus, Bifidobacterium, Enterococcus, and Bacillus (Kabir, 2009; Park et al., 2018). Each probiotic culture provides a range of benefits to the birds. However, Bacillus based probiotics have several advantages over other probiotic cultures. For example, Bacillus are facultative anaerobic spore formers, and their swift growth cycle makes their overall handling easier for production in industrial settings (Vazquez, 2016). Contrary to other commonly used probiotic bacteria, Bacillus can withstand high-temperature feed manufacturing processes. Bacillus are also resistant to low pH, bile salts, and other adverse intestinal conditions, which allows for higher concentrations of Bacillus to reach the gut (Barbosa et al., 2005; Shivaramaiah et al., 2011). Lastly, one of the major advantages of Bacillus species over other probiotics is their ability to exclude pathogens through the production of antimicrobial peptides (Stein, 2005; Santini et al., 2010; Shivaramaiah et al., 2011; Sumi et al., 2015).
The use of Bacillus based probiotics in the feed has yielded benefits to performance and gut health (Reis et al., 2017; Park et al., 2018), and the early delivery of these cultures through in ovo inoculation may provide earlier advantages to the bird. A single inoculated dose of a Bacillus based probiotic has the potential to become established in the small intestine and create an unfavorable environment for pathogenic bacteria that could become hazardous to the chick's health (De Oliveira et al., 2014). Similar to the effects obtained when adding probiotics to the feed, the early delivery of a probiotic could also promote earlier stimulation of the immune system to confer protection starting when the chicks are placed in a grow-out facility. Some studies that have evaluated the delivery of other probiotic bacteria in ovo have shown improvements in overall broiler health status and growth performance (Pender et al., 2017).
In previous research trials from our lab, Bacillus subtilis as well as other probiotic bacteria, were inoculated into the amnion of fertile eggs on day 18 of incubation. Different concentrations of Bacillus subtilis such as 104 and 105 cfu/ 50µL reduced hatchability to less than 10%. Nonetheless, the other probiotic Lactobacillus and Bifidobacterium, did not show any reduction (Triplett et al., 2018). The reduction in hatchability was unexpected because this probiotic culture was previously recognized as safe and has been shown to be advantageous for broiler health when included in the feed (Reis et al., 2017; Park et al., 2018). The detrimental result of B. subtilis in the previous study narrows the field of potential beneficial bacteria that can be used to provide an early benefit to the chick. Therefore, the objective of this study was to evaluate different Bacillus subtilis serotypes to determine if they are detrimental to broiler hatchability or if previously observed detrimental effects are associated with a particular serotype. If hatchability is not drastically reduced by the treatments, growth performance will be evaluated as well as any modulations in ileum and ceca microflora to determine the effectiveness of these cultures as beneficial bacteria.
MATERIALS AND METHODS
Incubation
Fertile broiler eggs were obtained from a commercial source when the breeder hens were approximately 55 weeks of age. Eggs were stored in a cooler at 18°C for three days prior to setting. While in the cooler, all eggs that were dirty, cracked, or misshapen were removed. A total of 2,160 eggs were randomly labeled according to egg number, flat and treatment. Eggs were removed from the cooler and allowed to acclimate to room temperature three hours prior to setting in the incubator to avoid moisture on the egg surface. The incubators (Model NMC-1080, Jacksonville, FL, USA) were sanitized with 70% ethanol prior to setting. For each treatment, 18 egg flats (30 eggs each) were weighed and randomly set throughout the two incubators, and each treatment was represented on each level within the incubator. The dry and wet bulb temperatures were set at 37.5°C ± 0.1 and 28.9°C ± 0.1, respectively. After 10 days of incubation, eggs were candled to discard any eggs that were infertile or presenting an early dead embryo. On day 18 of incubation, 1,886 eggs were inoculated. After in ovo inoculation, eggs belonging to each treatment were transferred into 18 previously sanitized hatching baskets. The baskets were equally distributed among three Georgia Quail Farm hatcher units according to each treatment to avoid cross contamination (6 flats in each hatcher, 3 hatchers for each treatment x 4 treatments = 12 total GQF MFG, 1,502 Digital Sportsman incubator; Savannah, GA) until day 21 of incubation. The hatcher dry and wet bulb temperatures were set at 36.9°C ± 0.1 and 30°C ± 0.1, respectively. Sterile water was added each day at the same time, to maintain the desired humidity level. Temperature and humidity data were recorded daily.
Treatments
The three B. subtilis serotypes evaluated in this study were obtained from ATCC. The bacterial cultures were reconstituted as directed, and the obtained stocks were stored at -80°C. To determine the bacterial concentration of each bacterial culture, 1 mL was inoculated into 9 mL of nutrient broth (BD Difco, Franklin Lanes, NJ) and incubated aerobically for 24 h at 37°C (VWR™ International, 1,535 incubator, Cornelius, OR, USA). The 24 h culture was 10-fold serially diluted and the dilutions were spread onto Mannitol Yolk Polymyxin B agar (MYP agar). Plates were incubated aerobically at 37°C for 24 h (VWR™ International, 1,535 incubator, Cornelius, OR, USA) and colonies present were counted, and log transformed. The desired concentration for in ovo inoculation was set to be approximately 106 cfu/50µL.
On the day of inoculation, a 24 h culture of each bacterial strain was diluted to obtain the desired concentration and centrifuged at 4,000 rpm for 5 min to obtain a pellet. The supernatant was removed, and the pellet was reconstituted with sterile diluent. All treatments were prepared on the day of inoculation and individually distributed into 800 mL bags of a commercial sterile diluent. A standard Herpesvirus of turkey (HVT) vaccine (16,000 doses/800 mL bag; Merial Select, Inc., Gainesville, GA) was aseptically added with a syringe to each diluent bag. All diluent bags containing each treatment were kept on ice until they were attached to the Inovoject machine. The applied treatments included: 1)50 µL HVT Marek's disease vaccine and no probiotic (MV), 2) Marek's disease vaccine (MV) + B. subtilis spp. subtilis (ATCC 6051), 3) MV + B. subtilis spp. subtilis (ATCC 8473), 4) MV + B. subtilis spp. subtilis (ATCC 9466). During the in ovo inoculation, 50 µL were collected from each treatment and spread onto the appropriate agar plates to confirm that the correct concentration of bacteria was delivered for each treatment. Plates were counted after 24 h, and counts were log-transformed.
Inoculation
After 18 days of incubation egg flats were weighed, one egg from each flat was set aside for embryo staging, and the remaining eggs were inoculated according to treatment. One flat of developing eggs was inoculated at a time, and each needle punctured the egg at a depth of 2.49 cm to deliver each 50 µL dose automatically. Cleaning and sanitization cycles were conducted between each applied treatment to avoid cross-contamination between bacterial cultures. After each cleaning cycle, sterile water was flushed to remove any remaining sanitizer from the injection line, and 50 µL were collected and spread onto Tryptic Soy Agar plates (TSA; Millipore Sigma, St. Louis, MO) to confirm that no bacterial contamination occurred between treatments. After all treatments were inoculated, the eggs that had been removed from each flat for embryo staging were in ovo inoculated with 50 µL of a Coomassie blue dye and immediately euthanized via CO2 asphyxiation. Each embryo was analyzed to confirm that the eggs were in the appropriate stage of development for 18 d of incubation. Also, the presence of the dye surrounding the embryo's body through the amniotic fluid confirmed that the inoculum was correctly delivered into the amniotic fluid and did not puncture the embryo's tissue.
Hatch and Grow-out
On d 21 of incubation, 1,421 hatched chicks were weighed to evaluate hatch of fertile eggs transferred and average chick weight. Unhatched embryos were removed from their hatching baskets, counted, and further evaluated through a hatch residue analysis to determine the growth stage of the embryo before its death. The egg number, treatment, and stage of each egg were recorded as: early, mid, or late dead as well as pipped and contaminated eggs. All embryos and chicks were treated under the Guide for the Care and Uses of Agricultural Animals in Research and Teaching (FASS, 2010) and the Mississippi State University Animal Care and Use Committee (IACUC #17-224).
Hatched chicks were feather sexed, and 100 males per treatment were moved to battery cages, within an environmentally controlled room, for a 21 d grow-out cycle. Female hatchlings were humanely euthanized. For the grow-out, there were 10 cages for each treatment, which were set within 10 blocks throughout the house. Male chicks were placed in cages where an empty cage was represented on either side in an attempt to avoid cross-contamination between treatments. The floor of each cage was covered with thin cardboard sheets, and each cage was equipped with 3 nipple drinkers and a single hanging feeder for ad libitum consumption. From d 0 to 7 of the grow-out, a separate tray was set in each cage and feed was added daily. A common corn and soybean meal diet formulated to meet or exceed Ross 708 guidelines was provided in two feeding phases: starter diet from d 0 to 14 and grower diet from d 14 to 21 following Ross 708 guidelines (Aviagen, 2014). For the lighting schedule, a 23L: 1D photoperiod was used from d 1 to 7 and a 20L: 4D photoperiod was used from d 8 to d 21 in the battery house. A commercial temperature program was followed as recommended by Aviagen (Aviagen, 2009). Feed intake (FI) and body weight gain (BW gain) were recorded on d 0, 7, d 14 and d 21. Daily mortality weight was recorded to calculate feed conversion ratio (FCR).
Sampling and Culture Based Microbial Analysis
On days 0, 7, 14 and 21 of the grow-out, one bird from each cage was randomly selected, humanely euthanized and necropsied to access their digestive tract. The ileum and cecum were aseptically collected, weighed, and placed in sterile whirl-Pak (Nasco, Saugerties, NY) bags which were kept on ice until further microbiological analysis. All tissues used for microbiology were homogenized (Stomacher 400 circulator, Seward, Worthing, UK) in Peptone Buffered Saline at a 1:10 wt. / vol (PBS, Fischer Scientific, Hampton, NH) and then serially diluted with the same buffer. Out of the dilution tubes, 100 µL were spread onto Tryptic Soy Agar (TSA, BD Difco, Franklin Lakes, NJ) and Eosin-Methylene Blue media (EMB, Oxoid, Thermo Fisher Scientific, Waltham, MA) to obtain total aerobic counts and total coliform counts, respectively. The plates were incubated for 24 h at 37°C aerobically (VWR International, 1,535 incubator, Cornelius, OR USA), and counts obtained were log-transformed according to BAM standards (FDA, 2001).
Statistical Analysis
All data collected were analyzed using SAS vs. 9.4 (SAS Institute, Cary, NC). Hatch of fertile eggs and hatch residue data were analyzed using a completely randomized design where each individual GQF hatcher served as the experimental unit (N = 3). Growth performance parameters such as BW gain, FCR, and feed intake were analyzed using a randomized complete block design (10 blocks) for each grow-out phase. Log coliform and log aerobic counts were analyzed using a randomized complete block design (10 blocks) with a split plot over the 4 selected days of grow-out (d 0, 7, 14, 21). Means were separated using Fisher's Protected LSD, and differences were considered significant when P ≤ 0.05 (Steel and Torrie, 1980).
RESULTS
In ovo Inoculation and Bacillus Concentration
Embryos obtained for embryo staging analysis on d 18 of incubation were confirmed to be in the right developmental stage, for in ovo inoculation. These embryos showed 3-lobed yolk sacs, and their intestines were mostly enclosed within the abdominal cavity. Embryos inoculated with Coomassie blue dye had dye surrounding their feathers and skin, which confirms that the Inovoject machine correctly delivered the dose into the amnion, and not puncturing the embryo's body. Plate counts obtained for each treatment during in ovo inoculation resulted in the following concentrations: MV (T1): no bacterial growth, as expected; T2: 9.7×106 cfu of B. subtilis ATCC 6051/50 µL; T3: 4.5×106 cfu of B. subtilis ATCC 8473/50 µL; and T4: 3.3×106 cfu of B. subtilis ATCC 9466/50 µL. All treatments were administered at ~ 106 cfu/50 µL. No growth was obtained on the TSA plates that were plated after each cleaning cycle, which indicates that there was no cross-contamination between the inoculated treatments.
Hatchability and Growth Performance
Hatch of transfer resulted in differences among treatments (Table 1). T2 reduced hatchability to 17.3%. However, it was determined that other B. subtilis serotypes evaluated did not have a negative impact on hatch of transfer, yielding hatch percentages higher than 94%, which were not different compared to the MV treatment (P < 0.0001). For hatch performance, T2 resulted in an increased percentage of late dead embryos as well as pipped eggs (P = 0.023 and P < 0.0001, respectively). Differences were also detected in average chick weight, where T2 hatched chicks had a lower weight compared to the chicks from T1, T3, and T4 (P = 0.0048). This difference was attributed to treatment effect and not to any difference in average egg weight (P = 0.454, Table 1). For all treatments, hatched bird weight agreed with the established Ross 708 performance objectives (Aviagen, 2019).
Table 1.
Effect of in ovo inoculation of a Marek's vaccine and three individual B. subtilis serotypes at ~ 106 cfu/ 50µL on hatch parameters1.
| Hatch parameters | MV (T1) | B. subtilis ATCC 6051 (T2) | B. subtilis ATCC 8473 (T3) | B. subtilis ATCC 9466 (T4) | P-value | SEM |
|---|---|---|---|---|---|---|
| Hatch of transfer | 94.7a | 17.3b | 96.1a | 96.2a | < 0.0001 | 1.215 |
| Early dead embryos | 0.45 | 0 | 0 | 0 | 0.0652 | 0.118 |
| Mid dead embryos | 0.58 | 0 | 0 | 0.21 | 0.1789 | 0.188 |
| Late dead embryos | 3.10b | 12.4a | 2.40b | 2.30b | 0.0023 | 1.401 |
| Pipped embryos | 1.15b | 69.5a | 1.32b | 0.85b | < 0.0001 | 0.551 |
| Contaminated embryos | 0 | 0.82 | 0.22 | 0.42 | 0.0628 | 0.182 |
| Avg. Egg Weight (g)2 | 63.63 | 63.97 | 64.23 | 64.04 | 0.4540 | 0.351 |
| Avg. Chick Weight (g)3 | 44.1a | 42.2b | 43.6a | 43.2a | 0.0048 | 0.374 |
a-bMeans in a row not sharing a common superscript are different (P ≤ 0.005).
N=3, observed means were calculated using each GQF hatcher unit as a replicate (∼180 eggs/GQF; ∼540 eggs/treatment).
Avg. egg weight on d 18 prior to the in ovo inoculation
Avg. chick weight on the day of hatch
Due to the drastically reduced hatchability induced by T2, there were not enough healthy chicks to be placed in the grow-out facility, and the remaining chicks that did hatch were immediately humanely euthanized. Chicks from T1, T3, and T4 that were placed in the grow-out facility showed no significant different in any growth parameter evaluated. BW gain from d 0 to 21 ranged between 0.758 kg to 0.776 kg (P = 0.593), feed intake ranged between 1.218 kg to 1.189 kg (P = 0.261), the FCR ranged from 1.693 to 1.750 (P = 0.481), and percent mortality ranged between 1% to 2.5% (P = 0.798). The performance data were compared to Ross 708 as-hatched performance objectives, and were approximately one day behind according to the established expectations (Aviagen, 2019). We attribute these differences birds reared in raised wire cages compared to floor pens.
Culture Based Bacterial Analysis for Ileum and Ceca
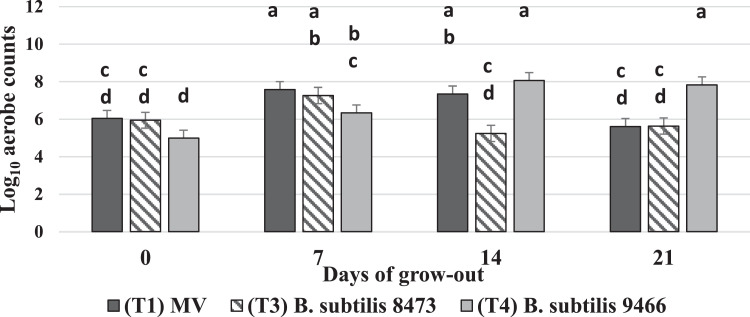
Treatment by day interactions were observed for total aerobic bacteria counts in the ileum (P = 0.0001, SEM = 0.429) (Figure 1). On the day of hatch, no differences were seen between treatments. By d 7, mean aerobic counts were reduced by T4 (6.33 log) compared to MV (7.57 log). However, by d 14, mean aerobic counts increased with T4 (8.05 log) compared to T3 only (5.24 log). By d 21, MV and T3 resulted in lower mean aerobic counts (5.61 and 5.63 log, respectively) compared to T4 (7.82 log).
Figure 1.
Treatment by day interactions observed for mean aerobic bacteria counts in the ileum. The in ovo inoculated treatments are represented as follows: Treatment 1, Marek's vaccine (MV) is denoted by the dark gray bar. Treatment 3, B. subtilis ATCC 8473, is denoted by the downward diagonal bar. Treatment 4, B. subtilis ATCC 9466, is denoted by the light gray bar. Treatment 2 did not yield enough birds to move to a grow-out facility and is not represented in the figure. Total bacterial counts in cfu/g are on the y-axis and the days of sampling during the grow-out are on the x-axis. Differences in mean bacterial count were considered significant at P ≤ 0.05. SEM = 0.429, P = 0.0001, and N = 10 (10 cages/ treatment, 1 chick was randomly sampled from each cage on each sampling day). Significant differences are distinguished according to alphabetical superscripts, where means not sharing a common superscript are significantly different.
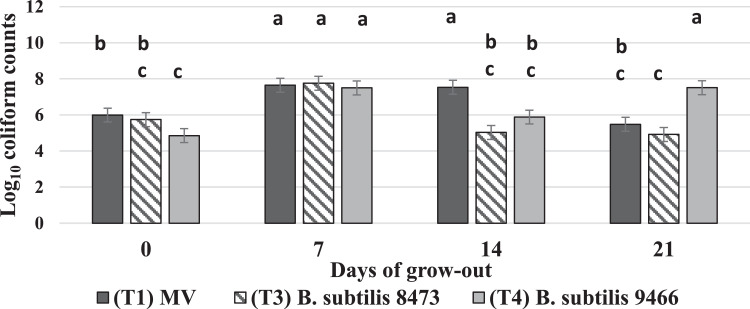
Treatment by day interactions were observed for total coliform counts in the ileum (P < 0.001, SEM = 0.386). On the d of hatch, T4 reduced log coliform counts (4.85 log) compared to the MV treatment (5.99 log). On d7, no reduction in coliform was detected according to treatment. As expected, coliform counts increased for all treatments as the chick aged. However, on d14, both B. subtilis T3 and T4 reduced coliform counts (5.03 and 5.88 log, respectively) compared to the MV treatment (7.53 log). By d21, neither the MV treatment nor T2 reduced coliform counts (5.47 and 4.91 log, respectively); on the contrary, T4 seemed to induce an increase in coliform counts (7.51 log) (Figure 2).
Figure 2.
Treatment by day interactions observed for mean coliform counts in the ileum. The in ovo inoculated treatments are represented as follows: Treatment 1, Marek's vaccine (MV) is denoted by the dark gray bar. Treatment 3, B. subtilis ATCC 8473, is denoted by the downward diagonal bar. Treatment 4, B. subtilis ATCC 9466, is denoted by the light gray bar. Treatment 2 did not yield enough birds to move to a grow-out facility and is not represented in the figure. Total bacterial counts in cfu/g are on the y-axis and the days of sampling during the grow-out are on the x-axis. Differences in mean bacterial count were considered significant at P ≤ 0.05. SEM= 0.386, P ≤ 0.0001, and N = 10 (10 cages/ treatment, 1 chick was randomly sampled from each cage on each sampling day). Significant differences are distinguished according to alphabetical superscripts, where means not sharing a common superscript are significantly different.
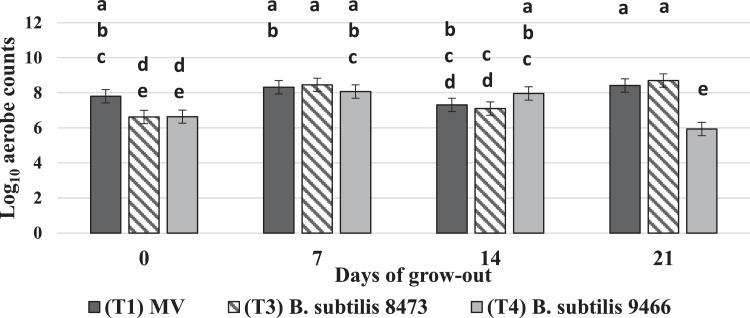
Treatment by day interactions were observed for mean aerobic counts in the ceca (P = 0.0001, SEM = 0.381). On the d of hatch, B. subtilis from both T3 and T4 reduced total aerobes (6.62 and 6.64 log, respectively) compared to the MV treatment (7.80 log). No further reduction in aerobic counts were seen for d 7 and 14 of the grow-out by any treatment. However, by d 21, aerobe counts seemed to be reduced again in the chicks in ovo inoculated with T4 (5.94 log) compared to the MV treatment and T3 (7.42 and 7.71 log, respectively) (Figure 3).
Figure 3.
Treatment by day interactions observed for mean aerobic bacteria counts in the ceca. The in ovo inoculated treatments are represented as follows: Treatment 1, Marek's vaccine (MV) is denoted by the dark gray bar. Treatment 3, B. subtilis ATCC 8473 is denoted by the downward diagonal bar. Treatment 4, B. subtilis ATCC 9466 is denoted by the light gray bar. Treatment 2 did not yield enough birds to move to a grow-out facility and is not represented in the figure. Total bacterial counts in cfu/g are on the y-axis and the days of sampling during the grow-out are on the x-axis. Differences in mean bacterial count were considered significant at P ≤ 0.05. SEM = 0.381, P ≤ 0.0001, and N = 10 (10 cages/ treatment, 1 chick was randomly sampled from each cage on each sampling day). Significant differences are distinguished according to alphabetical superscripts, where means not sharing a common superscript are significantly different.
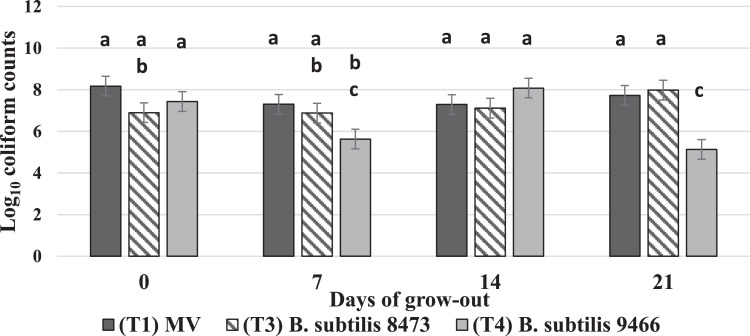
Treatment by day interactions were observed for total coliform counts in the ceca (P < 0.0001, SEM = 0.473). No differences in coliform counts were detected on d of hatch. However, on d 7, B. subtilis from T4 seemed to reduce total coliforms (5.63 log) compared only to the MV treatment (7.30 log). This reduction was lost by d 14 of the grow-out, and by d 21, T4 again caused a reduction of total coliforms (5.13 log) compared to T3 and the MV treatment (7.98 and 7.73 log, respectively) (Figure 4).
Figure 4.
Treatment by day interactions observed for mean coliform counts in the ceca. The in ovo inoculated treatments are represented as follows: Treatment 1, Marek's vaccine (MV) is denoted by the dark gray bar. Treatment 3, B. subtilis ATCC 8473, is denoted by the downward diagonal bar. Treatment 4, B. subtilis ATCC 9466 is denoted by the light gray bar. Treatment 2 did not yield enough birds to move to a grow-out facility and is not represented in the figure. Total bacterial counts in cfu/g are on the y-axis and the days of sampling during the grow-out are on the x-axis. Differences in mean bacterial count were considered significant at P ≤ 0.05. SEM = 0.473, P ≤ 0.0001, and N = 10 (10 cages/ treatment, 1 chick was randomly sampled from each cage on each sampling day). Significant differences are distinguished according to alphabetical superscripts, where means not sharing a common superscript are significantly different.
DISCUSSION
Hatchability and Growth Performance
The in ovo inoculation of different probiotic bacteria has been previously evaluated. However, most of these studies lack applicability in industrial settings, due to the use of manual in ovo inoculation procedures, which are highly variable and depend on the expertise of the person conducting the inoculation. Due to the intensive labor of inoculating one egg at a time, the number of replicates falls short compared to trials using commercial in ovo injection technology (Cox et al., 1992; Edens et al., 1997; Meijerhof and Hulet, 1997; de Oliveira et al., 2014). For these reasons, interest has developed in evaluating the in ovo administration of probiotics using an automated inoculation method such as the Inovoject technology. This technology is an industry-standard for the delivery of vaccines against Marek's disease and infectious bursal disease (Johnston et al., 1997). This method has been shown to increase the accuracy of inoculation to 83.8% compared to 36.1% obtained by manual inoculation (Wakenell et al., 2002), and several flats of eggs can be inoculated over a short period without impacting hatchability (Triplett et al., 2018; Beck et al., 2019; Castañeda et al., 2020).
Based on the negative results obtained in hatchability due to the in ovo administration of B. subtilis in a previous study by Triplett et al. (2018), it was of our interest to determine if other Bacillus subtilis serotypes have detrimental effects on broiler hatchability or if these effects are serotype-specific for B. subtilis ATCC 6051. In the current study, differences among the treatments were observed for percent hatch of transfer. Similar to the previous study by our lab, B. subtilis from T2 reduced hatchability to 17.3%. In agreement with this study, de Oliveira et al. (2014) also demonstrated a reduced hatchability as a result of the manual in ovo inoculation of a non-specified Bacillus subtilis serotype in broiler eggs. However, in the current study the detrimental effects were only seen for one of the B. subtilis serotypes (T2), while the other B. subtilis serotypes evaluated yielded hatch percentages higher than 94%, similar to the percentage obtained for the MV treatment. These results agree with Da Silva et al. (2017), who demonstrated no negative effects on hatchability when in ovo inoculating a non-specified Bacillus strain at 108 cfu/g. The positive results obtained for B. subtilis from T3 and T4 are promising for the use of these probiotic strains to promote further benefits in the chick's life. Nevertheless, it is important to emphasize that knowing not only the strain but also the serotype is relevant to obtain positive results from the use of probiotics. The concentration of the probiotic culture is also be an important factor to consider for egg inoculation, however, previous studies have shown no negative effects in concentrations ranging from 106 to 108 cfu/mL (Da Silva et al., 2017; Triplett et al., 2018; Beck et al., 2019; Castañeda et al., 2020).
B. subtilis ATCC 6051 (T2) was previously established to be safe for use as a probiotic culture, due to its lack of hemolytic activity in red blood cells (Dumitru et al., 2019). This strain is also Generally Recognized as Safe (GRAS) by the FDA, making it an attractive probiotic culture for live trials (Kabisch et al., 2013). However, according to the results obtained in the current study and in a previous study, this serotype is not safe for in ovo inoculation in broiler hatching eggs (Triplett et al., 2018). One key characteristic of this specific B. subtilis serotype (ATCC 6051), is the production of antimicrobial peptides as well as amylase and protease enzymes (Dumitru et al., 2019). When added to poultry feed, these enzymes are known to improve nutrient availability and absorption, thus inducing improvements in growth performance (Amerah et al., 2017; Alagawany et al., 2018). To our knowledge, the effects of these enzymes on broiler embryos have yet to be elucidated. However, previous research in fish revealed that proteases play an important role in the mobilization and hydrolysis of stored yolk proteins needed for embryonic development (Gwon et al., 2017). In broiler embryos, it is well known that during the last stage of incubation, the yolk is the main energy source used during the hatching process (Nangsuay et al., 2011; Şahan et al., 2014). There is a possibility that the enzymes produced specifically by B. subtilis (T2) may somehow lead to reduced energy availability for hatch, thus increasing the percentage of late dead and pipped embryos as seen in the current study. However, more research is needed to determine the exact effect of B. subtilis (T2) in the embryo to result in a drastically reduced hatchability.
Due to the reduced hatchability, B. subtilis from T2 did not yield healthy enough chicks to be placed in a grow-out facility. For the chicks from T1, T3 and T4 that were placed, no difference in growth parameters were detected among the treatments on any of the days evaluated. These results agree with another study, which evaluated the impact of the manual in ovo administration of a non-specified serotype of B. subtilis at a 107 cfu concentration and found no changes in growth performance (Majidi-Mosleh et al., 2017). A single in ovo dose of B. subtilis did not seem to affect the chick's growth performance. As previously mentioned, Bacillus has many advantages over other probiotic strains, however in a vegetative state as delivered in the current experiment, it may not persist in the chicken's intestinal epithelium for long periods (Barbosa et al., 2005; Latorre et al., 2014). After the bird has hatched and began the process of digestion, the vegetative Bacillus dose delivered may be transient in the chicken's gut (Bernardeau et al., 2017). Thus, possibly limiting the time available for Bacillus to exert its beneficial effects to approximately 6.5 hours as it passes through the gut (Latorre et al., 2014).
Bacterial Analysis in Ileum and Ceca
Bacillus species have become of great interest for the industry due to their ability to produce high quantities of enzymes and antimicrobial peptides (Abriouel et al., 2011; Sumi et al., 2015; Dumitru et al., 2019). In previous studies evaluating the inclusion of non-specified serotype of B. subtilis in the feed, reductions were obtained in Salmonella (Shivaramaiah et al., 2011) and Clostridium, which are two of the main pathogens of concern in the poultry industry (Sen et al., 2012). In the current study, the presence of total aerobic bacteria and total coliforms was quantified in the ileum and ceca, due to the high feed retention time and large bacterial density present in these segments (Svihus, 2014). Several modulations were detected in total aerobic bacteria counts in the ileum and in the ceca, caused mostly by T3 and T4 on different days of the grow-out. A similar pattern was detected for total coliforms, which were reduced mostly by T4 in the ileum and in the ceca on different days the grow-out. Based on these results, it seems as if the inoculated B. subtilis doses continued actively modulating the chick's microflora days after it was delivered into the egg. The reduction in total aerobic counts and coliform detected was most likely caused by B. subtilis's production of antimicrobial peptides such as subtilin, subpeptin, bacitracin, surfactin, bacisubin, among others (Stein, 2005; Sumi et al., 2015).
It is important to emphasize that in this study, B. subtilis for all treatments were inoculated as vegetative cells, and not as spores. Therefore, once the bird hatched, digestion and gastrointestinal tract conditions may have reduced B. subtilis viable cell counts, and thus, their effectiveness in promoting health benefits (Casula and Cutting, 2002; Barbosa et al., 2005; Cartman et al., 2008). In this study, the presence of Bacillus was reduced in chicks that were in ovo inoculated with the other Bacillus treatments when compared to the MV alone control (data not shown). It may be possible that B. subtilis has bacteriocin like activity against other naturally present Bacillus in the chicken gut after the first weeks of hatch, thus reducing the presence of Bacillus even further, as seen by Duc et al. (2004). However, the inoculation of beneficial B. subtilis serotypes in spore-form could potentially allow a higher concentration of Bacillus to be present, given their ability to germinate and sporulate in the gastrointestinal tract (Casula and Cutting, 2002). Bacillus inoculation in spore-form, and not in vegetative form, could therefore prolong the modulations in the microbial population, which may also result in improvements in growth performance. Nevertheless, the ability of a single in ovo probiotic dose even in a vegetative form, to start actively modulating the microflora has great implications for broiler management and a reduced incidence of infections.
Interestingly, T4 caused some modulations in total aerobe and coliform counts in the ceca on day 21. However, as previously established, the persistence of the inoculated Bacillus in the chick's intestinal tract may be limited by day 21. Therefore, at this stage of the chicken's life, these modulations could be due to the development of the immune system, which is known to become most active after week 3 of hatch (Nochi et al., 2018). However, this modulation seems to be exclusively caused by T4, B. subtilis (ATCC9466), given that no differences were observed for T3. Therefore, there is a possibility that the early presence of B. subtilis from T4 (ATCC 9466) in the chicken's gastrointestinal tract, had an early effect on the development of immune parameters that somehow contributed to the bacterial modulations on later days of the grow-out. However, further research in cytokine modulations, antibody titers, spleen and bursa morphology, and b- cell production need to be conducted to confirm these assumptions.
In conclusion, some Bacillus species and serotypes are not beneficial for broiler embryos, even if the serotype has been previously claimed to be safe for use in other hosts. However, other Bacillus subtilis serotypes do not affect hatchability and seem to be triggering early microflora modulations, which could have beneficial effects for pathogenic reduction through-out the bird's lifetime. There is a great possibility for the occurrence of immune stimulations even with a single in ovo Bacillus subtilis dose, which needs to be investigated further to determine its full potential for the control of infections. Further research evaluating the different non-detrimental B. subtilis as spores and the combination of in ovo with in feed applications are necessary to fully understand this probiotic and its potential for improving performance and overall broiler health.
ACKNOWLEDGEMENTS
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station, supported by the U.S. Department of Agriculture Hatch projects (Accession numbers of MIS-322,340 and specific Cooperative Agreements under accession number MIS-321,777).
The authors would like to thank Merial for providing the diluent and the HVT vaccine for treatment application, and Zoetis for the use of the Inovoject equipment and for their service during the injection process.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abriouel H., Franz C.M.A.P., Omar N.B, Galvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011;35:201–232. doi: 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iran. J. Vet. Res. 2018;19:157–164. [PMC free article] [PubMed] [Google Scholar]
- Al-Zenki S.F., Al-Nasser A.Y., Al-Saffar A.E., Abdullah F.K., Al-Bahouh M.E., Al-Haddad A.S., Alomirah H., Mashaly M. Effects of using a chicken-origin competitive exclusion culture and probiotic cultures on reducing Salmonella in broilers. J. Appl. Poult. Res. 2009;18:23–29. [Google Scholar]
- Amerah A.M., Romero L.F., Awati A., Ravindran V. Effect of exogenous xylanase, amylase, and protease as single or combined activities on nutrient digestibility and growth performance of broilers fed corn/soy diets. Poult. Sci. 2017;96:807–816. doi: 10.3382/ps/pew297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen Ross Broiler Management Manual. Aviagen, Huntsville, AL. 2009 [Google Scholar]
- Aviagen Ross 708 Nutrition Specifications. Aviagen, Huntsville, AL. 2014 [Google Scholar]
- Aviagen Ross 708 Performance Objectives. Aviagen, Huntsville, AL. 2019 [Google Scholar]
- Barbosa T.M., Serra C.R., La Ragione R.M., Woodward M.J., Henriques A.O. Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005;71:968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C.N., McDaniel C.D., Wamsley K.G.S., Kiess A.S. The potential for inoculating Lactobacillus animalis and Enterococcus faecium alone or in combination using commercial in ovo technology without negatively impacting hatch and post-hatch performance. Poult. Sci. 2019;98:7050–7062. doi: 10.3382/ps/pez441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardeau M., Lehtinen M.J., Forssten S.D., Nurminen P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Tech. 2017;54:2570–2584. doi: 10.1007/s13197-017-2688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartman S.T., La Ragione R.M., Woodward M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 2008;74:5254–5258. doi: 10.1128/AEM.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula G., Cutting S.M. Probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002;68:2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda, C. D., D. K. Dittoe, K. G. S. Wamsley, C. D. McDaniel, A. Blanch, D. Sandvang, and A. S. Kiess. 2020. In ovo inoculation of an Enterococcus faecium-based product to enhance broiler hatchability, live performance, and intestinal morphology. Poult Sci. 99:6163–6172. [DOI] [PMC free article] [PubMed]
- Cox N.A., Bailey J.S., Blankenship R.P., Gildersleeve R.P. Research note: in ovo administration of a competitive exclusion culture treatment to broiler embryos. Poult. Sci. 1992;71:1781–1784. doi: 10.3382/ps.0711781. [DOI] [PubMed] [Google Scholar]
- Da Silva, O. I.G., B.Vellano I.H., Moraes A.C., Lee I.M., Alvarenga B., Milbradt E.L., Hataka A., Okamoto A.S., Andreatti R.L.F. Evaluation of a probiotic and a competitive exclusion product inoculated in ovo on broiler chickens challenged with Salmonella heidelberg. Rev. Bras. Cienc. Avic. 2017;19:19–26. [Google Scholar]
- de Oliveira J.E., van der Hoeven-Hangoor E., van de Linde I.B., Montijn R.C., van der Vossen J.M.B.M. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Ding J., Dai R., Yang L., He C., Xu K., Liu S., Zhao W., Xiao L., Zhang Y., Meng H. Inheritance and establishment of gut microbiota in chickens. Front. Microbiol. 2017;8:1–11. doi: 10.3389/fmicb.2017.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc L.H., Hong H.A., Barbosa T.M., Henriques A.O., Cutting S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru M., Sorescu I., Habeanu M., Tabuc C., Idriceanu L., Jurcoane S. Preliminary characterisation of Bacillus subtilis strain use as a dietary probiotic bio-additive in weaning piglet. Food and Feed Res. 2019;45:203–211. [Google Scholar]
- Eckert N.H., Lee J.T., Hyatt D., Stevens S.M., Anderson S., Anderson P.N., Beltran R., Schatzmayr G., Mohnl M., Caldwell D.J. Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. J. Appl. Poult. Res. 2010;19:59–67. [Google Scholar]
- Edens F.W., Parkhurst C.R., Casas I., Dobrogosz W.J. Principles of ex ovo competitive exclusion and in ovo administration of Lactobacillus reuteri. Poult. Sci. 1997;76:179–196. doi: 10.1093/ps/76.1.179. [DOI] [PubMed] [Google Scholar]
- FAO/WHO . 2001. Health and Nutritional Properties of Pro-biotics in Food Including Powder Milk With Live Lactic Acid Bacteria, A Joint FAO/WHO Expert Consultation.http://www.fao.org/3/a-a0512e.pdf Cordoba, Argentina. 1-4 October. Accessed Oct. 2020. [Google Scholar]
- Federation of Animal Science Societies . Committees to Revise the Guide for the Care and Use of Agricultural Animals in Research and Teaching. 3rd Ed. 2010. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching.https://www.asas.org/docs/default-source/default-document-library/ag_guide_3rded.pdf?sfvrsn=4 Chater 9 Poultry. Accessed Aug. 2020. [Google Scholar]
- Gwon S.H., Kim H.K., Baek H.J., Lee Y.D., Kwon J.Y. Cathepsin b & d and the survival of early embryos in red spotted grouper, Ephinephelus akaara. Dev. Reprod. 2017;21:457–466. doi: 10.12717/DR.2017.21.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P.A, Liu H., Connell T.O., Phelps P., Bland M., Tyczkowski J., Kemper A., Harding T., Aviakan A., Haddad E., Whitfill C., Gildersleeve R., Ricks C.A. Applications in in ovo technology. Poult. Sci. 1997;76:165–178. doi: 10.1093/ps/76.1.165. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabisch J., Thürmer, A. A., Hübel T., Popper L., Daniel R., Schweder T. Characterization and optimization of Bacillus subtilis ATCC 6051 as an expression host. J. Biotechnol. 2013;163:97–104. doi: 10.1016/j.jbiotec.2012.06.034. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003;94:245–256. doi: 10.1016/s0378-1135(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kallapura G., Menconi A., Pumford N.R., Morgan M.J., Layton S.L., Bielken L.R., Hargis B.M., Tellez G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014;93:1793–1800. doi: 10.3382/ps.2013-03809. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Lee S.H., Bautista D.A., Siragusa G.R. Effect of Bacillus subtilis-based direct-fed microbials on immune status in broiler chickens raised on fresh or used litter. Asian-Australas. J. Anim. Sci. 2013;26:1592–1597. doi: 10.5713/ajas.2013.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., La T.M., Lee H.J., Choi I.S., Song C.S., Park S.Y., Lee J.B., Lee S.W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019;9:6838. doi: 10.1038/s41598-019-43280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yan H., Lv L., Xu Q., Yin C., Zhang K., Wang P., Hu J. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas. J. Anim. Sci. 2012;25:682–689. doi: 10.5713/ajas.2011.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi-Mosleh A., Sadeghi A.A., Mousavi S.N., Chamani M., Zarei A. Effects of in ovo infusion of probiotic strains on performance parameters, jejunal bacterial population and mucin gene expression in broiler chicken. Rev. Bras. Cienc. Avic. 2017;19:97–102. [Google Scholar]
- FDA . 2001. Bacteriological Analytical Manual. Aerobic Plate Count. Chapter 3.https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count Accesed Mar. 2020. [Google Scholar]
- Meijerhof R., Hulet R.M. In ovo injection of competitive exclusion culture in broiler hatching eggs. J. Appl. Poult. Res. 1997;6:260–266. [Google Scholar]
- Mountzouris K.C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Ruangpanit Y., Meijerhof R., Attamangkune S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci. 2011;90:2648–2655. doi: 10.3382/ps.2011-01415. [DOI] [PubMed] [Google Scholar]
- Nochi T., Jansen C.A., Toyomizu M., van Eden W. The well-developed mucosal immune systems of birds and mammals allow for similar approaches of mucosal vaccination in both types of animals. Front. Nutr. 2018;5:60. doi: 10.3389/fnut.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Yun H.M., Kim I.H. The effect of dietary Bacillus subtilis supplementation on the growth performance, blood profile, nutrient retention, and caecal microflora in broiler chickens. J. Appl. Anim. Res. 2018;46:868–872. [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017;96:1052–1062. doi: 10.3382/ps/pew381. [DOI] [PubMed] [Google Scholar]
- Reis M.P., Fassani E.J., Garcia A.A., Rodrigues P.B., Bertechini A.G., Barrett N., Persia M.E., Scmidt C.J. Effect of Bacillus subtilis (DSM 17299) on performance, digestibility, intestine morphology, and pH in broiler chickens. J. Appl. Poult. Res. 2017;26:573–583. [Google Scholar]
- Şahan U., Ipek A., Sozcu A. Yolk sac fatty acid composition, yolk absorption, embryo development, and chick quality during incubation in eggs from young and old broiler breeders. Poult. Sci. 2014;93:2069–2077. doi: 10.3382/ps.2013-03850. [DOI] [PubMed] [Google Scholar]
- Santini C., Baffoni L., Gaggia F., Granata M., Gasbarri R., Gioia D.D., Biavati B. Characterization of probiotic strains: An application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010;141:S98–108. doi: 10.1016/j.ijfoodmicro.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Schneitz C., Hakkinen M. The efficacy of a commercial competitive exclusion product on Campylobacter colonization in broiler chickens in a 5-week pilot-scale study. Poult. Sci. 2016;95:1125–1128. doi: 10.3382/ps/pew020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K., Chae B.J. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res.Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Shivaramaiah S., Pumford N.R., Morgan M.J., Wolfenden R.E., Wolfenden A.D., Torres-Rodríguez A., Hargis B.M., Téllez G. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult. Sci. 2011;90:1574–1580. doi: 10.3382/ps.2010-00745. [DOI] [PubMed] [Google Scholar]
- Steel R.G.D., Torrie J.H. McGraw-Hill; New York, NY: 1980. Principles and Procedures of Statistics: A Biometrical Approach. [Google Scholar]
- Stein T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Sumi C.D., Yang B.W., Yeo I.C., Hahm Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015;61:93–103. doi: 10.1139/cjm-2014-0613. [DOI] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. [Google Scholar]
- Torshizi M.A.K., Moghaddam A.R., Rahimi S., Mojgani N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010;51:178–184. doi: 10.1080/00071661003753756. [DOI] [PubMed] [Google Scholar]
- Triplett M.D., Zhai W., Peebles E.D., McDaniel C.D., Kiess A.S. Investigating commercial in ovo technology as a strategy for introducing probiotic bacteria to broiler embryos. Poult. Sci. 2018;97:658–666. doi: 10.3382/ps/pex317. [DOI] [PubMed] [Google Scholar]
- Vazquez A.P. Bacillus species are superior probiotic feed-additives for poultry. J. Bacteriol. Mycol. 2016;2:57–59. [Google Scholar]
- Wakenell P.S., Bryan T., Schaeffer J., Aviakin A., Williams C., Whitfill C. Effect of in ovo vaccine delivery route on herpesvirus of turkeys/sb-1 efficacy and viremia. Avian Dis. 2002;46:274–280. doi: 10.1637/0005-2086(2002)046[0274:EOIOVD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wine E., Gareau M.G., Johnson-Henry K., Sherman P.M. Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 2009;300:146–152. doi: 10.1111/j.1574-6968.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- Youssef G.A., Ezzeldeen N.A., Mostafa A.M., Sherif N.A. Effects of isolated Lactobacillus acidophilus as a probiotic on chicken vaccinated and infected with Salmonella typhimurium. Glob. Vet. 2011;7:449–455. [Google Scholar]
- Zhang L., Cao G., Zeng X.F., Zhou L., Ferket P.R., Xiao Y.P., Chen A.G., Yang C.M. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2014;9:46–53. doi: 10.3382/ps.2013-03412. [DOI] [PubMed] [Google Scholar]