Abstract
While surgical resection, local and cytotoxic therapies have long formed the basis of cancer care, immunotherapy now plays a key role in supplementing and even replacing these agents in the first line. Here we review the early success of programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 blockade and discuss biomarkers of therapeutic response. We next highlight a select group of novel targets in Phase III trials both as monotherapies and in combination with PD-1 inhibitors. Finally, we discuss innovations which seek to improve outcomes in therapy-resistant solid tumors.
Keywords: cancer, immunotherapy, solid tumors, tumor immunology
1 |. INTRODUCTION
Harnessing immunity against tumors has revolutionized the treatment of patients. Our growing understanding of the mechanisms by which cancer cells evade immunological attack has led to the discovery and targeting of a number of molecules to reinvigorate tumor immunity. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) were the among first of these molecules to achieve success and have since been at the forefront of cancer therapy. These targets have paved the way for discovery of novel pathways and innovation in addressing the limitations of current therapies and the complexities of the tumor-immune landscape.
Herein, we review the early and continued success of PD-1 and CTLA-4-based immunotherapies and their extensive application across solid tumors. We examine the most promising biomarkers and mechanisms that predict response to these therapies. While success with these targets has been vast, many combination therapies in development seek to extend efficacy to greater numbers of patients. Thus, we also review candidates farthest along in seeking Food and Drug Administration (FDA) approval as combination therapies. Finally, future immunotherapies beyond PD-1 and CTLA-4 are discussed, with commentary on the multitude of innovative combinations and the applicability of adoptive T cell therapies for patients refractory to standard checkpoint inhibitors. Overall, we review how far immunotherapies have come and where they will go to improve outcomes for patients with solid tumors.
1.1 |. Where we started: early immunotherapeutic targets
Our growing understanding of the tumor-immune interplay has led to a profound change in the approach to cancer therapy. T cell-bound negative regulatory proteins PD-1 and CTLA-4 interact with their associated ligands on a variety of cell types, including antigen presenting cells and regulatory T cells (Treg), leading to reduced T cell function and proliferation. As these receptors and their functions were elucidated, antibodies seeking to inhibit PD-1 and CTLA-4 became the first immunotherapies that achieved success in restoring antitumor immunity.
Ipilimumab, selectively targeting CTLA-4, was the first checkpoint inhibitor to demonstrate improved overall survival and durable response rates in patients with metastatic melanoma, a feat accomplished by no other therapies in this patient population.1 The first randomized Phase III trial, CA184-002, compared ipilimumab monotherapy with glycoprotein 100 (gp100) vaccine, known to induce weak antitumor responses, in patients with advanced melanoma. Ipilimumab as a single agent improved median overall survival to 10 months compared to 6.4 months in the gp100 cohort, with no added benefit seen with combination therapy. After a second Phase III trial, CA184-024, demonstrated extended survival with ipilimumab relative to standard chemotherapy with dacarbazine,2 FDA approval was granted for ipilimumab in 2011 as a first-line immunotherapeutic agent for advanced melanoma.
In 2014, the anti-PD-1 monoclonal antibody (mAb) nivolumab received accelerated FDA approval for patients with unresectable or metastatic melanoma. This decision was based on the CheckMate-037 Phase III trial, which demonstrated improved overall response rate with nivolumab compared to chemotherapy.3 In 2015, the anti-PD1 mAb pembrolizumab was approved as a first-line agent for the treatment of advanced metastatic melanoma. This was based on the KEYNOTE-006 randomized Phase III trial, in which pembrolizumab demonstrated superior progression-free survival and overall survival compared to ipilimumab monotherapy.4
Since these early approvals in melanoma, the number of indications and applications in other solid tumors have exploded, far extending the reach and impact of these drugs to cancer patients broadly (Table 1). Despite this widespread growth, surprisingly no other checkpoint inhibitory targets have received FDA approval since that time. As expanding the fraction of patients able to respond durably is important, we next discuss approaches to improve the efficacy of PD-1 and CTLA-4 therapy through our understanding of biological drivers of treatment response.
TABLE 1.
FDA-approved immunotherapeutic drugs by tumor type and associated landmark trials
| Drug/trade name | Target | Tumor type | Landmark trials |
|---|---|---|---|
| Pembrolizumab Keytruda | PD-1 | Advanced or unresectable melanoma | KEYNOTE 001 |
| Lymph node positive resecte melanoma | KEYNOTE 054 | ||
| Ipilimumab-refractory melanoma | KEYNOTE 002 | ||
| Advanced melanoma | KEYNOTE 006a | ||
| Metastatic PD-L1 positive NSCLC | KEYNOTE 010 | ||
| Metastatic PD-L1 positive NSCLC | KEYNOTE 024a | ||
| Metastatic non-squamous EGFR/ALK negative NSCLC | KEYNOTE 021a | ||
| Metastatic squamous NSCLC | KEYNOTE 407a | ||
| Stage III unresectable or metastatic NSCLC | NEYNOTE 042a | ||
| Advanced RCC | KEYNOTE 426a | ||
| Recurrent or metastatic HNSCC | KEYNOTE 012 | ||
| Unresectable or metastatic HNSCC | KEYNOTE 048a | ||
| Recurrent locally advanced or mestastic merkel cell carcinoma | KEYNOTE 017a | ||
| Locally advanced or metastatic TNBC | KEYNOTE 355 | ||
| Recurrent locally advanced or metastatic PD-L1 positive GEJ or gastric adenocarcinoma | KEYNOTE 059 | ||
| MSI-H or dMMR CRC | KEYNOTE 177a | ||
| Previously treated HCC | KEYNOTE 224 | ||
| MSI-H or dMMR solid tumors | NCT01876511 | ||
| Recurrent or metastatic PD-L1 positive cervical cancer | KEYNOTE 158 | ||
| Metastatic SCLC progressed on chemotherapy | KEYNOTE 028, KEYNOTE 158 | ||
| Recurrent or metastatic cutaneous SCC | KEYNOTE 629a | ||
| Locally advanced or metastatic urothelial carcinoma progressed on chemotherapy | KEYNOTE 045 | ||
| Locally advanced or metastatic urothelial carcinoma ineligible for chemotherapy | KEYNOTE 052a | ||
| BCG-unresponsive, high-risk NMIBC | KEYNOTE 057 | ||
| Locally recurrent unresectable or metastatic PD-L1 positive TNBC | KEYNOTE 355a | ||
| Advanced endometrial carcinoma progressed on chemotherapy | KEYNOTE 146 | ||
| Recurrent locally advanced or metastatic PD-L1 positive esophageal SCC | KEYNOTE 180 | ||
| Nivolumab Opdivo | PD-1 | Unresectable or metastatic melanoma | CheckMate-037 |
| Unresectable or metastatic BRAF600 wild-type melanoma | CheckMate-067a | ||
| Lymph node positive resected melanoma | CheckMate-238 | ||
| Advanced squamous NSCLC progressed on chemotherapy | CheckMate-017 | ||
| Metastatic NSCLC | Checkmate-057 | ||
| Metastatic or recurrent EGFR/ALK negative NSCLC | CheckMate-227, CheckMate-9LAa | ||
| Metastatic RCC | CheckMate-025 | ||
| Advanced, untreated RCC | CheckMate-214a | ||
| Recurrent or metastatic HNSCC | CheckMate-141 | ||
| MSI-H or dMMR metastatic CRC | CheckMate-142 | ||
| Previously treated HCC | CheckMate-040 | ||
| SCLC progressed on chemotherapy | CheckMate-032 | ||
| Locally advanced unresectable or metastatic urothelial carcinoma progressed on chemotherapy | CheckMate-275 | ||
| Unresectable advanced, recurrent or metastatic esophageal SCC | ATTRACTION-3 | ||
| Avelumab Bavencio | PD-1 | Merkell cell carcinoma | JAVELIN Merkel 200 |
| Advanced RCC | JAVELIN Renal 101a | ||
| Locally advanced or metastatic urothelial carcinoma progressed on chemotherapy | JAVELIN solid tumor | ||
| Locally advanced or metastatic urothelial carcinoma | JAVELIN bladder 100a | ||
| Cemiplimab Libtayo | PD-1 | Locally advanced or metastatic >50% PD-L1 NSCLC | EMPOWER-Lung1a |
| Locally advanced or metastatic cutaneous SCC | NCT02760498 | ||
| Atezolizumab Tecentriq | PD-L1 | Stage III NSCLC not progressed following platinum-based chemotherapy and radiation | PACIFIC |
| Metastatic EGFR/ALK negative NSCLC | IMpower130a | ||
| Metastatic PD-L1 high NSCLC | IMpower110a | ||
| BRAF600 unresectable or metastatic melanoma | IMspire150a | ||
| Unresectable or metastatic HCC | IMbrave150a | ||
| Extensive-stage SCLC | IMpower133a | ||
| Locally advanced or metastatic urothelial carcinoma | IMvigor210a | ||
| Locally advanced or metastatic PD-L1 positive TNBC | IMpassion100a | ||
| Durvalumab Imfinzi | PD-L1 | Extensive-stage SCLC | CASPIAN |
| Previously treated advanced bladder cancer | NCT01693562 | ||
| Ipilimumab Yervoy | CTLA-4 | Unresectable, advanced melanoma | MDX010-020 |
| BRAF V600 wild-type unresectable or metastatic melanoma | CheckMate-069 | ||
| Unresectable or metastatic melanoma | CheckMate-067 | ||
| Lymph node positive resected melanoma | CA 184-029 | ||
| Advanced, untreated RCC | CheckMate-214a | ||
| MSI-H or dMMR metastatic CRC | CheckMate-142 |
Abbreviations: CRC; hepatocellular carcinoma; CTLA-4, cytotoxic T-lymphocyte associated protein 4; dMMR, deficient mismatch repair; FDA, Food and Drug Administration; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; MSI-H, microsatellite instability-high; NSCLC, nonsmall-cell lung cancer; SCC, squamous cell carcinoma; SCLC, small-cell lung cancer; TNBC, triple negative breast cancer.
Denotes first-line therapy.
1.2 |. Mechanisms of response to checkpoint immunotherapies
While outcomes of checkpoint blockades are promising, it remains clear that only subsets of patients experience durable responses. Therefore, one major area of research focus is dedicated to elucidating why and how patients respond these therapies. We next discuss the current understanding of two main biomarkers of response to PD-1 and CTLA-4 blockade.
1.3 |. Programmed death ligand-1 tumor expression
Expression of programmed death ligand-1 (PD-L1), the canonical ligand for PD-1, has emerged as a practice-changing biomarker predictive of efficacy in a number of patients with solid tumors. PD-L1 expression on tumors is associated with treatment response in patients with nonsmall-cell lung cancer (NSCLC). In the landmark KEYNOTE-001 Phase I study in NSCLC, a more than 50% PD-L1 tumor proportion score correlated with improved outcomes following treatment with PD-1 inhibitor relative to tumors expressing lower PD-L1.5 These findings led to FDA approval of pembrolizumab as a first-line agent in this PD-L1-high population. Subsequent Phase III trials demonstrated increased overall survival, with an overall response rate of 44.8% with first-line pembrolizumab compared with standard of care platinum-based chemotherapy in patients with ≥50% PD-L1 tumor proportion score (KEYNOTE-024).6 Given that pembrolizumab monotherapy is a standard treatment option for high tumor PD-L1 expression in NSCLC, PD-L1 is now routinely tested in newly diagnosed patients to guide treatment plans.
In addition to pembrolizumab, the anti-PD-L1 mAb atezolizumab received FDA approval for first-line treatment of patients with metastatic NSCLC with PD-L1 expression in more than 50% of tumor cells or more than 10% tumor area with PD-L1 expressing immune cells. The POPLAR Phase II randomized trial demonstrated improved overall survival with atezolizumab compared with chemotherapy in patients with previously treated NSCLC without genetic driver mutations, which correlated with both tumor cell and tumor-infiltrating immune cell PD-L1 expression levels.7 Combination nivolumab plus ipilimumab was also effective in PD-L1 expressing tumors in the CHECKMATE-227 trial8; thus, nivolumab/ipilimumab was approved for first-line treatment of metastatic NSCLC in tumors with more than 1% PD-L1 expression.
In addition to NSCLC, patients with other types of solid tumors are showing improved outcomes in PD-L1 expressing cancers. In urothelial carcinoma, pembrolizumab (KEYNOTE-052) and atezolizumab (IMvigor210) demonstrated improved survival in locally advanced or metastatic disease either with tumor area more than 5% positive for PD-L1 expressing tumor-infiltrating immune cells or regardless of PD-L1 status9,10; however, these findings are in contrast to those from an early Phase I/II study investigating durvalumab, a direct PD-L1 inhibitor, which has shown durable disease response rates irrespective of PD-L1 expression in locally advanced or metastatic disease.11 Similar accelerated approvals have been granted for pembrolizumab in unresectable or metastatic triple-negative breast cancer with PD-L1 positive score more than 1012 and atezolizumab with PD-L1 immune cell positive-disease (IMpassion130),13 as well as for pembrolizumab in advanced PD-L1 positive cervical cancer (KEYNOTE-158).14
Despite the broad application of PD-L1 expression guiding disease management, its use as a reliable biomarker is not without limitations. Currently, there are a number of different validated FDA-approved antibody tests; however, one of these tests identified less than half of patients as PD-L1 expressers compared to the others.15 Further, there is a lack of standardization among scoring algorithms across tests. However, as testing becomes more standardized and is used with more patients, it is likely that the reliability of PD-L1 as a biomarker will continue to develop.
1.4 |. Tumor mutational burden
Tumor mutational burden (TMB) is emerging as a second biomarker of treatment response. Cancers with a higher frequency of mutations, such as melanoma and NSCLC,16 were among the first to show a benefit from CTLA-4 and PD-1 blockade. These therapies are also particularly effective in solid tumors with a high mutational load, including those with genomic instability (microsatellite instability-high or MSI-H) or deficiencies in mismatch repair (dMMR). Most recently in June of 2020, pembrolizumab joined the list of FDA approved therapies in the first-line for metastatic colorectal cancer with MSI-H or dMMR status. So why does mutational burden matter? It is posited that tumors with a high TMB may have a greater frequency of tumor-specific immune cells capable of responding to checkpoint blockade therapies.
Over several years, the reliability of TMB as a biomarker has been similar to PD-L1, in that for some trials, TMB was a clear predictor while in others the results were less clear. For example, TMB predicts better survival but not response to PD-1 therapy in melanoma patients,17 whereas it does predict response to PD-1 blockade in NSCLC.18 Combining trial data across multiple tumor types, an analysis of more than 100 trials concluded that TMB is significantly associated with response to PD-1, CTLA-4, and combination therapies, but did not correlate with toxicity of treatment.19 Remaining challenges to establish TMB as a robust biomarker include the method used to quantify mutations, appropriate cutoffs that determine “high” versus “low” TMB status, and whether the magnitude of mutations translates to longer survival. Further, does the quality or type of mutations matter? Improving our understanding of how specific mutations impact the response to immunotherapy may help disentangle these questions.
1.5 |. Other biomarkers in development
Because PD-L1 and TMB are not ideal biomarkers for all contexts, there has been a rigorous search for alternate biomarkers to stratify patients. Other examples which have been investigated on a smaller scale include T cell infiltration, presence of stem-like T cells, IFN-gamma response signatures, T cell receptor clonality, and microbiome. While an in-depth discussion of each of these biomarkers is beyond the scope of the current review, these biomarkers have been reviewed elsewhere.20
1.6 |. Where we are now: promising targets coming down the pipeline
The success of CTLA-4 and PD-1 targeted immunotherapies in the treatment of solid tumors ushered in an era of innovation, leading to the discovery of a number of exciting novel targets currently under investigation. Four novel agents in Phase III combination trials are summarized in Table 2 and discussed next, along with their varied success and implications for the future.
TABLEE 2.
Novel therapeutic targets in solid tumors currently under later phase clinical trial investigation
| Target | Other agents | Identifier | Phase | Tumor types | Sponsor/collaborator |
|---|---|---|---|---|---|
| LAG-3 | PD-1 | NCT03470922 | II/III | Untreated unresectable or metastatic melanoma | Bristol-Myers Squibb |
| PD-1 | NCT04082364 | II/III | HER2-positive gastric cancer or GEJ cancer | MacroGenics | |
| IDO1 | PD-1 | NCT03661320 | III | Muscle-invasive bladder cancer | Bristol-Myers Squibb |
| PD-1 | NCT03260894 | III | Metastatic RCC | Incyte Corporation | |
| IL-2 | PD-1 | NCT03635983 | III | Untreated unresectable or metastatic melanoma | Bristol-Myers Squibb |
| PD-1 | NCT04209114 | III | Muscle-invasive bladder cancer | Bristol-Myers Squibb | |
| PD-1 | NCT03729245 | III | Advanced metastatic RCC | Bristol-Myers Squibb | |
| PD-1 | NCT04410445 | III | Stage III/IV cutaneous melanoma | Bristol-Myers Squibb | |
| IL-15 | NCT03022825 | II/III | BCG-unresponsive high grade NMIBC | ImmunityBio, Inc. | |
| PD-1 | NCT03520686 | III | Stage III/IV advanced or metastatic NSCLC | Altor BioScience |
Abbreviations: GEJ, gastroesophageal junction; IDO1, indoleamine 2,3-deoxygenase 1; IL-2, interleukin-2; IL-15, interleukin-15; LAG-3, lymphocyte activation gene-3; NMIBC, nonmuscle invasive bladder cancer; NSCLC, nonsmall-cell lung carcinoma; PD-1, programmed cell death protein-1; RCC, renal cell carcinoma.
1.7 |. Lymphocyte activation gene-3: another promising checkpoint inhibitor
Lymphocyte activation gene-3 (LAG-3) is a promising novel target in the family of inhibitory receptors. LAG-3 is expressed on a number of cell types, including tumor-infiltrating lymphocytes, and is known to suppress CD4+ T cell effector function while simultaneously enhancing Treg suppressor activity (Figure 1).21,22 LAG-3 is often coexpressed with other inhibitory molecules, including PD-1, TIGIT, and TIM-3. Collectively, these molecules promote immune cell exhaustion, resulting in impaired effector function and decreased cytokine secretion.23 Further, LAG-3 coexpression with PD-1 in tumor infiltrating lymphocyte (TIL) CD4+ and CD8+ T cell populations has demonstrated a synergistic role in immune tolerance to tumor antigens.24 These findings suggest that combined blockade could offer a promising avenue for countering immune evasion.
FIGURE 1.
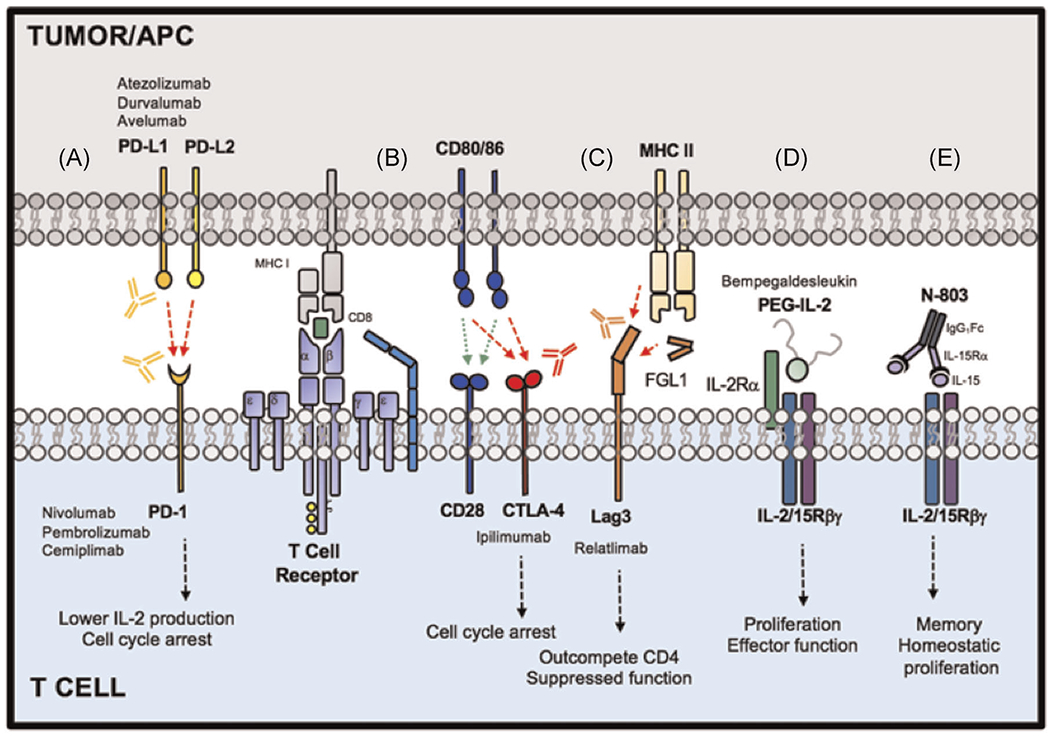
Select immunotherapeutic targets currently under Phase III investigation
Clinically, the combination of anti-LAG-3 with anti-PD-1 is now entering the forefront of combination candidates seeking FDA approval. Relatlimab is under investigation in a Phase II/III randomized trial in combination with nivolumab relative to nivolumab alone for patients with unresectable or metastatic melanoma (NCT03470922). Early phase studies suggest that this combination may be effective in patients regardless of prior immunotherapy; for example, the TACTI-mel Phase I study in metastatic melanoma found a 33% overall response rate to LAG-3/PD-1 combination therapy eftilagimod alpha and pembrolizumab in a cohort of pembrolizumab-refractory patients and 50% overall response rate in PD-1 blockade-naïve patients.25 Clinical outcomes and correlates from these trials will be critical to understand mechanisms of response to combined LAG-3/PD-1 therapy to identify patients who benefit most from this specific combination.
1.8 |. Metabolic pathways in the tumor microenvironment
The tumor microenvironment capitalizes on metabolic pathways directly responsible for immune suppression. One of these pathways involves IDO1 (indoleamine 2,3-deoxygenase 1), an enzyme responsible for the breakdown of the essential amino acid tryptophan.26 Depletion of tryptophan stores, a vital fuel source, via IDO1 results in impaired T cell proliferation. Further, kyneurenine, a catabolite of tryptophan degradation, induces apoptosis of Th1 cell populations and promotes Treg proliferation.27 Therefore, it was proposed that inhibition of IDO1 could be a new approach to restore T cell immunity in the tumor.
Epacadostat (INCB024360) is an orally available potent IDO1 inhibitor currently under investigation in a number of clinical trials. Encouraging results from early phase trials subsequently led to a Phase III randomized, double blind trial of epacadostat in combination with pembrolizumab in unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252). Surprisingly, this trial failed to show improved progression-free survival or overall survival for this combination compared with pembrolizumab alone, bringing to question the utility of IDO1 inhibitors. Despite these negative results, many studies with epacadostat in combination therapies are ongoing in patients with solid tumors. Combination IDO1 inhibitor and nivolumab has demonstrated early antitumor efficacy in advanced bladder cancer (NCT02658890)28 and is under continued Phase I/II investigation in other solid tumors, including HNSCC (NCT03854032) and NSCLC and melanoma (NCT02658890).
1.9 |. Bempegaldesleukin: the new face of IL-2
Interleukin-2 (IL-2) is a potent cytokine produced upon antigen stimulation, resulting in T cell growth, activation, and differentiation. Interestingly, in lower doses, IL-2 also promotes regulatory T cell production and maintenance. High-dose IL-2 therapy induced 6-8% complete cancer regression rates and up to 10% partial regression rates with durable responses in patients with metastatic melanoma and renal cell cancer.29 As a result, IL-2 received FDA approval for the treatment of metastatic renal cell cancer in 1992 and metastatic melanoma in 1998.30,31 However, the significant systemic toxicity and the short circulatory half-life of IL-2 greatly hindered its biological activity. Collectively, these limitations pushed the use of recombinant IL-2 out of favor with many physicians.
To address these obstacles and capitalize upon the effects of IL-2, bempegaldesleukin (NKTR-214) was developed as an IL-2 conjugated with polyethylene glycol. Bempegaldesleukin has a longer half-life and selectively induces activation and proliferation of CD8+ T cells and natural killer (NK) cells while avoiding Treg expansion. In the first in-human Phase I trial, bempegaldesleukin demonstrated durable antitumor efficacy in patients with pretreated advanced or metastatic solid tumors.32 Bempegaldesleukin in combination with nivolumab received breakthrough therapy designation by the FDA for patients with untreated unresectable or metastatic melanoma following the Phase I/II PIVOT-02 trial, which reported a 53% overall response rate and a 34% complete response rate,33 with the greatest responses seen among patients with PD-L1 expressing tumors. A Phase III trial (PIVOT IO 001) evaluating bempegaldesleukin in combination with nivolumab compared to nivolumab alone as first-line therapy for patients with advanced melanoma is currently ongoing (NCT03635983) (Table 2). This same combination regimen of bempegaldesleukin with nivolumab is being studied versus investigator’s choice of tyrosine kinase inhibitor therapy (sunitinib or cabozantinib monotherapy) in advanced metastatic renal cell carcinoma in a Phase III randomized open label trial (NCT03729245).34 The outcomes of these studies will impact the future of IL-2 as an immunotherapy and will continue to inform the design of novel targeted cytokines.
1.10 |. IL-15 superagonist
IL-15 is a homeostatic cytokine which supports survival and proliferation of CD8+ memory T cells and NK cells and has emerged as a promising immune activator for cancer immunotherapy.35 IL-15 signals to immune cells via the cell surface receptor, IL-2/IL-15Rβγ, shared between IL-2 and IL-15. Clinically, the agent ALT-803/N-803 was developed as an “IL-15 superagonist” equipped with potent IL-15Rβγ-activating ability (Figure 1). ALT-803 harnesses a high affinity IL-15 cytokine complexed to IL-15-Rα and fused to an IgG1 Fc domain, which armors the drug with enhanced potential to activate the receptor relative to endogenous or recombinant IL-15 cytokine.36 In a Phase 1b study in NSCLC, the combination of IL-15 superagonist and anti-PD-1 was safe with promising efficacy.37 Interestingly, the addition of ALT-803 could initiate objective antitumor responses in patients unresponsive to PD-1 therapy as a single agent.37 ALT-803 is now in Phase III trials for metastatic NSCLC in combination with PD-1 blockade as a first-line strategy relative to PD-1 blockade alone (NCT03520686) (Table 2). While the mechanism of immune reactivation is not fully understood, future study in patients will reveal the relative contributions of CD8+ T cells and NK cells to tumor immunity and whether this cytokine plays a role in preventing or delaying T cell exhaustion in the tumor.
2 |. WHAT’S NEXT FOR CLINICAL IMMUNOTHERAPY?
2.1 |. Combination therapies in development in early Phase I/II trials
While many therapies are currently in Phase III clinical trials, a multitude of novel agents are in development in hundreds of early phase trials. Other immune checkpoint inhibitors, targeting TIM-3 (T-cell immunoglobulin- and mucin-domain containing molecule-3), TIGIT (T-cell Ig and ITIM domain), and VISTA (V-domain Ig suppressor of T cell activation), are currently under investigation for their ability to promote tumor immunity. Antibodies which agonize costimulatory molecules, including ICOS (inducible costimulator), GITR (glucocorticoid-induced tumor necrosis facto receptor), and CD27, act to promote T cell activation, proliferation, and cytokine production, and have the potential to foster T cell memory to tumors.
In addition to targeting surface molecules, methods for manipulating the tumor and immune microenvironments are under investigation. Blocking the adenosine pathway, a key metabolic pathway known to hinder immune function and fuel cancer growth,38 is a promising strategy to alleviate suppression within the tumor microenvironment. A2AR inhibition with small molecules blocks the ability of adenosine to both support Treg activity and blunt effector T cell function. Several of these small molecules are currently under investigation as monotherapy or in combination with anti-PD-1/PD-L1 mAb.
Finally, manipulating immune cell access to the tumor microenvironment is another promising avenue for solid tumor therapies. Recently, combination nivolumab with pepinemab, a novel agent blocking SEMA4D, was reported at ASCO 2020 to promote immune cell infiltration for melanoma in the neoadjuvant setting and resulted in stable disease or better in a cohort of eight patients.39 Within a few years, investigators will be inundated with results from these early phase clinical trials, which will likely reveal the next generation of checkpoint inhibitors, costimulatory agonists, metabolic, and tumor microenvironment modulators which will be most impactful on patient outcomes.
2.2 |. Adoptive T cell therapy: a silver bullet or futile attempt postcheckpoint blockade?
Another strategy to augment the immune system’s response to cancer cells is adoptive T cell therapy. This approach harnesses a patient’s own T cells to kill tumors with the potential for inducing long-lived immunity40 and has shown great promise in patients with either solid tumors or hematological malignancies. T cell products are generated in two ways: (1) by selecting endogenous T cells from the tumor (i.e., TIL)41 or peripheral blood,42 or (2) by engineering T cells with antigen receptors (T cell receptor, TCR, or chimeric antigen receptor, CAR) against a desired tumor antigen. To date, there are three FDA approved T cell therapies (axicabtagene ciloleucel, tisagenlecleucel, and brexucabtagene autoleucel) which are all CD19-CAR T cell products approved for B cell malignancies.
While CAR T cells have been effective in hematologic malignancies, TIL therapies have shown efficacy in metastatic solid malignancies. The promise of this therapy is vast: up to 50% of metastatic melanoma patients have experienced an objective response to their own TIL in clinical trials.43–46 Such responses extend to other solid tumors as well.47–50 While promising, a large proportion of patients either relapse after treatment or do not benefit.
Given the challenges of T cell manufacture, use of T cell therapy may be most appropriate in the next line for patients who fail mAb therapies. Yet, there is conflicting evidence on whether TIL retain lytic activity against tumors after failing checkpoint immunotherapies. In a cohort of metastatic melanoma patients, response rates were similar compared to patients who failed prior immunotherapy to the whole cohort.46 Other reports also concluded no influence of prior CTLA-4 blockade on TIL therapy outcomes.45 However, some literature suggests that prior therapies negatively impact TIL therapy. In a cohort of 74 patients treated at MD Anderson, the overall response rate to TIL was lower in patients failing CTLA-4 blockade or CTLA-4/PD-1 coblockade.51 Additionally, new evidence suggests that patients who received TIL earlier on in their treatment course were more likely to respond than those receiving TIL in later lines.52 Further studies on the long-term impact of checkpoint blockade on T cell biology, particularly in patients with varied responses, will help elucidate whether TIL therapy is feasible and effective in this patient population.
3 |. CONCLUSION
The landscape of immunotherapy in the treatment of solid tumors is perpetually evolving.
Inhibitors of PD-1 and CTLA-4 achieved significant early success to restore antitumor immunity. While these checkpoint inhibitors have remained at the forefront of immunotherapeutics currently in practice, our understanding of the tumor-immune interface has ushered in an era of continued discovery and innovation. These ongoing efforts to isolate biomarkers that correlate with treatment responsiveness, identify novel targetable pathways, and harness the power of combination treatments and adoptive immunotherapy will continue to transform our approaches to these complex disease states.
REFERENCES
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. 10.1056/NEJMoa1003466. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. 10.1056/NEJMoa1104621. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. 10.1016/S1470-2045(15)70076-8. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. 10.1056/NEJMoa1503093. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. 10.1056/NEJMoa1501824. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. 10.1056/NEJMoa1606774. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 7.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. 10.1016/S0140-6736(16)00587-0. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 8.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. 10.1056/NEJMoa1910231. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 9.Vuky J, Balar AV, Castellano D, et al. Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol. 2020;38(23):2658–2666. 10.1200/JCO.19.01213. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016; 387(10031):1909–1920. 10.1016/S0140-6736(16)00561-4. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. 10.1001/jamaoncol.2017.2411. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. 10.1016/S0140-6736(20)32531-9. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 13.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. 10.1016/S1470-2045(19)30689-8. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 14.Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17): 1470–1478. 10.1200/JCO.18.01265. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 15.Rimm DL, Han G, Taube JM. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancerassessment of 4 assays for PD-L1 expression in NSCLC assessment of 4 assays for PD-L1 expression in NSCLC. JAMA Oncology. 2017;3(8):1051–1058. 10.1001/jamaoncol.2017.0013. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. 10.1038/nature12477. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. 10.1016/j.cell.2016.02.065. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.). 2015;348(6230):124–128. 10.1126/science.aaa1348. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osipov A, Lim SJ, Popovic A, et al. Tumor mutational burden, toxicity, and response of immune checkpoint inhibitors targeting PD(L) 1, CTLA-4, and combination: a meta-regression analysis. Clin Cancer Res. 2020;26(18):4842–4851. 10.1158/1078-0432.CCR-20-0458. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havel JJ, Chowell D, Chan TA The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3): 133–150. 10.1038/s41568-019-0116-x. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol. 2005;174(2): 688–695. 10.4049/jimmunol.174.2.688. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 22.Huang CT, Workman CJ, Flies D. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–513. 10.1016/jjmmuni.2004.08.010. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 23.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34: 539–573. 10.1146/annurev-immunol-032414-112049. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 24.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. 10.1158/0008-5472.CAN-11-1620. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson V, Khattak A, Haydon A, et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer. 2020;8(2): e001681. 10.1136/jitc-2020-001681. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Théate I, van Baren N, Pilotte L, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3(2):161–172. 10.1158/2326-6066.CIR-14-0137. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 27.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3): 193–207. 10.1016/j.it.2016.01.002. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luke JJ, Tabernero J, Joshua A, et al. BMS-986205, an indoleamine 2, 3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (nivo): updated safety across all tumor cohorts and efficacy in advanced bladder cancer (advBC). J Clin Oncol. 2019;37(7_suppl):358. 10.1200/JCO.2019.37.7_suppl.358. [published Online First: Epub Date]. [DOI] [Google Scholar]
- 29.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228(3):307–319. 10.1097/00000658-199809000-00004. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. 10.1158/1078-0432.CCR-11-0116. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. 10.1200/JCO.1999.17.7.2105. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 32.Bentebibel SE, Hurwitz ME, Bernatchez C, et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ—biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9(6):711–721. 10.1158/2159-8290.CD-18-1495. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 33.Diab A, Tykodi S, Daniels G, et al. 420 Progression-free survival and biomarker correlates of response with BEMPEG plus NIVO in previously untreated patients with metastatic melanoma: results from the PIVOT-02 study. J Immunother Cancer. 2020;8(Suppl 3):A446. 10.1136/jitc-2020-SITC2020.0420. [published Online First: Epub Date]. [DOI] [Google Scholar]
- 34.Tannir NM, Agarwal N, Pal SK, et al. PIVOT-09: a phase III randomized open-label study of bempegaldesleukin (NKTR-214) plus nivolumab versus sunitinib or cabozantinib (investigator’s choice) in patients with previously untreated advanced renal cell carcinoma (RCC). J Clin Oncol. 2020;38(6_suppl):TPS763. 10.1200/JCO.2020.38.6_suppl.TPS763. [published Online First: Epub Date]. [DOI] [Google Scholar]
- 35.Dwyer CJ, Knochelmann HM, Smith AS, et al. Fueling cancer immunotherapy with common gamma chain cytokines. Front Immunol. 2019;10:263. 10.3389/fimmu.2019.00263. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein MP, Kovar M, Purton JF. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proceedings of the National Academy of Sciences. 2006;103(24):9166–9171. 10.1073/pnas.0600240103. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrangle JM, Velcheti V, Patel MR, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19(5):694–704. 10.1016/s1470-2045(18)30148-7. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17(10):611–629. 10.1038/s41571-020-0382-2. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 39.Lowe MC, Olson B, Martinez A, et al. Integrated biomarker study of neoadjuvant pepinemab and nivolumab in patients with resectable metastatic melanoma. J Clin Oncol. 2020;38(15_suppl):10061. 10.1200/JCO.2020.38.15_suppl.10061. [published Online First: Epub Date]. [DOI] [Google Scholar]
- 40.Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. CAR T cells in solid tumors: blueprints for building effective therapies. Front Immunol. 2018;9:1740. 10.3389/fimmu.2018.01740. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudley M, Wunderlich J, Shelton T, Even J, Rosenberg S. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences. 2002;99(25):16168–16173. 10.1073/pnas.242600099. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. 10.1158/1078-0432.CCR-10-0041. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. 10.1158/1078-0432.CCR-11-0116. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19(17):4792–4800. 10.1158/1078-0432.CCR-13-0380. [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 46.Goff SL, Dudley ME, Citrin DE, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34(20):2389–2397. 10.1200/JCO.2016.66.7220. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus–targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543–1550. 10.1200/JCO.2014.58.9093. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran E, Robbins PF, Lu Y-C, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255–2262. 10.1056/NEJMoa1609279. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24:724–730. 10.1038/s41591-018-0040-8. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forget M-A, Haymaker C, Hess KR, et al. Prospective analysis of adoptive TIL therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. Clin Cancer Res. 2018;24:4416–4428. 10.1158/1078-0432.Ccr-17-3649. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borch TH, Andersen R, Ellebaek E, et al. Future role for adoptive T-cell therapy in checkpoint inhibitor-resistant metastatic melanoma. J Immunother Cancer. 2020;8(2):e000668. 10.1136/jitc-2020-000668. [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]


