Highlights
-
•
Diosgenin extraction from Trigonella foenum graecum seeds.
-
•
Analyzing better extract yield and diosgenin content from both methods.
-
•
Both yield of extract and diosgenin content is higher with ultrasound than microwave assisted extraction methods.
Keywords: Fenugreek, Diosgenin, Ultrasound assisted extraction, Microwave assisted extraction, Solvent, Treatment time
Abstract
From the recent market trend, there is a huge demand for the bioactive compounds from various food matrices that could be capable enough to combat the emerging health effects in day-to-day life. Fenugreek is a well-known spice from ancient times for its medicinal and health benefits. In the present study, two methods of green extraction microwave (MAE) and ultrasound (UAE) assisted were studied in regard of extraction of fenugreek diosgenin. In this study, solvent type (acetone, ethanol, hexane and petroleum ether), solvent concentration (40, 60, 80 and 100%) and treatment time (1.5, 3.0, 4.5 and 6.0 min and 30, 40, 50 and 60 min for MAE and UAE method respectively) was varied to observe the effect of these parameters over extract yield and diosgenin content. The results of this study revealed that treatment time, type of solvent and its concentration and method adopted for extraction of diosgenin has significant effect. In relation with better yield extract and diosgenin content, the yield of fenugreek seed extract was 7.83% with MAE and 21.48% with UAE of fenugreek seed powder at 80% ethanol concentration at 6 and 60 min respectively. The content of diosgenin was observed in fenugreek seed powder extract was 35.50 mg/100 g in MAE and 40.37 mg/100 g in UAE with 80% ethanol concentration at 6 and 60 min respectively. The overall range of yield of fenugreek extract was varied from 1.04% to 32.48% and diosgenin content was 15.82 mg/100 g to 40.37 mg/100 g of fenugreek seed powder including both extraction methods. This study revealed that UAE would impose better ways for preparing fenugreek extract and observing diosgenin content from fenugreek seeds.
1. Introduction
Fenugreek is one of the most known spices since old times. It also called as Trigonella foenum graceum which is a diploid legume that belongs to Leguminosae family. Initially, fenugreek seeds are best considered for usage, with the passage of time its shoots could be a better option for forage. The fenugreek seed contains tons of health beneficial properties like hypolipidemic effect [1], antioxidant- hypoglycemia effects [2], anticholesterolemic, antileprotic, antibronchitic, antimicrobial, anthelmintic effects [3], [4], [5], [6], anti-inflammatory and antipyretic properties [7], and better source of galactagogue aid for nursing mothers [8], [9]. The roasted fenugreek seeds could be a better remedy for groin pain and hernia [10].
There are few saponins naturally present in fenugreek seeds, out of which diosgenin prove more nutritionally beneficial. It is commercially procured from variety of yam, fenugreek, and bitter gourd [11]. In the face of pharmaceutical industry, diosgenin is synthetic steroidal drugs that could be extracted from some natural herbs like fenugreek. Diosgenin is spirostanol saponin which consists of hydrophilic sugar moiety and linked to a steroid aglycone that is hydrophobic in nature and its structure is similar to cholesterol [12]. The studies had been conducted related to the diosgenin and health effects. Diosgenin pose a better solution for the metabolic diseases [blood sugar, obesity, hyperlipidemia, and hypercholesterolemia], cancer and inflammation [13].
Diosgenin increasing market demand for its various health benefits lure researchers to enhance the production of diosgenin. Diosgenin bioactive properties are major and vibrant area for study in past few decades [14].
The diosgenin is majorly synthesized in pharmaceutical industries as it acts as precursor for hormones like progesterone, testosterone and glucocorticoids [15]. Along with the hidden benefits of diosgenin, yield is low that ultimately requires a huge set up to scale up production of diosgenin [16]. The extraction method consists of hydrolysis of ethanolic extract by hydrochloric acid. Another study conducted by Sauvaire et al. [17], reported that sulfuric acid hydrolysis yielded more saponin extraction from fenugreek seeds.
The diosgenin could also be extracted from the Dioscorea deltoidea which is an endangered species having tubers with good extract yield by green extraction method like MAE under shade drying conditions [18]. The extraction of diosgenin was also reported by Biswas et al. [19] from some endophytic fungi and bacteria to scale the production of diosgenin based drugs.
The extraction of diosgenin become more interesting day by day, as one of the studies reveals that sulfonated magnetic solid composites along with acid hydrolysis by 2.5 M hydrochloric acid aids in diosgenin extraction from Dioscorea nipponica Makino [20]. Hayat et al., [21] studied the effect of MAE treatment over the calcium content of citrus peels that was increased by 5.4% and 24.2% of kinnow and Feutrell’s early respectively. The study of valorization of Camellia sinensis branches with MAE and pressurized hot water extraction (autohydrolysis) to recover bioactive compounds from tea branches. It revealed that MAE produces much better results in respect of saving time and it also depicted the effect of temperature over the total phenolic content and antioxidant activity which were reduced with MAE and enhanced with autohydrolysis temperature [22]. Extraction of pectin from eggplant peel by UAE method generated a much better extraction yield of 33.64 g/100 g. The extracted peel pectin was rich in galacturonic acid (66.08 g/100 g) and total phenolic content (96.81 mg GAE/g pectin) [23]. The enhancement of the physical and nutritional quality of proso millet bran with the UAE method was studied. It revealed that the application of the UAE method during extraction, aids in limiting the browning of the sample, activation of polyphenol oxidase and enhanced dietary fibre by up to 38%. It imposes a better way to intend its usage in the bakery industry [24]. UAE method was also studied with mango peels for pectin extraction and it aids in enhancing over 50% pectin extraction yield without affecting its colour and quality attributes [25]. All these studies reported in the support of usage of green technology for the extraction of bioactive compounds from various sample matrices.
Out of these many techniques, for this work microwave and ultrasonic-assisted extraction methods were considered. Microwave-assisted extraction (MAE) is a non-contact method of heating that aids in reducing the thermal gradient and accelerating the energy transfer. It is generally used to extract the flavouring compound, antioxidants and essential oils [26]. This technique generates a quick and huge amount of heat in the solute–solvent mixture and requires less treatment time for extraction of the compounds [27].
Ultrasonic Assisted Extraction (UAE) method utilizes sound waves of 20 kHz to 100 MHz that were capable enough to generate the cavitation process which generates the much amount of extract by collapsing the bubble formed [28], [29]. These sound waves accelerate the heat and mass transfer of the entire process which leads to damage to the cell wall of the solute embedded cell and it results in the release of a much higher amount of bioactive compound [30]. Solvents, solvent concentration and extraction time affects the yield of extract and bioactive compounds [31]. The main eye-catching benefit of UAE is less consumption of solvent, less production of harmful chemicals back to the environment and less damage to the sample matrix.
UAE also provides a way to use GRAS solvents by improving their extraction performance [32].UAE proved to be more beneficial for the extraction of amino acid from grapes [33], pectin from sisal waste [34], anthocyanins from black chokeberry waste [35], geniposide from traditional Chinese medicine [36]. There are some more food matrices from which the extraction of bioactive was performed like carrageenan and alginates from Sargassum binderi, Turbinaria ornate, Kappaphycus alvarezii and Euchema denticulatum [37], fucose and glucan from L. digitata, L. hyperborea and A. nodosum [38] and phenolics and antioxidant activity from Hormosira banksii [39]. [32]UAE method proves to be a more sustainable, rapid, economic and environmentally friendly way to extract the bioactive compounds from sample matrices [40].
The objective of this study to see the effect of type of solvent, concentration of solvents and treatment time in MAE and UAE on extraction of diosgenin from fenugreek seed.
2. Material and method
2.1. Procurement of raw material
The Trigonella foenum-graecum seeds were selected of a particular variety HS-HM 57 which is commonly called as Hissar Sonali, and collected from Hissar district in Haryana, India. The seeds were cleaned from dirt, dust and small grit particles. The fenugreek seeds were oven dried at 80 °C for 4 h for the removal of moisture and followed by grinding with a lab scale grinder (Agrosaw Pvt. Ltd, India). The fenugreek seeds were ground and sieved with 80 mesh sieves (0.177 mm) to get uniform particle size of fenugreek seed powder.
2.2. Diosgenin extraction
The extraction of diosgenin was performed using fenugreek seed powder. The extraction carried with four types of solvents namely, acetone (AC), ethanol (ET), hexane (HX) and petroleum ether (PE) at varying concentrations (40, 60, 80 and 100%). Along with variation in the solvent type and its concentration, method of extraction and treatment time of extraction was also varied.
2.3. Microwave assisted extraction (MAE)
For preparation of fenugreek extract with MAE method, microwave system was used (IFB, India). In the extraction process, the sample-solvent ratio was kept 1:5 (w/v), with four selected solvents, AC, ET, HX and PE each. MAE was carried out at varied extraction treatment time (1.5, 3.0, 4.5 and 6.0 min) and at fixed power of 180 W.
2.4. Ultrasonic assisted extraction (UAE)
For the extraction of bioactive compound from the fenugreek seed powder by UAE, equipment used was ultrasonic bath (MSW-269, Macro Scientific Works, Pvt. Ltd., India). Similarly, like as MAE method, here also sample-solvent ratio was 1:5 (w/v), with four selected solvents, AC, ET, HX and PE each. The extraction treatment time was also varied (30, 40, 50 & 60 min). The temperature of ultrasonic bath was maintained at 30 °C in the entire process.
After the process of MAE and UAE, the extract obtained was centrifuged at 2500 rpm for 10 min to carry out separation of supernatant and residue. In continuation of centrifugation, filtration was done by Whatman filter paper 41 for removal of the extremely tiny particles left in supernatant. The filtered extract was dried by solvent recovery at 45 °C using rotary vacuum evaporator (Rotavapour, R-300 Buchi, Germany). The extract was concentrated and dried in hot air oven at 55 °C for 8–10 hr. After drying, the amber colored crystals were obtained which was stored at 4 °C for further studies [11]. In Fig. 1, the method of diosgenin extraction from fenugreek seeds was represented diagrammatically.
Fig. 1.
A systematic flow diagram for production of Diosgenin from fenugreek seed powder.
2.5. Extract yield
The yield of fenugreek seed extract was quantified with formula as below:
W1: weight of extract after solvent removal.
W2: weight of sample taken.
2.6. Diosgenin content
The content of diosgenin in extract of fenugreek seed powder was determined by the method of Chapagain and Weisman [41] with few modifications. In this procedure, two color developing reagent was used. First color developing reagent contains 50 mL conc. H2SO4 and 50 mL ethyl acetate and second color reagent contains 0.5 mL p-anisaldehyde and 99.5 mL ethyl acetate. Approximately, 10 mg extract was weighed precisely and dissolved in 5 mL methanol. About 0.2 mL extract in a capped test tube was taken followed by addition of 1 mL of each color reagent 1st and 2nd respectively. The test tubes were kept in water bath at 60 °C for 10 min and further at room temperature for 10 min. After cooling down of test tubes, 0.5 mL distill water was added. For the control, 2 mL ethyl acetate and remaining steps were kept same. In case of blank, only ethyl acetate was taken and absorbance was observed at 430 nm. For the preparation of standard diosgenin solution, different concentration of standard diosgenin was varied from (200 µg/mL to 1000 µg/mL), were prepared in ethyl acetate to generate a calibration curve. Diosgenin content is expressed as mg per 100 g of sample.
2.7. Statistical analysis
All the data in this research article was reported as mean of triplicates. The experimental data was analyzed using Duncan’s t-test.
3. Results and discussion
In this research, the fenugreek seeds were treated with various solvents to get the extract and diosgenin content fenugreek seed powder with MAE and UAE method.
3.1. Extract yield
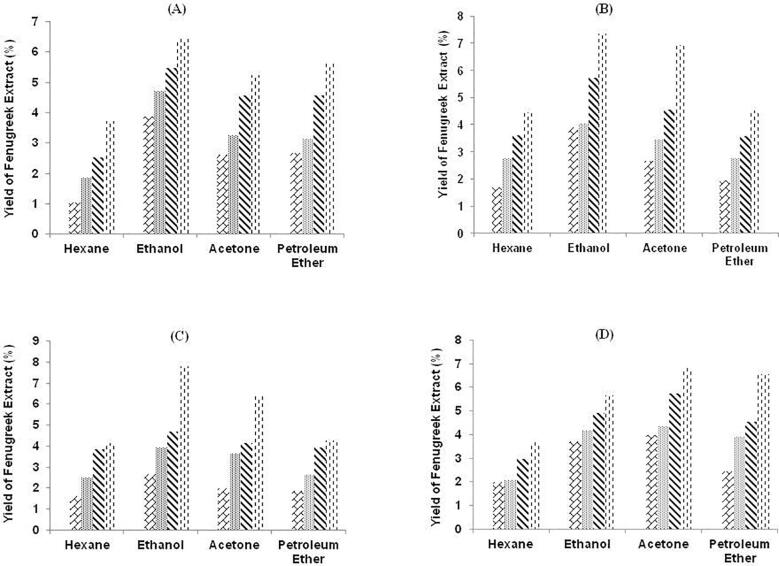
The measure of fenugreek extract was represented in percent of fenugreek seed powder and amount of fenugreek diosgenin was calculated with aid of UV- spectrophotometer in terms of standard diosgenin equivalent. In preparing the fenugreek extract, mixture of water and organic solvents (AC, ET, HX, and PE) was studied at different concentrations for their effects over extraction yield. Fig. 2, Fig. 3 depicts the graphical representation of the fenugreek extract at different pre-selected parameters. There was a significant (p < 0.05) difference in fenugreek extract yield by selected solvents.
Fig. 2.
Graphical representation of yield of fenugreek extract (%) by MAE method with four solvents at different solvent concentration A: 40%. B: 60%. C: 80% and D: 100%. Texture of the graphs represent treatment time (min) 1.5 , 3.0
, 3.0 , 4.5
, 4.5 and 6.0
and 6.0 respectively in each graph.
respectively in each graph.
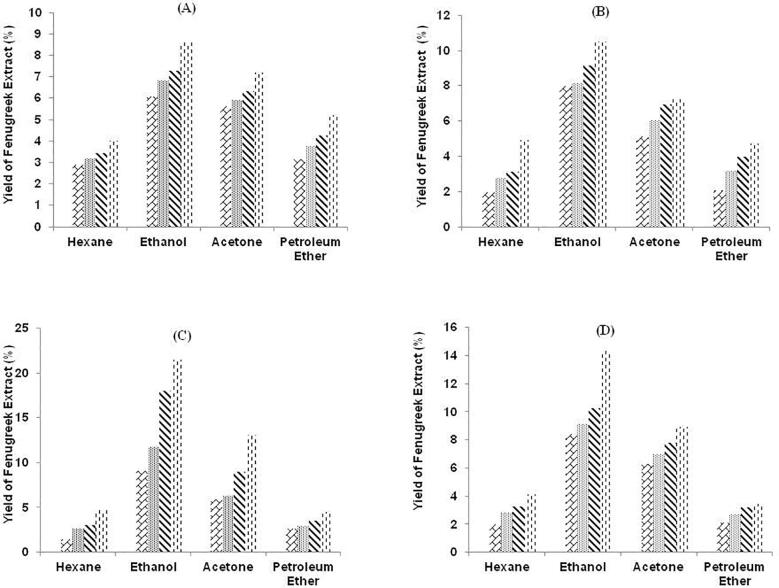
Fig. 3.
Graphical representation of yield of fenugreek extract (%) by UAE method with four solvents at different solvent concentration A: 40%. B: 60%. C: 80% and D: 100%. Texture of the graphs represents treatment time (min) 30 , 40
, 40 , 50
, 50 and 60
and 60 respectively in each graph.
respectively in each graph.
The effect of treatment time over extract yield was observed for extract yield in fenugreek seed powder with two methods. For the entire process, two ranges of treatment time were selected as 1.5 to 6.0 min for MAE and 30 to 60 min for UAE. Initially, range of extract yield at treatment time of 1.5 and 30 min was 1.04 to 3.96% and 1.45 to 9.14%, 3.0 and 40 min, extract yield was 1.86 to 4.72% and 2.63 to 11.72%, 4.5 and 50 min it was 2.54 to 5.71% and 3.04 to 17.94% and at 6.0 and 60 min, extract yield was 3.66 to 7.83% and 4.02 to 21.48% with MAE and UAE respectively. This indicates that extraction efficiency is favored by the polarity of the solvents as shown in Fig. 2, Fig. 3. A similar study was conducted by Bhukhari et al [42], that revealed the fenugreek extract was 25.32% with ethanol using soxhlet method.
The effect of solvent concentration and type was also studied for observing the extract yield in fenugreek seed powder from AC, ET, HX and PE and 40 to 100% solvent concentration and over. Initially, the non-polar solvents were selected to analyze extract yield with HX 1.04 to 4.17% and 1.45 to 4.75%, PE extract yield 1.89 to 4.28% and 2.16 to 4.59%. After non-polar solvents, polar solvents were studied to analyze the extract yield and it was observed with AC extract yield 2.03 to 5.25 and 5.89 to 13.02% and with ET extract yield 2.67 to 7.83% and 6.07 to 21.48%.
The extraction yield is directly affected by extraction (method & treatment time), solvent (type & concentration) and temperature [43], [44], [45]. As per previous studies, major effect of solvent type plays a crucial role. This research article rivets the gist of mixture of organic solvent and water over fenugreek seed powder extract. The outcomes revealed that different solvent yielded different percent of fenugreek seed powder extract due to variation in polarity of selected solvents. The highest yield was observed in ethanol extract as polarity of ethanol was the maximum in selected solvents. The possible reason that plant material contains huge number of polar compounds that are effectively soluble in high polarity solvents [46]. The comparison of MAE and UAE in regard of extract yield revealed that UAE yielded more amount of extract than with MAE. The continuous process of sound waves was beneficial to generate more extract than on–off process of MAE. In comparing MAE and UAE, extract yield was as 1.04 to 7.83% with MAE and 1.98 to 21.48% with UAE respectively. Therefore, UAE yielded more amount of extract as compared to MAE.
3.2. Diosgenin content in extract of fenugreek seed powder:
In this section, the major focus area was to study the effect of treatment time, type and concentration of the solvent and methods adopted (MAE, and UAE), on diosgenin content from fenugreek seed powder.
3.2.1. Effect of treatment time on diosgenin content
The effect of treatment time of two adopted methods played an important role in calculating the amount of diosgenin content. There were two treatment time were selected for as 1.5 to 6 min for MAE and 30 to 60 min for UAE. At first, there was antiphony raise in treatment time and at 1.5 and 30 min of solvent type and concentration, range of diosgenin content (15.82 to 22.86 and 15.78 to 26.80 mg/100 g FSP) was quite low. With the progression in treatment time at 3.0 and 40 min diosgenin content (20.12 to 24.95 and 19.97 to 25.94 mg/100 g FSP) was a bit high. As there was further increase in treatment time up to 4.5 and 50 min diosgenin content ranged from 22.12 to 29.98 to 25.58 to 34.45 mg/100 g FSP that was further higher. It was observed that at the maximum treatment time, 6 and 60 min, diosgenin content was 25.22 to 34.50 and 25.23 to 35.66 mg/100 g FSP with MAE and UAE respectively. With the increase in the treatment time, it was observed that the content of diosgenin was also increasing significantly (p < 0.05) and the maximum was at highest treatment time. The studies related to treatment time and its effect over extraction yield in Clinacanthus nutans Lindau [47]. It revealed that at maximum treatment time of 120 min, produced utmost extract yield that was observed by using response surface methodology as there was prolonged exposure of sample with solvent and allowed desired compound to migrate in to the solvent. Therefore, treatment time plays a crucial role in observing the content of diosgenin.
3.2.2. Effect of solvent concentration and type on diosgenin content
In comparing the four solvents concentration from 40 to 100% and solvent types as AC, ET, HX and PE, it was studied that the effect of solvent properties has a major concern with bioactive compound content.
The solvent concentration comes as a major factor in calculating the diosgenin content in fenugreek seed powder. Initially, the solvent concentration was varied from 40% to 100% with AC, ET, HX and PE. It was observed that there was gradual increase from 40% to 80% and decrease at 100% in the diosgenin content. The increase in diosgenin content was favored by the polarity and dielectric constant of the selected solvents and water. At 40 and 60% solvent concentration, the diosgenin content was quiet low as the high amount of water present in the solvent sample mixture caused the swelling of fenugreek gum and created much hindrance in extraction of bioactive compound [48]. The low concentration of solvents indicates low dissipation factor which produced less diosgenin content as the absorption of energy was more by water and less by solvent. When the solvent concentration was increased till 80%, the diosgenin content was increased significantly as the polarity of the entire solution was enhanced due to less amount of water and more amount of solvent at 80%. Proceeding with the last solvent concentration at 100%, it was observed that there was a decrease in diosgenin content as compared to 80% solvent concentration. The absence of water in solvent yielded diosgenin content as only solvent polarity was interacting with the sample matrix.
The maximum diosgenin content was observed at 80% solvent concentration as the polarity of both solvent and water were reacting efficiently. The overall polarity of the extracting solution was increased by “polarity versus polarity “principle. The progressive increase in the amount of solvent concentration yielded more extract. Similar findings are reported by Escribano-Bailon and Santos-Buelga[49] and founds that the combination of water and solvent in such a ratio where water is less and solvent is more could yield more content of bioactive compound instead of only solvent. In some plant extract, there was some swelling noticed that allows more interaction of water with sample matrix. This interaction affects the content of bioactive compound depending upon the amount of water [50]. The increased amount of water for extraction could possibly decrease the content of bioactive compound as high amount of water can enhance the polarity up to such an extent from where content of bioactive compound decreases significantly [51]. Another study reported that effect of solvent concentration and extraction treatment time over the flavonoid content by UAE method for fenugreek seeds and it revealed that utmost extraction was observed at 70% methanol concentration for duration of 50 min [52].
The variation in selection of type of solvents was done to study the effect of solvents over the diosgenin content in fenugreek seed powder. The solvent type was one of the major factors in considering the content of bioactive compound. In this research conducted, out of four solvents, two solvents were of polar nature (AC and ET) and two solvents were of non-polar nature (HX and PE). The polar and non-polar nature of the solvents comes as a major factor in analyzing the amount of compound extracted.
In case of polar solvents, the diosgenin content was observed more as compared to non-polar solvents because more polar solvent produces much more amount of bioactive compound. Along with the polarity of the selected solvent, dielectric constant also considered as one of the crucial factors. The dielectric constant is the physical property which interacts with the intermolecular and interatomic attractions of the selected solvents. Dielectric constant also causes the separation of electrolytes in to the ions by analyzing solvent efficiency. The dielectric constant of four selected solvents is ET = 80.1, AC = 20.7, PE = 2.2 and HX = 1.89 and water = 78.3 [53]. The solvents which have high dielectric constant aids in entire dissociation of the electrolytes and the bonds that cause high amount of extraction while the solvents which have low dielectric constant, ion pairing occurred that cause less amount of yield of extract [54].
The type of selected solvent (AC, ET, HX and PE) was varied with every solvent concentration (40 to 100%). It was studied that effect of type of solvent was also an important factor. At the beginning, non-polar solvents were used to study their effect over diosgenin content. In case of hexane non-polar nature and low dielectric constant was one of the major factors that produced less amount of diosgenin content 15.82 to 33.43 and 17.86 to 35.66 mg/100 g of FSP by MAE and UAE respectively. Proceeding with another non-polar pre-selected solvent, petroleum ether, and the dielectric constant was a bit high but much less than that of polar solvents. So, the diosgenin content 16.48 to 33.96 and 14.63 to 38.60 mg/100 g FSP by MAE and UAE respectively was a bit much better than hexane. As the analysis progressed with polar solvent acetone and it was observed that dielectric constant of acetone was bit higher than non-polar solvents. Diosgenin content 16.63 to 29.44 and 17.32 to 33.55 mg/100 g FSP by MAE and UAE respectively with AC was much better than HX and PE. The dielectric constant of ethanol is quite high that indicated that the more polar compound would be extracted easily. In the selected solvents, it was observed that maximum extract yield and diosgenin content was produced by ethanol. The polarity and dielectric constant of the ET produced maximum amount of diosgenin content was 18.75 to 34.50 and 19.97 to 40.37 mg/100 g FSP by MAE and UAE respectively. The concentration and polarity of selected solvents caused the variation in the extract yield and diosgenin content. It depicted that 80% ethanol generate much better extract yield and diosgenin content as compared to other solvent concentration and selected solvent types. The effect of solvent polarity over Phaseolus vulgaris seeds and it depicted that extract yield ranged from 0.24 to 7.23 g/100 g dry wt. with water which was higher than the non-polar solvent [55].
3.2.3. Effect of extraction methods on diosgenin content
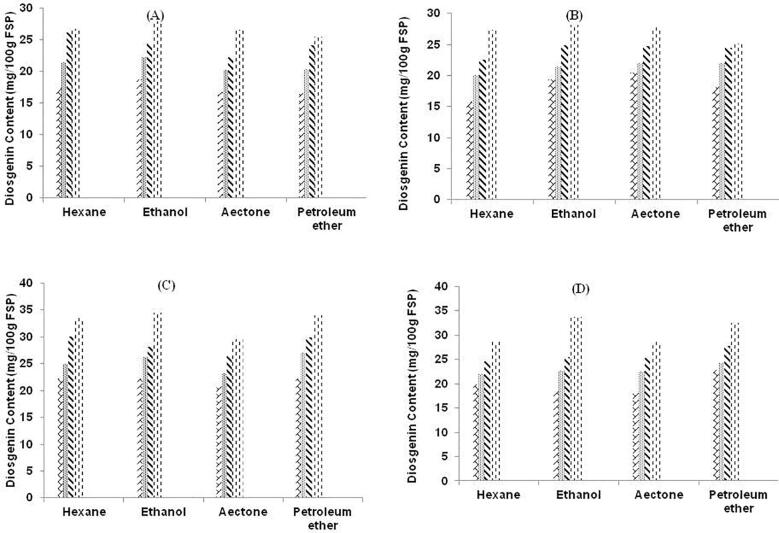
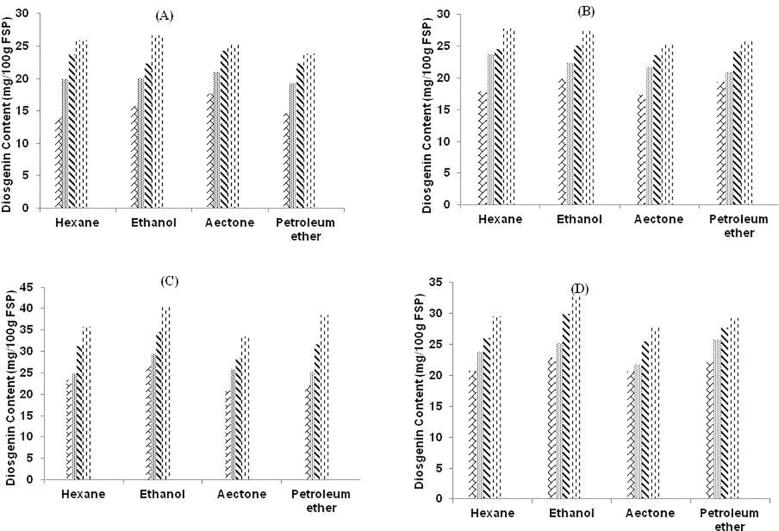
The comparison of two methods (MAE and UAE) was done for the extraction of diosgenin from fenugreek seed powder was studied. All the pre-selected parameter was varied with the method adopted to analyze effect of method over diosgenin content in fenugreek seed powder. Fig. 4 depicts the graphical representation of diosgenin content from four solvents (AC, ET, HX and PE) with variation in treatment time and solvent concentration and solvent type by MAE. This image depicts the highest concentration of diosgenin was detected in case of ET with 80% solvent concentration and at duration of 6 min.
Fig. 4.
Graphical representation of diosgenin content (mg/100 g FSP) by MAE with four solvents at different solvent concentration A: 40%. B: 60%. C: 80% and D: 100%. Texture of the graphs represent treatment time (min) 1.5 , 3.0
, 3.0 , 4.5
, 4.5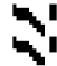 & 6.0
& 6.0 respectively in each graph. FSP: Fenugreek Seed Powder.
respectively in each graph. FSP: Fenugreek Seed Powder.
It was observed that the method adopted had an effect over content of diosgenin. In case of MAE, the less amount of diosgenin content was produced. In MAE, there was risk of loss of bioactive compound as there was a sudden rise in boiling point of solvent due to high temperature that generates solvent vapors quickly. This generates vapors that might catch fire and to avoid this hampering of the procedure, there is requirement to cool down the set-up for a while and then again re-treat it with after some time. As in general cases, the power of microwave is directly related to the heating of the solvents so this could be prevented by adopting the less power of microwave that generate slow heating of sample and also avoid fire incidents. In case of UAE, the extraction process took place with aid of cavitation’s process that aids in the more content of diosgenin as compared to MAE. Along with this, there was no such generation of vapors and much more treatment time. In UAE, the sample was in continuous process extraction and there was no such hindrance as in MAE. The effect of MAE over fenugreek seeds for analyzing the diosgenin content [56]. In MAE, due to high temperature in microwave, there was less amount of bioactive compound and more damage to quality and quantity of bioactive compound. This study also depicted that higher amount of time could extract more content of bioactive compound if the microwave power is kept low up to 25–50 W only, as increase in power cause a quick heating of the solute–solvent mixture that may cause harm to the content of bioactive compound. There was 0.159% of diosgenin was extracted with MAE in fenugreek seeds at 50% 2-propanol as solvent for 30 min of treatment time [56]. Fig. 5, represents the graphical image for content of diosgenin from fenugreek seed powder at different treatment time, solvent type and concentration by UAE. From the image, it could be concluded that the utmost content of diosgenin was observed with ET at 80% solvent concentration with 60 min of treatment time.
Fig. 5.
Graphical representation of diosgenin content (mg/100 g FSP) by UAE with four solvents at different solvent concentration A: 40%. B: 60%. C: 80% and D: 100%. Texture of the graphs represent treatment time (min) 30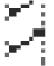 , 40
, 40 , 50
, 50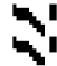 & 60
& 60 respectively in each graph. FSP: Fenugreek Seed Powder.
respectively in each graph. FSP: Fenugreek Seed Powder.
In this research study, the range of diosgenin content from MAE was (15.82 to 34.50 mg/100 g FSP) and with UAE it was (15.78 to 40.37 mg/100 g FSP). It could be observed that diosgenin content was much higher with UAE [32] and revealed the use of UAE in better extraction of bioactive compounds. So out of both extraction methods, UAE proved to be more beneficial as it produces much higher amount of extraction yield and diosgenin content from fenugreek seed powder.
4. Conclusion
There are various sources that could be enlisted for the diosgenin extraction but among these sources, fenugreek is an easily available and rich source of diosgenin content. The yield of diosgenin content from fenugreek seeds was also in to the consideration for the extraction of diosgenin. In this research, there was a comparison for the study of green method for extraction of diosgenin with better yield and diosgenin content among MAE and UAE. In between MAE and UAE, extract yielded as in range of 1.04 to 7.83% and 1.98 to 21.48% and diosgenin content as 15.82 to 34.50 and 15.78 to 40.37 mg/100 g FSP respectively.
The naturally extracted diosgenin could possibly be used as an alternative source for the various food matrices as compared to the synthetic diosgenin. The variation in the concentration of solvents could be a better option for the further selectivity for much higher yield of fenugreek extract and content of diosgenin with ethanol. The time always comes as crucial factor for the extraction of bioactive compounds, here with the progression in the treatment time, the extract yield and content of diosgenin was also enhanced. These methods could possibly generate future perspective in the sector of food for much easier extraction of diosgenin and its incorporation in various food matrices. Due to tremendous amount of health benefits of diosgenin, it might be a better way to treat the common people in day to day life with a better bite of food. This research could open the gate for better extraction of diosgenin in view of its utilization in different market food products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.P. Mukthamba, K. Srinivasan, Hypolipidemic and antioxidant effects of dietary fenugreek (Trigonella foenum-graecum) seeds and garlic (Allium sativum) in high-fat fed rats, Food Biosci. 14 (2016) 1–9. DOI10.1016/j.fbio.2016.01.002.
- 2.T. Zia, S. N. Hasnain, S. K. Hasan, Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L. in normal mice, J. Ethnopharmacol. 75 (2001) 191–195. DOI:10.1016/s0378-8741(01)00186-6. [DOI] [PubMed]
- 3.Belguith-Hadriche O., Bouaziz M., Jamoussi K., Simmonds M.S.J., El Feki A., Makni-Ayedi F. Comparative study on hypocholesterolemic and antioxidant activities of various extracts of fenugreek seeds. Food Chem. 2013;138:1448–1453. doi: 10.1016/j.foodchem.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 4.M. A. Bhatti, M. T. J. Khan, B. Ahmed, M. Jamshaid, W. Ammad, W, Antibacterial activity of Trigonella foenum-graecum seeds, Fitoterapia. 67 (1996) 372–374.Corpus ID: 88105970.
- 5.T. Devasena, V. P. Menon, Fenugreek affects the activity of beta-glucuronidase and mucinase in the colon, Phytother. Res. 17 (2003) 1088–1109. DOI:10.1002/ptr.1331. [DOI] [PubMed]
- 6.A. A. Mehrafarin, S. H. Rezazadeh, B. H. Naghdi, G. H. Noormohammadi, E. Zand, A. Qaderi, , A review on biology, cultivation, biotechnology of fenugreek as a valuable medicinal plant and multipurpose, J. Med. Plants Res. 10 (2011) 1–19.http://jmp.ir/article-1-228-en.html.
- 7.Ahmadiani A., Javan M., Semnanian S., Barat E., Kamalinejad M. M, Ant inflammatory and antipyretic effects of Trigonella foenum-graecum leave extract in the rat. J. Ethnopharmacol. 2001;75:283–286. doi: 10.1016/s0378-8741(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 8.Gaby M.P. Galactagogues: medications that induce lactation. J. Hum. Lact. 2002;18:274–279. doi: 10.1177/089033440201800311. [DOI] [PubMed] [Google Scholar]
- 9.A. A. Zuppa, P. Sindico, C. Orchi, C. Carducci, V. Cardiello, C. Romagnoli, P. Catenazzi, Safety and efficacy of galactogogues: Substances that induce, maintain and increase breast milk supply, J. Pharm. Pharm. Sci. 13 (2010) 162–174. DOI:10.18433/j3ds3r. [DOI] [PubMed]
- 10.Waqas N.B., Qazi T., Sabeera M., Farooq A., Masoodia I.W., Showkat A.G., Mohd M.B. Some nutraceutical properties of fenugreek seeds and shoots (Trigonella foenum-graecum L.) from the high Himalayan region. Food Biosci. 2018;23:31–37. [Google Scholar]
- 11.Wani S.A., Bishnoi S., Kumar P. Ultrasound and microwave assisted extraction of diosgenin from fenugreek seed and fenugreek-supplemented cookies. Food Measure. 2016;10:527–532. doi: 10.1007/s11694-016-9331-2. [DOI] [Google Scholar]
- 12.F. Roghani-Dehkordi, M. Roghani, T. Baluchnejadmojarad, Diosgenin mitigates streptozotocin diabetes-induced vascular dysfunction of the rat aorta: the involved mechanisms, J. Cardiovasc. Pharmacol. Ther. 66(6) (2015) 584-592. DOI:10.1097/fjc.0000000000000308. [DOI] [PubMed]
- 13.Raju J., Rao C. Diosgenin, a Steroid Saponin Constituent of Yams and Fenugreek: emerging evidence for applications in medicine. Bioactive Compd. Phytomed. 2012:1–21. doi: 10.5772/26700. [DOI] [Google Scholar]
- 14.M. K. Sangeetha, N. ShriShri Mal, K. Atmaja, V. K. Sali, H. R. Vasanthi, PPAR’s and Diosgenin a chemico biological insight in NIDDM, Chem. Biol. Interact. 206 (2013) 403-410. DOI: 10.1016/j.cbi.2013.08.014. [DOI] [PubMed]
- 15.S. Chaudhary, P. S. Chaudhary, S. K. Chikara, M. C. Sharma, M. Iriti, Review on fenugreek (Trigonella foenum-graecum L.) and its important secondary metabolite diosgenin, Not. Bot. Horti. Agribo.46 (2018) 22–31. DOI:10.15835/nbha46110996.
- 16.S. Aminkar, S. Shojaeiyan, A. S. Rashidi-Monfared, M. Ayyari, Quantitative assessment of diosgenin from different ecotypes of Iranian fenugreek (Trigonella foenum-graecum L.) by high-performance liquid chromatography, Int. J. Horti. Sci. 5 (2018) 115–121.DOI:10.22059/IJHST.2018.254988.230.
- 17.Sauvaire Y., Ribes G., Baccou J.C., Loubatieères-Mariani M.M. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. 1991;26(3):191–197. doi: 10.1007/BF02543970. [DOI] [PubMed] [Google Scholar]
- 18.Nazir R., Kumar V., Dey A., Pandey D.K. HPTLC quantification of diosgenin in Dioscorea deltoidea: evaluation of extraction efficacy, organ selection, drying method and seasonal variation. S. Afr. J. Bot. 2021;138:386–393. doi: 10.1016/j.sajb.2020.12.027. [DOI] [Google Scholar]
- 19.Biswas D., Nazir R., Biswas P., Kumar V., Nandy S., Mukherjee A., Dey A., Pandey D.K. Endophytic sources of diosgenin, a natural steroid with multiple therapeutic values. S. Afr. J. Bot. 2020;134:119–125. [Google Scholar]
- 20.Zhang F., Shen B., Jiang W., Yuan H., Zhou H. Hydrolysis extraction of diosgenin from Dioscorea nipponica Makino by sulfonated magnetic solid composites. J. Nanopart. Res. 2019;21:269. doi: 10.1007/s11051-019-4702-3. [DOI] [Google Scholar]
- 21.Hayat K., Zhang X., Abbas S., Hussain S., Hussain A., Tahir M.U. Effect of microwave treatment on the nutritional profile of the citrus mandarin cultivars peels. J. Food Process. Preserv. 2020;44(e14791):1–8. doi: 10.1111/jfpp.14791. [DOI] [Google Scholar]
- 22.Sanz V., Florez-Fernandez N., Domínguez H., Torres M.D. Valorisation of Camellia sinensis branches as a raw product with green technology extraction methods. Curr. Res. Food Sci. 2020;2:20–24. doi: 10.1016/j.crfs.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemi M., Khodaiyan F., Hosseini S.S. Eggplant peel as a high potential source of high methylated pectin: ultrasonic extraction optimization and characterization. LWT - Food Sci. Tech. 2019;105:182–189. doi: 10.1016/j.lwt.2019.01.060. [DOI] [Google Scholar]
- 24.N. Č. Mustač, B. Voučko, D. Novotni, S. Drakula, A. Gudelj, F. Dujmić, D. Ćurić Optimization of high intensity ultrasound treatment of proso millet bran to improve physical and nutritional quality, FTB Food Technol and Biotech. 57, (2019) 183-190 DOI:10.17113/ftb.57.02.19.6100. [DOI] [PMC free article] [PubMed]
- 25.B. B. V. Guandalini, N. P. Rodrigues, L. D. F. Marczak, Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound, Food Res. Int. 119 (2019) 455 461 DOI:10.1016/j.foodres.2018.12.011. [DOI] [PubMed]
- 26.Li Y., Fabiano-Tixier A.S., Vian M.A., Chemat F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. Trends Anal. Chem. 2013;47:1–11. doi: 10.1016/j.trac.2013.02.007ff. [DOI] [Google Scholar]
- 27.Oroian M., Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 2013;117(4):426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 29.Bhargava N., Mor R.S., Kumar R.K., Sharanagat V.S. Advances in application of ultrasound in food processing: a review. Ultrason. Sonochem. 2021;70:105293. doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.E. Roselló-Soto, M. Koubaa, A. Moubarik, R. P. Lopes, J. A. Saraiva, N. Boussetta, F. J. Barba, Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds, Trends Food Sci. Technol. 45(2) (2015) 296–310. DOI:10.1016/j.tifs.2015.07.003.
- 31.K. Kumar, S.Srivastav, V. S.Sharanagat, Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review, Ultrason. Sonochemi. 70 (2021) 105325. DOI:10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed]
- 32.Tiwari B.K. Ultrasound: a clean, green extraction technology. Trends Anal. Chem. 2015;71:100–109. [Google Scholar]
- 33.Carrera C., Ruiz-Rodriguez A., Palma M., Barroso C.G. Ultrasound-assisted extraction of amino acids from grapes. Ultrason. Sonochem. 2015;22:499–505. doi: 10.1016/j.ultsonch.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Maran J.P., Priya B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr. Polym. 2015;115:732–738. doi: 10.1016/j.carbpol.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 35.Albu S., Joyce E., Paniwnyk L., Lorimer J.P., Mason T.J. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004;11:261–265. doi: 10.1016/j.ultsonch.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Wang X.-S., Wu Y.-F., Dai S.-L., Chen R., Shao Y. Ultrasound-assisted extraction of geniposide from Gardenia jasminoides. Ultrason. Sonochem. 2012;19(6):1155–1159. doi: 10.1016/j.ultsonch.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Youssouf L., Lallemand L., Giraud P., Soulé F., Bhaw-Luximon A., Meilhac O., D'Hellencourt C.L., Jhurry D., Couprie J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017;166:55–63. doi: 10.1016/j.carbpol.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Vaquero M., Rajauria G., Tiwari B., Sweeney T., O'Doherty J. Extraction and yield optimisation of fucose, glucans and associated antioxidant activities from Laminaria digitata by applying response surface methodology to high intensity ultrasound-assisted extraction. Mar. Drugs. 2018;16(8):257. doi: 10.3390/md16080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ummat V., Sivagnanam S.P., Rajauria G., O’Donnell C., Tiwari B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021;110:90–106. doi: 10.1016/j.tifs.2021.01.018. [DOI] [Google Scholar]
- 40.McDonnell C., Tiwari B.K. Ultrasound: a clean, green extraction technology for bioactives and contaminants. Compr. Anal. Chem. 2017;76:1–18. doi: 10.1016/bs.coac.2017.03.005. [DOI] [Google Scholar]
- 41.Chapagain B., Wiesman Z. Variation in diosgenin level in seed kernels among different provenances of Balanites aegyptiaca Del (Zygophyllaceae) and its correlation with oil content. Afr. J. Biotechnol. 2005;4(11):1209–1213. [Google Scholar]
- 42.Bukhari S.B., Bhanger M.I., Memon S. Antioxidative activity of extracts from fenugreek seeds (Trigonella foenum-graecum) Pak. J. Anal. Environ. Chem. 2008;9(2):78–83. [Google Scholar]
- 43.Turkmen N., Sari F., Velioglu Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006;99(4):835–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- 44.McDonald S., Prenzler P.D., Antolovich M., Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73(1):73–84. doi: 10.1016/S0308-8146(00)00288-0. [DOI] [Google Scholar]
- 45.Ngo T.V., Scarlett C.J., Bowyer M.C., Ngo P.D., Vuong Q.V. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017:1–8. doi: 10.3390/separations6030044. [DOI] [Google Scholar]
- 46.P. Kuppusamy, M. M. Yusoff, N. R. Parine, N. Govindan, Evaluation of in-vitro antioxidant and antibacterial properties of Commelina nudiflora L. extracts prepared by different polar solvents, Saudi J. Biol. Sci. 22(3) (2015) 293–301. DOI:10.1016/j.sjbs.2014.09.016. [DOI] [PMC free article] [PubMed]
- 47.Sulaiman I.S.C., Basri M., Masoumi H.R.F., Chee W.J., Ashari S.E., Ismail M. Effects of temperature, time and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Curr. J. 2017;11:54–65. doi: 10.1186/s13065-017-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A. K. Harish, G. K. Ram, B. Singh, M. Phulwaria, N. S. Shekhawat, Molecular and biochemical characterization of different accessions of fenugreek (Trigonella foenum-graecum L.), Libyan Agricul. Res. Centre J. Int. (LARCJI) 2 (2011) 150-154. DOI:10.1016/j.sjbs.2015.09.015.
- 49.M. T. Escribano-Bailon, C. Santos-Buelga, Polyphenol Extraction from Foods. In: Santos-Buelga, C., Ed., Methods in Polyphenol Analysis, Royal Society of Chemistry, London, (2003) 1-12. DOI:10.1021/np030739x.
- 50.Veggi P.C., Martinez J., Meireles M.A.A. Fundamentals of microwave extraction. In: Chem F., Cravotto G., editors. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice, vol 4, Food engineering series. Cravotto (Springer Science & Business Media; New York: 2013. p. 15. [Google Scholar]
- 51.Mandal V., Dewanjee S., Mandal S.C. Microwave-assisted extraction of total bioactive saponin fraction from Gymnema sylvestre with reference to gymnemagenin: a potential biomarker. Phytochem. Anal. 2009;20(6):491–497. doi: 10.1002/pca.1151. [DOI] [PubMed] [Google Scholar]
- 52.S. Das, Optimization of ultrasound-assisted extraction of total flavonoids and antioxidant properties from Trigonella foenum-graecum seeds with response surface methodology, Int. J. Pharm. Sci. Res. 4 (2013) 4308-4318. DOI:10.13040/IJPSR.0975-8232.4(11).
- 53.Engineering Tool Box, Dielectric Constants of Liquids. Available at: https://www.engineeringtoolbox.com/liquid-dielectric-constants-d_1263.html. (2008).
- 54.Day R.A., Underwood A.L. 6th ed. Prentice-Hall; New Jersey: 1991. Quantitative Analysis. [Google Scholar]
- 55.H. Nawaz, M. A. Shad, N. Rehman, H. Andaleeb, N. Ullah, Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds, Braz. J. Pharm. Sci. 56, (2020) e17129. DOI:10.1590/s2175-97902019000417129.
- 56.B. Kaufmann, P. Christen, Recent extraction techniques for natural products: microwave-assisted extraction and pressurized solvent extraction, Phytochem. Anal. 13(2) (2002) 105-113. DOI:10.1002/pca.631. [DOI] [PubMed]