Abstract
This cohort study examines the association of BRCA1 and BRCA2 with tumor mutation burden and response to immune checkpoint inhibitors.
Introduction
Immune checkpoint inhibitors (ICIs) have achieved impressive success in a subset of malignant tumors. Tumor mutation burden (TMB), programmed cell death ligand 1 expression, and deficient DNA mismatch repair are the few biomarkers known to be associated with response to ICIs. Breast cancer type 1 or 2 susceptibility gene (BRCA1 [OMIM 113705] and BRCA2 [OMIM 600185]) play critical roles in DNA repair. However, the roles of BRCA1/2 alteration in tumor immunotherapy remains uncharacterized, to our knowledge. Several early phase clinical trials are examining the combination of ICIs and poly(ADP-ribose) polymerase inhibitor in BRCA1/2 altered tumors. The role of BRCA1/2 alteration in immunotherapy remains controversial across different tumor types. We hypothesized that BRCA1/2 alteration is associated with TMB and may serve as a novel indicator associated with better treatment outcomes of ICIs.
Methods
This cohort study was deemed exempt from institutional review board approval and informed consent by the University of Oklahoma Health Sciences Center ethics committee, as only deidentified human samples were used. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
BRCA alteration status data were obtained from the cBioPortal platform.1 The included alteration types are missense, nonsense, nonstart, fusion, frame-shift deletion or insertion, in-frame deletion, and splice. Patients in the Memorial Sloan Kettering Cancer Center (MSKCC) cohort who received ICIs and genome sequencing were enrolled in the survival analysis.2 TMB was determined by normalizing the number of nonsynonymous alterations based on the sequenced genome.2 High TMB was defined as the top 10% of TMB in each tumor type, while the bottom 90% was regarded as low TMB.2
The primary outcome was overall survival (OS), started from the first day receiving ICIs. Kaplan-Meier analysis was performed to compare OS in patients with or without BRCA1/2 alteration, and log-rank test was applied. The significance level was α = .05 for a 2-sided test. Statistical analysis was performed in SPSS version 20.0 (IBM), Prism version 5.0 (GraphPad) and R version 3.6.3 (R Project for Statistical Computing). Data were analyzed from July to November 2020.
Results
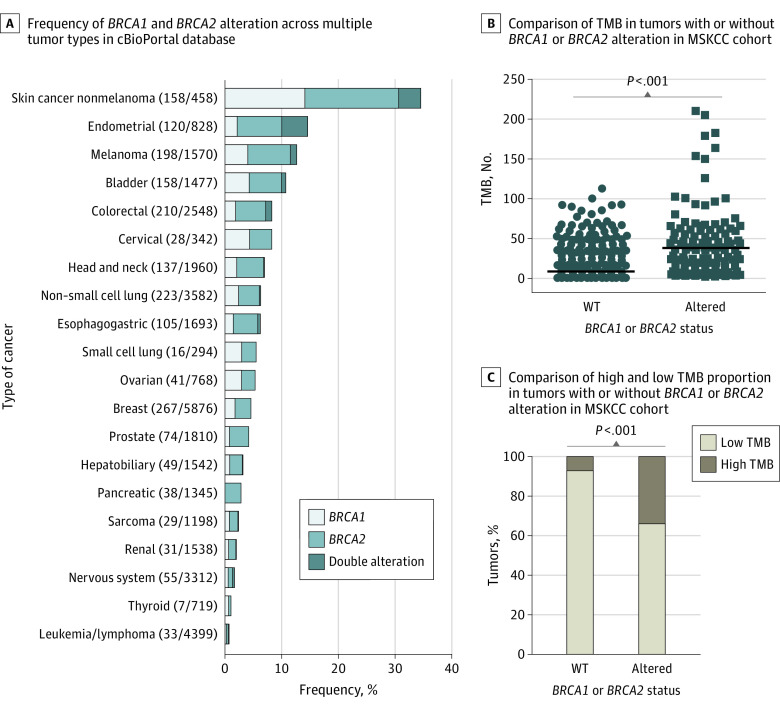
A total of 39 307 tumor samples from 37 259 patients were included in the study, including 1977 patients (5.3%) with a BRCA1/2 alteration. Among them, 164 patients (0.4%) had double alteration, 662 patients (1.8%) had BRCA1 single alteration, and 1151 patients (3.1%) had BRCA2 single alteration. The prevalence of BRCA1/2 alteration across multiple tumor types is presented in Figure 1A. BRCA1/2 altered tumors had higher median (interquartile range [IQR]) TMB (24.59 [9.84-52.14]) than that in wild-type (WT) tumors (5.90 [2.95-10.00]; P < .001) (Figure 1B). A total of 49 patients (34.8%) with BRCA1/2 alteration also had high TMB, while 1399 patients (92.0%) with WT BRCA1/2 had low TMB (P < .001) (Figure 1C).
Figure 1. Frequency of BRCA1/2 Alteration Across Multiple Tumors and Correlation With Tumor Mutation Burden (TMB).
MSKCC indicates Memorial Sloan Kettering Cancer Center; WT, wild type. B, unpaired t test was applied with 2-tailed P value. C, Cochran-Mantel-Haenszel test was applied.
A total of 1661 patients in the Memorial Sloan Kettering Cancer Center (MSKCC) cohort who received ICIs and genome sequencing were enrolled in the survival analysis.2 There were 141 patients (8.5%) with BRCA1/2 alteration in the MSKCC immunotherapy cohort. BRCA1 alteration was not associated with OS in the MSKCC cohort (Figure 2A). Patients with BRCA2 altered tumors had better OS than those without (median [IQR] OS, 31.0 [10.0-80.0] months vs 18.0 [6.0-58.0] months; P = .02) (Figure 2B). Patients with low TMB BRCA2 altered tumors had comparable OS with patients with high TMB tumors (median [IQR] OS, 44.0 [10.0-67.0] months vs 41.0 [13.0-80.0] months), and both groups had better OS than patients with low TMB WT BRCA2 tumors (median [IQR] OS, 16.0 [6.0-57.0] months; P < .001) (Figure 2C).
Figure 2. Prognostic Association of Tumor Mutation Burden (TMB) and BRCA1/2 Alteration in Patients Receiving Immune Checkpoint Inhibitors.
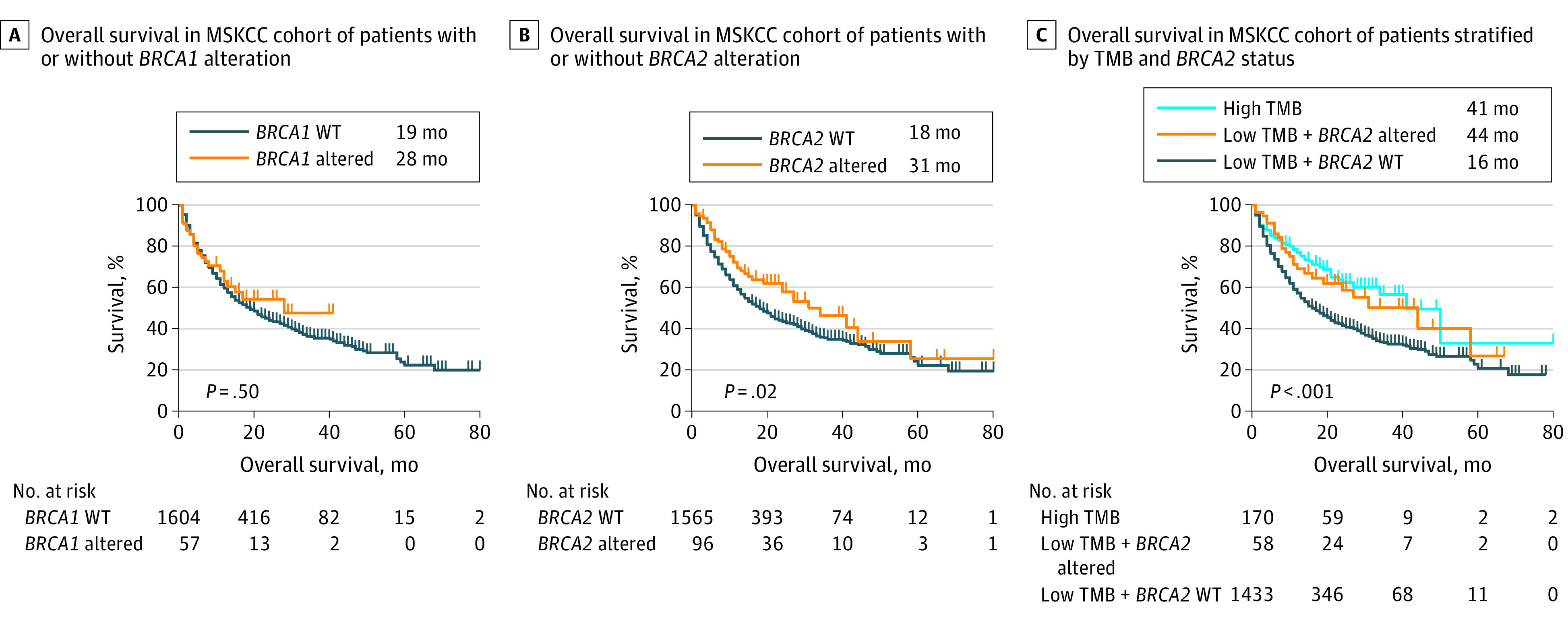
MSKCC indicates Memorial Sloan Kettering Cancer Center; WT, wild type.
Discussion
The findings of this cohort study suggest that BRCA2 alteration in combination with TMB was a potential biomarker associated with response to ICIs. BRCA1/2 altered tumors have shown enhanced immunosurveillance in several preclinical studies, but their correlation with ICI treatment outcomes remains uncharacterized. Early phases of randomized clinical trials have shown promising results of combining poly(ADP-ribose) polymerase inhibitor with ICIs in BRCA1/2 altered tumors.3,4
This study has some limitations, such as that the role of BRCA2 alteration in immunotherapy in specific tumor type warrants further study. This study includes both pathogenic and undefined variants, which may result in higher alteration rates. Previous studies from our group and others have reported that CXC chemokine receptor 2 (CXCR2) plays critical roles in tumor immune evasion and progression.5,6 Further studies are warranted to pinpoint the specific tumor types that are responsive to ICIs when BRCA2 is altered, and to verify whether the addition of CXCR2 inhibition can further improve survival in these patients.
References
- 1.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401-404. doi: 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-206. doi: 10.1038/s41588-018-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155-1164. doi: 10.1016/S1470-2045(20)30324-7 [DOI] [PubMed] [Google Scholar]
- 4.Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2019.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Z, Xia G, Xiang Z, et al. A C-X-C chemokine receptor type 2-dominated cross-talk between tumor cells and macrophages drives gastric cancer metastasis. Clin Cancer Res. 2019;25(11):3317-3328. doi: 10.1158/1078-0432.CCR-18-3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele CW, Karim SA, Leach JDG, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832-845. doi: 10.1016/j.ccell.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]