Abstract
Current advances of immunotherapy have greatly changed the way of cancer treatment. At the same time, a great number of nanoparticle-based cancer immunotherapies (NBCIs) have also been explored to elicit potent immune responses against tumors. However, few NBCIs are nearly in the clinical trial which is mainly ascribed to a lack understanding of in vivo fate of nanoparticles (NPs) for cancer immunotherapy. NPs for cancer immunotherapy mainly target the immune organs or immune cells to enable efficient antitumor immune responses. The physicochemical properties of NPs including size, shape, elasticity and surface properties directly affect their interaction with immune systems as well as their in vivo fate and therapeutic effect. Hence, systematic analysis of the physicochemical properties and their effect on in vivo fate is urgently needed. In this review, we first recapitulate the fundamentals for the in vivo fate of NBCIs including physio-anatomical features of lymphatic system and strategies to modulate immune responses. Moreover, we highlight the effect of physicochemical properties on their in vivo fate including lymph nodes (LNs) drainage, cellular uptake and intracellular transfer. Challenges and opportunities for rational design of NPs for cancer immunotherapy are also discussed in detail.
KEY WORDS: Physicochemical properties, Nanoparticle-based cancer immunotherapies, Cancer treatment, In vivo fate, Immune responses, Lymph nodes drainage, Cellular uptake, Intracellular transfer
Graphical abstract
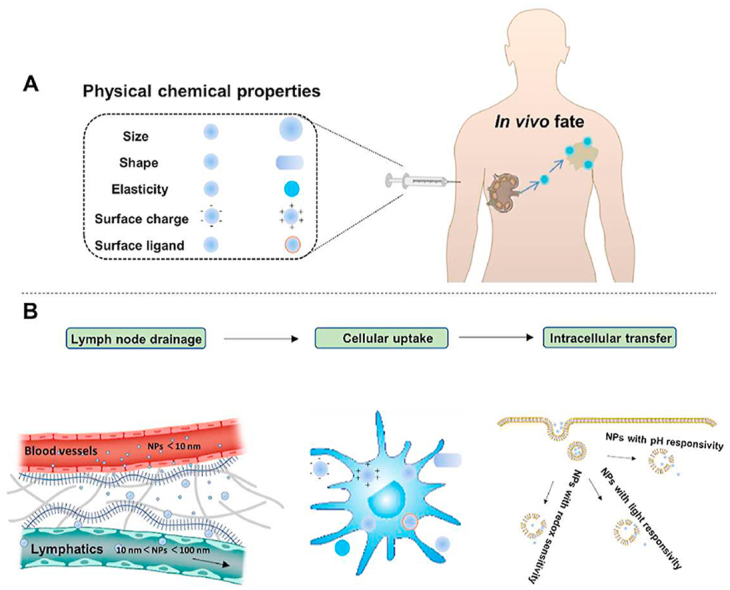
Physicochemical properties including size, shape, elasticity, surface charge and surface ligand influence the in vivo fate of NBCIs, including LNs drainage, cellular uptake and intracellular transfer.
1. Introduction
Immunotherapies have been utilized for the treatment of various diseases, such as infectious diseases1,2, autoimmunity3,4, and cancer5,6. Among these various diseases, immunotherapies for cancer recently experience significant advances and shift the paradigm of cancer treatment. In the past few years, several types of cancer immunotherapies including cancer vaccines7,8, checkpoint inhibitors9,10 and chimeric antigen receptor (CAR) T-cell therapies11,12 have demonstrated promising anticancer effect. However, immunotherapies for cancer treatment still faces challenges. For example, Sipuleucel-T13, the first approved therapeutic vaccine for prostate cancer, only extended overall survival by four months in clinical trials and failed to improve progression free survival. Moreover, its clinical translation is challenged by high cost and complicated manufacturing process14. As for checkpoint blockade therapy, monotherapy with checkpoint blockade leads to low response rate15, while combination with other therapies caused severe immune related side effects, including gastrointestinal toxicity, pruritus and fatigue16, 17, 18. Furthermore, resistance to checkpoint inhibitors has also been observed which caused poor efficiency and disease progression19,20. For CAR T-cell therapies, the antitumor effect is only significant for small part of hematological cancers but limited for the much more prevalent solid tumors21,22. In addition, CAR T-cell therapies may cause life-threatening cytokine release syndrome (CRS) which result in fever, neurologic symptoms and organ dysfunction23,24. Therefore, approaches to safely and effectively elicit immune responses against cancer remain an important unmet need.
In recent years, nanotechnology has experienced rapid development and emerged as a promising approach to targeted deliver therapeutic agents to designated sites for efficient diagnosis and treatment25, 26, 27. Various NPs including inorganic NPs, liposomes and polymer micelles have been exploited as vehicles to deliver therapeutic agents28,29. Due to their unique physicochemical properties, nanomaterials protect therapeutic agents from degradation and promote enhanced tumor accumulation which result in improved efficiency and decreased side effects25. Several nanomaterial based drugs have already been approved for cancer therapy, such as liposomal doxorubicin (Doxil)30, albumin-bound paclitaxel (Abraxane)31 and liposomal daunorubicin and cytarabine (Vyxeos)32. In recent years, nanotechnology-based strategies for immunotherapy have also been extensively investigated33, 34, 35. For instance, antigens and adjuvants can be loaded inside or conjugated to outside of the NPs and targeted delivered to lymphoid organs to achieve efficient antitumor immunity36, 37, 38. NPs encapsulating checkpoint inhibitors have also been widely investigated to improve their stability and tumor accumulation for enhanced antitumor efficacy39,40. Recombinant cytokines challenged by its systemic toxicity can also be engineered into NPs to increase their accumulation at the designated sites and internalization by specific immune cells, and thereby dramatically improve their potency and reduce systemic toxicity41,42
Despite the advances, most nanoparticle based delivery systems for cancer immunotherapy are still under the academic or preclinical investigate stage, few delivery systems are nearly in the clinical trial43. This disappointing clinical translation might result from insufficient studies of the pharmacokinetics of the delivery system for immunotherapy. There have been several studies44, 45, 46 focusing on the pharmacokinetics of drug delivery systems carrying chemotherapeutics and targeting the tumor environment and tumor cells. Going further, several studies47, 48, 49 find that the physicochemical properties of drug delivery system loading chemotherapeutics have a great influence on biodistribution, intratumoral penetration, and internalization, and thus significantly affect their efficiency. Different from the NPs carrying chemotherapeutics, NBCIs mainly modulate immune responses by targeted delivering therapeutic agents to immune organs and immune cells. The physicochemical properties of NPs including size, shape, elasticity and surface properties directly influence their interactions with immune system, and thus affect their in vivo fate and therapeutic effects. For example, NPs with smaller size tend to enter into the lymph nodes (LNs) more efficiently than NPs with larger size which contribute to different antitumor immunity50. Apart from size, shape of NPs may affect their internalization as well as intracellular transportation by immune cells51. However, the interactions between NPs and immune systems are rarely studied. Therefore, systematic analysis of the physicochemical properties and their effect on LNs drainage, cellular uptake and intracellular transfer is urgently needed. Herein, we first summarized fundamentals for the in vivo fate of NBCIs including physio-anatomical features of lymphatic system and strategies to modulate immune responses. Then we highlight the effect of physicochemical properties on LNs drainage, cellular uptake and intracellular traffic after administration. Finally, challenges and future perspectives for rational design of NBCIs are also discussed in detail.
2. Fundamentals for the in vivo fate of NBCIs
2.1. Physio-anatomical features of lymphatic system
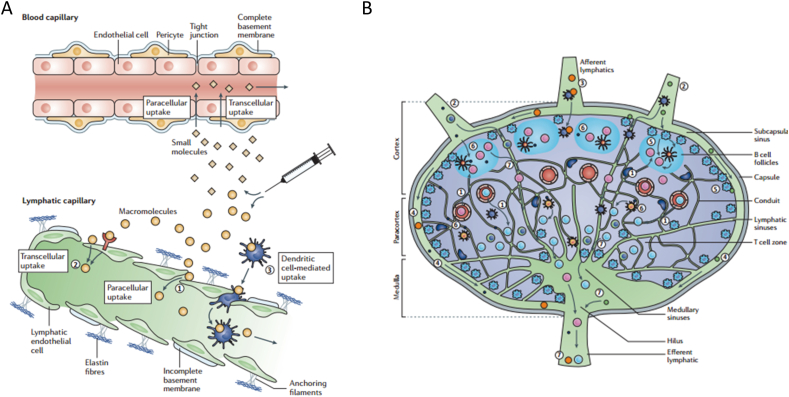
Lymphatic system including lymphoid organs and lymphatic vessels are the main target to modulate for efficient immune responses, especially the LNs. Hundreds of LNs which are connected by a network of lymphatic vessels disperse all over the human body52. The lymphatic vessels which comprises initial and larger collecting lymphatic vessels stretch into the interstitium. The lymphatic vessels facilitate interstitial fluid, antigens and lymphocytes to enter into the LNs which play a critical role in tissue fluid balance and immune response53. The interior region of LNs can be divided into three zones including cortex, paracortex and medulla zone. B cells and specialized follicular dendritic cells (DCs) make up a surrounding cortex zone. DCs and T cells comprise the paracortex zone54. Blood vessels termed high endothelial venules (HEVs) are found in the paracortex of LNs for the recirculation of lymphocytes from blood to LNs53. Soluble antigens or pathogens phagocytosed by antigen presentation cells (APCs) from interstitium enter into the lymphatic vessels, then transport to LNs. The antigens entering the LNs are internalized and processed by the APCs, then presented to the naïve T cells and B cells to generate antigen specific immune cells and antibodies55,56. Upon activation, antigen specific immune cells and antibodies exit the LN through medulla efferent lymphatic, enter into the circulation through the thoracic duct and traffic to diseased site to clear antigens or combat pathogens (Fig. 1)57. Therefore, lymphatic systems are primary site that therapeutic agents must reach to generate antigen-specific responses for cancer immunotherapy.
Figure 1.
Schematic illustration of lymphatic vessels and lymph node. (A) Initial lymphatic vessels paralleled with blood capillary stretched into the interstitium. Initial lymphatic vessels with blind-ended structures and discontinuous basement membrane, while the blood capillary with tight junction of endothelial cells. (B) The structure of lymph node which can be divided into cortex, paracortex and medulla zone. Reproduced with the permission from Ref. 57. Copyright © 2015 Springer Nature.
2.2. Strategies for NCBIs to modulate immune responses
Instead of direct killing cancer cells, NBCIs mainly modulate immune organs or immune cells to eradicate cancer cells. Strategies for NCBIs to modulate immune responses have been well developed. Firstly, NCBIs enable efficient immune priming by targeted delivering immunotherapeutic agents to lymph node. For example, nanovaccines, carrying antigens and adjuvants, targeted enter into LNs and activate APCs for improved antigen presentation and T cell immune responses. Secondly, NCBIs could improve the antitumor efficiency via efficiently delivering immune stimulants or checkpoint inhibitors to regulate the viability of T cells. Furthermore, NCBIs could reprogram tumor immune microenvironment for improved cancer treatment. For instance, cytokines engineered into NPs can be protected and targeted delivered to tumor microenvironment to increase the antitumor activity or decrease the pro-tumor activity of immune cells.
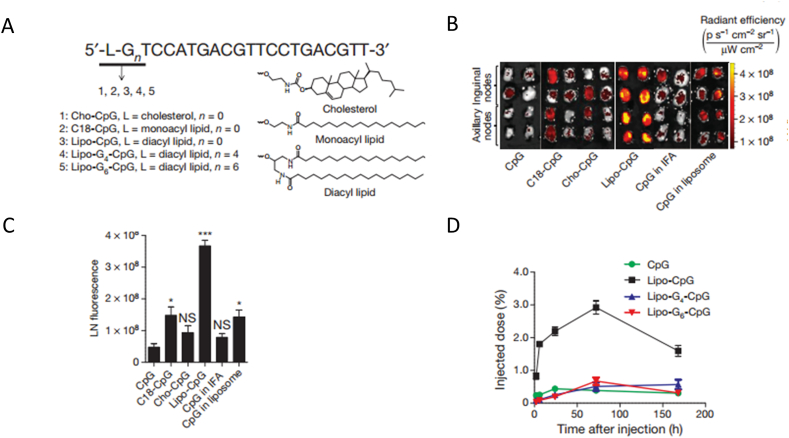
As for immune priming, lymph node is the primary site for immunotherapeutic agents to modulate immune priming and activation. DCs resident in the lymph node are the critical cells for antigen processing and presentation. Nanomaterials-based drug delivery systems such as liposomes, polymeric NPs and inorganic NPs have been exploited to deliver antigens and adjuvants to LNs, which efficiently promote antigen presentation and T cell activation58,59. For example, adjuvant CpG or peptide antigen was conjugated to albumin-binding lipids to construct an amphiphilic nanovaccine for LNs delivery. Compared with the free adjuvant or antigen, the albumin based nanovaccine achieved a much higher increase in LNs accumulation which promoted the T cell proliferation and antitumor efficiency in a murine model of melanoma (Fig. 2)60. In another study, antigens were reversibly conjugated to a synthetic glyco-adjuvant polymer for DCs targeting and activating. Results demonstrated that synthetic glyco-adjuvant polymer showed enhanced accumulation in LNs compared with the non-targeted polymers which lead to robust humoral and cellular immunity61. Inorganic nanomaterials have also been developed to modulate the lymph node immune responses for cancer immunotherapy. For example, a mesoporous silica rod (MSR) vaccine was manufactured by absorbing polyethylene mine (PEI) on the surface. The PEI absorbing vaccines was developed in a simple and convenient preparation way. Particularly, the vaccine with PEI efficiently promoted the dendritic cell activation and antigen specific T cell responses. Results showed that the MSR-PEI vaccine loading a single antigen efficiently inhibited the growth of TC-1 tumors. Furthermore, the MSR-PEI vaccine loading multi-antigen enabled robust immune response for personalized cancer vaccination62.
Figure 2.
Nanoparticle based immunotherapy target lymph node and DC cells. Adjuvant CpG was engineered into NPs for enhanced lymph node accumulation. (A) CpG was conjugated to different type of lipid to form NPs. (B) Fluorescent image of lymph node removed from mice injected with different formulations. (C) Quantification of the fluorescent image of lymph node. (D) CpG retention in the lymph node of different formulation 7 days after injection. Reproduced with the permission from Ref. 60. Copyright © 2014 Springer Nature.
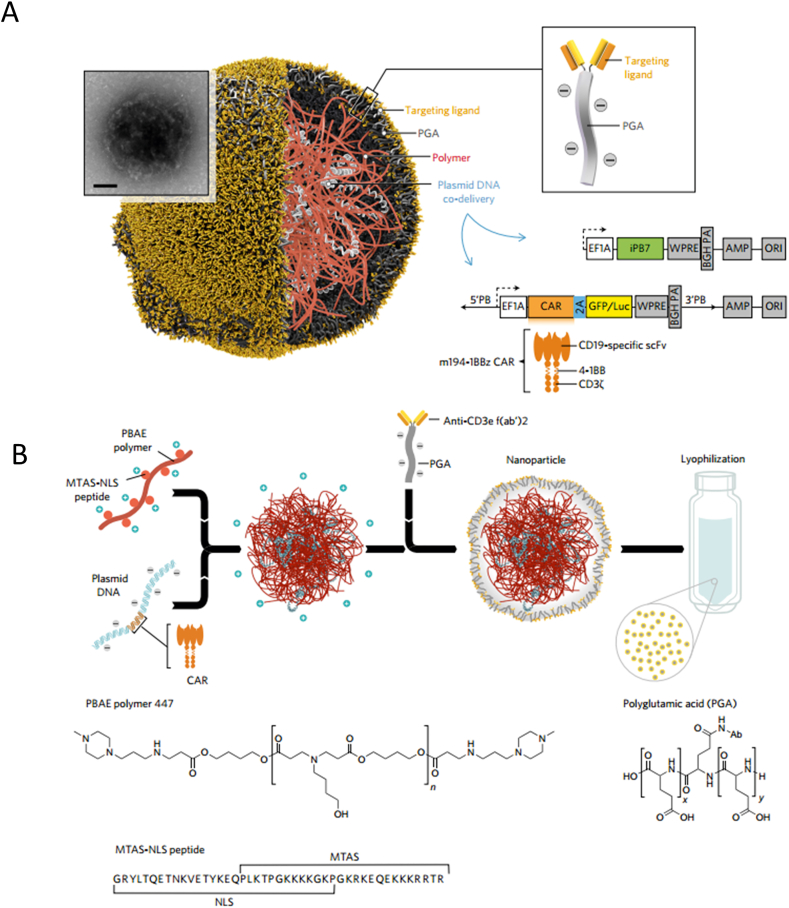
After effectively priming and presentation by DCs, the activation and proliferation of T cells is of great importance for cancer immunotherapy63, 64, 65. Nanomaterials loading with immune stimulating agents can be used to enhance endogenous T cell activity66, 67, 68. Schmid and coworkers69 synthesized poly(lactic-co-glycolic acid) (PLGA) and PEG (PLGA–PEG) based NPs functionalized with T cell targeting antibody and encapsulating transforming growth factor-β (TGF-β) inhibitor to restore T cell activity. Additionally, they targeted delivered a Toll-like receptor 7/8 (TLR7/TLR8) agonist to programmed cell death protein 1 (PD-1) expressing T cells to improve the viability of T cells. Results clarified that the way of efficiently delivering stimulating agents to endogenous T cells induced efficient eradication of cancer cells and significantly extended survival in a colorectal mouse model. Other than improving endogenous T cell activity, nanomaterials have also shown great potential to maintain the viability of adoptively transferred T cells in vivo70, 71, 72. For instance, liposomes and liposome-like synthetic NPs encapsulating adjuvant drugs were conjugated to the therapeutic T cells via maleimide-thiol coupling. The adjuvant drugs released from the NPs provide sustained stimulation to the therapeutic T cells, resulting in enhanced T cell therapy. This approach boosted the viability and proliferation of hematopoietic stem cell grafts compared with free adjuvants70. Apart from strengthening T cells activity by delivery immune stimulants, T cell function can be up regulated by blocking the inhibitory receptors via checkpoint inhibitors. Nanomaterials can be used to deliver checkpoint inhibitors locally73 or systemically74 to enhance the T cell function and reduce side effects75. Furthermore, T cells circulated in the blood can be modified to recognize and attack cancer cells. In one study, A DNA-carrying polymer nanoparticle was developed to target T cells circulated in the blood and program them with leukemia-recognizing CAR genes (Fig. 3)76. These polymer NPs enabled robust antitumor effect. Particularly they provided a practical preparation way for CAR T-cell therapy which avoided laborious and complicated manufacturing procedures.
Figure 3.
Nanoparticle based immunotherapy target T cells in circulation. (A) Schematic illustration of the NPs structure targeting and modifying the T cells in circulation. (B) Components and preparation of the polymer-based NPs for T cells targeting. Reproduced with the permission from Ref. 76. Copyright © 2017 Springer Nature.
Apart from DCs and T cells, other immune cells, such as tumor-associated macrophages (TAMs), natural killer (NK) cells and myeloid-derived suppressor cells (MDSCs) also play a critical role in the antitumor immunity77,78. For instance,TAMs infiltrating in the tumor environment can be classified into M1-type macrophages and M2-type macrophages which exert antitumor and pro-tumor effects respectively79. As tumor progresses, TAMs predominately exist in the phenotype of M2 which contribute to form an immunosuppressive and pro-tumor microenvironment resulting in invasion of tumor cells. Nanomaterials delivering therapeutic agents to eliminate80,81 or modulate82,83 the phenotype of TAMs have been well studied to achieve efficient antitumor immune responses. For example, a bisphosphonate–glucomannan conjugate was developed to targeted deplete M2 macrophages. The conjugate efficiently targeted the macrophages and caused a 84.5% reduction of TAMs in the tumor tissue compared with 17.0% reduction of TAMs treated with free bisphosphonate80. In addition, NK cells, as an innate effector lymphocytes, can directly recognize and attack cancer cells at an early stage of tumor progress which lead to a much faster immune response84. Nanomaterials have been investigated to modulate the viability of NK cells for cancer treatment85,86. Loftus and coworkers85 prepared a graphene oxide-based nanoscale clusters functionalized with antibodies on the outside surface to mimic an immune cell to interact with NK cells. These NGO-templated nanoclusters significantly stimulated NK cells activity via interacting with the CD16 receptor compared with soluble antibodies. MDSCs, as an immunosuppressive immune cell, have also been a target cell to deplete or block its function for efficient antitumor immunity87, 88, 89. For example, gemcitabine was found to be effective to inhibit the proliferation of MDSCs at a low concentration. Maria and coworkers developed a lipid-based NPs encapsulating lauroyl-modified gemcitabine. This lipid NPs efficiently targeted and depleted MDSCs which resulted in reduced percentage of MDSCs in the spleen and tumor for enhanced antitumor efficacy of lymphoma and melanoma89.
Strategies for NCBIs to modulate immune responses have experienced great progress. The developed NCBIs were summarized and listed in Table 1. To achieve efficient antitumor immunity, the in vivo fate of NCBIs which was greatly influenced by the physicochemical properties of NPs and their interaction with immune system should be fully investigated.
Table 1.
Strategies for nanoparticle-based cancer immunotherapies (NCBIs) to modulate immune responses.
| Target cell | NPs platform | Immunotherapeutic agent | Function | Ref. |
|---|---|---|---|---|
| DCs | Ionizable lipid-like materials | mRNA or antigens | Efficient mRNA delivery and STING activation | 58 |
| pH-sensitive polymers | Antigens | Efficient antigen cytosolic delivery and STING activation | 59 | |
| Albumin-binding lipids | CpG or peptide | Enhanced lymph node delivery and T Cell activation | 60 | |
| Polymers | Antigens and adjuvants | Robust humoral and cellular immunity | 61 | |
| Mesoporous silica rod absorbing PEI | CpG, antigens and GM-CSF | Promote dendritic cell activation and antigen specific T cell responses | 62 | |
| T cells | Nanoscale polymeric gels | TGF-β inhibitor and IL-2 | Facilitate the CD8+ T-cell infiltration into tumors | 68 |
| PLGA/PEG-based NPs | TGFβR1 inhibitor and TLR7/TLR8 agonist | Improve the viability of endogenous T cells and its antitumor efficiency | 69 | |
| liposomes and liposome-like synthetic NPs | Adjuvant drugs | Boosted the viability and proliferation of T cells | 70 | |
| Reactive oxygen species–responsive scaffold | Gemcitabine and checkpoint inhibitor | Improve the activity of CD8+ T cells for efficient cancer eradication | 73 | |
| Gold NPs | Checkpoint inhibitors | Enhanced antitumor effect and rapid prediction of therapeutic response | 74 | |
| Other immune cells | Bisphosphonate-glucomannan conjugate | Bisphosphonate and glucomannan | Efficiently reduced the percentage of TAMs | 80 |
| Ferumoxytol | Ferumoxytol | Inhibit tumor growth by converting M2-like TAM into M1-like TAM | 82 | |
| Graphene oxide-based nanoscale clusters | Antibodies | Significantly stimulate NK cells activity | 85 | |
| Phosphonate capped dendrimers | Phosphonate capped dendrimers | Efficient proliferation of human NK cells | 86 | |
| Lipid NPs | Lauroyl-modified gemcitabine | Reduce the percentage of MDSCs in the spleen and tumor | 87 | |
| Heparin-tocopherol succinate nanoparticle | Heparin-tocopherol Succinate nanoparticle | Inhibit the recruitment of MDSCs and expression of MMP-9 in MDSCs | 88 |
DCs, dendritic cells; GM-CSF, granulocyte–macrophage colony-stimulating factor; MDSCs, myeloid-derived suppressor cells; MMP-9, matrix metalloprotein 9; mRNA, messenger RNA; NK cells, natural killer cells; NPs, nanoparticles; PEI, polyethylenimine; TAM, tumor-associated macrophages; TGF-β, transforming growth factor-β; TGFβR1, transforming growth factor beta-receptor 1; TLR7/TLR8, toll-like receptor 7/8.
3. Effect of NPs physicochemical properties on lymph node drainage
Lymph node, as one of the most important lymphoid organs provides a primary site for immunotherapeutic agents to modulate immune priming and activation53,90. Most therapeutic agents are delivered to the lymph node trough interstitial administration including subcutaneous, intramuscular or intradermal injection54,91. Immunotherapeutic agents can be loaded inside or conjugated to the outside of the NPs. Physicochemical properties of the NPs such as size, shape, elasticity and surface properties significantly influence their interaction with interstium and lymphatic system which determine their biological fate as well as antitumor immune responses. In this part, we highlight the effect of major physicochemical properties of NPs on their interaction with interstium and LNs drainage.
3.1. Size
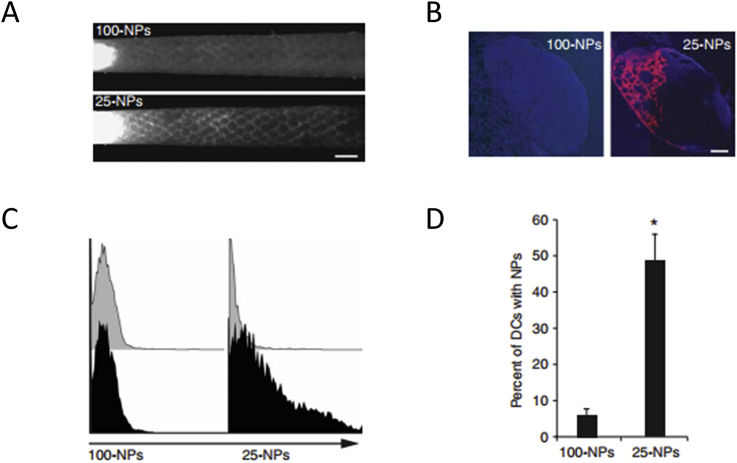
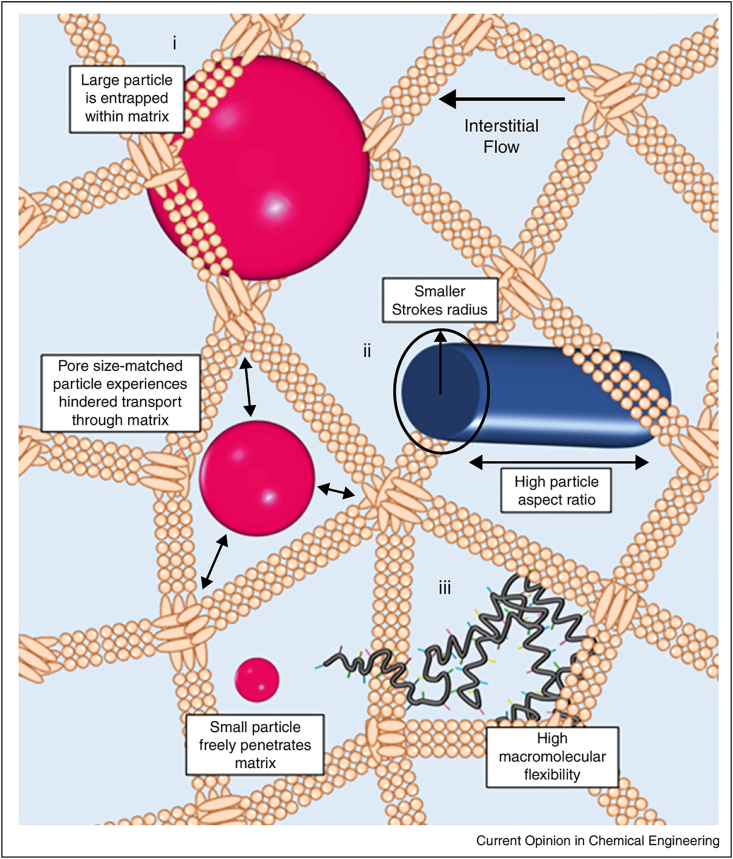
The size of NPs which predominantly affects the contact area of NPs with physiological environments is a critical physicochemical property that influence their in vivo fate92. Studies have shown that small NPs can readily enter into lymphatic vessels, then transfer to LNs50,93,94. In one critical study, small NPs (25 nm) and large NPs (100 nm) was used to study the effect of size on LNs drainage. The results revealed that the 25 nm NPs entered into lymphatic vessels more quickly and efficiently than the 100 nm NPs, then transferred to the LNs. Particularly, more than half of DCs resident in the lymph node were found with the 25 nm NPs compared with 6% DCs with 100 nm NPs (Fig. 4)50. Similarly, poly (propylene sulfide) NPs with diameter of 20, 45, and 100 nm was constructed to investigate their delivery efficiency toward LNs. The results showed that 20 nm NPs transferred into the lymphatic vessels more efficiently and stay in the LNs for longer time than 45 nm and 100 nm NPs93. Large NPs tend to be difficult to enter into lymphatic vessels and transfer to LNs95, 96, 97. Manolova and coworkers95 domonstated that large NPs with diameter of 500–1000 nm were mainly trapped in the interstitial matrix and phagocytized by macrophages and DCs resident in the interstitial matrix. Moreover, Ryan96 demonstrated that large NPs with diameter larger than 100 nm could not readily transfer to lymphatic vessels because the water channels formed by interstitial extracellular matrix were typically 100 nm in diameter which limited the size of molecules transfer to lymphatic vessels (Fig. 5)55. Therefore, NPs larger than 100 nm cannot efficiently enter into the lymph node. However, the size of the NPs drain to LNs cannot be too small98, 99, 100. Kobayashi and colleagues100 demonstrated that NPs smaller than 9 nm were more likely to enter into the vascular capillaries and rapidly cleared away from the blood via urinary excretion, while the NPs above this size predominantly drained into the lymphatic vessels. Another study57 suggested that NPs smaller than 10 nm penetrated into both vascular capillaries and lymphatic vessels freely. While the small NPs predominantly entered into vascular capillaries owing to the vascular capillaries with a high blood flow rate than the lymph fluid in the lymph capillaries. In general, NPs with diameter ranging from 10 to 100 nm may be preferable to efficiently transfer through interstitial matrix, enter into the lymphatic vessels and ultimately drain into the LNs.
Figure 4.
Small NPs are more readily accumulate in the lymph node. (A) Fluorescence microlymphangiography of lymphatic vessels after injection of 100 nm and 25 nm NPs. (B) Location and retention of NPs in the lymph node. (C) CD11c + cells with 100 nm and 25 nm NPs analyzed by flow cytometer. (D) Quantification of CD11c + cells with 100 nm and 25 nm NPs. Reproduced with the permission from Ref. 50. Copyright © 2007 Springer Nature.
Figure 5.
Schematic illustration of small and large NPs transferred in the interstitial matrix. Small NPs can transfer through the interstitial freely, while the large NPs is entrapped within the matrix. Reproduced with the permission from Ref. 55. Copyright © 2015 Elsevier.
3.2. Surface charge
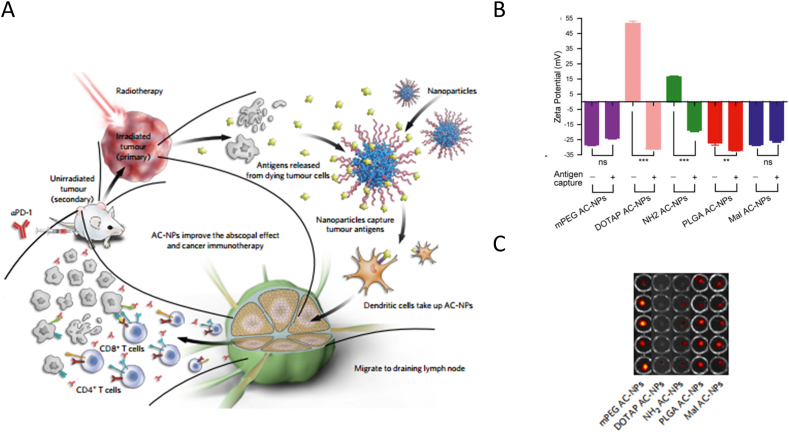
Surface charge has a significant impact on the interaction between NPs and interstitial matrix which influence the in vivo fate of NPs. Interstitial matrix is consist of polysaccharides, glycoproteins and collagen fibers which carries negative charge101. Thus, NPs with positive charge tend to be trapped in the interstitium owing to electrostatic interactions102. While neutral or negative charged NPs usually transfer through the interstitium more easily. Doddapaneni and coworkers103 studied the charge effect on LNs distribution and anti-lymphatic metastasis effect using three types of NPs constructed by PEG‒PCL polymers. The surface charge state of three NPs was neutral, partially charged and fully charged by modifying PEG block with different ratio of anionic and neutral groups. They observed that the neutral and partially charged NPs could efficiently target the lymph node and combat the lymph node metastatic of melanocytes compared with the fully-charged NPs. Additionally, Huang's group104 developed negatively-charged and positively-charged lipid–calcium phosphate NPs for LNs imaging. They found that the negatively-charged NPs drained more readily to the LNs compared with the positively charged LCP NPs. In another study, Min and coworkers105 modified poly(lactic-co-glycolic acid) NPs with different surface charge to deliver antigens released by radiotherapy to LNs for enhanced immunotherapy. Their results showed that mPEG AC-NPs, PLGA and Mal AC-NPs with negative charge accumulated at higher rates in the LNs compared with DOTAP AC-NPs and NH2 AC-NPs with positive charge post administration (Fig. 6). Other than experimental evidences, Stylianopoulos and coworkers106 investigate the charge effect of macromolecules and NPs on their transfer through the interstitial matrix of physiological tissues using a mathematical framework. Their results showed that positively-charged NPs were typically more avidly trapped at the injection site due to the electrostatic interactions with negative charged collagen fibers and hyaluronic acid57 filled in the interstitium. Therefore, neutral or negative charged NPs are preferable to drain freely to lymphatic vessels, then arrive at LNs.
Figure 6.
NPs with different charge state for lymph node targeting. (A) Schematic illustration of NPs with different charge absorbing antigens released from radiotherapy drained to lymph node. (B) Charge state of different NPs absorbing antigens or not. (C) Fluorescence image of lymph node injected with different formulations. Reproduced with the permission from Ref. 105. Copyright © 2017 Springer Nature.
3.3. Hydrophobicity
Surface hydrophobicity/hydrophilicity balance plays an important role in the transportation of NPs from interstitium to LNs. As the interstitium101 is primarily composed of hydrophilic collagen fibers and glycosaminoglycans, transfer of hydrophilic NPs through interstitium is thought to be more easily than hydrophobic NPs. Rao and coworkers107 studied the effect of hydrophobicity/hydrophilicity balance on LNs accumulation by using higher hydrophobic PS NPs and lower hydrophobic PP (PLGA-PMA:PLA-PEG) NPs of the similar size. Their results showed that PP NPs with low hydrophobicity had much higher LNs accumulation than the PS NPs after subcutaneous injection. Furthermore, they suggested that this was probably due to the rapid aggregation of the PS NPs at the injection site for their high hydrophobicity which prevent their drainage to LNs. These studies thus highlight that hydrophilic NPs tend to more efficiently transfer to the lymphatic vessels and drain into the LNs than hydrophobic NPs.
3.4. Elasticity
Elasticity reflects the property of a nanoparticle to be deformable which is characterized by certain physical parameters such as Young's moduli108. The effect of nanoparticle elasticity on biodistribution and uptake by tumor cells after intravenous administration has been well studied108, 109, 110. Apart from influencing the in vivo fate of nanoparticle following intravenous administration, elasticity has been shown to influence the interstium retention time and antigen LNs drainage after interstium administration. Xia and co-workers111 studied the pliability and lateral mobility of particles for its immune response by evaluating antigen LNs drainage and immune cellular uptake. Their results suggested that pickering emulsion particles with pliability compared with rigid particles showed enhanced antigen LNs drainage by increased droplet cell contact area induced by particle deformation (Fig. 7). On the contrary, Christensen et al.112 compared the LNs drainage and immune activation capacity of liposome prepared by rigid dimethyldioctadecylammonium (DDA) and highly fluid dimethyldioleoylammonium (DODA). They found that the rigid DDA-based liposomes easily formed a depot at the injection site which resulted in a more efficient LNs drainage and antigen cellular uptake than the highly fluid DODA-based liposomes. Thus, further investigations are still needed to study the effect of nanoparticle elasticity on LNs drainage and immune responses.
Figure 7.
Effect of particle elasticity on cellular uptake and lymph node accumulation. (A) Particles with pliability and lateral mobility deform on the cell surface for enhanced cellular uptake. (B) Rigid particles did not deform on the cell surface with reduced cellular uptake. (C) Comparison of antigen presentation of nanoparticle with different elasticity. (D) Fluorescence image of lymph node accumulation after injected with different formulations. Reproduced with the permission from Ref. 111. Copyright © 2018 Springer Nature.
4. Effect of NPs physicochemical properties on cellular uptake
Upon drainage into the LNs, NPs need to be internalized by immune cells to exerts antitumor immune responses113. When NPs reach to the outside of the immune cells, they interact with the plasma membrane and then was engulfed by membrane invagination to form endocytic vesicles114 for internalization. Physicochemical properties of NPs including size, shape, elasticity and surface properties significantly affect the adsorption, contact area and interaction strain between NPs and cell membrane110,115. Therefore, the effect of physicochemical properties on cellular uptake by immune cells need to be well analyzed.
4.1. Size
The size of NPs determines the contact area of particle surface and cell membrane which greatly influenced the cellular uptake by immune cells. The uptake of NPs through endocytosis can be classified into several different mechanisms including phagocytosis and macropinocytosis116, 117, 118. With the size increase, the surface to volume ratio of NPs is decreased, which decrease their interactions area with immune cells resulting in less uptake119. For example, with the size of NPs exceeding 500 nm, the cellar uptake by DCs was less efficiently118. While small NPs of 10 and 50 nm were engulfed more readily by DCs. Furthermore, a significantly higher cellular uptake of 10 nm AuNPs than 50 nm AuNPs was observed120. Additionally, large NPs are generally internalized through phagocytosis mechanisms by macrophages, neutrophils, or DCs resident in the tissue which are response for the host defense and pathogen clearance. While small NPs usually enter into cells via the cooperation of several endocytic mechanisms. For instance, polystyrene NPs with diameter of 40 and 600 nm was used to investigate the size effect on cellular uptake mechanism by immune cells. Their results demonstrated that 40 nm polystyrene NPs were internalized by multiple uptake mechanisms including clathrin-mediated endocytosis, phagocytosis and macropinocytosis. In comparison, 600 nm polystyrene NPs were internalized mainly through phagocytosis by J774.1A macrophages121. Going further, Gu et al.122 investigated the cellular uptake pathway of super paramagnetic iron oxide NPs by endocytic inhibitor analysis. Their results demonstrated that small superparamagnetic iron oxide NPs of 10 nm were internalized by Raw 264.7 macrophage cells through multiple endocytic pathways involved clathrin-dependent endocytosis, caveolae-dependent endocytosis and macropinocytosis. Therefore, small NPs with diameter ranging from 10 to 50 nm tend to be more easily internalized by immune cells through multiple uptake mechanisms including clathrin-mediated endocytosis, phagocytosis and micropinocytosis, while large NPs with diameter exceeding 500 nm show less efficient cellular uptake.
4.2. Shape
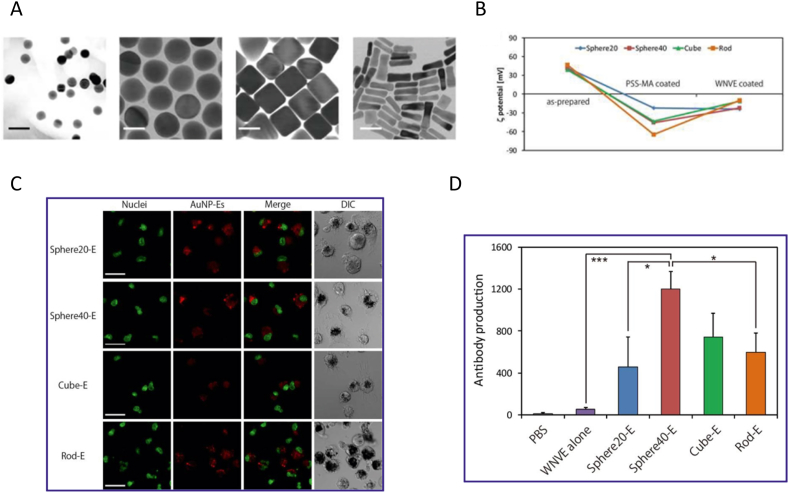
Shape of NPs characterized by aspect ratios and edge curvature affects the orientation and contact angle of NPs being internalized by cells, thus being a critical physicochemical property influencing cellular uptake123,124. It has been reported that rod-shaped NPs showed lower cellular uptake than spherical NPs by cancer cells125,126. Consistent with cancer cells, several studies demonstrated that immune cells internalized spherical NPs more efficiently than rod-shaped NPs127. For instance, disc-shaped and rod-shaped NPs with similar volume were incubated with mouse bone marrow dendritic cells (BMDCs). Results showed that disc-shaped NPs could be internalized more efficiently than rod-shaped NPs by BMDCs at all time points127. Similarly, spherical (20 nm and 40 nm in diameter), rod (40 nm × 10 nm), and cubic (40 nm × 40 nm × 40 nm) AuNPs was prepared to study the shape effect on immune response by evaluating the efficiency of cellular uptake by RAW264.7 macrophages. Results from their study revealed that the spherical AuNPs were more efficiently internalized by macrophages than rod AuNPs in terms of the weight of NPs (Fig. 8)128. The underline mechanism of shape effect on internalization was explained through theoretical simulation approaches123,129. A theoretical model was established by Yi's group129 to evaluate the process of membrane wrapping of different particles. Keeping other physical chemical parameters constant, they found that rod-shaped particles require more energy for cell membrane wrapping than spherical particles. Additionally, rod-shaped particles underwent an orientation change during wrapping which also increased energy expenditure and decreased its cellular uptake129. Other than that, NPs of different shape were also proved to be internalized through different uptake pathways. In one example, spherical and cylindrical micelles of varied lengths was constructed to investigate the uptake pathway by RAW 264.7 macrophages. Results showed that spherical micelles had a higher accumulation in the macrophages through clathrin- and caveolin-dependent endocytosis than cylindrical micelles which mostly through clathrin mediated endocytosis130. Additionally, shape may influence the immune responses toward immune cells51,131. For instance, Wang et al.131 developed inorganic TiO2 particles with rough surface to investigate their immune responses. They observed that only TiO2 particles with spiky surface elicited K+ efflux and inflammasome priming in macrophages and DCs. Furthermore, the spiky particles enabled enhanced DC maturation which resulted in robust humoral and adaptive immune responses against tumor and influenza. Thus, spherical NPs could be internalized more efficiently by immune cells because less energy is required for cell membrane wrapping during the process of cellular uptake.
Figure 8.
Effect of shape on cellular uptake by RAW264.7 macrophages. (A) TEM images of spherical, cubic and rod Au NPs. (B) Characterization of ζ potential of Au-NPs after modification. (C) Cellular uptake of Au-NPs with different shape observed by CLSM. (D) Antibody production treated with different formulations. Reproduced with the permission from Ref. 128. Copyright © 2013 American Chemical Society.
4.3. Surface charge
Surface charge which is typically measured by zeta potential of NPs dispersed in aqueous media directly affects the interaction between NPs and cell membrane117,132. NPs can be engineered with positive, negative, or neutral surface charge by different compositions or surface modifications. Cell membrane mainly comprise of phospholipid-based bilayer leading to an overall negative charge. Therefore, positively charged NPs show higher internalization due to the enhanced electrostatic attraction between NPs and negatively charged cell membrane133, 134, 135. For example, Yue et al.136 fabricated three kinds of Chitosan-based NPs with similar size but different surface charge to study the surface charge effect on cellular uptake. Their results demonstrated that positive charged NPs showed higher cellular uptake than the neutral and negative charged NPs in eight representative cell lines including immune cells. Similarly, Fytianos and coworkers137 reported that positive PVA-NH2 AuNPs were taken up higher than the negative PVA-COOH NPs by both the monocyte-derived macrophages and monocyte-derived dendritic cells. In another study, Mou and coworkers138 compared the cellular uptake of positively charged Fe2O3 NPs and negatively charged Fe2O3 NPs. They demonstrated that positively charged Fe2O3 NPs showed higher cellular uptake by DCs and enhanced cross-presentation, while the negatively charged Fe2O3 NPs with lower cellular uptake and showed activation of autophagy. Therefore, NPs with positive charge exhibited higher internalization due to the enhanced electrostatic attraction between NPs and negatively charged cell membrane.
4.4. Elasticity
NPs elasticity also significantly influences the internalization of NPs by immune cells. For example, Palomba and coworkers139 demonstrated that the rigid NPs showed 5 times more efficiently internalization than the soft NPs in bone marrow derived monocytes independent of the size and shape. The interactions of NPs with macrophage cell membrane was studied by high-resolution live cell microscopy. The results indicated that the time of soft NPs interact with the cell membrane was shorter than that of rigid NPs which may decrease the cellular recognition and internalization. However, Xia and co-workers111 demonstrated that pickering emulsions with pliability and lateral mobility compared with solid particles showed enhanced uptake by BMDCs. They suggested that pickering emulsions showed deformation on the cell membrane and increased droplet–cell contact area, then promoted the cellular uptake. While deformation was not observed on the cells treatment with solid particles which inhibited the landing of actions and prevented the interaction of BMDCs and NPs111. Therefore, the effect of elasticity on cellular uptake by immune cells is still need to be studied by further investigation.
4.5. Modifying ligand
The surface of NPs can be engineered with specific ligands for enhanced cellar targeting and uptake. For example, Yang and coworkers constructed a poly(d,l-lactide-co-glycolide) nanoparticle coated with tumor cell membranes which was modified by mannose for efficient APCs targeting. Due to the overexpression of mannose receptors on APCs, they observed that mannose-modified NP@M–M enabled much higher cellular uptake by BMDCs and macrophages than NP@M without mannose modification. Furthermore, they observed that the mannose-modified NP@M–M NPs combined with checkpoint blockade therapy showed efficient antitumor efficiency140. Similarly, Cruz and coworkers studied the uptake of non-targeted NPs and targeted NPs modified with antibody CD40, DEC-205, and CD11c overexpressed by DCs. Their results demonstrated that targeted NPs showed higher dendritic cell uptake and efficient T cell priming capacity compared with non-targeted NPs141. Similarly, AuNPs modified with DC-SIGN antibody which targeting dendritic cell surface showed significantly increased uptake by DCs which result in robust T cell activation compared with non-modified control AuNPs137. Therefore, NPs modified with targeting ligand could efficiently promote the cellular uptake by immune cells.
5. Effect of NPs physicochemical properties on intracellular transfer
Following cellular internalization, NPs are usually trapped in endocytic vesicles142. The intracellular trafficking of endocytic vesicles is mediated by a serial of cellular endosomes including lysosome, Golgi apparatus, and endoplasmic reticulum116,143. The endocytic vesicles experience a maturation process and eventually fuses with lysosome compartments to digest and degrade the immunotherapeutic agents which compromise the antitumor effect144. Furthermore, antigens escaped from the endosomes and presented through class I MHC molecules to CD8+ T cells is necessary to elicit efficient adaptive antitumor immunity for cancer treatment145,146. Therefore, strategies that functionalized NPs with stimulus responsivity will facilitate endosomal escape and cytosolic delivery for efficient antitumor immunity147,148.
5.1. pH responsivity
NPs with pH responsivity have been utilized to induce endosomal escape and antigen cytoplasmic delivery149, 150, 151. For example, Luo and colleagues59 developed an serial of pH-sensitive polymers comprising of linear or cyclic tertiary amines in the side chains for cytosolic antigen delivery. These NPs escaped from the endosome and facilitated the cytosolic delivery of tumor antigens due to the proton sponge effect of the polymer under an acidic environment. The results showed that the pH-sensitive copolymer based nanovaccine enable efficient antigen cytosolic delivery and robust antitumor immunity in various tumor models (Fig. 9). Similarly, polymer micelles were also developed for efficient cytosolic delivery of tumor antigens for efficient antitumor effect152. This polymer micelle comprises a tercopolymer ampholytic core forming block with pH-sensitive activity for endosome escape and an N-(2-hydroxypropyl) methacrylamide block to conjugate with antigens. Results demonstrated that this pH-sensitive polymer micelles promoted much higher cytoplasmic antigen levels in murine DCs than soluble antigens. Following subcutaneous immunization, mice with antigens conjugated to the pH-sensitive polymer micelle achieve efficient CD8+ cytotoxic T cell (CTL) responses compared with free antigens. Other than efficient cytosolic delivery of antigens, Hu's group153 developed a pH-responsive core−shell segregated polymer for efficient delivery of small molecules and proteins which was membrane impermeable for DCs. The polymer was constructed by a pH-responsive core for endosome escape and a hydrophilic shell for drug loading. Results showed that the polymer achieved efficient cytosolic delivery of membrane impermeable molecules by disrupt the endosome membrane due to the proton sponge effect, resulting efficient CD8+ T cell priming153. Hence, NPs with pH responsivity enable efficient endosomal escape and cytoplasmic delivery.
Figure 9.
NPs with pH responsivity for intracellular delivery of antigens. (A) Schematic illustration of polymer based nanovaccine for efficient antigen cytosolic delivery and robust tumor inhibition. (B) Chemical structure of polymers with linear or cyclic tertiary amines in the side chains. (C) Antigen presentation with free OVA or OVA encapsulated in the pH responsive polymers. (D) Tumor growth inhibition rate of mice bearing B16-OVA after immunized by different formulations. Reproduced with the permission from Ref. 59. Copyright © 2017 Springer Nature.
5.2. Light responsivity
Light-responsive NPs have also been utilized to promote endosome escape for efficient cytosolic delivery. For example, a photosensitive mixture composed of antigen and photosensitizer Amphinex was constructed to test the efficiency of photochemical mediated endosomes disruption and cytosolic delivery of antigens. Upon light exposure, the photosensitive mixture enabled enhanced cytosolic antigen delivery and MHC class I antigen presentation than free antigen by DCs. Additionally, autologous immunization with DCs that had been treated with the mixture and light exposure generated efficient antigen-specific CD8+ T cell proliferation in mice154. In another study, copolymers with light responsivity were developed for efficient endosome disruption and cargo release into the cytosol. Ethyl eosin was used as the hydrophobic part and acts as a photosensitizer responsive to light. Upon light exposure, the copolymer destabilized the endosome membrane to enable efficient antigen cytosolic delivery. Furthermore, MHC I antigen presentation was observed in DCs treated with copolymers encapsulating a model antigen after light exposure155. Therefore, NPs with light responsivity promote endosomal escape and cytosolic delivery for efficient immune responses.
5.3. Redox sensitivity
NPs with redox sensitivity tend to disassemble and release their encapsulating cargoes in the bio-reducible environment including cytosol and cell nucleus as well as late endosomes156, 157, 158. NPs with redox sensitivity have been exploiting as a promising platform to enhance chemotherapeutic agents and gene therapeutics delivery by promoting cytoplasmic drug release159,160. As a means to facilitate endosome escape and cytosolic delivery, NPs with redox sensitivity have also been utilized for efficient cytoplasmic delivery of antigens and immunotherapeutic agents. For example, Li et al.161 synthesized alginate-poly(ethylene imine) nanogels with bio-reducible responsiveness for antigen cytosolic delivery. The nanogels was constructed by branched PEI electrostatic interacting with negatively charged alginate and cross-linked by disulfide to obtain bio-reducible ability. The nanogels achieved efficient endosome escape via proton sponge effect of PEI, then degraded and release the encapsulating antigens in the reducing cytosol environment. The nanogels enabled efficient endosome escape and cytosolic release of the loading antigens which resulted in robust immune responses compared with non-reducible nanogels.
6. Conclusion and future perspectives
With the rapid development of nanotechnology and immunotherapy, nanomaterials have been increasingly exploited as the delivery systems of immunotherapeutic agents to improve immune efficiency and reduce toxicity. The physicochemical properties of NPs like size, charge, shape, hydrophobicity, elasticity and surface modifications determine their interactions with physiological environment which significant affect their in vivo fate and efficiency. Thus, systematic analysis of the physicochemical properties and their interaction with physiological environment is urgently needed. In this review, we first summarized fundamentals for the in vivo fate of NBCIs including physio-anatomical features of lymphatic system and strategies to modulate immune responses. Then we highlighted the effect of physicochemical properties of NPs on LNs drainage, immune cellular uptake and intracellular traffic after administration. For rational design of NBCIs, several general rules are suggested. Firstly, the size of NPs should be controlled within the range of 10–100 nm which will be preferable to efficiently enter into the lymphatic vessels and ultimately drain into the LNs. Secondly, NPs with high hydrophilicity and negative charge is preferred to transfer freely through interstitial matrix and then drain into the LNs. Furthermore, NPs with spherical shape and targeting ligand is more efficiently internalized by immune cells. At last, NPs with pH responsivity, light responsivity and redox sensitivity enable efficient endosomal escape and cytosolic delivery which elicit potent antitumor immunity. Through regulating the physicochemical properties of NPs, efficient immune responses against tumors can be achieved.
Despite the advances, great efforts still need to be made to understand the in vivo fate of NBCIs. Firstly, more efforts should be made to understand the potential influence of delivery barriers for immunotherapeutic agents. It has been reported that immunotherapeutic agents for cancer immunotherapy are mainly targeted delivered to lymphoid tissues or specific immune cells through interstitial administration which is quite different from the target of chemotherapeutics through intravenous or intratumoral administration. Therefore, systematic investigation of the delivery barriers confronted immunotherapeutic agents from interstitium to lymphoid tissues is urgently needed. Moreover, the microenvironment of lymphoid organs such as LNs and spleens need to be studied to elucidate their interaction with immunotherapeutic agents for immune responses. Secondly, several physiochemical properties have different effect on the in vivo fate of NPs for immunotherapy. For instance, Palomba demonstrated that rigid NPs showed higher cellular uptake than the soft NPs by BMDCs. While Xia and co-workers111 demonstrated that pickering emulsions with pliability and lateral mobility showed enhanced uptake than solid particles by BMDCs. They explained that pickering emulsions deformed on the cell membrane and increased droplet cell contact area, then promoted the cellular uptake. Thus, more efforts should be made to optimize the physicochemical parameters for efficient LNs targeting and antitumor immunity. Additionally, some physicochemical parameters influence the activation of immune responses. For example, rigid liposomes encapsulating antigen Ag85B-ESAT-6 enabled much more efficient Th-1 immune responses than soft liposomes. Future investigation that explain the mechanism and interaction between physicochemical parameters and immune response will be helpful for the rational design of drug delivery systems for cancer immunotherapy.
Overall, it has been shown that physicochemical properties significantly influence the interaction of NPs with immune system which affects the in vivo fate and antitumor immunity. We believe that in-depth investigation of the physicochemical properties of NPs and their interaction with immune systems will accelerate the development of NBCIs and facilitate its medicine translation.
Acknowledgments
This work was supported by National Key Research & Development Program of China (Grant No. 2018YFE0117800, China), the National Natural Science Foundation of China (NSFC) key projects (grant No. 31630027, 32030060, 51773227 and 81701815, China), NSFC international collaboration key project (Grant No. 51861135103, China) and NSFC-German Research Foundation (DFG) project (Grant No. 31761133013, China). The authors also appreciate the support by “the Beijing-Tianjin-Hebei Basic Research Cooperation Project” (19JCZDJC64100, China), and the Youth Thousand-Talents Program of China.
Author contributions
Yongchao Wang, Jinjin Wang, Dandan Zhu, Yufei Wang, Guangchao Qing, Yuxuan Zhang, Xiaoxuan Liu and Xing-Jie Liang conceived the manuscript. Yongchao Wang wrote the manuscript and designed the figures. Yongchao Wang, Jinjin Wang, Dandan Zhu, Yufei Wang, Guangchao, Qing, Yuxuan Zhang, Xiaoxuan Liu and Xing-Jie Liang edited the manuscript.
Conflicts of interest
The authors declare no competing financial interests.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Xiaoxuan Liu, Email: xiaoxuanliucpu@163.com.
Xing-Jie Liang, Email: liangxj@nanoctr.cn.
References
- 1.Armstrong-James D., Brown G.D., Netea M.G., Zelante T., Gresnigt M.S., van de Veerdonk F.L. Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect Dis. 2017;17:e393–e402. doi: 10.1016/S1473-3099(17)30442-5. [DOI] [PubMed] [Google Scholar]
- 2.Look M., Bandyopadhyay A., Blum J.S., Fahmy T.M. Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv Drug Deliv Rev. 2010;62:378–393. doi: 10.1016/j.addr.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caspi R.R. Immunotherapy of autoimmunity and cancer: the penalty for success. Nat Rev Immunol. 2008;8:970–976. doi: 10.1038/nri2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblum M.D., Gratz I.K., Paw J.S., Abbas A.K. Treating human autoimmunity: current practice and future prospects. Sci Transl Med. 2012;4:10. doi: 10.1126/scitranslmed.3003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanmamed M.F., Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Burg S.H., Arens R., Ossendorp F., van Hall T., Melief A.J.M. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.June C.H., Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L., Dichwalkar T., Chang J.Y.H., Cossette B., Garafola D., Zhang A.Q. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 14.Graff J.N., Chamberlain E.D. Sipuleucel-T in the treatment of prostate cancer: an evidence-based review of its place in therapy. Core Evid. 2014;10:1–10. doi: 10.2147/CE.S54712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maleki Vareki S., Garrigós C., Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol. 2017;116:116–124. doi: 10.1016/j.critrevonc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Friedman C.F., Proverbs-Singh T.A., Postow M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncology. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 17.Naidoo J., Wang X., Woo K.M., Iyriboz T., Halpenny D., Cunningham J. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutros C., Tarhini A., Routier E., Lambotte O., Ladurie F.L., Carbonnel F. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 19.Koyama S., Akbay E.A., Li Y.Y., Herter-Sprie G.S., Buczkowski K.A., Richards W.G. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sade-Feldman M., Jiao Y.J., Chen J.H., Rooney M.S., Barzily-Rokni M., Eliane J.P. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8:1136. doi: 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newick K., Moon E., Albelda S.M. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 25.Peer D., Karp J.M., Hong S., FaroKhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 26.Chen G., Roy I., Yang C., Prasad P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 27.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 28.Shi J.J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W.J.M., Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Gradishar W.J. Albumin-bound paclitaxel: a next-generation taxane. Expet Opin Pharmacother. 2006;7:1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 32.Lancet J.E., Cortes J.E., Hogge D.E., Tallman M.S., Kovacsovics T.J., Damon L.E. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milling L., Zhang Y., Irvine D.J. Delivering safer immunotherapies for cancer. Adv Drug Deliv Rev. 2017;114:79–101. doi: 10.1016/j.addr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Mooney D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat Mater. 2018;17:761–772. doi: 10.1038/s41563-018-0147-9. [DOI] [PubMed] [Google Scholar]
- 36.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon J.J., Suh H., Bershteyn A., Stephan M.T., Liu H., Huang B. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynn G.M., Laga R., Darrah P.A., Ishizuka A.S., Balaci A.J., Dulcey A.E. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu S., Wang C., Yu J., Wang J., Lu Y., Zhang Y. Injectable bioresponsive gel depot for enhanced immune checkpoint blockade. Adv Mater. 2018;30 doi: 10.1002/adma.201801527. [DOI] [PubMed] [Google Scholar]
- 40.Galstyan A., Markman J.L., Shatalova E.S., Chiechi A., Korman A.J., Patil R. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10:3850. doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu T., Kishida T., Hasegawa U., Ueda Y., Imanishi J., Yamagishi H. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367:330–335. doi: 10.1016/j.bbrc.2007.12.112. [DOI] [PubMed] [Google Scholar]
- 42.Mishra P., Nayak B., Dey R.K. PEGylation in anti-cancer therapy: an overview. Asian J Pharm Sci. 2016;11:337–348. [Google Scholar]
- 43.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auría-Soro C., Nesma T., Juanes-Velasco P., Landeira-Viñuela A., Fidalgo-Gomez H., Acebes-Fernandez V. Interactions of nanoparticles and biosystems: microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials. 2019;9:1365. doi: 10.3390/nano9101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B., He X., Zhang Z., Zhao Y., Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res. 2013;46:761–769. doi: 10.1021/ar2003336. [DOI] [PubMed] [Google Scholar]
- 46.Huo D., Jiang X., Hu Y. Recent advances in nanostrategies capable of overcoming biological barriers for tumor management. Adv Mater. 2020;32 doi: 10.1002/adma.201904337. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Z.M., Ukidve A., Krishnan V., Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Deliv Rev. 2019;143:3–21. doi: 10.1016/j.addr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Ernsting M.J., Murakami M., Roy A., Li S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172:782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S., Anselmo A.C., Banerjee A., Zakrewsky M., Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220:141–148. doi: 10.1016/j.jconrel.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill N.A., Eppler H.B., Jewell C.M., Bromberg J.S. Harnessing the lymph node microenvironment. Curr Opin Organ Transplant. 2018;23:73–82. doi: 10.1097/MOT.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girard J.-P., Moussion C., Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 54.Andorko J.I., Hess K.L., Jewell C.M. Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. AAPS J. 2015;17:323–338. doi: 10.1208/s12248-014-9708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas S.N., Schudel A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr Opin Chem Eng. 2015;7:65–74. doi: 10.1016/j.coche.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bahmani B., Vohra I., Kamaly N., Abdi R. Active targeted delivery of immune therapeutics to lymph nodes. Curr Opin Organ Transplant. 2018;23:8. doi: 10.1097/MOT.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trevaskis N.L., Kaminskas L.M., Porter C.J.H. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 58.Miao L., Li L., Huang Y., Delcassian D., Chahal J., Han J. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 59.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H., Moynihan K.D., Zheng Y., Szeto G.L., Li A.V., Huang B. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson D.S., Hirosue S., Raczy M.M., Bonilla-Ramirez L., Jeanbart L., Wang R. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat Mater. 2019;18:175–185. doi: 10.1038/s41563-018-0256-5. [DOI] [PubMed] [Google Scholar]
- 62.Li A.W., Sobral M.C., Badrinath S., Choi Y., Graveline A., Stafford A.G. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17:528–534. doi: 10.1038/s41563-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Daniel S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Hong E., Dobrovolskaia M.A. Addressing barriers to effective cancer immunotherapy with nanotechnology: achievements, challenges, and roadmap to the next generation of nanoimmunotherapeutics. Adv Drug Deliv Rev. 2019;141:3–22. doi: 10.1016/j.addr.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Yu J.X., Hubbard-Lucey V.M., Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18:899–900. doi: 10.1038/d41573-019-00167-9. [DOI] [PubMed] [Google Scholar]
- 66.Li J., Burgess D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm Sin B. 2020;10:2110–2124. doi: 10.1016/j.apsb.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y., Yu X., Thamphiwatana S.D., Zheng Y., Pang Z. Nanomedicines modulating tumor immunosuppressive cells to enhance cancer immunotherapy. Acta Pharm Sin B. 2020;10:2054–2074. doi: 10.1016/j.apsb.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park J., Wrzesinski S.H., Stern E., Look M., Criscione J., Ragheb R. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid D., Park C.G., Hartl C.A., Subedi N., Cartwright A.N., Puerto R.B. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8:1747. doi: 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stephan M.T., Moon J.J., Um S.H., Bershteyn A., Irvine D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y., Stephan M.T., Gai S.A., Abraham W., Shearer A., Irvine D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J Control Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang L., Zheng Y., Melo M.B., Mabardi L., Castaño A.P., Xie Y.Q. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol. 2018;36:707–716. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C., Wang J., Zhang X., Yu S., Wen D., Hu Q. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan3682. [DOI] [PubMed] [Google Scholar]
- 74.Meir R., Shamalov K., Sadan T., Motiei M., Yaari G., Cohen C.J. Fast image-guided stratification using anti-programmed death ligand 1 gold nanoparticles for cancer Immunotherapy. ACS Nano. 2017;11:11127–11134. doi: 10.1021/acsnano.7b05299. [DOI] [PubMed] [Google Scholar]
- 75.Francis D.M., Thomas S.N. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv Drug Deliv Rev. 2017;114:33–42. doi: 10.1016/j.addr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W., Reiman D. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhan X., Jia L., Niu Y., Qi H., Chen X., Zhang Q. Targeted depletion of tumour-associated macrophages by an alendronate–glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35:10046–10057. doi: 10.1016/j.biomaterials.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Shen S., Li H.J., Chen K.G., Wang Y., Wang J. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemo-immunotherapy. Nano Lett. 2017;17:3822–3829. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 82.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Lin Y.X., Qiao S.L., An H.W., Ma Y., Qiao Z.Y. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–163. doi: 10.1016/j.biomaterials.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 84.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 85.Loftus C., Saeed M., Davis D.M., Dunlop I.E. Activation of human natural killer cells by graphene oxide-templated antibody nanoclusters. Nano Lett. 2018;18:3282–3289. doi: 10.1021/acs.nanolett.8b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Griffe L., Poupot M., Marchand P., Maraval A., Turrin C.O., Rolland O. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew Chem Int Ed. 2007;46:2523–2526. doi: 10.1002/anie.200604651. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki E., Kapoor V., Jassar A.S., Kaiser L.R., Albelda S.M. Gemcitabine selectively eliminates splenic GR-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 88.Long Y., Lu Z.Z., Xu S.S., Li M., Wang X.H., Zhang Z.R. Self-Delivery Micellar Nanoparticles prevent premetastatic niche formation by interfering with the early recruitment and vascular destruction of granulocytic myeloid-derived suppressor cells. Nano Lett. 2020;20:2219–2229. doi: 10.1021/acs.nanolett.9b03883. [DOI] [PubMed] [Google Scholar]
- 89.Sasso M.S., Lollo G., Pitorre M., Solito S., Pinton L., Valpione S. Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials. 2016;96:47–62. doi: 10.1016/j.biomaterials.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Swartz M.A. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 91.Schudel A., Francis D.M., Thomas S.N. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4:415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joshi V.B., Geary S.M., Salem A.K. Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013;15:85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reddy S.T., Rehor A., Schmoekel H.G., Hubbell J.A., Swartz M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Nishioka Y., Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47:55–64. doi: 10.1016/s0169-409x(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 95.Manolova V., Flace A., Bauer M., Schwarz K., Saudan P., Bachmann M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 96.Ryan G.M., Kaminskas L.M., Porter C.J.H. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J Control Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 97.Irvine D.J., Swartz M.A., Szeto G.L. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Irvine D.J., Hanson M.C., Rakhra K., Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaminskas L.M., Porter C.J.H. Targeting the lymphatics using dendritic polymers (dendrimers) Adv Drug Deliv Rev. 2011;63:890–900. doi: 10.1016/j.addr.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi H., Kawamoto S., Bernardo M., Brechbiel M.W., Knopp M.V., Choyke P.L. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Control Release. 2006;111:343–351. doi: 10.1016/j.jconrel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 101.Wiig H., Swartz M.A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 102.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J Control Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Doddapaneni B.S., Kyryachenko S., Chagani S.E., Alany R.G., Rao D.A., Indra A.K. A three-drug nanoscale drug delivery system designed for preferential lymphatic uptake for the treatment of metastatic melanoma. J Control Release. 2015;220:503–514. doi: 10.1016/j.jconrel.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Tseng Y.-C., Xu Z., Guley K., Yuan H., Huang L. Lipid–calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials. 2014;35:4688–4698. doi: 10.1016/j.biomaterials.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Min Y., Roche K.C., Tian S., Eblan M.J., McKinnon K.P., Caster J.M. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stylianopoulos T., Poh M.Z., Insin N., Bawendi M.G., Fukumura D., Munn Lance L. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic Interactions. Biophys J. 2010;99:1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao D.A., Forrest M.L., Alani A.W.G., Kwon G.S., Robinson J.R. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharmacol Sci. 2010;99:2018–2031. doi: 10.1002/jps.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo P., Liu D.X., Subramanyam K., Wang B.R., Yang J., Huang J. Nanoparticle elasticity directs tumor uptake. Nat Commun. 2018;9 doi: 10.1038/s41467-017-02588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anselmo A.C., Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv Drug Deliv Rev. 2017;108:51–67. doi: 10.1016/j.addr.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Donahue N.D., Acar H., Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi: 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Xia Y.F., Wu J., Wei W., Du Y.Q., Wan T., Ma X.W. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat Mater. 2018;17:187–194. doi: 10.1038/nmat5057. [DOI] [PubMed] [Google Scholar]
- 112.Christensen D., Henriksen-Lacey M., Kamath A.T., Lindenstrøm T., Korsholm K.S., Christensen J.P. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release. 2012;160:468–476. doi: 10.1016/j.jconrel.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 113.Kumari S., Mg S., Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20:256–275. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao F., Zhao Y., Liu Y., Chang X., Chen C., Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 115.Conner S.D., Schmid S.L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 116.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46:4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu M., Nie G., Meng H., Xia T., Nel A., Zhao Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc Chem Res. 2013;46:622–631. doi: 10.1021/ar300031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Foged C., Brodin B., Frokjaer S., Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y.C., Hardie J., Zhang X.Z., Rotello V.M. Effects of engineered nanoparticles on the innate immune system. Semin Immunol. 2017;34:25–32. doi: 10.1016/j.smim.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tomić S., Ðokić J., Vasilijić S., Ogrinc N., Rudolf R., Pelicon P. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096584. e96584-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuhn D., Vanhecke D., Michen B., Blank F., Gehr P., Fink A. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol. 2014;5:1625–1636. doi: 10.3762/bjnano.5.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu J., Xu H., Han Y., Dai W., Hao W., Wang C. The internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. Sci China Life Sci. 2011;54:793–805. doi: 10.1007/s11427-011-4215-5. [DOI] [PubMed] [Google Scholar]
- 123.Dasgupta S., Auth T., Gompper G. Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Lett. 2014;14:687–693. doi: 10.1021/nl403949h. [DOI] [PubMed] [Google Scholar]
- 124.Toy R., Peiris P.M., Ghaghada K.B., Karathanasis E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine. 2014;9:121–134. doi: 10.2217/nnm.13.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arnida, Malugin A., Ghandehari H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres. J Appl Toxicol. 2010;30:212–217. doi: 10.1002/jat.1486. [DOI] [PubMed] [Google Scholar]
- 126.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 127.Agarwal R., Singh V., Jurney P., Shi L., Sreenivasan S.V., Roy K. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc Natl Acad Sci U S A. 2013;110:17247–17252. doi: 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 129.Yi X., Gao H. Phase diagrams and morphological evolution in wrapping of rod-shaped elastic nanoparticles by cell membrane: a two-dimensional study. Phys Rev. 2014;89 doi: 10.1103/PhysRevE.89.062712. [DOI] [PubMed] [Google Scholar]
- 130.Li Z., Sun L., Zhang Y., Dove A.P., O'Reilly R.K., Chen G. Shape effect of glyco-nanoparticles on macrophage cellular uptake and immune response. ACS Macro Lett. 2016;5:1059–1064. doi: 10.1021/acsmacrolett.6b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang J., Chen H.-J., Hang T., Yu Y., Liu G., He G. Physical activation of innate immunity by spiky particles. Nat Nanotechnol. 2018;13:1078–1086. doi: 10.1038/s41565-018-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vold M.J. Zeta potential in colloid science. Principles and applications. J Colloid Interface Sci. 1982;88 608-08. [Google Scholar]
- 133.Vigderman L., Manna P., Zubarev E.R. Quantitative replacement of cetyl trimethylammonium bromide by cationic thiol ligands on the surface of gold nanorods and their extremely large uptake by cancer cells. Angew Chem Int Ed. 2012;51:636–641. doi: 10.1002/anie.201107304. [DOI] [PubMed] [Google Scholar]
- 134.Ngamcherdtrakul W., Morry J., Gu S., Castro D.J., Goodyear S.M., Sangvanich T. Cationic polymer modified mesoporous silica nanoparticles for targeted sirna delivery to HER2+ breast cancer. Adv Funct Mater. 2015;25:2646–2659. doi: 10.1002/adfm.201404629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang Y., Huo S., Mizuhara T., Das R., Lee Y.W., Hou S. The interplay of size and surface functionality on the cellular uptake of sub-10 nm gold nanoparticles. ACS Nano. 2015;9:9986–9993. doi: 10.1021/acsnano.5b03521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yue Z.G., Wei W., Lv P.P., Yue H., Wang L.Y., Su Z.G. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules. 2011;12:2440–2446. doi: 10.1021/bm101482r. [DOI] [PubMed] [Google Scholar]
- 137.Fytianos K., Chortarea S., Rodriguez-Lorenzo L., Blank F., von Garnier C., Petri-Fink A. Aerosol delivery of functionalized gold nanoparticles target and activate dendritic cells in a 3D lung cellular model. ACS Nano. 2017;11:375–383. doi: 10.1021/acsnano.6b06061. [DOI] [PubMed] [Google Scholar]
- 138.Mou Y., Xing Y., Ren H., Cui Z., Zhang Y., Yu G. The effect of superparamagnetic iron oxide nanoparticle surface charge on antigen cross-presentation. Nanoscale Res Lett. 2017;12:52. doi: 10.1186/s11671-017-1828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Palomba R., Palange A.L., Rizzuti I.F., Ferreira M., Cervadoro A., Barbato M.G. Modulating phagocytic cell sequestration by tailoring nanoconstruct softness. ACS Nano. 2018;12:1433–1444. doi: 10.1021/acsnano.7b07797. [DOI] [PubMed] [Google Scholar]
- 140.Yang R., Xu J., Xu L., Sun X., Chen Q., Zhao Y. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 141.Cruz L.J., Rosalia R.A., Kleinovink J.W., Rueda F., Löwik C.W.G.M., Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: a comparative study. J Control Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 142.Chou L.Y.T., Ming K., Chan W.C.W. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 143.Stewart M.P., Sharei A., Ding X., Sahay G., Langer R., Jensen K.F. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 144.Stewart M.P., Lorenz A., Dahlman J., Sahay G. Challenges in carrier-mediated intracellular delivery: moving beyond endosomal barriers. WIREs Nanomed Nanobiotechnol. 2016;8:465–478. doi: 10.1002/wnan.1377. [DOI] [PubMed] [Google Scholar]
- 145.Huang L.Y., Germain R.N. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992;356:796–798. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 146.Neefjes J., Jongsma M.L.M., Paul P., Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 147.Ackerman A.L., Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–684. doi: 10.1038/ni1082. [DOI] [PubMed] [Google Scholar]
- 148.Joffre O.P., Segura E., Savina A., Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 149.Gong N., Zhang Y., Teng X., Wang Y., Huo S., Qing G. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2020;15:1053–1064. doi: 10.1038/s41565-020-00782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu J., Lv J., Zhuang Q., Yang Z., Cao Z., Xu L. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat Nanotechnol. 2020;15:1043–1052. doi: 10.1038/s41565-020-00781-4. [DOI] [PubMed] [Google Scholar]
- 151.Liu S., Jiang Q., Zhao X., Zhao R., Wang Y., Wang Y. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater. 2021;20:421–430. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 152.Keller S., Wilson J.T., Patilea G.I., Kern H.B., Convertine A.J., Stayton P.S. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8+ T cell responses. J Control Release. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu Y., Litwin T., Nagaraja A.R., Kwong B., Katz J., Watson N. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core−shell nanoparticles. Nano Lett. 2007;7:3056–3064. doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- 154.Waeckerle-Men Y., Mauracher A., Håkerud M., Mohanan D., Kündig T.M., Høgset A. Photochemical targeting of antigens to the cytosol for stimulation of MHC class-I-restricted T-cell responses. Eur J Pharm Biopharm. 2013;85:34–41. doi: 10.1016/j.ejpb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 155.Vasdekis A.E., Scott E.A., O'Neil C.P., Psaltis D., Hubbell J.A. Precision intracellular delivery based on optofluidic polymersome rupture. ACS Nano. 2012;6:7850–7857. doi: 10.1021/nn302122h. [DOI] [PubMed] [Google Scholar]
- 156.Huo M., Yuan J., Tao L., Wei Y. Redox-responsive polymers for drug delivery: from molecular design to applications. Polym Chem. 2014;5:1519–1528. [Google Scholar]
- 157.Liu L., Rui L., Gao Y., Zhang W. Self-assembly and disassembly of a redox-responsive ferrocene-containing amphiphilic block copolymer for controlled release. Polym Chem. 2015;6:1817–1829. [Google Scholar]
- 158.Pillay C.S., Elliott E., Dennison C. Endolysosomal proteolysis and its regulation. Biochem J. 2002;363:417–429. doi: 10.1042/0264-6021:3630417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sauer A.M., Schlossbauer A., Ruthardt N., Cauda V., Bein T., Bräuchle C. Role of endosomal escape for disulfide-based drug delivery from colloidal mesoporous silica evaluated by live-cell imaging. Nano Lett. 2010;10:3684–3691. doi: 10.1021/nl102180s. [DOI] [PubMed] [Google Scholar]
- 160.Wu M., Meng Q., Chen Y., Zhang L., Li M., Cai X. Large pore-sized hollow mesoporous organosilica for redox-responsive gene delivery and synergistic cancer chemotherapy. Adv Mater. 2016;28:1963–1969. doi: 10.1002/adma.201505524. [DOI] [PubMed] [Google Scholar]
- 161.Li P., Luo Z., Liu P., Gao N., Zhang Y., Pan H. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Control Release. 2013;168:271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]