Abstract
As one of the most important components of caveolae, caveolin-1 is involved in caveolae-mediated endocytosis and transcytosis pathways, and also plays a role in regulating the cell membrane cholesterol homeostasis and mediating signal transduction. In recent years, the relationship between the expression level of caveolin-1 in the tumor microenvironment and the prognostic effect of tumor treatment and drug treatment resistance has also been widely explored. In addition, the interplay between caveolin-1 and nano-drugs is bidirectional. Caveolin-1 could determine the intracellular biofate of specific nano-drugs, preventing from lysosomal degradation, and facilitate them penetrate into deeper site of tumors by transcytosis; while some nanocarriers could also affect caveolin-1 levels in tumor cells, thereby changing certain biophysical function of cells. This article reviews the role of caveolin-1 in tumor prognosis, chemotherapeutic drug resistance, antibody drug sensitivity, and nano-drug delivery, providing a reference for the further application of caveolin-1 in nano-drug delivery systems.
KEY WORDS: Caveolin-1, Cancer, Drug resistance, Transcytosis, Nano-drug delivery systems, Biofate
Abbreviations: 5-FU, 5-fluorouracil; ADC, antibody drug conjugates; BBB, blood–brain barrier; CAFs, cancer-associated fibroblasts; CPT, camptothecin; CSD, caveolin scaffolding domain; CTB, cholera toxins B; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; ERK, extracellular regulated protein kinases; FGF2, fibroblast growth factor 2; GGT, γ-glutamyl transpeptidase; GPI, glycosylphosphatidylinositol; HER2, human epidermal growth factor receptor 2; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; HSA, human serum albumin; IBC, infiltrating breast cancer; IR, insulin receptor; MAPK, mitogen-activated protein kinase; MDR, multidrug resistance; MSV, multistage nanovectors; NPs, nanoparticles; PC, prostate cancer; PDGF, platelet-derived growth factor; PFS, progression free survival; ROS, reactive oxygen species; SCLC, small cell lung cancer; cell SMA, styrene maleic acid; SV40, simian virus 40
Graphical abstract
Caveolin-1 is involved in caveolae-mediated internalization pathways, and could be used to predict the prognostic effect of tumor treatment. Caveolin-1 in tumor prognosis, chemotherapeutic drug resistance, antibody drug sensitivity and nano-drug delivery were reviewed.
1. Introduction
Caveolin-1 is a biomarker of lipid microdomains (including lipid rafts and caveolae) on the cell membrane, and is involved in the generation and function of caveolae1, such as caveolae-mediated endocytosis and transcytosis2, cholesterol transport between cytoplasm and membrane to maintain lipid homeostasis3. Moreover, caveolin-1 mediated signal transduction is also reported to affect many bio-effects, such as cell cycle regulation4, proliferation, invasion and cell death5, 6, 7, 8. Here, in this review, we describe some findings in clinical and basic researches, including the expression level of caveolin-1 in tumor tissues, as well as the role of caveolin-1 in the prognosis of tumor treatment, chemo-resistance and antibody drugs resistance, and nano-based drug delivery. Moreover, as an important entry route of biological macromolecule and nano-drugs, we will focus on the role of caveolin-1 in the nano-drugs entry pathway and intracellular biofate and their subsequent anti-tumor efficacy. Finally, the effect of nanomaterials on caveolin-1 expression and distribution, and subsequent biological effects will also be discussed.
2. Caveolin-1
2.1. Caveolae
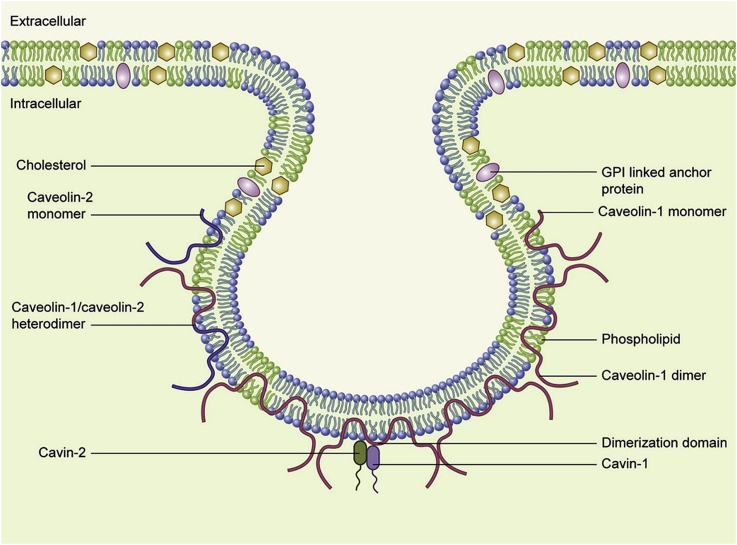
Caveolae is a highly hydrophobic membrane structure with a vesicular hollow on the cell membrane of about 50–100 nm in diameter, which occupies 20% of the area on the cell membrane9. Caveolae exists in the most cell types of vertebrates, and its density is depended on cell types, external factors, and subcellular organelles in the cells2. The components include cholesterol, sphingolipids and other lipid components. Because it is consistent with the lipid composition of lipid rafts, it is also considered to be a special type of lipid raft, excepting caveolae encapsulated by caveolin to form a morphologically discontinuous structure1. Caveolin is a unique protein family. Among them, caveolin-1 is a key protein molecule in caveolae biogenesis and functions. In addition, cavin supports the formation of complete caveolae by regulating the composition and function of caveolin, which acts as a scaffold in caveolae (Fig. 1)10,11. Caveolae involves in a classic process of mediating endocytosis. At the same time, caveolae has also revealed its functions in cholesterol transport and homeostasis, as well as signal transduction1.
Figure 1.
Schematic illustration of caveolae. Caveolin-1 and caveolin-2 could exist as monomers, homodimers or heterodimers. Cavins facilitate caveolins stabilization and caveolae formation. Reprinted with the permission from Ref. 11. Copyright © 2014 Elsevier B.V. and Société française de biochimie et biologie Moléculaire (SFBBM).
2.2. Functions of caveolin-1
There are three isomers in the caveolin family, caveolin-1, caveolin-2 and caveolin-3. Among them, caveolin-1 is widely distributed in various cell types except skeletal muscle, and is widely expressed in endothelial cells, epithelial cells, adipocytes, lung type I cells. It plays a vital role in the biological occurrence of caveolae9.
The molecular weight of caveolin-1 is about 21–24 kDa, which is the most studied member of the caveolin family and the most commonly needed part of caveolae assembly. It is enriched in caveolae on the cell membrane, intracellular endoplasmic reticulum (ER) and Golgi apparatus, and can shuttle back and forth between the cytoplasm and the cell membrane as needed12. Once caveolae is established, it will maintain stable structure for a long time, and caveolin-1 and cavins will not frequently undergo rapid turnover. But when caveolae mediates endocytosis, caveolin-1 will participate in the fusion and division process on the plasma membrane13.
2.2.1. Caveolin-1 in caveolae-mediated endocytosis
Caveolae-mediated endocytosis is a clathrin-independent endocytosis pathway. It is widely accepted that cargoes including glycosylphosphatidylinositol (GPI) anchor proteins, insulin receptors, Shigella and Cholera toxins B (CTB), membrane-bound simian virus 40 (SV40), and cholesterol and albumin are endocytosed through the caveolae-mediated pathway14.
Caveolin-1 is involved in caveolae-mediated endocytosis. The steps of caveolae-mediated endocytosis are not fully understood. It is generally believed that the interaction between caveolae and some specific cargoes (such as SV40 and CTB) would induce a rapid caveolae-mediated endocytosis. Actin filaments (F-actin) and microtubules are also involved in this process. At the beginning, actin cytoskeleton appears to confine caveolae to the cell surface, preventing caveolae from detachment as a simple physical barrier, and microtubules serve as tracks for the transport of caveolae to the cytoplasm. While when the local de-polymerization or disassembly of F-actin occurs, the endocytosis by caveolae happens15. With the help of dynamin, the concave vesicles containing caveolin-1 are formed and pinched off from the plasma membrane. In addition, F-actin is also involved in the intracellular transport of caveolin-1 containing vehicles13.
Different cargoes experience different intracellular biofate after being internalized by caveolin-1. After endocytosis of iron and 5-methyltetrahydrofolate through this route, they are delivered to the cytoplasm, and caveolin-1 will be recycled to the surface; However, after endocytosis of SV40, CTB, calcium, cholesterol, fatty acids, these cargoes will reach the ER and caveolin-1 will be circulated to the cell membrane; moreover, after endocytosis of albumin, transthyretin and various membrane proteins, these cargoes will bypass the lysosome degradation and release the cargo to the other side of the cell membranes by transcytosis, and caveolin-1 is fused with the other side membrane; in addition, after endocytosis, such as alkaline phosphatase, bradykinin receptor, endothelin, ligands and receptors are all sequestered in the caveolin-1 containing vesicles16.
2.2.2. Caveolin-1 in caveolae-mediated transcytosis
Transcytosis is one of the endocytosis pathways, which occurs widely in different cells of the body and is used to exchange substances between two different environments. It refers to the direct transport of cargo from one side of the cell to the other through the caveolae-mediated endocytosis pathway, which can be divided into receptor-mediated transcytosis and adsorption-mediated transcytosis17. For example, in the blood–brain barrier (BBB), vascular endothelium, and gastrointestinal tract, materials such as albumin, lipids, antibodies could be endocytosed from the apical side to the basal side of endothelial cells2. Many studies have shown that caveolin-1 is involved in the caveolae-mediated transcytosis pathway, which is also currently exploited to deliver biological macromolecules and functionalized nanoparticles18.
2.2.3. Cholesterol homeostasis maintenance
In vitro, caveolin-1 prefers to bind to cholesterol-containing membranes, and then moves in caveolae and inner membranes3. The high cholesterol level in the cells leads to increased levels of caveolin-1 mRNA. Therefore, caveolin-1 appears to be part of the intracellular lipid transport system, and they are involved in transporting sterols between ER and caveolae16.
2.2.4. Signal transduction
There are many membrane receptors in caveolae and many signaling pathways happen there. It has been reported that caveolin-1 is directly related to epidermal growth factor receptor (EGFR) through its caveolin scaffolding domain (CSD), inhibiting its tyrosine kinase activity, and that EGFR phosphorylation and activity are related to leaving from caveolae. Similar to EGFR, caveolin-1 directly interacts with platelet-derived growth factor (PDGF) through interaction with CSD in vesicles, thereby inhibiting the activity of PDGF10. Unlike other receptors, the beta subunit of insulin receptor (IR) directly binding to caveolin-1 has been shown to enhance insulin signaling. The role of caveolin-1 in transforming growth factor-β (TGF-β) receptor internalization may explain the anti-fibrotic function of caveolin-1 in different organs2.
3. The role of caveolin-1 in clinical anticancer therapies
3.1. The relationship between caveolin-1 expression and tumor prognosis
3.1.1. Caveolin-1 expression in cancer-associated fibroblasts and tumor prognosis
Extracellular matrix (ECM) is one of the important components of tumor microenvironment. In there, tumor cells metabolically crosstalk with fibroblasts in the surrounding extracellular matrix. Fibroblasts in the matrix transform into cancer-associated fibroblasts (CAF), which in turn promote the growth, invasion and metastasis of tumor cells19. This “metabolic coupling” phenomenon occurs during the growth and proliferation of tumor cells. Tumor cells secrete a large amount of reactive oxygen species (ROS) to the outside and affected peripheral fibroblasts to undergo oxidative stress, and then, the down-regulation of caveolin-1 expression is induced on these fibroblasts20,21. The phenotype of fibroblasts under oxidative stress is gradually transformed into CAFs, and the metabolic mode is transformed into aerobic glycolysis, and produced high-energy metabolites such as pyruvate and lactic acid to the tumor cells through the “lactic acid shuttle” pathway between their cell membranes22,23. Therefore, CAFs provide energy for the mitochondrial respiratory metabolism in tumor cells and promote the growth of tumor tissue. This effect is called the “reverse Warburg effect”24,25. Therefore, from the perspective of metabolic coupling, the decrease of caveolin-1 expression in CAFs is a sign of oxidative stress and beneficial to tumor growth.
Clinically, it is also observed that the downregulation or deletion of caveolin-1 on stromal CAFs leads to poor tumor prognosis. Taking breast cancer as an example, it is reported that the absence of matrix caveolin-1 is a new biomarker that can predict all the most common subtypes of human breast cancer26, and for the first time, they found that compared with normal fibroblasts, CAFs are more numerous in human breast cancer, more narrow and elongated, and accompanied by hyperplasia. Western blot analysis confirmed that in 11 human breast cancer patients, 8 of them showed significant decreasing level of caveolin-1 in CAFs. Treatment of caveolin-1 deficient CAFs with caveolin-1 mimetic peptides could reverse the phenotype of CAF hyper-proliferation27. Immunostaining of 429 human breast cancer samples also found that matrix caveolin-1 expression is a powerful prognostic marker for clinical outcome. The existence of caveolin-1 positive matrix components in the primary tumor microenvironment is related to the improvement of breast cancer prognosis, and the expression of caveolin-1 in the tumor-related matrix is related to the lower tumor grade and improved diagnosis stage28. In another study, it is showed that breast cancer patients who lost the stromal caveolin-1 showed a 5-year survival rate of 20%, while patients with high expression of stromal caveolin-1 showed an 80% 5-year survival rate. In triple-negative breast cancer patients, the 5-year survival rate of high stroma caveolin-1 is 75.5%, and the 5-year survival rate of lacking stroma caveolin-1 is 9.4%29. Moreover, the loss of stromal caveolin-1 also predicts the progression of ductal carcinoma in situ to invasive tumors, indicating that the loss of caveolin-1 also regulates tumor progression.
Similarly, the results of clinical studies on the expression level of caveolin-1 in the matrix have also been found in gastric cancer30, malignant melanoma31, and prostate cancer32,33. In patients with gastric cancer, the decrease in matrix caveolin-1 is significantly associated with poor prognosis, early relapse, and lower five-year survival rates30. Among patients with malignant melanoma, compared with the group with high matrix caveolin-1 expression, the group with low matrix caveolin-1 expression showed shorter survival time. Caveolin-1 expression in CAFs is also closely related to disease-free survival (P = 0.029) and overall survival (P = 0.013)30. Among prostate cancer (PC) patients, a large sample of 724 PC patients showed that there was a significant correlation in a decrease in stromal cell caveolin-1 levels and an increase in the gleason score (P = 0.012) and a reduction in relapse-free survival (P = 0.009)32. Therefore, these researches indicate that high matrix caveolin-1 expression is beneficial for inhibiting cancer progression and metastasis, and loss of stromal caveolin-1 predicts bad clinical outcomes.
3.1.2. Caveolin-1 expression in tumor cells and tumor prognosis
The low-level expression of matrix caveolin-1 in the tumor microenvironment has a relatively clear correlation with the poor prognosis of cancer. However, in tumor cells, the role of caveolin-1 expression in tumor development is currently controversial. The expression level of caveolin-1 depends on tumor types, tumor stages and grades, and other situations34. For example, in sarcoma and malignant adenoma, caveolin-1 in tumor cells is downregulated; in other cases, such as prostate cancer, advanced squamous cell carcinoma or metastatic or multidrug resistance (MDR) tumor cell lines, caveolin-1 is upregulated9, and the caveolin-1 expression level is positively correlated with tumor invasiveness.
CAV-1 gene could perform a tumor suppressor gene. CAV-1 is reported to locate on human chromosome 7q31.1, where mutations and deletions often occurs in tumor patients, indicating their suppressor role35. The mechanisms of caveolin-1 inhibiting tumorigenesis mainly include these below: caveolin-1 could inhibit the activity of cyclinD1 gene promoter to block cell malignantly proliferation36; in addition, caveolin-1 could suppress the activation of ERK which is the crucial member of MAPK family, and inhibit MAPK pathway37; moreover, the phosphorylation of SRC-protein tyrosine kinase could be inhibited by caveolin-1 and block the downstream signal transduction. The loss of caveolin-1 expression is closely associated with the methylation of caveolin-1 promoter. Taking SCLC tumor patients as an example, it is reported that 93% of caveolin-1 promoter are methylated, which contribute to the loss of caveolin-1 expression, further affecting corresponding signal pathways related to caveolin-138.
On the other hand, in prostate cancer and in the late and advanced stages of most tumors, the caveolin-1 level of tumor cells is increased compared with that in normal cells, which promotes tumor invasion and metastasis. The increase in caveolin-1 level is thought to be related to the down-regulation of the methylation level of the caveolin-1 promoter39. Taking prostate cancer cells as an example, up-regulation of caveolin-1 could activate the PI3K-AKT signaling pathway and interact with metastasis-promoting molecules such as VEGF, TGF-β1 and FGF2 to promote cancer cell metastasis40. The clinical data also shows that overexpression of caveolin-1 is associated with adverse pathological features in prostate cancer41. The conversion of caveolin-1 from a tumor suppressor gene to a cancer-promoting gene is mainly related to its mutation and the phosphorylation of tyrosine-14 and serine-80 in caveolin-142,43. These changes could induce cell transformation and activate MAPK signaling pathways, thereby promoting tumorigenesis and development44. These not only happen in prostate cancers, but also in the late and advanced stages of most tumors. Although the clinical significance of caveolin-1 expression in different types of tumors is still controversial in most cancers, the up-regulation of caveolin-1 in tumor cells serves as a tumor promoter21, and the level of stromal caveolin-1 has no obvious correlation with the level of tumor cell caveolin-1 (P = 0.751)30.
3.2. The relationship between caveolin-1 expression and drug treatment response
3.2.1. Caveolin-1 and small molecular chemotherapy drugs resistance
In various cancer types, chemo-drug resistance is related to the overexpression of caveolin-1. It is showed that caveolin-1 is highly expressed in 5-fluorouracil (5-FU)-resistant colon cancer cells45, cisplatin-resistant ovarian cancer cells46, paclitaxel-resistant breast cancer cells and lung cancer cells47, T-DM1-resistant gastric cancer cells and human epidermal growth factor receptor 2 (HER2)-positive breast cancer cells48. Therefore, by reducing the expression level of caveolin-1, the therapeutic effect of small molecule chemotherapy drugs may be improved. For example, caveolin-1 could be inhibited by β-cyclodextrin on 5-FU-resistant colon cancer cells, thereby drug-resistant cells are re-sensitized to 5-FU45; in addition, caveolin-1 could be silenced through gene transfection on cisplatin-resistant ovarian cancer cells46 and paclitaxel-resistant lung cancer cells47, enhancing the toxicity and effectiveness of cisplatin and paclitaxel, respectively.
Caveolin-1 is associated with drug resistance to gastric cancer49. Researchers founded that in the presence of caveolin-1, when treating with cisplatin, the cell viability of gastric cancer cells increased and showed drug resistance, and overexpression of caveolin-1 even could inhibit cisplatin-induced apoptosis. The related mechanism may be that caveolin-1 enhanced the cisplatin resistance of gastric cancer cells by activating the WNT/β-CATENIN signaling pathway and MET-HER2 crosstalk.
Caveolin-1 is associated with drug resistance to lung cancer50. Results showed that after exposure to cisplatin, caveolin-1 levels in A549 lung cancer cells were significantly inhibited. Knockout of caveolin-1 further promoted the death of A549 cells triggered by cisplatin. Studies have shown that inhibiting caveolin-1 caused mitochondrial reactive oxygen burst, cell metabolism destruction, and mitochondrial membrane potential reduction, which amplified cisplatin-induced mitochondrial stress signaling. At the molecular level, caveolin-1 increased cisplatin-mediated mitochondrial damage by inhibiting the mitochondrial autophagy associated with PARKIN. These results indicated that by down-regulating or silencing caveolin-1 on A549 cells, mitochondrial autophagy and the activity of the ROCK1 pathway could be inhibited, thereby improving the resistance of A549 cells to cisplatin therapy.
3.2.2. Caveolin-1 and antibody drugs resistance
It is reported that HER2 is located in caveolae in cell membranes51. In breast cancer cells, resistance to anti-HER2 antibodies is related to the expression level of caveolin-1 on the cell membrane. Herceptin® (Trastuzumab, Roche) is a monoclonal antibody used to treat HER2-positive breast cancer52. It specifically binds to HER2 overexpressed on breast cancer cells with high affinity, thereby preventing epidermal growth factor (EGF) from binding to HER2 and slowing tumor division and proliferation53. Therefore, gene amplification or protein overexpression of HER2 in tumors was a prerequisite for trastuzumab to initiate treatment and trastuzumab needed to effectively bind to HER2 on the surface of the cell membrane to function.
It was reported that caveolin-1 expression was negatively correlated with HER2 expression on the breast cancer cell surface. If caveolin-1 was depleted, the internalization of HER2 was inhibited and more HER2 were maintaining on the membrane, which showed that caveolin-1 was involved in the endocytosis of HER254. When trastuzumab were treated to HER2-positive breast cancer patients, caveolin-1 expression level may be a predictive marker to distinguish whether tumor cells could respond to trastuzumab treatment55. Therefore, in the clinical application of trastuzumab, low expression or defect of caveolin-1 on tumor cells may be a better choice for effective therapy.
Lovastatin is a kind of cholesterol-lowering drug, and it competitively inhibits the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) in the synthesis of cholesterol in the body and reduce cholesterol synthesis. Further research founded that the short-term consumption of caveolin-1 by lovastatin would increase the half-life and the availability of HER2 on the tumor cell membranes, and also reduced the shedding rate of HER2 without affecting the activity of HER2. Consequently, trastuzumab binding on the membrane and therapy efficacy for HER2-positive tumors was increased54. These all indicated that caveolin-1 played an important role in the efficacy of trastuzumab in targeting HER2-positive tumors by regulating the distribution of HER2 on the cell membrane. Therefore, caveolin-1 depletion by lovastatin may have direct therapeutic significance for trastuzumab-mediated HER2 targeted therapy.
In addition to monoclonal antibodies, the anti-tumor effect of antibody drug conjugates (ADC) was also related to caveolin-1 expression level. Kadcyla® (Trastuzumab-emtansine, T-DM1, Genetech) was a compound covalently linking cytotoxic agent DM1 and HER2 monoclonal antibody trastuzumab, and was the only ADC approved by FDA for the treatment of solid tumor. T-DM1 is approved for the treatment of HER2-positive breast cancer previously treated with trastuzumab and taxanes, and was also considered to be effective in patients with incomplete response to neoadjuvant therapy. However, the problems of internalization and acquired drug resistance of T-DM1 are still challenging.
Firstly, T-DM1 needed to bind to HER2 and be internalized into the cell through receptor-mediated endocytosis, and then transported to lysosomes through the endosomal maturation pathway, and catabolized in lysosomes. Then catabolism products containing DM1 were released to bind to tubulin, resulting in mitotic arrest and cell death. Therefore, different from trastuzumab, T-DM1 internalization via receptor binding was the core mechanism to function56,57. However, in breast cancers, the relationship between the level of caveolin-1 and the sensitivity of ADC is still controversial.
On the one hand, researchers discussed the relationship between the expression of caveolin-1 and the chemo-sensitivity of HER2-positive breast cancer cells to T-DM1. In breast cancer patients, it was showed in the analysis of immunohistochemistry that the caveolin-1 could mediate endocytosis and promote the internalization of T-DM1 into HER2-positive cells. Therefore, caveolin-1 expression was considered to be an effective predictor of response to T-DM1 treatment in breast cancer58. Researchers further founded that increased expression of caveolin-1 could promote the internalization of T-DM1 and enhance the sensitivity of T-DM1. In this study, the diabetes drug metformin was exploited to induce the expression of caveolin-1 in breast cancer cells, and the results showed enhanced T-DM1 efficacy. Caveolin-1 was overexpressed in BT474 cells previously treated with metformin, which further promoted the drug efficacy by enhancing the internalization of T-DM1. Therefore, the increase of caveolin-1 on the cell membrane improved the internalization of T-DM1 and promoted the effectiveness of T-DM1 in breast cancer59.
But at the same time, some researchers believed that even if lovastatin depleted caveolin-1 to prevent HER2 internalization, it may not immediately reduce the therapeutic effect of T-DM154. They thought that the temporary depletion of caveolin-1 may prolong the half-life of HER2 on tumor cell membrane and then enhanced the affinity of T-DM1 for these HER2-positive tumors, and may improve the pharmacokinetics of T-DM1 in vivo. These may in turn facilitated targeted-mediated drug treatment, resulting in a significant increase in T-DM1 in the early stages of tumors. However, due to the temporary depletion of caveolin-1, once caveolin-1 returned to normal, HER2 would also return to normal distribution on the cell membrane, and receptor-mediated antibody internalization will return to normal.
However, on the other hand, by establishing a cell model of acquired T-DM1 resistance in vitro, researchers believed that caveolin-1-mediated endocytosis of T-DM1 was a new mechanism of T-DM1 resistance48. Researchers hold the idea that although caveolin-mediated endocytosis could help T-DM1 internalization, the intracellular pathway may reduce the opportunity of T-DM1 into lysosomes, which was thought to be a necessary step for DM1 to action. In this in vitro study, caveolin-1 expression was elevated in T-DM1-resistant NCI-N87 cells and there was also increasing co-localization of T-DM1 and caveolin-1 intracellularly. While compared with parental cells, the lysosomal retaining of T-DM1 was decreased. However, when caveolin-1 was knock out by shRNA, the sensitivity to T-DM1 in resistant NCI-N87 cells was still not recovered. These results showed the controversies in the relationship between caveolin-1 expression and T-DM1 resistance, and further researches need to be done.
4. The effect of caveolin-mediated endocytosis in nanomedicine
Nano-drugs include nano-scale drugs such as nanocrystals, as well as various nano-materials based drug delivery systems. They all have a nano-scale particle size, and through their special physical and chemical properties and targeting capabilities, they enter the cell through a biological barrier to perform their functions. At present, they are widely used in tumor-related biomedical fields, including anti-tumor drug delivery, gene therapy and imaging diagnosis.
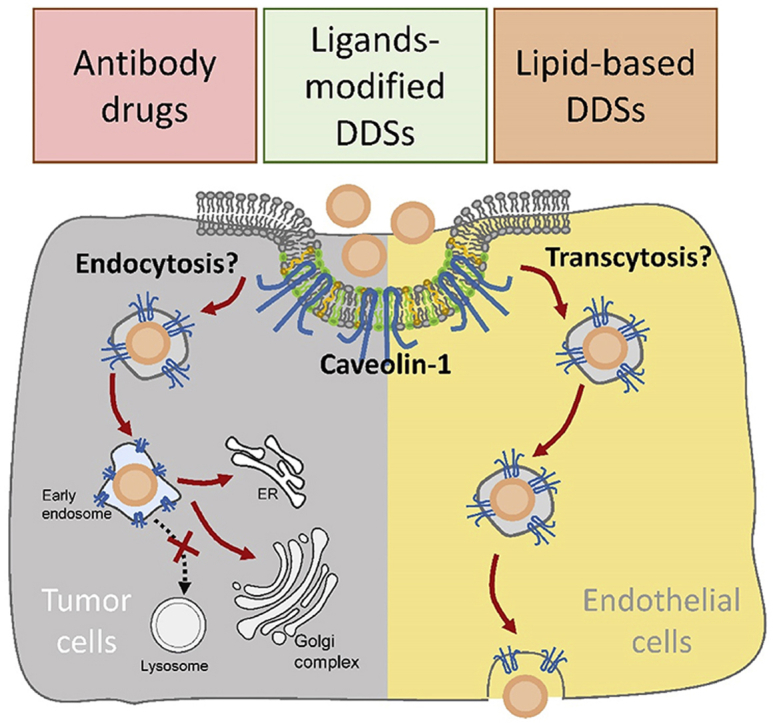
Nano-drugs entered cells through various endocytosis pathways, such as clathrin-dependent endocytosis and caveolae-dependent endocytosis, clathrin/caveolae-independent endocytosis and micropinocytosis60. The size, shapes, charge and other biophysical properties of nano-drugs affected and determined their internalization routes and the trafficking routes within cells, which were key factors in determining their biological distributions, biomedical functions, intracellular biological effects and toxicity, ultimately affecting the treatment efficacy. Clathrin-dependent endocytosis was the classic routes for uptake of nano-drugs61, but the final intracellular destination of this route were lysosomes with high acidity and various enzymes, which contributed the degradation of nano-drugs62 and limited the efficacy.
Caveolin-mediated endocytosis could provide alternative intracellular trafficking routes for nano-drugs, depending on cell types. It was easy to understand that this endocytosis pathway could not be adopted by the cells without caveolin-1 expression, such as HepG263. In addition, different caveolae density and distribution in different cell types also had an effect on the caveolar endocytosis of nanoparticles64. Compared with epithelial cells, endothelial cells have rather higher caveolin-1 expression levels; compared with normal tumor cells, some drug-resistant tumor cells and invasive tumor cells have higher caveolin-1 expressions. Therefore, when targeting to endothelial cells, with the help of caveolin-1 pathway, some nano-drugs could transport directly across the cell by transcytosis from blood vessel to tumor interstitium, thereby increasing drug accumulation in tumor sites (Fig. 2A); when targeting to certain tumor cells with high caveolin-1 expression, caveolin-mediated endocytosis pathway facilitates nano-drugs to avoid lysosomal entrapment and degradation, achieving efficient antitumor drug release in cytoplasm or other organelles such as ER and Golgi complex and help increase drug entrance and enhance antitumor response (Fig. 2B). Therefore, the strategies for caveolin-1 targeting nano-drugs may be beneficial to antitumor efficacy.
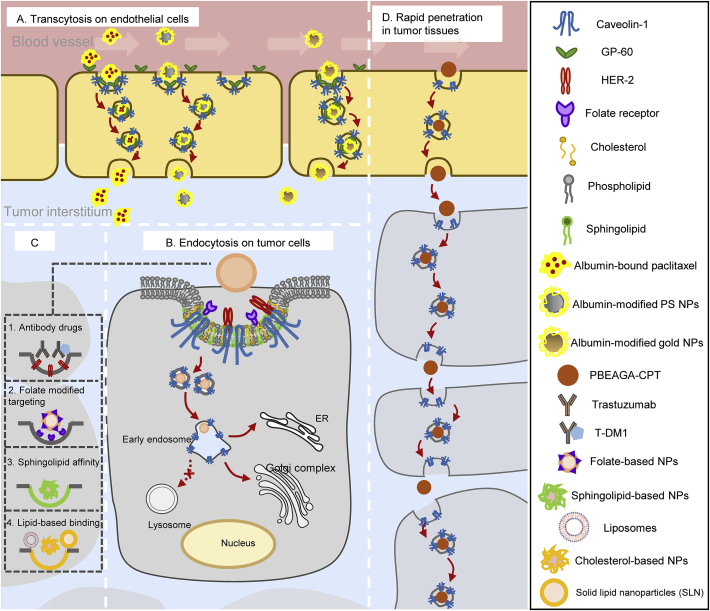
Figure 2.
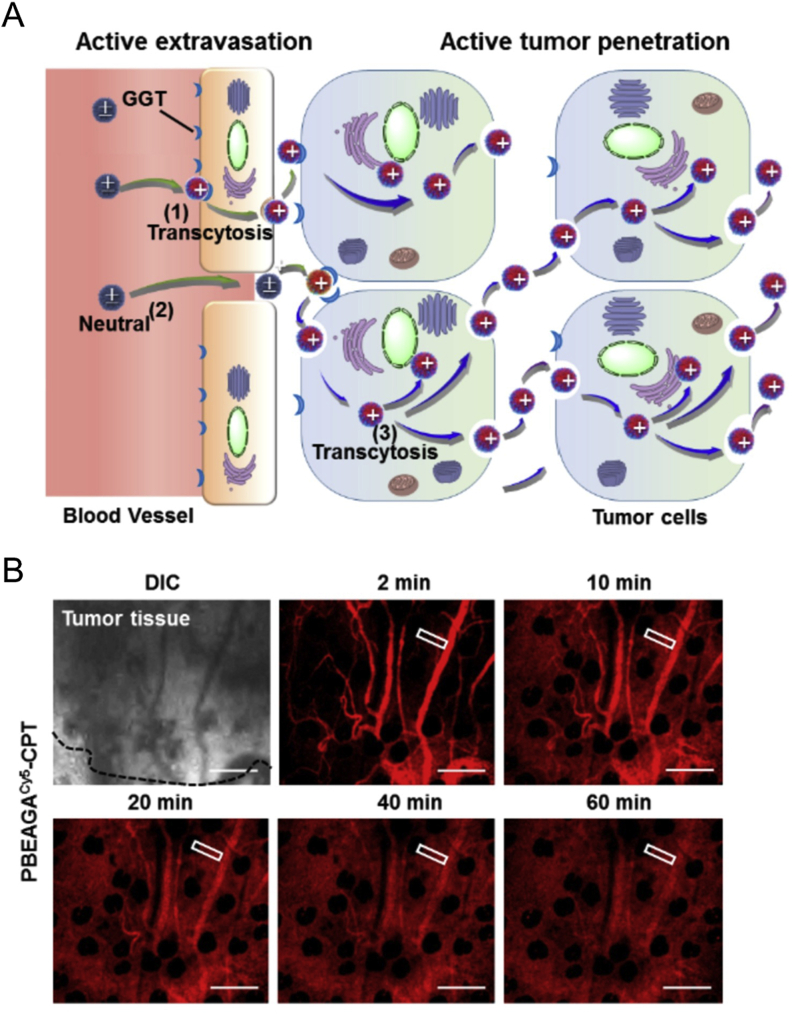
Nano-drug delivery systems and antibody drugs involved caveolin-1-mediated pathways in endothelial cells and tumor cells. (A) Albumin-related nano-drugs pass through adjacent endothelial cells by transcytosis to increase tumor accumulation; (B) Examples of antibody drugs and nano-based drug delivery systems for caveolin-mediated endocytosis to increase antitumor effects. Anti-tumor drugs or certain siRNA or DNA could be encapsulated in specific nanocarriers to enter tumor cells through caveolin-mediated pathways and arrive in ER or Golgi complex and bypass lysosomal degradation during intracellular trafficking. (C) Intracellular biofate of some nano-drugs in caveolin-mediated pathway; (D) Functional polymeric nanoparticles (PBEAGA-CPT) possess rapid extravasation on endothelial cells and rapid transcytosis in tumor cells to achieve deep penetration in tumor cells.
What kind of nano-drugs is preferred to enter cells via caveolin-mediated pathway? Here we summarized some characteristics for nano-drug delivery systems via caveolin-mediated endocytosis or transcytosis (As shown in Table 165, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78). Firstly, some receptors such as folate receptor65, albondin (60-kDa glycoprotein, GP60)66 mainly existed in caveolae microdomains, so nanocarriers modified with folic acid and albumin were preferred to enter cells by receptor-ligand caveolin-mediated endocytosis (Fig. 2A); secondly, nanoparticles modified with materials that have high affinity with caveolae would help, such as cholesterol67, sphingolipid68, and certain lipophilic polyelectrolytes69; in addition, in certain situations, some nanocarriers such as liposomes and solid lipid nanoparticles were also preferred to pass through blood vessels by transcytosis or enter tumor cells by caveolin-mediated endocytosis (Fig. 2B and C). Moreover, researchers also developed functional polymeric nanoparticles to pass across endothelial cells and tumor cells by rapid endocytosis and exocytosis in caveolin-1-mediated pathway, thereby achieving deep tumor penetration79 (Fig. 2D).
Table 1.
Nano-based strategies for caveolin-targeting drug delivery systems.
| Strategy | Example | Description | Targeting cells | Mechanism | Ref. |
|---|---|---|---|---|---|
| Folic acid | Folate-modified polyplex | By combining folic acid with PEI, these polyplex would target to caveolae in Hela cells and enhance the gene delivery efficacy | Tumor cells | Bypasses lysosomes and helps gene delivery | 65 |
| Cholesterol | Cholesterol-modified PLGA nanoparticles encapsulating curcumin | Tumor targeting and antitumor efficacy are enhanced by caveolin-mediated endocytosis | Tumor cells | Bypass lysosomes and increase antitumor efficacy | 67 |
| Sphingolipid | Sphingolipid-decorated self-micellizing antitumor nanoparticles | High affinity between sphingolipid with caveolae enhances nanoparticles to actively target tumor cells without ligands modification | Tumor cells | Increases targeting ability and bypass lysosomes | 68 |
| Lipophilic polyelectrolytes | Polyelectrolytes with aromatic sulfonic acid backbone | Negatively charged aromatic sulfonic backbone modified polyelectrolytes would be selectively internalized by endothelial cells which are highly express caveolin-1 compared with epithelial cells | Endothelial cells | Transcytosis | 69 |
| Albumin | Albumin-bound paclitaxel | Increases the binding with endothelial cells in blood vessels and helps transport to tumor tissues for drug accumulation. | Endothelial cells | Transcytosis | 70, 71, 72 |
| Albumin-coated gold nanoparticles | 20 nm-sized albumin-coated gold NPs bind to gp60 to internalize into cells via caveolin-mediated pathway | Endothelial cells | Transcytosis | 73,74 | |
| Albumin-modified PS nanoparticles | Albumin-modified PS nanoparticles have highest co-localization with caveolin-1 in endothelial cells and pass through cells | Endothelial cells | Transcytosis | 75 | |
| Albumin-modified polyplex | Albumin-modified polyplex delivering siRNA enters cells via caveolin-mediated endocytosis and increases the silencing effect | Tumor cells | Bypasses lysosomes and increases gene transduction efficacy | 76 | |
| Liposomes | Liposomes-loaded paclitaxel | By caveolae-targeting transcytosis in VEGFR2-inhibited blood vessels, the accumulation of paclitaxel to tumor tissues is enhanced by loading paclitaxel into liposomes | Endothelial cells | Transcytosis | 77 |
| Solid lipid nanoparticles | SLN-encapsulated paclitaxel | The overexpression of caveolin-1 in drug-resistant tumor cells helps SLN/paclitaxel internalization by caveolin-mediated endocytosis | Tumor cells | Bypasses lysosomes and increases drug response | 78 |
4.1. Albumin-related nano-drugs
Albumin were reported to involve in many nano-based drug delivery systems due to its biocompatibility and non-immunogenicity80. Albumin and their related nano-drugs were mainly transported into adjacent tissue through transcytosis of endothelial cells in blood vessels. By binding to GP60 receptor in the caveolae on endothelial cells, caveolin-1 was subsequently activated and then caveolar vehicles were formed from the invagination of the cell membrane. Therefore, caveolar vehicles containing albumin-related drugs transported intracellularly or arrived at basal side in a transcellular route. This process also occurred in tumor sites (Fig. 2A).
Albumin-bounded paclitaxel nanoparticles were transported from the endothelial cells to the tumor tissue through this caveolin-1-involved pathway70,71. High expression of caveolin-1 could be utilized and even enhanced on drug-resistant cells to improve the therapeutic effectiveness. Nab-paclitaxel (Abraxane®, NJ, USA) are nanoparticles with a particle size of about 130 nm, which were used in patients with advanced breast cancer and patients receiving neoadjuvant therapy in breast cancer (patients who did not receive chemotherapy) and was approved by the FDA in 2005. It showed better safety profile than Taxol® which caused severe allergic reactions related to a solubilization excipient Cremophor EL®. Moreover, in a preclinical research, compared with paclitaxel, the endothelial binding of nab-paclitaxel increased 9.9-fold, and the transport of paclitaxel across the endothelium increased 4.2-fold compared with Taxol®70,72.
It was reported that caveolin-1 was essential for the uptake of albumin-bound paclitaxel nanoparticles in tumors, and the caveolin-1 levels was directly related to the sensitivity of albumin-bound paclitaxel81. If caveolin-1 expression was interfered and attenuated by RNAi, the uptake of albumin-bound paclitaxel on the tumor cell would be reduced and caused the tumor cell to develop resistance to albumin-bound paclitaxel-induced apoptosis. In a mouse xenograft model with down-regulated caveolin-1 expression, tumor cells showed resistance to the anti-tumor effect of albumin paclitaxel treatment. Therefore, the level of caveolin-1 was considered to be a predictive marker in response to albumin-bound paclitaxel treatment. Up-regulating the level of caveolin-1 on the tumor cells facilitated albumin-bound paclitaxel nanoparticles to enter the cells and exert its efficacy.
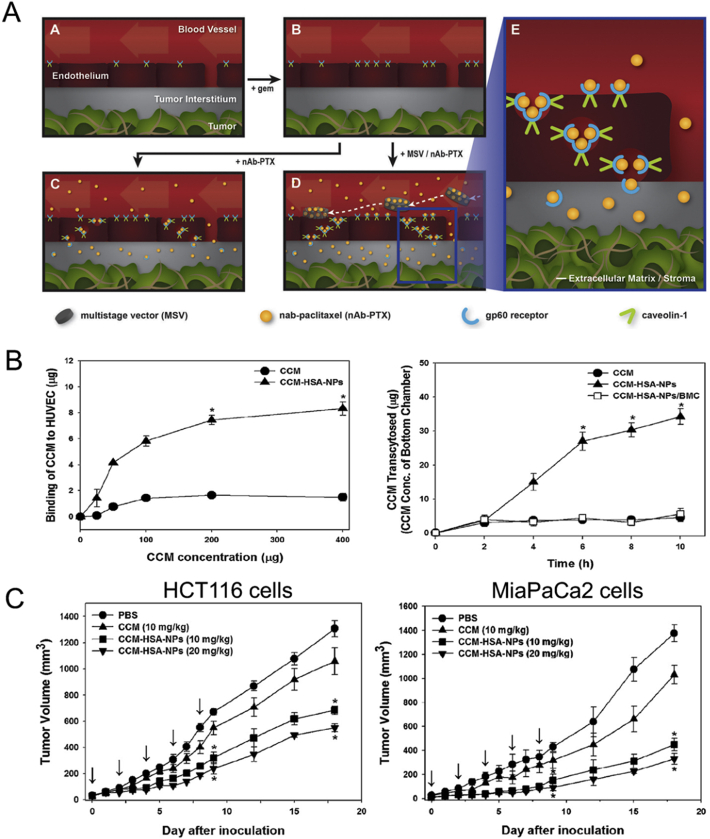
In addition, it was reported that nab-paclitaxel treatment in gemcitabine-resistant pancreatic ductal adenocarcinoma could be enhanced in vitro and in vivo by re-administration of gemcitabine because it could increase caveolin-1 expression (Fig. 3A)82. Gemcitabine was reported to inhibit DNA methyltransferase and reduce hypermethylation of caveolin-1 promoter, which may reverse the downregulated caveolin-1 expression83. Moreover, by encapsulating nab-paclitaxel in multistage nanovectors (MSV), local accumulation and uptake of albumin-bounded paclitaxel could be further increased to improve anti-tumor efficacy82.
Figure 3.
(A) The mechanism illustration of gemcitabine re-treatment improving caveolin-1 expression and nab-paclitaxel uptake, and it is further enhanced by encapsulation in MSV to improve targeting ability. Reprinted with the permission from Ref. 82. Copyright © 2017, Elsevier B.V. (B) The effects of CCM-HSA-NPs in HUVEC binding and transcytosis in cells, and the (C) anti-tumor efficacy of CCM-HAS-NPs in xenograft HCT116 (left) and MiaPaCa2 models (right). Reprinted with the permission from Ref. 88. Copyright © 2010 Elsevier B.V.
Clinically, there was a phase II clinical trial to evaluate the effectiveness of the weekly four-week treatment regimen of albumin-bound paclitaxel and gemcitabine for patients with metastatic breast cancer, as well as the relationship between caveolin-1 in tumor tissues and treatment efficacy84. The study analyzed caveolin-1 immunohistochemistry samples from 45 patients with metastatic breast cancer in tumor cells and stromal tissue. The results showed that 27 patients were tested for low expression of caveolin-1 in tumor cells, 18 patients were tested for high expression of caveolin-1 in tumor cells; 24 patients were tested for low expression of caveolin-1 in stromal matrix, and 21 patients had high expression of caveolin-1 in stromal fibroblasts. The results showed that metastatic breast cancer patients who possess high tumor caveolin-1 levels and low stromal caveolin-1 levels had the longest progression free survival (PFS) and best efficacy after administration of this regimen. The authors thought that breast cancer patients with high caveolin-1 expression in tumor cells could transport more albumin-bound paclitaxel through caveolin-1 involved transcytosis, thus having a higher concentration of albumin-binding paclitaxel and better efficacy in tumor cells. If the stromal matrix caveolin-1 expression was higher, it would cause more albumin paclitaxel uptake into the matrix than tumor cells, competitively reducing uptake of albumin paclitaxel into tumor cells. Therefore, patients with metastatic breast cancer with high stroma caveolin-1 levels were relatively ineffective. The conclusion of this study was contrary to many previous studies about caveolin-1's prognosis value for tumors. In almost all types of breast cancer, the down-regulation of caveolin-1 in the stroma has shown a role in tumor promotion and chemotherapy resistance85, 86, 87. Nonetheless, the efficacy of albumin-bound paclitaxel predicted by CAF caveolin-1 does not seem to be affected by the tumor-promoting function of CAF caveolin-1.
When applying albumin to nanoparticles, the enhanced endothelial binding could also help poorly soluble drugs such as curcumin to pass across the blood vessels to accumulate in tumors. Human serum albumin (HSA) was bound to curcumin to form nanoparticles (CCM-HSA-NPs) with size of 130–150 nm88. In vitro, CCM-HSA-NPs showed increased tumor accumulation of 14 times higher than that achieved by CCM alone 1 h after injection. Moreover, endothelial binding of CCM-HSA-NPs were also increased 5.5-fold and the content of CCM transcytosed across Caco-2 cells were significantly elevated in CCM-HSA-NPs (Fig. 3B). In vivo experiments showed maximally 66% tumor growth inhibition compared with CCM of 18% inhibition rate in xenograft HCT116 models and MiaPaCa2 models with non-toxicity (Fig. 3C).
Furthermore, caveolin-mediated endocytosis and transcytosis were also involved in other albumin-modified nano-drug delivery systems, such as gold nanoparticles73,74, polystyrene nanoparticles75 (Fig. 2A), which could increase their transcellular transport through epithelial cells to accumulate in tumor tissues.
4.2. Nano-based biomacromolecules delivery through caveolin-mediated endocytosis
Except for small molecular drugs, it was reported that caveolin-mediated entry pathways would also improve the efficacy of nano-based biomacromolecules delivery, such as therapeutic peptides, proteins and nuclei acids which were encapsulated in nanocarriers. For instance, by caveolin-mediated endocytosis, nano-delivery systems encapsulated insulin could bypass lysosomal degradation and increase their intracellular stability89. Furthermore, when targeting to tumor cells, the caveolae targeting ability would be amplified with the active-ligands modification of albumin or folate in these nano-drugs.
For example, nanocarriers carrying siRNA or DNA could enhance their gene transfection efficacy by caveolin-mediated endocytosis. It was reported that the gene delivery vectors which took up by caveolae endocytosis have the highest nuclear delivery efficiency90, 91, 92. In metastatic breast cancer cells, when albumin was incorporated in branched polyethylenimine (PEI)-siRNA polyplexes76, albumin helped polyplexes internalization in caveolin-mediated way. Their internalization into epithelial cells and silencing efficiency was enhanced, and also protect siRNA from extracellular endonucleases and lysosomal degradation.
Folic acid was also reported to be internalized in caveolae by binding with folate receptor expressed here, and it is also believed to be trafficked to Golgi complex rather than lysosomes. Therefore, by exploiting folate-modified nanoparticles, PEI-mediated transfection could also be enhanced by caveolin-mediated endocytosis. Gabrielson et al.65 reported the conjugation of folate to PEI to establish a caveolae-targeting polyplex delivery in HeLa cells, and the results showed caveolar pathways yielded successful gene delivery. While as a comparison, transferrin-modified PEI-containing vehicles had low gene delivery efficiency, due to their transport routes to acidic lysosomes.
Moreover, except for increasing active targeting by ligands modification, high expression of caveolin-1 on specific tumor cells such as inflammatory infiltrating breast cancer (IBC) cells also inspired nano-drug delivery system design, which may show potential ability to utilize caveolar endocytosis to gain tumor targeting93. Histone peptide (H3)-modified DNA polyethyleneimine (PEI) complexes (polyplexes) were designed to efficiently transported by caveolar endocytosis pathway followed by the Golgi apparatus and the ER, and finally targeted the perinuclear compartments and accumulate within nucleus93. Therefore, H3-targeted polyplexes increased activity in caveolin-1-overexpressing infiltrating breast cancer cells; moreover, this caveolin-1-related targeting effect could be used to help IBC-specific transfection efficiency in the presence of normal breast epithelial cells. The results of co-cultivation experiments with IBC SUM149 and MCF10A cells showed that compared to normal breast epithelial cells MCF10A, H3-targeted polyplexes are selectively taken up by IBC SUM149 cells and reached the nucleus through the Golgi and ER bypassing the lysosome, which increase the IBC transfection efficiency to 4 times. These results showed that the difference in the expression of caveolin-1 on cell membrane could be exploited to promote the selectivity of gene delivery, which was beneficial to the treatment of invasive tumors.
4.3. Caveolin-targeting nano-drug delivery by high affinity binding
By utilizing specific materials which have a high affinity for caveolin-1 on the tumor cell membranes, nanocarriers were designed to actively target the highly expressed caveolin-1 to increase the drug entrance into tumor cells or transcytosis in endothelial cells, thereby increasing antitumor therapeutic effect (Fig. 2B and C).
The progression of colorectal cancer is positively correlated with caveolin-1 levels. Because of the difference in the distribution density and expression level of caveolin-1 in colon cancer tissues and normal tissues, a biological lipid-sphingolipid that has high affinity for caveolin-1 and could induce tumor cell apoptosis by generating ROS was introduced to prepare nano-micelles68. Researchers chose lauramide derivative SMAL102 and phospholipid (PL) as the nanoparticle lipid materials, and by self-micellization, an anticancer lipid based nanoparticle (SMAL-OL)-containing oxaliplatin was designed. The caveolin-mediated endocytic pathway of SMAL-OL was proved by significant uptake inhibition when applying M-β-CD (inhibitor for caveolae-mediated uptake) and sucrose (inhibitor for lipid raft-mediated endocytosis) in HCT116 and HT29 cells, which indicate the role of caveolin-mediated pathway in SMAL-OL internalization, and researchers believed that these may help nano-micelles bypass the lysosomal pathway and reduce drug degradation. With particle size of around 100 nm, on the one hand, the high affinity and specific binding of sphingolipids and caveolae facilitated effective accumulation and uptake of oxaliplatin in HCT116 and HT29 cells; on the other hand, as the component of SMAL-OL, sphingolipids and oxaliplatin could both elevate anticancer effect via different pathways without being toxic to healthy tissues. In vitro, anti-apoptotic proteins BCL-XL and BID were down-regulated, and pro-apoptotic proteins P53, BAX, CASPASE-3 and CASPASE-9 were up-regulated. Moreover, SMAL-OL induced apoptosis through PI3K/AKT/mTOR signaling pathway.
In addition, by introduction of cholesterol to classic polymeric materials, tumor accumulation could also be enhanced. Researchers67 reported a PLGA-cholesterol nanoparticle encapsulating curcumin (PLGA-C/CUR NPs), compared with PLGA NPs loaded with curcumin, PLGA-C/CUR NPs showed an improved cellular accumulation efficiency in Hep-2 cells and enhanced in vivo tumor target ability in Hep-2 tumor-xenografted mouse model. Researchers believed that cholesterol in caveolae was involved in endocytosis of materials and cancer proliferation and metastasis, so cholesterol-bearing PLGA NPs may be more easily uptaken into tumor cells in caveolin-mediated endocytosis.
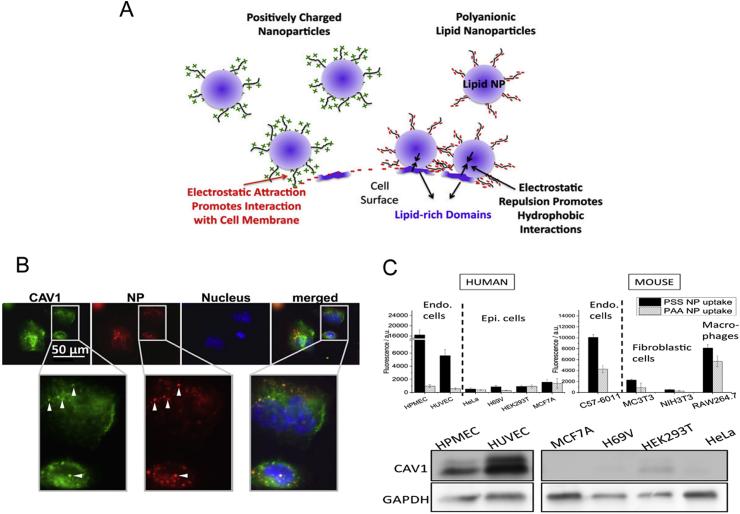
By modifying nanoparticles with highly lipophilic anionic polyelectrolytes, the caveolin-mediated endocytosis would be significantly improved (Fig. 4)69. Researchers found that compared with epithelial cells, synthetic polyelectrolytes with aromatic sulfonic acid backbone showed natural specific affinity for caveolin-overexpressed endothelial cells HUVEC and increase transport and accumulation there. Their targeting mechanisms mainly include, on the one hand, lipophilic groups provided a high affinity for cell membrane lipids on caveolae, on the other hand, the highly negatively charged surface helped nanoparticle recognize target cells by lipophilic affinity, rather than charge–charge interactions, thereby enabling the targeting of caveolae. This method also provided a new strategy for non-ligand modified active targeting delivery.
Figure 4.
(A) The interaction mechanism between polyanionic lipid nanoparticles and cell surface. (B) The colocalization between caveolin-1 and polyelectrolytes in Confocal Laser Scanning Microscopy observation; (C) Endothelial cells both in human and mouse show better uptake ability and HUVEC cells show highest caveolin-1 expression. Reprinted with the permission from Ref. 69. Copyright © 2014 freely available online through the PNAS open access option.
In addition, lipid-based nanocarriers could be applied to change the initial entry pathway of small-molecule chemotherapeutics to caveolin-1-mediated endocytosis which enhance drug uptake and decrease drug resistance. For example, in caveolin-1 highly expressed multidrug resistance (MDR) breast cancer cell line MDR-MCF-7, paclitaxel as a substrate for P-gP, would be effluxed to reduce the intracellular content, resulting in paclitaxel resistance. Compared with normal MCF-7 cells, caveolin-1 was highly expressed on MDR-MCF-7. To solve the problem, researchers encapsulated paclitaxel with egg yolk lecithin and DSPE-mPEG2000 to prepare paclitaxel-loaded solid lipid nanoparticles (PTX-SLN). The results showed that in MDR-MCF-7, PTX-SLN changed the original uptake pathway of paclitaxel and were taken up into cells by the caveolin-1 endocytosis pathway, thereby reducing the efflux of free drugs and improving drug resistance77.
Moreover, in certain situations, liposomes were also reported to involve in caveolin-mediated transcytosis. Taking advantage of the high expression of caveolin-1 on vascular epithelial cells, the accumulation of long-circulating liposomes at the tumor sites could be enhanced by transcytosis, thus improving drug efficacy78. Researchers explored the reason why the tumor accumulation of liposomes increased significantly after knocking out vascular endothelial cell growth factor receptor 2 (VEGFR2) of endothelial cells. A cyclic RGD peptide modified liposome (RGD-MEND)-encapsulating siRNA against VEGFR2 was designed to target vascular endothelial cells. In the renal cell carcinoma model, as RGD-MEND was administered intravenously, the knockdown of VEGFR2 caused vasculature maturation and more complete, accompany with the increased pericytes coverage and endothelial cell adhesion. However, the accumulation of liposomes in tumor tissue was improved rather than decreased. This result was inconsistent with the theory of EPR effect—“nanoparticles are expected to leak from irregular vascular pores to accumulate in tumor tissue”. Immunofluorescence staining results showed that after RGD-MEND knocked out the VEGF signal, the expression of caveolin-1 on endothelial cells was significantly enhanced. It showed that caveolin-1 on tumor endothelial cells played an important role in tumor accumulation of liposomes, at least when VEGF signaling was suppressed. Moreover, it was proved in the renal cell carcinoma model that liposomes accumulated into tumor tissue through transcellular way rather than paracellular route. However, the authors cautiously suggested that this increase in tumor accumulation of nanomaterials through caveolin-1-mediated transcytosis was currently only proved in liposomes, and further research was needed to clarify caveolin-1-mediated delivery in various cancers types and nanoparticle types.
Furthermore, through efficient caveolin-1-mediated transcytosis, nano-drug delivery system was designed to endocytose and transport to the Golgi apparatus and avoid lysosomal degradation, and finally be rapidly exocytosed to another cells, which achieved rapid active penetration and transcellular drug transport in tumor tissues. In a recent study, due to the high expression of γ-glutamyl transpeptidase (GGT) on the endothelial cells of tumor blood vessels and nearby tumor cells, Zhou et al.79 developed a GGT-response polymer PBEAGA and its conjugate PBEAGA-CPT with the chemotherapy drug camptothecin (CPT, Fig. 5). After reaching the tumor site, the γ-glutamyl groups on PBEAGA were hydrolyzed to amine groups by GGT on the cell surface, and then the positive-charged polymers would be rapidly taken up by adsorption and mediated by caveolae-mediated endocytosis, and further induce caveolae-mediated transcytosis, while avoiding the lysosomal pathway and retaining effective drug loading. In this way, the rapid endocytosis and the intact exocytosis facilitated the transcellular route in tumor cells, and PBEAGA-CPT verified its significant in vivo tumor-inhibiting effect in various tumor-bearing animal models including pancreatic cancer.
Figure 5.
(A) Schematic illustration of PBEAGA-CPT with rapidly transcytosis ability and deep tumor penetration ability. (B) In vivo real-time permeability of PBEAGA-Cy5-CPT from the blood vessel to HepG2 xenograft tumor in a mouse ear after intravenous injection. Reprinted with the permission from Ref. 70. Copyright © 2020, Elsevier Ltd.
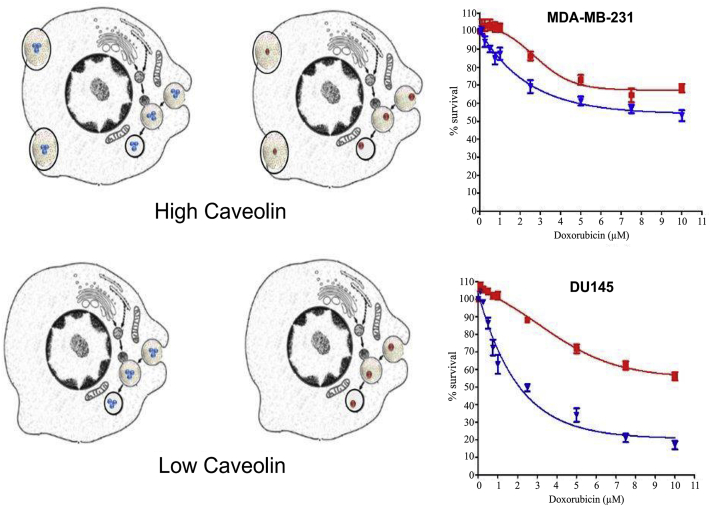
However, different caveolin-1 levels expressed on the tumor cell membrane would also affect the efficacy of nanoparticles with different drug loadings which endocytosed by the caveolae pathway. The listed doxorubicin-containing nanoparticles including Doxil® and Myocet® have been clinically less effective than expected. To analyze this problem, styrene maleic acid (SMA) was utilized by researchers as the doxorubicin-containing micelle material, and three micelles were designed with different drug loadings of 4.4%, 14.5%, and 28.4% respectively, and evaluate the relative toxicity in different breast cancer cell lines (MDA-MB-231 and MCF-7) and on prostate cancer cell lines (PC3 and DU-145). Moreover, the relationship between relative toxicity and caveolin-1 levels on these cell lines were also compared94. Result showed that the pattern of cytotoxicity varies on different cell lines. Cytotoxicity of MDA-MB-231 cells at any concentration had little dependence on micellar drug loading, while the difference in cytotoxicity of PC3 cells was smaller in higher concentrations. MCF-7 and DU-145 cells showed significant differences in cytotoxicity at different concentrations and different drug loadings, and the higher the micellar drug loading, the stronger the cytotoxicity to the cell line. Caveolin-1 level results showed that MDA-MB-231 had the highest caveolin-1 level, followed by PC3 and DU-145, while MCF-7 had almost no caveolin-1 expression. Researchers believed that the differences in cytotoxicity observed in different cell lines may be related to the level of caveolin-1 and the ability of cells to take up nanoparticles (Fig. 6). High caveolin-1 expression may indicate that MDA-MB-231 cells are capable of rapidly taking up a sufficient amount of micelles, so regardless of the drug loading, the maximum effect can be achieved at each concentration. MCF-7 cells with low or without caveolin-1 expression may not be able to take up a large number of micelles at the same time, so their cytotoxicity depended on the drug loading in the micelles, because the cells could not take up enough micelles to achieve the maximum efficacy. But the authors also pointed out that in addition to the internalization of micelles, the factors that affect the efficacy were also involved in the sensitivity of the cell line to free doxorubicin.
Figure 6.
The relationship between caveolin-1 levels and cytotoxicity with SMA-doxorubicin micelles with high loading (blue) and low loading (red) against MDA-MB-231 (high caveolin-1 levels) and DU-145 cells (low caveolin-1 levels), when they are endocytosed in caveolin-mediated pathways. Reprinted with the permission from Ref. 94. Copyright © 2014 Nehoff H et al.
5. The effects of nanocarriers on caveolin-1
Caveolin-1 affects the cellular pathways and intracellular biofate of nano-drug, in turn, the distribution and expression of caveolin-1 on the cell membrane would also be affected by nano-drugs, further affecting subsequent intracellular biological effects related to caveolin-1, although there is limited information available in this field.
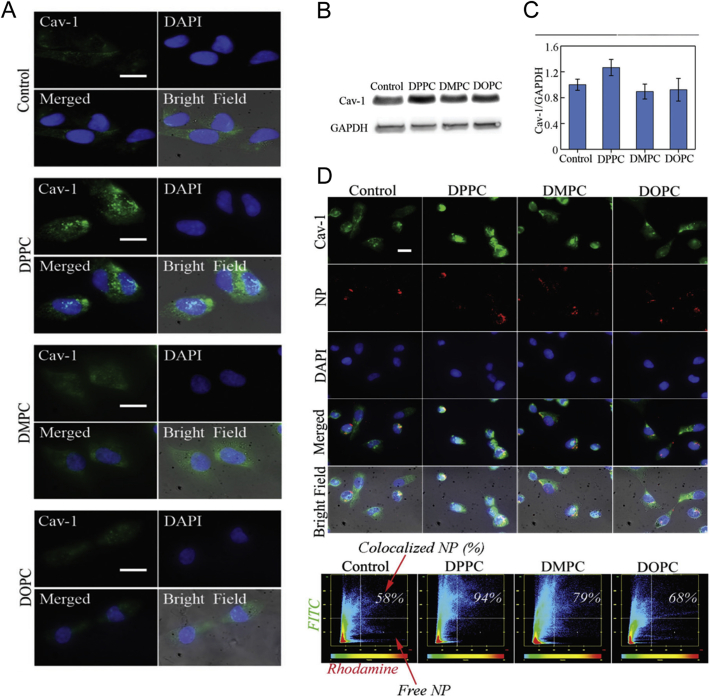
It was reported that compared with soft tumor extracellular matrix, tumor matrix with higher stiffness could elevate caveolin-1 expression levels of adjacent tumor cells, and modulate the uptake efficiency of PEG–PLGA nanoparticle and promote drugs enter cells95. Similarly, this effect could also be achieved by specific nanocarriers. In this study, liposomes with high stiffness changed the cell membrane that uptake them, and further promoted the uptake of PLGA nanoparticles through caveolin-1 pathway96. Three liposomes with different degrees of stiffness (DPPC > DOPC > DMPC) were compared for the bio-effects on breast cancer cell line MDA-MB-231. The results showed that pretreatment with hardest DPPC liposomes would increase the rigidity of the cell membrane and promote the expression and recruitment of caveolin-1 on the surface (Fig. 7A–C). Moreover, this pre-treatment significantly increases PLGA nanoparticles endocytosis via caveolin-1 mediated pathway (Fig. 7D). These results indicated the influence of liposomes on the stiffness of tumor cell membranes and the further effect on the endocytosis pathway of subsequent polymer nanoparticles mediated by caveolin-1. This method could be used to locally deliver lipids with high stiffness to improve the rigidity of breast cancer cell membranes, thereby optimizing the entry pathway of drug-loaded polymer nanoparticles to achieve better antitumor efficacy.
Figure 7.
(A) Liposome treatment impacts recruitment of caveolin-1 by influencing stiffness of plasma membrane. (B) and (C) Caveolin-1 expression is elevated most in the DPPC-treated groups in comparison to both control and DMPC and DOPC-treated groups. (D) Fluorescent microscopy show intracellular biological fate of PLGA nanoparticles (Red), and caveolin-1 in DPPC-treated cells show most colocalization with PLGA nanoparticles. Reprinted with the permission from Ref. 96. Copyright © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Dixit et al.97 reported a PLA-PEG based nano-vaccine which could be endocytosed via caveolin-1 mediated pathway, and could cause caveolin-1 increase vice versa. In vitro experiment, the antigen Chlamydial M278 peptide showed a strong co-localization with caveolin-1 in the endocytic route, which indicated the endocytosis of the nano–vaccine was mediated by caveolin-1. At the same time, during this caveolin-1 mediated endocytosis, the transcription and protein expression of caveolin-1 in the cell were further increased and obtained a continuous and enhanced CD4+ T cells-mediated Chlamydia-specific immune effector response.
6. Conclusions and prospects
With the development of nanomedicine research, understanding of nanomedicine in anti-tumor therapy is also constantly improving. In the beginning, researchers focused on how nanocarriers could overcome the in vivo barrier and reach the tumor tissue through the EPR effect. However, they were faced with the dilemma that clinically marketed nanomedicine could only improve safety but without efficacy elevating. Now, researchers are gradually paying more attention to that after nano-drugs and anti-tumor drugs reach tumor tissues, how they interact with various cells in the tumor microenvironment, and even how they interact with substructures and proteins on cell membranes, moreover, the biological effects of tumor. People hope that by more systematic and detailed researches in cell level and molecular level, the problem of clinical efficacy of nanomedicine would finally get improved.
Caveolin-1, as a protein in lipid microdomain on cell surface, has attracted attentions for a long time. Besides playing its basic role, they were also used as a tumor treatment and prognosis predictor, as well as affecting the resistance and effectiveness of antitumor drugs, and they also play an increasingly important role in the delivery of antibody drugs, ADCs and nano-drugs (Fig. 2). When involved in tumor prognosis prediction, although the effect of the caveolin-1 expression on various tumor cells is still controversial, stromal matrix caveolin-1 levels in tumor microenvironments may be predictive. In the future, by combining the observation and statistics of clinical tumor status to study the difference in the expression of caveolin-1 in tumor CAFs and tumor cells, a more reasonable and realistic in vitro tumor model based on this could be established, and rational and effective nanocarriers according to needs may be developed, thereby providing support for the delivery of clinically effective nano-drugs. In addition, in view of the important role of caveolin-1 in the endocytosis of nanomedicine, whether the expression or quantity of caveolin-1 on the cell membrane will be affected by the endocytosis of a large number of nanoparticles, is also worth further discussing.
Moreover, in view of the ongoing challenge of the EPR effect, the active transcytosis mediated by caveolin-1 and its role in the accumulation of various nano-drugs in tumor tissues has gradually attracted much attention. In the transcytosis mechanism, does the expression level of caveolin-1 on different tumor cells affect the efficacy of the same nano-drug? It is also noticeable that not all nanocarriers endocytosed in caveolin-mediated pathway would bypass lysosomal degradation, therefore, what kind of characteristics that nano-drugs should possess to reach Golgi or ER to achieve cytosolic delivery, or perform transcytosis and rapidly exocytosis to improve the penetration efficiency in tumor? What factors determine the intracellular biofate of transcytosis of nanocarriers? In the future, researchers could develop nanocarriers transcytosed by caveolin-1 and study their intracellular transport process, which may provide some reference for improving the clinical effectiveness of nanomedicine.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81872809, 81690264 and 31671017); and the National Key Research and Development Program of China (2017YFA0205600).
Author contributions
Canyu Yang wrote the manuscript. Bing He, Wenbing Dai, Hua Zhang and Ying Zheng provided suggestions and modified the article layout, Xueqing Wang and Qiang Zhang designed and revised the manuscript.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Xueqing Wang, Email: wangxq@bjmu.edu.cn.
Qiang Zhang, Email: zqdodo@bjmu.edu.cn.
References
- 1.Sotgia F., Martinez Outschoorn U.E., Howell A., Pestell R.G., Pavlides S., Lisanti M.P. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol: Mechanisms of Disease. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 2.Cheng J.P., Nichols B.J. Caveolae: one function or many?. Trends Cell Biol. 2016;26:177–189. doi: 10.1016/j.tcb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Li X.A., Everson W.V., Smart E.J. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15:92–96. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Galbiati F., Volonte D., Liu J., Capozza F., Frank P.G., Zhu L. Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent mechanism. Mol Biol Cell. 2001;12:2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelman J.A., Wykoff C.C., Yasuhara S., Song K.S., Okamoto T., Lisanti M.P. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 6.Torres V.A., Tapia J.C., Rodríguez D.A., Párraga M., Lisboa P., Montoya M. Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J Cell Sci. 2006;119:1812–1823. doi: 10.1242/jcs.02894. [DOI] [PubMed] [Google Scholar]
- 7.Xu L., Qu X.J., Li H.M., Li C., Liu J., Zheng H.C. Src/caveolin-1-regulated EGFR activation antagonizes TRAIL-induced apoptosis in gastric cancer cells. Oncol Rep. 2014;32:318–324. doi: 10.3892/or.2014.3183. [DOI] [PubMed] [Google Scholar]
- 8.Meyer C., Liu Y., Kaul A., Peipe I., Dooley S. Caveolin-1 abrogates TGF-β mediated hepatocyte apoptosis. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2012.204. e466-e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgermeister E., Liscovitch M., Röcken C., Schmid R.M., Ebert M.P. Caveats of caveolin-1 in cancer progression. Cancer Lett. 2008;268:187–201. doi: 10.1016/j.canlet.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Lamaze C., Prior I. Springer; New York: 2018. Endocytosis and signaling. [Google Scholar]
- 11.Gupta R., Toufaily C., Annabi B. Caveolin and cavin family members: dual roles in cancer. Biochimie. 2014;107:188–202. doi: 10.1016/j.biochi.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Fielding P.E., Fielding C.J. Intracellular transport of low density lipoprotein derived free cholesterol begins at clathrin-coated pits and terminates at cell surface caveolae. Biochemistry. 1996;35:14932–14938. doi: 10.1021/bi9613382. [DOI] [PubMed] [Google Scholar]
- 13.Hayer A., Stoeber M., Ritz D., Engel S., Meyer H.H., Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191:615–629. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muley H., Fadó R., Rodríguez Rodríguez R., Casals N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem Pharmacol. 2020;177:1139–1159. doi: 10.1016/j.bcp.2020.113959. [DOI] [PubMed] [Google Scholar]
- 15.Echarri A., Del Pozo M.A. Caveolae-mechanosensitive membrane invaginations linked to actin filaments. J Cell Sci. 2015;128:2747–2758. doi: 10.1242/jcs.153940. [DOI] [PubMed] [Google Scholar]
- 16.Anderson R.G. Annual Reviews; USA: 1998. The caveolae membrane system. 4139 El Camino Way, PO Box 10139, Palo Alto, CA 94303-0139. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q., Dong C.Y., Fan W.F., Jiang H.P., Xiang J.J., Qiu N.S. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: the current status and transcytosis strategy. Biomaterials. 2020;240:1199–1202. doi: 10.1016/j.biomaterials.2020.119902. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Huo Y.Y., Yao L., Xu Y.W., Meng F.Q., Li H.F. Transcytosis of nanomedicine for tumor penetration. Nano Lett. 2019;19:8010–8020. doi: 10.1021/acs.nanolett.9b03211. [DOI] [PubMed] [Google Scholar]
- 19.Wu D.J., Zhuo L.Y., Wang X.D. Metabolic reprogramming of carcinoma-associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin Cell Dev Biol. 2017;64:125–131. doi: 10.1016/j.semcdb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Martinez Outschoorn U.E., Pavlides S., Whitaker Menezes D., Daumer K.M., Milliman J.N., Chiavarina B. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 21.Chen D.L., Che G.W. Value of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression. Oncol Lett. 2014;8:1409–1421. doi: 10.3892/ol.2014.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avagliano A., Granato G., Ruocco M.R., Romano V., Belviso I., Carfora A. Metabolic reprogramming of cancer associated fibroblasts: the slavery of stromal fibroblasts. BioMed Res Int. 2018 doi: 10.1155/2018/6075403. https://doi/10.1155/2018/6075403 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing Y.Z., Zhao S.M., Zhou B.H., Mi J. Metabolic reprogramming of the tumour microenvironment. FEBS J. 2015;282:3892–3898. doi: 10.1111/febs.13402. [DOI] [PubMed] [Google Scholar]
- 24.Penkert J., Ripperger T., Schieck M., Schlegelberger B., Steinemann D., Illig T. On metabolic reprogramming and tumor biology: a comprehensive survey of metabolism in breast cancer. Oncotarget. 2016;7:67626. doi: 10.18632/oncotarget.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilde L., Roche M., Domingo Vidal M., Tanson K., Philp N., Curry J. Metabolic coupling and the Reverse Warburg Effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44:198–203. doi: 10.1053/j.seminoncol.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witkiewicz A.K., Casimiro M.C., Dasgupta A., Mercier I., Wang C., Bonuccelli G. Towards a new “stromal-based” classification system for human breast cancer prognosis and therapy. Cell Cycle. 2009;8:1654–1658. doi: 10.4161/cc.8.11.8544. [DOI] [PubMed] [Google Scholar]
- 27.Mercier I., Casimiro M.C., Wang C., Rosenberg A.L., Quong J., Minkeu A. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 down-regulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloan E.K., Ciocca D.R., Pouliot N., Natoli A., Restall C., Henderson M.A. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witkiewicz A.K., Dasgupta A., Sammons S., Er O., Potoczek M., Guiles F. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X.D., He Y.Y., Gao J., Fan L.F., Li Z.H., Yang G.F. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu K.N., Queenan M., Brody J.R., Potoczek M., Sotgia F., Lisanti M.P. Loss of stromal caveolin-1 expression in malignant melanoma metastases predicts poor survival. Cell Cycle. 2011;10:4250–4255. doi: 10.4161/cc.10.24.18551. [DOI] [PubMed] [Google Scholar]
- 32.Ayala G., Morello M., Frolov A., You S., Li R., Rosati F. Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse—free survival and is functionally relevant to tumour progression. J Pathol. 2013;231:77–87. doi: 10.1002/path.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Vizio D., Morello M., Sotgia F., Pestell R.G., Freeman M.R., Lisanti M.P. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease spread and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J., Bae E., Lee C., Yoon S.S., Chae Y.S., Ahn K.S. RNA interference-directed caveolin-1 knockdown sensitizes SN12CPM6 cells to doxorubicin-induced apoptosis and reduces lung metastasis. Tumor Biol. 2010;31:643–650. doi: 10.1007/s13277-010-0081-1. [DOI] [PubMed] [Google Scholar]
- 35.Parat M.O., Riggins G.J. Caveolin-1, caveolae, and glioblastoma. Neuro Oncol. 2012;14:679–688. doi: 10.1093/neuonc/nos079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulit J., Bash T., Fu M., Galbiati F., Albanese C., Sage D.R. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275:21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 37.Fiucci G., Ravid D., Reich R., Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 38.Sunaga N., Miyajima K., Suzuki M., Sato M., White M.A., Ramirez R.D. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004;64:4277–4285. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- 39.Cui J., Rohr L.R., Swanson G., Speights V.O., Maxwell T., Brothma A.R. Hypermethylation of the caveolin-1 gene promoter in prostate cancer. Prostate. 2001;46:249–256. doi: 10.1002/1097-0045(20010215)46:3<249::aid-pros1030>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Li L., Ren C., Yang G., Goltsov A.A., Tabata K., Thompson T.C. Caveolin-1 promotes autoregulatory, Akt-mediated induction of cancer-promoting growth factors in prostate cancer cells. Mol Cancer Res. 2009;7:1781–1791. doi: 10.1158/1541-7786.MCR-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathieu R., Klatte T., Lucca I., Mbeutcha A., Seitz C., Karakiewicz P.I. Prognostic value of Caveolin-1 in patients treated with radical prostatectomy: a multicentric validation study. BJU Int. 2016;118:243–249. doi: 10.1111/bju.13224. [DOI] [PubMed] [Google Scholar]
- 42.Lee H., Volonte D., Galbiati F., Iyengar P., Lublin D.M., Bregman D.B. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 43.Tahir S.A., Yang G., Ebara S., Timme T.L., Satoh T., Li L. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–3885. [PubMed] [Google Scholar]
- 44.Bonuccelli G., Casimiro M.C., Sotgia F., Wang C., Liu M., Katiyar S. Caveolin-1 (P132L), a common breast cancer mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signature. Am J Pathol. 2009;174:1650–1662. doi: 10.2353/ajpath.2009.080648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z.Y., Wang N., Huang C.X., Bao Y.H., Jiang Y.Q., Zhu G.T. Downregulation of caveolin-1 increases the sensitivity of drug-resistant colorectal cancer HCT116 cells to 5-fluorouracil. Oncol Lett. 2017;13:483–487. doi: 10.3892/ol.2016.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou W., Ma X.D., Hua W., Chen B.L., Cai G.Q. Caveolin-1 mediates chemoresistance in cisplatin-resistant ovarian cancer cells by targeting apoptosis through the Notch-1/Akt/NF-κB pathway. Oncol Rep. 2015;34:3256–3263. doi: 10.3892/or.2015.4320. [DOI] [PubMed] [Google Scholar]
- 47.Yang C.P.H., Galbiati F., Volonté D., Horwitz S.B., Lisanti M.P. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998;439:368–372. doi: 10.1016/s0014-5793(98)01354-4. [DOI] [PubMed] [Google Scholar]
- 48.Sung M., Tan X., Lu B., Golas J., Hosselet C., Wang F. Caveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1) Mol Cancer Therapeut. 2018;17:243–253. doi: 10.1158/1535-7163.MCT-17-0403. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Lu B., Dai C.Y., Fu Y.F., Hao K., Zhao B. Caveolin-1 promotes chemoresistance of gastric cancer cells to cisplatin by activating WNT/β-catenin pathway. Front Oncol. 2020;10:46. doi: 10.3389/fonc.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Fu Y.L., Hu X.X., Chen S., Miao J.B., Wang Y. Caveolin-1 knockdown increases the therapeutic sensitivity of lung cancer to cisplatin-induced apoptosis by repressing Parkin-related mitophagy and activating the ROCK1 pathway. J Cell Physiol. 2020;235:1197–1208. doi: 10.1002/jcp.29033. [DOI] [PubMed] [Google Scholar]
- 51.Nagy P., Vereb G., Sebestyén Z., Horváth G., Lockett S.J., Damjanovich S. Lipid rafts and the local density of ErbB proteins influence the biological role of homo-and heteroassociations of ErbB2. J Cell Sci. 2002;115:4251–4262. doi: 10.1242/jcs.00118. [DOI] [PubMed] [Google Scholar]
- 52.Steven S. Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin multinational investigator study group. Semin Oncol. 1999;4:71–77. [PubMed] [Google Scholar]
- 53.Nahta R., Esteva F.J. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–138. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 54.Pereira P.M., Sharma S.K., Carter L.M., Edwards K.J., Pourat J., Ragupathi A. Caveolin-1 mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat Commun. 2018;9:5137. doi: 10.1038/s41467-018-07608-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekhar S.C., Kasai T., Satoh A., Shigehiro T., Mizutani A., Murakami H. Identification of caveolin-1 as a potential causative factor in the generation of trastuzumab resistance in breast cancer cells. J Cancer. 2013;4:391–401. doi: 10.7150/jca.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritchie M., Tchistiakova L., Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs. 2013;5:13–21. doi: 10.4161/mabs.22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barok M., Joensuu H., Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209–221. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung Y.C., Kuo J.F., Wei W.C., Chang K.J., Chao W.T. Caveolin-1 dependent endocytosis enhances the chemosensitivity of HER-2 positive breast cancer cells to trastuzumab emtansine (T-DM1) PLoS One. 2015;10 doi: 10.1371/journal.pone.0133072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung Y.C., Chang C.M., Wei W.C., Chang T.W., Chang K.J., Chao W.T. Metformin-induced caveolin-1 expression promotes T-DM1 drug efficacy in breast cancer cells. Sci Rep. 2018;8:3930. doi: 10.1038/s41598-018-22250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahay G., Alakhova D.Y., Kabanov A.V. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Roy C., Wrana J.L. Clathrin-and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 62.Luo D., Saltzman W.M. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 63.Fujimoto T., Kogo H., Nomura R., Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113 Pt 19:3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- 64.Sahay G., Kim J.O., Kabanov A.V., Bronich T.K. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials. 2010;31:923–933. doi: 10.1016/j.biomaterials.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabrielson N.P., Pack D.W. Efficient polyethylenimine-mediated gene delivery proceeds via a caveolar pathway in HeLa cells. J Control Release. 2009;136:54–61. doi: 10.1016/j.jconrel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Petrelli F., Borgonovo K., Barni S. Targeted delivery for breast cancer therapy: the history of nanoparticle-albumin-bound paclitaxel. Expet Opin Pharmacother. 2010;11:1413–1432. doi: 10.1517/14656561003796562. [DOI] [PubMed] [Google Scholar]
- 67.Lee J.J., Lee S.Y., Park J.H., Kim D.D., Cho H.J. Cholesterol-modified poly (lactide-co-glycolide) nanoparticles for tumor-targeted drug delivery. Int J Pharm. 2016;509:483–491. doi: 10.1016/j.ijpharm.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Sundaramoorthy P., Ramasamy T., Mishra S.K., Jeong K.Y., Yong C.S., Kim J.O. Engineering of caveolae-specific self-micellizing anticancer lipid nanoparticles to enhance the chemotherapeutic efficacy of oxaliplatin in colorectal cancer cells. Acta Biomater. 2016;42:220–231. doi: 10.1016/j.actbio.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Voigt J., Christensen J., Shastri V.P. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc Natl Acad Sci Unit States Am. 2014;111:2942–2947. doi: 10.1073/pnas.1322356111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desai N., Trieu V., Yao Z., Louie L., Ci S., Yang A. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 71.Schettini F., Giuliano M., De Placido S., Arpino G. Nab-paclitaxel for the treatment of triple-negative breast cancer: rationale, clinical data and future perspectives. Cancer Treat Rev. 2016;50:129–141. doi: 10.1016/j.ctrv.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Hawkins M.J., SoonShiong P., Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 73.Komarova Y., Malik A.B. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 74.Predescu S.A., Predescu D.N., Malik A.B. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293:L823–L842. doi: 10.1152/ajplung.00436.2006. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z., Tiruppathi C., Minshall R.D., Malik A.B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3:4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicolì E., Syga M.I., Bosetti M., Shastri V.P. Enhanced gene silencing through human serum albumin-mediated delivery of polyethylenimine-siRNA polyplexes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu W., Bae E.J., Lee M.K. Enhanced anticancer activity and intracellular uptake of paclitaxel-containing solid lipid nanoparticles in multidrug-resistant breast cancer cells. Int J Nanomed. 2018;13:7549–7563. doi: 10.2147/IJN.S182621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakurai Y., Kato A., Harashima H. Involvement of caveolin-1-mediated transcytosis in the intratumoral accumulation of liposomes. Biochem Biophys Res Commun. 2020;525:313–318. doi: 10.1016/j.bbrc.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Q., Shao S.Q., Wang J.Q., Xu C.H., Xiang J.J., Piao Y. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 80.Mariam J., Sivakami S., Dongre P.M. Albumin corona on nanoparticles—a strategic approach in drug delivery. Drug Deliv. 2016;23:2668–2676. doi: 10.3109/10717544.2015.1048488. [DOI] [PubMed] [Google Scholar]
- 81.Chatterjee M., BenJosef E., Robb R., Vedaie M., Seum S., Thirumoorthy K. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017;77:5925–5937. doi: 10.1158/0008-5472.CAN-17-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borsoi C., Leonard F., Lee Y., Zaid M., Elganainy D., Alexander J.F. Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma. Cancer Lett. 2017;403:296–304. doi: 10.1016/j.canlet.2017.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gray S.G., Baird A.M., O'Kelly F., Nikolaidis G., Almgren M., Meunier A. Gemcitabine reactivates epigenetically silenced genes and functions as a DNA methyltransferase inhibitor. Int J Mol Med. 2012;30:1505–1511. doi: 10.3892/ijmm.2012.1138. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Y.N., Lv F.F., Chen S., Wang Z.H., Zhang J., Zhang S. Caveolin-1 expression predicts efficacy of weekly Nab-paclitaxel plus gemcitabine for metastatic breast cancer in the phase II clinical trial. BMC Cancer. 2018;18:1019. doi: 10.1186/s12885-018-4936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El Gendi S.M., Mostafa M.F., El Gendi A.M. Stromal caveolin-1 expression in breast carcinoma. Correlation with early tumor recurrence and clinical outcome. Pathol Oncol Res. 2012;18:459–469. doi: 10.1007/s12253-011-9469-5. [DOI] [PubMed] [Google Scholar]
- 86.Elsheikh S., Green A., Rakha E., Samaka R., Ammar A., Powe D. Caveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99:327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witkiewicz A.K., Dasgupta A., Sotgia F., Mercier I., Pestell R.G., Sabel M. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim T.H., Jiang H.H., Youn Y.S., Park C.W., Tak K.K., Lee S. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403:285–291. doi: 10.1016/j.ijpharm.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 89.Zheng Y., Wu J., Shan W., Wu L., Zhou R., Liu M. Multifunctional nanoparticles enable efficient oral delivery of biomacromolecules via improving payload stability and regulating the transcytosis pathway. ACS Appl Mater Interfaces. 2018;10:34039–34049. doi: 10.1021/acsami.8b13707. [DOI] [PubMed] [Google Scholar]
- 90.Fichter K.M., Ingle N.P., McLendon P.M., Reineke T.M. Polymeric nucleic acid vehicles exploit active interorganelle trafficking mechanisms. ACS Nano. 2013;7:347–364. doi: 10.1021/nn304218q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Munsell E.V., Ross N., O Sullivan M. Journey to the center of the cell: current nanocarrier design strategies targeting biopharmaceuticals to the cytoplasm and nucleus. Curr Pharmaceut Des. 2016;22:1227–1244. doi: 10.2174/1381612822666151216151420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rejman J., Bragonzi A., Conese M. Role of clathrin-and caveolae-mediated endocytosis in gene transfer mediated by lipo-and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 93.Ross N.L., Sullivan M.O. Overexpression of caveolin-1 in inflammatory breast cancer cells enables IBC-specific gene delivery and prodrug conversion using histone-targeted polyplexes. Biotechnol Bioeng. 2016;113:2686–2697. doi: 10.1002/bit.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nehoff H., Parayath N.N., Taurin S., Greish K. The influence of drug loading on caveolin-1 mediated intracellular internalization of doxorubicin nanomicelles in vitro. J Nanomed Nanotechnol. 2014;5:197. [Google Scholar]
- 95.Wei X., Wei R., Jiang G.Y., Jia Y.J., Lou H., Yang Z.Y. Mechanical cues modulate cellular uptake of nanoparticles in cancer via clathrin-mediated and caveolae-mediated endocytosis pathways. Nanomedicine. 2019;14:613–626. doi: 10.2217/nnm-2018-0334. [DOI] [PubMed] [Google Scholar]
- 96.Xiang S., Sarem M., Shah S., Shastri V.P. Liposomal treatment of cancer cells modulates uptake pathway of polymeric nanoparticles by altering membrane stiffness. Small. 2018;14:17042–17045. doi: 10.1002/smll.201704245. [DOI] [PubMed] [Google Scholar]
- 97.Dixit S., Sahu R., Verma R., Duncan S., Giambartolomei G.H., Singh S.R. Caveolin-mediated endocytosis of the Chlamydia M278 outer membrane peptide encapsulated in poly (lactic acid)-poly (ethylene glycol) nanoparticles by mouse primary dendritic cells enhances specific immune effectors mediated by MHC class II and CD4+ T cells. Biomaterials. 2018;159:130–145. doi: 10.1016/j.biomaterials.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]