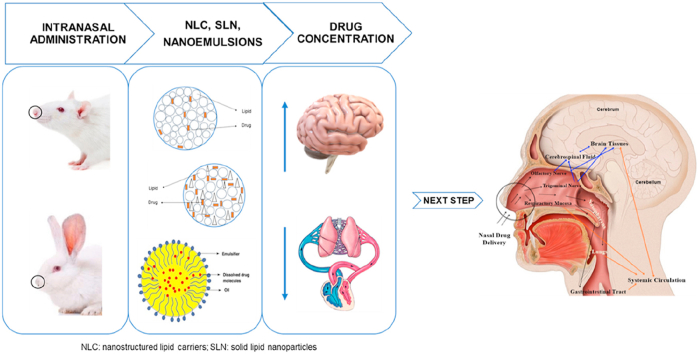
Abstract
The management of the central nervous system (CNS) disorders is challenging, due to the need of drugs to cross the blood‒brain barrier (BBB) and reach the brain. Among the various strategies that have been studied to circumvent this challenge, the use of the intranasal route to transport drugs from the nose directly to the brain has been showing promising results. In addition, the encapsulation of the drugs in lipid-based nanocarriers, such as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) or nanoemulsions (NEs), can improve nose-to-brain transport by increasing the bioavailability and site-specific delivery. This review provides the state-of-the-art of in vivo studies with lipid-based nanocarriers (SLNs, NLCs and NEs) for nose-to-brain delivery. Based on the literature available from the past two years, we present an insight into the different mechanisms that drugs can follow to reach the brain after intranasal administration. The results of pharmacokinetic and pharmacodynamics studies are reported and a critical analysis of the differences between the anatomy of the nasal cavity of the different animal species used in in vivo studies is carried out. Although the exact mechanism of drug transport from the nose to the brain is not fully understood and its effectiveness in humans is unclear, it appears that the intranasal route together with the use of NLCs, SLNs or NEs is advantageous for targeting drugs to the brain. These systems have been shown to be more effective for nose-to-brain delivery than other routes or formulations with non-encapsulated drugs, so they are expected to be approved by regulatory authorities in the coming years.
KEY WORDS: Nose-to-brain delivery, Intranasal administration, Nanostructured lipid carriers, NLC, Solid lipid nanoparticles, SLN, Nanoemulsions, In vivo studies, Pharmacokinetic, Pharmacodynamics
Graphical abstract
Intranasal delivery of nanostructured lipid carriers (NLC), solid lipid nanoparticles (SLN) and nanoemulsions in rats and rabbits resulted in higher drug concentration in the brain, compared to blood, proving the effectiveness of nose-to-brain delivery.
1. Introduction
The recent focus of research on alternative routes to improve drug targeting for the management of central nervous system (CNS) disorders has become essential, due to the unsatisfactory results of other routes, such as the parenteral and oral. In this context, the nose-to-brain route emerges as a promising alternative that allows drug passage directly from the nose to the brain, avoiding the need to cross the blood‒brain barrier (BBB)1. The latter is a complex barrier formed by tightly connected endothelial capillary cells, pericytes, astroglia and perivascular mast cells, which protects the brain from pathogens and xenobiotics and prevents the passage of about 98% of the molecules, from the blood to the brain2, 3, 4. Lipophilic and low molecular weight molecules are the only molecules that can easily cross BBB endothelial cells5,6. According to the US Food and Drug Administration (FDA), more than 90% of new drugs used to treat CNS diseases have not been approved due to the difficulty of crossing the BBB7. Besides, the CNS has other barriers, including the blood-cerebrospinal fluid (CSF), the blood‒spinal cord and the blood‒retinal barrier8,9.
Thereby, the intranasal route was suggested as a promising approach to improve the delivery of drugs to the CNS avoiding the need to cross the BBB. This route has shown several advantages, including high drug bioavailability, non-invasiveness, high blood flow, large surface area available for drug absorption, easiness of application, rapid onset of action, avoidance of gastrointestinal and hepatic metabolism and, as mentioned, possibility to circumvent the BBB10, 11, 12. However, several factors can limit the maintenance of the drug in the nasal mucosa and must be considered when developing an intranasal formulation10, including short residence time (15–30 min) due to the mucociliary clearance mechanism, low drug permeability in the nasal mucosa, small volume available for administration (up to 200 μL per nostril), and enzymatic degradation. Furthermore, intranasal formulations should have physiological tonicity, adequate viscosity and pH compatible with the nasal mucosa (5.0–6.5), using biocompatible and odorless excipients1,6,7,11,13. Accordingly, permeation and absorption enhancers, enzyme inhibitors, mucoadhesive agents and hydrogel systems have been included in nasal formulations to improve drug absorption and permeability, and increase the residence time in the nasal mucosa13. In addition, the use of lipid-based nanocarriers also improves drug permeability and absorption and protects drug from enzymatic degradation14.
The inclusion of intranasal formulations in specific devices that direct the formulation to the appropriate region of the nasal cavity, specifically, to the upper and posterior part where the olfactory region is located, has also been used to improve the residence time of the formulations, preventing the loss of drug that may occur after administration10,15. There are several marketed devices used to target drugs from the nose to the brain, such as OptiMist™, ViaNasa™, Optinose®, Breath Powered™, SipNose and Precision Olfactory Delivery (POD®)16, 17, 18, 19, 20, 21, 22, 23. This review provides a state of the art of in vivo studies with lipid-based nanocarriers [solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) and nanoemulsions (NEs)] for the delivery of drugs from the nose to brain, through the analysis of the pharmacokinetic, pharmacodynamics and brain targeting efficiency studies published in the past two years. The first sections describe the anatomy and physiology of the human nasal cavity and the mechanisms involved in nose-to-brain transport. Subsequently, the main characteristics of NLCs, SLNs and NEs for intranasal administration are described, followed by an extensive analysis of the most relevant outcomes of in vivo studies with these nanocarriers. Finally, the number of ongoing clinical studies involving intranasal medicines is presented.
2. Intranasal route
The anatomy, physiology and defence mechanisms of the nasal cavity should be considered when developing an intranasal formulation.
2.1. Anatomy of the nasal cavity
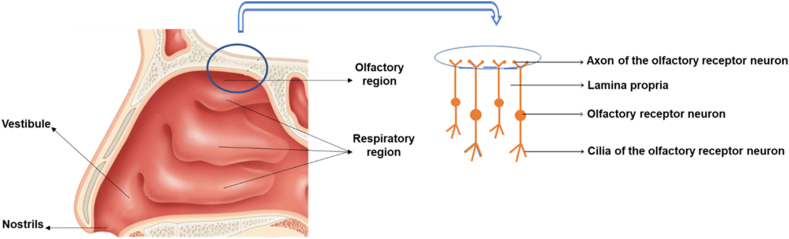
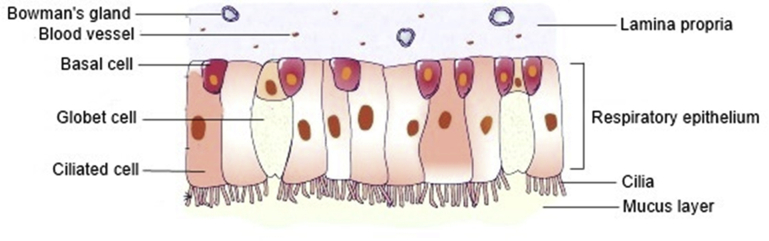
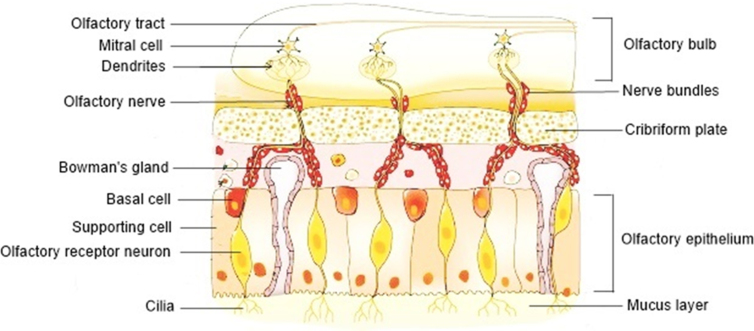
The nose is responsible for olfaction, regulation of temperature and humidity of the inhaled air and removal of external pathogens. The nasal cavity presents a total surface area of about 160 cm2, which has a volume of 13.0 mL and a length of 12–14 cm, from the nostrils to the nasopharynx, being one of the smallest organs in the human body15,21,24. The nasal septum divides the nose in two cavities that are subdivided in three different regions: vestibule, respiratory region and olfactory region (Fig. 1)24,25. The nasal cavity is lined by squamous, transitional, respiratory and olfactory epithelia1,15. The frontal part of this cavity is the vestibule, which is the first defence to the entrance of pathogens into the body. It is the less permeable region, due to the low vasculature, small surface area (about 0.6 cm2) and presence of mucus and nasal hairs (vibrissae) that filter large air particles. This area is covered by a squamous epithelium that contains sweat and sebaceous glands6,12,21. This nasal region is of little interest for drug administration studies26,27. In contrast, the respiratory region is the biggest of the nasal cavity, with a surface area of approximately 130 cm2, being the most vascularized and permeable region, which is attractive for drug absorption. This region contains the inferior, middle and superior turbinates that are lined by the respiratory epithelium, also known as ciliated pseudostratified columnar epithelium, which is composed by the basal, goblet, ciliated and non-ciliated columnar cells (Fig. 2)15,16,26,28. The goblet cells are responsible for mucin secretion, whereas the basal cells are defence cells responsible for epithelial recovery upon injury, being capable to differentiate in other cell types within the respiratory epithelium25. About 15%–20% of these respiratory cells are covered by cilia, which move co-ordinately and drive the mucus from the anterior part of the nasal cavity to the pharynx that further eliminates this mucus through the gastrointestinal tract27,29. This mechanism is called mucociliary clearance, being responsible for protecting the respiratory tract from harmful exogenous substances that are entrapped in the mucus and eliminated through this renewal process each 15–30 min6. In addition, the numerous microvilli present in this region provide a large surface area, increasing drug absorption to the systemic circulation. The lamina propria situated above the respiratory epithelium is responsible for the drug absorption, due to the presence of blood vessels, capillaries and nerves. Additionally, this region is innervated by the ophthalmic and maxillary branches of the trigeminal nerves, which represents a direct pathway to the CNS25. The olfactory region (Fig. 1) is located in the upper part of the nasal cavity, above the superior nasal turbinate of the respiratory region and under the cribriform plate of the skull, being the unique region that directly connects the nasal mucosa to the brain, representing a direct access for the drugs entering the CNS via the olfactory bulb. This region, with a surface area of 10 cm2, is also a direct pathway for the CSF1,10,30. The cribriform plate, a bone structure composed by little pores with neuronal bundles, allows the drug passage from the olfactory epithelium into the CNS24. The olfactory epithelium contains three types of cells, including basal cells, supporting cells and olfactory receptor neurons (Fig. 3)15. The latter are responsible for the connection between the nasal cavity and the olfactory bulb12. The lamina propria, located under the epithelial membrane, contains the olfactory axon bundles, lymphatics vessels, mucus secreting Browman's glands and the maxillary branch of the trigeminal nerve28.
Figure 1.
Longitudinal perspective of the human nasal cavity (left) and configuration of the olfactory region (right).
Figure 2.
Structure of the respiratory region of the nasal cavity. Adapted from Ref. 28 with permission from Elsevier.
Figure 3.
Structure of the olfactory region of the nasal cavity. The olfactory receptor neurons are embedded in the supporting cells, while the basal cells and the Bowman's glands are embedded in the lamina propria. Bowman's gland produces and secrets the mucus. The nerve bundles penetrate the cribriform plate and extend until the olfactory bulb. Adapted from Ref. 28 with permission from Elsevier.
2.2. Pathways of drug delivery
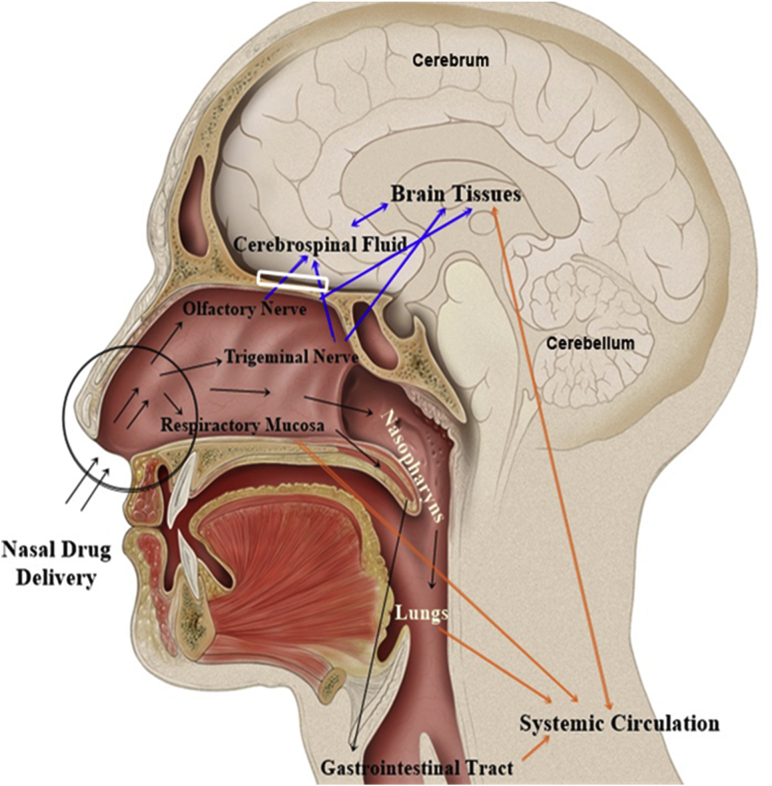
The mechanisms of drug delivery from the nose to the brain have been extensively investigated. Much evidence has shown that the drug can reach the brain by one of three different pathways or by a combination of these (Fig. 4). In the indirect route (also called systemic pathway), the drug is absorbed by the nasal mucosa into the systemic circulation before reaching the brain. In the direct route, the drug passes through the olfactory and/or trigeminal nerves and reaches the brain. The drug permeation in the nasal mucosa depends on several characteristics, such as drug solubility, formulation residence time, metabolic stability and rate of mucociliary clearance10,31. Besides, it is extremely important to evaluate the safety and toxicity of the drug in the nasal mucosa to avoid irritation, ciliotoxicity and/or tissue damage. Furthermore, after intranasal administration, the drug needs to circumvent the mucociliary clearance mechanism before reaching the olfactory region. This process can lead to the loss of some drug before reaching the therapeutic effect (Fig. 4)16.
Figure 4.
Schematic representation of the different drug pathways to the brain, following intranasal administration. After nasal drug instillation (black arrows), drug clearance can occur at a certain extent by the mucociliary clearance mechanism in the vestibular region (black circle). The drug that remains in the nasal cavity (black arrows) diffuses to the posterior region. Here, the drug that reaches the olfactory region becomes available for transport to the brain via the olfactory nerves through the olfactory bulb (white rectangle), following a direct route (blue arrows). The drug that reaches the respiratory region is available for transport to the brain via trigeminal nerve (blue arrows), or diffuse into the systemic circulation, following an indirect route (orange arrows). The drug that reaches the systemic circulation needs to go through the BBB to reach the brain. Adapted from Ref. 16 with permission from Elsevier.
2.2.1. Nose-to-brain transport
There are two pathways for the direct drug transport from the nose to the brain (Fig. 4): via the olfactory region (olfactory pathway) and via the trigeminal region (trigeminal pathway). Herein, the olfactory pathway leads to a faster brain targeting of the drug (approximately 0.33 h), when compared to the trigeminal pathway (approximately 1.7 h)21.
2.2.1.1. Olfactory pathway
The olfactory pathway includes the olfactory epithelium, anterior olfactory nucleus, olfactory tract, amygdala, hypothalamus and piriform cortex31,32. This region is lined by a mucosal layer of olfactory receptor neurons, supporting cells and basal cells33. The supporting cells surround the olfactory receptor neurons, providing mechanical support to the olfactory epithelial cells and have microvilli that have an important function in the sensory stimulation of the olfactory mucosa28. The basal cells are responsible for the maintenance and recovery of olfactory mucosa21,28. The olfactory receptor neurons originate in the nasal olfactory epithelium, pass through the holes in the cribriform plate of the ethmoid bone and reach the olfactory bulb where, through the axons, facilitate a continuous communication between the bulb and the nasal cavity12,34. The olfactory receptor neurons are responsible for mediating the sense of smell in the nasal cavity, transmitting sensorial information to the CNS26,32.
Fig. 5 describes the different routes of nose-to-brain delivery in the olfactory mucosa. Briefly, the drug passes along the olfactory nerves through the olfactory bulb via intraneuronal and extraneuronal transport into the olfactory cortex and, from here, to the CNS, specifically, to the cortex, cerebrum and cerebellum15. The intraneuronal transport requires passage through the axons and is slow, taking hours or even days for the drug to reach its target site. In contrast, the extraneuronal transport is faster and follows paracellular and transcellular transport, taking a few min for the drug to reach the olfactory bulb and the CNS1,15. The drug can also cross the olfactory mucosa through the supporting cells (transcellular transport) or along with the supporting cells (paracellular transport). In both cases, the drug passes through the lamina propria25. In the paracellular transport, the drug passes through the tight junctions, such as occludin, claudin and zonula occludens21. Generally, hydrophilic drugs undergo this transport, while lipophilic drugs pass by transcellular transport, through receptor-mediated endocytosis or passive diffusion27. Thereby, the drug transport from the olfactory region to the CNS can occur within the nerves or outside of them, although it is more likely that the transport of the drug results from a combination of the different routes instead of taking a single route25.
Figure 5.
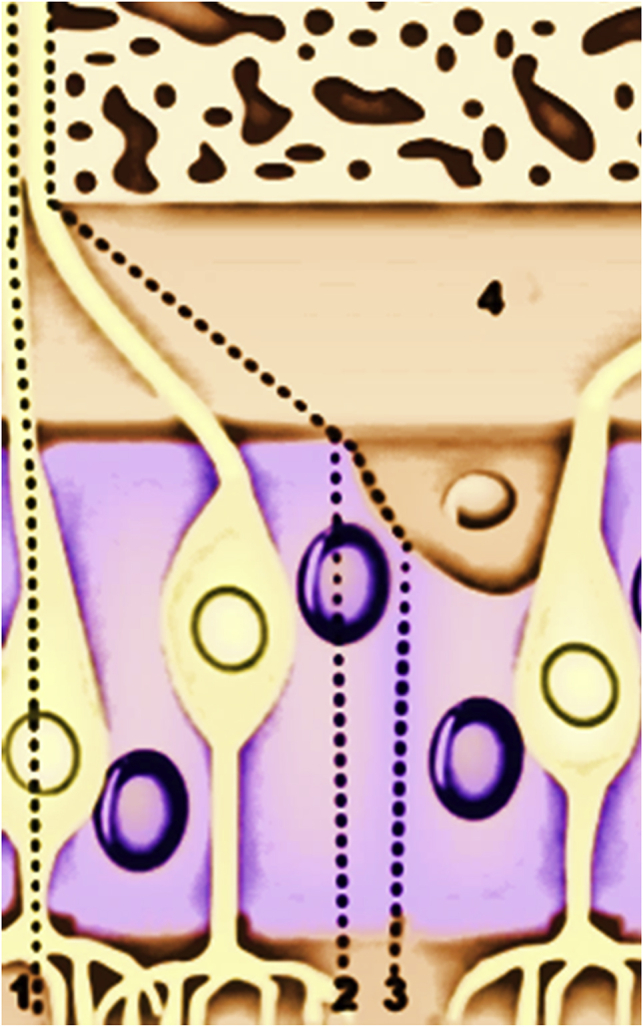
Different routes of nose-to-brain delivery in the olfactory mucosa. (1) Drug transport through the olfactory receptor neuron (intraneuronal transport); (2) Drug transport through the supporting cells (extraneuronal transcellular transport); (3) Drug transport along the supporting cells (extraneuronal paracellular transport); (4) Lamina propria.
2.2.1.2. Trigeminal pathway
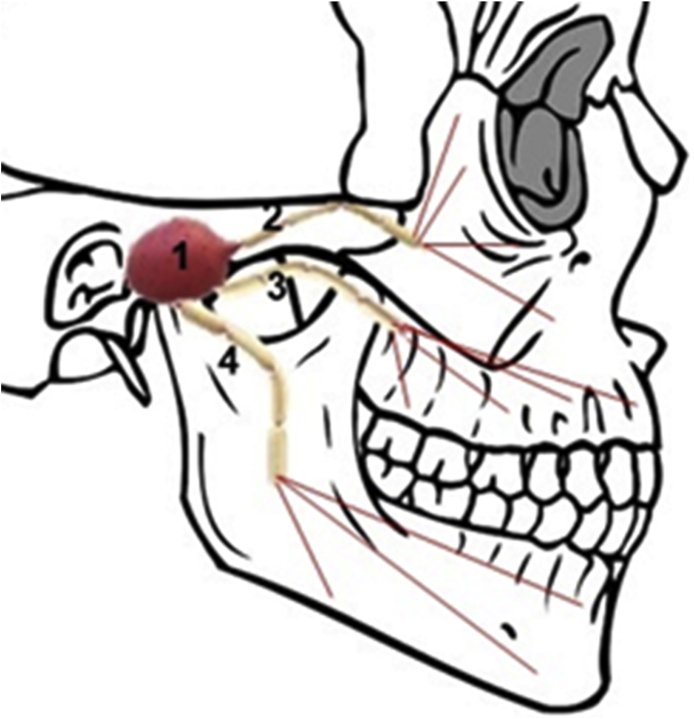
The trigeminal pathway comprises the transport through the trigeminal nerve, which follows endocytic and axonal transport (i.e., intracellular transport)15. The trigeminal nerve (Fig. 6) is the largest cranial nerve that innervates the olfactory and respiratory epithelium31,32 and has three different branches (mandibular, ophthalmic and maxillary), which merge in the trigeminal ganglion, enter in the CNS and finishes in the brainstem31. The maxillary and ophthalmic branches of the trigeminal nerve are very important for the nose-to-brain delivery, since they connect the nasal cavity to the CNS5,15. The drugs can reach the brain through the trigeminal nerve via respiratory epithelium, entering to the brainstem, and via dorsal olfactory epithelium, through the cribriform plate, translocating the drug to the rostral and caudal parts of the brain1,5,32. The trigeminal nerve is responsible to transport the sensorial information from the nasal cavity to the cornea, eyelids, oral cavity and CNS, via mandibular, ophthalmic and maxillary divisions32. Thorne et al.35 used a rat model to demonstrate the delivery of the insulin-like growth factor 1 factor (IGF-1) through this pathway to the spinal cord and to the brainstem. Johnson et al.36 also showed that intranasal lidocaine achieved the brain within 10 min via the trigeminal pathway.
Figure 6.
Anatomy of the trigeminal nerve and its terminal branches. (1) Trigeminal nerve; (2) Ophthalmic branch; (3) Maxillary branch; (4) Mandibular branch.
2.2.2. Systemic pathway
The systemic pathway is an indirect route, where the drugs pass to the lungs and to the blood circulation before reaching the brain (Fig. 4). Therefore, in this route, the drugs have to cross the BBB to attain the brain, which increases the time needed to achieve the therapeutic effect and limits the amount of drug that effectively reaches the brain11,31. Furthermore, the amount of drug in the brain following intranasal administration differs between patients and suffers elimination via renal and hepatic mechanisms6,15. Thereby, drugs can reach the brain via the direct route and via the systemic route, which depends largely on the properties of the drug. For instance, some lipophilic drugs enter the brain by the systemic pathway after intranasal administration6.
2.2.3. Challenges of the nose-to-brain drug delivery
Following intranasal administration, the transport of the drugs from the nose to the brain is challenging, due to the anatomical and physiological characteristics of the nasal cavity. Herein, several questions remain unanswered, such as “How can we effectively target drugs from the nose to the brain?” “How do we know which pathway (direct, indirect or both) drugs take from the nose to the brain?”
Among the problems to be solved is the volume of the nasal cavity, which only allows the administration of a small volume of formulation, restricting the amount of drug that can reach the brain. The mechanism of mucociliary clearance, which reduces the residence time of the drug in the nasal mucosa, directly affects the success of nose-to-brain transport. Other barriers of the nasal mucosa that reduce the amount of drug absorbed, such as enzymes and transport mechanisms. To avoid these drawbacks, it is necessary to take into account different factors, including the nasal delivery device and the drug molecular weight and solubility. The use of a suitable nasal device, the positioning of that device at a correct angle in conjunction with the proper position of the head during administration can influence the deposition of the drug within the nasal cavity. This facilitates the targeting of the drug to the upper region of the nasal cavity, where it can reach the trigeminal nerve and the olfactory bulb, thus promoting the passage to the brain. In addition, more in vivo studies should be carried out to monitor the transport of the drug to the brain after nasal administration, so that it is possible to verify the path they follow6,16,17,37,38.
3. Main features of intranasal NLCs, SLNs and NEs
Regarding their features, NLCs, SLNs and NEs have been showing high potential to improve the nose-to-brain drug delivery (Table 1). These systems have high stability and ability to penetrate biological membranes, which are related to the presence of emulsifiers and their small sizes, respectively. Moreover, they provide sustained drug release, drug protection from chemical and/or enzymatic degradation and increased bioavailability11,39. It is possible to add bioadhesive excipients (e.g., gelling agents and viscosity enhancers) to the formulations of NLCs, SLNs or NEs to increase their residence time within the nasal mucosa and, and therefore, the drug transport through the olfactory neurons, and also modulators to open the tight junctions between the nasal cells, improving the drug passage7,10. Despite the advantages described in Table 1 for SLNs, NLCs and NEs, these nanocarriers have disadvantages, including limited capacity to transport hydrophilic drugs; in vitro and in vivo instability; poor stability during storage, due to polymorphic transitions of lipids that cause the release of encapsulated drugs, the occurrence of aggregation of the nanoparticles and phase separation6,51,61. These disadvantages were identified as the main obstacles to the scaling-up and approval of the SLN, NLC and NE formulations. Several strategies have been studied to overcome these disadvantages. For example, to avoid the aggregation of nanoparticles during storage, the use of cationic or anionic surfactants that increase the absolute value of ZP, led to an increase in the electrostatic repulsion of the nanoparticles. The coating of the nanoparticles with polyethylene glycol (PEG) or its derivatives to increase the circulation time of the nanoparticles in the blood stream, which improves the bioavailability of the drug41,48.
Table 1.
Characteristics of lipid-based nanocarriers to improve nose-to-brain drug delivery.
| Lipid-based nanocarrier | General characteristic to improve drug delivery | Specific characteristic to improve nose-to-brain drug delivery | Ref. |
|---|---|---|---|
| 1. Lipid nanoparticles |
|
|
39, 40, 41, 42 |
1.1. Solid lipid nanoparticles (SLNs) 
|
|
40, 43, 44 | |
1.2. Nanostructured lipid carriers (NLCs) 
|
|
40, 45, 46 | |
2. Nanoemulsions (NEs) 
|
|
47 |
To guarantee the quality of the final pharmaceutical products containing NLCs, SLNs and NEs is important the implementation of a robust control system. For this, the critical quality attributes (CQAs) of the formulation must be evaluated, such as particle/droplet size, polydispersity index (PDI), zeta potential (ZP) and encapsulation efficiency (EE)49, 50, 51. These CQAs can change with the type and concentration of lipid(s) and emulsifier(s) and with the production techniques used, including emulsification speed and time, sonication amplitude and time and high-pressure homogenization cycles and pressure40,43. In this area, the Quality-by-Design (QbD) approach is a useful tool to define the critical steps required to obtain the best CQAs that facilitate the scaling up of the manufacturing processes. Examples of typical QbD attributes used are the critical material attributes (CMAs), which are related to the optimization of the excipients, such as lipid(s) and emulsifier(s); and the critical process parameters (CPPs), which are related to the parameters of the equipment used during the production of the formulations50,51.
Regarding the CQAs of intranasal delivery, the particle/droplet size, which corresponds to the mean hydrodynamic diameter of the particles/droplets, should have a narrow nanometric range, since this is a crucial parameter to guarantee the transport of encapsulated drugs via olfactory neurons. In this sense, it has been described that the size of the intranasal nanocarriers must be lower than 200 nm10,52. The PDI that shows the size distribution of the particles in the formulations ranges between 0 and 1. In a monodisperse distribution, the PDI value is close or equal to 0, while in a polydisperse distribution the PDI value is close or equal to 1. Low PDI and particle size values promote a uniform drug absorption through the nasal mucosa. Usually, the PDI values of intranasal nanocarriers are less than 0.3, although NLC formulations generally have higher values than NE, due to the asymmetry of the non-spherical lipid nanoparticles14,43,52,53. However, it is important to note that formulations with the same PDI values can behave differently, since they can have different particle sizes. For example, if two formulations have the same PDI, but the particle size is 100 nm for one formulation and 234 nm for the other formulation, their behaviour will be different after intranasal administration. Thus, it is important to analyse together the particle size and the PDI. The ZP corresponds to the surface charge of the nanoparticles/nanodroplets that predicts the long-term physical stability of the formulation, and depends on environmental conditions, such as pH and ionic strength. It has been described that positively charged nanoparticles/nanodroplets originate higher drug accumulation in the brain, due to the occurrence of electrostatic bonds with the negatively charged membrane of the nasal mucosa45,54, 55, 56. In general, a ZP value of ±30 mV indicates good stability of the nanocarriers43,52,57. The EE estimates the amount of drug encapsulated in the nanoparticles/nanodroplets and is used to confirm the suitability of the nanocarriers to incorporate the drugs. Generally, an EE higher than 80% is required for intranasal lipid-based nanocarriers46. Other important challenge is to maintain the CQAs values stable during storage, which enables the estimation of the expiration date of the final pharmaceutical formulation. It is also extremely important to evaluate the toxicity of the nanoparticles/nanodroplets in the brain and the drug release profile, which is useful to predict the in vivo performance7. The drug release depends on the medium, temperature and some characteristics of the nanocarriers, such as size, shape, surface charge, type of lipids and emulsifiers, drug location in the nanoparticles/nanodroplets, partition coefficient and production method54.
4. In vivo experiments with intranasal formulations
Before starting clinical studies, it is necessary to evaluate the effectiveness of intranasal formulations in animal models. However, it is difficult to extrapolate the data from these studies (e.g., drug absorption and pharmacokinetics/pharmacodynamics parameters) for humans, due to differences in the nasal anatomy and physiology between the species.
4.1. Characteristics of the nasal cavity of the different animal models
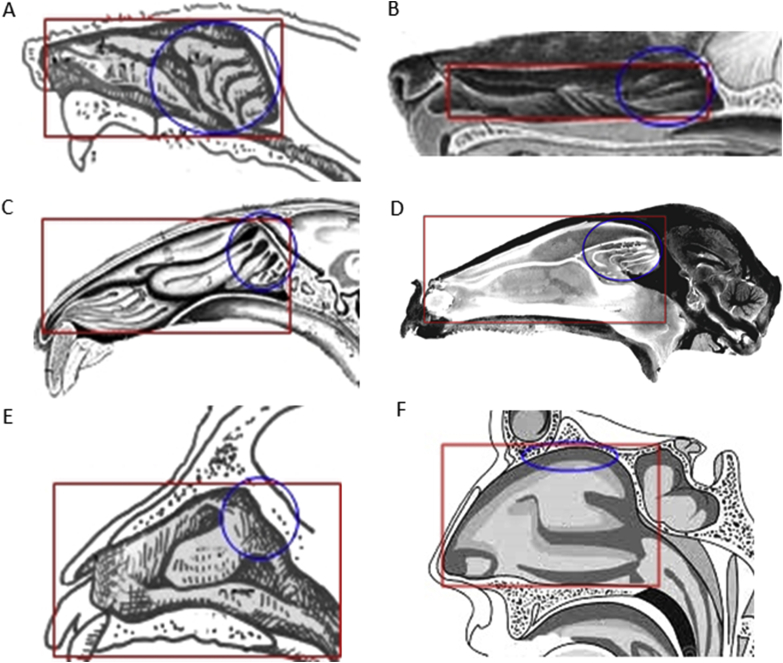
Selecting a suitable in vivo model is crucial for an efficient evaluation of a nasal formulation and its respective delivery system. For this, it is important to know the animal nasal anatomy and physiology before starting the studies (Table 2 and Fig. 76,58, 59, 60).
Table 2.
| Species | Mean nasal surface area (cm2) | Mean nasal cavity volume (mL) | Conchae structure |
|---|---|---|---|
| Mouse | 2.8 | 0.03 | Double scroll |
| Rat | 14 | 0.4 | Double scroll |
| Rabbit | 61 | 6 | Branched conchae |
| Monkey | 62 | 8 | Single scroll |
| Human | 160 | 20 | Single scroll |
| Dog | 210 | 20 | Branched conchae |
| Sheep | 327 | 114 | Double scroll |
Figure 7.
Schematic representation of the differences in the surface area of the nasal cavity and in the olfactory region between the animals most used in in vivo studies and humans. (A) rat; (B): dog; (C): rabbit; (D) sheep; (E) monkey and (F) human. The olfactory region (blue circles) occupies 10% of the nasal cavity (red rectangles) of rabbits, sheep, monkeys and humans, while in rats and dogs, it occupies most of the nasal cavity. Adapted from Refs. 6, 58, 59 with permission from Elsevier and Ref. 57 with permission from John Wiley and Sons.
Rats were the first model used for in vivo studies and, afterwards, mice, dogs, rabbits, monkeys and sheep were used. Nonetheless, most reported in vivo studies were conducted in rats. The most common animal types for in vivo studies with intranasal formulations are Wistar and Sprague–Dawley rats, BALB/c and CD1 (mice), New Zealand and Japanese white rabbits, Labrador and Retriever dogs, Harley guinea pigs and Karaman and Sufolk sheep25. The volume administered per nostril differs between animal types. For example, Wistar rats can be administered with a volume of 40 μL, whereas Sprague–Dawley rats bear 50 μL25. The tools for administration also differ between species. For small size animals, formulations are usually administered using a pipette/micropipette or an intranasal canula, while in large size animals are used nasal devices, such as atomizers and sprays25. Furthermore, the animal head position at the moment of the administration is extremely important61,62. For rats and mice, the supine position seems to increase the probability of the drug reaching the olfactory region63,64.
Based on anatomical nasal differences between different types of animals, rats are the ones that most differ from humans, not being a good model for pharmacokinetic and pharmacodynamics studies. However, most published studies have been carried out on rats and mice. The main reasons for this are the low cost and easy access to these animals, compared to other species. Sometimes the choice of the animal depends on the study type and the monetary capacity of the laboratory. For toxicity studies, the small animals, as rats and mice, are suitable. However, large animals, such as rabbits and dogs, are more useful for pharmacokinetic and pharmacodynamics studies25.
The extrapolation of the in vivo results from animals to humans is directly affected by the differences in the olfactory region between species, such as the metabolic activity and permeability of the nasal mucosa. Apart from the nasal anatomical differences between animals and humans, the nasal absorption site, drug distribution and deposition in the nasal mucosa also interfere33.
The olfactory region comprises 10% of the nasal cavity in humans and represents 50% and 45% of the total nasal surface area in rats and mice, respectively12,34. In addition, the drug administration in rats and mice is difficult, due to the small nasal orifices. Rabbits, dogs, sheep, and monkeys are more suitable for pharmacokinetic and pharmacodynamics studies compared to the rats and mice, due to the larger nose orifice that facilitates intranasal administration10. Furthermore, in these models, it is possible to perform pharmacokinetic and pharmacodynamics studies in the same animal, after a washing and recovery period, without anesthesia, which is not possible with rats and mice. Usually, rabbits are preferred to the dogs, sheep and monkeys, due to the relatively small cost33. Monkeys have an olfactory region similar to humans, which is advantageous. However, these animals are extremely expensive and contested by animal rights groups25,33,65. Rabbits's olfactory region area is also similar to humans66. However, the volume administered per nostril in rabbits and monkeys is approximately 58 μL, which is three times smaller than the volume administered to humans. In contrast, dogs have a volume of administration per nostril (207 μL) higher than humans25,33. Concerning the olfactory region, dogs have a surface area of 150 cm2, which is 15 times higher than that of humans, occupying 77% of the nasal cavity of dogs, against 10% in humans27,67. It was also reported that this region represents half of the nasal cavity in rats and mice12,34.
4.2. Pharmacokinetic and pharmacodynamics studies and translation to humans
In vivo studies allow predicting the pharmacokinetic, pharmacodynamics and absorption profiles in humans of intranasal drugs25. These studies are performed before clinical experiments and are used to observe the drug fate in the brain after nose-to-brain transport and to evaluate the safety and toxicity of intranasal formulations68. Pharmacokinetics includes the effects of the body in the drug, describing absorption, distribution, metabolization and elimination, while pharmacodynamics are related to the effects of the drug in the body69, 70, 71. Data from pharmacokinetic studies report the amount of drug in the brain and plasma, which is calculated by measuring drug concentrations at predetermined time intervals. These studies also evaluate the therapeutic action and toxicity of the drug in the target-site72. The in vivo drug nasal absorption is affected by several biological factors, such as physiological conditions (secretions, mucociliary clearance, pH and blood supply), pathological conditions, biochemical changes and environmental factors (temperature and humidity). Besides, drug absorption is affected by some physicochemical properties of the nasal formulations, including pH, viscosity, osmolality, drug distribution, type of pharmaceutical dosage form, amount of formulation administered, and drug molecular weight, lipophilicity, solubility, partition and dissociation coefficients. As mentioned, the characteristics of the nanocarriers (i.e., particle/droplet size, PDI, ZP and EE) and the deposition site in the nasal cavity also influence the nasal absorption of the drug33,73. Thereby, all of these factors affect the pharmacokinetic and pharmacodynamics results.
4.3. Relevant outcomes with nanostructured lipid carriers (NLC), solid lipid nanoparticles (SLN) and nanoemulsions (NE)
The most relevant outcomes of in vivo studies with intranasal NLC, SLN and NE published in the last 2 years are summarized in Supporting Information Table 1, through the results of pharmacokinetic and pharmacodynamics studies in different animal models. Diverse parameters were evaluated in the in vivo studies, such as the Cmax (maximum drug concentration), the Tmax (time required to reach the maximum drug concentration); the AUC (area under the curve); the mean retention time of the formulation in the brain and the brain/blood ratio (i.e., the bioavailability of the drug in the brain). In addition, the drug targeting efficiency (DTE), which measures the accumulation in the brain after intranasal administration compared to intravenous administration, and the drug targeting potential (DTP), which measures the amount that reaches the brain by olfactory pathway and/or trigeminal pathways (i.e., direct nose-to-brain transport) were also calculated through the following Eqs. (1), (2) 14,30,62,74,75:
| (1) |
| (2) |
where, the (AUCbrain)IN is the drug concentration in brain following intranasal (IN) administration; (AUCbrain)IV is the drug concentration in brain following intravenous (IV) administration; the (AUCblood)IN is the drug concentration in blood following IN administration; the (AUCblood)IV is the drug concentration in blood following IV administration.
DTE and DTP values range between 0 to +∞ and ‒∞ to 100, respectively. DTE higher than 100 means more effective drug brain targeting through intranasal administration versus intravenous administration, while DTP values above 0 mean the occurrence of drug brain targeting through the olfactory and/or trigeminal pathways6,14. A DTP value of 100 means that no drug was absorbed through the indirect route (i.e., from the systemic circulation) after intranasal administration14.
From Table 3 it can be observed that most studies with intranasal formulations of NLCs, SLNs and NEs were conducted in rats, although experiments with mice and rabbits have also been reported. In some of these studies, the animals had free access to water and food, while in other studies the animals were fasted overnight. All animals were anesthetized before the instillation of the formulation with a micropipette. Due to the small size of the nostrils of rats and mice, some researchers attached a polyethylene cannula to the micropipette to facilitate insertion of the formulation into the nostrils. The animals were sacrificed at predefined intervals and blood and vital organs were collected to quantify the amount of drug. For the experiments, the animals were divided into two or more groups to facilitate the observation of the differences in distribution of the NLCs, SLNs and NEs in the brain and plasma after intranasal administration, over other administration routes, different pharmaceutical dosage forms or placebo formulations. From Table 3 it can also be observed that most researches aimed to improve the treatment of neurological diseases via nose-to-brain transport. In general, the data showed that, upon intranasal administration of NLCs, SLNs and NEs, was obtained a higher brain concentration of the drug, when compared to other routes or to the intranasal administration of free drug. These results show the advantage of using intranasal lipid-based nanocarriers to target the drugs to the brain over other administration routes, which is related to several factors63,76, 77, 78, 79: i) drug reaches the brain via direct route, avoiding hepatic metabolism and degradation through the gastrointestinal tract; ii) lipids promote the partition of the nanoparticles/nanodroplets in the nasal mucosa, increasing the retention time; iii) protection of the encapsulated drugs from nasal glycoprotein-P efflux proteins and enzymatic degradation; iv) electrostatic interactions between the nanocarriers and the nasal mucosa minimizes drug elimination through the mucociliary clearance mechanism; v) use of formulations with mucoadhesive agents increases the drug concentration in the brain, due to the improve on the residence time in the nasal mucosa. In contrast, when the drug was administered intravenously, intraperitoneally or orally, a higher drug concentration was observed in the blood over the brain.
Among the referred lipid-based nanocarriers, the NLCs were the most studied over the last 2 years, which could be attributed to its higher stability and ability to accommodate drugs, when compared to SLNs and NEs.
Before starting clinical studies, it is important to keep in mind that drug deposition in a specific area of the nasal cavity depends on several factors, including particle size, type of pharmaceutical dosage form, delivery device, head position at the time of the administration and spray angle. These factors also interfere with the amount of drug that reaches the brain after intranasal administration12,32.
In the following subsections a more detailed description of the most relevant studies presented in Table 3 is provided.
4.3.1. NLCs
In the past two years, about ten studies regarding NLCs have been published for nose-to-brain drug delivery, mainly for the treatment of neurological disorders. For instance, Pardeshi et al.62 evaluated the efficacy for the treatment of Parkinson's disease of a ropinirole-dextran sulfate with a mucoadhesive agent, after intravenous and intranasal administration, and a ropinirole hydrochloride suspension, after intranasal administration, to mice. Brain concentration of intranasal mucoadhesive NLCs (29.04 ± 0.04 μg/mL) was higher than intravenous mucoadhesive NLCs (17.37 ± 1.0 μg/mL) and intranasal ropinirole hydrochloride suspension (1.67 ± 0.02 μg/mL). AUC of intranasal NLCs was 181.17 ± 1.32 h∙µg/mL, while AUC of intravenous NLCs was 120.00 ± 3.55 h∙µg/mL and intranasal suspension was 13.18 ± 0.25 h∙µg/mL. Plasma concentrations of ropinirole were notably elevated for intravenous mucoadhesive NLCs (20.89 ± 0.81 μg/mL) with a Tmax of 30 min, compared to intranasal mucoadhesive NLCs (12.08 ± 0.06 μg/mL) and intranasal suspension (1.717 ± 0.02 μg/mL), both with a Tmax of 8 h. The AUC in the plasma was higher for intranasal NLCs (108.33 ± 2.2 h∙µg/mL) and intravenous NLCs (98.76 ± 1.45 h∙µg/mL), than for intranasal suspension (18.10 ± 0.15 h∙µg/mL). The mean retention time in the brain was similar for the three formulations, with values from 5.17 up to 5.93. However, in the plasma, the mean retention time was significantly higher for intranasal NLCs (7.28 ± 0.71 h) and suspension (6.13 ± 1.15 h) than intravenous NLCs (4.93 ± 0.94 h). DTE and DTP values for mucoadhesive NLCs were 221.02% and 20.71%, respectively. These results suggested that the inclusion of a mucoadhesive agent increases the brain targeting efficiency.
Rajput et al.80 studied the brain and plasma concentration of oral resveratrol suspension and intranasal in situ gel of gellan gum containing resveratrol-loaded NLC for the treatment of Alzheimer's disease in rats. The results showed that the brain/blood ratio after intranasal in situ gel of resveratrol-loaded NLC was 2.5-fold higher than oral suspension, with a Cmax in the brain of 1030 ± 135 and 402 ± 41 ng/mL, respectively. The Cmax in the plasma was much lower for the intranasal formulation (167 ± 20 ng/mL), compared to the brain concentration. The Tmax for the intranasal formulation was 30 min, whereas for the oral formulation was 2 h, indicating a faster resveratrol transport to the brain after intranasal administration. The AUC values were also higher in the brain for intranasal NLC (2572 ± 338 h∙ng/mL) and oral suspension (1809 ± 206 h∙ng/mL), compared to plasma concentration (558 ± 62 and 816 ± 94 h∙ng/mL, respectively). The drug biodistribution of both formulations showed a higher concentration of the in situ gel of resveratrol-loaded NLC in the brain, followed by spleen, lungs, kidney, heart and liver. In contrast, the resveratrol suspension exhibited higher concentration in the liver, followed by lungs, spleen, brain, heart and kidney, which could be attributed to the hepatic metabolism of the drug. The authors of this study that the addition of a gellan gum to prepare the in situ gel increased the residence time of the NLC in the nasal cavity80. Jojo et al.63 also studied the brain targeting of intranasal NLC for the treatment of Alzheimer's disease. In their study, the authors compared the brain targeting of pioglitazone-loaded NLC and pioglitazone solution after intranasal and intravenous administration and the results were similar to the ones obtained by Rajput et al80.
In other study, Singh et al.77 evaluated the efficacy of intravenous asenapine solution, intranasal asenapine solution and intranasal asenapine-loaded NLC coated with glycol chitosan for the treatment of schizophrenia in rats. The glycol chitosan, a junction between ethylene glycol and chitosan, acts as a steric stabilizer for NLCs and has a positive charge that interacts with mucin. The intranasal asenapine-loaded NLCs coated with glycol chitosan showed higher brain concentration, compared to intranasal and intravenous asenapine solution, with a Cmax of 94.93 ± 11.73, 53.34 ± 10.76 and 41.46 ± 7.57 ng/mL, respectively. Moreover, the time to attain the maximum concentration was faster for asenapine-loaded NLCs coated with glycol chitosan with 1 h, whereas for the others was 2 h. As expected, the opposite results were observed in the plasma, with a Cmax of 82.76 ± 14.78 ng/mL for intravenous asenapine, followed by intranasal asenapine-loaded NLCs coated with glycol chitosan (44.12 ± 2.92 ng/mL) and intranasal asenapine (33.65 ± 15.52 ng/mL). In the brain, the AUC was 4-fold higher for intranasal asenapine-loaded NLCs coated with glycol chitosan formulation (826.81 ± 78.29 h∙ng/mL), compared to intranasal asenapine (209.42 ± 42.48 h∙ng/mL) and intravenous asenapine (202.70 ± 35.65 h∙ng/mL). Besides, in the plasma the AUC was also higher for intranasal asenapine-loaded NLC coated with glycol chitosan, followed by intravenous asenapine and intranasal asenapine, with values of 163.62 ± 10.79, 115.63 ± 25.53 and 68.25 ± 21.34 h∙ng/mL, respectively. The NLC formulation demonstrated a higher ability to improve drug brain targeting, showing the highest DTE (2.88%) compared to the free drug (1.75%). Furthermore, the absolute bioavailability of the drug in the brain was nearly four times more for NLC formulation (407.89%) in comparison to free asenapine (103.31%) after intranasal administration. Based on these results, the authors concluded that the use of NLC coated with glycol chitosan increases the contact time of the formulation with the nasal mucosa and, consequently, increases the asenapine absorption showing a higher brain concentration. In addition, based on the absolute bioavailability of the drug in the blood and in the brain, the intranasal route has been shown to be more effective in improving the cerebral targeting of the drug, compared to the intravenous route77. Other authors have also demonstrated the effectiveness of using NLC intranasal formulations for the treatment of schizophrenia, showing similar results81,82.
The pharmacodynamics behavior of intranasal NLC formulations compared to other routes or other intranasal formulations was investigated. For example, Abbas et al.83 studied the use of an in situ gel of poloxamer 407 and sodium alginate to improve the brain delivery of clonazepam for epilepsy therapy. For the study, two different groups of mice were administered intranasally with an in situ gel of clonazepam-loaded NLC or with an in situ gel of clonazepam and superparamagnetic iron oxide nanoparticles-loaded NLC. Five min after the intranasal administration, pentylenetetrazol was injected in mice to induce clonic convulsions. The onset of time of the first clonic seizure and the time of death were recorded for the mice administered with both formulations and for the ones administered with pentylenetetrazol alone (control group). The results showed that the control presented 41.7 ± 5.6 s for the first clonic seizure and 113.5 ± 24.6 s to die. However, the group of animals administered with the in situ gel of clonazepam-loaded NLC exhibited a prolonged onset time of first clonic seizure (64.9 ± 6.3 s) and death (552.6 ± 102.3 s). Moreover, the incorporation of the superparamagnetic iron oxide nanoparticles in the clonazepam-loaded NLCs, prolonged 7.5 times the onset time of the first clonic seizure (313.6 ± 49.5 s) and delayed 14 times the onset time of death (1574.6 ± 272.8 s) compared to the control. These results suggest that the use of the intranasal route together with the nanoparticles are a promising combination for the treatment of epileptic seizures83. Hammad et al.84 evaluated the efficacy of oral mosapride tablets, intranasal mosapride suspension and intranasal mosapride loaded surface-modified NLC for gastroesophageal reflux disease in rabbits. The intranasal mosapride-loaded surface-modified NLC originated higher drug concentration in plasma, compared to intranasal suspension and oral tablets, with Cmax values of 13.19 ± 7.24, 8.62 ± 4.2 and 5.91 ± 1.84 μg/mL, respectively. Furthermore, the AUC of intranasal NLC formulation was 1.41-fold higher than intranasal suspension and 2.44-fold higher than oral tablets (22.78 ± 1.02, 16.18 ± 0.724 and 9.345 ± 0.418 h∙µg/mL, respectively). For the pharmacodynamics study, the estimating gastric emptying and rate of duod7enal contraction were measured in the rabbits before (control) and after 3 h of the administration. The results showed that the contraction per minute in rabbits before treatment was 8.84 ± 0.61. Furthermore, the contractions per minute were 8.9 ± 0.72 for oral tablets, 10.02 ± 0.62 for intranasal suspension and 21.54 ± 1.88 for intranasal NLC. From these results, it was concluded that the use of intranasally administered NLC improves the clinical efficacy of mosapride84. Other authors have also demonstrated the greater efficacy of administering NLC formulations intranasally, compared to other routes, such as oral and intraperitoneal. In both studies, it was concluded that the intranasal use of NLCs promoted the activity of the drugs in the brain85,86.
4.3.2. SLNs
Since 2018, about two studies using SLN for nose-to-brain drug delivery have been published30,76. This reduced number is probably due to the emergence of NLC that have overcome some of the limitations of SLN. For example, Youssef et al.30 evaluated the brain and plasma concentration of almotriptan malate for the management of migraine headache using three different formulations: intravenous almotriptan malate solution, intranasal in situ gel of almotriptan malate and intranasal in situ gel of almotriptan malate-loaded SLNs. Up to 6 h, the brain/blood ratio was 5-fold higher for intranasal in situ gel of almotriptan malate-loaded SLNs, compared to intravenous almotriptan malate solution, which indicates higher drug distribution in the brain. The brain Cmax was 1.23 ± 0.02 μg/mL for intravenous almotriptan malate, 1.43 ± 0.02 μg/mL for intranasal in situ gel of almotriptan malate and 2.41 ± 0.04 μg/mL for intranasal in situ gel of almotriptan malate-loaded SLNs, with a Tmax of 0.5, 2 and 0.17 h, respectively. In the brain, the AUC was higher for the intranasal in situ gel of almotriptan malate-loaded SLN (7.87 ± 0.09 h∙µg/mL), followed by intranasal in situ gel of almotriptan malate (6.25 ± 0.03 h∙µg/mL) and intravenous almotriptan malate (3.32 ± 0.04 h∙µg/mL). As expected, in the plasma, the Cmax and AUC values were higher for intravenous almotriptan malate (3.2 ± 0.06 μg/mL; 12.43 ± 0.09 h∙µg/mL), followed by intranasal free drug (3.09 ± 0.05 μg/mL; 9.15 ± 0.07 h∙µg/mL) and intranasal almotriptan malate-loaded SLN (2.69 ± 0.02 μg/mL; 8.77 ± 0.08 h∙µg/mL). DTE and DTP values were also higher for intranasal almotriptan malate-loaded SLN (335.69 ± 8.37% and 70.21 ± 0.72%), compared to intranasal non-encapsulated almotriptan malate (255.1 ± 3.68% and 60.80 ± 0.56%). These findings show the efficacy of the intranasal almotriptan malate-loaded SLN for brain targeting. In addition, the use of the mucoadhesive polymer poloxamer 407 increases the contact time of the formulation in the nasal mucosa, which further improves the targeting of the drug to the brain30.
4.3.3. NEs
In the past two years, about nine studies have been published that have investigated the use of NEs to improve drug delivery from nose to brain. For example, Gaba et al.87 demonstrated the efficacy of intranasal naringenin-loaded NEs, compared to intranasal naringenin solution and intravenous naringenin-loaded NEs, for the treatment of Parkinson's disease. Following intranasal administration, the Cmax of naringenin was higher in the brain for NE formulation (1148.64 ± 3.3 ng/mL) and solution (870.77 ± 5.4 ng/mL), compared to the plasma (794.33 ± 7.1 and 775.44 ± 2.5 ng/mL, respectively). Similarly, the brain AUC were higher for intranasal NE (5345.13 ± 7.5 h∙ng/mL) and intranasal solution (3352.86 ± 8.9 h∙ng/mL) over the plasma (3777.63 ± 5.3 and 1919.734 ± 6.7 h∙ng/mL, respectively). Following intravenous administration, the Cmax of naringenin was higher in the plasma for the naringenin-loaded NE (2502.74 ± 4.2 ng/mL) over the brain (381.67 ± 3.1 ng/mL), while the AUC values were 7533.91 ± 4.5 h∙ng/mL in the plasma and 1599.36 ± 4.5 h∙ng/mL in the brain. In addition, the brain/blood ratio of naringenin for the intranasal NE was 3.69 ± 0.25, which was 1.4-fold higher than the intranasal solution (2.66 ± 0.68), and 52.7-fold higher than intravenous NE (0.07 ± 0.25). The DTE and DTP were slightly higher for intranasal NE in comparison to the intranasal solution (822.71 ± 9.14% and 666.51 ± 8.95%; and 72.14 ± 5.87% and 68.41 ± 7.42%, respectively). For the pharmacodynamics study, only the intranasal naringenin-loaded NE was tested with or without oral levodopa, administered at the same time. The rats were divided in five groups, which received normal saline intranasal solution, intravenous 6-OHDA (inductor of the Parkinson's disease symptoms), oral levodopa, intranasal naringenin-loaded NE or oral levodopa and intranasal naringenin-loaded NEs. Afterwards, the rats were submitted to different tests to assess behavioral activities, and the group treated with intranasal NEs and oral levodopa showed a marked improvement, reverting the effects induced by the 6-OHDA (muscle coordination, swimming activity and grip strength)87. Other authors have also demonstrated the efficacy of drug-loaded intranasal NEs compared to intravenous drug solutions. The results were similar and showed that the use of NEs can increase the amount of drug that reaches the brain via intranasal88,89.
Ahmad et al.74 evaluated the brain and plasma concentration of intranasal amiloride-loaded NEs, intranasal amiloride solution, intravenous amiloride-loaded NEs and intravenous amiloride solution, and their efficacy to avoid clonic seizures. The results showed that the Cmax of amiloride in the brain was 449.64 ± 24.67 ng/mL for intranasal amiloride-loaded NEs, 104.84 ± 8.19 ng/mL for intravenous amiloride-loaded NEs, 28.40 ± 4.63 ng/mL for intranasal amiloride solution and 19.46 ± 2.64 ng/mL for intravenous amiloride solution. The AUC values were also higher for intranasal amiloride-loaded NEs (7937.46 ± 101.19 min∙ng/mL), followed by intravenous amiloride-loaded NEs (1254.37 ± 25.94 min∙ng/mL), intranasal amiloride solution (264.63 ± 19.13 min∙ng/mL) and intravenous amiloride solution (187.06 ± 18.17 min∙ng/mL). The plasma Cmax was 1852.49 ± 41.19 ng/mL for intravenous amiloride solution, 469.26 ± 18.48 ng/mL for intravenous amiloride-loaded NEs, 29.14 ± 2.74 ng/mL for intranasal amiloride-loaded NEs and 10.40 ± 1.02 ng/mL for intranasal amiloride solution. In the plasma, the AUC for the intravenous solution was 18718.37 ± 411.36 min∙ng/mL and for intravenous NEs was 8201.57 ± 135.63 min∙ng/mL, while the AUC for intranasal NE was 265.53 ± 36.56 min∙ng/mL and 103.48 ± 16.16 min∙ng/mL for the intranasal solution. The brain/plasma ratio was significantly higher for intranasal NE, compared to the intranasal solution, intravenous NEs and intravenous solution, with values of 15.43, 2.73, 0.22 and 0.01, respectively. In addition, the DTE and DTP values were also higher for the intranasal NEs (1992.67 ± 45.63% and 586.18 ± 11.63%) over the intranasal solution (99.63 ± 3.78% and 54.15 ± 1.15%). These findings are in accordance with the results of the pharmacodynamics study, where the mice were firstly injected with pentylenetetrazol to induce clonic seizures and myoclonic jerks, and later administered with amiloride solution, amiloride-loaded NEs or saline solution (control). The amiloride-loaded NEs showed better results compared to amiloride solution and saline solution, leading to a higher reducing the onset of myoclonic jerks and clonic seizures. In addition, the increasing current electroshock test was performed and the results were similar to the previous ones, which means that intranasal amiloride-loaded NEs gave greater protection against epileptic seizures than amiloride solution and control solution, being a system of direct administration to the brain safe in the treatment of epilepsy74. Other authors have also studied the potential of using NEs loaded with intranasal topiramate for the treatment of epilepsy. The results of the pharmacodynamics and pharmacokinetic studies showed that the use of NEs loaded with intranasal topiramate improves the bioavailability of the drug in the brain90.
The use of a mucoadhesive agent (chitosan) in NEs loaded with quercetin for intravenous and intranasal administration has been studied, in comparison with intranasal and intravenous quercetin solutions, to improve the treatment of cerebral ischemia in rats. The results showed that the inclusion of a mucoadhesive agent originated a higher quercetin concentration in the brain through the intranasal and intravenous routes, presenting a Cmax of 1788.68 ± 3.67 and 374.59 ± 19.60 ng/mL, followed by the intranasal solution (202.10 ± 11.27 ng/mL) and intravenous solution (109.86 ± 12.01 ng/mL). In contrast, the maximum concentration of quercetin in plasma was 1852.49 ± 46.92 ng/mL for the intravenous solution, 1828.00 ± 24.87 ng/mL for the intravenous mucoadhesive NEs, 404.22 ± 6.98 ng/mL for the intranasal mucoadhesive NEs and 61.59 ± 3.92 ng/mL for the intranasal solution. The brain AUC values were intranasal quercetin mucoadhesive NEs (24893.11 ± 368.85 min∙ng/mL) > intravenous quercetin mucoadhesive NEs (4704.76 ± 188.56 min∙ng/mL) > intranasal solution (2540.60 ± 199.76 min∙ng/mL) > intravenous solution (1075.80 ± 71.92 min∙ng/mL). The plasma AUC was higher for intravenous mucoadhesive NEs, followed by intravenous solution, intranasal mucoadhesive NEs and intranasal solution, with values of 24988.47 ± 235.67, 18718.37 ± 388.76, 6350.80 ± 166.56 and 453.02 ± 28.10 min∙ng/mL, respectively. The brain/blood ratio was significantly higher for intranasal NEs (4.43) and intranasal solution (3.28), compared to intravenous NEs (0.20) and intravenous solution (0.06). Intranasal quercetin mucoadhesive NEs presented the highest DTE (9333.33 ± 39.39%) and DTP (2181.83 ± 15.69%), compared to intranasal solution (DTE: 2063.63 ± 5.98% and DTP: 546.75 ± 1.05%). For the pharmacodynamics studies, the rats were divided in control solution, control NEs, MCAO (middle cerebral artery occlusion that affects the behavioral activity, including locomotor and grip strength) groups. The rats first received the MCAO and were further treated with different intranasal or intravenous formulations. The results showed an improvement in the locomotor activity and grip strength, and a reduction in the infarction volume and the tissue damage, for the rats treated with quercetin solution and quercetin NEs, proving that both formulations provided a protective role in stroke, although the NEs formulation presented better results. Based on the pharmacokinetic and pharmacodynamics results, the authors concluded that the addition of chitosan to the NEs improved the residence time of the formulations in the nasal mucosa, increasing the drug uptake into the brain, being an effective system for the treatment of cerebral ischemia75.
Shobo et al.61 compared the brain and plasma concentration of intranasal pretomanid-loaded NEs, intranasal pretomanid solution, intraperitoneal pretomanid solution and oral pretomanid solution, with a pretomanid concentration of 20 mg/kg, for the treatment of tuberculosis in rats. The results showed that intranasal NEs originated a higher concentration of drug in the brain compared to the other formulations tested. Briefly, the intranasal pretomanid-loaded NEs showed a higher Cmax value in the brain (12062.3 ng/g), compared to the intranasal pretomanid solution (3060.3 ng/g), intraperitoneal pretomanid solution (236.8 ng/g) and oral pretomanid solution (194.9 ng/g), with a Tmax of 8, 4, 2 and 8 h, respectively. The AUC value was also higher for intranasal pretomanid-loaded NEs (183465.8 h∙ng/mL), followed by intranasal pretomanid solution (36589.0 h∙ng/mL), intraperitoneal pretomanid solution (1069.9 h∙ng/mL) and oral pretomanid solution (<limit of quantification). Additionally, the plasma Cmax and AUC values for intranasal pretomanid-loaded NEs (3616.9 ng/mL; 47908.1 h∙ng/mL) and intranasal pretomanid solution (109.3 ng/mL; 679.2 h∙ng/mL) were lower compared to the values obtained in the brain. The opposite was observed for the intraperitoneal (1146.6 ng/mL; 3988.5 h∙ng/mL) and oral pretomanid (626.7 ng/mL; 3724.8 h∙ng/mL) solutions. From these results, the authors concluded that the higher drug concentration in the brain obtained for intranasal pretomanid-loaded NEs (4-fold higher), in comparison to the intranasal pretomanid solution, is related to the higher ability of NEs to protect and target the drug to the brain61. Other authors have also studied the use of intranasal NEs for the treatment of Alzheimer's disease and erectile dysfunction. In both studies, it was concluded that the use of the intranasal route increases the bioavailability of the drug in the brain, while the use of drug-loaded NEs improves absorption in the nasal cavity64,91.
5. Clinical studies with nasal formulations
William H. Frey II patented the concept of nose-to-brain delivery in 198916,26. This finding was the first step towards the beginning of clinical studies that use the intranasal route to reach the CNS. In 1996, Pietrowski et al.92 conducted a clinical trial in 15 healthy men who received intranasal and intravenous arginine-vasopressin. In this study, the brain wave activity was measured, being concluded that, after intranasal administration, the brain wave activity increased, in contrast with the intravenous administration. In 1998, Derad et al.93 conducted a clinical study in 12 healthy adults who received intranasal and intravenous angiotensin II and observed similar results.
According to the US National Library of Medicine, there are 284 ongoing clinical trials with nasal formulations94. Among these ongoing trials, there are 12 in phase 0 (i.e., first conducted to investigate if the drug affects the body, involving very limited human exposure to the drug), 51 in phase 1, 95 in phase 2, 44 in phase 3, 37 in phase 4 and 61 without specific phase94,95. From the 37 approved nasal medicines, 1 is a TrueTear™ device for neuropathic corneal pain, 2 contain influenza vaccines and 34 contain different drugs, being midazolam, ketamine and dexmedetomidine the most prevalent. However, to our knowledge, there are no nasal formulations of SLNs, NLCs and NEs marketed, although the use of these nanocarriers has been studied to improve the drug deliver through other routes of administration, such as oral, parenteral, cutaneous, ocular, rectal and pulmonary, and for cosmetics45,48,96. Despite the several investigations that have been carried out, only cosmetic products with SLNs and NLCs have been approved37,41,42,97,98.
The prospects and challenges of the clinical translation of lipid-based nanocarriers include the use of new GRAS excipients to promote the nasal bioavailability of drugs, the use of robust optimization processes of the formulations, to increase the drug loading capacity, avoid the occurrence of aggregation and phase separation phenomena during storage. Despite the easy scaling-up of lipid-based nanocarriers formulations, it is important to prove the viability of their large-scale production methods before applying for regulatory approval38,48,51,99.
6. Conclusions
In recent decades, research on the intranasal route and how it can avoid the need for drugs to cross the BBB has attracted great attention. In vivo studies reported much evidences about the existence of a direct transport route from the nose to the brain via olfactory and trigeminal nerves, providing a fast onset of action. However, the exact mechanism of drug transportation is not yet fully understood and its efficacy in humans is unclear.
From the reported in vivo studies, we conclude that the intranasal route together with the use of lipid-based nanocarriers, such as NLCs, SLNs and NEs, is advantageous for targeting drugs to the brain. These systems have demonstrated higher effectiveness upon nose-to-brain delivery than through other administration routes or intranasal administration of solutions or suspensions of free drug. When developing a lipid-based nanocarrier formulation it is important to identify the factors that influence the nasal absorption of the drug and ensure that it reaches the brain with minimal losses arising from mucociliary clearance, enzymatic degradation or absorption in the systemic circulation. In addition, a deeper understanding of the nose-brain transport mechanism is crucial to develop a suitable device that allows the drug to be targeted to the upper region of the nasal cavity.
Although there are encouraging results of preclinical studies, the nasal anatomy differences between animal species and incorrect extrapolation of the drug dose from animals to humans, have been pointed as the main reasons for the failure of clinical trials with intranasal formulations. To tackle these challenges, the use of advanced mathematical models has been suggested. Despite the challenges, lipid-based nanocarriers encompass a great potential to facilitate intranasal drug administration.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) (SFRH/136177/2018, Portugal), by the Applied Molecular Biosciences Unit-UCIBIO which is financed by national funds from FCT (UIDP/04378/2020 and UIDB/04378/2020).
Author contributions
Cláudia Pina. Costa and Ana Catarina Silva designed the research and wrote the manuscript. Cláudia Pina Costa performed data analysis. Ana Catarina Silva, João Nuno Moreira and José Manuel Sousa Lobo revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of Interest
The authors have no conflicts of interests to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.02.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alexander A., Agrawal M., Bhupal Chougule M., Saraf S., Saraf S. Chapter 9 - nose-to-brain drug delivery: an alternative approach for effective brain drug targeting. In: Shegokar R., editor. Nanopharmaceuticals. Elsevier; 2020. pp. 175–200. [Google Scholar]
- 2.Pottoo F.H., Sharma S., Javed M.N., Barkat M.A., Harshita, Alam M.S. Lipid-based nanoformulations in the treatment of neurological disorders. Drug Metab Rev. 2020;52:185–204. doi: 10.1080/03602532.2020.1726942. [DOI] [PubMed] [Google Scholar]
- 3.Weiss N., Miller F., Cazaubon S., Couraud P.O. The blood‒brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Kaur I.P., Bhandari R., Bhandari S., Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127:97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Khan A.R., Liu M., Khan M.W., Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268:364–389. doi: 10.1016/j.jconrel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Costa C., Moreira J.N., Amaral M.H., Sousa Lobo J.M., Silva A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J Control Release. 2019;295:187–200. doi: 10.1016/j.jconrel.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Islam S.U., Shehzad A., Ahmed M.B., Lee Y.S. Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules. 2020;25:1929. doi: 10.3390/molecules25081929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraiva C., Praça C., Ferreira R., Santos T., Ferreira L., Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood‒brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni A.D., Patel H.M., Surana S.J., Belgamwar V.S., Pardeshi C.V. Brain‒blood ratio: implications in brain drug delivery. Expet Opin Drug Deliv. 2016;13:85–92. doi: 10.1517/17425247.2016.1092519. [DOI] [PubMed] [Google Scholar]
- 10.Sabir F., Ismail R., Csoka I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook. Drug Discov Today. 2020;25:185–194. doi: 10.1016/j.drudis.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Alavian F., Shams N. Oral and intra-nasal administration of nanoparticles in the cerebral ischemia treatment in animal experiments: considering its advantages and disadvantages. Curr Clin Pharmacol. 2020;15:20–29. doi: 10.2174/1574884714666190704115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali J., Ali M., Baboota S., Sahani J.K., Ramassamy C., Dao L. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr Pharmaceut Des. 2010;16:1644–1653. doi: 10.2174/138161210791164108. [DOI] [PubMed] [Google Scholar]
- 13.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pires P.C., Santos A.O. Nanosystems in nose-to-brain drug delivery: a review of non-clinical brain targeting studies. J Control Release. 2018;270:89–100. doi: 10.1016/j.jconrel.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Selvaraj K., Gowthamarajan K., Karri V.V.S.R. Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif Cells Nanomed Biotechnol. 2018;46:2088–2095. doi: 10.1080/21691401.2017.1420073. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal M., Saraf S., Saraf S., Antimisiaris S.G., Chougule M.B., Shoyele S.A. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Xiong G., Tsang W.C., Schätzlein A.G., Uchegbu I.F. Nose-to-brain delivery. J Pharmacol Exp Therapeut. 2019;370:593–601. doi: 10.1124/jpet.119.258152. [DOI] [PubMed] [Google Scholar]
- 18.Djupesland P.G., Skretting A. Nasal deposition and clearance in man: comparison of a bidirectional powder device and a traditional liquid spray pump. J Aerosol Med Pulm Drug Deliv. 2012;25:280–289. doi: 10.1089/jamp.2011.0924. [DOI] [PubMed] [Google Scholar]
- 19.Warnken Z.N., Smyth H.D.C., Watts A.B., Weitman S., Kuhn J.G., Williams R.O. Formulation and device design to increase nose to brain drug delivery. J Drug Deliv Sci Technol. 2016;35:213–222. [Google Scholar]
- 20.Shahaf D, Hadash J, Inventors; SIPNOSE Ltd., assignee. Nasal delivery device. United States patent US9227031B2. 2016 Jan 5.
- 21.Gänger S., Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10:116. doi: 10.3390/pharmaceutics10030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djupesland P.G., Skretting A., Winderen M., Holand T. Breath actuated device improves delivery to target sites beyond the nasal valve. Laryngoscope. 2006;116:466–472. doi: 10.1097/01.MLG.0000199741.08517.99. [DOI] [PubMed] [Google Scholar]
- 23.Djupesland P.G., Messina J.C., Mahmoud R.A. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5:709–733. doi: 10.4155/tde.14.41. [DOI] [PubMed] [Google Scholar]
- 24.Mistry A., Stolnik S., Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379:146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Erdő F., Bors L.A., Farkas D., Bajza Á., Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Crowe T.P., Greenlee M.H.W., Kanthasamy A.G., Hsu W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Pardeshi C.V., Belgamwar V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood‒brain barrier: an excellent platform for brain targeting. Expet Opin Drug Deliv. 2013;10:957–972. doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- 28.Bourganis V., Kammona O., Alexopoulos A., Kiparissides C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur J Pharm Biopharm. 2018;128:337–362. doi: 10.1016/j.ejpb.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharmaceut Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 30.Youssef N.A.H.A., Kassem A.A., Farid R.M., Ismail F.A., El-Massik M.A.E., Boraie N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: preparation, characterization and in vivo evaluation. Int J Pharm. 2018;548:609–624. doi: 10.1016/j.ijpharm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Kozlovskaya L., Abou-Kaoud M., Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–140. doi: 10.1016/j.jconrel.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 32.Dhuria S.V., Hanson L.R., Frey W.H. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharmaceut Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 33.Ugwoke M.I., Verbeke N., Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol. 2001;53:3–21. doi: 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- 34.Graff C.L., Pollack G.M. Nasal drug administration: potential for targeted central nervous system delivery. J Pharmaceut Sci. 2005;94:1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 35.Thorne R.G., Pronk G.J., Padmanabhan V., Frey W.H., 2nd Delivery of insulin-like growth factor-i to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Johnson N.J., Hanson L.R., Frey W.H. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm. 2010;7:884–893. doi: 10.1021/mp100029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha S., Amaral M.H., Lobo J.M.S., Silva A.C. Lipid nanoparticles for nasal/intranasal drug delivery. Crit Rev Ther Drug Carrier Syst. 2017;34:257–282. doi: 10.1615/CritRevTherDrugCarrierSyst.2017018693. [DOI] [PubMed] [Google Scholar]
- 38.Mittal D., Ali A., Md S., Baboota S., Sahni J.K., Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21:75–86. doi: 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad E., Feng Y., Qi J., Fan W., Ma Y., He H. Evidence of nose-to-brain delivery of nanoemulsions: cargoes but not vehicles. Nanoscale. 2017;9:1174–1183. doi: 10.1039/c6nr07581a. [DOI] [PubMed] [Google Scholar]
- 40.Tapeinos C., Battaglini M., Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release. 2017;264:306–332. doi: 10.1016/j.jconrel.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva A.C., Amaral M.H., Lobo J.M.S., Almeida H. Editorial: applications of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): state of the art. Curr Pharmaceut Des. 2017;23:6551–6552. doi: 10.2174/1381612824666171214102607. Available from: https://doi.org/10.2174/1381612824666171214102607. [DOI] [PubMed] [Google Scholar]
- 42.Garcês A., Amaral M.H., Sousa Lobo J.M., Silva A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharmaceut Sci. 2018;112:159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Khosa A., Reddi S., Saha R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. doi: 10.1016/j.biopha.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 44.Silva A.C., González-Mira E., Lobo J.M.S., Amaral M.H. Current progresses on nanodelivery systems for the treatment of neuropsychiatric diseases: Alzheimer's and schizophrenia. Curr Pharmaceut Des. 2013;19:7185–7195. doi: 10.2174/138161281941131219123329. [DOI] [PubMed] [Google Scholar]
- 45.Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13:288–303. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das S., Ng W.K., Tan R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded slns and nlcs? Eur J Pharmaceut Sci. 2012;47:139–151. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Karami Z., Saghatchi Zanjani M.R., Hamidi M. Nanoemulsions in CNS drug delivery: recent developments, impacts and challenges. Drug Discov Today. 2019;24:1104–1115. doi: 10.1016/j.drudis.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Battaglia L., Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expet Opin Drug Deliv. 2012;9:497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 49.Shirodkar R.K., Kumar L., Mutalik S., Lewis S. Solid lipid nanoparticles and nanostructured lipid carriers: emerging lipid based drug delivery systems. Pharm Chem J. 2019;53:440–453. [Google Scholar]
- 50.Ragelle H., Danhier F., Préat V., Langer R., Anderson D.G. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expet Opin Drug Deliv. 2017;14:851–864. doi: 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]
- 51.Cunha S., Costa C.P., Moreira J.N., Sousa Lobo J.M., Silva A.C. Using the quality by design (qbd) approach to optimize formulations of lipid nanoparticles and nanoemulsions: a review. Nanomedicine. 2020;28:102206. doi: 10.1016/j.nano.2020.102206. [DOI] [PubMed] [Google Scholar]
- 52.Cunha S., Costa C.P., Loureiro J.A., Alves J., Peixoto A.F., Forbes B. Double optimization of rivastigmine-loaded nanostructured lipid carriers (NLC) for nose-to-brain delivery using the quality by design (QbD) approach: formulation variables and instrumental parameters. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danaei M., Dehghankhold M., Ataei S., Hasanzadeh Davarani F., Javanmard R., Dokhani A. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordillo-Galeano A., Mora-Huertas C.E. Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release. Eur J Pharmaceut Sci. 2018;133:285–308. doi: 10.1016/j.ejpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Blasi P., Giovagnoli S., Schoubben A., Puglia C., Bonina F., Rossi C. Lipid nanoparticles for brain targeting I. Formulation optimization. Int J Pharm. 2011;419:287–295. doi: 10.1016/j.ijpharm.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y., Qi J., Wu W. Chapter 20 - lipid nanoparticles: In vitro and in vivo approaches in drug delivery and targeting. In: Grumezescu A.M., editor. Drug targeting and stimuli sensitive drug delivery systems. William Andrew Publishing; 2018. pp. 749–783. [Google Scholar]
- 57.Haider M., Abdin S.M., Kamal L., Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 2020;12:288. doi: 10.3390/pharmaceutics12030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barrios A.W., Sanchez Quinteiro P., Salazar I. The nasal cavity of the sheep and its olfactory sensory epithelium. Microsc Res Tech. 2014;77:1052–1059. doi: 10.1002/jemt.22436. [DOI] [PubMed] [Google Scholar]
- 59.Johnson-Delaney C.A., Orosz S.E. Rabbit respiratory system: clinical anatomy, physiology and disease. Vet Clin: Exot Anim Pract. 2011;14:257–266. doi: 10.1016/j.cvex.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Harkema J.R., Carey S.A., Wagner J.G., Dintzis S.M., Liggitt D. 6 - Nose, sinus, pharynx, and larynx. In: Treuting P.M., Dintzis S.M., editors. Comparative anatomy and histology. Academic Press; San Diego: 2012. pp. 71–94. [Google Scholar]
- 61.Shobo A., Pamreddy A., Kruger H.G., Makatini M.M., Naicker T., Govender T. Enhanced brain penetration of pretomanid by intranasal administration of an oil-in-water nanoemulsion. Nanomedicine. 2018;13:997–1008. doi: 10.2217/nnm-2017-0365. [DOI] [PubMed] [Google Scholar]
- 62.Pardeshi C.V., Belgamwar V.S. Improved brain pharmacokinetics following intranasal administration of N,N,N-trimethyl chitosan tailored mucoadhesive NLCS. Mater Technol. 2020;35:249–266. [Google Scholar]
- 63.Jojo G.M., Kuppusamy G., De A., Karri V. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer's disease using box-behnken design. Drug Dev Ind Pharm. 2019;45:1061–1072. doi: 10.1080/03639045.2019.1593439. [DOI] [PubMed] [Google Scholar]
- 64.Elbardisy B., Galal S., Abdelmonsif D.A., Boraie N. Intranasal tadalafil nanoemulsions: formulation, characterization and pharmacodynamic evaluation. Pharmaceut Dev Technol. 2019;24:1083–1094. doi: 10.1080/10837450.2019.1631846. [DOI] [PubMed] [Google Scholar]
- 65.Chamanza R., Wright J.A. A review of the comparative anatomy, histology, physiology and pathology of the nasal cavity of rats, mice, dogs and non-human primates. Relevance to inhalation toxicology and human health risk assessment. J Comp Pathol. 2015;153:287–314. doi: 10.1016/j.jcpa.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 66.Ruigrok M.J., de Lange E.C. Emerging insights for translational pharmacokinetic and pharmacokinetic-pharmacodynamic studies: towards prediction of nose-to-brain transport in humans. AAPS J. 2015;17:493–505. doi: 10.1208/s12248-015-9724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Illum L. Nasal drug delivery--possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 68.Wright B. Chapter 2—clinical trial phases. In: Shamley D., Wright B., editors. A comprehensive and practical guide to clinical trials. Academic Press; 2017. pp. 11–15. [Google Scholar]
- 69.Turfus S.C., Delgoda R., Picking D., Gurley B.J. Chapter 25—pharmacokinetics. In: Badal S., Delgoda R., editors. Pharmacognosy. Academic Press; Boston: 2017. pp. 495–512. [Google Scholar]
- 70.Lea-Henry T.N., Carland J.E., Stocker S.L., Sevastos J., Roberts D.M. Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol. 2018;13:1085–1095. doi: 10.2215/CJN.00340118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glassman P.M., Muzykantov V.R. Pharmacokinetic and pharmacodynamic properties of drug delivery systems. J Pharmacol Exp Therapeut. 2019;370:570–580. doi: 10.1124/jpet.119.257113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meibohm B. The role of Pharmacokinetics and pharmacodynamics in the development of biotech drugs. In: Meibohm B., editor. Drug development. Wiley-VCH Verlag GmbH; 2006. [Google Scholar]
- 73.Agu R. UM. In situ and ex vivo nasal models for preclinical drug development studies. In: Ehrhardt C.K.K., editor. vol. III. Springer; Boston, MA: 2008. (Drug absorption studies. Biotechnology: pharmaceutical aspects). [Google Scholar]
- 74.Ahmad N., Ahmad R., Alam M.A., Ahmad F.J., Amir M. Impact of ultrasonication techniques on the preparation of novel amiloride-nanoemulsion used for intranasal delivery in the treatment of epilepsy. Artif Cells Nanomed Biotechnol. 2018;46:S192–S207. doi: 10.1080/21691401.2018.1489826. [DOI] [PubMed] [Google Scholar]
- 75.Ahmad N., Ahmad R., Naqvi A.A., Alam M.A., Ashafaq M., Abdur Rub R. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif Cells Nanomed Biotechnol. 2018;46:717–729. doi: 10.1080/21691401.2017.1337024. [DOI] [PubMed] [Google Scholar]
- 76.Yasir M., Sara U.V.S., Chauhan I., Gaur P.K., Singh A.P., Puri D. Solid lipid nanoparticles for nose to brain delivery of donepezil: formulation, optimization by box–behnken design, in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol. 2018;46:1838–1851. [Google Scholar]
- 77.Singh S.K., Hidau M.K., Gautam S., Gupta K., Singh K.P., Singh S.K. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: pharmacokinetic and teratogenic assessment. Int J Biol Macromol. 2018;108:1092–1100. doi: 10.1016/j.ijbiomac.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 78.Muntimadugu E., Dhommati R., Jain A., Challa V.G., Shaheen M., Khan W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: a potential brain targeting strategy for Alzheimer's disease. Eur J Pharmaceut Sci. 2016;92:224–234. doi: 10.1016/j.ejps.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Rajput Apb S. Fabrication of an ion-sensitive in situ gel loaded with nanostructured lipid carrier for nose to brain delivery of donepezil. Asian J Pharm. 2018;12 [Google Scholar]
- 80.Rajput A.P., Butani S.B. Resveratrol anchored nanostructured lipid carrier loaded in situ gel via nasal route: formulation, optimization and in vivo characterization. J Drug Deliv Sci Technol. 2019;51:214–223. [Google Scholar]
- 81.Jazuli I., Annu Nabi B., Moolakkadath T., Alam T., Baboota S., Ali J. Optimization of nanostructured lipid carriers of lurasidone hydrochloride using Box-Behnken design for brain targeting: In vitro and in vivo studies. J Pharmaceut Sci. 2019;108:3082–3090. doi: 10.1016/j.xphs.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Gadhave D.G., Tagalpallewar A.A., Kokare C.R. Agranulocytosis-protective olanzapine-loaded nanostructured lipid carriers engineered for cns delivery: optimization and hematological toxicity studies. AAPS PharmSciTech. 2019;20:22. doi: 10.1208/s12249-018-1213-y. [DOI] [PubMed] [Google Scholar]
- 83.Abbas H., Refai H., El Sayed N. Superparamagnetic iron oxide-loaded lipid nanocarriers incorporated in thermosensitive in situ gel for magnetic brain targeting of clonazepam. J Pharmaceut Sci. 2018;107:2119–2127. doi: 10.1016/j.xphs.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Hammad R.W., Sanad R.A.B., Abdelmalk N.S., Aziz R.L., Torad F.A. Intranasal surface-modified mosapride citrate-loaded nanostructured lipid carriers (MOS-SMNLCs) for treatment of reflux diseases: in vitro optimization, pharmacodynamics, and pharmacokinetic studies. AAPS PharmSciTech. 2018;19:3791–3808. doi: 10.1208/s12249-018-1142-9. [DOI] [PubMed] [Google Scholar]
- 85.Du W., Li H., Tian B., Sai S., Gao Y., Lan T. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf, B. 2019;183:110446. doi: 10.1016/j.colsurfb.2019.110446. [DOI] [PubMed] [Google Scholar]
- 86.Gadhave D.G., Kokare C.R. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: optimization and in vivo studies. Drug Dev Ind Pharm. 2019;45:839–851. doi: 10.1080/03639045.2019.1576724. [DOI] [PubMed] [Google Scholar]
- 87.Gaba B., Khan T., Haider M.F., Alam T., Baboota S., Parvez S. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-ohda Parkinson's disease model. BioMed Res Int. 2019;2019:2382563. doi: 10.1155/2019/2382563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar S., Dang S., Nigam K., Ali J., Baboota S. Selegiline nanoformulation in attenuation of oxidative stress and upregulation of dopamine in the brain for the treatment of Parkinson's disease. Rejuvenation Res. 2018;21:464–476. doi: 10.1089/rej.2017.2035. [DOI] [PubMed] [Google Scholar]
- 89.Arora A., Kumar S., Ali J., Baboota S. Intranasal delivery of tetrabenazine nanoemulsion via olfactory region for better treatment of hyperkinetic movement associated with huntington's disease: pharmacokinetic and brain delivery study. Chem Phys Lipids. 2020;230:104917. doi: 10.1016/j.chemphyslip.2020.104917. [DOI] [PubMed] [Google Scholar]
- 90.Patel R.J., Parikh R.H. Intranasal delivery of topiramate nanoemulsion: pharmacodynamic, pharmacokinetic and brain uptake studies. Int J Pharm. 2020;585:119486. doi: 10.1016/j.ijpharm.2020.119486. [DOI] [PubMed] [Google Scholar]
- 91.Jiang Y., Liu C., Zhai W., Zhuang N., Han T., Ding Z. The optimization design of lactoferrin loaded hupa nanoemulsion for targeted drug transport via intranasal route. Int J Nanomed. 2019;14:9217–9234. doi: 10.2147/IJN.S214657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pietrowsky R., Strüben C., Mölle M., Fehm H.L., Born J. Brain potential changes after intranasal vs. Intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol Psychiatr. 1996;39:332–340. doi: 10.1016/0006-3223(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 93.Derad I., Willeke K., Pietrowsky R., Born J., Fehm H.L. Intranasal angiotensin ii directly influences central nervous regulation of blood pressure. Am J Hypertens. 1998;11:971–977. doi: 10.1016/s0895-7061(98)00095-8. [DOI] [PubMed] [Google Scholar]
- 94.Medicine USNLo Intranasal ongoing studies. https://clinicaltrials.gov/ct2/results?term=Intranasal&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt= Available from:
- 95.Medicine USNLo Intranasal ongoing studies (without a specific phase) https://clinicaltrials.gov/ct2/results?term=Intranasal&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=&phase=5&Search=Apply Available from:
- 96.Deshpande A., Mohamed M., Daftardar S.B., Patel M., Boddu S.H.S., Nesamony J. Chapter 12 - Solid lipid nanoparticles in drug delivery: opportunities and challenges. In: Mitra A.K., Cholkar K., Mandal A., editors. Emerging nanotechnologies for diagnostics, drug delivery and medical devices. Elsevier; Boston: 2017. pp. 291–330. [Google Scholar]
- 97.Pardeike J., Hommoss A., Müller R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Battaglia L., Ugazio E. Lipid nano- and microparticles: an overview of patent-related research. J Nanomater. 2019;2019:2834941. [Google Scholar]
- 99.Gasco M.R. Lipid nanoparticles: perspectives and challenges. Adv Drug Deliv Rev. 2007;59:377–378. doi: 10.1016/j.addr.2007.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.