Mutations in ATP1A3, which encodes the α3 subunit of Na, K-ATPase, produce various neurologic and psychological disorders that are increasingly believed to be on a continuum, from severe infantile presentations to adult-onset movement disorders. We present evidence that a single codon deletion can nonetheless produce a typical syndrome of rapid onset dystonia-parkinsonism (RDP, DYT/PARK-ATP1A3, OMIM 128235).1 The novel heterozygous mutation p.Phe297del (c.889-891delTTC in NM_152296) was identified in 4 patients in 3 different countries with different genetic backgrounds, European, Japanese, and mixed. This supports the idea that there are discrete mutation-related syndromes underlying the continuum of ATP1A3 phenotypes.
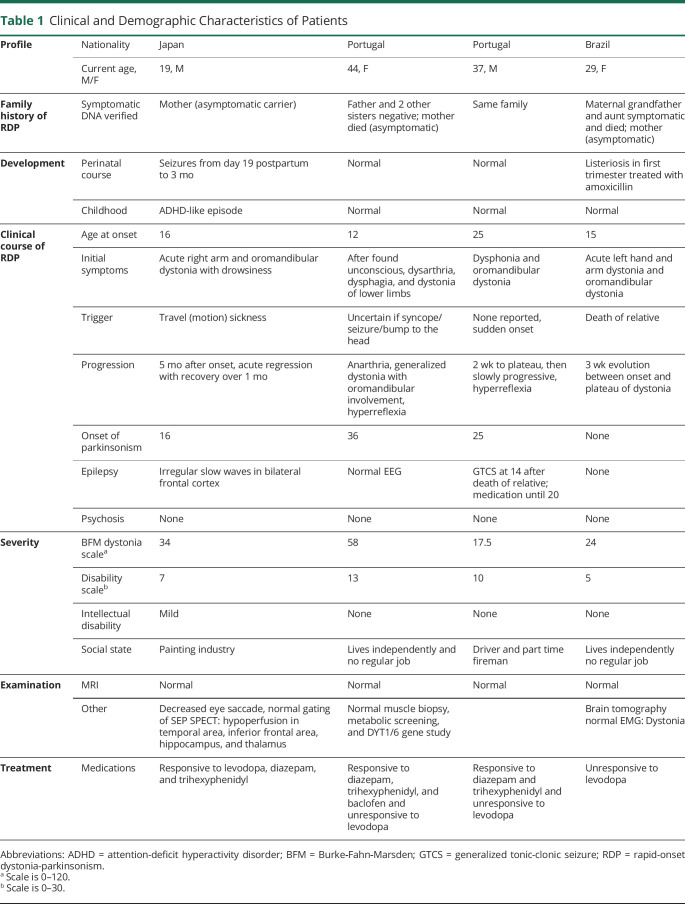
A 19-year-old Japanese man, 44-year-old and 37-year-old Portuguese siblings (older sister and younger brother), and a 29-year-old Brazilian woman were investigated clinically and genetically. Subjects underwent next-generation sequencing panel or Sanger sequencing under research protocols. All 4 cases had typical1 and mild-to-moderate symptoms of RDP. Details are in table 1. All cases were familial according to family history and/or genetic testing. Rapid onset of oromandibular and upper extremity dystonia occurred in adolescence in 3 patients and at age 25 in 1. There were triggers in 3. Symptoms appeared immediately or over 3 weeks. Three developed mild parkinsonism within a decade. All had mild-to-moderate scores in the Burke-Fahn-Marsden dystonia scale; both the Japanese and Portuguese men work. None suffered from severe psychiatric disorders or intellectual disability. The Japanese man revealed abnormal SPECT, EEG, and memory-guided saccades (e-Methods case 1, figures e-1–5 and table e-1, links.lww.com/NXG/A392, and video 1). The Portuguese woman had normal muscle biopsy and metabolic screening. The Brazilian woman had normal brain tomography.
Table 1.
Clinical and Demographic Characteristics of Patients
Clinical exam of the Japanese patient. Segment 1. The right fingers and arm exhibit stiffness and dystonic posture. Rotation of the outstretched forearms from pronation to supination is slow. The patient has limited facial movement, and his jaw is retracted. Segment 2. Mouth opening and tongue protrusion are restricted because of oromandibular dystonia. His face shows slight hypomimia. Segment 3. The patient speaks at a low volume with a squeezing voice, probably because of laryngeal dystonia. Segment 4. The right fifth finger exhibits a dystonic posture, but the patient can write and draw spirals. His writing is micrographic. Segment 5. Striatal or dystonic toe is noted while he uses his smart phone. Segment 6. Myerson's sign is present. Segment 7. The patient has no postural instability. Segment 8. Arm swing is limited during walking and running. His posture is slightly stooped and stiff. An overall rostro-caudal (face>arm>leg) gradient of involvement is evident.Download Supplementary Video 1 (12.3MB, mp4) via http://dx.doi.org/10.1212/000562_Video_1
The presence of consistent symptoms in independent patients with the same recurrent variant is itself strong evidence for pathogenicity. Supporting the pathogenicity of the shared variant, p.Phe297del in ATP1A3 corresponds to p.Phe305del in ATP1A2, which was reported in a case of hemiplegic migraine with symptoms typical of other mutations in that gene (FHM2, OMIM 602481).2 In all 4 Na, K-ATPase catalytic subunit genes, there are 2 adjacent phenylalanines with the same codons, TTCTTC in ATP1A1, ATP1A2, and ATP1A3 and TTTTTT in ATP1A4. The deletion of 3 bases is so far the only mutation found at the site. p.Phe297del in ATP1A3 produced a uniform syndrome on different genetic backgrounds here, suggesting a mutation-phenotype relationship. The position in the protein is near the extracellular surface, not close to the ion binding sites or to domains essential for ATP hydrolysis (figure e-6, links.lww.com/NXG/A392). Deleting 1 residue of a helix will change the positions of amino acids around it, and in this case, this has the potential to distort the pathway for K+ entry and Na+ exit at the extracellular surface.3 The deletion will slightly shorten transmembrane helix M3, which means shifting the short extracellular linker between M3 and M4 inward. There is good reason to predict a functional consequence: movement of the extracellular segment of M4 controls the opening and closing of the ion pathway.3 The shortening is also likely to affect the orientation of Glu309, which is on M4 approximately opposite Phe297. In one of the most intriguing laboratory studies of ATP1A3 disease mutations, the secondary mutation p.Glu309Asp was shown to correct the reduced Na+ affinity of the human mutation p.Asp923Asn, 30 Å distant on the cytoplasmic side of M8.4 Aspartate is only slightly smaller than glutamate, and the affinity increase was believed to be due to adjustment of the position of M4, which contributes part of the ion binding pocket.3,5 In this context, p.Phe297del may produce ATP1A3 and ATP1A2 neurologic disorders by altered kinetic properties or by inactivation of enzymatic activity.3,5
Different ATP1A3 mutations produce a range of symptoms with considerable overlap,6 but there seem to be discrete mutation-related syndromes underlying the continuum of phenotypes, and early indications of structure-phenotype relationships.7 Why mutations of ATP1A3 produce 1 syndrome and not others is of paramount importance for development of therapies. Factors that can impact phenotype are the level of inactivation (loss of activity or loss of membrane delivery); alteration of neuronal physiology by changing ion affinity, intracellular Na+, and membrane potential; and whether the protein is stable. Each ATP1A3 variant will have an intrinsic propensity to each form of damage, resulting in a tendency to produce milder or more severe syndromes. A few mutations, such as p.Asp923Asn, have been shown to produce 2 different syndromes even in the same family, and in such cases, other factors must contribute to symptom differences.
Ethical Standards
The authors hereby declare that the research documented in the submitted study has been carried out in accordance with ethical standards laid down in the 1964 declaration of Helsinki and approved by the Ethics Committee of the Segawa Memorial Neurological Clinic for Children and the institutional review boards of the Tokushima University; Hospital de Clínicas de Porto Alegre; and Hospital de Santo António, Centro Hospitalar Universitário do Porto, Porto, Portugal.
Acknowledgment
The authors thank Prof. Keiko Ikeda, Murayama Medical Center, for useful discussion and collaboration on the early stages of this work.
Appendix. Authors
Contributor Information
Kathleen J. Sweadner, Email: sweadner@helix.mgh.harvard.edu.
Toshitaka Kawarai, Email: tkawarai@tokushima-u.ac.jp.
Jonas Alex Saute, Email: jsaute@hcpa.edu.br.
Karina C. Donis, Email: kcdonis@hcpa.edu.br.
Kazue Kimura, Email: kimura@segawa-clinic.jp.
Hideki Fukuda, Email: fukuda.sac@gmail.com.
Masaharu Hayashi, Email: hayashi-ms@igakuken.or.jp.
Tetsuya Higuchi, Email: tetsuyah92md@gmail.com.
Yoshio Ikeda, Email: ikeday006@gunma-u.ac.jp.
Laurie J. Ozelius, Email: laurie.ozelius@mgh.harvard.edu.
Ryuji Kaji, Email: rkaji@tokushima-u.ac.jp.
Study Funding
NIH grant NS058949 to Allison Brashear (K.J.S. and L.J.O.). Health and Labour Science Research Grants for Research on Rare and Intractable Diseases, Clinical Research for Establishment of Evidence Based Guidelines for Hereditary Dystonias and Huntington Disease, Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, the Ministry of Health, Labour and Welfare of Japan (grant to R.K.), and by the Japan Agency for Medical Research and Development for Grants-in-Aid for core hospitals for the Initiative on Rare and Undiagnosed Diseases (to T.K.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
References
- 1.Haq IU, Snively BM, Sweadner KJ, et al. Revising rapid-onset dystonia-parkinsonism: broadening indications for ATP1A3 testing. Mov Disord 2019;34,1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riant F, Ducros A, Ploton C, et al. De novo mutations in ATP1A2 and CACNA1A are frequent in early-onset sporadic hemiplegic migraine. Neurology 2010;75,967–972. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa H, Cornelius F, Hirata A, et al. Sequential substitution of K+ bound to Na+,K+-ATPase visualized by X-ray crystallography. Nat Commun 2015;6,8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holm R, Einholm AP, Andersen JP, et al. Rescue of Na+ affinity in aspartate 928 mutants of Na+,K+-ATPase by secondary mutation of glutamate 314. J Biol Chem 2015;290,9801–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holm R, Toustrup-Jensen MS, Einholm AP, et al. Neurological disease mutations of α3 Na+,K+-ATPase: structural and functional perspectives and rescue of compromised function. Biochim Biophys Acta 2016;1857,1807–1828. [DOI] [PubMed] [Google Scholar]
- 6.Brashear A, Sweadner KJ, Cook JF, et al. ATP1A3-related neurologic disorders. In: Gene Reviews [Internet]. Seattle: University of Washington, Seattle; 2018; ncbi.nlm.nih.gov/books/NBK1115/. [PubMed] [Google Scholar]
- 7.Sweadner KJ, Arystarkhova E, Penniston JT, et al. Genotype-structure-phenotype relationships diverge in paralogs ATP1A1, ATP1A2, and ATP1A3. Neurol Genet 2019;5:e303. doi: 10.1212/NXG.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical exam of the Japanese patient. Segment 1. The right fingers and arm exhibit stiffness and dystonic posture. Rotation of the outstretched forearms from pronation to supination is slow. The patient has limited facial movement, and his jaw is retracted. Segment 2. Mouth opening and tongue protrusion are restricted because of oromandibular dystonia. His face shows slight hypomimia. Segment 3. The patient speaks at a low volume with a squeezing voice, probably because of laryngeal dystonia. Segment 4. The right fifth finger exhibits a dystonic posture, but the patient can write and draw spirals. His writing is micrographic. Segment 5. Striatal or dystonic toe is noted while he uses his smart phone. Segment 6. Myerson's sign is present. Segment 7. The patient has no postural instability. Segment 8. Arm swing is limited during walking and running. His posture is slightly stooped and stiff. An overall rostro-caudal (face>arm>leg) gradient of involvement is evident.Download Supplementary Video 1 (12.3MB, mp4) via http://dx.doi.org/10.1212/000562_Video_1