Abstract
Objective
We performed a cross-sectional analysis to determine whether nonsustained ventricular tachycardia (NSVT) and premature ventricular contractions (PVCs) were associated with dementia in a population-based study.
Methods
We included 2,517 (mean age 79 years, 26% Black) participants who wore a 2-week ambulatory continuous ECG recording device in 2016 to 2017. NSVT was defined as a wide-complex tachycardia ≥4 beats with a rate >100 bpm. We calculated NSVT and PVC burden as the number of episodes per day. Dementia was adjudicated by experts. We used logistic regression to assess the associations of NSVT and PVCs with dementia.
Results
The mean recording time of the Zio XT Patch was 12.6 ± 2.6 days. There were 768 (31%) participants with NSVT; prevalence was similar in White and Black participants. There were 134 (6.5%) dementia cases (5% in White, 10% in Black participants). After multivariable adjustment, there was no overall association between NSVT and dementia; however, there was a significant race interaction (p < 0.001). In Black participants, NSVT was associated with a 3.67 times higher adjusted odds of dementia (95% confidence interval [CI] 1.92–7.02) compared to those without NSVT, whereas in White participants NSVT was not associated with dementia (odds ratio [95% CI] 0.64 [0.37–1.10]). In Black participants only, a higher burden of PVCs was associated with dementia.
Conclusions
Presence of NSVT and a higher burden of NSVT and PVCs are associated with dementia in elderly Black people. Further research to confirm this novel finding and to elucidate the underlying mechanisms is warranted.
Nonsustained ventricular tachycardia (NSVT) and premature ventricular contractions (PVCs) are heart rhythm conditions that affect individuals encompassing the entire health spectrum, from apparently healthy individuals to patients with severe heart disease.1 In the presence of certain comorbid conditions, NSVT is a marker of increased risk of myocardial infarction2 and death.2,3 The prevalence of PVCs increases with age, and PVCs have been associated with an increased risk of stroke, particularly embolic stroke, in normotensive and nondiabetic people.4,5 Collectively, existing data suggest that NSVT and PVCs are not benign and that their impact on health may be wide-ranging.
Dementia is a significant public health problem that will continue to be more challenging as the population ages. Many studies have documented higher prevalence and incidence of dementia, including Alzheimer disease dementia, among Black than among White participants.6 However, currently available evidence regarding differences in dementia by race is incomplete and difficult to interpret. On one hand, some studies indicate that social, educational, and behavioral factors are the major drivers of race-based difference in dementia prevalence and incidence.7–9 On the other hand, Black individuals have a higher prevalence of cardiovascular risk factors, including hypertension10 and diabetes,11 with earlier onset of risk, greater severity, and more poorly controlled conditions,12 all of which are considered major risk factors for dementia and cognitive impairment.13,14 Yet another conundrum is the lower prevalence and incidence of atrial fibrillation (AF)—an established risk factor for dementia—in Black than White individuals but a higher prevalence and incidence of dementia. In this regard, although the evidence for the association of AF with poorer cognitive function is compelling,15–17 little is known about whether ventricular arrhythmias such as NSVT and PVCs are associated with dementia.
Using data from a 2-week continuous ambulatory ECG recording device, the Zio XT Patch (iRhythm Technologies, Inc, San Francisco, CA), in the setting of a community-based cohort study, the Atherosclerosis Risk in Communities (ARIC) study, we assessed the cross-sectional association of NSVT and PVCs with dementia.
Methods
Study Population
The ARIC study is a prospective cohort study of cardiovascular disease (CVD) and atherosclerosis risk factors.18 Participants at baseline (1987–1989) included 15,792 men and women 45 to 64 years of age, mostly White or Black race, recruited from 4 communities in the United States (Washington County, Maryland [majority White participants]; the northwest suburbs of Minneapolis, MN [majority White participants]; Jackson, MS [all Black participants]; and Forsyth County, North Carolina [both Black and White participants]). Thus far, 6 study visits have been completed, with visit 6 occurring in 2016 to 2017. In addition, ARIC participants have received annual follow-up calls (semiannual since 2012), with response rates of ≥90% among survivors.
This analysis is based on participants who attended visit 6 and wore the Zio XT Patch, a noninvasive, single-lead ECG monitor that provides up to 2 weeks of continuous ECG data. All participants who attended visit 6 were invited to wear the Zio XT Patch, which was applied to the upper chest, over the heart, by a trained ARIC staff member. Further details have been published.19 The longer wear time provides a more precise measurement of NSVT and PVC burden in the population compared to a 24-hour Holter monitor. Of the 4,003 participants who attended visit 6, 2,616 wore the Zio XT Patch. Of those, we excluded participants who wore the Zio XT Patch <2 days (n = 68), those missing cognitive scores (n = 26), and those missing covariates (n = 5). After exclusions, our study population included 2,517 participants (58% female, 26% Black race). We ran a secondary analysis in a subset of ARIC participants that in addition had a brain MRI performed at visit 5 (2011–2013) (n = 874) to assess the impact of cerebral vascular disease.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by institutional review boards at each participating center, and all study participants provided written informed consent.
Dementia Ascertainment
The methodology used to define dementia among ARIC participants has been described in detail elsewhere.20 Briefly, information was obtained from a comprehensive neurocognitive battery, followed by a detailed neurologic history, interviews, and informant interviews, and that information was reviewed by a panel of neurologists and neuropsychologists. This panel classified participants as having dementia following an algorithm based on the National Institute on Aging–Alzheimer's Association working group formulations of dementia21 and DSM-5.22
NSVT and PVC Ascertainment
Participants were asked to wear the Zio XT Patch for up to 14 days. At the end of the recording period, participants removed the device and mailed the heart rhythm monitor to iRhythm Technologies Inc. Data collected from the Zio XT Patch are processed with a proprietary algorithm (Zio ECG Utilization Service System) that provides information on the number of PVCs and NSVT episodes during the wearing period.23 NSVT was defined as the presence of at least 1 wide-complex tachycardia ≥4 beats with a rate >100 bpm (yes/no). We explored the burden of NSVT by dividing the number of NSVT episodes by Zio XT Patch wear time. PVC count was calculated from the number of isolated PVCs divided by wear time to obtain a mean PVC count per day. We also determined the percentage of beats that were isolated PVCs during each participant's wear time.
Covariate Measures
Detailed procedures on covariate measure have been previously published.18 In brief, body mass index was calculated as weight in kilograms divided by height in meters squared. Study staff measured blood pressure using a random-zero sphygmomanometer after 5 minutes of rest in the sitting position and used the average of the second and third measurements. The use of antihypertensive medications was confirmed by standardized review of medications brought to each visit by the patient. ARIC participants had APOE genotyping performed with the TaqMan assay (Applied Biosystems, Foster City, CA) using blood samples and were categorized into 0, 1, or 2 alleles. We defined diabetes mellitus as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, treatment for diabetes mellitus, or self-reported physician diagnosis of diabetes. Participants self-reported current cigarette smoking status. A participant was considered to have heart failure (HF) on the basis of use of HF medication in the previous 2 weeks, presence of HF according the Gothenburg criteria (baseline ARIC visit only), or presence of HF ICD codes in any hospitalization or during follow-up before their visit 6 examination.24,25 ARIC defined coronary heart disease (CHD) as prior cardiovascular revascularization, physician-diagnosed myocardial infarction, presence of a previous myocardial infarction by ECG, or by CHD ascertained by the ARIC Morbidity and Mortality Classification Committee using data from follow-up calls and hospitalization records.26 We ascertained AF using 3 sources in this analysis: 12-lead ECGs performed at previous study visits, hospital discharge codes during follow-up, or captured while wearing the Zio XT Patch.27 ARIC defined stroke as a self-reported physician diagnosis of a stroke before visit 1, and after visit 1, stroke was adjudicated from diagnosis codes indicative of cerebrovascular disease using criteria adapted from the National Survey of Stroke.28 All covariates listed above were assessed at visit 6 except for race/center, sex, and education, which were assessed at visit 1. Finally, we assessed measures of left ventricular ejection fraction (LVEF), left atrial volume index (LAVI), E/A ratio, and E/Eʹ lateral ratio using 2-dimensional echocardiography.29 Echocardiography was performed at visit 5 (2011–2013), which was, on average, 5 years before Zio XT Patch application and cognitive assessments for this study. At the ARIC–Neurocognitive Study at visit 5, brain MRI scans were performed on selected individuals by using 3T brain MRI following identical protocols at each study site, as previously described.30 Data were processed by the ARIC MRI Reading Center at the Mayo Clinic (Rochester, MN). Subclinical cerebrovascular disease, including white matter hyperintensity (WMH) volume and infarcts, was assessed on T2 fluid-attenuated inversion recovery sequences, and microbleeds were assessed on T2* gradient recalled echo sequences.
Statistical Analysis
To describe NSVT burden and to assess the association with dementia, we initially explored associations using restricted cubic splines and found the median burden value to be a natural split. Therefore, we stratified those with NSVT by the number of NSVT episodes per week on the median (0.59 episode per week) into low and high burden. We explored the relationship between PVC burden and dementia using a restricted cubic spline and found it to be linear. Because there are no established clinical cut points for PVC burden, we present results by tertiles and per 1-SD increase in the number of PVCs per day. As a secondary analysis, we grouped the percentage of beats that were isolated PVCs into 3 categories (<0.1%, 0.1%–4.99%, ≥5%) to look at associations in those with a high burden of PVCs (≥5%).
We used multivariable logistic regression to estimate the cross-sectional associations of NSVT and PVCs with dementia. For all analyses, we further tested for effect modification by race and sex using a multiplicative term in the models. Models were adjusted for the baseline variables of age (continuous), sex, race/center (5 levels), APOE genotype (0, 1 or 2 alleles), education (high school graduate vs not), cigarette smoking (current vs not current), body mass index (continuous), systolic and diastolic blood pressures (continuous), antihypertensive medication use (yes/no), diabetes (yes/no), CHD (yes/no), HF (yes/no), AF (yes/no), stroke (yes/no), and antiarrhythmic medication use. We performed a secondary analysis limited to participants with echocardiographic measures at visit 5 (89% of participants in our study had echocardiography measures). In those participants, a second model adjusts for LVEF, LAVI, E/A ratio, and E/Eʹ lateral ratio, all as continuous measures. We performed a sensitivity analysis excluding those with prevalent CVD, which consisted of CHD, HF, and stroke. We performed an additional secondary analysis to assess cerebral vascular disease, and this analysis was limited to participants who in addition underwent brain MRI measures at visit 5. We used multivariable logistic regression models adjusted for the covariates listed above plus cerebral infarcts, microbleeds, and WMH volume. All statistical analyses were performed with SAS version 9.4 (SAS Inc, Cary, NC) and Stata 14.0 (StataCorp LP, College Station, TX).
Data Availability
ARIC data are available through the NIH National Heart, Lung, and Blood Institute–sponsored Biologic Specimen and Data Repository Information Coordinating Center at biolincc.nhlbi.nih.gov/
Results
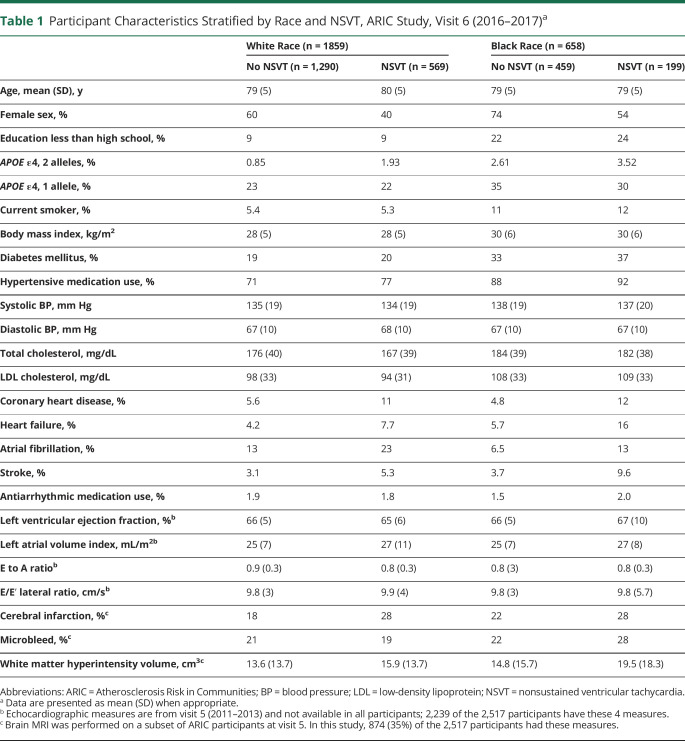
Of the 2,517 participants (mean age 79 ± 5 years, 58% female, 26% Black race) who wore the Zio XT Patch, the mean recording time was 12.6 ± 2.5 days. There were 768 (31%) participants with at least 1 episode of NSVT recorded, ranging from 0.5 to 526 episodes per week (median 0.58, 25th–75th percentile 0.51–1.51). Baseline characteristics of ARIC participants by both the presence and burden of NSVT are shown in table 1. Participants with NSVT detected were slightly older, were more likely to be male, and had a higher prevalence of CHD, HF, AF, and stroke compared to those without NSVT. Those with a higher burden of NSVT were more likely to be male and had a higher prevalence of CHD, HF, AF, and stroke compared to those with a lower burden of NSVT. The prevalence of NSVT was similar in White (31%) and Black (30%) participants, and burden was the same in White and Black individuals.
Table 1.
Participant Characteristics Stratified by Race and NSVT, ARIC Study, Visit 6 (2016–2017)a
NSVT and NSVT Burden
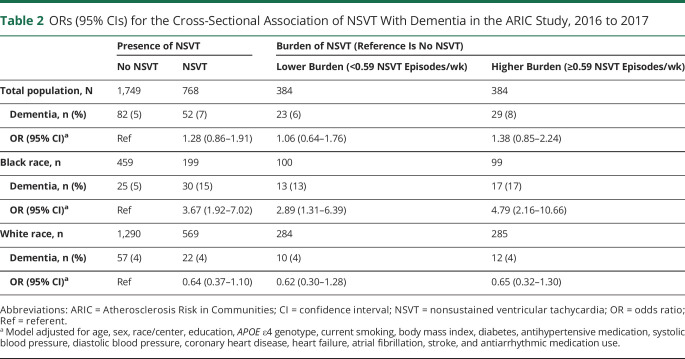
A total of 134 (6.5%) dementia cases were identified at visit 6, and the prevalence of dementia was 5.2% in White and 9.9% in Black participants. The multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of dementia by NSVT status and burden are given in table 2. Overall, there was no significant association between dementia and NSVT status (OR [95% CI] 1.28 [0.86–1.91]). However, there was a significant interaction by race (p = 0.0004); therefore, we stratified the dementia results by Black and White race. In White participants, there was no association between dementia by NSVT status (OR [95% CI] 0.64 [0.37–1.10]). In Black participants, NSVT was associated with a 3.67 times higher adjusted odds of dementia (95% CI 1.92–7.02) compared to those without NSVT. Furthermore, this association was stronger in Black participants with a higher burden (OR [95% CI] 4.79 [2.16–10.66]) than it was in Black participants with a lower burden (OR [95% CI] 2.89 [1.31–6.39]) compared to Black participants without NSVT. There were no significant associations in White participants by NSVT status and dementia. Interaction by sex was not significant. In a sensitivity analysis limited to those without prevalent CVD, the estimates were similar.
Table 2.
ORs (95% CIs) for the Cross-Sectional Association of NSVT With Dementia in the ARIC Study, 2016 to 2017
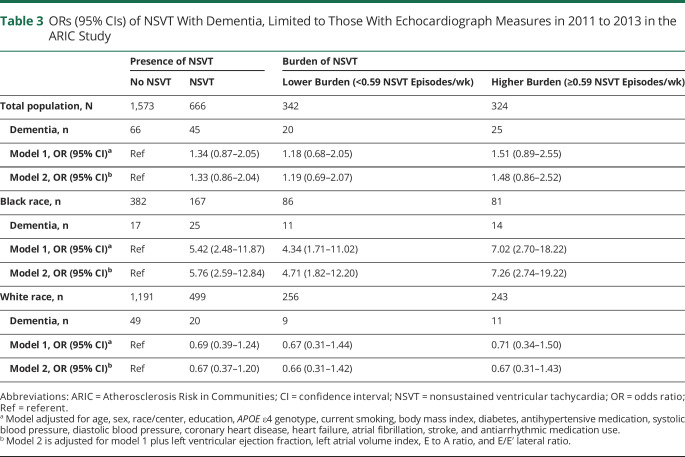
To account for potential confounding by cardiac structure and function, a secondary analysis was performed limited to participants with echocardiographic measures performed at visit 5, ≈5 years before the baseline for this study. The results are presented in table 3. Further adjustment for the variables of LVEF, LAVI, E to A ratio, and E/Eʹ lateral ratio slightly strengthened the associations of NSVT and NSVT burden with dementia in Black individuals, although estimates in this table are less precise due to a smaller sample size. In contrast, effect estimates were largely unchanged in White participants after adjustment for echocardiographic measures.
Table 3.
ORs (95% CIs) of NSVT With Dementia, Limited to Those With Echocardiograph Measures in 2011 to 2013 in the ARIC Study
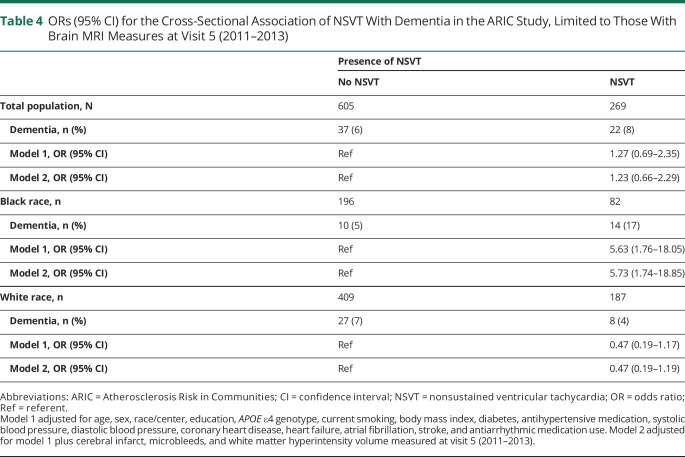
To account for cerebral vascular disease, a secondary analysis was performed that was limited to subset of participants who had a brain MRI at visit 5. These results are presented in table 4. Further adjustment for the variables of cerebral infarcts, microbleeds, and WMH volume had little effect on the estimates of association between NSVT and dementia; however, power was very limited in this subset analysis.
Table 4.
ORs (95% CI) for the Cross-Sectional Association of NSVT With Dementia in the ARIC Study, Limited to Those With Brain MRI Measures at Visit 5 (2011–2013)
Premature Ventricular Contractions
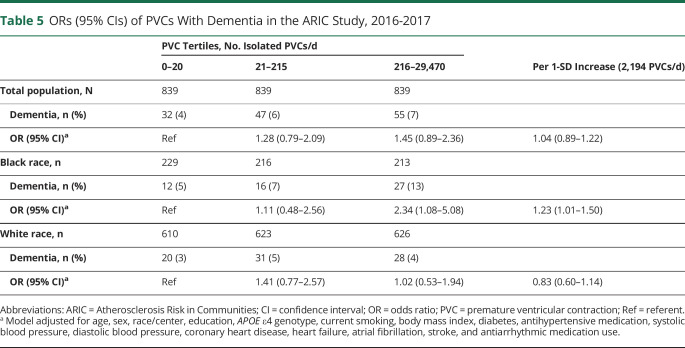
In this elderly cohort, 98.9% of the participants had PVCs detected by the Zio XT Patch. The mean (SD) number per day was 716 (2,194) and the median (25th-75th percentile) was 64 (9.99–406). The mean (SD) in Black participants was 648 (2,183) and in White participants was 740 (2,197). In Black participants, for every 1% increase in the number of beats that were PVCs, the odds of dementia was 2 times higher (OR [95% CI] 2.03 [1.14–3.60]). There was no association in White participants. Results by PVC tertiles are presented in table 5. Overall, there was no significant association between PVCs and dementia. However, there was a significant interaction by race (p = 0.04). Black participants in the highest tertile burden of PVCs had a 2.34 higher odds (95% CI 1.08–5.08) of dementia compared to those in the lowest tertile burden. Interaction by sex was not significant. In a sensitivity analysis limited to those without prevalent CVD, the estimates were similar.
Table 5.
ORs (95% CIs) of PVCs With Dementia in the ARIC Study, 2016-2017
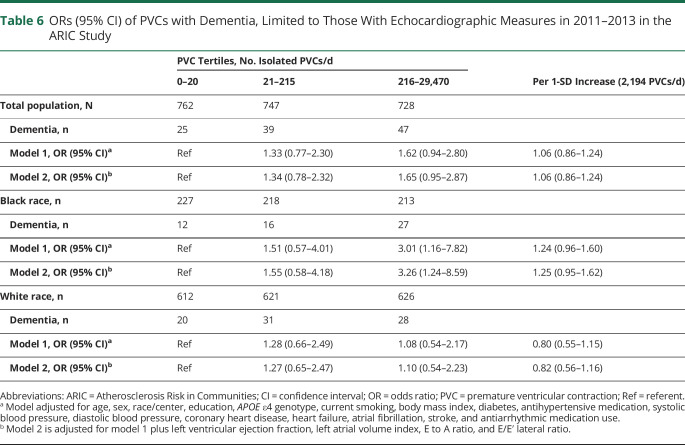
The association of PVCs with dementia limited to those with echocardiographic variable measures is presented in table 6. Associations were similar to the main analysis with further adjustment for echocardiographic variables.
Table 6.
ORs (95% CI) of PVCs with Dementia, Limited to Those With Echocardiographic Measures in 2011–2013 in the ARIC Study
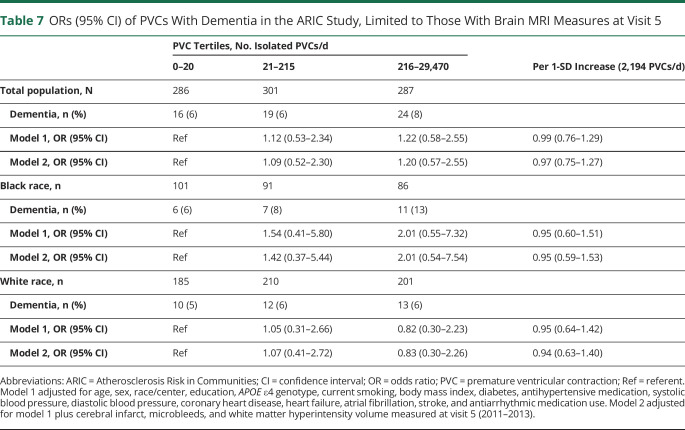
The results for a secondary analysis limited to subset of participants who had a brain MRI at visit 5 are presented in table 7. Further adjustment for the variables of cerebral infarcts, microbleeds, and WMH volume had little effect on the estimates of association between PVCs and dementia; however, power was very limited in this subset analysis.
Table 7.
ORs (95% CI) of PVCs With Dementia in the ARIC Study, Limited to Those With Brain MRI Measures at Visit 5
Discussion
In this population-based cross-sectional study of Black and White individuals, we observed the following principal findings: (1) the prevalence of NSVT in an elderly population (mean age 79 years) was high (31%), with a similar prevalence in White and Black participants; and (2) the association of NSVT and PVCs with dementia was modified by race. Presence of NSVT and higher burden of NSVT and PVCs were independently associated with dementia in elderly Black but not White individuals.
Limited information is available from general population studies about the prevalence of ventricular arrhythmias with extended recording, both overall and by race. The prevalence rates of NSVT and PVCs in our study in ARIC are comparable to those in a similar study that was conducted in Multi-Ethnic Study of Atherosclerosis (MESA), a community-based study with a selected sample of older individuals (mean age 75 years in MESA) who wore the Zio XT Patch. We report an overall prevalence of 31% with NSVT and 98.9% with PVCs, whereas MESA reported a prevalence of 34.6% with NSVT and 99.5% with PVCs.31 In our study, the prevalence of NSVT was similar in White and Black participants (31% and 30%, respectively) on the basis of up to 2 weeks of continuous ECG recording. Previous studies, including those at prior ARIC visits,6,8 have indicated that PVCs are more prevalent in Black individuals compared to White individuals4,32,33; however, we did not find that to be the case in our study because White participants had, on average, ≈90 more PVCs per day. One reason for the discrepancy could be that in previous ARIC studies, the prevalence was reported in middle-aged populations with a 2-minute rhythm ECG, whereas this study consists of an elderly population and uses 2-week ambulatory monitoring.
Our study advances the field on several fronts. We evaluated the relationship of ventricular arrhythmias to dementia in a community-based cohort who used continuous ECG monitoring. We observed a race-based difference in the association: in elderly Black but not in White participants, presence of NSVT and higher burden of NSVT and PVCs were associated with higher odds of dementia. These associations were independent of concomitant cardiovascular conditions and cardiac structure and function. This consideration is critical because cardiovascular conditions such as CHD, HF, AF, valvular disorder, aortic stiffening, left ventricular hypertrophy, and left ventricular systolic/diastolic dysfunction34–39 are associated with brain hypoperfusion and cognitive impairment. These conditions can lower cardiac output and with time will reduce regional cerebral blood flow.40–42 It has been shown that even a subtle but persistent drop in cardiac output can affect cerebral perfusion homeostasis and increase the risk of cognitive decline in the elderly.41,42
Several mechanisms may explain the relationship of ventricular arrhythmias to dementia. First, subclinical cerebrovascular disease could potentially explain the relationship of ventricular arrhythmias to dementia. To explore this possibility, we evaluated a subset of participants who wore the Zio XT Patch at visit 6 and who had brain MRI measures at visit 5. Those with NSVT had a higher prevalence of cerebral infarcts and higher volumes of WMH compared to those without NSVT. Our findings in this limited subset were similar to findings in the broader sample: ventricular arrhythmias were associated with higher odds of dementia in Black people. In addition, the effect estimates were minimally affected after adjustment for markers of subclinical cerebrovascular disease. Thus, these findings suggest that subclinical cerebrovascular disease does not completely explain the relationship of ventricular arrhythmias to dementia. Second, NSVT causes ventricular dyssynchrony and may contribute to mechanical abnormalities in the left atrial appendage, which might explain the potential for enhanced stroke/thromboembolic events and cognitive impairment. Furthermore, ventricular dyssynchrony asymmetrically increases wall thickness and alters blood flow in the myocardium,43 which could lead to cerebral hypoperfusion and cognitive decline.44,45 Although we controlled for echocardiogram measures in a sensitivity analysis, it is possible that we are not capturing all functional measures of the heart. Third, changes in heart rate, blood pressure, and stroke volume during and after PVCs—a consequence of changes in ventricular filling, ventricular contractility, and baroreflex activity—are well described, and reductions in blood flow to the brain may contribute to dementia and cognitive impairment.44,45 Moreover, frequent PVCs are associated with impaired ventricular relaxation and have the potential to remodel the heart.46–48 Finally, ventricular arrhythmias could be related to many reported noncardiac systemic diseases, including metabolic, liver disease, and electrolyte imbalance,1 which could affect cognitive function, although we adjusted for many of these contributing factors in our analysis.
The race-based difference in association warrants further explanation. There are several potential considerations: (1) differences in severity of ventricular arrhythmias, (2) variability at the brain tissue level in response to ventricular arrhythmias, and (3) differences in genetic susceptibility to the effect of ventricular arrhythmias. In regard to the first consideration, we evaluated NSVT and PVC burden to account for severity of ventricular arrhythmias. However, we could not account for the duration of exposure to NSVT and high PVC burden because we do not have data on the onset of ventricular arrhythmias. Whether the onset of ventricular arrhythmias (hence, longer duration of exposure) is earlier in Black than White individuals is unknown. With respect to the last 2 considerations, previous ARIC studies have shown that certain risk factors such as diabetes may differentially affect the brain in Black vs White individuals.14 Whether ventricular arrhythmias have greater effect on cardiac hemodynamics and brain perfusion in Black than White people is unknown and deserves further attention. Due to the cross-sectional nature of this study, we cannot rule out a bidirectional relationship whereby cognitive or autonomic dysfunction results in a higher risk of cardiac arrhythmias.
Strengths of this study include the large number of Black and White men and women from a community-based sample of elderly participants, the excellent analyzable time achieved with the monitoring device, and the rigorous adjudication of dementia in ARIC. Our analysis is not without limitations. First, our analysis is cross-sectional; therefore, temporality of conditions cannot be established. Next, we do not have echocardiographic measures of atrial and ventricular function at the time of the Zio XT Patch and cognitive assessment. However, we adjusted for echocardiographic measures at visit 5, which was only ≈4 years before visit 6, and associations remained significant. Third, Black participants in this study are located mainly in Jackson, MS (91%), which has a high rate of CHD, stroke, and vascular risk factors. Although we are underpowered to detect a site-based difference, the estimates of association in Black participants at the Jackson site appeared similar to that in Black participants at the Forsyth County, North Carolina, site, where both White and Black individuals have been included. Finally, although we adjusted extensively for potential confounders such as cardiovascular risk factors and conditions, we cannot completely exclude residual confounding as an explanation for our observations.
In this population-based cross-sectional study of elderly Black and White men and women, both the presence of NSVT and a higher burden of NSVT and PVCs were independently associated with dementia in elderly Black participants. These associations remained significant after adjustment for many cardiovascular risk factors, including echocardiographic variables of left atrial and left ventricular function. The mechanisms of this novel association are unclear and warrant further investigation. Further research with a larger sample size and a prospective design is warranted.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- AF
atrial fibrillation
- ARIC
Atherosclerosis Risk in Communities
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, 5th edition
- HF
heart failure
- ICD
International Classification of Diseases
- LAVI
left atrial volume index
- LVEF
left ventricular ejection fraction
- MESA
Multi-Ethnic Study of Atherosclerosis
- NSVT
nonsustained ventricular tachycardia
- OR
odds ratio
- PVC
premature ventricular contraction
- WMH
white matter hyperintensity
Appendix. Authors
Study Funding
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data are collected by U01 2U01HL096812, U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 from the NIH (National Heart, Lung, and Blood Institute, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders) and with previous brain MRI examinations funded by R01-HL70825 from the National Heart, Lung, and Blood Institute. This work was also supported by grants from the National Heart, Lung, and Blood Institute (R01HL126637-01A1 [L.Y.C.], R01HL141288 [L.Y.C.], K24 HL148521 [A.A.], T32HL007779 [M.R.R.], and T32HL007024 [M.R.R.]), the American Heart Association (16EIA26410001 [A.A.]), and the National Institute on Aging (K24 AG052573 [R.F.G.]).
Disclosure
F.L. Norby, A. Alonso, M.R. Rooney, A. Maheshwari, R.J. Koene, M. Zhang, E.Z. Soliman, L.R. Loehr, and T. Mosley report no disclosures relevant to the manuscript. R.F. Gottesman is an associate editor for Neurology. She reports no other disclosures relevant to the manuscript. J. Coresh and L.Y. Chen report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Katritsis DG, Zareba W, Camm AJ. Nonsustained ventricular tachycardia. J Am Coll Cardiol 2012;60:1993–2004. [DOI] [PubMed] [Google Scholar]
- 2.Frishman WH, Heiman M, Karpenos A, et al. Twenty-four-hour ambulatory electrocardiography in elderly subjects: prevalence of various arrhythmias and prognostic implications (report from the Bronx Longitudinal Aging Study). Am Heart J 1996;132:297–302. [DOI] [PubMed] [Google Scholar]
- 3.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med 1992;117:990–996. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Heiss G, Rautaharju PM, Shahar E, Massing MW, Simpson RJ Jr. Premature ventricular complexes and the risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2010;41:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ofoma U, He F, Shaffer ML, Naccarelli GV, Liao D. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc 2012;1:e002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013;80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev 2008;18:223–254. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, D'Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez J, Williams OA. A decade of racial and ethnic stroke disparities in the United States. Neurology 2014;82:1080–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2017 [online]. Available at: cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf. Accessed March 8, 2020.
- 12.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic and Racial Differences in Stroke study. Stroke 2006;37:1171–1178. [DOI] [PubMed] [Google Scholar]
- 13.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain aging in African-Americans: the Atherosclerosis Risk in Communities (ARIC) experience. Curr Alzheimer Res 2015;12:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LY, Lopez FL, Gottesman RF, et al. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: the Atherosclerosis Risk in Communities study. Stroke 2014;45:2568–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LY, Agarwal SK, Norby FL, et al. Persistent but not paroxysmal atrial fibrillation is independently associated with lower cognitive function: ARIC study. J Am Coll Cardiol 2016;67:1379–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A, Knopman DS, Gottesman RF, et al. Correlates of dementia and mild cognitive impairment in patients with atrial fibrillation: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J Am Heart Assoc 2017;6:e006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives: the ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Rooney MR, Soliman EZ, Lutsey PL, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC study. Circ Arrhythmia Electrophysiol 2019;12:e007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's Disease. Alzheimers Demen 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. DSM_5: Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 23.Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015;38:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea: validation of a scoring test for clinical-epidemiological use: the study of men born in 1913. Eur Heart J 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 25.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 26.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years' experience. J Clin Epidemiol 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 27.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in Whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities-Neurocognitive Study. Stroke 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heckbert SR, Austin TR, Jensen PN, et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the Multi-Ethnic Study of Atherosclerosis. J Electrocardiol 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerola T, Dewland TA, Vittinghoff E, Heckbert SR, Stein PK, Marcus GM. Modifiable predictors of ventricular ectopy in the community. J Am Heart Assoc 2018;7:e010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal SK, Simpson RJ, Jr., Rautaharju P, et al. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2012;109:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol 2014;11:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flicker L. Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clin Geriatr Med 2010;26:17–27. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso A, Mosley TH Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009;80:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norby FL, Chen LY, Soliman EZ, Gottesman RF, Mosley TH, Alonso A. Association of left ventricular hypertrophy with cognitive decline and dementia risk over 20 years: the Atherosclerosis Risk in Communities-Neurocognitive Study (ARIC-NCS). Am Heart J 2018;204:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson AL, Beiser AS, Himali JJ, et al. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation 2015;131:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bursi F, Rocca WA, Killian JM, et al. Heart disease and dementia: a population-based study. Am J Epidemiol 2006;163:135–141. [DOI] [PubMed] [Google Scholar]
- 42.Oh JE, Shin JW, Sohn EH, et al. Effect of cardiac function on cognition and brain structural changes in dementia. J Clin Neurol 2012;8:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis 2006;49:26–41. [DOI] [PubMed] [Google Scholar]
- 44.de la Torre J. The vascular hypothesis of Alzheimer's disease: a key to preclinical prediction of dementia using neuroimaging. J Alzheimers Dis 2018;63:35–52. [DOI] [PubMed] [Google Scholar]
- 45.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topaloglu S, Aras D, Cagli K, et al. Evaluation of left ventricular diastolic functions in patients with frequent premature ventricular contractions from right ventricular outflow tract. Heart Vessels 2007;22:328–334. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003;19:295–299. [DOI] [PubMed] [Google Scholar]
- 48.Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 2005;45:1259–1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ARIC data are available through the NIH National Heart, Lung, and Blood Institute–sponsored Biologic Specimen and Data Repository Information Coordinating Center at biolincc.nhlbi.nih.gov/